Abstract
In mice homozygous for the gene mutation for type I and type II macrophage scavenger receptors (MSR-A), MSR-A−/−, the formation of hepatic granulomas caused by a single intravenous injection of heat-killed Corynebacterium parvum was delayed significantly for 10 days after injection, compared with granuloma formation in wild-type (MSR-A+/+) mice. In the early stage of granuloma formation, numbers of macrophages and their precursor cells were significantly reduced in MSR-A−/− mice compared with MSR-A+/+ mice. In contrast to MSR-A+/+ mice, no expression of monocyte chemoattractant protein-1, tumor necrosis factor-α, and interferon-γ mRNA was observed in MSR-A−/− mice by 3 days after injection. Also in MSR-A−/− mice, uptake of C. parvum by Kupffer cells and monocyte-derived macrophages in the early stage of granuloma formation was lower and elimination of C. parvum from the liver was slower than in MSR-A+/+ mice. In the livers of MSR-A+/+ mice, macrophages and sinusoidal endothelial cells possessed MSR-A, but this was not seen in the livers of MSR-A−/− mice. In both MSR-A−/− and MSR-A+/+ mice, expression of other scavenger receptors was demonstrated. These data suggest that MSR-A deficiency impairs the uptake and elimination of C. parvum by macrophages and delays hepatic granuloma formation, particularly in the early stage.
Scavenger receptors are one of the essential receptors for macrophages, together with Fc receptors and complement receptors, and include type I and type II macrophage scavenger receptors (MSR-A), 1,2 macrophage receptor with a collagenous structure (MARCO), 3-5 CD36, 6,7 SR-BI, 7,8 dSR-CI, 9 FcγII-B2, 10 and CD68/macrosialin. 11 These scavenger receptors are divided into three classes: A, B, and C. 1-11 MSR-A and MARCO receptors are included in class A. MSR-A are trimeric membrane glycoproteins consisting of six domains: a C-terminal domain, a collagen-like domain, an α-helical coiled coil domain, a spacer domain, a transmembrane domain, and a cytoplasmic domain. 1,2 Although the C-terminal domain is type-specific (ie, different for each type of receptor), the collagen-like domain is common to both receptor types and is important for binding to ligands. 1,2,12 The α-helical coiled coil domain is involved in dissociation from ligands within endosomes and mediates cation-independent macrophage adhesion in vivo. 13 The MARCO receptor is similar in molecular structure to MSR-A but different in that it has a long collagen-like domain and an extremely short α-helical coiled coil domain. 3-5 CD36 and SR-BI are class B receptors. 6-8 CD36 is an 88-kd glycoprotein expressed on monocyte/macrophages, platelets, and endothelial cells; it serves as a receptor for both the adhesive glycoprotein thrombospondin and collagen. 6,7 SR-BI is a CD36-related receptor and is regarded as a high density lipoprotein (HDL) receptor. 7,8 CD68/macrosialin is expressed in the endolysosomal compartments and partly on the cell surface of macrophages. 11
Among these various scavenger receptors, MSR-A is the most important and can bind to a diverse array of negatively charged macromolecules, including modified low density lipoproteins (LDLs) such as acetylated LDL, oxidized LDL, advanced glycation end products, negatively charged collagen, and asbestos bodies. 14-18 MSR-A is known to be implicated in the removal of these macromolecules, in atherogenesis, 14-16 and in host defense mechanisms. 14,15
Granulomatous inflammation occurs predominantly as a focal collection of mononuclear phagocytes in response to a variety of microbial agents, foreign particulate matter, and etiologically unknown factors. For the experimental production of hepatic granulomas in animals such as mice, rats, guinea pigs, or rabbits, various microbes and particulate matter such as Corynebacterium parvum, bacillus Calmette-Guérin (BCG), Listeria monocytogenes, glucan, and zymosan have been used. 19-26 During hepatic granuloma formation, monocyte influx into the liver and differentiation of monocytes into exudate macrophages in loco occur after infiltration of neutrophils and focal collection and accumulation of monocyte/macrophages are induced in hepatic sinusoids, together with focal proliferation of Kupffer cells. 23-26 Recently, we generated mice deficient in type I and type II MSR-A by disrupting exon 4 of an MSR-A gene, which is essential for the formation of the trimeric receptor. 14,15 In these MSR-A-deficient mice, we demonstrated a significant reduction in the size of atherosclerotic lesions in mice with decreased apolipoprotein-E and in mice made deficient in LDL receptors via diet. 14-16 In addition, MSR-A-deficient mice show an increased susceptibility to infection with L. monocytogenes or herpes simplex virus-1, indicating that MSR-A may play a role in host defense against pathogens. 14,15 Although we reported a preliminary study on glucan-induced hepatic granuloma formation in MSR-A-deficient mice, 15 the role of MSR-A in hepatic granuloma formation in mice remains uncharacterized.
In the present study, MSR-A-deficient and wild-type mice were injected intravenously with heat-killed C. parvum to produce granulomas in the liver. In these hepatic granulomas, we examined the behavior and kinetics of monocyte/macrophages and their precursors, the uptake and elimination of C. parvum by macrophages, the local proliferation of macrophages, and the expression of scavenger receptors and cytokines at the message level to elucidate the role of MSR-A in hepatic granuloma formation.
Materials and Methods
Animals
Mice deficient in type I and type II MSR-A were generated by disrupting exon 4 of the MSR-A gene, which is essential for the formation of MSR-A. 14,15 A3–1 embryonic stem cells 27 containing the disrupted alleles were injected into C57BL/6J (CLEA, Hamamatsu, Japan) blastocysts. The embryos were transferred into the uteri of ICR recipient mice (CLEA). To obtain heterozygous mutants, chimeras were mated with ICR females. Brother-sister mating of heterozygotes was carried out to generate homozygous mutants. From 6- to 7-month-old homozygous MSR-A-deficient (MSR-A−/−) mice and wild-type (MSR-A+/+) littermates were examined. We excluded mice heterozygous for the MSR-A mutation (MSR-A−/+). Heat-killed C. parvum (P. acnes-WC; RIBI, Hamilton, MT), 0.5 mg, was injected into the tail vein of MSR-A−/− and MSR-A+/+ mice. All mice were killed using ether anesthesia at 1, 3, 5, 7, 10, 14, 21, or 28 days after injection and their livers were removed. Body and liver weights were measured for each mouse. Some liver tissues were frozen in liquid nitrogen and stored for mRNA analysis; others were fixed for morphological studies.
Blood Cell Count
For cell counts, a small amount of blood was sampled from the retro-orbital plexus of each animals and 1,000 white blood cells were counted on a blood film to obtain a differential count.
Light Microscopy
Liver tissues were fixed in 10% formaldehyde and embedded in paraffin. Paraffin sections 3 μm thick were prepared and stained with hematoxylin and eosin for light microscopy.
Monoclonal Antibodies
For immunohistochemistry, the following monoclonal antibodies were used: anti-mouse monoclonal antibodies against mouse macrophage F4/80, 28 against monocytic cells ER-MP20, 28 or against myeloid macrophage precursors ER-MP58 (BMA Biomedicals, August, Switzerland), 28 against murine scavenger receptors 2F8, 13-16 against murine MARCO receptor ED31, 4,5 against murine macrosialin FA/11, 29,30 against murine Fcγ II receptor 2.4G2, 10,31 against T lymphocytes Thy-1.2, 32 against murine natural killer (NK) cells pan-NK, and against murine B lymphocytes monoclonal antibody B220 (Pharmingen, San Diego, CA). 33 Dr. Siamon Gordon (Sir William Dunn School of Pathology, University of Oxford, Oxford, UK) kindly supplied 2F8 and FA/11, 13,30 and ED31 was kindly supplied by Dr. Luc J. W. van der Laan (Free University, Amsterdam, The Netherlands). 4,5 Table 1 ▶ shows the antigen specificities and immunoreactive cells of the monoclonal antibodies used in the present study. Among these antibodies, F4/80 can also be applied to paraffin sections.
Table 1.
Immunoreactive and Antigen Specificities of Monoclonal Antibodies Used in this Study
| Monoclonal antibody | Isotype | Immunoreactive cells or receptor proteins | Reference(s) |
|---|---|---|---|
| F4/80 | IgG2b | Promonocytes, monocytes, free or tissue-fixed macrophages, Kupffer cells, histocytes, synovial A cells, microglial cells, phagocytes on the periosteal and endosteal bone surfaces, epidermal Langerhans cells | 28 |
| ER-MP20 | IgG2a | Macrophage colony-forming cells, monoblasts, promonocytes, monocytes, immature macrophages | 28 |
| ER-MP58 | IgM | Myeloid precursors (granulocyte/macrophage colony-forming cells) | 28 |
| Thy-1.2 | IgG2b | T lymphocytes | 32 |
| B220 | IgG2a | B lymphocytes | 33 |
| pan-NK | IgM | Natural killer cells and T lymphocytes | |
| 2F8 | IgG2b | MSR-A | 13–16 |
| ED31 | IgG1 | MARCO recepter | 4, 5 |
| FA/11 | IgG2a | macrosialin | 29, 30 |
| 2.4G2 | IgG2b | Fc γ receptor II/III | 10, 31 |
Immunohistochemistry
Liver tissues were fixed in 2% periodate-lysine-paraformaldehyde solution at 4°C for 4 hours, embedded in OCT compound (Miles, Elkhart, IN), frozen in liquid nitrogen, and cut by a cryostat into 6-μm-thick sections. After inhibition of endogenous peroxidase activity by the method of Isobe et al, 34 immunoperoxidase studies using the above-mentioned monoclonal antibodies were performed. As a secondary antibody, we used anti-rat immunoglobulin-horseradish peroxidase-linked F(ab′)2 fragment (Amersham, Poole, UK). After visualization with 3,3′-diaminobenzidine, the sections were stained with hematoxylin for nuclear staining and mounted with resin. As negative controls, the same procedures were performed but the primary antibodies were omitted. After 0.1% trypsin treatment, paraffin sections were stained with gram stain and then immunostained with F4/80 for detection of gram-positive bacteria in macrophages.
Evaluation of Hepatic Granulomas
According to the previous studies, 23-25 hepatic granulomas were defined as being composed of more than 10 cells. The number of granulomas per 1-mm 2 section was counted. In each section, 100 granulomas were randomly selected, their diameters were measured, and their average areas were calculated.
Autoradiography with [3H]thymidine
The [3H]thymidine (specific activity, 0.3 to 0.5 MBq/mmol) was purchased from Amersham (Poole, UK) and stored at 4°C. At 3, 5, 7, 10, and 14 days after C. parvum injection, mice were injected intraperitoneally with [3H]thymidine at 1 MBq per mouse and killed 60 minutes after pulse labeling. After immunohistochemical staining with F4/80, slides were dipped in a Sakura NR-M2 liquid emulsion (Konica, Tokyo, Japan) diluted 1:2 with water, exposed at 4°C for 7 days, and developed. Cells with 10 or more grains on their nuclei above the background level were determined to be labeled.
DNA Nick End-Labeling
DNA nick end-labeling was performed with ApopTag Plus In Situ Apoptosis Detection Kit (Oncor, Gaithersburg, MD) to detect apoptotic cells in the granulomas. Briefly, formalin-fixed, paraffin-embedded tissue sections were deparaffinized with xylene and stripped of proteins by incubation with 20 mg/ml proteinase K (Sigma, St. Louis, MO) for 15 minutes at room temperature. The tissues were washed in distilled water and endogenous peroxidase was inactivated by covering the sections with 2% H2O2 for 5 minutes. Sections were then rinsed twice with phosphate-buffered saline for 5 minutes and were reacted with terminal deoxynucleotidyl transferase (TdT) with digoxigenin-nucleotide for 60 minutes at 37°C. After sections were washed with distilled water, they were incubated with anti-digoxigenin antibody with a peroxidase conjugate, visualized with 3,3′-diaminobenzidine, and treated with hematoxylin for nuclear staining.
Reverse Transcriptase-Polymerase Chain Reaction
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as described in this section. Total cellular RNA was isolated from liver tissues by the acid guanidinium thiocyanate-phenol-chloroform method. 35 Total RNA (4 mg) was mixed with 50 ng of a random primer (Gibco BRL, Life Technologies, Rockville, MD). The mixture was incubated at 95°C for 10 minutes and chilled on ice for 4 minutes. Five microliters of RT buffer, 2.5 μl of 10 nmol/L deoxyribonucleotide 3-phosphates mix (dNTPs mix) (Perkin-Elmer Corp., Branchburg, NJ), and 0.25 μl of reverse transcriptase (RT) (200 U/ml, Gibco BRL) were added with distilled water used for adjustment to the final volume of 25 μl. The mixture was incubated at 37°C for 90 minutes and at 95°C for 10 minutes. PCR amplification was performed by using a Gene Amplification PCR System 2400 (Perkin-Elmer). The reaction mixture consisted of 2 μl of cDNA, 5 μl of PCR amplification buffer, 2 μl of 25 mmol/L MgCl2, 1 μl of 10 mmol/L dNTPs mix, 2 μl of 20 mmol/L primer, 0.3 μl of Taq polymerase (5 U/ml, Perkin-Elmer), and 37.7 μl of sterile distilled water to bring the final volume to 50 μl. The primers used in this study are shown in Table 2 ▶ . The mixture was first incubated at 94°C for 5 minutes and then cycled 30 times at 95°C for 1 minute, at 58°C for 1 minute, and at 72°C for 1 minute with a final elongation step at 72°C for 5 minutes. As negative controls, samples from the livers of untreated mice were used. All samples were separated on a 2% agarose gel containing ethidium bromide at 0.3 mg/ml and bands were visualized and photographed by ultraviolet transillumination.
Table 2.
Sequences of Oligonucleotide Primers Used for RT-PCR
| mRNA | Primers | Sequences (5′ to 3′) | Products (bp) |
|---|---|---|---|
| IL-1 | Sense | TGGAATCCAGGGGAAACACTG | 288 |
| Anti-sense | CTCTAGAGCACCATGCTACAGAC | ||
| TNF-α | Sense | ACATTCGAGGCTCCAGTGAATTCGG | 309 |
| Anti-sense | GGCAGGTCTACTTTGGAGTCATTGC | ||
| IFN-γ | Sense | GTCACAGTTTTCAGCTGTATAGGG | 213 |
| Anti-sense | AGCGGCTGACTGAACTCAGATTGTAG | ||
| MCP-1 | Sense | GCATGAGGTGGTTGTGAAAAA | 350 |
| Anti-sense | CTCACCTGCTGCTACTCATTC | ||
| M-CSF | Sense | ATGAGCAGGAGTATTGCCAAGG | 187 |
| Anti-sense | ATCTCGGCTAGAGCACTTAGC | ||
| MSR-A | Sense | AGAATTTCAGCATGGCAACTG | 166 |
| Anti-sense | ACGGACTCTGACATGCAGTG | ||
| MARCO | Sense | GAAACAAAGGGGACATGGG | 280 |
| Anti-sense | TTCACACCTGCAATCCCTG | ||
| Macrosialin | Sense | TCCAAGATCCTCCACTGTTG | 364 |
| Anti-sense | CATTGTATTCCACCGCCATG | ||
| CD36 | Sense | GTGACGTGGCAAAGAACAG | 543 |
| Anti-sense | AAAGGAGGCTGCGTCTGTG | ||
| G3PDH* | Sense | GGAAAGCTGTGGCGTGATG | 382 |
| Anti-sense | CTGTTGCTGTAGCCGTATTC |
*G3PDH, glyceraldehyde-3-phosphate dehydrogenase
RT Reaction with Nested PCR
For the expression of granulocyte/macrophage colony-stimulating factor (GM-CSF) mRNA, the RT reaction was performed using a random primer and nested PCR was then done using the following primers. The primer pairs for mouse GM-CSF were designed using the published cDNA sequence. 36 The sequences of outer and inner primers were as follows: outer primer, sense: 5′-GAGGAGGATGTGGCTGCA-3′, antisense: 5′-CAGGCACAAAAGCAGCAGTG-3′, size of the amplified product, 487 bp; inner primer, sense: 5′-TATGGTCTACAGCCTCTCAGCAC-3′, antisense: 5′-CAAAGGGGATATCAGTCAGAAAGGT-3′, size of the amplified product, 367 bp.
Cell Enumeration in Tissues
In immunostained frozen sections, numbers of F4/80+, ER-MP20+, ER-MP58+, and Thy-1.2+ cells within or outside the granulomas were counted per 1-mm 2 section. In paraffin sections doubly stained with gram stain and F4/80, numbers of F4/80+ macrophages per 1-mm 2 section ingesting gram-positive C. parvum were counted using light microscopy.
Cell Culture
Phagocytosis of C. parvum by macrophages was assayed according to the method described previously, 37 with minor modifications. Instead of the quantitative fluorometric assay, we used gram staining to detect C. parvum. Mice were injected intraperitoneally with 10 ml of heparinized Hanks’ balanced salt solution (10 U/ml) and peritoneal cells were harvested and centrifuged at 200 × g for 5 minutes. After removing supernatant fluid, pellets were dissolved in 2 ml of 0.2% NaCl. After adding 2 ml of 1.6% NaCl 2 and 6 ml of Hanks’ balanced salt solution, sediments were centrifuged and pellets were resuspended in 4 ml of RPMI 1640 medium containing 10% fetal calf serum. The final peritoneal cell suspensions were adjusted to contain 2 × 10 6 cells/chamber and preincubated in a CO2 incubator at 37°C for 90 minutes. After adding 20 μg of C. parvum per chamber, the supernates were removed and washed 4 times with phosphate-buffered saline at 30 minutes and 1, 3, and 6 hours. The adhering peritoneal cells were fixed in acetone and stained with both gram stain and immunohistochemistry with F4/80. Numbers of cells ingesting C. parvum were counted.
Peritoneal macrophages harvested and prepared as above were preincubated with antibody 2.4G2 10 μg/ml to block Fcγ II receptor. One hour after incubation with opsonized C. parvum, the cells were fixed and stained as above. Numbers of macrophages ingesting C. parvum were counted. For opsonization of C. parvum, sera obtained from mice were diluted 1:2, incubated with C. parvum for 10 minutes, and centrifuged. For the blocking experiment with MARCO receptor, mice were injected intraperitoneally with 100 μg of lipopolysaccharides (LPS) to induce the emergence of MARCO-expressing macrophages, and peritoneal macrophages were harvested and cultured as above 24 hours after LPS injection. For blocking MARCO receptor, the macrophages were preincubated with ED31 10 μg/ml as described previously. 4 One hour after incubation with C. parvum, numbers of macrophages ingesting C. parvum were counted. In all three experiments, more than three animals were examined.
Statistics
Statistical significance of the data was evaluated by ANOVA with post hoc testing. P values <0.05 were considered significant.
Results
Changes in Numbers of White Blood Cells and Monocytes in Peripheral Blood after Intravenous Injection of C. parvum
After intravenous injection of heat-killed C. parvum, numbers of white blood cells in peripheral blood of MSR-A+/+ and MSR-A−/− mice increased until they peaked at 5 days, then declined. Peripheral blood leukocyte counts were lower in MSR-A−/− mice than in MSR-A+/+ mice. However, no significant differences in peripheral blood monocyte counts were found between the two types of mice.
Hepatic Granuloma Formation of MSR-A−/− Mice after Intravenous Injection of C. parvum
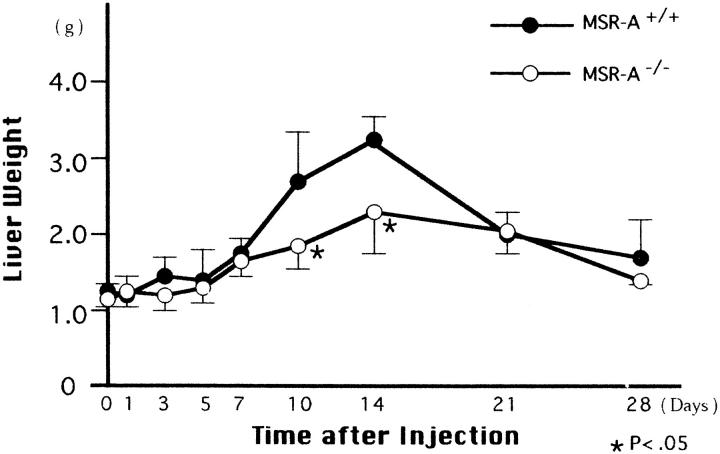
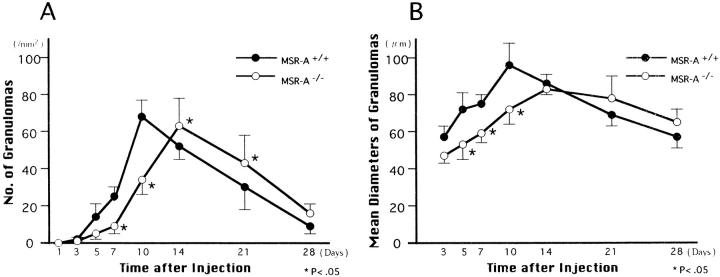
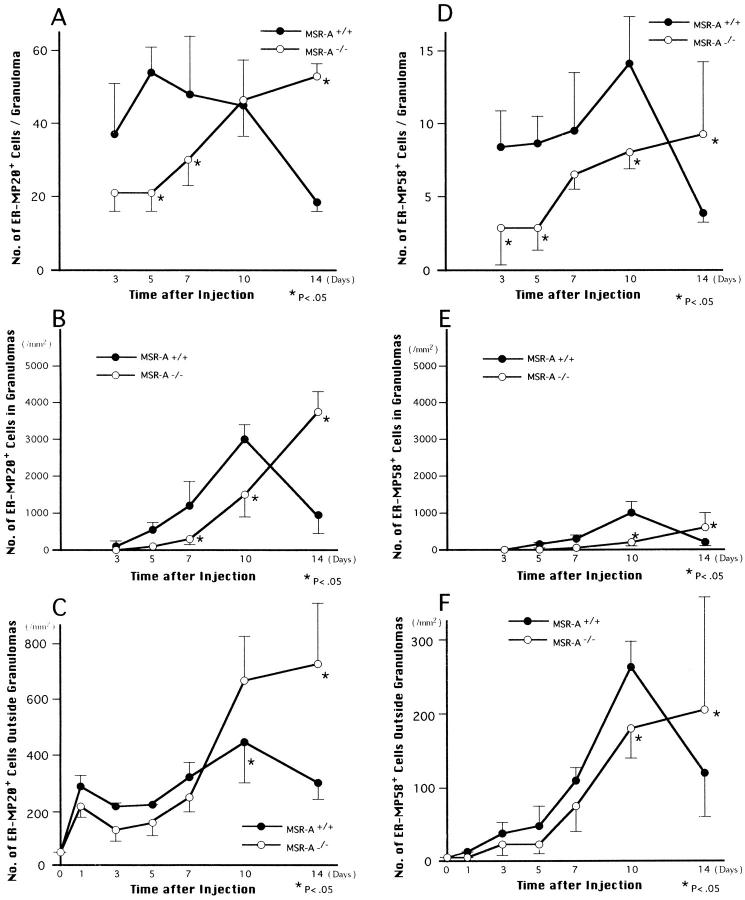
The liver started to swell 3 days after injection of C. parvum in both MSR-A−/− and MSR-A+/+ mice. The swelling peaked at 14 days and decreased thereafter (Figure 1) ▶ . Compared with that of MSR-A+/+ mice, the liver weight of MSR-A−/− mice was significantly reduced at 10 and 14 days after injection (Figure 1) ▶ . At 1 day after injection, ER-MP20+ monocytes infiltrated the hepatic sinusoids of MSR-A−/− and MSR-A+/+ mice after polymorphonuclear leukocyte infiltration (Figure 2, A and B) ▶ . In wild-type mice, F4/80+ cells aggregated and collected in the hepatic sinusoids at 3 days after injection, when granulomas started to form (Figure 2C) ▶ . The number and mean diameters of hepatic granulomas increased until they peaked at 10 days and declined thereafter (Figures 2C, 2E, 3A, and 3B) ▶ ▶ . Compared with granuloma formation in MSR-A+/+ mice, the development of hepatic granulomas in MSR-A−/− mice was delayed until 14 days after C. parvum injection and their number and mean diameters were smaller (Figures 2D, 2F, 3A, and 3B) ▶ ▶ . A comparable difference was found in granuloma area for MSR-A−/− and MSR-A+/+ mice (data not shown). During this time, numbers of F4/80+ cells within and outside the granulomas were reduced in MSR-A−/− mice compared with MSR-A+/+ mice (Figures 2E, 2F, and 4A ▶ ▶ -C). The influx of ER-MP20+ monocytic cells in the livers of MSR-A−/− mice was lower at 7 days after injection and higher thereafter than in MSR-A+/+ mice (Figures 2G, 2H, and 5A-C ▶ ▶ ). The influx of ER-MP58+ myeloid macrophage precursor cells into the hepatic granulomas and outside the granulomas peaked in both types of mice at 10 days after injection, but it was lower in MSR-A−/− mice than in MSR-A+/+ mice (Figures 2I, 2J, and 5D-F ▶ ▶ ). These data show that liver granuloma formation induced by intravenous injection of C. parvum is delayed in its early phase in the MSR-A−/− mice and that this delay results in a reduced influx of monocytic cells and myeloid macrophage precursors into the liver, particularly within the granulomas, and in their slow differentiation into macrophages within granulomas.
Figure 1.
Changes in liver weight of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of five mice.
Figure 2.
Infiltration and granuloma formation of F4/80+ macrophages (C-F), ER-MP20+ monocytic cells (A, B, G, H), and ER-MP58+ myeloid macrophage precursor cells (I, J) in the livers of homozygous mutant (MSR-A−/−) (B, D, F, H, J) and wild-type (MSR-A+/+) (A, C, E, G, I) mice after injection of C. parvum. A and B: At 1 day after injection, infiltration of ER-MP20+ monocytic cells in the liver is more marked in an MSR-A+/+ mouse (A) than in an MSR-A−/− mouse (B). C and D: At 3 days after injection, focal collections of F4/80+ macrophages and granuloma formation are seen in the liver of an MSR-A+/+ mouse (C) but not in MSR-A−/− mouse (D). E and F: At 10 days, hepatic granulomas are much more developed and larger in an MSR-A+/+ mouse (E) than in an MSR-A−/− mouse (F). G and H: At 7 days, ER-MP20+ cells in the hepatic granulomas are more numerous in an MSR-A+/+ mouse (G) than in an MSR-A−/− mouse (H). I and J: At 10 days, infiltration of ER-MP58+ cells in the liver is more marked in an MSR-A+/+ mouse (I) than in an MSR-A−/− mouse (J). A-I: Indirect immunoperoxidase method with F4/80 C-F, ER-MP20 (A, B, G, and H), and ER-MP58 (I and J). Magnification, ×200
Figure 3.
Changes in the number (A) and mean diameters (B) of granulomas in livers of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of five mice.
Figure 4.
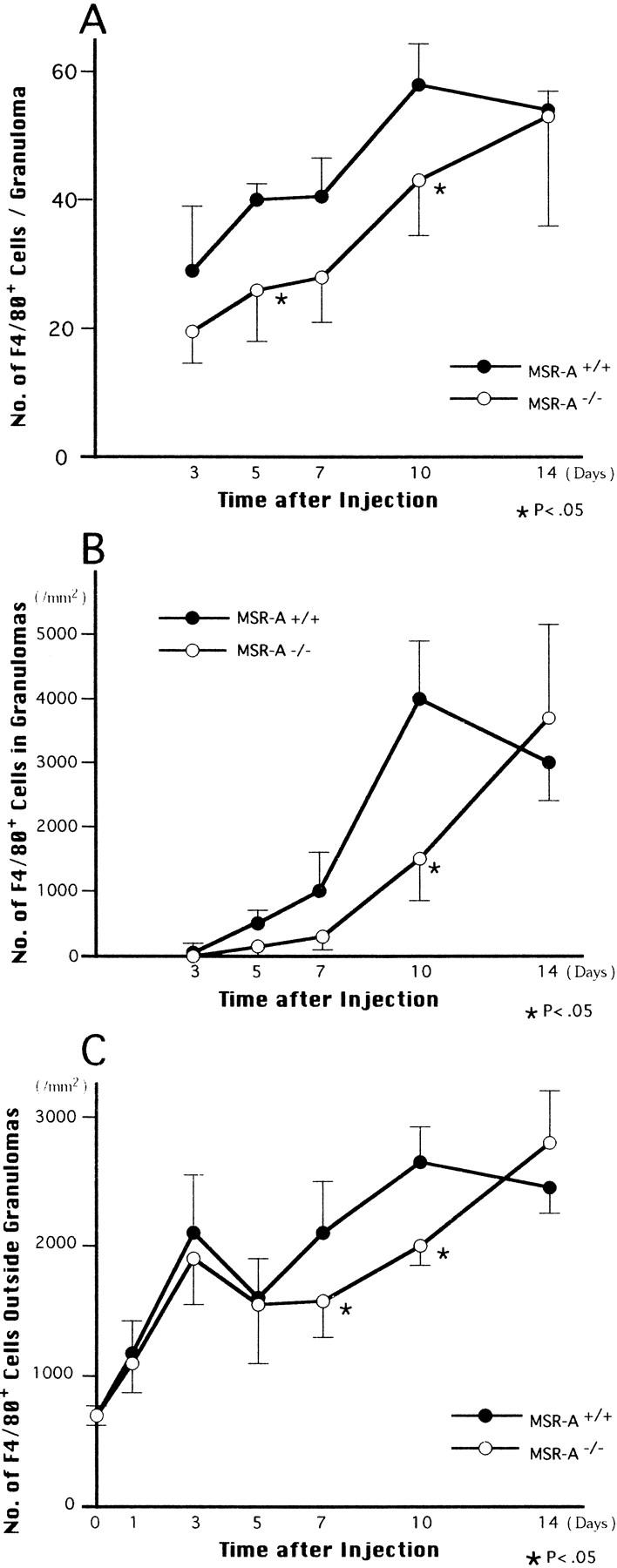
Changes in the number of F4/80+ macrophages per granuloma (A) and per 1 mm 2 within (B) and outside (C) granulomas in livers of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of five mice.
Figure 5.
Changes in the number of ER-MP20+ monocytic cells (A-C) and ER-MP58-+ myeloid macrophage precursor cells (D-F) per granuloma (A and D) and per 1 mm 2 within (B and E) and outside (C and F) granulomas in livers of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of five mice.
Local Proliferation and Cell Death of Macrophages During Hepatic Granuloma Formation
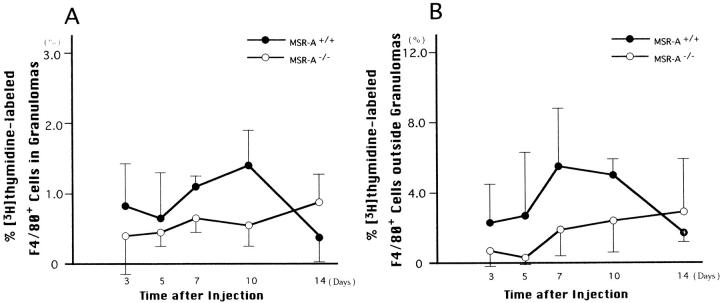
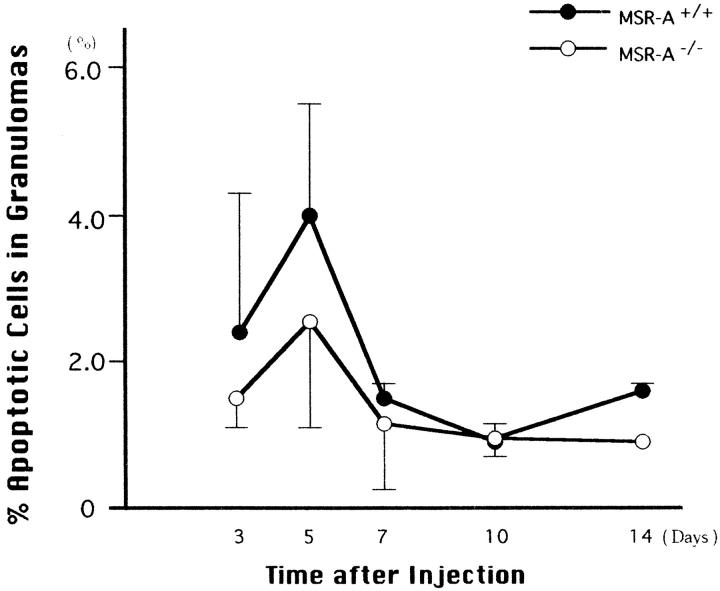
Figure 6 ▶ shows [3H]thymidine-labeling rates of macrophages within and outside hepatic granulomas of MSR-A−/− mice and MSR-A+/+ mice. The proliferative potential of macrophages within and outside the granulomas was lower in MSR-A−/− mice than in MSR-A+/+ mice 10 days after C. parvum injection. As shown in Figure 7 ▶ , the percentages of apoptotic cells in the granulomas were lower in MSR-A−/− mice than in MSR-A+/+ mice. However, these differences are not statistically significant (P > 0.05).
Figure 6.
[3H]thymidine labeling of F4/80+ cells within (A) and outside (B) granulomas in livers of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of three mice.
Figure 7.
Percentages of apoptotic cells in hepatic granulomas of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of three mice.
Changes in Numbers of Lymphocytes During Hepatic Granuloma Formation
A small number of lymphocytes were usually present in livers of MSR-A+/+ and MSR-A−/− mice. After C. parvum injection, lymphocytes infiltrated livers of both MSR-A+/+ and MSR-A−/− mice; most of these lymphocytes were Thy-1.2+ T cells. Approximately 10 to 30 Thy-1.2+ T cells were seen within a granuloma in both MSR-A+/+ and MSR-A−/− mice (Figure 8A) ▶ , but the numbers of T cells per 1 mm 2 increased within and outside granulomas in both types of mice in parallel with increases in the numbers and diameters of the granulomas (Figures 3, 8B, and 8C) ▶ ▶ . In addition, NK cells were observed in the hepatic granulomas. However, the numbers of T cells per 1 mm 2 within and outside the granulomas were significantly lower in MSR-A−/− mice 10 days after injection than in MSR-A+/+ mice and exceeded the latter at 14 days (Figure 8, B and C) ▶ . These data indicate that T cell infiltration into the liver during granuloma formation in response to injected C. parvum is lower in MSR-A−/− mice than in MSR-A+/+ mice.
Figure 8.
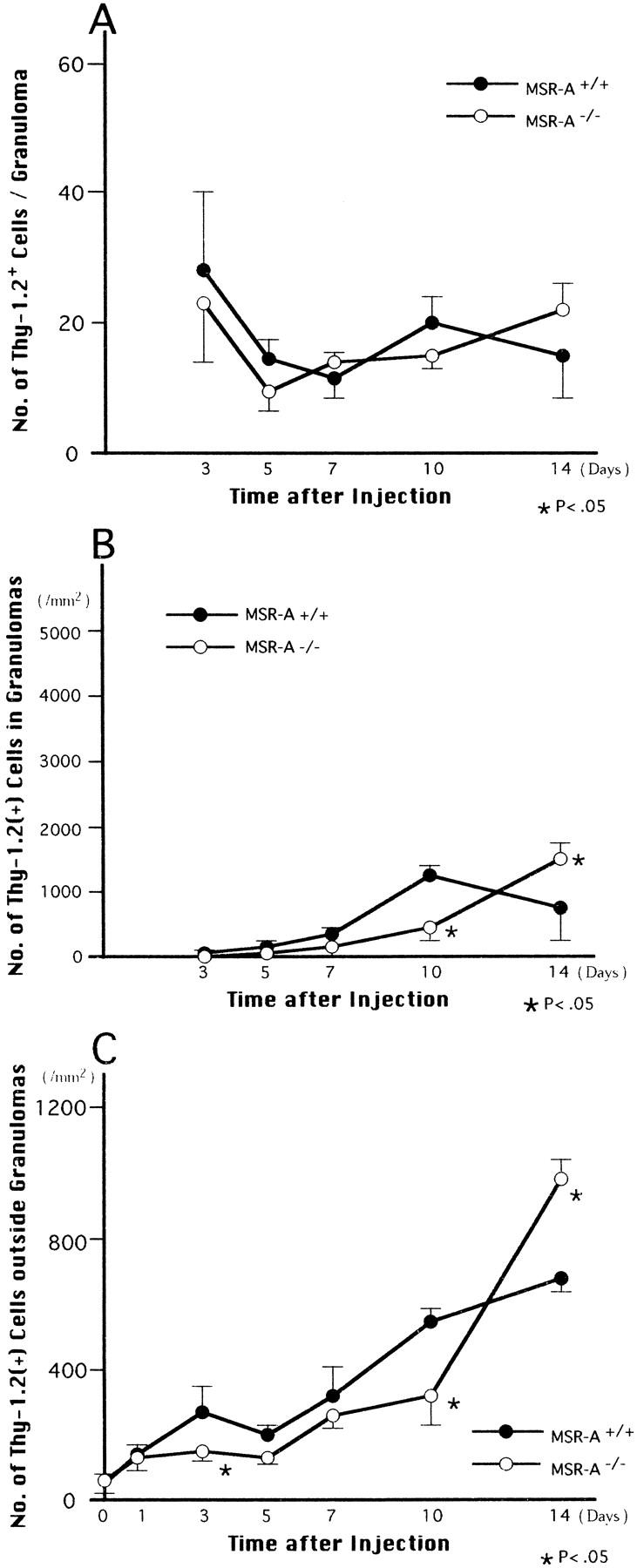
Changes in the number of Thy-1.2+ T cells per granuloma (A) and per 1 mm 2 within (B) and outside (C) granulomas in livers of thomozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of five mice.
Expression of Proinflammatory Cytokine mRNA During Hepatic Granuloma Formation
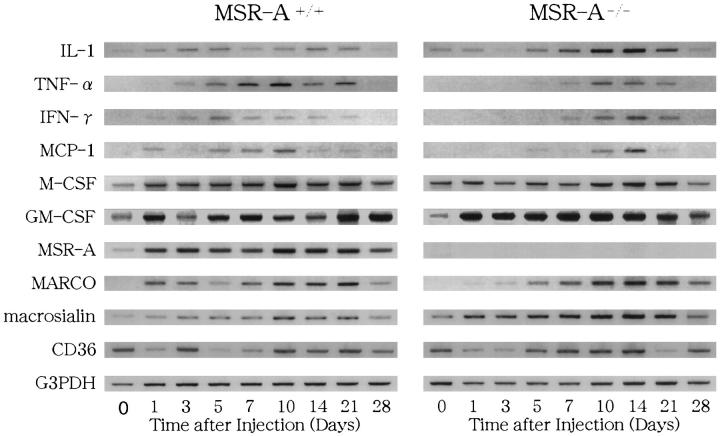
Figure 9 ▶ shows the expression of interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), macrophage colony-stimulating factor (M-CSF), and GM-CSF mRNA in the livers of MSR-A−/− and MSR-A+/+ mice revealed by RT-PCR or RT-nested PCR using cDNA primers for these cytokines. As shown in Figure 9 ▶ , IL-1, M-CSF, and GM-CSF mRNAs were expressed in both MSR-A−/− and MSR-A+/+ mice throughout the experimental period. Although TNF-α mRNA was expressed in MSR-A+/+ mice from 1 to 28 days after injection, it was not expressed in MSR-A−/− mice from 1 to 3 days after injection and was seen from 5 days onward (Figure 9) ▶ . MSR-A+/+ mice showed expression of IFN-γ mRNA in the liver, but IFN-γ mRNA was not detected in livers of MSR-A−/− mice from 1 to 3 days after injection (Figure 9) ▶ . MCP-1 mRNA was not expressed in livers of MSR-A−/− mice 1 to 3 days after injection, it was expressed in MSR-A−/− mice from 5 to 28 days and in MSR-A+/+ mice from 1 to 28 days after injection (Figure 9) ▶ .
Figure 9.
Expression of IL-1, TNF-α, IFN-γ, MCP-1, M-CSF, GM-CSF, MSR-A, MARCO, macrosialin, and CD36 mRNA in the livers of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. The result from one of two similar experiments is shown.
Expression of Scavenger Receptors in the Hepatic Granuloma Formation
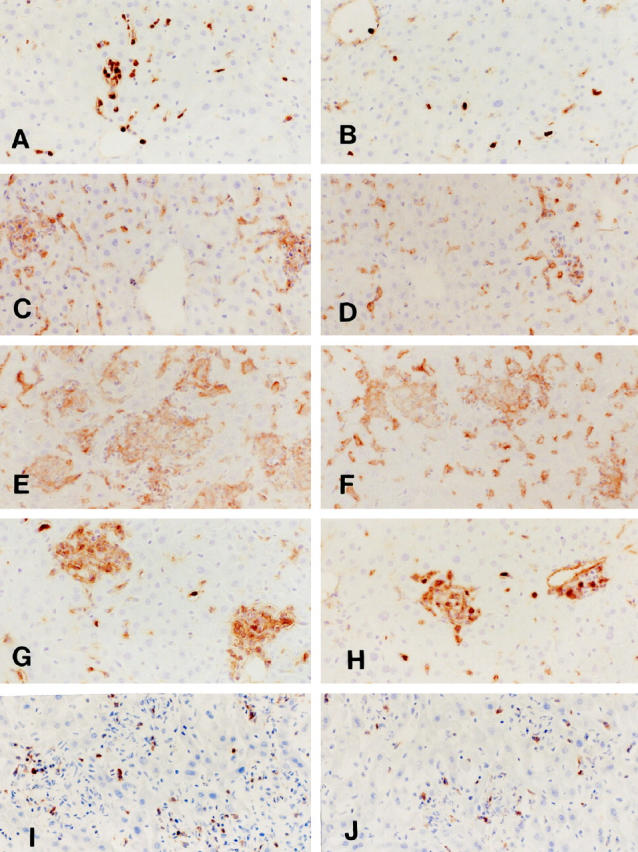
Previous findings showed that Kupffer cells and sinusoidal endothelial cells were positive for 2F8 in MSR-A+/+ mice, whereas they showed a negative reaction in the MSR-A−/− mice. 13,14 Within and outside liver granulomas, macrophages and sinusoidal endothelial cells also showed an intense positive reaction for 2F8 in MSR-A+/+ mice throughout the experimental period (Figure 11A) ▶ , whereas all such cells were negative for 2F8 in MSR-A−/− mice (Figure 11B) ▶ . In both MSR-A+/+ and MSR-A−/− mice, the sinusoidal cells, including Kupffer cells, did not express MARCO before injection of C. parvum. From 1 day after injection, Kupffer cells expressed MARCO, and the number of ED31+ macrophages increased within and outside liver granulomas in both MSR-A+/+ and MSR-A−/− mice (Figure 11, C and D) ▶ ▶ . In MSR-A+/+ and MSR-A−/− mice, Kupffer cells were positively stained with FA/11 before C. parvum injection, and macrophages within and outside hepatic granulomas were intensely positive for FA/11 after stimulation (Figure 11, E and F) ▶ ▶ . In MSR-A+/+ and MSR-A−/− mice, Kupffer cells and sinusoidal endothelial cells before injection, as well as macrophages within and outside granulomas after injection, were positive for monoclonal antibody against Fcγ receptor II, 2.4G2 (Figure 11, G and H) ▶ . As shown in Figure 9 ▶ , expression of MSR-A, MARCO, and macrosialin mRNA was consistent with the immunohistochemical expression of these scavenger receptors in both MSR-A+/+ and MSR-A−/− mice. Also, expression of CD36 mRNA was proved in both MSR-A+/+ and MSR-A−/− mice throughout the experimental period (Figure 9) ▶ .
Figure 11.

Immunohistochemical expression of various types of scavenger receptors during hepatic granuloma formation in homozygous mutant (MSR-A−/−) (B, D, F, and H) and wild-type (MSR-A+/+) (A, C, E, and G) mice at 10 days after injection of C. parvum. A and B: Macrophages within and outside granulomas and sinusoidal endothelial cells express immunoreactivity for 2F8 in MSR-A+/+ mice (A) but not in MSR-A−/− mice (B). C-F: Macrophages within and outside hepatic granulomas of MSR-A+/+ and MSR-A−/− mice are positive for ED31 (C and D) and FA/11 (E and F). G and H: Macrophages within and outside granulomas and sinusoidal endothelial cells express Fcγ II receptor in livers of MSR-A+/+ (G) and MSR-A−/− (H) mice. A-H: Indirect immunoperoxidase method with 2F8 (A and B), ED31 (C and D), FA/11 (E and F), and 2.4G2 (G and H). Magnification, ×200
Uptake and Elimination of C. parvum by Macrophages in the Liver of MSR-A−/− Mice
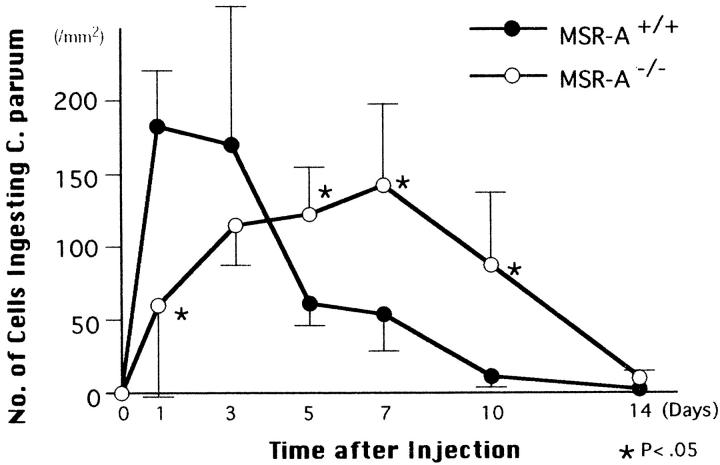
Figure 10 ▶ shows changes in numbers of F4/80+ macrophages ingesting gram-positive C. parvum in the livers of MSR-A−/− and MSR-A+/+ mice. At 1 day after injection of C. parvum into MSR-A+/+ mice, the number of cells that had ingested gram-positive bacteria peaked in the liver; this number decreased thereafter. At 1 day, approximately 14% of Kupffer cells ingested gram-positive bacteria and distributed throughout the hepatic lobules of MSR-A+/+ mice. In MSR-A−/− mice, the numbers of macrophages that had ingested gram-positive bacteria were low at 1 day, increased to a peak at 7 days, and declined thereafter. At 14 days, most gram-positive bacteria were eliminated from the livers of both MSR-A−/− and MSR-A+/+ mice. During the process of hepatic granuloma formation, macrophages ingesting gram-positive bacteria were collected in the granulomas, with such macrophages in MSR-A+/+ mice tending to show greater ingestion of bacteria than those in MSR-A−/− mice. However, numbers of gram-positive bacteria ingested by macrophages varied from cell to cell.
Figure 10.
Changes in the number of F4/80+ macrophages ingesting gram-positive C. parvum in livers of homozygous mutant (MSR-A−/−) and wild-type (MSR-A+/+) mice after injection of C. parvum. Data are mean ±SD of five mice
In Vitro Uptake of C. parvum by Peritoneal Macrophages via Scavenger Receptor
Figure 12 ▶ shows the percentages of peritoneal macrophages ingesting C. parvum in total adherent peritoneal cells in MSR-A+/+ and MSR-A−/− mice in the early stage of phagocytosis in vitro. The percentages of macrophages ingesting C. parvum were reduced in MSR-A−/− mice compared with MSR-A+/+ mice: the differences at 3 and 6 hours after injections were significant (Figure 12A) ▶ . These data provide direct evidence of the involvement in uptake of C. parvum by macrophages via MSR-A.
Figure 12.
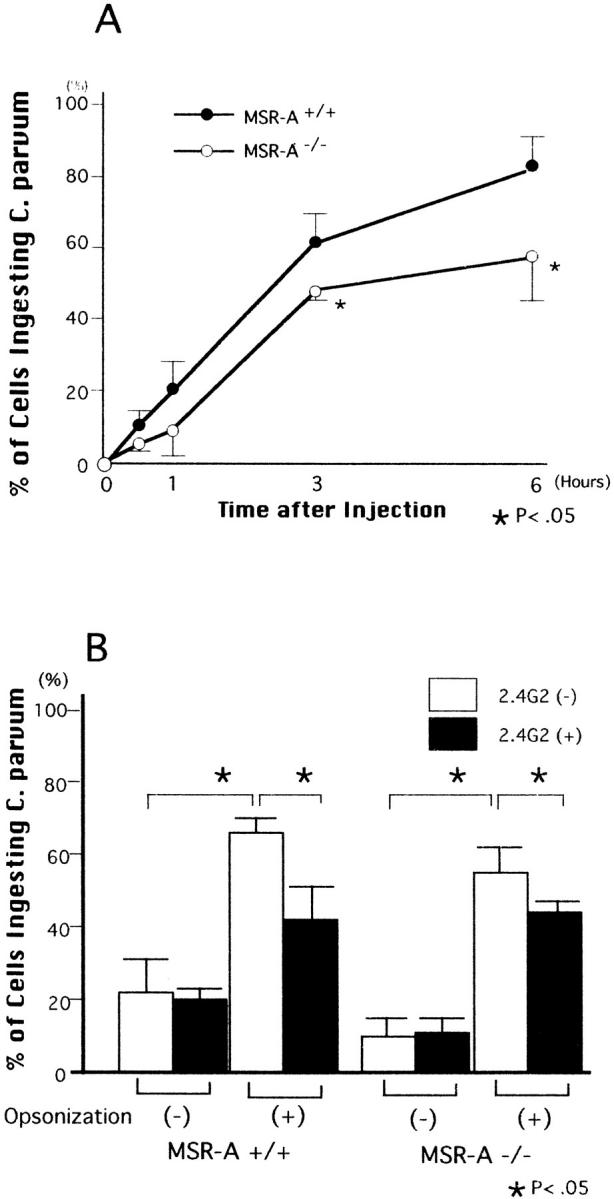
Shows in vitro uptake of C. parvum by peritoneal macrophages via scavenger receptors in the early stage of phagocytosis. A: Compared with MSR-A-expressing macrophages, the uptake of C. parvum by MSR-A-deficient macrophage was significantly reduced. B: MSR-A-expressing and MSR-A-deficient macrophages show no difference in uptake of nonopsonized C. parvum. Compared with untreated macrophages, macrophages with Fcγ II receptor blocking show significant reductions in uptake of opsonized C. parvum in both MSR-A+/+ and MSR-A−/− mice. □, untreated; ▪, blocked with 2.4G2. Data are mean ±SD of three assays.
Further, we performed two in vitro experiments to examine whether scavenger receptors such as Fcγ II receptor or MARCO receptor can participate in binding to and uptake of C. parvum by macrophages. In MSR-A+/+ and MSR-A−/− mice, marked increases in the uptake of opsonized C. parvum by macrophages were demonstrated, compared with the uptake of non-opsinized C. parvum. (Figure 12B) ▶ . Uptake of non-opsinized C. parvum by untreated macrophages was not different from that of Fcγ II receptor-blocked macrophages. In contrast, the uptake of opsinized C. parvum by untreated macrophages was significantly higher than that of macrophages with Fcγ II receptor blocking (P < 0.05, Figure 12B ▶ ).
In untreated wild-type mice, peritoneal macrophages did not express MARCO receptor in a normal steady state condition. However, MARCO was expressed on the macrophages at 24 hours after LPS injection. We examined in vitro uptake of C. parvum by MARCO-expressing macrophages with or without preincubation with ED31, a monoclonal MARCO antibody. As the results indicate, we could not find any significant differences in the number of MARCO-positive macrophages ingesting C. parvum between macrophages preincubated with ED31 and those without ED31 preincubation (data not shown).
Discussion
The present study has revealed that liver granuloma formation in mice homozygous for the MSR-A gene mutation, compared with that in wild-type mice, is impaired and delayed for 10 days after a single injection of heat-killed C. parvum. During the early stage of granuloma formation, the numbers of F4/80+, ER-MP20+, and ER-MP58+ cells in the granulomas are reduced in mutant mice compared with wild-type mice. During this processes, the mRNA expression of proinflammatory cytokines such as MCP-1, TNF-α, and IFN-γ is impaired in the livers of mutant mice, whereas M-CSF, GM-CSF, and IL-1 are expressed at the message level in both mutant and wild-type mice. In mutant mice, MSR-A is deficient on Kupffer cells, sinusoidal endothelial cells, and infiltrated macrophages in the granulomas, but other scavenger receptors such as MARCO, macrosialin, CD36, and Fcγ II receptors are expressed immunohistochemically and/or at the message level, as in wild-type mice.
In granulomatous inflammation in the liver, Kupffer cells respond first to exogenously invading pathogens, induce infiltration of polymorphonuclear leukocytes and monocytes into the liver, and promote monocyte differentiation into macrophages. These processes were clearly demonstrated in previous studies in mice depleted of Kupffer cells by administration of liposome-encapsulated dichloromethylene diphosphonate. 26 Hepatic granuloma formation was markedly delayed in the Kupffer cell-depleted mice in that it began to occur at 5 days after stimulation, followed by a marked delay of monocyte influx into the liver and of differentiation of monocytes into macrophages. 26 In previous studies, marked impairments of hepatic granuloma formation were also demonstrated in mice depleted of blood monocytes by administration of strontium-89 (89Sr) 23,24 and in mice homozygous for the osteopetrosis (op) mutation. 25 In the 89Sr-induced, severely monocytopenic mice, a marked delay of glucan-induced hepatic granuloma formation was caused by a lack of supply and mobilization of monocytes from the bone marrow into the liver; hepatic granulomas were formed exclusively by local proliferation of Kupffer cells. 23,24 In op/op mice defective in the production of functional M-CSF protein, hepatic granuloma formation was impaired by an insufficient supply of monocytes and defective differentiation of monocytes into macrophages; granulomas were formed mainly by local proliferation and M-CSF-independent maturation of immature Kupffer cells. 25 In contrast to these three mouse models, however, the numbers of Kupffer cells in the liver and of monocytes in peripheral blood were normal in homozygous MSR-A-deficient mice under normal steady-state conditions. During the processes of C. parvum-induced hepatic granuloma formation, local proliferation of Kupffer cells was not significantly different between homozygous mutant and wild-type mice.
MSR-A exhibits broad binding specificities for negatively charged polyanionic ligands such as modified lipoproteins, lipoteichoic acid of gram-positive bacteria, and lipopolysaccharides of gram-negative bacteria. 38,39 Because C. parvum is also negatively charged, macrophages are able to ingest C. parvum via MSR-A. In our in vitro study, we confirmed a reduced uptake of C. parvum by peritoneal macrophages from MSR-A-deficient mice in the early stage of phagocytosis, compared with those from wild-type mice. In the present in vivo study, we further demonstrated reduced uptake of C. parvum by Kupffer cells and monocyte-derived macrophages in the liver of MSR-A-deficient mice compared with wild-type mice. The elimination of C. parvum by macrophages in the liver was delayed in mutant mice compared with wild-type mice. The delay of hepatic granuloma formation in the mutant mice seems to be caused by the reduced uptake of C. parvum by Kupffer cells and monocyte-derived macrophages in hepatic granulomas due to MSR-A deficiency.
A study of Kupffer cell-depleted mice showed a marked delay of zymosan-induced granuloma formation and suppressed expression of IL-1, MCP-1, TNF-α, and IFN-γ mRNA, suggesting that these cytokines are produced mainly by Kupffer cells and monocyte-derived macrophages. 26 Recently, IFN-γ, TNF-α, MCP-1, macrophage inflammatory protein-1 (MIP-1), and IL-6 have been demonstrated to play a role in the development of hepatic and pulmonary granulomas in humans and animals. 19,21,22,40-48 In a study of a viable BCG infection, MSR-A-deficient mice infected with BCG recruited macrophages to the sites of granuloma formation, activated them in situ, and restricted BCG replication. 49 The present study revealed a marked delay in the expression of MCP-1, TNF-α, and IFN-γ mRNA in the early stage of C. parvum-induced granuloma formation in MSR-A-deficient mice, suggesting that such a delay is caused mainly by marked reductions in the uptake and elimination of heat-killed C. parvum by macrophages and in intracellular signaling for the production of these cytokines. It is known that multiple signal transduction pathways, including protein kinase C, or tyrosine phosphorylation are involved in the production of proinflammatory cytokines such as MCP-1, IL-1, TNF-α, or IFN-γ by monocyte/macrophages. 50-55 Although knowledge regarding the signal transduction pathways induced by ligand binding to MSR-A is limited, 56,57 a recent study demonstrated that binding of both modified LDL and non-LDL ligands to MSR-A induced tyrosine phosphorylation and increased protein kinase C activity that led to up-regulated urokinase-type plasminogen activator expression by macrophages, suggesting that MSR-A acts as a signaling receptor. Considering these results together, it is speculated that MSR-A deficiency impairs signal transduction through protein kinase-dependent pathways common to those for the production of proinflammatory cytokines in macrophages.
MCP-1 belongs to the CC subfamily of chemokines on the basis of adjacent position of first two cysteines. 58 Its main biological action is chemotactic activity for monocytes and T cells in tissues. 59 MCP-1 is produced by Kupffer cells and endothelial cells in response to inflammatory stimuli. 60,61 In inflammation, infiltrating leukocytes and monocyte-derived macrophages are also able to produce MCP-1 in the liver. 62 Among these cells, Kupffer cells respond first to circulating pathogens and are the major cells for production of MCP-1 in the liver. 26 Thus, no expression of MCP-1 mRNA in the liver of MSR-A-deficient mice in the early stage of granuloma formation seems to be caused by impaired binding to and uptake of C. parvum by Kupffer cells due to MSR-A deficiency, induced insufficient supply and mobilization of monocytes from peripheral blood into the liver, or delays in the infiltration into and accumulation of macrophages in hepatic granulomas. In addition, the present study demonstrated a reduced number of T cells in the liver of MSR-A-deficient mice. These findings suggest the cause of the delayed IFN-γ mRNA expression, because T cells and NK cells and macrophages are the major cells for the production of IFN-γ. 63,64
In contrast to the Kupffer cell-depleted mice, 26 MSR-A-deficient mice showed expression of IL-1 mRNA in the liver before and after C. parvum injection, as did wild-type mice. Because tissue macrophages, including Kupffer cells in the liver, are known to produce IL-1 mRNA in a normal steady-state condition, 22,65 expression of IL-1 mRNA in the liver of both homozygous MSR-A-deficient and wild-type mice is suggested to occur regardless of stimuli or MSR-A deficiency.
The present study also demonstrated the expression of M-CSF and GM-CSF mRNA in both MSR-A-deficient and wild-type mice before and after C. parvum injection. This result indicates that both macrophage growth factors are expressed at the message level regardless of MSR-A deficiency, because M-CSF and GM-CSF are known to be produced by hepatocytes, Kupffer cells, and sinusoidal endothelial cells 66,67 and because expression of M-CSF mRNA was also reported in Kupffer cell-depleted and control mice. 26
The present investigation has demonstrated that MSR-A deficiency impairs the uptake of C. parvum by Kupffer cells and monocyte-derived macrophages and thus induces a marked delay in hepatic granuloma formation in MSR-A-deficient mice. In addition, the elimination of C. parvum by Kupffer cells and monocyte-derived macrophages from the liver of homozygous mutant mice was delayed, and the bacteria remained longer in macrophages, mainly within the hepatic granulomas, compared with the situation in the wild-type mice. Because several types of scavenger receptors besides type I and type II MSR-A are also known, 1-18 we examined the expression of MARCO receptor, macrosialin/CD68, Fcγ receptor II-B2, and CD36 in the process of the hepatic granuloma formation in homozygous mutant mice and demonstrated the immunohistochemical expression of these proteins and/or their expression at the message level. Among these membrane proteins, MARCO is in the same class as type I and type II MSR-A and is known to be deeply involved in the uptake of bacterial antigens and neutral polysaccharides, and it is expressed in marginal zone macrophages in the spleen and macrophages in lymphatic sinuses of lymph nodes, but not in other tissues, including the liver, in unstimulated normal mice. 3-5 However, this receptor is expressed not only on Kupffer cells but also on infiltrated monocyte-derived macrophages in the livers of mice infected with BCG 5 or suffering from endotoxic shock induced by Klebsiella pneumonia. 4 In MSR-A-deficient and wild-type mice, we detected no expression of MARCO and its mRNA in the liver before C. parvum injection by immunohistochemistry with anti-MARCO monoclonal antibody ED31 and by RT-PCR using a primer for MARCO. After injection, however, expression of MARCO receptor was demonstrated on Kupffer cells and monocyte-derived macrophages immunohistochemically, as was the expression of MARCO mRNA in the liver. However, C. parvum was not evidently demonstrated in MARCO-expressing marginal zone macrophages in the present study (data not shown). Furthermore, to clarify whether MARCO receptor can participate in the uptake of C. parvum, we examined the uptake of the bacteria by MARCO-expressing peritoneal macrophages in vitro. However, we could not confirm any data supporting the uptake of C. parvum by macrophages via MARCO receptor.
The present study demonstrated the expression of Fcγ II receptors in Kupffer cells and sinusoidal endothelial cells before and after C. parvum injection, as well as in monocyte-derived macrophages and granuloma macrophages after C. parvum injection, in the liver of homozygous mutant and wild-type mice. In previous studies, the expression of Fcγ II receptors on splenic macrophages and significant increases in phagocytic cells with the receptors were induced by intraperitoneal injection of C. parvum, 68 antibody-coated bacteria were recognized by Fcγ I and II receptors and were taken up by macrophages after cross-linking with the receptors, 31,69 and the intracellular killing of gram-positive bacteria (Staphylococcus aureus) by monocytes after cross-linking with Fcγ I and II receptors occurred in a phospholipase C-dependent process. 70 In our present in vitro assays for the binding of Fcγ II receptor to C. parvum, marked increases in the uptake of the opsonized bacteria by peritoneal macrophages compared with the uptake of non-opsonized bacteria were demonstrated in both types of mice. We found a marked reduction in the uptake of opsonized C. parvum by peritoneal macrophages after blocking the receptor with neutralizing monoclonal antibody 2.4G2. Our present data suggest that immune receptors including Fcγ II receptor are also involved in uptake of C. parvum by macrophages in the process of immune phagocytosis during hepatic granuloma formation in mutant mice lacking MSR-A.
Our recent studies demonstrated the expression of macrosialin and CD36 at protein and message levels in spontaneous and diet-induced atherosclerosis of double knockout mice lacking MSR-A and apolipoprotein-E or LDL receptor, suggesting that both receptor proteins are implicated in the accumulation of oxidized LDL in atherosclerotic lesions. 14-16 Although the role of these scavenger receptors in the uptake of C. parvum by macrophages and their involvement in hepatic granuloma formation are not clear, the present study showed the expression of macrosialin and CD36 in hepatic granulomas. This may reflect a possible role for these receptors at certain steps in the process of hepatic granuloma formation. The functional roles of macrosialin and CD36 receptors, together with complement receptors or mannosyl-fucosyl receptors, in granulomatous inflammation should be elucidated in subsequent studies.
In conclusion, MSR-A is important for the binding to gram-positive bacteria such as C. parvum, uptake of the bacteria by macrophages, hepatic granuloma formation, and expression of certain proinflammatory cytokines such as IFN-γ, TNF-α, and MCP-1.
Acknowledgments
We thank Dr. Siamon Gordon of the Sir William Dunn School of Pathology, University of Oxford, for kindly supplying monoclonal antibodies against murine scavenger receptors 2F8 and FA/11. We also thank Mr. Takenobu Nakagawa and Ms. Emi Miyata, Second Department of Pathology, Kumamoto University School of Medicine, for their skillful technical assistance and Ms. Judith Gandy for her reading of the manuscript, suggestions, and editing.
Footnotes
Address reprint requests to Kiyoshi Takahashi, M.D., Second Department of Pathology, Kumamoto University School of Medicine, 2–2-1 Honjo, Kumamoto 860-0811, Japan.
Supported in part by Grants-in-Aid for scientific research from the Ministry of Education, Science, and Culture, Japan (08457071 and 09877048).
References
- 1.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M: Type I macrophage scavenger receptor contains α-helical, and collagen-like coiled coils. Nature 1990, 343:531-535 [DOI] [PubMed] [Google Scholar]
- 2.Rohrer L, Freeman M, Kodama T, Penman M, Krieger M: Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature 1990, 343:570-572 [DOI] [PubMed] [Google Scholar]
- 3.Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen S, Liakka A, Thesleff I, Kraal G, Tryggvason K: Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 1995, 80:603-609 [DOI] [PubMed] [Google Scholar]
- 4.van der Laan LJW, Kangas M, Döpp EA, Holub E, Elomaa O, Kraal G, Tryggvason K: Macrophage scavenger receptor MARCO: In vitro and in vivo regulation and involvement in the anti-bacterial host defense. Immunol Lett 1997, 57:203-208 [DOI] [PubMed] [Google Scholar]
- 5.van der Laan LJW, Döpp EA, Haworth R, Pikkarainen T, Kangas M, Elomaa O, Dijkstra CD, Gordon S, Tryggvason K, Kraal G: Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J Immunol 1999, 162:939-947 [PubMed] [Google Scholar]
- 6.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA: CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 1993, 268:11811-11816 [PubMed] [Google Scholar]
- 7.Acton SL, Scherer PE, Lodish HF, Krieger M: Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem 1994, 269:21003-21009 [PubMed] [Google Scholar]
- 8.Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH: Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest 1996, 98:984-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson A, Lux A, Krieger M: Expression cloning of dSR-CI, a class C macrophage-specific scavenger receptor from Drosophila melanogaster. Proc Natl Acad Sci USA 1995, 92:4056-4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanton LW, White RT, Bryant CM, Protte AA, Endemann G: A macrophage Fc receptor for IgG is also a receptor for oxidized low density lipoprotein. J Biol Chem 1992, 267:22446-22451 [PubMed] [Google Scholar]
- 11.Ramprasad MP, Fischer W, Witztum JL, Sambrano GR, Quehenberger O, Steinberg D: The 94- to 97-kd mouse macrophage membrane protein that recognizes oxidized low density lipoprotein, and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc Natl Acad Sci USA 1995, 92:9580-9584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emi M, Asaoka H, Matsumoto A, Itakura H, Kurihara Y, Wada Y, Kanamori H, Yazaki Y, Takahashi E, Lepert M, Lalouel J, Kodama T, Mukai T: Structure, organization, and chromosomal mapping of the human macrophage scavenger receptor gene. J Biol Chem 1993, 268:2120-2125 [PubMed] [Google Scholar]
- 13.Fraser I, Hughes D, Gordon S: Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature 1993, 364:343-346 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Horiuchi S, Takahashi K, Kodama T: A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 1997, 386:292-296 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Sakaguchi H, Kruijt JK, Higashi T, Suzuki T, Berkel TJC, Horiuchi S, Takahashi K, Yazaki Y, Kodama T: The multiple roles of macrophage scavenger receptors (MSR) in vivo: resistance to atherosclerosis and susceptibility to infection in MSR knockout mice. J Atheroscler Thromb 1997, 4:1-11 [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi H, Takeya M, Suzuki H, Hakamata H, Kodama T, Horiuchi S, Gordon S, van der Laan LJW, Kraal G, Ishibashi S, Kitamura N, Takahashi K: Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest 1998, 78:423-434 [PubMed] [Google Scholar]
- 17.Krieger M, Acton S, Ashkenas J, Person A, Penman M, Resnick D: Molecular flypaper, host defense, and atherosclerosis. J Biol Chem 1993, 268:4569-4572 [PubMed] [Google Scholar]
- 18.Mori T, Takahashi K, Higashi T, Takeya M, Kume S, Kawabe Y, Kodama T, Horiuchi S: Localization of advanced glycation end products of Maillard reaction in bovine tissues and their endocytosis by macrophage scavenger receptors. Exp Mol Pathol 1995, 63:135-152 [DOI] [PubMed] [Google Scholar]
- 19.Senaldi G, Yin S, Shaklee CL, Piguet PF, Mak TW, Ulich TR: Corynebacterium parvum-and Mycobacterium bovis bacillus Calmette-Guérin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice, and by treatment with soluble TNF-RI. J Immunol 1996, 157:5022-5026 [PubMed] [Google Scholar]
- 20.Tsuji N, Kawada N, Ikeda K, Kinoshita H, Kaneda K: Immunohistochemical and ultrastructural analyses of in situ activation of hepatic stellate cells around Propionibacterium acnes-induced granulomas in the rat. J Submicrosc Cytol Pathol 1997, 29:1892-1898 [PubMed] [Google Scholar]
- 21.Gordon S, Keshav S, Stein M: BCG-induced granuloma formation in murine tissues. Immunobiology 1994, 191:369-377 [DOI] [PubMed] [Google Scholar]
- 22.Wagner RD, Czuprynski CJ: Cytokine mRNA expression in liver of mice infected with Listeria monocytogenes. J Leukoc Biol 1993, 53:527-531 [DOI] [PubMed] [Google Scholar]
- 23.Yamada M, Naito M, Takahashi K: Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukoc Biol 1990, 47:195-205 [PubMed] [Google Scholar]
- 24.Naito M, Takahashi K: The role of Kupffer cells in glucan-induced granuloma formation in the liver of mice depleted of blood monocytes by administration of strontium-89. Lab Invest 1991, 64:664-674 [PubMed] [Google Scholar]
- 25.Takahashi K, Naito M, Umeda S, Shultz LD: The role of macrophage colony-stimulating factor in hepatic glucan-induced granuloma formation in the osteopetrosis mutant mouse defective in the production of macrophage colony-stimulating factor. Am J Pathol 1994, 144:1381-1392 [PMC free article] [PubMed] [Google Scholar]
- 26.Moriyama H, Yamamoto T, Takatsuka H, Umezu H, Tokunaga K, Nagano T, Arakawa M, Naito M: Expression of macrophage colony-stimulating factor and its receptor in hepatic granulomas of Kupffer cell-depleted mice. Am J Pathol 1997, 150:2047-2060 [PMC free article] [PubMed] [Google Scholar]
- 27.Azuma A, Toyoda Y: Production of germ-line chimeric mouse derived from newly established embryonic stem cells. Jpn J Anim Reprod 1991, 37:37-43 [Google Scholar]
- 28.Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W: Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods 1992, 174:5-19 [DOI] [PubMed] [Google Scholar]
- 29.Smith MJ, Koch GL: Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes. J Cell Sci 1987, 87:113-119 [DOI] [PubMed] [Google Scholar]
- 30.Rabinowitz SS, Gordon S: Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med 1991, 174:827-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurlander RJ, Ellison DM, Hall J: The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J Immunol 1984, 133:855-862 [PubMed] [Google Scholar]
- 32.Seaman WE, Wofsy D, Greenspan JS, Ledbetter JA: Treatment of autoimmune MRL/lpr mice with monoclonal antibody to Thy-1.2: a single injection has sustained effects on lymphoproliferation and renal disease. J Immunol 1983, 130:1713-1718 [PubMed] [Google Scholar]
- 33.Ballas ZK, Rasmussen W: Lymphokine-activated killer cells. VII. IL-4 induces an NK1.1+CD8α+β− TCR-αβ B220+ lymphokine-activated killer subset. J Immunol 1993, 150:17-30 [PubMed] [Google Scholar]
- 34.Isobe Y, Nakane PK, Brown WR: Studies on translocation of immnoglobulins across intestinal epithelium. I. Improvements in the peroxidase-labeled antibody method for application to study of human intestinal mucosa. Acta Histochem Cytochem 1977, 10:161-171 [Google Scholar]
- 35.Greenberg ME: Preparation and analysis of RNA. Ausubel FM Brent R Kingston RE eds. Current Protocols in Molecular Biology. 1989, :pp 4.1-4.5 Greene Publishing Associates and Wiley-Interscience, New York [Google Scholar]
- 36.Miyatake S, Otsuka T, Yokota T, Lee F, Arai K: Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J 1985, 10:2561-2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oda T, Maeda H: A new simple fluorometric assay for phagocytosis. J Immunol Methods 1986, 88:175-183 [DOI] [PubMed] [Google Scholar]
- 38.Hapton RY, Golenbock DT, Penman M, Krieger M, Paetz CRH: Recognition and plasma clearance of endotoxin by scavenger receptors. Nature 1991, 352:342-344 [DOI] [PubMed] [Google Scholar]
- 39.Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA: The type I macrophage scavenger receptor binds to Gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA 1994, 91:1863-1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL: Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-γ and IL-4 knockout mice. J Immunol 1997, 159:3565-3573 [PubMed] [Google Scholar]
- 41.Kamijo R, Le J, Shapiro D, Havell EA, Huang S, Aguet M, Bosland M, Vilcek J: Mice that lack the interferon-γ receptor have profoundly altered responses to infection with bacillus Calmette-Guérin and subsequent challenge with lipopolysaccharide. J Exp Med 1993, 178:1435-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kindle V, Sappino A-P, Grau GE, Piquet P-F, Vassali P: The inducing role of tumor necrosis factor in the development of bacterial granulomas during BCG infection. Cell 1989, 56:731-740 [DOI] [PubMed] [Google Scholar]
- 43.Marino M, Dunn A, Grail D, Ingelese M, Noguchi Y, Richards E, Jungblyth A, Wada H, Morre M, Williamson B, Basu S, Ild LJ: Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA 1997, 94:8093-8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones ML, Warren JS: Monocyte chemoattractant protein 1 in a rat model of pulmonary granulomatosis. Lab Invest 1992, 66:498-503 [PubMed] [Google Scholar]
- 45.Lukacs NW, Chensue SW, Smith RE, Strieter RM, Warington K, Wilke C, Kundel SL: Production of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α by inflammatory granuloma fibroblasts. Am J Pathol 1994, 144:711-718 [PMC free article] [PubMed] [Google Scholar]
- 46.Flory CM, Jones ML, Miller BF, Warren JS: Regulatory roles of tumor necrosis factor-α and interleukin-1β in monocyte chemoattractant protein-1-mediated pulmonary granuloma formation in the rat. Am J Pathol 1995, 146:450-462 [PMC free article] [PubMed] [Google Scholar]
- 47.Chensue SW, Warington KS, Ruth JH, Sangli PS, Lincoln P, Kundle SL: Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation. J Immunol 1996, 157:4602-4608 [PubMed] [Google Scholar]
- 48.Kilgore KS, Imlay MM, Szaflarski JP, Silverstein FS, Malani AN, Evans VM, Warren JS: Neutrophils and reactive oxygen intermediates mediate glucan-induced pulmonary granuloma formation through the local induction of monocyte chemoattractant protein-1. Lab Invest 1997, 76:191-201 [PubMed] [Google Scholar]
- 49.Haworth R, Platt N, Kreshav S, Highes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S: The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med 1997, 186:1431-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shyy Y-J, Li Y-S, Kolattukudy PE: Activation of MCP-1 gene expression is mediated through multiple signaling pathways. Biochem Biophys Res Commun 1993, 192:693-699 [DOI] [PubMed] [Google Scholar]
- 51.Zeh K, Matsuda J, Sasaguchi T, Kosaka C, Ogata J: Gene expression of monocytes is regulated by cell density through protein tyrosine kinase and protein kinase. Exp Cell Res 1994, 215:172-179 [DOI] [PubMed] [Google Scholar]
- 52.Scholl PR, Trede N, Chatla TA, Ceha RS: Role of protein tyrosine phosphorylation in monokine induction by the staphyloccccal superantigen toxic shock syndrome toxin-1. J Immunol 1992, 70:137-144 [PubMed] [Google Scholar]
- 53.Trede NS, Morio T, Scholl PR, Chatila T: Early activation events induced by the staphylococcal superantigen toxic shock syndrome toxin-1 in human peripheral blood monocytes. Clin Immunol Immnunopath 1994, 70:137-144 [DOI] [PubMed] [Google Scholar]
- 54.Xu L, Kim S, Chen M, Rockow S, Yi SE, Wagner AJ, Hay N, Weichselbaum RR, Li W: Blockage of the early events mitogenic signaling by interferon-γ in macrophages in response to colony-stimulating factor-1. Blood 1995, 86:2774-2788 [PubMed] [Google Scholar]
- 55.Chakrabari R, Erickson KL: Tyrosine kinase but not phospholipid/Ca2+ signaling pathway is involved in interferon-γ stimulation of Ia expression in macrophages. J Cell Biochem 1996, 60:235-245 [DOI] [PubMed] [Google Scholar]
- 56.Sano H, Higashi T, Matsumoto K, Melkko, Jinnouchi Y, Ikeda K, Ebia Y, Makino H, Smedsrod B, Horiuchi S: Insulin enhances macrophage scavenger receptor-mediated endocytic uptake of advanced glycation end products. J Biol Chem 1998, 273:8630-8637 [DOI] [PubMed] [Google Scholar]
- 57.Hsu H-Y, Hajjar DP, Khan KMF, Falcone DJ: Ligand binding to macrophage scavenger receptor-A induces urokinase-type plasminogen activator expression by protein kinase-dependent signaling pathway. J Biol Chem 1998, 273:1240-1246 [DOI] [PubMed] [Google Scholar]
- 58.Baggiolini M, Dewald B, Moser B: Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Adv Immunol 1994, 55:97-179 [PubMed] [Google Scholar]
- 59.Yamashiro S, Takeya M, Kuratsu J, Ushio Y, Takahashi K, Yoshimura T: Intradermal injection of monocyte chemoattractant protein-1 induces emigration and differentiation of blood monocytes in rat skin. Int Arch Allergy Immunol 1998, 115:15-23 [DOI] [PubMed] [Google Scholar]
- 60.Rollins BJ, Yoshimura T, Leonard EJ, Pober JS: Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol 1990, 136:1229-1233 [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi Y, Matsumura F, Takeya M, Ichiguchi O, Kuratsu J, Horiuchi T, Akizuki E, Matsuda T, Okabe K, Ohshiro H, Liang J, Mori K, Yamada S, Takahashi K, Ogawa M: Monocyte chemoattractant protein-1 enhances expression of intercellular adhesion molecule-1 following ischemia-reperfusion of the liver in rats. Hepatology 1998, 27:727-734 [DOI] [PubMed] [Google Scholar]
- 62.Sakanashi Y, Takeya M, Yoshimura T, Feng L, Morioka T, Takahashi K: Kinetics of macrophage subpopulations and expression of monocyte chemoattractant protein-1 (MCP-1) in bleomycin-induced lung injury of rats studied by a novel monoclonal antibody against rat MCP-1. J Leukoc Biol 1994, 56:741-750 [DOI] [PubMed] [Google Scholar]
- 63.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G: Induction of interferon γ production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med 1991, 173:869-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marzio PD, Puddu P, Conti L, Belardelli F, Gessani S: Interferon-γ upregulates its own gene expression in mouse peritoneal macrophages. J Exp Med 1994, 179:1731-1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takacs L, Kovacs EJ, Smith MR, Young HA, Durum SK: Detection of IL-1α and IL-1β gene expression by in situ hybridization: tissue localization of IL-1 mRNA in the normal C57BL/6 mouse. J Immunol 1988, 141:3081-3095 [PubMed] [Google Scholar]
- 66.Tukui T, Kikuchi K, Sudo T, Sakamoto T, Sato N: Production of macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes). J Leukoc Biol 1992, 52:383-389 [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto T, Mabuchi A, Kuriya S, Sudo T, Aida T, Asano G: Production of granulocyte-macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes). Reg Immunol 1991, 3:260-267 [PubMed] [Google Scholar]
- 68.Shibata Y, Volkman A: The effect of bone marrow depletion on prostaglandin E-producing suppressor macrophages in mouse spleen. J Immunol 1985, 135:3897-3904 [PubMed] [Google Scholar]
- 69.Gomez F, Rutz P, Scheinber AD: Impaired function of macrophage Fc γ receptors and bacterial infection in alcocholic cirrhosis. N Engl J Med 1994, 331:1122-1128 [DOI] [PubMed] [Google Scholar]
- 70.Zheng L, Zomerdijk TP, Arnoudse C, van Furth R, Nibbering PH: Role of protein kinase C isozymes in Fc γ receptor-mediated intracellular killing of Staphylococcus aureus by human monocytes. J Immunol 1995, 155:776-784 [PubMed] [Google Scholar]