Abstract
Trisomy 8 and trisomy 20 are nonrandom aberrations in desmoid tumors. The presence of these trisomies in related benign fibrous lesions of bone has not been previously addressed. In this study, 22 specimens from 19 patients diagnosed with desmoid tumor, desmoplastic fibroma, periosteal desmoid tumor, osteofibrous dysplasia, or fibrous dysplasia were examined by cytogenetic analysis of short-term cultures and bi-color fluorescence in situ hybridization of cytological touch preparations or paraffin-embedded tissue with centromeric probes for chromosomes 8 and 20. Trisomy 8 and trisomy 20 were detected by molecular cytogenetic methodologies in 15 specimens, including 10 primary bone lesions. Traditional cytogenetic analysis revealed trisomy 8 in two cases of osteofibrous dysplasia. Our findings demonstrate that trisomy 8 and trisomy 20 are also nonrandom aberrations in histologically similar, but clinically distinct, benign fibrous lesions of bone.
Desmoid tumor, desmoplastic fibroma, periosteal desmoid tumor, osteofibrous dysplasia and fibrous dysplasia are benign mesenchymal lesions arising in soft tissue or bone. Desmoid tumors, also known as aggressive fibromatosis or fibromatosis of soft tissue, may occur in extra-abdominal, abdominal, or intra-abdominal locations. Desmoplastic fibroma and periosteal desmoid tumor, identical histologically, differ only by location. Desmoplastic fibroma and periosteal desmoid tumor are considered to represent the bone counterparts of desmoid tumors of soft tissue. A tumor-like proliferation of fibro-osseous tissue is characteristic of osteofibrous dysplasia and fibrous dysplasia. The former is distinguished histologically by osteoblastic rimming of the bone trabeculae. Cytogenetic and molecular cytogenetic analyses of desmoid tumors are few and are particularly sparse or are nonexistent for desmoplastic fibroma, periosteal desmoid tumor, osteofibrous dysplasia, and fibrous dysplasia. 1-17 To determine the frequency of trisomy 8 and trisomy 20 in additional desmoid tumor cases and to examine their presence in related fibrous lesions of bone, both standard karyotypic analysis and molecular cytogenetic analysis were performed on 22 representative specimens.
Materials and Methods
Cytogenetic Analysis
Twenty-two specimens (to include three desmoplastic fibromas, two periosteal desmoid tumors, nine desmoid tumors, four osteofibrous dysplasias, and four fibrous dysplasias) from 19 different patients were examined by traditional cytogenetic and molecular cytogenetic methodologies. A 0.5- to 1.0-cm 3 sample of each specimen was received for cytogenetic analysis. Standard culture and harvesting procedures were used that have been described previously. 4 Briefly, the tissues were disaggregated mechanically and enzymatically and then cultured in RPMI 1640 medium supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin-l-glutamine (Irvine Scientific, Santa Ana, CA) for 3 to 5 days. Two to four hours before harvest, cells were exposed to Colcemid (0.02 μg/ml). After hypotonic treatment (0.074 mol/L KCl for 30 minutes for flasks and 0.8% sodium citrate for 25 minutes for coverslips), the preparations were fixed three times with methanol/glacial acetic acid (3:1). Metaphase cells were banded with Giemsa trypsin. The karyotypes were expressed according to the International System for Human Cytogenetic Nomenclature 1995. 18
Fluorescence in Situ Hybridization (FISH)
FISH studies were performed using chromosome 8 and chromosome 20 specific centromeric probes (D8Z1 and D20Z1, ONCOR, Gaithersburg, MD) on cytological touch preparations or on nuclei extracted from paraffin-embedded tissue. With respect to the paraffin-embedded material, 50-μm-thick sections were deparaffinated, rehydrated, and incubated with pepsin for 10 to 20 minutes for tissue disaggregation, and the disaggregated nuclei were resuspended in 1X PBS for slide preparation.
Hybridization was performed as previously described. 19 The number of hybridization signals for each specimen was assessed in 200 interphase nuclei with strong and well delineated signals by two different individuals. To exclude the possibility of triploidy, the biotinylated probe, D7Z1, which binds to the centromeric region of chromosome 7, was used. As an additional control, normal peripheral blood lymphocytes were simultaneously hybridized with chromosomes 7, 8, and 20 probes. A specimen was interpreted as trisomic for chromosomes 8 and/or 20 if three clearly separate signals for each respective probe could be detected in greater than 10% of the cells evaluated (greater than two standard deviations above the average false positive rate).
Results
Cytogenetics
A summary of the clinical, the cytogenetic, and the molecular cytogenetic findings is provided in Table 1 ▶ . Clonal chromosomal abnormalities were detected by traditional cytogenetic methodologies in eight specimens, including three desmoid tumors (cases 5, 6, and 11), four osteofibrous dysplasias (cases 13, 14A (Figure 1) ▶ , 14B, and 15), and one fibrous dysplasia (case 16, Figure 2 ▶ ). Cytogenetic analysis revealed trisomy 8 as a clonal aberration in three osteofibrous dysplasias (cases 13, 14A, and 14B) and in a single metaphase cell of case 6 (desmoid tumor). Trisomy 20 was observed in a single metaphase cell of case 12 (desmoid tumor). Loss of 5q21-22 (gene location for familial adenomatous polyposis and Gardner syndrome) 20-22 was observed in two desmoid tumors (one patient with and one without Gardner syndrome; cases 5 and 11, respectively). Loss of 4p and rearrangement of 12q12-13 were detected in two desmoid tumor cases and a case of fibrous dysplasia (cases 6, 11, and 16, respectively).
Table 1.
Clinical Data and Cytogenetic and FISH Results
| Case | Age/sex | Diagnosis (P or R) | Karyotype | % of trisomy 8 cells | % of trisomy 20 cells | % of cells with trisomy 8 and 20 |
|---|---|---|---|---|---|---|
| 1 | 32 /M | Desmoplastic fibroma (P) | 46,XY[20] | 33 | 21 | 16 |
| 2 | 42 /M | Desmoplastic fibroma (P) | 46,XY[24] | 6 | 14 | 4 |
| 3 | 58 /F | Desmoplastic fibroma (P) | 46,XX[20] | 5 | 5 | 4 |
| 4a* | 12 /M | Periosteal desmoid tumor (P) | 46,XY[20] | 15 | 18 | 10 |
| 4b* | 12 /M | Periosteal desmoid tumor (P) | 46,XY[20] | 8 | 11 | 5 |
| 5† | 23 /M | Desmoid tumor (P) | 46,XY,del(5)(q13q33)[10]/ 46,XY[8]46,XY[8] | 7 | 20 | 4 |
| 6‡ | 30 /M | Desmoid tumor (P) | 47,XY,+mar[5]/46,XY,del(4) (p15)[2]/46,XY[29] | 15 | 25 | 8 |
| 7a* | 26 /M | Desmoid tumor (R) | 46,XY[20] | 4 | 24 | 1 |
| 7b* | 26 /M | Desmoid tumor (R) | 46,XY[14] | 5 | 7 | 3 |
| 8 | 28 /F | Desmoid tumor (P) | 46,XX[20] | 11 | 19 | 7 |
| 9 | 15 /F | Desmoid tumor (P) | 46,XX[20] | 3 | 6 | 2 |
| 10 | 42 /M | Desmoid tumor (R) | 46,XY[24] | 7 | 4 | 1 |
| 11 | 10 /F | Desmoid tumor (P) | 46,XX,der(1)t(1;12)(q25;q13), der(5)t(1;5)(q25;q13),der(12)t (5;12)(q31;q13)[15]/46,XX[5] | 3 | 2 | 3 |
| 12§ | 37 /F | Desmoid Tumor (P) | 46,XX[23] | 0 | 10 | 0 |
| 13 | 19 /M | Osteofibrous dysplasia (R) | 49,XY,+7,+8,+22[8]/46,XY[12] | 34 | <1 | <1 |
| 14a | 18 /F | Osteofibrous dysplasia (P) | 52,XX,+5,+7,+7,+8,+21,+21[10] | 27 | 8 | 5 |
| 14b | 18 /F | Osteofibrous dysplasia (R) | 52,XX,+5,+7,+7,+8,+21,+21[10] | 10 | 3 | 0 |
| 15 | 11 /M | Osteofibrous dysplasia (R) | 47,XY,+12[20] | 33 | 30 | 14 |
| 16 | 15 /M | Fibrous dysplasia (P) | 47-49,XY,−2,i(2)(p10),del (4)(p14,+6,+7,+11,add(11) (p15),der(12)inv(p13;q12) ins(12;?)(p13,?),−17,−21,+1-3mar[cp14] | 3 | 5 | 1 |
| 17 | 23 /M | Fibrous dysplasia (P) | 46,XY[20] | 3 | 14 | 2 |
| 18 | 28 /F | Fibrous dysplasia (P) | 46,XX,t(14;21)(q23;q21)[1]/46,XX[19] | 4 | 2 | 1 |
| 19¶ | 33 /M | Fibrous dysplasia (P) | 46,XY[20] | 12 | 3 | 1 |
The cytogenetic findings of cases 5, 11, 13, and 14A have been previously described. 6,15 P, primary lesion; R, recurrent lesion; M, male; F, female.
* Biopsy and definitive surgical specimens.
† Patient has Gardner syndrome.
‡ Numerous random structural abnormalities were seen in this case, including telomeric associations. Notably, trisomy 8 and a del(5)(q21q31) were observed in a single metaphase cell each.
§ Trisomy 20 was observed in a single metaphase cell.
¶ Polyostotic fibrous dysplasia; remaining cases of fibrous dysplasia (cases 16, 17, and 18 were all monostotic).
Figure 1.
Representative karyotype from case 14A (osteofibrous dysplasia) illustrating trisomy 8 as well as extra copies of chromosomes 5, 7, 21, and 22.
Figure 2.
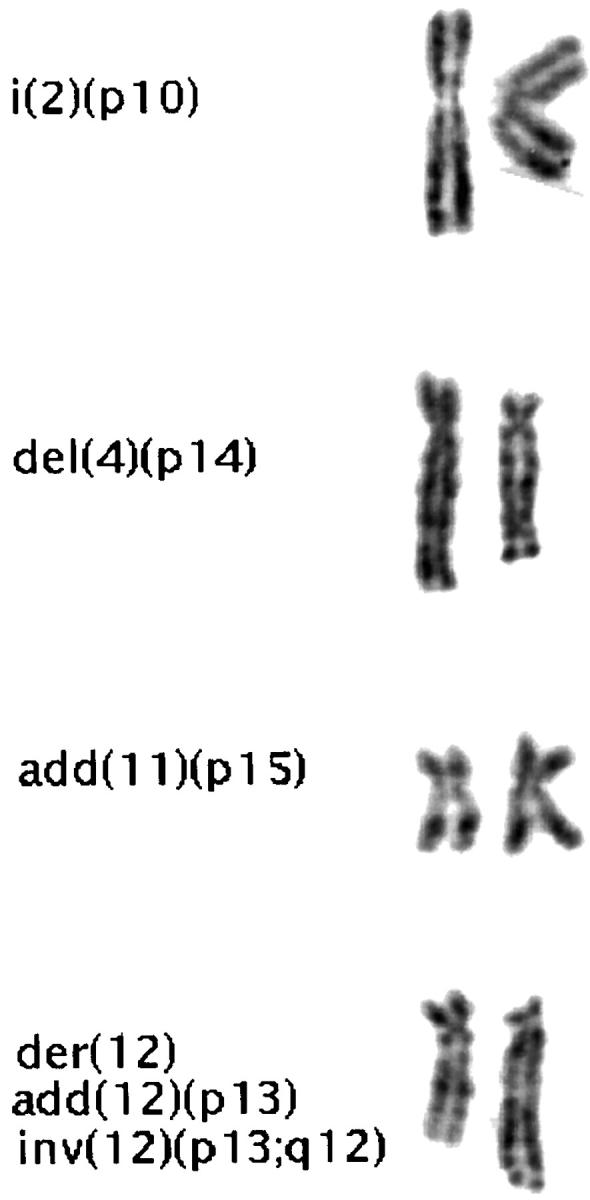
Partial karyotype of case 16 (fibrous dysplasia) illustrating an isochromosome 2p, a deleted 4p, additional material of unknown origin on 11p, and a derivative 12 with complex rearrangements.
Fluorescence in Situ Hybridization
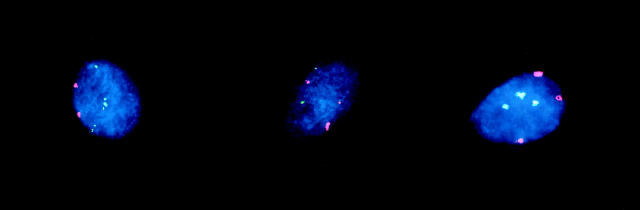
Trisomy 8 and trisomy 20 were observed in both the bone and soft tissue lesions (Figure 3) ▶ . Trisomy 8 was detected in 9 specimens (cases 1, 4, 6, 8, 13, 14A, 14B, 15, and 19), and trisomy 20 was detected in 11 specimens (cases 1, 2, 4A, 4B, 5, 6, 7, 8, 12, 15, and 17) by molecular cytogenetic methodologies. Three cases (cases 1, 4, and 15) exhibited trisomies 8 and 20 concurrently in addition to independent clones of trisomy 8 and trisomy 20. Extra copies of chromosomes 7 and 8, but not chromosome 20, were observed in both cases of osteofibrous dysplasia that demonstrated trisomy or tetrasomy 7 and trisomy 8 karyotypically. (The chromosome 20 α-satellite probe served as the control for excluding triploidy in these two cases.) Extra copies of chromosome 7 were not observed in the remaining osteofibrous dysplasia lesion, case 15.
Figure 3.
FISH analyses performed on cytological touch preparations or on disaggregated paraffin-embedded cells using the α-satellite probe D8Z1 to the centromeric region of chromosome 8 (green) and the α-satellite probe D20Z1 to the centromeric region of chromosome 20 (red). Left: FISH analysis of case 6 (recurrent desmoid tumor) showing three hybridization signals for chromosome 8 and two for chromosome 20. Center: FISH analysis of case 8 (primary desmoid tumor) showing three hybridization signals for chromosome 20 and two for chromosome 8. Right: FISH analysis of case 1 (desmoplastic fibroma) illustrating trisomy 8 and trisomy 20 within the same interphase cell.
Discussion
Trisomy 8 and/or 20 has been reported as clonal aberrations in Dupuytren’s contracture, plantar fibromatosis, Peyronie’s disease, infantile fibrosarcoma, solitary fibrous tumor, and most recently in a subset of desmoid tumors arising in soft tissue supporting a close pathogenetic relationship. 7-10,14,16,17,23-25 Desmoplastic fibroma and periosteal desmoid tumor are histologically identical to desmoid tumor of soft tissue and are considered to represent the bone counterparts of this neoplasm. Osteofibrous dysplasia and fibrous dysplasia are also benign fibrous lesions of bone. Only two cases of osteofibrous dysplasia, four cases of fibrous dysplasia, and an unusual case of desmoplastic fibroma arising in fibrous dysplasia have reportedly shown clonal chromosomal abnormalities. 1,11-15 There are no cytogenetic descriptions of periosteal desmoid tumor, and there are no molecular cytogenetic reports of any of these benign fibrous lesions of bone regarding chromosomes 8 and 20 copy numbers.
In the present study, cytogenetic and molecular cytogenetic analysis of 13 benign fibrous lesions of bone revealed chromosomally abnormal cell populations in 11 specimens, including trisomy 8 and/or trisomy 20 in 10 of them. These findings indicate that, analagous to soft tissue fibrous lesions, trisomy 8 and trisomy 20 contribute to aberrant cell proliferation in primary fibrous lesions of bone, supporting a common or related mechanism of pathogenesis.
These data also provide further evidence of clonality in these tumors. The cytogenetic findings of two cases of osteofibrous dysplasia were remarkably similar, both exhibiting extra copies of chromosomes 7 and 8 (cases 13 and 14). Although the third case of osteofibrous dysplasia (case 15) did not show extra copies of these chromosomes cytogenetically, FISH studies revealed trisomy 8 as well as trisomy 20 in approximately one-third of the cells evaluated. Additionally, several of the chromosomal breakpoints aberrant in one case of fibrous dysplasia (case 16) are similar to those reported in other cases. 1,11-13 Specifically, loss of 2q (particularly 2q31-35) and structural rearrangements of 11p15 and 12p13 have been observed in other fibrous dysplasias demonstrating clonal structural abnormalities. Interestingly, one of the fibrous dysplasias exhibiting trisomy 8 in the current study (case 19) was from a patient with polyostotic fibrous dysplasia. All previous cytogenetic reports of fibrous dysplasia are of monostotic lesions. 1,11-13 Notably, trisomy 8 was also observed cytogenetically in one of these previously described fibrous dysplasias. 11
Desmoid tumor, an infiltrative and frequently locally recurrent lesion, may occur sporadically or as a feature of Gardner syndrome (an autosomal dominant trait that is also characterized by intestinal polyposis, osteomata, and cutaneous cysts). 20 The gene for Gardner syndrome and familial adenomatous polyposis has been localized to the long arm of chromosome 5 (5q21-22). 21,22 We, and others, have identified abnormalities of 5q resulting in loss of 5q21-22 in a subset of desmoid tumors from patients with and without Gardner syndrome. 3-6 In this study, two of the eight cases diagnosed as desmoid tumors showed clonal loss of 5q21-22. 6 An additional case showed random loss of this region; a single metaphase cell with del(5)(q21q31) was detected in case 6.
Trisomy 8 and trisomy 20 were observed by FISH methodologies in five of nine desmoid tumor specimens, confirming the presence of these anomalies in a subset of these tumors. Trisomies 8 and 20 were not detectable as clonal abnormalities by traditional cytogenetic techniques in any of the currently evaluated tumors, but trisomy 8 was seen in a single metaphase cell of one case (case 6), and trisomy 20 was seen in a single metaphase of a separate case (case 12). The disparity between cytogenetic and molecular cytogenetic approaches in detecting trisomy 8 and trisomy 20 in desmoid tumors has been addressed in previous studies. Several authors have suggested that failure to detect these trisomies by conventional cytogenetic methodologies may be explained by rapid overgrowth of the trisomic cells by diploid populations. 7,8 Fletcher et al 8 and Kouho et al 17 have also observed a correlation between the presence of trisomy 8 and an increased risk of recurrence. In the current study, trisomy 8 was detected in two desmoid tumors; one was a recurrent lesion, and the other was from a patient who was lost to follow-up. Trisomy 8 was not observed in cases 7 and 10, however, both of which were recurrent lesions.
Similar to desmoid tumors, trisomy 8 and trisomy 20 were not present in all of the fibrous lesions of bone. In addition, not all of the anomalies were observed in separate specimens from the same patient (cases 4 and 7). Dal Cin et al 7 have suggested that the fact that trisomy 8 is not present in all cases of desmoid tumors may be due to genuine heterogeneity among desmoid tumors or possibly sampling error. This explanation is applicable to the current study of desmoid tumors and histologically similar, but clinically distinct, benign fibrous lesions of bone.
The trisomic populations in the fibrous lesions evaluated in this study, as well as those of other authors, have all constituted less than one-third of the total cells examined. A possible explanation for these relatively small subclones is that these trisomies are acquired during neoplastic progression (representing secondary changes). The primary anomaly may not be recognizable at the cytogenetic or molecular cytogenetic level such as an APC (adenomatous polyposis coli) gene mutation in desmoid tumors. Certainly, trisomy 8 is a well recognized secondary abnormality in a number of other mesenchymal neoplasms, such as Ewing’s sarcoma, dermatofibrosarcoma protuberans, and clear-cell sarcoma. 14 Additional studies are needed to determine the precise role of these trisomies in fibrous lesions of bone and soft tissue.
In conclusion, trisomy 8 and trisomy 20 were present in a significant subset of the desmoid tumors (56%) and the fibrous lesions of bone (76%) examined in this study. These findings demonstrate that trisomy 8 and trisomy 20 are nonrandom aberrations in benign fibrous conditions of bone as well as soft tissue, indicating a shared or similar mechanism of pathogenesis.
Footnotes
Address reprint requests to Dr. Julia A. Bridge, University of Nebraska Medical Center, 985440 Nebraska Medical Center, Omaha, NE 68198-5440. E-mail: jbridge@mail.unmc.edu.
Supported by the Nebraska State Department of Health, LB 506 and LB595, and the John A. Wiebe Children’s Health Care Fund.
References
- 1.Bridge JB, Rosenthal H, Sanger WG, Neff JR: Desmoplastic fibroma arising in fibrous dysplasia: chromosomal analysis and review of the literature. Clin Orthop Rel Res 1989, 247:272-278 [PubMed] [Google Scholar]
- 2.Karlsson I, Mandahl N, Heim S, Rydholm A, Willén H, Mitelman F: Complex chromosome rearrangements in an extraabdominal desmoid tumor. Cancer Genet Cytogenet 1988, 34:241-245 [DOI] [PubMed] [Google Scholar]
- 3.Yoshida MA, Ikeuchi T, Iwama T, Miyaki M, Mori T, Ushijima Y, Hara A, Miyakita M, Tonomura A: Chromosome changes in desmoid tumors developed in patients with familial adenomatous polyposis of the colon. Jpn J Cancer Res 1991, 82:916-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridge JA, Sreekantaiah C, Mouron B, Neff JR, Sandberg AA, Wolman SR: Clonal chromosomal abnormalities in desmoid tumors: implications for histopathogenesis. Cancer 1992, 69:430-436 [DOI] [PubMed] [Google Scholar]
- 5.Dangel A, Meloni AM, Lynch HT, Sandberg AA: Deletion (5q) in a desmoid tumor of a patient with Gardner’s syndrome. Cancer Genet Cytogenet 1994, 78:94-98 [DOI] [PubMed] [Google Scholar]
- 6.Bridge JA, Meloni AM, Neff JR, DeBoer J, Pickering D, Dalence C, Jeffrey B, Sandberg AA: Deletion 5q in desmoid tumor and fluorescence in situ hybridization for chromosome 8 and/or 20 copy number. Cancer Genet Cytogenet 1996, 92:150-151 [DOI] [PubMed] [Google Scholar]
- 7.Dal Cin P, Sciot R, Aly MS, Stas M, De Wever I, Van Damme B, Van den Berghe H: Some desmoid tumors are characterized by trisomy 8. Genes Chromosomes & Cancer 1994, 10:131-135 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JA, Naeem R, Xiao S, Corson JM: Chromosome aberrations in desmoid tumors. Trisomy 8 may be a predictor of recurrence. Cancer Genet Cytogenet 1995, 79:139-143 [DOI] [PubMed] [Google Scholar]
- 9.Dal Cin P, Sciot R, Van Damme B, De Wever I, Van den Bergh H: Trisomy 20 characterizes a second group of desmoid tumors. Cancer Genet Cytogenet 1995, 79:189. [DOI] [PubMed] [Google Scholar]
- 10.Qi H, Dal Cin P, Hernández JM, Garcia JL, Sciot R, Fletcher C, Van Eyken P, De Wever I, Van den Berghe H: Trisomies 8 and 20 in desmoid tumors. Cancer Genet Cytogenet 1996, 92:147-149 [DOI] [PubMed] [Google Scholar]
- 11.Tarkkanen M, Kaipainen A, Karaharju E, Böhling T, Szymanska J, Heliö H, Kivioja A, Elomaa I, Knuutila S: Cytogenetic study of 249 consecutive patients examined for a bone tumor. Cancer Genet Cytogenet 1993, 68:1-21 [DOI] [PubMed] [Google Scholar]
- 12.Dal Cin P, Sciot R, Speleman F, Samson I, Laureys G, De Potter C, Meire F, Van Damme B, Van den Berghe H: Chromosome aberrations in fibrous dysplasia. Cancer Genet Cytogenet 1994, 77:114-117 [DOI] [PubMed] [Google Scholar]
- 13.Mertens F, Albert A, Heim S, Lindholm J, Brosjö O, Mitelman F, Mandahl N: Clonal structural chromosome aberrations in fibrous dysplasia. Genes Chromosomes & Cancer 1994, 11:271-272 [DOI] [PubMed] [Google Scholar]
- 14.Sandberg AA, Bridge JA: The Cytogenetics of Bone and Soft Tissue Tumors. 1994:pp 35-36 TX, RG Landes, Austin
- 15.Bridge JA, Dembinski A, DeBoer J, Travis J, Neff JR: Clonal chromosomal abnormalities in osteofibrous dysplasia: implications for histopathogenesis and its relationship with adamantinoma. Cancer 1994, 73:1746-1752 [DOI] [PubMed] [Google Scholar]
- 16.Mertens F, Willén H, Rydholm A, Brosjö O, Carlén B, Mitelman F, Mandahl N: Trisomy 20 is a primary chromosome aberration in desmoid tumors. Int J Cancer 1995, 63:527-529 [DOI] [PubMed] [Google Scholar]
- 17.Kouho H, Aoki T, Hisaoka M, Hashimoto H: Clinicopathological and interphase cytogenetic analysis of desmoid tumors. Histopathology 1997, 31:336-341 [DOI] [PubMed] [Google Scholar]
- 18.ISCN: An International System for Human Cytogenetic Nomenclature. Edited by F Mitelman, Basel, Karger, 1995
- 19.Bridge JA, Bhatia PS, Anderson JR, Neff JR: Biological and clinical significance of cytogenetic and molecular cytogenetic abnormalities in benign and malignant cartilaginous lesions. Cancer Genet Cytogenet 1993, 69:79-90 [DOI] [PubMed] [Google Scholar]
- 20.Gardner EJ, Richards RC: Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953, 5:139-147 [PMC free article] [PubMed] [Google Scholar]
- 21.Bodmer WF, Bailey CJ, Bodner J, Bussey HJR, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P, Sheer D, Solomon E, Spurr NK: Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature 1987, 328:614-616 [DOI] [PubMed] [Google Scholar]
- 22.Leppert M, Dobbs M, Scamber P, O’Connell P, Nakamura Y, Stauffer D, Woodward S, Burt R, Hughes J, Gardner E, Lathrop M, Wasmuth J, Lalouel JM, White R: The gene for familial polyposis coli maps to the long arm of chromosome 5. Science 1987, 238:1411-1413 [DOI] [PubMed] [Google Scholar]
- 23.Breiner JA, Nelson M, Bredthauer BD, Neff JR, Bridge JA: Trisomy 8 and trisomy 14 in plantar fibromatosis. Cancer Genet Cytogenet 1999, 108:176-177 [DOI] [PubMed] [Google Scholar]
- 24.Dal Cin P, DeSmet L, Sciot R, Van Damme B, Van den Berghe H: Trisomy 7 and trisomy 8 in dividing and nondividing tumor cells in Dupuytren’s disease. Cancer Genet Cytogenet 1999, 108:137-140 [DOI] [PubMed] [Google Scholar]
- 25.Miettinen MM, el-Rifai W, Sarlomo-Rikala M, Andersson LC, Knuutila S: Tumor size-related DNA copy number changes occur in solitary fibrous tumors but not in hemangiopericytoma. Mod Pathol 1997, 10:194-200 [PubMed] [Google Scholar]