Abstract
Murine cytomegalovirus (MCMV), which causes acute, latent, and persistent infection of the natural host, is used as an animal model of human cytomegalovirus (HCMV) infection. Transcription of MCMV immediate-early (IE) genes is required for expression of the early and late genes and is dependent on host cell transcription factors. Cell-type-specific expression activity of the MCMV IE promoter was analyzed in transgenic mice generated with the major IE (MIE) enhancer/promoter involving nucleotides −1343 to −6 (1338 bp) connected to the reporter gene lacZ. Distinct expression was observed in the brain, kidneys, stomach, and skeletal muscles. Weak expression was observed in a portion of the parenchymal cells of the salivary glands and pancreas, and expression was hardly detected in the lungs, intestine, or immune and hematopoietic organs such as the thymus, spleen, lymph nodes, and bone marrow. The spectrum of organs positive for expression was narrower than that of the HCMV MIE promoter-lacZ transgenic mice reported previously and showed a greater degree of cell-type specificity. Interestingly, astrocyte-specific expression of the transgene was observed in the brain and primary glial cultures from the transgenic mice by combination of β-galactosidase (β-Gal) expression and immunostaining for cell markers. However, the transgene was not expressed in neurons, oligodendroglia, microglia, or endothelial cells. Furthermore, the β-Gal expression in glial cultures was stimulated significantly by MCMV infection or by addition of calcium ionophore. These observations indicated that expression activity of the MCMV IE promoter is strictly cell-type specific, especially astrocyte-specific in the brain. This specific pattern of activity is similar to that of natural HCMV infection in humans.
Human cytomegalovirus (HCMV), a member of the herpes virus group, is the most significant infectious cause of congenital anomalies of the central nervous system (CNS) caused by intrauterine infection in humans. 1-4 In adults, infection with HCMV is usually asymptomatic in immunocompetent hosts, but the virus can cause severe or fatal diseases in immunocompromised patients. 5 Cytomegalovirus (CMV) demonstrates a strict host cell type and species specificity in terms of infection. 6,7 Due to the limitations of studying HCMV in an animal host, murine CMV (MCMV) has been used as a model of human infection for pathogenesis, tissue tropism, and latency studies in the laboratory mouse. 8-11 Similar to HCMV and other herpesviruses, MCMV genes are expressed in three sequential phases: immediate-early (IE), early, and late. 6,7 The expression of IE genes is dependent on appropriate cellular transcription factors that bind to the DNA sequence of the CMV major IE enhancer/promoter. 12,13 However, by DNA transfection, the major IE (MIE) promoter activates heterogeneous genes at high levels in most cultured cell lines. Two groups, Koedood et al 14,15 and Baskar et al, 16,17 reported that expression of HCMV MIE promoter-lacZ genes in transgenic mice was cell type specific during embryogenesis. In adult tissues, the transgenes were expressed in neurons, choroid plexus cells, and endothelial cells but not in astrocytes unless the cells were stimulated. 15
In the present study, we examined cell-type-specific activity of the MCMV MIE enhancer/promoter (hereafter referred as MCMV MIE promoter) in adult transgenic mice. As MCMV can infect mice as a host, transcriptional activity of the MCMV MIE promoter in transgenic mice seems to be more natural in terms of using host cellular factors than that of the HCMV MIE promoter. We used a longer enhancer/promoter sequence (nucleotides −1343 to −6; 1338 bp) of the MCMV MIE gene rather than the HCMV promoter for transgenic mice reported previously. 14-18 We showed that expression activity of the MCMV IE promoter was more strictly cell type specific than that of the HCMV MIE promoter in transgenic mice, and the expression was astrocyte specific but not neuron specific.
Materials and Methods
Construction and Preparation of Transgene
The coding region for abundant IE transcription of MCMV was reported to be restricted to a region between map units 0.769 and 0.817 of the genome. 19,20 MCMV (Smith strain) DNA 21 was digested with BamHI and cloned into pACYC184. The plasmid clone containing the major IE enhancer/promoter of ie1 and ie3 (MCMV-MIE pro1) was selected using PCR with primers specific for the ie1 gene. The fragment was further digested with PstI, and the 2.3-kb fragment was subcloned into pUC18. The MCMV-MIE pro1 was excised as a KpnI-HpaI fragment covering nucleotides −1343 to −6 (1338 bp) and then inserted into the pnlacF vector containing the nuclear localization signal from SV40, Escherichia coli β-galactosidase (β-Gal) coding sequence, and poly(A) and intron from mouse protamine, provided by Dr. R. D. Palmiter, University of Washington. 22 The resulting plasmid was designated as pMCMV-MIEpro1-lacZ. The transgene fragment (5.1 kb) for microinjection was isolated from the digested plasmid DNA with KpnI and BglII.
Generation of Transgenic Mice
Transgenic mice were produced by standard techniques. 23 Purified linearized DNA of the MCMV-MIEpro1-lacZ DNA was injected into the pronuclei of fertilized ova derived from BDF1 (C57BL/6 female × DBA/2 male) mice. After injection, ova were transferred to oviducts of pseudopregnant females of ICR mice. Transgenic founders were identified by PCR of genomic DNA prepared from tail tips using standard procedures. 24 PCR was performed with MCMV-MIEpro1-lacZ-specific primers (5′ primer, 5′-GGCACGCATTCTATTGGCT-3′, and 3′primer, 5′-TTTGAGGGGACGACGACAG-3′; Figure 1, A and B ▶ ) and with the mouse apolipoprotein (apo)E internal control primers (5′ primer, 5′-AACCGCTTCTGGGATTACCT-3′; 3′ primer, 5′-CATAGTGTCCTCCATCAGTG-3′) for 30 cycles of 1 minute at 94°C, 1 minute at 60°C, and 1 minute at 74°C. The reaction products were run on a 2.0% agarose gel that was then stained with ethidium bromide and photographed. Transgenic founders identified by PCR were confirmed by Southern blotting. The genomic DNA and control DNA containing nontransgenic mouse tail DNA with 1 or 10 genomic equivalents of MCMV-MIEpro1-lacZ were digested with BamHI, which cuts twice in the transgene, run on 0.9% agarose gels, and transferred on nylon membranes (MSI, Westborough, MA). The filters were then probed with 33P-labeled transgene BamHI fragment and analyzed with a Bio Imaging Analyzer (BAS1000Mac, Fuji Photo Film). Founder lines were established from transgenic mice that transmitted the MCMV-MIEpro1-lacZ to progeny. Expression analysis was performed on F1 mice obtained by mating hemizygous transgenic animals with outbred C57BL/6Cr.
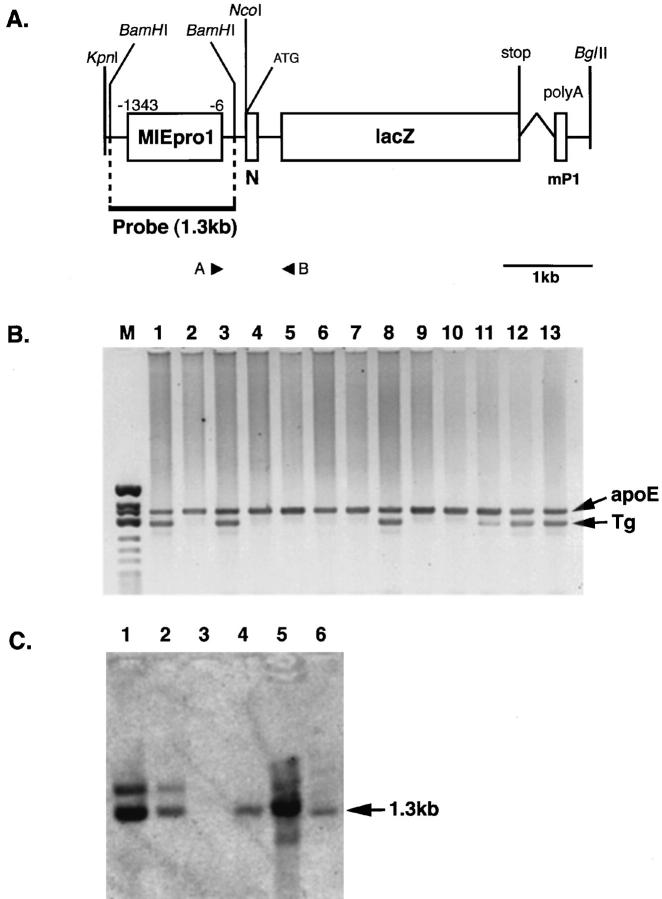
Figure 1.
A: Schema of the MCMV-MIEpro1-lacZ transgene DNA fragment (transgene; 5.1 kb) used to generate transgenic mice. The MCMV major IE promoter (MIEpro1) (nucleotides −1343 to −6) was inserted into the pnlacF vector. 22 N, nuclear localization signal. Splice and polyadenylation signals were from the mouse protamine (mP1) gene. B: Screening of integrated transgene DNA by multiplex PCR. DNA from the tails of mice was tested for the presence of the transgene and host gene by PCR using specific primers (primer A and B) and the mouse apoE internal control primers, respectively. PCR amplification was performed, and the reaction products were run on 2% agarose gels, which were then stained with ethidium bromide. Lanes 10 to 13 show 0, 0.1, 1, and 10 copies of the MCMV-MIEpro1-lacZ plasmid, respectively. C: Southern blot of the tail DNA from the transgenic mice. Aliquots of 15 μg of tail DNA were digested with BamHI, run in 0.9% agarose gels, transferred on nylon membranes, and probed with 33P-labeled BamHI fragment (1.3 kb; A). Control lanes show 10 copies (lane 1), 1 copy (lane 2), and 0 copies (lane 3) of MCMV-MIEpro1-lacZ with 15 μg of normal mouse tail DNA. Lane 4, Tg-1; lane 5, Tg-2; lane 6, Tg-3.
Histochemistry and Immunohistochemistry
For histochemical analysis, adult mice (1 to 2 months old) were anesthetized with diethyl ether and perfused with 4% paraformaldehyde (PFA) and 0.1% glutaraldehyde (GA) in 0.1 mol/L phosphate buffer (PB). After perfusion, tissues were removed and fixed in freshly prepared 4% PFA and 0.1% GA in PB for 1 hour at 4°C with shaking. The tissues were incubated in a reaction mixture containing 1 mg of 5-bromo-4-chloro-3-indolyl-β-galactosidase (X-Gal) per ml, 5 mmol/L K4Fe(CN)6, 5 mmol/L K3Fe(CN)6 in PB, pH 7.3, overnight at room temperature. 22 After staining, each tissue was embedded in paraffin and sectioned. Sections were lightly stained with hematoxylin. For immunohistochemical analysis, adult mice were anesthetized and perfused with 4% PFA in 0.1 mol/L PB. Tissues were removed, fixed with 4% PFA in 0.1 mol/L PB again for 1 to 2 hours, embedded in paraffin, and sectioned. After deparaffinization and rehydration, sections were pretreated with 0.3% hydrogen peroxidase and incubated with goat serum blocking solution for 10 minutes. The sections were incubated with rabbit anti-β-galactosidase antibody (anti-β-Gal; Cappel, Durham, NC) for 30 minutes at room temperature, sequentially incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, and then colored with 3-amino-9-ethyl carbazole (AEC; DAKO, Tokyo, Japan). 25
Primary Glial Cell Cultures
Primary cortical glial cell cultures were prepared according to the methods of Togashi et al 26 and Norris et al 27 with some modifications. Briefly, brains were removed from postnatal day 1 transgenic mice. The cortex was dissected out under a microscope and dissociated in plating medium (Eagle’s minimal essential medium (MEM); Gibco BRL, Grand Island, NY) supplemented with 10% fetal calf serum (FCS), 10% horse serum, 20 mmol/L glucose, 2 mmol/L glutamine, 30 mmol/L NaHCO3, 100 U/ml penicillin, and 50 μg/ml streptomycin). After digestion with 0.125% trypsin (Gibco BRL) in calcium-, magnesium-free phosphate buffered-saline (PBS) and 30 mmol/L glucose at 30°C for 30 minutes, the cells were suspended in the plating medium and plated on coverslips (18 × 12 mm) in 12-multiwell plates (Becton-Dickinson, Franklin Lakes, NJ), precoated with poly-d-lysine (0.1 mg/ml; Sigma), and placed in a 5% CO2, humidified 37°C incubator. The cultures were allowed to grow to confluence, and the cells were maintained in growth medium that was similar to the plating medium but lacked FCS.
For stimulation of the transgene in glial culture, MCMV (Smith strain) was infected at a multiplicity of infection of 5 or calcium ionophore (A23187; Sigma) 27 was added in the medium at the concentration of 5 μmol/L.
Combination of X-Gal Staining and Immunohistochemical Staining
After perfusion and fixation with 4% PFA, the brains were sliced and incubated in X-Gal solution overnight as described above. The slices were embedded in paraffin, sectioned, and subjected to immunohistochemical staining as described below. For the glial cell cultures, the coverslips were fixed in 4% PFA in 0.1 mol/L phosphate buffer at 4°C for 10 minutes and stained with X-Gal. After X-Gal staining, the cultures were post-fixed with 4% PFA in 0.1 mol/L PB. Immunohistochemical staining of sections of X-Gal-reacted brains or coverslips of the cultured glial cells was performed as previously described. 25
After pretreatment as described above, the samples were first reacted with rabbit anti-γ-neuron-specific enolase (NSE) antibody (Dr. K. Kato, Aichi Colony, Aich, Japan) for neurons, rabbit anti-glial fibrillary acidic protein antibody (GFAP; Dako Corp., Carpinteria, CA) for astrocytes, rat anti-microglia/macrophage antibody (F4/80; Serotec, Oxford, UK) for microglia, or mouse anti-myelin-associated glycoprotein (MAG; Boehringer Mannheim Biochemica, Mannheim, Germany) for oligodendroglia, incubated with biotinylated secondary antibody and with HRP-conjugated streptavidin, and colored with AEC for brain sections or colored with 3,3′-diaminobenzidine (DAB). 25
Chemiluminescent Assay of β-Galactosidase
For the chemiluminescent reporter assay, the Aurora Gal-XE kit was obtained from ICN Pharmaceuticals (Costa Mesa, CA), and a chemiluminescent assay for β-Gal of glial cultures was performed according to the kit’s manufacturer. Extracts of glial cells were prepared in the lysate solution of the kit and centrifuged at 12,500 × g for 5 minutes, and 20 μl of supernatant fluid was assayed. 28 Chemiluminescent emission was measured for 5 seconds using a Lumicounter 700 (Microtech Nichion, Chiba, Japan).
Results
Generation of Transgenic Mice
Transgenic mice that expressed lacZ gene under transcriptional control of the MCMV-MIE promoter (nucleotides −1343 to −6; Figure 1A ▶ ) were generated for analysis of the tissue-specific regulation of this promoter. The linearized, purified MCMV-MIEpro1-lacZ transgene was microinjected into pronuclei of fertilized embryos (BDF1BDF1) at the one-cell stage. Founder mice were screened by PCR using tail DNA. PCR amplification was performed in the presence of primers for the transgene or for mouse apoE as an internal control. Several founder mice bearing the MCMV-MIEpro1-lacZ transgene were detected, and three of them are shown in Figure 1B ▶ (lanes 1, 3, and 8). Among positive founder mice, three lines were found to express the transgene by X-Gal staining (as shown below). Southern blotting analysis of the three transgenic lines is shown in Figure 1C ▶ . BamHI fragment (1.3 kb) including MCMV-MIEpro1 was detected in these transgenic lines after digestion with BamHI (Figure 1C ▶ , lanes 4 to 6). Control lanes contained 10 (lane 1) or 1 copy (lane 2) with normal mouse tail DNA. The transgenic lines Tg-1 (lane 4) and Tg-3 (lane 6) had one copy, and the Tg-2 (lane 5) had ∼10 copies. As the transgene (5.1 kb) can be cleaved at one site by NcoI (Figure 1A) ▶ , the 5.1-kb band was not detected in Tg-1 and Tg-3 after digestion with NcoI, because they had one copy (not shown). The male founder was crossed with nontransgenic C57Bl/c mice and transmitted the gene to ∼50% of the offspring. These hemizygous males were mated with female C57Bl/c, and the offspring were used for experiments.
Cell Type-Specific Expression in Transgenic Mice
Among several founder mice bearing the MCMV-MIEpro1-lacZ transgene, three lines (Tg-1, -2, and -3) were found to express the transgene by X-Gal staining. The expression pattern in terms of cell specificity was basically the same among the three transgenic lines. As intensity of expression in the brain was the strongest in the Tg-1, cell type specificity of expression of the MCMV MIE promoter was shown using the Tg-1 line. Expression of the transgene in 2-month-old transgenic mice was assayed by either X-Gal or immunohistochemical staining using an antibody specific to β-galactosidase (anti-β-Gal). In the brain, cells positive by either X-Gal staining or β-Gal immunostaining were scattered sparsely in the cerebral cortex (Figure 2A) ▶ , hippocampus (Figure 2B) ▶ , cerebellum (Figure 2C) ▶ , basal ganglia, and brainstem (not shown). The distribution of the positive cells was not in accordance with that of neuronal cells. In the kidneys, strong expression was observed in the epithelial cells of the distal tubules. No staining was observed in the glomeruli or in the proximal tubules (Figure 2D) ▶ . The salivary glands showed weak expression in a portion of acinar cells (Figure 2E) ▶ . Only sporadic positive cells were observed in hepatic cells around central veins (Figure 2F) ▶ and acinar cells of the pancreas (not shown). Clear expression was observed in the chief cells of the gastric mucosa (Figure 2G) ▶ . A few positive cells were observed among epithelial cells of the small intestine (Figure 2H) ▶ , but expression was hardly observed in the large intestine or esophagus (not shown). Lymphoid organs such as the thymus, spleen, and lymph nodes (Figure 1) ▶ were negative for expression (Table 1) ▶ . The spectrum of expression activity of the MCMV IE promoter was much narrower than that of the HCMV IE promoter in transgenic mice reported by Baskar et al 16 and Schmid et al 18 (Table 1) ▶ . Although positive expression driven by the HCMV IE promoter was reported in the skin, esophagus, testis, spleen, and bone marrow in their transgenic mice, these organs were negative for expression from the MCMV IE promoter (Table 1) ▶ . Even in the organs positive for MCMV MIEpro1-lacZ expression, only one type of cell was positive in each organ.
Figure 2.
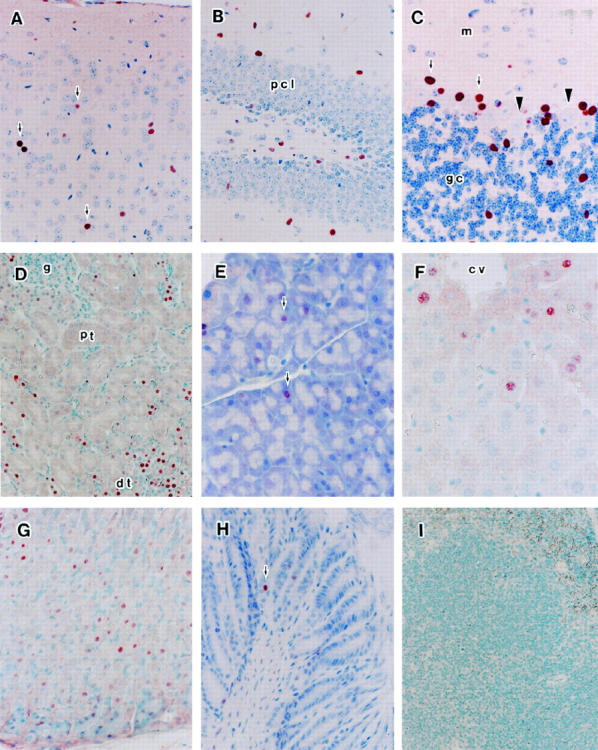
Immunohistochemical staining of expression of the MCMV MIEpro1-lacZ transgenic mice. Transgenic mice (Tg-1) generated by the MCMV-MIEpro1-lacZ transgene were examined by immunohistochemical staining. Tissues from 2-month-old Tg-1 mice were fixed in 4% PFA and embedded in paraffin. Deparaffinized sections were reacted with the anti-β-galactosidase antibody (anti-β-Gal), followed by goat anti-rabbit IgG horseradish peroxidase (HRP), and then colored with 3-amino-9-ethyl carbazole (AEC). A: Cerebral cortex (arrow, β-Gal-positive cells); B: Hippocampus (pcl, pyramidal cell layer); C: Cerebellum (m, molecular layer; gc, granular cells; arrows, β-Gal-positive cells (Bergmann glia); arrowheads, Purkinje cells); D: Kidney (g, glomerulus; pt, proximal tubules; dt, distal tubules); E: Salivary gland (arrow, slight β-Gal-positive cells); F: Parenchymal cells around the central vein of the liver (cv, central vein); G: Gastric mucosa; H: Small-intestinal mucosa (arrow, β-Gal-positive cells); I: Spleen.
Table 1.
MCMV-MIE-lacZ Expression in Transgenic Mice with Reference to HCMV-MIE-lacZ Transgenic Mice
| System and organ | MCMV | HCMV | |
|---|---|---|---|
| −1343/−6 | −670/+54 | −584/−16 | |
| Brain | |||
| Cerebrum | + | + | + |
| Cerebellum | + | + | + |
| Gastrointestinal | |||
| Esophagus | − | + | +/− |
| Stomach | + | +/− | +/− |
| Small intestine | − | − | + |
| Colon | − | − | +/− |
| Liver | −∼+ | − | − |
| Salivary gland | −∼+ | + | +/− |
| Pancreas | −∼+ | + | − |
| Genitourinary | |||
| Kidney | + | + | + |
| Bladder | − | + | + |
| Testes | − | + | + |
| Ovary | − | + | +/− |
| Respiratory | |||
| Lung | − | − | − |
| Circulatory | |||
| Heart | −∼+ | + | + |
| Lymphoid and Blood | |||
| Spleen | − | + | + |
| Thymus | − | +/− | +/− |
| Bone marrow | − | + | |
| Blood | − | − | − |
| Lymph node | − | +/− | + |
| Endocrine gland | |||
| Adrenal gland | − | +/− | |
| Thyroid | − | +/− | +/− |
| Others | |||
| Smooth muscle | − | + | |
| Skeletal muscle | + | + |
Expression activity of MCMV MIE promoter (−1343/−6) was detected by x-Gal staining or immunostaining with anti β-Gal Ab. Expression activity of HCMV MIE promoter (−670/+54) was detected by x-Gal staining or immunostaining with anti β-Gal Ab. 16 Transcription activity of the HCMV IE promoter (−584/−16) was detected by Northern blotting. 18 +, positive; −, negative; −∼+, a portion of cells were positive; +/−, positive or negative.
Expression of MCMV-IE-Promoter-lacZ in the CNS
In contrast with the HCMV MIE-promoter-lacZ transgenic mice in which the transgene was intensely expressed in cells morphologically resembling neurons rather than astrocytes, expression of the MCMV MIEpro1-lacZ in transgenic mice was predominantly in glial-like cells but not in neurons as described above (Figure 2, A–C) ▶ . Furthermore, no expression was observed in the endothelial cells of the brain vessels (not shown), although positive expression was reported in the transgenic mice with HCMV MIE promoter (Table 2) ▶ . In the cerebellum, positive cells were mainly located in the junction between the molecular and granular layers and were adjacent to the Purkinje cells (arrowheads, Figure 2C ▶ ); these were considered to be Bergmann glia (arrows). No staining was observed in Purkinje cells or granular cells (Figure 2C) ▶ .
Table 2.
MCMV-MIEprol-lacZ Expression in Transgenic Mice with Reference to HCMV IE-lacZ Transgenic Mice
| System and organ | MCMV | HCMV | |
|---|---|---|---|
| −1343/−6 | −524/+13 | −670/+54 | |
| Cerebrum | |||
| Neurons | − | + | + |
| Astrocytes | + | − | |
| Oligodendrocytes | − | ||
| Microglia | − | − | |
| Ependymal cells | + | + | |
| Endothelial cells | − | + | + |
| Cerebellum | |||
| Molecular cells | − | − | |
| Purkinje cells | − | − | + |
| Bergmann cells | + | ||
| Granular cells | − | + |
Expression activity of MCMV-MIE promoter (−1343/−6) was detected by x-Gal staining or immunostaining with anti β-Gal Ab. Expression activity of HCMV-MIE promoter (−524/+13) was detected by x-Gal staining. 15 Expression activity of HCMV MIE promoter (−670/+54) was detected by x-Gal staining or immunostaining with anti β-Gal Ab. 16 +, positive; −, negative.
Combined Staining for β-Galactosidase Activity with Immunohistochemical Staining of Neural Cell Markers
To further characterize the glial cells expressing MCMV MIE-promoter-lacZ, the positive cells were double stained immunohistochemically for neuronal, glial, oligodendroglial, and microglial markers. After X-Gal staining of whole brains or brain slices, paraffin-embedded or frozen sections were subjected to immunohistochemical staining for cell type markers. The lacZ-expressing cells were not double stained with anti-NSE antibody (Ab) (Figure 3A) ▶ but partially double stained with anti-GFAP Ab (Figure 3, B and C) ▶ , probably because only a portion of astrocytes may be stained by anti-GFAP Ab; fibrous astrocytes in the upper cerebral layers (Figure 3B) ▶ and the white matter (Figure 3C) ▶ were stained with anti-GFAP Ab, but protoplasmic astrocytes were not stained. 29 It was difficult to define the cell types by staining for oligodendroglial and microglial markers in brain sections. Therefore, primary culture of the glial cells from transgenic mice with MCMV-promoter-lacZ was performed. More than 50% of β-Gal-positive cells were double stained with anti-GFAP Ab (Figure 3D) ▶ . Microglial cells detected by staining with the F4/80 Ab were not double stained with X-Gal (Figure 3E) ▶ . Relatively fewer oligodendroglia in the cultures were stained with anti-AMS Ab, but these cells were not double stained with X-gal (Figure 3F) ▶ . These results suggested that the MCMV MIE promoter in our transgenic mice showed astrocyte-specific expression in the brain.
Figure 3.
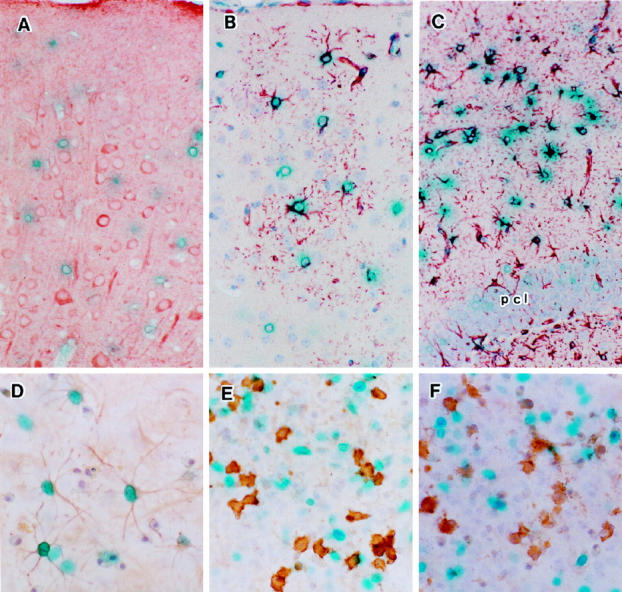
Combination of X-Gal staining and immunohistochemical staining. A: Slices of the cerebral cortex of the Tg-1 mice were first subjected to the X-Gal reaction and then embedded in paraffin. Deparaffinized sections were reacted with anti-NSE Ab and colored with AEC. B: Section from the same slice with X-Gal reaction but immunostained with anti-GFAP Ab . C: Fibrous astrocytes in the white matter around the hippocampus. pcl, pyramidal cell layer. D to F: Primary glial culture from the cerebral cortex of 3-day-old Tg-1 mice. After X-gal staining, the cells were stained with the GFAP Ab (D), with F4/80 Ab (microglia/macrophages; E), or with MAG Ab for oligodendroglia (F).
MCMV-IE-Promoter Activity in Glial Cultures of the Transgenic Mice Can Be Stimulated
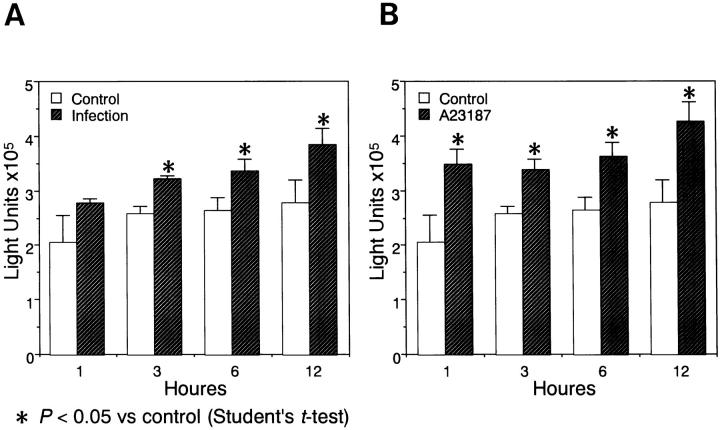
Glial cultures from the transgenic mice cultured in 12-well plates were infected with MCMV (Smith strain) at a multiplicity of infection of 5. MCMV MIE promoter activity measured by chemiluminescent assay for β-Gal was significantly stimulated by viral infection when compared with that of uninfected cultures (Figure 4A) ▶ . As HCMV MIE promoter was reported to be stimulated via induction of NF-κB, 30 calcium ionophore (A23187) 27 was added in the culture medium (5 mol/L). MCMV MIE promoter activity was also significantly stimulated (Figure 4B) ▶ .
Figure 4.
Chemiluminescent assay for β-galactosidase of glial cultures from the transgenic mice. A: Glial cells plated in 12-well plates were infected or mock-infected with MCMV (Smith strain) and assayed 1 to 12 hours after infection. B: Glial cells were added with calcium ionophore (A23187) at a concentration of 5 mol/L or not added and cultured until 12 hours.
Discussion
In the present study, we showed that expression activity of the MCMV MIE promoter in transgenic mice was cell type specific as reported in the HCMV MIE promoter transgenic mice by Koedood et al, 14 Fritschy et al, 15 and Baskar et al, 16,17 although some early studies reported this promoter to be pantropic in its expression 18,31 or to have unusual cell type specificity. 32 However, the spectrum of organs showing expression from the MCMV MIE promoter in the adult transgenic mice was narrow, and the expression activity was further strictly restricted to particular cells in these organs when compared with that in transgenic mice carrying the HCMV IE promoter-lacZ (Tables 1 and 2) ▶ ▶ . There are two possible explanations for the more specific expression activity of the MCMV IE than the HCMV IE promoter reported recently in transgenic mice. 15,16 Host cellular transcription factors may interact with the MCMV MIE promoter more naturally in transgenic mice because of the homogeneity of this combination, although transcription factors are thought to be evolutionarily well preserved across the species. 33 Alternatively, these observations may have been because the MCMV MIE promoter region used here was 1338 bp in length (−1343 to −6), far longer than the HCMV IE promoter used in previous transgenic studies (−524/+13 14,15 and −670/+54. 16,17 It has been reported that the HCMV MIE promoter may contain a modulator region located between nucleotides 750 and −1145 of the the HCMV MIE promoter region that negatively regulates expression of the major IE gene in undifferentiated cells but positively influences expression in differentiated cells. 13,34 Although whether there is a similar modulator region in the MCMV MIE promoter has not been determined, it is possible that the MCMV MIE promoter used in the present study contained such a region.
It is noteworthy that expression of the MCMV MIEpro1-lacZ in the brain of the transgenic mice in the present study was predominantly observed in glial cells. According to the combination of X-Gal staining and immunostaining for cell markers, the positive cells were astrocytes, because they were positive for GFAP but negative for neuronal, oligodendroglial, and microglial markers. This result was in contrast with the expression pattern of the HCMV MIE promoter-lacZ used in previous transgenic studies, which was observed in neurons and endothelial cells but not astrocytes. 15,16
Interestingly, the MCMV MIE promoter-lacZ was expressed only in the Bergmann glia in the cerebellum in the present study based on the specific distribution of the cells adjacent to Purkinje cells. The transcription factors binding to the cAMP response element (CRE)-binding protein (CREB)-1 are constitutively expressed in periventricular glia and Bergmann glia. 35,36 It was also shown that the major enhancer of HCMV can be regulated by cAMP treatment in a cell-type-specific manner, 37 and the sequence for the CREB was detected between nucleotides −78 and −56 of the HCMV MIE promoter (UL112/113), 38 corresponding to the region shown in MCMV. 39
The cell type specificity of MCMV MIE promoter-lacZ expression in the transgenic mice was consistent with target cells of HCMV infection in various organs such as the brain, kidneys, stomach, salivary glands, and pancreas, 2-4,40 although the levels of expression in the latter two organs were low. Interestingly, astrocyte-specific expression of the MCMV MIE pro1-lacZ constructs in the transgenic mouse brain was consistent with the observation that these cells are among the targets in HCMV encephalitis in humans. 41 We have previously shown that the glial cells expressing GFAP expressed the major IE 89-kd antigen in the neonatal mouse brain after intrauterine infection, and the glial cells were preferentially transferred to lytic infection with expression of the late gene, 25 although the viral early nuclear antigen was expressed in various mesodermal cell types in MCMV-infected embryos 42 and neuronal cells in the postnatal mouse brain. 43
Unexpectedly, we did not detect expression of the MCMV MIE promoter-lacZ in the lung, intestine, or immune and hematopoietic organs such as the thymus, spleen, lymph nodes, or bone marrow, although these organs are known to be susceptible to HCMV infection. To explain the lack of MIE promoter activity in the susceptible organs, Baskar et al 16 suggested that expression may be below the limits of detection, this promoter may require other viral or virally modified host factors, or the cell may revert back to fetal type in these organs. In addition, it is possible that some factors may suppress the MIE promoter activity in these organs, resulting in latent infection, because latent infection has been reported in the these organs, including the salivary glands. 44-47
The MCMV MIE promoter was stimulated in the glial cultures from the transgenic mice by infection with MCMV or by adding calcium ionophore in culture medium, although the stimulation was not extensive. Calcium ionophore was reported to activate NF-κB via phosphorylation of protein kinase C. 27 HCMV IE promoter was stimulated by induction of the NF-κB. 30 Conversely, activity of NF-κB was stimulated by infection with MCMV 48 and with HCMV. 49 However, HCMV IE gene products were involved in negative autoregulation of the HCMV IE promoter. 50 Further investigation is to be scheduled for the mechanism of the stimulation of the transgene in glial culture.
In conclusion, we have shown that expression activity of the MCMV MIE promoter (−1343 to −6) is highly cell type specific in transgenic mice. The spectrum of organs and cells positive for the activity was narrower than that of the HCMV MIE promoters previously reported in transgenic mice. In the brain, expression activity of the promoter was restricted to astrocytes with no expression in neurons, oligodendroglia, microglia, or endothelial cells.
Acknowledgments
We thank Miss M. Kawashima and Mr. M. Kaneta for their excellent technical assistance. We also thank Dr. Richard D. Palmiter, University of Washington, Seattle, WA, for providing the pnlacF vector.
Footnotes
Address reprint requests to Dr.Yoshihiro Tsutsui, Second Department of Pathology, Hamamatsu University School of Medicine, 3600 Handa-cho, Hamamatsu 431-3192, Japan. E-mail: ytsutsui@hama-med.ac.jp.
Supported in part by a grant (8A-8–23) from the National Center of Neurology and Psychiatry (NCNP) of the Ministry of Health and Welfare, Japan.
References
- 1.Weller TH: The cytomegalovirus: ubiquitous agents with protean clinical manifestation. N Engl J Med 1971, 285:203-214 [DOI] [PubMed] [Google Scholar]
- 2.Becroft DMO: Prenatal cytomegalovirus infection: epidemiology, pathology, pathogenesis. Perspectives in Pediatric Pathology, Vol. 6: Infectious Diseases. Edited by HS Rosenberg and J Bernstein. New York, Masson Publishing USA, 1981, pp 203–241 [PubMed]
- 3.Bale JF: Human cytomegalovirus infection and disorders of the nervous system. Arch Neurol 1984, 41:310-320 [DOI] [PubMed] [Google Scholar]
- 4.Ho M: Congenital and perinatal human cytomegalovirus infections. ed 2 Cytomegalovirus: Biology and Infection, 1991, :pp 205-227 Plenum Press, New York [Google Scholar]
- 5.Britt WJ, Alford CA: Cytomegalovirus. Virology, ed 3. Edited by Fiels BN, Knipe DM, Howley PM. Philadelphia, Lippincott-Raven, 1996 pp 2493–2523
- 6.Griffith PD, Grundy JE: Molecular biology and immunology of cytomegalovirus. Biochem J 1987, 241:313-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocarski ES, Jr: Cytomegalovirus and their replication. ed 3 Fiels BN Knipe DM Howley PM eds. Virology, 1996, :pp 2447-2492 Lippincott-Raven, Philadelphia [Google Scholar]
- 8.Mocarski ES, Jr, Kemble GW: Recombinant cytomegaloviruses for study of replication and pathogenesis. Intervirology 1996, 39:320-330 [DOI] [PubMed] [Google Scholar]
- 9.Staczek J: Animal cytomegaloviruses. Microbiol Rev 1990, 54:247-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsutsui Y, Kashiwai A, Kawamura N, Kadota C: Microphthalmia and cerebral atrophy induced in mouse embryos by infection with murine cytomegalovirus in midgestation. Am J Pathol 1993, 143:804-812 [PMC free article] [PubMed] [Google Scholar]
- 11.Tsutsui Y: Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection. Pathol Int 1995, 45:91-102 [DOI] [PubMed] [Google Scholar]
- 12.Nelson JA, Gnann JW, Wahren B: Regulation and tissue-specific expression of human cytomegalovirus. Curr Top Microbiol Immunol 1990, 154:75-100 [DOI] [PubMed] [Google Scholar]
- 13.Ghazal P, Nelson JA: Transcription factors and viral regulatory proteins as potential mediators of human cytomegalovirus pathogenesis. Becker Y Darai G Huang E-S eds. Molecular Aspects of Human Cytomegalovirus Diseases. 1993, :pp 360-383 Springer-Verlag, Berlin [Google Scholar]
- 14.Koedood M, Fichtel A, Meier P, Mitchell PJ: Human cytomegalovirus (HCMV) immediate-early enhancer/promoter specificity during embryogenesis defines target tissues of congenital HCMV infection. J Virol 1995, 69:2194-2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritschy J-M, Brandner S, Aguzzi A, Koedood M, Lüscher B, Mitchell PJ: Brain cell type specificity and gliosis-induced activation of the human cytomegalovirus immediate-early promoter in transgenic mice. J Neurosci 1996, 16:2275-2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baskar JF, Smith PP, Nilaver G, Jupp RA, Hoffmann S, Peffer NJ, Tenney DJ, Colberg-Poley AM, Ghazal P, Nelson JA: The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol 1996, 70:3207-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskar JF, Smith PP, Ciment GS, Hoffmann S, Tucker C, Tenney DJ, Colberg-Poley AM, Nelson JA, Ghazal P: Developmental analysis of the cytomegalovirus enhancer in transgenic animals. J Virol 1996, 70:3215-3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid EV, Christoph G, Zeller R, Leder P: The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol Cell Biol 1990, 10:4406-4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marks JR, Mercer JA, Spector DH: Transcription of mouse embryo cells permissively infected by murine cytomegalovirus. Virology 1983, 131:247-254 [DOI] [PubMed] [Google Scholar]
- 20.Keil GM, Ebeling-Keil A, Koszinowski UH: Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol 1984, 50:784-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebihara K, Minamishima Y: Protective effects of biological response modifiers on murine cytomegalovirus infection. J Virol 1984, 51:117-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercer EH, Hoyle GW, Kapur RJ, Brinster RL, Palmiter LD: The dopamine β-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron 1991, 7:703-716 [DOI] [PubMed] [Google Scholar]
- 23.Hogan B, Costantini F, Lacy E: Manipulating the Mouse Embryo: A Laboratory Manual. 1986:pp 153-186 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY,
- 24.Innis MA, Gelfand DH, Sninsky JJ, White TJ: PCR Protocols: A Guide to Methods and Applications. 1990. Academic Press, San Diego
- 25.Shinmura Y, Aiba-Masago S, Kosugi I, Li R-Y, Baba S, Tsutsui Y: Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with a murine cytomegalovirus. Am J Pathol 1997, 151:1331-1340 [PMC free article] [PubMed] [Google Scholar]
- 26.Togashi H, Sasaki M, Frohman E, Taira E, Ratan RR, Dawson TM, Dawson VL: Neuronal (type I) nitric oxide synthase regulates nuclear factor κB activity and immunologic (type II) nitric oxide synthase expression. Proc Natl Acad Sci 1997, 94:2676-2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norris JG, Tang L, Sparacio SM, Benveniste EN: Signal transduction pathways mediating astrocyte IL-6 induction by IL-1β and tumor necrosis factor-α. J Immunol 1994, 152:841-850 [PubMed] [Google Scholar]
- 28.Jain VK, Magrath IT: A chemiluminescent assay for quantitation of β-galactosidase in the femtogram range: application to quantitation of β-galactosidase in lacZ-transfected cells. Anal Biochem 1991, 199:119-124 [DOI] [PubMed] [Google Scholar]
- 29.Miller RH, Raff MC: Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 1984, 4:585-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prösch S, Staak K, Stein J, Liebenthal C, Stamminger T, Volk H-D, Krüger DH: Stimulation of the human cytomegalovirus IE enhancer/promoter in HL-60 cells by TNF-α is mediated via induction of NF-κB. Virology 1995, 208:197-206 [DOI] [PubMed] [Google Scholar]
- 31.Furth PA, Henninghausen L, Baker C, Beatty B, Woychick R: The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res 1991, 19:6205-6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kothary R, Barton SC, Franz T, Norris ML, Hettle S, Surani MAH: Unusual cell specific expression of a major human cytomegalovirus immediate early gene promoter-lacZ hybrid gene in transgenic mouse embryos. Mech Dev 1991, 35:25-31 [DOI] [PubMed] [Google Scholar]
- 33.Palmiter SD, Brinster RL: Germ-line transformation of mice. Annu Rev Genet 1986, 20:465-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang TH, Oka T, Asai T, Okada T, Merrills BW, Gertson PN, Whitson RH, Itakura K: Repression by a differentiation-specific factor of the human cytomegalovirus enhancer. Nucleic Acids Res 1996, 24:1695-1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrer I, Blanco R, Rivera R, Carmona M, Ballabriga J, Olivé M, Planas AM: CREB-1 and CREB-2 immunoreactivity in the rat brain. Brain Res 1996, 712:159-164 [DOI] [PubMed] [Google Scholar]
- 36.Kara CJ, Liou H-C, Ivashkiv LB, Glimcher LH: A cDNA for a human cyclic AMP response element-binding protein which is distinct from CREB and expressed preferentially in brain. Mol Cell Biol 1990, 10:1347-1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamminger T, Fickenscher H, Fleckenstein B: Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J Gen Virol 1990, 71:105-113 [DOI] [PubMed] [Google Scholar]
- 38.Lang D, Gebert S, Arlt H, Stamminger T: Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J Virol 1995, 69:6030-6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawlinson WD, Farrell HE, Barrell BG: Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol 1996, 70:8833-8849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlman JM, Argyle C: Lethal cytomegalovirus infection in preterm infant: clinical, radiological, and neurological findings. Ann Neurol 1992, 31:64-68 [DOI] [PubMed] [Google Scholar]
- 41.Wiley CA, Nelson JA: Role of human immunodeficiency virus and cytomegalovirus in AIDS encephalitis. Am J Pathol 1988, 133:73-81 [PMC free article] [PubMed] [Google Scholar]
- 42.Kashiwai A, Kawamura N, Kadota C, Tsutsui Y: Susceptibility of mouse embryo to murine cytomegalovirus infection in early and mid-gestation. Arch Virol 1992, 127:37-48 [DOI] [PubMed] [Google Scholar]
- 43.Tsutsui Y, Kashiwai A, Kawamura N, Aiba-Masago S, Kosugi I: Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch Virol 1995, 140:1725-1736 [DOI] [PubMed] [Google Scholar]
- 44.Balthesen M, Messerle M, Reddehase MJ: Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol 1993, 67:5360-5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins T, Pomeroy C, Jordan MC: Detection of latent cytomegalovirus DNA in diverse organs of mice. J Infect Dis 1993, 168:725-729 [DOI] [PubMed] [Google Scholar]
- 46.Manning WC, Stoddart CA, Lagenaur LA, Abenes GB, Mocarski ES, Jr: Cytomegalovirus determination of replication in salivary glands. J Virol 1992, 66:3794-3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddehase MJ, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski UH: The combination of primary infection define the load of recurrent cytomegalovirus disease. J Exp Med 1993, 179:185-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gribaudo G, Ravaglia S, Gaboli M, Gariglio M, Cavallo R, Landolfo S: Interferon-α inhibits the murine cytomegalovirus immediate-early gene expression by down-regulating NF-κB activity. Virology 1995, 211:251-260 [DOI] [PubMed] [Google Scholar]
- 49.Yurochko AD, Kowalik TF, Huong S-M, Huang E-S: Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol 1995, 69:5391-5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hermiston TW, Malone CL, Stinski MF: human cytomegalovirus immediate-early two protein region involved in negative regulation of the major immediate-early promoter. J Virol 1990, 64:3532-3536 [DOI] [PMC free article] [PubMed] [Google Scholar]