Abstract
This study demonstrates a novel role for the Fas pathway in the promotion of local tumor growth by inducing apoptotic cell death in normal hepatocytes at the tumor margin in colorectal hepatic metastases. Our results show that >85% of lymphocytes infiltrating colorectal liver cancer express high levels of Fas-ligand (Fas-L) by flow cytometry. Using immunohistochemistry of tumor tissue we showed strong Fas expression in noninvolved hepatocytes, whereas Fas-L expression was restricted to tumor cells and infiltrating lymphocytes at the tumor margin. Apoptosis was observed in 45 ± 13% of the Fashigh hepatocytes at the tumor margin whereas only 7 ± 3% tumor cells were apoptotic (n = 10). In vitro, primary human hepatocytes expressed Fas receptor and crosslinking with anti-Fas antibody induced apoptosis in 44 ± 5% of the cells compared with 4.6 ± 1.0% in untreated controls (P = 0.004). Both tumor-infiltrating lymphocytes (TIL) and human metastatic colon cancer cells cells are able to induce Fas-mediated apoptosis of primary human hepatocytes in coculture cytotoxic assays. TIL induced apoptosis in 47 ± 9% hepatocytes compared with control 4.3 ± 1.0% (P = 0.009) and this effect was reduced by anti-human Fas-L mAb (18.7 ± 1.3%, P = 0.009). SW620 cells induced apoptosis in 26 ± 2% hepatocytes compared with control 5.6 ± 1.7% (P = 0.004) and this was reduced to 11.2 ± 1.8% (P = 0.004) in the presence of anti-human Fas-L mAb. These data suggest that the inflammatory response at the margin of colorectal liver metastases induces Fas expression in surrounding hepatocytes, allowing them to be killed by Fas-L-bearing TIL or tumor cells and facilitating the invasion of the tumor into surrounding liver tissue.
The liver is the commonest site for development of metastatic colorectal cancer. Approximately 25% of patients with colorectal cancer have liver metastases at the time of diagnosis and in another 25%, liver metastases will develop within 2 years. 1 The remarkably high incidence of liver metastases in patients with colorectal cancer suggests that the liver provides an environment conducive to the development of metastases. For metastatic tumor cells to become established and develop into overt disease, they must evade immune surveillance and invade and replace local tissue at the secondary site. Several mechanisms are implicated in cancer invasion, including release of metalloproteinases that degrade extracellular matrix, secretion of growth factors to promote neovascularization, and changes in adhesion molecules that promote tumor cell migration. 2,3 However, although the replacement of normal tissue is a prerequisite for tumor growth, the precise mechanisms by which colorectal cancer displaces or destroys resident liver cells are not known.
There is substantial evidence that lymphocyte-mediated mechanisms are important in controlling tumor growth in some cancers. 4-6 However, despite being infiltrated by lymphocytes, the growth of colorectal hepatic metastases is relentless, suggesting that the local antitumor immune response is impaired. 7,8 Most mononuclear cells infiltrating colorectal hepatic metastases are confined to the peritumoral margin between the advancing edge of the tumor and the adjacent uninvolved liver tissue. 8 Although the infiltrate contains activated T cells, the cells are unable to lyse autologous tumor targets, even following in vitro activation with rIL-2, 8,9 suggesting that the tumor deletes antitumor effector lymphocytes. It has been proposed recently that one mechanism responsible for this is Fas-mediated killing of lymphocytes by Fas ligand (Fas-L)-expressing tumor cells. 10 Activated cytolytic lymphocytes (CTL) express Fas-L, a member of the tumor necrosis superfamily, which can trigger apoptosis in target cells that bear its cell surface receptor Fas (CD95/Apo-1). 11 Fas-L/Fas interactions are involved in CTL killing of target cells, including virally infected hepatocytes in viral hepatitis. 12 However, in addition to Fas-L, activated CTL also express Fas. Lymphocyte-lymphocyte interactions involving Fas and Fas-L trigger apoptosis and provide a mechanism for down regulating immune responses in vivo. 11,13 The potent ability of Fas-L-bearing cells to kill Fas-expressing lymphocytes means that Fas-L expression is tightly controlled in vivo and tissues in which Fas-L is constitutively expressed, such as the eye and testis, are immune-privileged sites. 14,15 Several tumor types, including primary colorectal cancer and hepatocellular carcinoma, express Fas-L and exploit its ability to kill Fas-expressing lymphocytes to evade immune recognition. 10,16 In the present studies we have extended these observations to colorectal hepatic metastases and propose that the Fas pathway not only is responsible for killing infiltrating lymphocytes, but also facilitates local tumor growth by inducing apoptotic cell death in normal hepatocytes at the tumor margin.
Materials and Methods
Patient Characteristics and Tissues Studied
Surgically resected colorectal hepatic metastases were obtained from 15 patients (6 males and 9 females; median age 59 years, range, 48–79 years) treated at the Liver Unit, Queen Elizabeth Hospital (Birmingham, UK). Six were histologically well differentiated, 5 moderately differentiated, and 4 poorly differentiated adenocarcinoma. None of the patients had received immunosuppressive drugs, radiotherapy, or chemotherapy prior to surgery. Tissue containing both tumor and adjacent, macroscopically normal liver and a sample of noninvolved liver tissue distant from the tumor were snap-frozen in liquid nitrogen and stored at −70°C until analyzed. A separate sample of fresh tumor tissue was used for isolation of lymphocytes. Peripheral blood lymphocytes (PBL) were isolated from venous blood obtained from the same patients immediately before surgery.
Lymphocyte Isolation
Tumor-infiltrating lymphocytes (TIL) were isolated from fresh tumor tissues removed at surgery as described previously. 8 Tumor tissues were immediately cut into small pieces, washed, and digested using RPMI-1640 (Gibco/Life Technologies, Paisley, UK) supplemented with 0.2% (w/v) collagenase type IV (Sigma, Poole, UK) and 20% fetal calf serum (Gibco) for 2–3 hours with continuous stirring at room temperature. The tumor digest was then passed through a nylon mesh to obtain a single cell suspension that was washed with phosphate-buffered saline (PBS) until the supernatant became clear. The single cell suspension was layered onto Ficoll Hypaque (Lymphoprep, Nycomed, Oslo, Norway) and centrifuged at 1600 rpm for 30 minutes at room temperature. TIL and tumor cells were recovered from the interface. Autologous PBL were isolated from heparinized venous blood obtained from the same patients immediately prior to surgery. PBL were separated by Ficoll Hypaque centrifugation at 1600 rpm for 30 minutes at room temperature and then washed twice with PBS.
Isolation and Culture of Primary Human Hepatocytes
Primary human hepatocytes were isolated from surplus liver tissue obtained from adult donor livers that had been reduced in size for transplantation into pediatric recipients as described previously. 17 Approximately 150 g of human liver tissue were treated with segmental perfusion with 5 mmol/L calcium chloride, 0.05% Collagenase H (Life Technologies), 0.025% dispase (Boehringer-Mannheim, Mannheim, Germany), 0.0125% type 1-S hyaluronidase (Sigma), and 0.005% DNase (Boehringer Mannheim) in 10% Hanks’ balanced salt solution. The resulting cell suspension was filtered and centrifuged at 28 × g to pellet hepatocytes. The hepatocytes were further purified by Percoll density gradient centrifugation and their viability determined by trypan blue dye exclusion. Immediately after isolation, hepatocytes were resuspended in Williams E medium containing hydrocortisone, insulin, and glutamine and seeded onto collagen-coated eight-well tissue culture plates (Costar Corp., Cambridge, MA), which were then incubated at 37°C in humidified air and 5% CO2. The hepatocytes were allowed to adhere for 2 hours, following which the medium was replaced with fresh culture medium and the cells allowed to recover for 24 hours before further studies began.
Culture of SW620
The human colon cancer cell line SW620 was obtained from American Type Culture Collection (Manassas, VA) and grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (FCS) in a humidified 5% CO2 at 37°C.
Immunohistochemistry
Localization of Fas and Fas-L expression in peritumoral tissue was carried out on 6-μm cryostats that contained both tumor and adjacent normal hepatocytes, using standard immunohistochemistry as described previously. 18,19 Tissue sections were fixed in acetone for 10 minutes at room temperature and then incubated with rabbit polyclonal anti-human Fas- or Fas-L-specific IgG (Santa Cruz Biotechnology, Santa Cruz, CA) at 2 μg/ml. The primary antibody binding was localized by mouse anti-rabbit (Dako, High Wycombe, UK) followed by rabbit anti-mouse (Dako) secondary antibodies, developed by the indirect alkaline phosphatase-anti-alkaline phosphatase (Sigma) technique, and the resulting enzyme complex visualized with napthol-AX (Sigma) and Fast Red TR (Sigma). Sections were counterstained with Meyer’s haematoxylin. Incubations were done at room temperature for 45 minutes and sections were washed for 5 minutes with 2 changes of Tris buffer between incubations. Normal tonsil sections were used as positive controls. Negative controls consisted of sections in which the primary antibody was omitted. In addition, the specificity of anti-Fas or anti-Fas-L antibody was confirmed by the addition of a competitive inhibitor, either a Fas or Fas-L peptide (20 μg/ml, Santa Cruz Biotechnology), during the primary antibody incubation. The results of immunohistochemical studies for Fas and Fas-L expression were assessed by scoring the intensity of immunoreactivity for a given antigenic determinant relative to control staining, using a scale of 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The mean (± SE) was then calculated for all individual scores. We have previously validated this quantitative scoring system using confocal microscopy. 20
Cellular proliferation was determined by immunostaining of paraffin-embedded sections with mouse anti-human Ki-67 (nuclear proliferation antigen; mib-1 clone of IgG1 isotype from Coulter Electronics, Luton, UK) at 1 μg/ml. Sections were dewaxed with xylene/alcohol and treated with 3% hydrogen peroxide in methanol for 10 minutes to block endogenous peroxidase, followed by microwaving for 30 minutes in citric acid (2.1 g/L adjusted to pH 6.0 with NaOH) to enhance antigen retrieval. Anti-Ki-67 was detected by biotinylated goat anti-mouse (Dako) and streptavidin-avidin-biotin complex/horseradish (Dako) and developed using diaminobenzidine tetrahydrochloride (Sigma). The sections were counterstained with Mayer’s haematoxylin. Sections stained with an irrelevant mouse mAb were used as negative control. Normal tonsil sections stained with anti-Ki-67 were employed as positive controls. Approximately 100 tumor cells, mononuclear cells, and hepatocytes were enumerated separately in each high power field (×400) and 10 random high-power fields were counted on each section using light microscopy. The results were expressed as mean ± SE of 10 samples.
The proliferative index was calculated as follows:
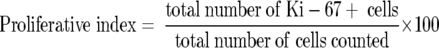 |
Determination of Apoptosis
Apoptosis was assessed by three methods: cellular morphology according to Kerr’s criteria (cytoplasmic shrinkage, pyknosis, and/or nuclear fragmentation), 21 in situ end-labeling (ISEL) to detect DNA fragmentation on paraffin-embedded tumor sections, and immunofluorescent staining of cryostats using mouse anti-human APO 2.7-PE (2.7A6A3 clone, IgG1 isotype, Coulter Electronics) at 4 μg/ml. ISEL was performed on paraffin-embedded tissue sections as described elsewhere. 22 Sections 6 μm in width were dewaxed, dehydrated, and air-dried for 15 minutes, followed by incubation for 30 minutes at 37°C with 5 μg/ml proteinase K (Sigma) diluted in 50 mmol/L TBS, pH 8.2, containing 1 mmol/L EDTA. The sections were then washed 3× in distilled water, dehydrated with alcohol, and air-dried. One hundred microliters of the following labeling mixture were applied to each section: 0.01 mmol/L aATP, dCTP, dGTP, and digoxigenin-labeled dUTP (Boehringer Mannheim, Lewes, UK) made up in 50 mmol/L TBS, pH 7.4, containing 5 mmol/L MgCl2, 10 mmol/L beta-mercaptoethanol, 5 mg/ml BSA, and 20 units/ml Klenow DNA polymerase fragment (Boehringer Mannheim). Siliconized coverslips were placed on each section to minimize drying and spread of nucleotide mixture and sections were further incubated for 1 hour at 37°C. The reaction was terminated by 3 washes in distilled water. DNA polymerase was omitted in negative control sections. Digoxigenin-labeled cells were identified using alkaline phosphatase-conjugated F(ab)2 fragments of a polyclonal sheep anti-digoxigenin antibody (Boehringer Mannheim) diluted 1:200 in TBS, pH 7.4, and incubated for 1 hour at room temperature. Sections were then washed 3× in TBS, pH 8.2, and developed with alkaline phosphatase/Fast Red working solution. Approximately 100 tumor cells, mononuclear cells, and hepatocytes were enumerated separately in each high-power field (×400) and 10 random high-power fields were counted on each section using light microscopy. Results were expressed as mean ± SE of ten samples.
The apoptotic index was calculated as follows:
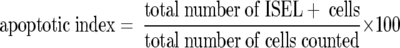 |
Flow Cytometry
The expression of Fas and Fas-L on TIL, primary human hepatocytes, and tumor cells was assessed by immunofluorescent staining with rabbit polyclonal anti-human Fas- or Fas-L-specific antibodies (Santa Cruz Biotechnology) at 2 μg/ml followed by 1:20 dilution of FITC-labeled goat anti-rabbit immunoglobulin (Dako). The phenotypic composition of freshly isolated TIL and autologous PBL was analyzed by two-color flow cytometry using standard techniques. 23 Single-cell suspensions (10 6 cells/ml) were incubated with 5 μl of primary unconjugated mouse mAb followed by 1:20 dilution of FITC-conjugated F(ab)2 fragments of rabbit anti-mouse immunoglobulin (Dako). Thereafter, the cell suspension was incubated with normal mouse serum to saturate the free binding sites on the F(ab)2 fragments before a final incubation with PE-conjugated anti-CD3 (Dako). All incubations were carried out at 4°C for 30 minutes and cells were washed twice with PBS (0.02% w/v sodium azide and 2% v/v FCS) between incubations. The cell suspension was fixed with 1% paraformaldehyde and analyzed using the FACS 440 (Becton Dickinson). A lymphocyte gate was set to exclude dead cells and debris and at least 10 4 cells were analyzed in each sample. Irrelevant isotype-matched mAb or nonimmune serum was used as a control. In addition, the specificity of the anti-Fas or anti-Fas-L antibody was confirmed by the addition of a competitive inhibitor, either a Fas or Fas-L peptide (20 μg/ml, Santa Cruz Biotechnology), during the primary antibody incubation.
Induction of Fas-Mediated Apoptosis of Primary Human Hepatocytes
Cell surface Fas receptor expression on isolated primary human hepatocytes was studied by flow cytometric analysis of single-cell suspensions of confluent hepatocyte monolayers before and after treatment with varying doses of IL-1 for 24 hours at 37°C. The effect of Fas ligation on human hepatocytes was evaluated by treating the cells with 20 ng/ml anti-Fas antibody for 12, 24, and 48 hours at 37°C, following which apoptosis was determined by ISEL staining of cytospin sections. The ability of TIL derived from colorectal liver metastases and SW620 human colon cancer cells to induce apoptosis of human hepatocytes was investigated by a cell coculture cytotoxicity assay. Effector TIL or SW620 tumor cells were cocultured with confluent monolayers of target human hepatocytes for 24 hours at an effector:target ratio of 5:1 (TIL) or 2:1 (SW620). In some experiments, 40 ng/ml of antibody to human Fas-L (mAb NOK-1, Pharmingen, San Diego, CA) were added to the hepatocyte culture medium before coculturing the hepatocytes with TIL or SW620. This mAb inhibits Fas-mediated apoptosis by specifically binding to human Fas-L on effector cells. 24 Each experiment was carried out in triplicate. Hepatocytes were harvested by trypsinization for 5 minutes with 0.25% v/v bovine pancreatic trypsin and then neutralized by 10% FCS. Apoptosis of hepatocytes was determined by ISEL of DNA fragmentation and quantified on cytospin sections by calculating approximately 200 hepatocytes on ten random high-power fields (×400) on each cytospin section using light microscopy. The enumeration of apoptotic hepatocytes is calculated as follows:
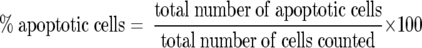 |
and expressed as the mean ± SE of results from 6 experiments.
Statistical Analysis
Kruskal-Wallis analysis of variance was used to determine variation between groups of samples. Differences between unpaired samples were analyzed by the Mann-Whitney U test for nonparametric data. Differences between paired samples were analyzed by the nonparametric Wilcoxon’s rank sum test. The flow cytometric data of Fas expression on primary human hepatocytes were analyzed by the unpaired Student’s t-test assuming unequal variance. The level of significance was set at P < 0.05.
Results
We have previously reported that the majority of lymphocytes that infiltrate liver metastases from colorectal cancers are confined to the margin between the advancing tumor edge and the surrounding normal hepatocytes, with very few lymphocytes detected centrally in the tumor. 8 In the present study lymphocytes at the tumor margin were mainly activated T cells (mean CD4/CD8 ratio of 15 TIL cases, 2.3 ± 0.3) that expressed the activation markers CD69 and HLA-DR (Figure 1) ▶ . A large subset of these T cells also expressed both Fas and Fas-L (Figure 1) ▶ . Despite their activated phenotype, these tumor-derived lymphocytes are unable to lyse autologous or allogeneic tumor cell targets in vitro. 8
Figure 1.
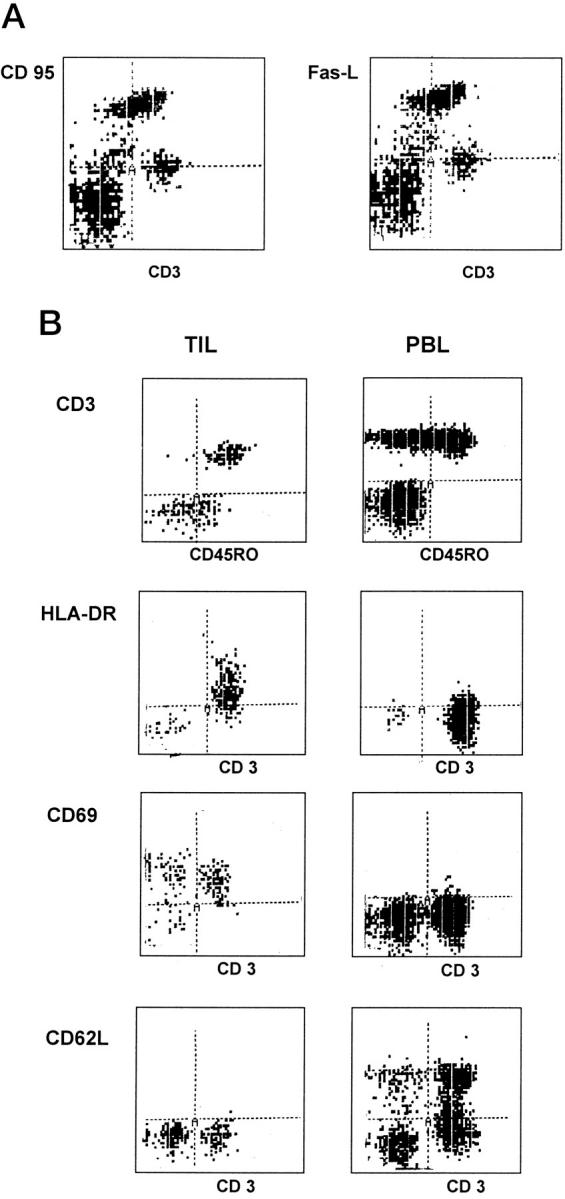
Lymphocytes infiltrating colorectal hepatic metastases comprise mainly activated memory T cells expressing Fas (CD95) and Fas-L. A: Phenotype of freshly isolated TIL showing that they include a subset of activated lymphocytes that are CD95high and Fas-Lhigh. B: The majority of the TIL are CD45RO+, CD69high, HLA-DRhigh, and CD62L (L-selectin)low compared with autologous peripheral blood lymphocytes (PBL). Cells were analyzed by flow cytometry gated on CD3+ population. Figure ▶ shows one representative experiment of ten.
Fas and Fas-L Expression in the Peritumoral Margin of Liver Metastases from Colorectal Cancer
Immunohistochemistry was used to analyze the pattern and distribution of Fas and Fas-L protein expression in the different cellular compartments at the margin of the tumor. Ten of the cases we studied were moderately or well differentiated colorectal hepatic metastases consisting of glandular structures with two distinct domains: the basolateral membrane, which is in contact with the extracellular matrix and the peritumoral stroma, and a luminal (apical) domain, which is not. Fas expression was restricted to the luminal surface of carcinoma cells (Figure 2a) ▶ , whereas Fas-L protein was detected on the membrane throughout the tumor cells (Figure 2b) ▶ . Fas and Fas-L proteins were also detected on lymphocytes infiltrating the stroma between the tumor cells and adjacent uninvolved hepatocytes (Figure 2, a and b) ▶ . The strongest expression of Fas was on the uninvolved hepatocytes, which showed intense membranous and cytoplasmic staining for Fas protein extending for several cell layers from the interface with the peritumoral stroma (Figure 2a) ▶ . Fas-L protein was not detected in either the peritumoral or more distant hepatocytes (Figure 2b) ▶ . The specificity of the anti-Fas or anti-Fas-L antibody was confirmed by absent immunostaining in the presence of a competitive inhibitor, Fas or Fas-L peptide, in the primary antibody incubation.
Figure 2.
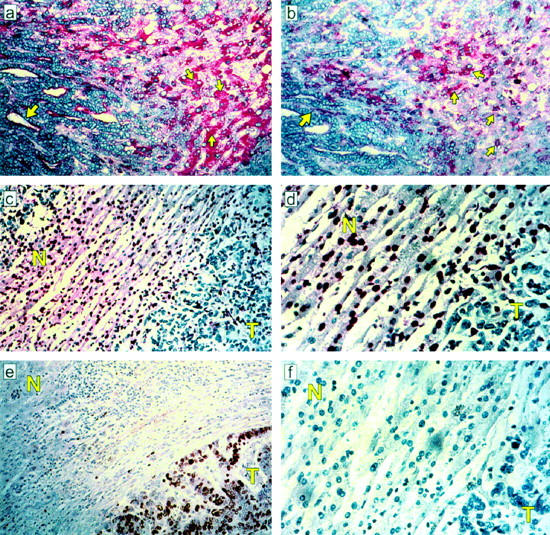
Relationship between Fas and Fas-L expression and apoptosis in different cellular compartments in the peritumoral margin of colorectal hepatic metastases. a: Fas immunoreactivity detected by APAAP and Fast Red on luminal aspect of tumor cells (big arrows) and hepatocytes (small arrows) (original magnification, ×400). b: Fas-L immunostaining on a serial section detected by APAAP and Fast Red on tumor cells (big arrows) and infiltrating mononuclear cells (small arrows) (original magnification, ×400). c: Positive ISEL staining visualized as dark brown can be seen in the nuclei of adjacent hepatocytes and infiltrating mononuclear cells (N, normal liver, T, tumor; original magnification, ×200). d: Positive ISEL staining in the nuclei of adjacent hepatocytes and infiltrating mononuclear cells at a higher magnification, ×400 (N, normal liver, T, tumor). e: Ki-67 immunostaining detected by peroxidase and DAB is localized mainly to the nuclei of tumor cells (original magnification, ×200), f: control section (original magnification, ×400).
The Majority of Normal Hepatocytes and Infiltrating Lymphocytes Surrounding the Peritumoral Margin Are Apoptotic
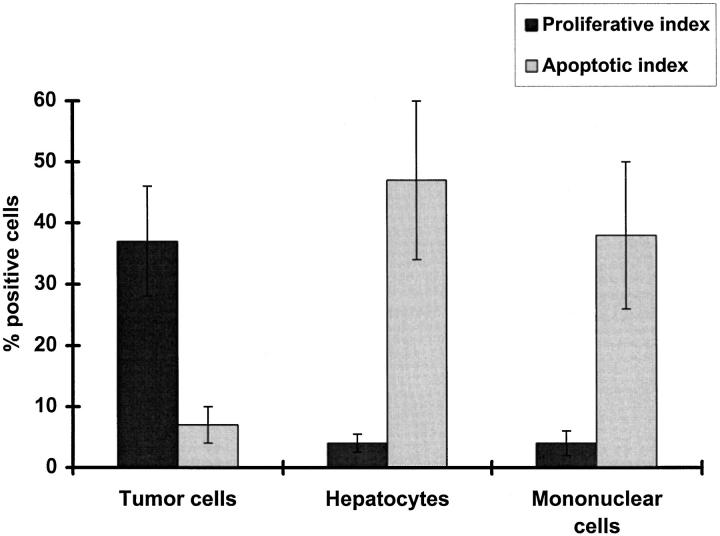
To determine which of the Fas-expressing cells were targets of Fas-L attack, we looked for apoptosis in the different cellular compartments at the tumor margin, ie, tumor cells, inflammatory cells, and hepatocytes. Many Fashigh hepatocytes at the tumor margin as well as many infiltrating lymphocytes were undergoing apoptosis as defined by morphology, Apo 2.7 immunostaining, and ISEL of DNA fragmentation (Figures 2, c and d ▶ , 3, and 4). Hepatocytes farther from the tumor margin were Faslow and very few (<3%) were apoptotic. Apoptotic lymphocytes were detected in lymphoid aggregates as well as among mononuclear cells in direct contact with tumor cells, suggesting that lymphocytes were being killed by neighboring Fas-L-expressing lymphocytes via a bystander effect as well as by Fas-L on tumor cells (Figure 2, c and d) ▶ . In contrast, very few tumor cells were apoptotic (Figure 2, c and d) ▶ and many were in active cell cycle as determined by staining for the nuclear proliferation antigen Ki-67 (Figures 2e and 4) ▶ ▶ . Thus, both the infiltrating lymphocytes and the surrounding hepatocytes were targets for Fas-mediated apoptosis.
Figure 4.
Quantitative analysis of proliferative index determined by Ki-67 immunostaining and apoptotic index determined by ISEL of DNA fragmentation in different cellular compartments in the peritumoral margin of colorectal hepatic metastases from 10 patients. Data represent the percentage of positive cells shown as mean ± SE. P < 0.01 (2-tailed Mann-Whitney U-test) for Proliferative index versus Apoptotic index for each cell compartment.
Isolated Primary Human Hepatocytes Are Susceptible to Fas-Mediated Induction of Apoptosis
Increased Fas expression has been reported on hepatocytes during liver inflammation in vivo. 12,25 Thus, the increased Fas expression detected on hepatocytes at the tumor margin in the present study could be a consequence of the inflammatory response at this site. In support of this we detected the proinflammatory cytokines IL-1 and TNF-α in the peritumoral infiltrate by immuno-histochemistry (data not shown). Furthermore, although the level of surface Fas on human hepatocytes was high after 24 hours in culture (85% of cells positive) it could be increased further by treatment with exogenous IL-1 (Figure 5) ▶ .
Figure 5.
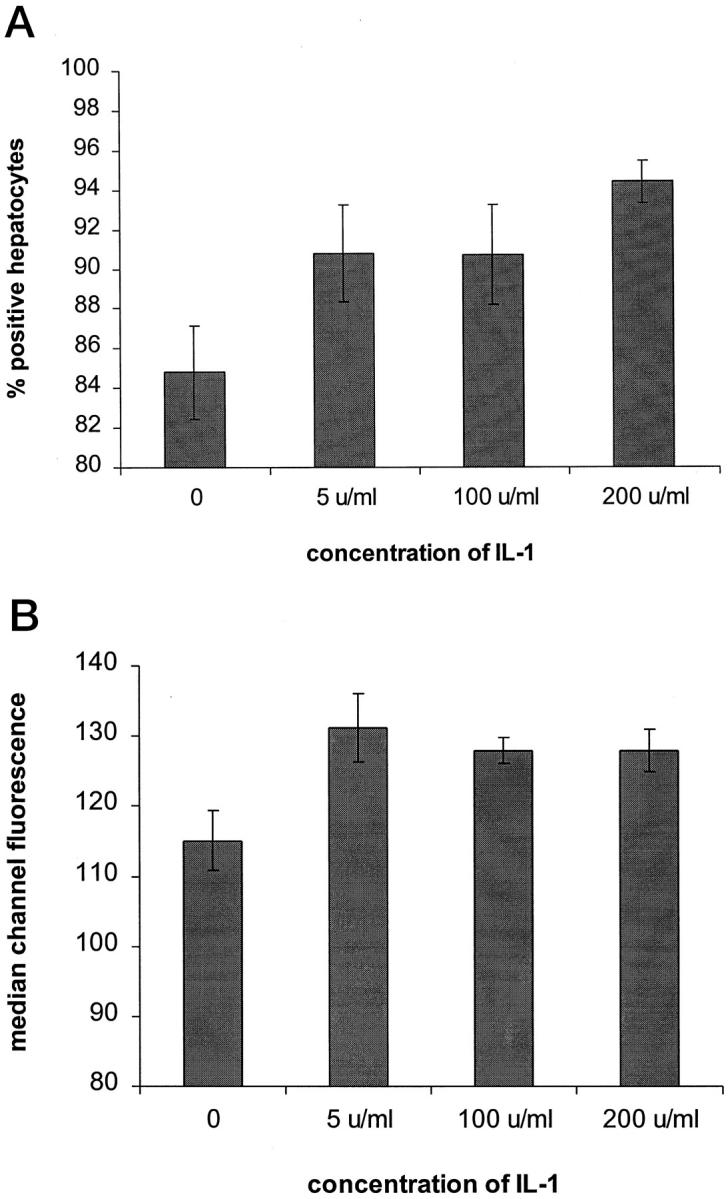
Effect of exogenous IL-1 on Fas expression in isolated primary human hepatocytes. Cells were treated with different concentrations of IL-1 for 24 hours at 37°C and cell surface Fas expression was determined by flow cytometry. Results are expressed as percentage of positive hepatocytes (A) and median channel fluorescence (B) and represent the mean ± SE of 5 samples. The median channel fluorescence is expressed as the difference between the test and the control antibody. P = NS for 5 u/ml versus control, P = NS for 100 u/ml versus control, P < 0.005 for 200 u/ml versus control in A. P < 0.02 for 5 u/ml, 100 u/ml, and 200 u/ml versus control in B.
We subsequently demonstrated that Fas expressed by human hepatocytes is functional in vitro because treatment of hepatocytes with an agonistic anti-Fas antibody resulted in apoptosis of 44 ± 5% of hepatocytes compared to 4.6 ± 1.0% apoptosis in cell treated with an irrelevant antibody (P = 0.004, Figure 6 ▶ ).
Figure 6.
Induction of apoptosis in isolated primary human hepatocytes in vitro following incubation of cells with different concentrations of anti-Fas antibody for 24 hours at 37°C. Control wells contained hepatocytes treated with a nonimmune serum. Apoptosis of hepatocytes was determined by ISEL staining of cytospin sections and quantified as described in Materials and Methods. Results are expressed as percentage of apoptotic hepatocytes and represent the mean ± SE of 6 individual experiments. P = 0.004 (2-tailed Mann-Whitney U-test) for 10 ng/ml and 20 ng/ml anti-Fas Ab versus control.
Lymphocytes Infiltrating Colorectal Hepatic Metastases Can Induce Fas-Mediated Apoptosis of Human Hepatocytes in Vitro
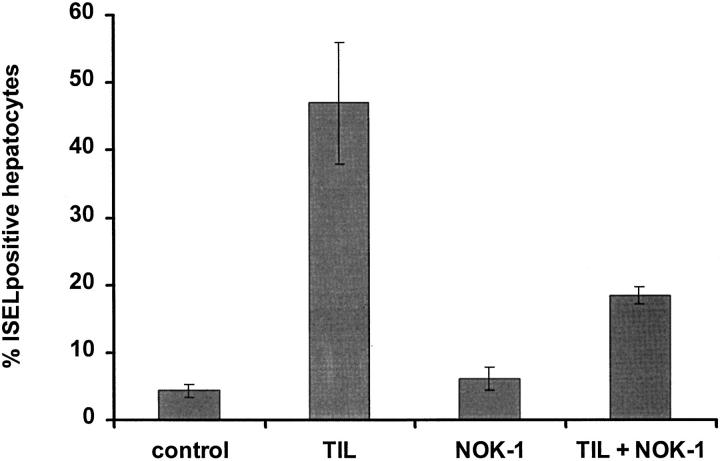
Flow cytometric and immunohistochemical studies show that lymphocytes infiltrating colorectal hepatic metastases express cell surface Fas-L (Figures 1 and 2) ▶ ▶ . In order to determine if the cell surface Fas-L was functional, TIL isolated from colorectal hepatic metastases were cocultured for 24 hours with cell surface Fas-positive primary human hepatocytes at a TIL:hepatocyte ratio of 5:1. At the end of the coculture 47 ± 9% of hepatocytes were apoptotic as determined by ISEL of DNA fragmentation when cocultured with TIL compared to 4.3 ± 1.0% apoptosis of hepatocytes in control wells treated with medium alone (P = 0.009, Figure 7 ▶ ).
Figure 7.
Results of coculture cytotoxic assay using colorectal liver tumor-derived lymphocytes and human hepatocytes. Induction of apoptosis in isolated primary human hepatocytes in vitro following incubation of cells with TIL or NOK-1 (anti-human Fas-L mAb) alone, or a combination of TIL and NOK-1 mAb for 24 hours at 37°C. The effector-to-target (TIL-to-hepatocyte) ratio was 5:1. To determine if TIL killing of hepatocytes was Fas-mediated, pretreatment with NOK-1 mAb (40 ng/ml) to block human Fas-L was carried out prior to the coculture of TIL and hepatocytes. Each experiment was conducted in triplicate wells. Control wells contained hepatocytes treated with culture medium alone. Apoptosis of hepatocytes was determined by ISEL staining of cytospin sections and quantified as described in Materials and Methods. Results are expressed as percentage of apoptotic hepatocytes and represent the mean ± SE of 5 individual experiments. P = 0.009 (2-tailed Mann-Whitney U-test) for TIL versus control, TIL versus NOK-1, TIL versus TIL + NOK-1, TIL + NOK-1 versus control, and TIL + NOK-1 versus NOK-1.
In order to determine whether TIL-induced apoptosis of hepatocytes is Fas-mediated, pretreatment with an anti-human Fas-L mAb, NOK-1, at 40 ng/ml was carried out before coculture of TIL and hepatocytes. NOK-1 treatment specifically inhibited apoptosis by 62% compared with hepatocytes cocultured with TIL in the absence of NOK-1 (P = 0.009, Figure 7 ▶ ). The NOK-1 monoclonal antibody alone did not effect apoptosis of hepatocytes, as we have reported similar observations previously. 24 Thus the Fas receptor and Fas-L on human hepatocytes and TIL, respectively, are functional in vitro.
Tumor Cells from Colon Cancer Metastases Can Induce Fas-Mediated Apoptosis of Human Hepatocytes in Vitro
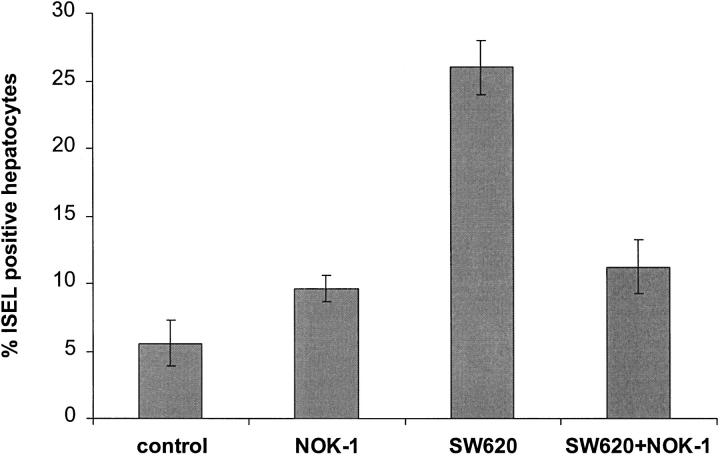
Because tumor cells express Fas-L it is possible that these cells also induce apoptosis of normal hepatocytes. In order to test this we used SW620, a human colonic tumor cell line derived from a lymph node metastasis of a colon cancer and previously shown to express Fas-L at both the mRNA and protein levels. 10 The expression of surface Fas-L on SW620 was confirmed by flow cytometric analysis, which showed that 87% of the tumor cells used for coculture experiments were positive for cell surface Fas-L (Figure 8) ▶ . In order to determine the ability of metastatic colon cancer cells to kill hepatocytes, SW620 cells were cocultured with primary human hepatocytes for 24 hours at 37°C using a ratio of 2 SW620 cells to 1 hepatocyte. Coculture resulted in apoptosis of 26 ± 2% of hepatocytes as determined by ISEL compared with only 5.6 ± 1.7% apoptosis in control wells in which the hepatocytes were treated with culture medium alone (P = 0.004) (Figure 9) ▶ .
Figure 8.
Flow cytometric analysis of cell surface Fas-L expression on SW620 tumor cells. Immunofluorescent staining was carried out as described in Materials and Methods. Fas-L expression is shown by the shaded histogram and control antibody staining by the open histogram.
Figure 9.
Results of coculture cytotoxic assay using metastatic colon cancer cells and human hepatocytes. Induction of apoptosis in isolated primary human hepatocytes in vitro following incubation of cells with SW620, NOK-1 (anti-Fas-ligand mAb, 40 ng/ml), or anti-Fas mAb (20 ng/ml) alone, or a combination of NOK-1 and SW620 for 24 hours at 37°C. The effector to target ratio of SW620 to hepatocytes was 2:1. In addition, pretreatment with NOK-1 (40 ng/ml) to specifically block human Fas-ligand was carried out prior to the coculture of SW620 and hepatocytes. Control wells contained hepatocytes treated with culture medium alone. Each experiment was carried out in triplicate. Apoptosis of hepatocytes was determined by ISEL staining of cytospin sections and quantified as described in Materials and Methods. Results are expressed as percentage of apoptotic hepatocytes and represent the mean ± SE of 6 individual experiments. P = 0.004 (2-tailed Mann-Whitney U-test) for SW620 versus control and SW620 versus SW620 + NOK-1. P = NS for NOK-1 versus control, SW620 + NOK-1 versus control, and NOK-1 versus SW620 + NOK-1.
Evidence that apoptosis was Fas-mediated was again provided by experiments in which NOK-1, an anti-human Fas-L mAb, was used specifically to inhibit human Fas-L before coculture of SW620 and hepatocytes. Blocking the Fas-L with 40 ng/ml of NOK-1 in the coculture assay resulted in a significant reduction in the percentage of apoptotic hepatocytes (11.2 ± 1.8%) compared to hepatocytes cocultured with untreated SW620 (26 ± 2%, P = 0.004) (Figure 9) ▶ . There was no significant difference between the apoptosis in hepatocytes cocultured with NOK-1-treated SW620 (11.2 ± 2%) and in those treated with NOK-1 alone (9.6 ± 1.6%, Figure 9 ▶ ). Thus, SW620 colon cancer cells can induce apoptosis of human hepatocytes in vitro in a Fas-dependent manner.
Discussion
The results of this study suggest that although colorectal hepatic metastases provoke an inflammatory response, strategies have developed that prevent the tumor from being destroyed by the immune system. One of these strategies is down-regulation of Fas and up-regulation of Fas-L on tumor cells, which enables the tumor to kill Fas-positive CTLs at the tumor margin. A similar mechanism has been shown previously to operate in primary colorectal cancer. 16 In the present study we report data that extend the role of Fas and Fas-L from one of immune protection to one of promoting local tumor invasion. We show that Fas expression is markedly up-regulated on hepatocytes at the margin of colorectal metastases and that many of the hepatocytes are undergoing apoptosis. The peritumoral infiltrate contains large numbers of Fas-Lhigh CTLs that are unable to gain access to Fas on tumor targets and instead induce killing of bystander Fas-expressing hepatocytes. Thus, the tumor subverts the inflammatory response to promote local invasion through adjacent normal tissue.
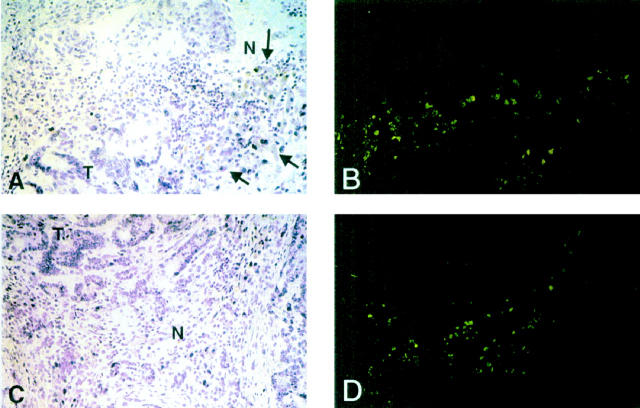
One reason for the failure of the immune response against colorectal metastases is the lack of lymphocyte entry into tumor tissue. The inflammatory infiltrate is confined to the peritumoral margin, with very few lymphocytes detected in the tumor parenchyma (Figure 2) ▶ . Moreover, <5% of the lymphocytes at the tumor margin were proliferating, as determined by expression of the nuclear proliferation antigen Ki-62, whereas up to 50% of them were dying by apoptosis (Figures 3 and 4) ▶ ▶ .
Figure 3.
Micrograph shows immunofluorescent staining of the peritumoral margin of colorectal hepatic metastases with anti-Apo 2.7. Panels A and B are from one patient and panels C and D from another patient. A and C show two sections under H&E staining and B and D show the same two sections after immunofluorescent staining for Apo 2.7. It can be seen that Apo 2.7 staining is largely confined to hepatocytes adjacent to the tumor (arrows) and infiltrating mononuclear cells. Original magnification, ×200. N, normal adjacent liver; T, tumor.
Colorectal hepatic metastases use several strategies to avoid being destroyed by the immune system. First, as we have recently reported, tumor endothelium in colorectal hepatic metastases down-regulates adhesion molecule expression. Tumor vessels express very little ICAM-1 and no VCAM-1 or E-selectin and they fail to support the adhesion of lymphocytes in tissue binding assays. 26 Failure to support lymphocyte adhesion will prevent lymphocyte access to the tumor. Second, as reported here, human metastatic colon cancer cells express Fas-L, thus allowing them to induce apoptosis via activation of Fas on the few cytotoxic T cells that succeed in entering the tumor. A similar mechanism has been demonstrated by O’Connell et al for primary colorectal tumors 16 and by other groups for hepatocellular carcinoma. 10 Third, we show in the present study that in vivo Fas expression is polarized to the luminal (apical) aspect of the tumor cells, with very little expression detected on the basolateral aspect where interactions with infiltrating lymphocytes are likely to take place. The polarization of Fas may be due either to shedding from the basolateral cell surface or to redistribution within the cell membrane. 27 In either case the net result is that Fas is inaccessible to CTLs at the tumor margin. In addition, recent in vitro studies indicate that the Fas receptor on the human metastatic colon cancer cell line, SW620, does not deliver an apoptotic signal following ligation by an agonistic anti-Fas mAb, suggesting that the receptor is not functional. 10
The outcome of these strategies is that activated lymphocytes accumulate in the stroma at the tumor periphery but cannot penetrate the tumor itself. In the absence of Fas-bearing tumor targets, the Fas-Lhigh lymphocytes might interact with other Fas-bearing cells including neighboring lymphocytes or adjacent hepatocytes (see below). Thus, lymphocyte death occurs either by suicide, mediated by interactions between Fas and Fas-L within the same cell, or by fratricide, in which Fas-L-positive lymphocytes kill neighboring Fas-positive lymphocytes. Similar mechanisms of fratricidal lymphocyte killing are believed to be responsible for resolving inflammation and maintaining lymphocyte homeostasis at sites of inflammation after the offending antigen has been removed. 13,28,29
We propose not only that colorectal metastases protect themselves by eliminating activated CTLs but also that they utilize the frustrated immune response at the tumor margin to enhance local invasion into the adjacent uninvolved liver. The peritumoral infiltrate, which includes large numbers of activated monocytes as well as CTLs, 8 is associated with the expression of proinflammatory cytokines, including IL-1, at the tumor margin. Proinflammatory cytokines can increase the expression of Fas on hepatocytes in vitro (Figure 5) ▶ and the marked up-regulation of Fas we detected on hepatocytes at the tumor margin is thus likely to be a consequence of the adjacent inflammatory infiltrate (Figure 2A) ▶ . In the absence of Fas-expressing tumor targets it is possible that Fas-Lhigh CTLs at the tumor margin will engage Fas on adjacent hepatocytes and thereby induce them to undergo apoptosis, allowing the tumor to expand into the surrounding hepatic tissue. A similar mechanism of bystander hepatocyte killing has been proposed to explain hepatocyte destruction in alcoholic hepatitis. 25 We provide evidence that TIL derived from human colorectal liver metastases can kill hepatocytes because they were able to induce apoptosis in primary human hepatocytes when cocultured with these cells in vitro. Much of the apoptosis could be prevented by blocking Fas-L with the NOK-1 monoclonal antibody, confirming a role for Fas-mediated killing of hepatocytes by TIL. However, the failure of NOK-1 to completely inhibit apoptosis of hepatocytes by TIL suggests that other apoptotic pathways might also be involved.
We used SW620, a colon cancer cell line, as a model for metastatic colon cancer cells because this cell line was derived from a lymph mode metastasis of a human colon cancer. Our flow cytometric analysis show that approximately 87% of SW620 cells express surface Fas-L and this finding is consistent with a previous study by O’Connell et al, who reported that Fas-L is expressed by SW620 at both the mRNA and protein levels. 10 The fact that tumor cells also express Fas-L suggests the possibility that the Fas-L-positive tumor cells can also kill Fas-bearing hepatocytes directly. Further evidence for this mechanism is provided by the experiments in which we have shown that SW620 can also kill primary human hepatocytes in vitro in a Fas-dependent manner. In these experiments the Fas-mediated pathway could account for all of the hepatocyte apoptosis because anti-human Fas-L mAb could completely inhibit the killing of hepatocytes by tumor cells. Despite these intriguing observations, tumor killing of hepatocytes is likely to be less important than TIL-mediated killing because the peritumoral stroma separates the tumor from the surrounding hepatocytes, whereas the CTL are in direct contact with surrounding normal tissue. An alternative source for Fas-L would be soluble ligand secreted or shed from the tumor or the activated lymphocytes, although recent evidence suggests that soluble Fas-L may actually inhibit apoptosis induced by cell-bound Fas-L. 30
These studies suggest that an inflammatory response does not always protect the host against an infiltrating tumor and may help to explain the paradoxical observation that some tumors occur less frequently and are less aggressive in patients who are immunosuppressed. 31 To our knowledge this is the first time a metastatic tumor has been shown to promote Fas-dependent apoptosis of surrounding uninvolved tissue. These findings may also explain why the liver is a common site for the development of metastatic spread from colon cancers. It is noteworthy that not all primary colon cancers express Fas-L, but all of the colorectal liver metastases we have studied so far are positive for Fas-L (Yoong and Adams, unpublished observation). The Fas pathway would allow Fas-L-expressing cancer cells to establish at the secondary site and grow into adjacent normal liver tissue in an orderly manner. Thus we suggest that the Fas system is pivotal in facilitating local tumor growth of colon cancer cells in the liver. Whether these observations are unique to the liver or are more widely applicable mechanism of tumor invasion in other tissues remains to be determined.
Table 1.
Results of Immunolocalization of Fas and Fas-L Protein Expression
| Cell type | Fas | Fas-L |
|---|---|---|
| Peritumoral margin | ||
| Tumor cells | 0.7 ± 0.26 | 1.2 ± 0.20 |
| Mononuclear cells | 1.4 ± 0.16 | 1.6 ± 0.16 |
| Hepatocytes | 2.7 ± 0.15 | 0 |
Results shown are for the different cellular compartments constituting the peritumoral margin of colorectal hepatic metastases (n = 10). Intensity of immunoreactivity scored as follows: 0 = absent, 1 = weak, 2 = moderate, 3 = strong expression for a given antigenic determinant. Results represent the mean ± SE for all individual scores.
Acknowledgments
We thank the nursing and medical staff on the Liver and Hepatobiliary Unit at the Queen Elizabeth Hospital for help with sample collection and our surgical colleagues, J.A.C. Buckels and D. Mayer, for allowing us to study their patients. This work was supported by grants from the Endowment Fund of the United Birmingham Hospitals and a Fellowship to KFY from the Royal College of Surgeons (England). We also thank J. Neuberger and L. Young for helpful discussions.
Footnotes
Address reprint requests to K. F. Yoong, Liver Research Laboratories, University of Birmingham, Queen Elizabeth Hospital, Birmingham, B15 2TH, United Kingdom.
Supported by grants from the Endowment Fund of the United Birmingham Hospitals and a research fellowship to KFY from the Royal College of Surgeons (England).
References
- 1.Hughes KS: Resection of the liver for colorectal-carcinoma metastases: a multi-institutional study of indications for resection. Surgery 1988, 103:278-288 [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KJ, Li B, Winer J: Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993, 362:841-844 [DOI] [PubMed] [Google Scholar]
- 3.Hardan I, Weiss L, Hershkoviz R: Inhibition of metastatic cell coloni-zation in murine lungs and tumor-induced morbidity by nonpeptidic arg-gly-asp mimetics. Int J Cancer 1993, 55:1023-1028 [DOI] [PubMed] [Google Scholar]
- 4.Robbins PF, Kawakami Y: Human tumor antigens recognised by T cells. Curr Opin Immunol 1996, 8:628-636 [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yannelli JR, Yang JC: Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin-2. J Natl Cancer Inst 1994, 86:1159-1166 [DOI] [PubMed] [Google Scholar]
- 6.Straten P, Becker JC, Seremet T, Brocker EB, Zeuthen J: Clonal T-cell responses in tumor-infiltrating lymphocytes from both regressive and progressive regions of primary human-malignant melanoma. J Clin Invest 1996, 98:279-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu Y, Iwatsuki S, Herberman RB, Whiteside TL: Clonal analysis of tumour infiltrating lymphocytes from human primary and metastatic liver tumours. Int J Cancer 1990, 46:878-883 [DOI] [PubMed] [Google Scholar]
- 8.Yoong KF, Adams DH: Interleukin-2 restores CD3 zeta chain expression but fails to generate specific cytolytic activity in tumor infiltrating lymphocytes from patients with hepatic colorectal metastases. Br J Cancer 1998, 77:1072-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takagi S, Chen K, Schwarz R, Iwatsuki S, Herberman RB, Whiteside TL: Functional and phenotypic analysis of tumor infiltrating lymphocytes isolated from human primary and metastatic liver tumours and cultured in recombinant interleukin-2. Cancer 1989, 63:102-111 [DOI] [PubMed] [Google Scholar]
- 10.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: Fas-mediated T-cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996, 184:1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 12.Mita E, Hayashi N, Iio S: Role of Fas ligand in apoptosis induced by hepatitis-c virus infection. Biochem Biophy Res Comm 1994, 204:468-474 [DOI] [PubMed] [Google Scholar]
- 13.Ju ST, Panka DJ, Cui HL: Fas(CD95) FasL interactions required for programmed cell-death after t-cell activation. Nature 1995, 373:444-448 [DOI] [PubMed] [Google Scholar]
- 14.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- 15.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC: A role for CD95 ligand in preventing graft rejection. Nature 1995, 377:630-632 [DOI] [PubMed] [Google Scholar]
- 16.Strand S, Hofmann WJ, Hug H, Galle PR: Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells: a mechanism of immune evasion? Nature Med 1996, 2:1361-1366 [DOI] [PubMed] [Google Scholar]
- 17.Strain AJ, Ismail T, Tsubouchi H, McMaster P, Neuberger JM: Native and recombinant human hepatocyte growth factor are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J Clin Invest 1991, 87:1853-1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams DH, Hubscher SG, Fisher NC, Williams A, Robinson M: Expression of E-selectin (CD62E) and E-selectin ligands in human liver inflammation. Hepatology 1996, 24:533-538 [DOI] [PubMed] [Google Scholar]
- 19.Adams DH, Burra P, Hubscher SG, Elias E, Newman W: Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology 1994, 19:588-594 [DOI] [PubMed] [Google Scholar]
- 20.Adams DH, Hubscher SG, Shaw J, Neuberger JM: Increased expression of ICAM-1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology 1991, 14:426-432 [PubMed] [Google Scholar]
- 21.Wyllie AH, Kerr JFR, Currie AR: Cell death: the significance of apoptosis. Int Rev Cytol 1980, 68:251-306 [DOI] [PubMed] [Google Scholar]
- 22.Afford SC, Hubscher S, Strain AJ, Adams DH, Neuberger JM: Apoptosis in the human liver during allograft-rejection and end-stage liver-disease. J Pathol 1995, 176:373-380 [DOI] [PubMed] [Google Scholar]
- 23.Adams DH, Yannelli J, Newman W, Rosenberg SA, Shaw S: Adhesion of tumour-infiltrating lymphocytes to endothelium: a functional and phenotypic analysis. Br J Cancer 1997, 75:1421-1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH: CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface FasL expression and amplifies Fas mediated hepatocyte death during allograft rejection. J Exp Med 1999, 189:441-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galle PR, Hofmann WJ, Wlaczak H: Involvement of the CD95 (APO1/FAS) receptor and ligand in liver damage. J Exp Med 1995, 182:1223-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoong KF, Hubscher SG, Adams DH: Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J Immunol 1998, 160:3978-3988 [PubMed] [Google Scholar]
- 27.Cheng J, Zou T, Liu C: Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 1994, 263:1759-1762 [DOI] [PubMed] [Google Scholar]
- 28.Alderson MR, Tough TW, Davis-Smith T: Fas ligand mediates activation-induced cell-death in human T-lymphocytes. J Exp Med 1995, 181:71-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunner T, Mogil RJ, Laface D: Cell-autonomous Fas (CD95) Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995, 373:441-444 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Itai T, Adachi M, Nagata S: Downregulation of Fas ligand by shedding. Nature Med 1998, 4:31-36 [DOI] [PubMed] [Google Scholar]
- 31.Stewart T, Tsai SC, Grayson H, Henderson R, Opelz G: Incidence of de-novo breast cancer in women chronically immunosuppressed after organ transplantation. Lancet 1995, 346:796-798 [DOI] [PubMed] [Google Scholar]