Abstract
The establishment of persisting Helicobacter pylori infection in laboratory animals has been difficult, but in 1996 Hirayama reported the development of a successful Mongolian gerbil model. The present study was undertaken with two aims: to better characterize the normal histological structure and histochemical properties of the gastric mucosa of the Mongolian gerbil; and to evaluate the progression of the histopathological features of H. pylori-induced gastritis in this animal model for one year after the experimental infection. Seventy-five Mongolian gerbils were used. Mongolian gerbils were sacrificed at 2, 4, 8, 12, 26, 38, and 52 weeks after H. pylori inoculation. Sections prepared from stomachs immediately fixed in Carnoy’s solution were stained with hematoxylin and eosin and Alcian blue at pH 2.5/periodic acid-Schiff, a dual staining consisting of the galactose oxidase-cold thionin Schiff reaction and paradoxical Concanavalin A staining, and with immunostaining for H. pylori and BrdU. H. pylori infection induced in the Mongolian gerbil a chronic active gastritis, in which a marked mucosal infiltration of neutrophils on a background of chronic inflammation became detectable 4 weeks after inoculation and continued up to 52 weeks. Intestinal metaplasia and gastric ulcers appeared after 26 weeks in some of the animals, whereas others developed multiple hyperplastic polyps. The Mongolian gerbil represents a novel and useful model for the study of H. pylori-induced chronic active gastritis and may lend itself to the investigation of the epithelial alterations that lead to intestinal metaplasia and gastric neoplasia.
Helicobacter pylori is the most important etiological agent of chronic active gastritis and peptic ulcer disease. H. pylori infection is also epidemiologically related to gastric carcinoma and has been implicated in the pathogenesis of primary gastric marginal zone lymphomas (also known as lymphomas of the mucosa-associated lymphoid tissue).
The pathogenic mechanisms leading from chronic active inflammation of the gastric mucosa to the development of the epithelial and lymphoid alterations that may result in ulceration, metaplasia, cancer, and lymphoma remain poorly understood. Progress in our understanding of these areas has been hampered in part by the lack of a well-characterized animal model. Several animal models have been developed to help understand the pathogenesis of H. pylori infection. 1,2,3,4,5 However, none of these models has been found to reproduce completely either the basic features of human H. pylori infection (an intense, mostly antral or diffuse chronic active gastritis), its later complications (mucosal atrophy and intestinal metaplasia), or its associated diseases (peptic ulcer, gastric carcinoma, and lymphoma).
In an effort to develop a model that would parallel as closely as possible the course of the infection as it occurs in the human host, Hirayama et al, 6 Matsumoto et al, 7 and Takahashi et al 8 inoculated a human strain of H. pylori into the stomach of Mongolian gerbils. After a short period most H. pylori-infected Mongolian gerbils developed chronic active gastritis. Later, intestinal metaplasia and chronic gastric ulcer developed in some of the infected Mongolian gerbils.
More recently, Tatematsu et al 9 have established a Mongolian gerbil model in which gastric carcinogenesis is induced by N-methyl-N-nitorosourea (MNU), and Sugiyama et al 10 have demonstrated that H. pylori has both co-initiating and promoting effects on MNU-induced gastric carcinogenesis in this model. However, the evolution of the histopathological changes taking place in the gastric mucosa of experimentally infected Mongolian gerbils has not been described in a systematic fashion.
The present study was undertaken with two related aims. The first was to better characterize the histological and histochemical properties of the gastric mucosa of the normal Mongolian gerbil. The second goal was to describe the chronological sequence of histopathological changes occurring in this animal model after experimental infection with H. pylori. Our results indicate that both the histopathological and histochemical alterations found in the inflamed mucosa of experimentally infected Mongolian gerbils closely resemble those found in the H. pylori-infected human stomach. Therefore, the Mongolian gerbil may represent a useful model improve our understanding of the pathogenesis of H. pylori-related human gastric disease.
Materials and Methods
Animals
The study was approved by the Animal Experiment Committee of Shinshu University School of Medicine. Seventy-five 7-week-old, specific-pathogen-free male Mongolian gerbils (MGS/Sea, Seac Yoshitomi, Fukuoka, Japan) were used in this study. They were housed in an air-conditioned biohazard room designed for infectious animals, with a 12-hour light/12-hour dark cycle. They were given food (CE-2, Clea Japan, Tokyo) and water ad libitum. The animals were divided into nine groups consisting of two control groups and seven H. pylori-inoculated groups (Table 1) ▶ .
Table 1.
Summary of Pathological Responses in Mongolian Gerbils Infected with H. pylori During First 12 Weeks
| Age (weeks) | Length of coloni- zation (weeks) | No. of gerbils | Body weight (g) | Antibody titer (AI) | Fundic mucosa/ Total mucosa (%) | Antrum | Corpus | Poly | Mono | Number of intestinal metaplasia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BrdU index | Proliferative zone thickness/Mucosal thickness (%) | Mucosal thickness (mm) | SMGL (mm) | BrdU index | Proliferative zone thickness/Mucosal thickness (%) | Mucosal thickness (mm) | SMGL (mm) | |||||||||
| 7 | 0 | 5 | 30 | 0.3 | 62.1 | 9 | 10 | 0.29 | 0.07 | 2 | 5 | 0.66 | 0 | 0 | 0 | 0 |
| 9 | 2 | 10 | 42.2 | 0.3 | 61.6 | 13 | 21 | 0.2 | 0.1 | 5 | 8 | 0.57 | 0.05 | 0.5 | 1.2 | 0 |
| 11 | 4 | 10 | 61.4 | 1.1 | 50.5 | 37 | 70 | 0.74 | 0.34 | 10 | 8 | 0.7 | 0.04 | 1.4 | 2 | 0 |
| 15 | 8 | 10 | 63.2 | 15.7 | 31.9 | 28 | 64 | 0.9 | 0.2 | 6 | 15 | 0.65 | 0.11 | 2.4 | 2.8 | 0 |
| 19 | 12 | 10 | 38.9 | 38.9 | 27.7 | 33 | 71 | 0.73 | 0.41 | 6 | 17 | 0.76 | 0.01 | 2.6 | 3 | 0 |
AI, arbitrary index; BrdU, 5′-bromo-2′deoxyuridine; Poly, polymorphonuclear neutrophil; Mono, mononuclear cell; SMGL, surface mucous gel layer.
Bacterial Strains and Inoculation
The bacterial strains used in this study were H. pylori ATCC43504 (American Type Culture Collection, Manassas, VA). This strain was grown in Brucella broth (Becton Dickinson, Cockeysville, MD) containing horse serum 10% v/v for 40 hours at 35°C under microaerobic conditions (15% CO2) and high humidity, with shaking at 150 rpm. Each animal was subjected to a 24-hour fasting period before being administered, by means of a feeding needle, a single intragastric dose of a 1 × 10 9 CFU/ml suspension containing H. pylori in Brucella broth. Four hours after administration, animals were again allowed free access to water and food.
Time Course and Sacrifice
Infected gerbils were killed by cervical dislocation at 2, 4, 8, 12, 26, 38, and 52 weeks after H. pylori inoculation. Noninfected control gerbils were killed at 7 weeks of age (when the other gerbils were inoculated with H. pylori) or at 33 weeks of age (to serve as controls for the infected animals 26 weeks after H. pylori inoculation). At each time point the body weight of all gerbils was measured. Thirty minutes before being sacrificed, gerbils were given 200 mg/kg of 5′-bromo-2′deoxyuridine (BrdU) intraperitoneally.
Cultures
At necropsy, the stomachs were opened along the greater curvature, beginning at the gastroesophageal junction and ending at the proximal portion of duodenum, and observed macroscopically. A piece of gastric mucosa (1 mm2) obtained from the posterior wall of the antrum of each gerbil was used for culture of H. pylori. These fragments were minced with Brucella broth and placed on H. pylori plate (Eiken Kagaku, Tokyo). Cultures were incubated under microaerobic condition and high humidity at 35°C for seven days. The remaining portions of the stomach were fixed as described below.
Serology
Before sacrifice, blood samples were obtained from the orbital plexus using hematocrit tubes. Using sera thus obtained, anti-H. pylori IgG antibody was measured by the enzyme-linked immunosorbent assay (GAP-IgG, Biomerica, Newport Beach, CA). An ELISA system was developed in this laboratory to quantitate serum anti-H. pylori IgG of Mongolian gerbils using anti-Mongolian gerbil IgG antibody. The titers of antibody were expressed as arbitrary index. An arbitrary index value > 1.5 indicated the presence of H. pylori antibodies. To define this index, a reference serum was prepared by pooling the sera of several anti-H. pylori IgG-positive gerbils. The reference serum was diluted serially from 1:100 to 1:3200 with phosphate-buffered saline (pH 7.4) containing 4% bovine serum albumin, and the amount of anti-H. pylori IgG corresponding to 1:3200 was expressed as the reference arbitrary index value of 1.0. Microwell strips coated with H. pylori antigens of GAP-IgG kit (Biomerica) were used. Aliquots of 100 μl of reference serum or 1:200 of diluted serum were added to the wells and the plates incubated for 1 hour at room temperature. After washing, 100 μl of horseradish peroxidase-conjugated anti-gerbil IgG (diluted 1:1500 in phospate-buffered saline containing 0.05% Tween 20) was then added and the plates incubated for 30 minutes at room temperature. The plates were washed and incubated with 100 μl of substrate (3,3′,5,5′-tetramethylbenzisine 0.35 mg, H2O2 0.15 mg/ml) for 10 minutes. After stopping the reaction with 1N HCl, the color was read at 450 nm. The anti-H. pylori IgG value of each serum was determined from a standard curve of calibrators.
Histopathology and Immunohistochemistry
For histological examination, immediately after collection the stomachs were placed in Carnoy’s solution for two hours at 4°C, taking special care not to disturb the surface mucous gel layer (SMGL). Stomachs were then sliced along the longitudinal axis into 9 slips of equal width except for stomachs obtained at 52 weeks, which were considerably enlarged and were therefore sliced into 18 slips. All tissue sections were dehydrated in 100% ethyl alcohol, cleared in xylene, and embedded in paraffin. For histological and immunohistochemical examinations, serial paraffin sections 3 μm thick were prepared. They were stained with hematoxylin and eosin (H&E) for morphological observation, and with Alcian blue at pH 2.5/periodic acid-Schiff (AB/PAS) for the detection of mucin-containing cells. The high iron-diamine stain (HID), which stains sulfated mucins dark brown, has been a useful marker for incomplete intestinal metaplasia in human stomachs. However, our pilot study showed that in Mongolian gerbils HID-positive mucins were present in pyloric gland mucous cells, surface mucous cells in the antrum, and some mucous neck cells in the fundic mucosa close to cardia. Therefore, the HID stain could not be used reliably to distinguish complete from incomplete intestinal metaplasia in the gastric mucosa of gerbils.
To acquire some insights into the biochemical alterations of mucous cells, tissue sections were stained by a previously described dual staining consisting of the galactose oxidase-cold thionin Schiff reaction (GOTS) and the paradoxical Concanavalin A staining (PCS). This method was used to distinguish two types of gastric mucins, surface mucous cell-mucin and gland mucous cell-mucin. 11 In addition, tissue sections were immunostained for H. pylori by using the indirect immunoperoxidase method, with rabbit anti-H. pylori polyclonal antibody (1:20, DAKO, Glostrup, Denmark), and peroxidase-labeled polyclonal anti-rabbit IgG antibody (1:50, DAKO). Furthermore, BrdU labeling (an indicator of epithelial proliferation) was visualized by using mouse monoclonal anti-BrdU antibody (1:50, DAKO) as described previously. 12
To evaluate the relative ratio of fundic-type to pyloric-type mucosa, the length of each type of mucosa was measured on H&E-stained slides of the 9 or 18 tissue sections obtained from the stomach of each gerbil. The thickness of the SMGL and the mucosa and the labeling indices in BrdU-stained slides were measured at 10 different arbitrarily selected points in the mid-antrum and mid-body. Because the inflammatory component of the mucosa was virtually identical to that found in human H. pylori gastritis, the degree of inflammation could be graded according to the Updated Sydney System. 13
Statistical significance was evaluated by Student’s t-test after analysis of variance. P values <0.01 were considered to be significant. Results are expressed as the mean.
Results
The serial changes of body weight are shown in Tables 1, 2, and 3 ▶ ▶ ▶ . As described below, two groups were observed 26 weeks after H. pylori inoculation. One group consisted of animals that had developed ulcers and will be collectively referred to as the ulcer group. The other group consisted of animals that had developed hyperplastic polyps without ulcer and is referred to as the hyperplastic group. In the ulcer group, the average body weight remained almost unchanged from 12 weeks to 52 weeks, whereas it increased steadily in the hyperplastic group. The body weight of gerbils in hyperplastic group was significantly higher than those in ulcer group (P < 0.01).
Table 2.
Summary of the Pathological Responses in Mongolian Gerbils of Ulcer Group Infected with H. pylori from 33 to 59 Weeks
| Age (weeks) | Length of coloni- zation (weeks) | No. of gerbils | Body weight (g) | Antibody titer (AI) | Fundic mucosa/ Total mucosa (%) | Antrum | Corpus | Poly | Mono | Number of intestinal metaplasia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BrdU index | Proliferative zone thickness/Mucosal thickness (%) | Mucosal thickness (mm) | SMGL (mm) | BrdU index | Proliferative zone thickness/Mucosal thickness (%) | Mucosal thickness (mm) | SMGL (mm) | |||||||||
| 33 | 26 | 2 | 80.7 | 648 | 29.6 | 28 | 49 | 0.45 | 0.07 | 25 | 19 | 1.14 | 0.07 | 2 | 2.5 | 3.5 |
| 45 | 38 | 1 | 76.6 | 746.6 | 28 | 30 | 75 | 0.43 | 0.13 | 27 | 18 | 1.02 | 0.08 | 2 | 3 | 4 |
| 59 | 52 | 2 | 72.8 | 491.6 | 29.8 | 17 | 94 | 0.55 | 0.19 | 3 | 12 | 0.98 | 0.07 | 2 | 3 | 7 |
AI, arbitrary index; BrdU, 5′-bromo-2′deoxyuridine; Poly, polymorphonuclear neutrophil; Mono, mononuclear cell; SMGL, surface mucous gel layer.
Table 3.
Summary of the Pathological Responses in Mongolian Gerbils of Hyperplastic Group Infected with H. pylori from 33 to 59 Weeks
| Age (weeks) | Length of coloni- zation (weeks) | No. of gerbils | Body weight (g) | Antibody titer (AI) | Fundic mucosa/ Total mucosa (%) | Antrum | Corpus | Poly | Mono | Number of intestinal metaplasia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BrdU index | Proliferative zone thickness/Mucosal thickness (%) | Mucosal thickness (mm) | SMGL (mm) | BrdU index | Proliferative zone thickness/Mucosal thickness (%) | Mucosal thickness (mm) | SMGL (mm) | |||||||||
| 33 | 26 | 3 | 95.9 | 182.7 | 28.7 | 23 | 65 | 1.17 | 0.09 | 7 | 12 | 0.98 | 0.03 | 1.7 | 1.7 | 1.7 |
| 45 | 38 | 4 | 106.1 | 209.2 | 28 | 25 | 72 | 1.52 | 0.25 | 6 | 12 | 1.1 | 0.05 | 1.7 | 1.3 | 10.3 |
| 59 | 52 | 13 | 116.2 | 168.8 | 24.3 | 24 | 89 | 1.63 | 0.21 | 3 | 11 | 1.18 | 0.06 | 1.5 | 1.8 | 11.8 |
AI, arbitrary index; BrdU, 5′-bromo-2′deoxyuridine; Poly, polymorphonuclear neutrophil; Mono, mononuclear cell; SMGL, surface mucous gel layer.
Bacteriology
Bacteriological examination showed no detectable H. pylori in control gerbils and in the infected animals at two weeks after inoculation. H. pylori grew from all 10 experimentally inoculated gerbils at 4 weeks, in 9 of 10 gerbils at both 8 and 12 weeks, in 4 of 5 gerbils at 26 weeks, in 2 of 3 gerbils at 38 weeks, and in 3 of 10 gerbils at 52 weeks. Overall, all gerbils in the ulcer group were positive by culture, whereas only 4 of 13 in the hyperplastic group were positive.
Serology
In both groups, the titer of anti-H. pylori IgG antibody increased constantly after inoculation. After 26 weeks the IgG antibody titer in the ulcer group became significantly higher in than that in the hyperplastic group (P < 0.01) (Tables 1, 2, and 3) ▶ ▶ ▶ .
Pathology: Macroscopic Findings
The gastric mucosa of all noninfected gerbils and of those infected for 2 weeks showed no visible changes (Figure 1a) ▶ . At 4 weeks after inoculation the antral mucosa appeared slightly expanded and thickened and was covered by abundant mucus (Figure 1b) ▶ . At 12 weeks after inoculation erosive lesions with bleeding appeared, but no ulcer was detected. From week 26 through 52 after inoculation two groups of animals could be distinguished. In one group the stomach showed ulcers, located distally close to the transitional zone between fundic and pyloric mucosa (Figure 1c) ▶ . In the other group the stomach showed many sessile hyperplastic polyps with occasional apical erosions (Figure 1d) ▶ .
Figure 1.
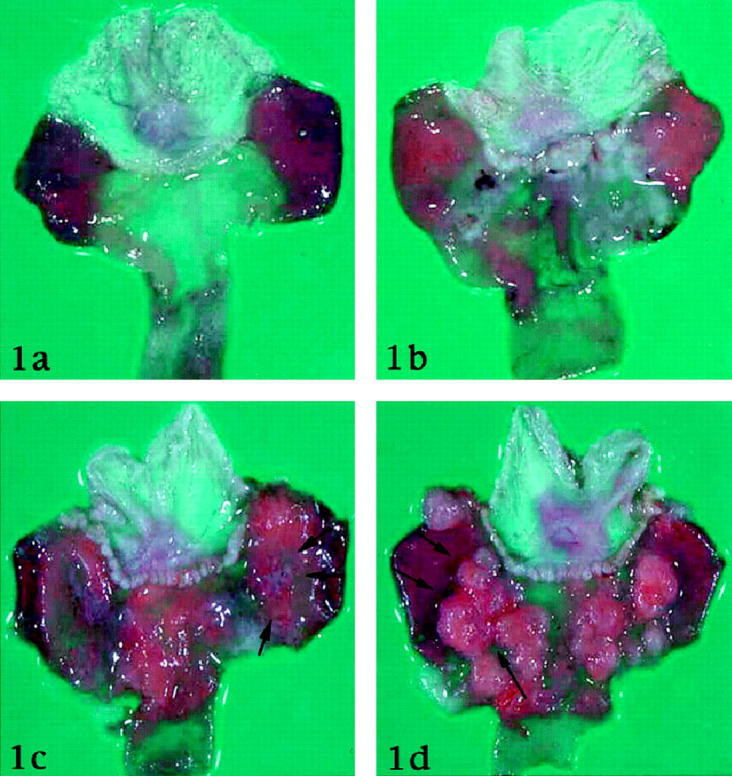
Macroscopic findings of the Mongolian gerbil stomach. a: Normal control. b. 4 weeks after inoculation of H. pylori. Antral mucosa slightly expands. c: 26 weeks after inoculation of H. pylori. Ulcer is formed at the border between pyloric mucosa and fundic mucosa (arrows). d: 26 weeks after inoculation of H. pylori. Many sessile polyps are found around the border between pyloric mucosa and fundic mucosa (arrows).
Pathology: Histological Findings
The gastric mucosa of normal Mongolian gerbils resembles that of other rodents and consists of a forestomach lined by squamous epithelium and a glandular stomach. The mucosa of the glandular stomach consists of cardiac, fundic, and pyloric mucosa (Figures 2a, 2b, and 2c ▶ , respectively). In our control animals the fundic mucosa contained psammoma-like small particles in the lamina propria (Figure 2b) ▶ . Inflammatory cell infiltration in the lamina propria was almost negligible (Figures 2a, 2b, and 2c) ▶ . AB/PAS staining revealed that surface mucous cells contained neutral mucins, regardless of the region in the stomach, and that the mucins in the gland mucous cell mucins were acid. The reactivity properties to GOTS/PCS of gerbils’ stomach resembled those of the gastric mucosa of other mammals (Figure 2d) ▶ . 11 Thus, surface mucous cells stained blue with GOTS and gland mucous cells stained brown with PCS (Figure 2d) ▶ . The mucosal surface was mostly covered by a thin layer of mucus in which gland mucous cell mucins predominated and surface mucous cell mucins were hardly identified (Figure 2d) ▶ . Immunostaining for BrdU showed that most reactive cells were located at the neck portion of glands. BrdU labeling indices in the normal mucosa were 2% in the mid-fundic mucosa, and 9% in the mid-pyloric mucosa (Table 1) ▶ .
Figure 2.
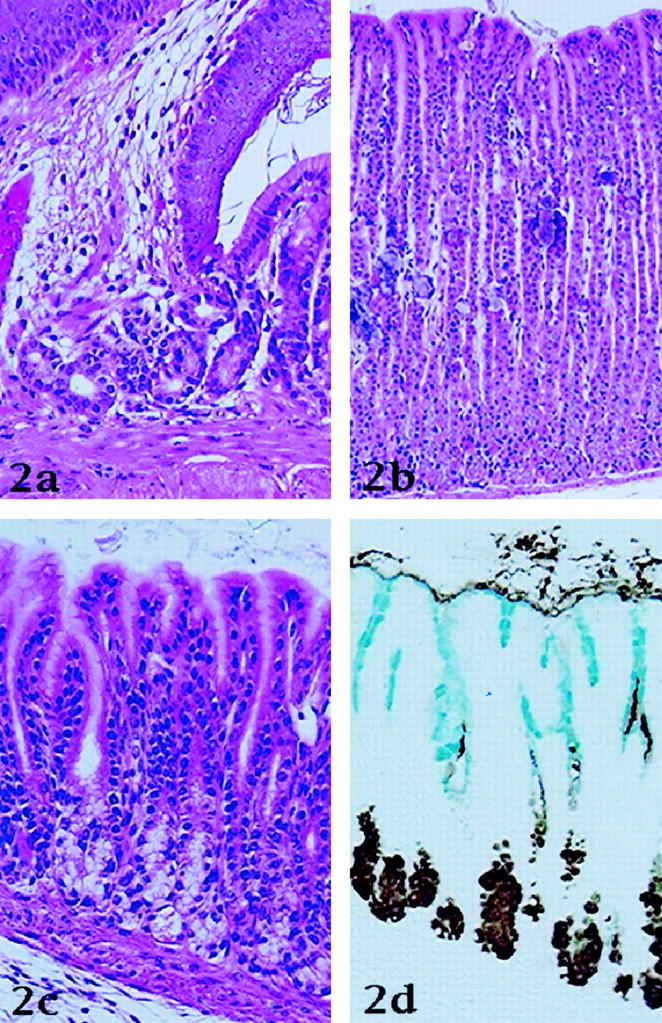
Histology of normal stomach of Mongolian gerbil. a: Cardiac mucosa. Cardiac glands are recognized around the border between forestomach and gland stomach (H&E stain). b: Fundic mucosa. Many psammoma-like small particles are present in the lamina propria (H&E stain). c: Pyloric mucosa (H&E stain). d: Histochemical findings in the pyloric mucosa. In the serial section of c, surface mucous cells stain blue with galactose oxidase thionine Schiff reaction (GOTS) and pyloric gland cells stain brown with PCS (GOTS/PCS).
At 2 weeks after inoculation, the gastric mucosa showed almost no histological changes.
At 4 weeks after inoculation, as shown in Table 1 ▶ , the length of fundic mucosa decreased significantly in parallel with an expansion of the pyloric mucosa. In the transitional zone from fundic to pyloric mucosa, parietal and chief cells disappeared, leaving elongated pseudopyloric glands with small amounts of mucins (Figure 3a) ▶ . This finding was observed in all H. pylori-inoculated gerbils. Mild to moderate infiltration of inflammatory cells appeared in the lamina propria of the pyloric mucosa, especially in the proximal half along the small curvature. The inflammatory infiltrate consisted predominantly of neutrophilic polymorphonuclear cells, frequently found between epithelial cells and in aggregates within pits (foveolar microabscesses) (Figure 3b) ▶ . In all animals H. pylori was located mostly in the SMGL and in the pits; bacterial adhesion to surface mucous cells was seen only rarely (Figure 3c) ▶ . At this time, BrdU labeling indices reached their peak both in the antrum and in the corpus; in the antrum, BrdU-labeled epithelial nuclei were distributed throughout almost the entire thickness of the mucosa (Tables 1, 2, and 3) ▶ ▶ ▶ . After this time, BrdU labeling indices decreased gradually (Tables 1, 2, and 3) ▶ ▶ ▶ . However the ratio of proliferative zone to mucosal thickness continued to increase in the pyloric mucosa until the end of the experiment (Tables 1, 2, and 3) ▶ ▶ ▶ .
Figure 4.
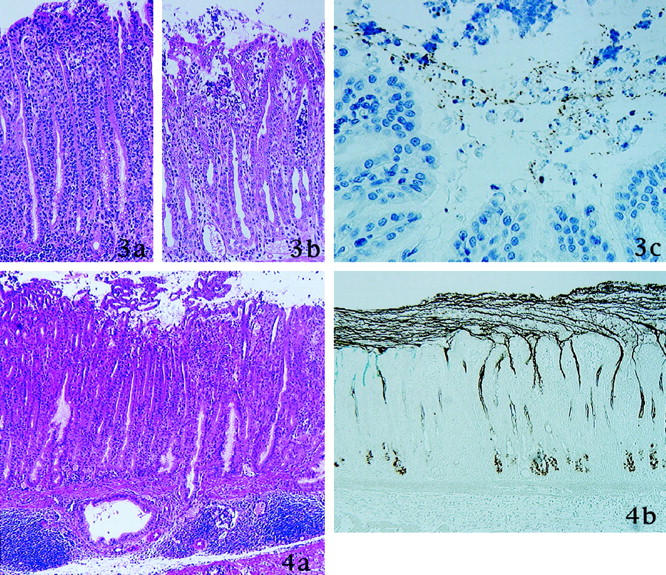
8 weeks after inoculation of H. pylori. a: Erosion is observed. Small clusters of surface mucous cells protruded into the lumen. Dilated mucous gland and accumulation of lymphocytes are present in the submucosa. b: Mucins in the surface mucous cells and in the pyloric glands are depleted markedly. Surface mucous gel layer (SMGL) was thickened and consist of two type of gastric mucins. Thin strands of mucins from pyloric gland cells are evident and join the SMGL (GOTS/PCS).
Figure 3. 4 weeks after inoculation of H. pylori. a: In the transitional zone between pylorus and fundus, elongated pseudopyloric glands emerge (H&E stain). b: Neutrophils infiltrate the epithelium and form intrafoveolar microabscess (H&E stain). c: H. pylori stained brown with immunostaining for H. pylori and are present in the surface mucous gel layer (immunoperoxidase method for H. pylori).
At 8 and 12 weeks after inoculation, histological and histochemical changes reached their peak (Tables 1, 2, and 3) ▶ ▶ ▶ . Chronic inflammation was confined to the pyloric mucosa, where small erosions were frequently observed (Figure 4a) ▶ . The erosions were mostly the size of several foveolae and were covered by a mixture of exfoliated cells and gland mucous cell mucins (Figure 4a) ▶ . In all cases examined, small clusters of surface mucous cells frequently protruded into the lumen (Figure 4a) ▶ . Dilated pyloric glands containing more neutral mucins than normal pyloric glands were frequently seen. Similar mucous glands were also located in the submucosa, surrounded by interstitium with a marked infiltration of inflammatory cells, mostly lymphocytes (Figure 4a) ▶ . Lymphoid follicles were especially conspicuous in the submucosa, but they were also found in the deep portion of the mucosa (Figure 4a) ▶ .
AB/PAS and GOTS/PCS revealed marked depletion of mucins in the surface mucous cells as well as in the glandular mucous cells in the inflamed pyloric mucosa (Figure 4b) ▶ . On the other hand, the thickness of the SMGL increased remarkably in the inflamed mucosa (Tables 1, 2, and 3) ▶ ▶ ▶ . Similarly to what we have observed in the human stomach, 14 the SMGL consisted of two types of gastric mucins and contained numerous small clusters of epithelial cells and neutrophils (Figure 4b) ▶ . Thin strands of mucins originating from the surface mucous cells and from the gland mucous cells were also detected in the SMGL (Figure 4b) ▶ .
At 26, 38, and 52 weeks after inoculation, infected Mongolian gerbils could be classified into two groups. One group (5 gerbils) had a gastric ulcer (ulcer group); the other group (20 gerbils) had hyperplastic polyps but no ulcers (hyperplastic group) (Tables 2 and 3) ▶ ▶ . The ulcers were located near the transitional zone and their depth reached to the muscularis propria. Their base consisted of granulation tissue with numerous inflammatory cells, including neutrophils, lymphocytes, plasma cells, and macrophages (Figure 5) ▶ . The mucosa surrounding the ulcers showed active inflammation of a degree comparable to that observed in animals at 8 and 12 weeks. Two large erosions, which involved the whole mucosal thickness, were observed in the vicinity of transitional mucosa between pylori and fundic mucosa. In this study, no lesions of intermediate depth that could prove the transition from superficial to deep erosions were detected. Animals in the hyperplastic group had many sessile hyperplastic polyps but a less dense inflammatory cell infiltrate, consisting mostly of lymphocytes (Figure 6) ▶ . The hyperplastic lesions occurred in more frequently in the pyloric mucosa, but could also be found in the fundic mucosa. Both foveolar and glandular epithelia contributed to the hyperplastic growth (Figure 6) ▶ , but proliferative cells labeled with BrdU were located mostly in the neck region of the hyperplastic polyps, in an area corresponding to the proliferative zone of the nonhyperplastic surrounding mucosa.
Figure 8.
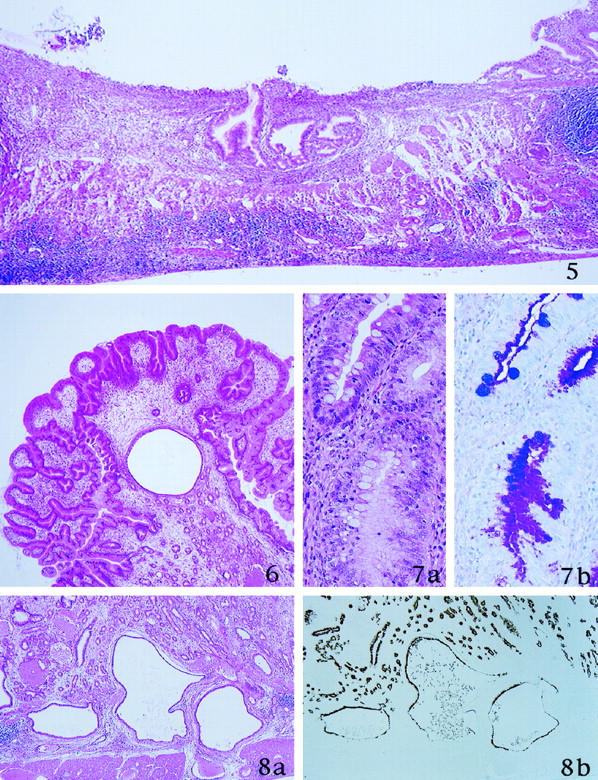
52 weeks after inoculation of H. pylori. a: Dilated mucous glands are distributed in the lamina propria and submucosa (H&E stain). b: These mucous glands stain brown with PCS (GOTS/PCS).
Figure 5. 26 weeks after inoculation of H. pylori. Ulcer is formed. Granulation tissue with inflammatory cells and necrotic materials are present in the ulcer base (H&E stain).
Figure 6. 26 weeks after inoculation of H. pylori. Histological finding of the sessile polyps around the border between pylorus and fundus. Hyperplastic foveolae with widened stromal tissue are evident (H&E stain).
Figure 7. 26 weeks after inoculation of H. pylori. a: Incomplete intestinal metaplasia is recognized. b: Metaplastic goblet cells show alcianophilia (AB/PAS stain).
Intestinal metaplasia was found in all infected gerbils at 26, 38, and 52 weeks (Figure 7) ▶ . H. pylori was absent in metaplastic glands. The metaplasia occurred frequently in the transitional zone between pyloric and fundic mucosa, and to a lesser degree in the pyloric mucosa. Intestinal metaplasia was of the incomplete type: 15 metaplastic tubules were lined by goblet cells, columnar cells, and immature absorptive cells without a well defined microvillous brush border, but consistently lacked Paneth cells (Figure 7) ▶ . Goblet cells contained acid mucins that could not be stained by GOTS/PCS. The number of metaplastic foveolae increased steadily from 26 weeks to 52 weeks (Tables 2, and 3) ▶ ▶ . This phenomenon occurred more frequently in the hyperplastic group, but the difference was not statistically significant (Tables 2 and 3) ▶ ▶ . Both groups showed numerous irregularly branched, dilated mucous glands in the lower portion of the mucosa and in the submucosa, and rarely also in the proper muscle layer (Figure 8a) ▶ . The mucous cells in these glands were cytologically bland and stained with PCS, indicating that they belonged to the gland mucous cell lineage (Figure 8b) ▶ .
Discussion
In this study we have described the histological and histochemical changes taking place in the gastric mucosa of Mongolian gerbils during the first year following their experimental inoculation with a human strain of H. pylori. The significance of these observations with regard to the potential use of this model may be best appreciated when these changes are compared with the histological and histochemical features known to occur in infected humans.
In our experimental animals marked infiltration of neutrophils on the background of chronic inflammation appeared 4 weeks after H. pylori inoculation and persisted up to 52 weeks. This indicates that in these animals, neutrophilic infiltration is not a transient phenomenon; rather, similarly to what is observed in humans, an active inflammatory activity persists for the entire duration of H. pylori infection. 16
In infected Mongolian gerbils the area occupied by pyloric-type mucosa widened as the infection progressed and the transitional zone gradually expanded up into the fundic mucosa. A similar phenomenon was reported by Kimura et al in a classic study in which patients with chronic gastritis were followed up for several years by sequential mapped gastric biopsies. 17 In a proportion of these patients the antrum expanded progressively at the expenses of the corpus, an observation that was pivotal in shaping up the concept of the atrophic border in the progression of atrophy.
Numerous superficial erosions, usually confined to the upper foveolae, appeared at 8 weeks after inoculation. A small number of larger erosions involving the whole mucosal thickness was observed at 26 weeks after inoculation, the same time the ulcers appeared. The contemporaneous appearance of these lesions suggests that deep erosions and ulcerations may represent different degrees of expression of the same phenomenon. In our study, however, we did not detect lesions of intermediate depth that might provide evidence of the transition from superficial to deep erosions.
Intestinal metaplasia appeared at 26 weeks after inoculation of H. pylori and increased steadily to the 52 weeks. Intestinal metaplasia is one of the characteristic features of chronic atrophic gastritis in the human stomach and is considered the most important risk factor for the development of intestinal-type carcinoma. The development of intestinal metaplasia has not been observed in any other animals infected either experimentally or naturally with any species of Helicobacter. Thus, the Mongolian gerbil could provide an ideal model through which to explore the histogenesis of metaplasia and its relationship with carcinogenesis. In this respect, it is interesting to note that Paneth cells were rarely found in metaplastic foci in the gerbils, indicating that the metaplasia was mostly of the incomplete type that, in humans, has been associated with an increased malignant potential. 18
After 26 weeks of infection, two different patterns of gastritis became apparent in these gerbils. The ulcer group, which consisted of a smaller number of animals, had higher anti-H. pylori antibody titers and showed more severe active inflammation, less frequent intestinal metaplasia, and lighter body weight. A larger number of animals developed hyperplastic polyps similar to those found in humans with atrophic gastritis. Animals in this group were heavier in body weight and had lower titers of anti-H. pylori antibodies. Histologically, they had a type of gastritis that might be called atrophic, with more extensive intestinal metaplasia and lesser degrees of inflammation (both active and chronic). Because the animals were all infected with the same strain of H. pylori and were subjected to identical experimental conditions, including the diet, individual host factors may have played a role in determining the different direction of the gastritis in these two groups.
The increase in proliferative activity in the mucosa is another feature of interest. At 4 weeks after inoculation, BrdU-labeled cells represented almost 40% of the total epithelial cells, whereas in the antrum they occupied almost the entire thickness of the mucosa. This phenomenon corresponded to numerous small clusters of the surface mucous cells in the SMGL and small papillary projections from the mucosal surface, indicating a marked increase of exfoliation of lining cells. Although the labeling indices began to decrease after 8 weeks, the ratio of proliferative zone to mucosal thickness continued to increase until the end of the experiment. Fan et al reported a similar increase of Ki-67-positive cells in H. pylori-associated gastritis in humans. 19 In the fundic mucosa the BrdU labeling indices increased slightly but significantly at 4 weeks after inoculation, although the inflammatory cell infiltration remained almost negligible in the corpus. One could speculate that the inflammation in the pyloric mucosa might influence the cell turnover in the fundus.
If there were many intriguing similarities between H. pylori gastritis in gerbils and humans, there were also some notable differences. Acute H. pylori infection in human adults is believed to induce acute neutrophilic gastritis, in which mucosal lesions are sometimes accompanied by multiple bleeding erosions, ulcers, and marked neutrophilic infiltration. Takahashi et al reported that initial changes caused by H. pylori infection were edema and congestion in the antrum at 1 week and that mild superficial damage occurred in the antrum without inflammatory infiltration at 2 weeks after H. pylori infection. In this study, no acute responses were observed in the gerbils when they were sacrificed 2 weeks after inoculation. One unlikely possibility is that this interval was too long and the acute lesions were missed. More plausibly, lesions may develop gradually in gerbils and there is no acute phase.
Contrary to our expectations, the numbers of H. pylori found in histological preparations from the stomach of experimentally infected Mongolian gerbils were much lower than those found in infected human gastric mucosa. In the gerbils most H. pylori was found in the SMGL; only rarely were they seen attached to the apical surface of the surface mucous cells. In the human stomach, H. pylori was first described to adhere to the apical surface of the surface mucous cells, 20 and its ability to adhere was considered an essential factor for the stable infection by H. pylori. 21 In addition, several studies using in vitro experimental systems have suggested that this attachment accelerates the production of interleukin-8, which induces neutrophil infiltration. 22,23 In the human stomach, as shown by Shimizu et al, 24 this organism actively colonizes not only the apical surface but also the SMGL. Our findings in Mongolian gerbils seem to support this notion. Studies are underway to determine whether the attachment of H. pylori is an indispensable factor to induce inflammation.
The thickness of the gastric mucosa of H. pylori-infected gerbils increased after 4 weeks and remained significantly thicker until the end of the experiments. In some human populations a considerable proportion of subjects with H. pylori chronic active gastritis develop atrophy and reduced thickness of the gastric mucosa. Before meaningful comparison can be made between the development of atrophy in humans and Mongolian gerbils, however, the respective life spans of the species must be taken into account. Most biopsy or surgical resection specimens used for studies on human H. pylori-related disease have been obtained from middle-aged or elderly patients. Because most people are infected in childhood, it is likely that atrophic changes take several decades to develop. The average life span of a Mongolian gerbil is about 3 years. 25 If a crude extrapolation can be made, seven weeks would correspond to a human age of 3 to 4 years, 26 weeks to 14 years, and 1 year to 25 years. Observation of H. pylori-inoculated Mongolian gerbils for longer than 1 year may be needed to demonstrate the development of a true chronic atrophic gastritis with intestinal metaplasia. However, changes in that direction appeared to have already developed in the group of animals with hyperplastic polyps.
In humans the SMGL is thinner in the inflamed than in the normal gastric mucosa. 24 In contrast, the thickness of the SMGL of Mongolian gerbils increased markedly between 4 and 12 weeks after infection. After 26 weeks, however, it decreased considerably, although it remained significantly thicker than that of the normal mucosa. It is also possible that in humans the earlier stages of H. pylori infection are accompanied by an increase in the thickness of the SMGL, which may then become progressively thinner as the infection enters its chronic phase. These apparent dissimilarities may be accounted for by our comparatively short observation period in the animal model (ie, the SMGL might have become thinner if the gerbils had been infected longer) or they may represent another consequence of the different life spans of Mongolian gerbils and humans.
Two possible sources of mucins contribute to form the SMGL: small clusters of exfoliated surface mucous cells provide one source; mucins secreted by surface mucous cells and gland mucous cells represents the other one. Many thin strands of mucins originating from both surface mucous cells and gland mucous cells were detected in the inflamed gastric mucosa and deposited in the thickened SMGL, thus confirming that in the infected gerbils there was hypersecretion of mucins.
Studies to enhance our understanding of the systemic and mucosal immune system of the Mongolian gerbil and its gastric function are needed to achieve a better understanding of the interaction of this host with H. pylori. As such investigations are being pursued, the relatively low cost of purchase and husbandry of the Mongolian gerbil, its susceptibility to infection with human strains of H. pylori, and the remarkably similar histopathological aspects of gastritis in the two species suggest that this animal may eventually become the model of choice for the experimental investigation of H. pylori infection.
Footnotes
Address reprint requests to Atsushi Sugiyama, M.D., The First Department of Surgery, Shinshu University School of Medicine, Asahi 3–1-1, Matsumoto 390-8621, Nagano, Japan.
References
- 1.Krakowka S, Morgan DR, Kraft WG, Leunk RD: Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun 1987, 55:2789-2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karita M, Kouchiyama T, Okita K, Nakazawa T: New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroenterol 1991, 86:1596-1603 [PubMed] [Google Scholar]
- 3.Shuto R, Fujioka T, Kubota T, Nasu M: Experimental gastritis induced by Helicobacter pylori in Japanese monkeys. Infect Immun 1993, 61:933-939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karita M, Li Q, Cantero D, Okita K: Establishment of a small animal model for human Helicobacter pylori infection using germ-free mouse. Am J Gastroenterol 1994, 89:208-213 [PubMed] [Google Scholar]
- 5.Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF: A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 1997, 112:1386-1397 [DOI] [PubMed] [Google Scholar]
- 6.Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y: Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol 1996, 31(Suppl IX):24-28 [PubMed] [Google Scholar]
- 7.Matsumoto S, Washizuka Y, Matsumoto Y, Tawara F, Ikeda F, Yokota Y, Karita M: Induction of ulceration and severe gastritis in Mongolian gerbil by Helicobacter pylori infection. J Med Microbiol 1997, 46:391-397 [DOI] [PubMed] [Google Scholar]
- 8.Takahashi S, Keto Y, Fujita H, Muramatsu H, Nishino T, Okabe S: Pathological changes in the formation of Helicobacter pylori-induced gastric lesions in Mongolian gerbils. Dig Dis Sci 1998, 43:754-765 [DOI] [PubMed] [Google Scholar]
- 9.Tatematsu M, Yamamoto M, Shimizu N, Yoshikawa A, Fukami H, Kaminishi M, Oohara T, Sugiyama A, Ikeno T: Induction of glandular stomach cancers in Helicobacter pylori-sensitive Mongolian gerbils treated with N-methyl-N-nitrosourea and N-methyl-N′-nitro-N-nitrosoguanidine in drinking water. Jpn J Cancer Res 1998, 89:97-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M: Helicobacter pylori infection enhances MNU (N-methyl-N-nitrosourea)-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res 1998, 58:2067-2069 [PubMed] [Google Scholar]
- 11.Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y: A dual staining method for identifying mucins of different gastric epithelial mucus cells. Histochem J 1991, 23:22-28 [DOI] [PubMed] [Google Scholar]
- 12.Maeyama H, Furuwatari C, Ota H, Akamatsu T, Nakayama J, Katsuyama T: Histon H3 messenger RNA in situ hybridization for identifying proliferating cells in formalin-fixed rat gastric mucosa. Histochem J 1997, 29:1-7 [DOI] [PubMed] [Google Scholar]
- 13.Dixon MF, Genta RM, Yardley JH, Correa P: Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol 1996, 20:1161-1181 [DOI] [PubMed] [Google Scholar]
- 14.Ota H, Katsuyama T: Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J 1992, 24:86-92 [DOI] [PubMed] [Google Scholar]
- 15.Inada K, Nakanishi H, Fujimitsu Y, Shimizu N, Ichinose M, Miki K, Nakamura S, Tatematsu M: Gastric and intestinal mixed and solely intestinal types of intestinal metaplasia in the human stomach. Pathol Int 1997, 47:831-841 [DOI] [PubMed] [Google Scholar]
- 16.Kimura K, Takemoto T: An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 3:87-97 [Google Scholar]
- 17.Kimura K: Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology 1972, 63:584-592 [PubMed] [Google Scholar]
- 18.Filipe MI, Munoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, Teuchmann S, Benz M, Prijon T: Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer 1994, 57:324-329 [DOI] [PubMed] [Google Scholar]
- 19.Fan XG, Kelleher D, Fan XJ, Xia HX, Keeling PW: Helicobacter pylori increases proliferation of gastric epithlial cells. Gut 1996, 38:19-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall BJ, Wallen JR: Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, i:1311-1315 [DOI] [PubMed] [Google Scholar]
- 21.Hessey SJ, Spencer J, Wyatt JI, Sobala G, Rathbone BJ, Axon AT, Dixon MF: Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut 1990, 31:134-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, Primrose JN, Lindley IJ: Interleukin-8 expression in Helicobacter pylori infected, normal and neoplastic gastroduodenal mucosa. J Clin Pathol 1994, 47:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowe SE, Alvarez L, Dytoc M, Hunt RH, Muller M, Sherman P, Oatel J, Jin Y, Ernst PB: Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology 1995, 108:65-74 [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Akamatsu T, Ota H, Katsuyama T: Immunohistochemical detection of Helicobacter pylori in the surface mucous gel layer and its clinicopathological significance. Helicobacter 1996, 1:197-206 [DOI] [PubMed] [Google Scholar]
- 25.Dillon WG, Glomski CA: The Mongolian gerbil: qualitative and quantitative aspects of the cellular blood picture. Lab Anim 1975, 9:283-287 [DOI] [PubMed] [Google Scholar]


