Abstract
Expression of the proteasome subunits LMP2 and LMP7, the MHC-encoded transporter subunits TAP1 and TAP2, and HLA Class I antigens was examined by immunoperoxidase staining in 10 nevi and 98 melanoma lesions (60 primary and 38 metastatic), because these molecules play an important role in the presentation of melanoma-associated peptide antigens to cytotoxic T cells. LMP2 was less frequently expressed than LMP7 in primary and metastatic melanoma lesions. TAP1, TAP2, and HLA Class I antigen expression was more frequently (P < 0.05) down-regulated in metastatic than in primary melanoma lesions and in nevi. A synchronous TAP1, TAP2, and HLA Class I antigen down-regulation was observed in 58% of primary and 52% of metastatic lesions. TAP and HLA Class I antigen down-regulation in primary lesions was significantly associated with lesion thickness, stage of disease, reduced time to disease progression, and reduced survival. These results suggest that TAP down-regulation plays a role in the clinical course of malignant melanoma, probably by providing melanoma cells with a mechanism to escape from cytotoxic T lymphocyte recognition during disease progression.
HLA Class I antigens play a major role in the immune response against viral infections and transformed cells by presenting peptide antigens to cytotoxic T lymphocytes (CTL). 1 Peptide antigens are derived from degradation of mostly endogenous proteins in the nucleus and cytosol by the proteasome complex. 2 Peptides generated in this way are then translocated via the transporter associated with antigen processing (TAP) into the endoplasmic reticulum, 3 where they are loaded onto newly synthesized HLA Class I heavy chain-β2 microglobulin (β2m) dimers. The resulting trimolecular HLA Class I complex is then expressed on the cell surface.
The TAP molecule is composed of two noncovalently associated subunits, TAP1 and TAP2, encoded by closely related genes located in the MHC Class II region. 4 TAP activity is required for efficient peptide loading and proper assembly of MHC Class I molecules. Mutant cells that have deleted the TAP genes are unable to load peptides onto MHC Class I molecules in the endoplasmic reticulum and have a severe defect in MHC Class I antigen cell surface expression. 5-7 Closely linked to the TAP1 and TAP2 genes within the MHC are two genes encoding the IFN-γ inducible proteasome subunits, LMP2 and LMP7. 8,9 Incorporation of LMP2 and/or LMP7 subunits into the proteasome complex confers subtle effects on its proteolytic activities enhancing the production of antigenic peptides that are capable of binding to MHC Class I molecules. 10,11 The up-regulation of both LMP and TAP subunits by IFN-γ suggests that increased expression of these molecules is important in the host immune response.
The potential role of immunological events in the pathogenesis and clinical course of malignant melanoma has influenced the emergence of T cell-based immunotherapy for the treatment of this disease. 12 The critical role played by HLA Class I antigens in the success of T cell-based immunotherapy has led to a growing interest in analyzing the expression and function of these molecules by melanoma cells. A large number of surgically removed melanoma lesions have been analyzed by immunohistochemical techniques for HLA Class I antigen expression. Malignant transformation of melanocytes has been found to be frequently associated with abnormalities in HLA Class I antigen expression, which range from complete loss of HLA Class I antigens to selective loss of one HLA Class I allospecificity. 13 These abnormalities appear to be clinically relevant because they have been reported to be associated with disease progression in patients with Stage 2 melanoma 14 and with recurrence of the disease in a few patients following an initial response to T cell-based immunotherapy. 15 These findings have been suggested 13 to reflect the escape of melanoma cells from recognition by melanoma-associated antigen (MAA)-specific, HLA Class I antigen-restricted CTL. In contrast to the large body of evidence demonstrating HLA Class I antigen down-regulation in melanoma lesions, there is no information about the expression of antigen-processing molecules responsible for generating peptides from MAA and for their transport and loading on HLA Class I molecules. Therefore, in the present study we have investigated LMP2, LMP7, TAP1, and TAP2 expression in primary and metastatic melanoma lesions. Furthermore, we have correlated the expression of the TAP subunits in melanoma cells with that of HLA Class I antigens and with the histopathological characteristics of the lesions as well as with the clinical course of the disease. The information from these studies contributes to our understanding of the clinical significance of abnormalities in the antigen processing machinery of melanoma cells.
Materials and Methods
Tissues
Benign and malignant lesions of melanocyte origin were obtained from surgical patients. Tissues were processed within 15 minutes after their surgical removal. Whole or partial tissue samples were fixed in 10% neutral buffered formaldehyde, routinely processed, and embedded in paraffin. The remaining tissue was snap-frozen in liquid nitrogen and stored at −80°C until use. Cryostat sections 4 μm thick were dried and fixed in absolute acetone for 1 minute. Under these fixation conditions, cryostat sections could be stored for at least 3 months at −20°C without loss of reactivity.
Monoclonal and Polyclonal Antibodies
The affinity-purified rabbit anti-human LMP2, 16 anti-human LMP7, 16 anti-human TAP1 17 and anti-human TAP2 antibodies, 17 and the mAb HC-10 to a determinant preferentially expressed on β2m-free HLA-B and C heavy chains 18 were developed and characterized as described. The Vectastain ABC kit, biotinylated anti-rabbit Ig xenoantibodies, and biotinylated anti-mouse Ig xenoantibodies were purchased from Vector Laboratories (Burlingame, CA).
Indirect Immunoperoxidase Staining
Indirect immunoperoxidase staining of frozen and formalin-fixed tissues was performed using the Vectastain ABC kit according to the manufacturer’s instructions with minor modifications as described elsewhere. 19 Briefly, 4-μm-thick paraffin sections were deparaffinized with xylene, rehydrated by passage through decreasing concentrations of ethanol (100 to 80%), and pretreated for 10 minutes at 90°C with Target Unmasking Fluid (Kreatech, Amsterdam, The Netherlands). Paraffin-embedded and frozen sections were preincubated with 5% normal goat or horse serum for 30 minutes at room temperature followed by an overnight incubation at 4°C with primary antibody. Negative control staining reactions were performed by replacing primary antibody with normal rabbit serum or with supernatant from the murine myeloma cell line P3-X63-Ag8.653. The percentage of stained melanoma cells in each lesion was evaluated independently by two investigators. A melanoma lesion was scored as positive when the percentage of stained melanoma cells in the entire lesion was > 75%, heterogeneous when the percentage was between 25 and 75%, and negative when the percentage was < 25%. Variations in the percentage of stained cells enumerated by the two investigators were within a 10% range.
Statistical Analysis
Differences in the expression of LMP2, LMP7, TAP1, TAP2, and HLA Class I antigens in terms of positive, heterogeneous, or negative staining of nevi, primary and metastatic lesions were analyzed using the χ 2 test. The disease-free and survival curves were calculated by using the method of Kaplan and Meier. Differences in time to disease progression and survival curves were analyzed with a log rank test.
Results
Patients
Ten pigmented nevi lesions were obtained from two male and eight female patients with an average age of 30.9 years (range, 15–54 years). Two lesions were compound type and 8 were intradermal nevi. Sixty primary melanoma lesions were obtained from 28 male and 32 female patients with an average age of 66.4 years (range, 26–93 years). Thirty-three patients suffered from acral lentiginous melanoma, seven from superficial spreading melanoma, nine from nodular melanoma, four from mucosal melanoma and two from lentigo malignant melanoma. Nine patients had Stage 1, 17 Stage 2, 31 Stage 3, and 3 Stage 4 disease. Tumor staging was based on the histopathological TNM classification system. The mean thickness of the 60 primary lesions was 4.3 mm (range, 0.4–17.0 mm). Ten lesions had a thickness <1.5 mm, 25 between 1.5–4.0 mm and 25 >4.0 mm. Patients were monitored every 2 to 6 months by an attending physician of the Department of Dermatology, Kumamoto University School of Medicine, or by the referring physician. All 60 patients in this study were followed for time to disease progression and survival. Time to disease progression was calculated as the interval, in months, between surgical removal of the primary lesion and detection of a skin metastasis by patient or physician and/or of a metastasis in visceral organs by radiography, ultrasound echography, CT scan, MRI, and/or gallium scintigraphy. Forty patients were disease-free between 6 and 65 months following removal of primary lesions; the remaining 20 patients had experienced recurrence of the disease between 2 and 55 months after surgery. Forty-two patients were alive between 6 and 65 months following removal of primary lesions; the remaining 18 patients were dead between 5 and 50 months after surgery.
Thirty-eight metastatic lesions were obtained from 20 male and 18 female patients with an average age of 63.1 years (range, 14–91 years). Twenty-one lesions were obtained from skin metastases and the remaining 17 from lymph nodes.
Immunohistochemical Staining Patterns of Formalin-Fixed and Paraffin-Embedded versus Frozen Sections of Primary Melanoma Lesions with Anti-LMP and Anti-TAP Antibodies
Formalin-fixed, paraffin-embedded tissue specimens were chosen for the present study to increase the number of available melanoma lesions and to provide the opportunity for retrospective analyses. Preliminary experiments were performed to determine whether the fixation procedure affected the immunohistochemical staining of tissue sections with anti-LMP and anti-TAP antibodies. To this end, sixteen primary melanoma lesions were divided into two aliquots. One was frozen and the other one was formalin-fixed and paraffin-embedded. The staining patterns of frozen and formalin-fixed, paraffin-embedded tissue sections with anti-LMP and anti-TAP antibodies were compared. As summarized in Table 1 ▶ , the staining patterns of formalin-fixed, paraffin-embedded tissue sections with both anti-LMP and anti-TAP antibodies were not significantly different from those of frozen tissue sections (P > 0.05). Anti-LMP2 antibodies stained more than 75% of melanoma cells in 63% of both tissue substrates. In contrast, the remaining antibodies stained a lower percentage of formalin-fixed, paraffin-embedded tissue sections than of frozen tissue sections. Specifically, anti-LMP7, anti-TAP1 and anti-TAP2 antibodies stained with a positive score 75, 69, and 62%, respectively, of formalin-fixed, paraffin-embedded tissue sections and 88, 75, and 81%, respectively, of frozen tissue sections. Furthermore, the anti-LMP2 antibodies stained two and the anti-TAP1 and anti-TAP2 antibodies stained three of the formalin-fixed, paraffin-embedded tissue sections with a negative score. In contrast, the three antibodies stained all frozen tissue sections with a positive score. Anti-LMP7 antibodies stained neither formalin-fixed, paraffin-embedded sections nor frozen tissue sections with a negative score. These results indicate that formalin-fixed, paraffin-embedded tissue sections are only slightly less reactive than frozen tissue sections in immunohistochemical assays.
Table 1.
Immunoperoxidase Staining of Frozen and Formalin-Fixed Sections of Primary Melanoma Lesions with Anti-LMP2, Anti-LMP7, Anti-TAP1 and Anti-TAP2 Antibodies
| Lesions | Score | LMP2 | LMP7 | TAP1 | TAP2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | no. | % | no. | % | no. | % | ||
| Frozen | + | 10 | (63) | 14 | (88) | 12 | (75) | 13 | (81) |
| +/− | 6 | (37) | 2 | (12) | 4 | (25) | 3 | (19) | |
| − | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | |
| Formalin-Fixed | + | 10 | (63) | 12 | (75) | 11 | (69) | 10 | (62) |
| +/− | 4 | (25) | 4 | (25) | 2 | (12) | 3 | (19) | |
| − | 2 | (12) | 0 | (0) | 3 | (19) | 3 | (19) |
+, positive (>75%); +/−, heterogeneous (25–75%); −, negative (<25%)
LMP2 and LMP7 Expression in Benign and Malignant Lesions of Melanocyte Origin
To analyze the expression of the proteasome subunits LMP2 and LMP7 in transformed melanocytes, surgically removed nevi and melanoma lesions were stained with rabbit anti-LMP2 and anti-LMP7 antibodies. The results of immunohistochemical staining of 10 nevi and 60 primary and 38 metastatic melanoma lesions are summarized in Table 2 ▶ . Examples of positive, heterogeneous, and negative LMP staining are shown in Figure 1 ▶ . Both anti-LMP2 and anti-LMP7 antibodies stained 60% of the nevi lesions with a positive score and 40% with a heterogeneous score. Anti-LMP2 and anti-LMP7 antibodies stained 37 and 55%, respectively, of primary melanoma lesions with a positive score. The remaining primary lesions were stained with a heterogeneous score by anti-LMP2 and anti-LMP7 antibodies. LMP2 and LMP7 displayed only slight differences in their expression in primary lesions of different histotypes. The highest percentage of lesions stained with a positive score by anti-LMP2 and anti-LMP7 antibodies was found in superficial spreading melanoma, while the highest percentage of lesions stained by these antibodies with a negative score was found in lentigo malignant melanoma.
Table 2.
LMP2, LMP7, TAP1, TAP2, and HLA Class I Antigen Expression in Nevi and in Primary and Metastatic Melanoma Lesions
| Lesions | Score | LMP2 | LMP7 | TAP1 | TAP2 | HLA Class I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | % | no. | % | no. | % | no. | % | no. | % | ||
| Nevi | + | 6 | (60) | 6 | (60) | 7 | (70) | 5 | (50) | 5 | (50) |
| +/− | 4 | (40) | 4 | (40) | 3 | (30) | 5 | (50) | 2 | (20) | |
| − | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 3 | (30) | |
| Primary | + | 18 | (37) | 27 | (55) | 29 | (48) | 26 | (44) | 25 | (42) |
| +/− | 20 | (41) | 20 | (41) | 19 | (32) | 23 | (38) | 24 | (40) | |
| − | 11 | (22) | 2 | (4) | 12 | (20) | 11 | (18) | 11 | (18) | |
| Metastatic | + | 7 | (19) | 20 | (53) | 5 | (13) | 9 | (24) | 7 | (18) |
| +/− | 21 | (55) | 14 | (37) | 26 | (69) | 21 | (55) | 16 | (42) | |
| − | 10 | (26) | 4 | (10) | 7 | (18) | 8 | (21) | 15 | (40) |
+, positive (>75%); +/−, heterogeneous (25–75%); −, negative (<25%)
Figure 1.
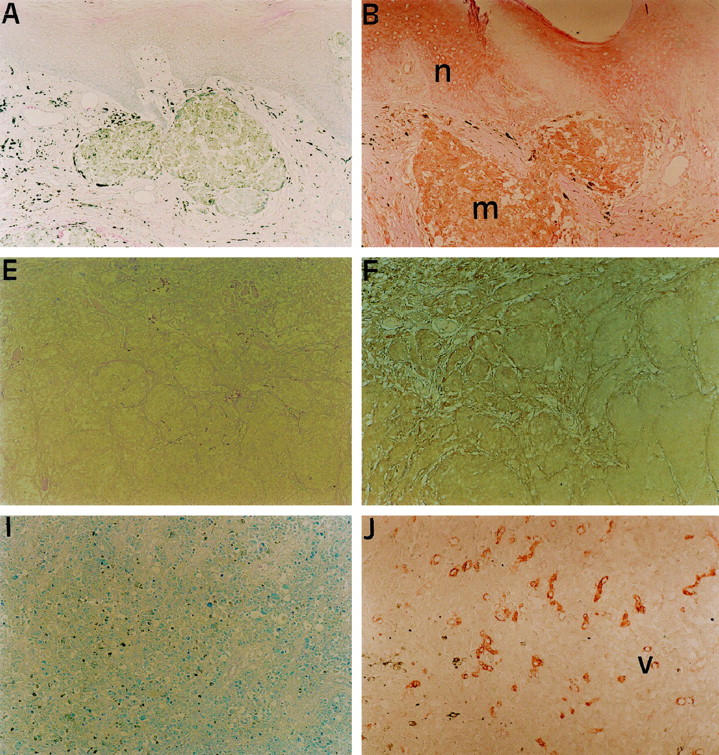
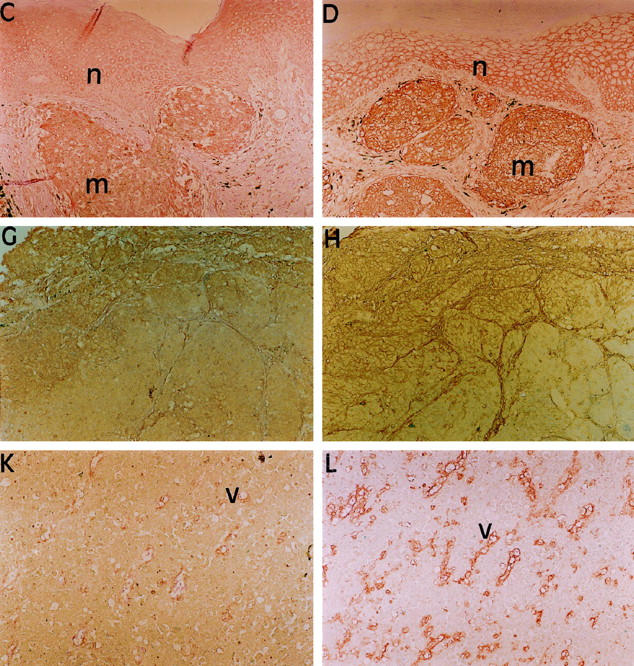
LMP2, TAP1 and HLA Class I antigen expression in melanoma lesions. Serial sections of a formalin-fixed, paraffin-embedded primary (A-D) and a metastatic (E-L) melanoma lesion were stained in the immunoperoxidase reaction with control (A, E, I), anti-LMP2 (B, F, J), anti-TAP1 (C, G, K) or anti-HLA Class I (D, H, L) antibodies. Positive or heterogeneous staining for LMP2, TAP1 and HLA Class I antigens is shown in primary melanoma lesions (m) as well as normal (n) epidermis (B-D). Melanoma cells in metastatic lesions stained with a heterogeneous (F-H) or negative (J-L) score for LMP2, TAP1, and HLA Class I antigens. Blood vessels (v) in metastatic lesions (J-L) stained with a positive score for the three markers. Original magnification, ×100.
Only 19% of metastatic lesions were stained with a positive score by anti-LMP2 antibodies. More than 50% of the lesions were stained with a heterogeneous score. The difference in LMP2 expression between primary and metastastic lesions did not reach the level of statistical significance. The expression of LMP7 in metastases was also not significantly different from that in primary lesions, as 53% of the lesions were stained with a positive score.
TAP1, TAP2, and HLA Class I Antigen Down-Regulation in Primary and Metastatic Melanoma Lesions
To determine whether TAP1 and TAP2 expression is down-regulated in melanoma lesions, surgically removed nevi and primary and metastatic melanoma lesions were stained with rabbit anti-TAP1 and anti-TAP2 antibodies. Furthermore, the expression of HLA Class I heavy chains was assessed by staining the lesions with mAb HC-10. Examples of positive, heterogeneous, and negative staining for TAP and HLA Class I heavy chains are shown in Figure 1 ▶ and results are summarized in Table 2 ▶ .
Anti-TAP1 and anti-TAP2 antibodies stained 70 and 50% of nevi, respectively, with a positive score and the remaining nevi with a heterogeneous score. Anti-TAP1 and anti-TAP2 antibodies stained 48 and 44%, respectively, of primary melanoma lesions with a positive score. Most of the remaining primary lesions were stained by the two antibodies with a heterogeneous score. The two TAP subunits were coordinately down-regulated in 26 of the 34 lesions with reduced TAP1 and/or TAP2 expression. TAP1 and TAP2 were similarly expressed in primary lesions of different histotypes.
TAP1 and TAP2 expression was down-regulated in metastatic lesions. Only 13 and 24% of the metastatic lesions were stained with a positive score by anti-TAP1 and anti-TAP2 antibodies, respectively. The majority of the remaining lesions were stained by both antibodies with a heterogeneous score and weak intensity. The two TAP subunits were coordinately down-regulated in 25 of the 33 metastatic lesions with reduced TAP1 and/or TAP2 expression. The difference in TAP1 expression between primary and metastatic lesions was statistically significant (P < 0.001), while that of TAP2 expression was not.
Anti-HLA Class I mAb HC-10 stained 50% of nevi with a positive score, 20% with a heterogeneous score, and 30% with a negative score. Anti-HLA Class I mAb HC-10 stained 42% of primary melanoma lesions with a positive score and 40% with a heterogeneous score. HLA Class I antigen expression was markedly reduced in metastatic lesions. Only 18% of the lesions were stained with a positive score. The difference in HLA Class I antigen expression between primary and metastatic lesions, as measured by the percentage of lesions stained with a positive score, was statistically significant (P = 0.02). HLA Class I antigens displayed only slight differences in their expression in primary lesions of different histotypes.
Staining of serial sections of primary and metastatic lesions revealed synchronous TAP1 and/or TAP2 and HLA Class I antigen down-regulation in many of the lesions with TAP or HLA Class I antigen down-regulation (Figure 2) ▶ . The same areas of melanoma lesions that were not stained by anti-TAP1 and/or TAP2 antibodies were also not stained by anti-HLA Class I mAb in 20 and 20, respectively, of 35 primary lesions and in 18 and 16, respectively, of 31 metastases.
Figure 2.
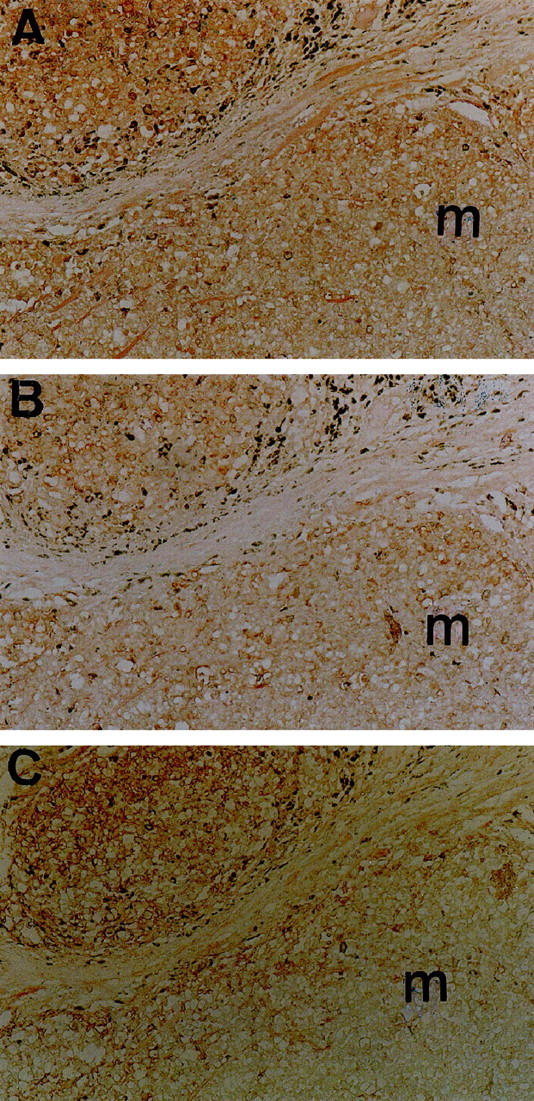
Synchronous TAP1, TAP2, and HLA Class I antigen down-regulation in metastatic melanoma lesions. Serial sections of a formalin-fixed, paraffin-embedded metastatic melanoma lesion were stained in the immunoperoxidase reaction with rabbit anti-TAP1 (A) and anti-TAP2 (B) antibodies and with anti-HLA Class I mAb HC-10 (C). Heterogeneous staining for TAP1, TAP2, and HLA Class I antigens is shown in nests of melanoma cells (m) with synchronous loss of each antigen. Original magnification, ×100.
Differential LMP2, TAP1, TAP2, and HLA Class I Antigen Expression in Primary and Autologous Metastatic Lesions
To establish that the differences in LMP subunit, TAP subunit, and HLA Class I antigen expression between primary and metastatic lesions reflect an association with disease progression and not variability among patients, the expression of LMP and TAP subunits and HLA Class I antigens in 24 metastases and 21 autologous primary lesions removed from 21 patients was compared. Expression of all of the markers analyzed, with the exception of LMP7, was lower in metastases than in primary lesions. It is noteworthy that the expression of LMP2, TAP1, TAP2, and HLA Class I antigens was higher in metastases than in the autologous primary lesions in six, seven, nine, and six cases, respectively. In most of them, the primary lesion thickness was >4 mm. The negative staining patterns for LMP2, TAP1, TAP2, and HLA Class I antigens in these cases are likely to be due to lesion thickness as described below.
Relationship of LMP and TAP Subunit and HLA Class I Antigen Expression in Primary Lesions with Lesion Thickness, Stage of Disease, and Clinical Course
LMP, TAP, and HLA Class I antigen expression in primary lesions showed a relationship with lesion thickness (Table 3) ▶ . Specifically, the percentage of lesions with a thickness >4 mm stained by anti-LMP2 and anti-LMP7 antibodies with a positive score was significantly (P < 0.05) lower than that of lesions with a thickness <4 mm. Moreover, the percentage of lesions with a thickness >4 mm stained by anti-LMP2 (P ≤ 0.001), anti-TAP1 (P = 0.129), anti-TAP2 (P = 0.036), and anti-HLA Class I (P = 0.007) antibodies with a negative score was higher than that of lesions with a thickness <4 mm.
Table 3.
Relationship of LMP2, LMP7, TAP1, TAP2, and HLA Class I Antigen Expression in Primary Melanoma Lesions with Their Thickness
| Thickness (mm) | LMP2 | LMP7 | TAP1 | TAP2 | HLA Class I | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | +/− | − | + | +/− | − | + | +/− | − | + | +/− | − | + | +/− | − | ||
| <1.5 | no. | 1 | 7 | 1 | 6 | 3 | 0 | 6 | 3 | 1 | 4 | 6 | 0 | 6 | 4 | 0 |
| (%) | (11) | (78) | (11) | (67) | (33) | (0) | (60) | (30) | (10) | (40) | (60) | (0) | (60) | (40) | (0) | |
| 1.5–4.0 | no. | 14 | 5 | 1 | 14 | 6 | 0 | 14 | 9 | 2 | 13 | 10 | 2 | 12 | 12 | 1 |
| (%) | (70) | (25) | (5) | (70) | (30) | (0) | (56) | (36) | (8) | (52) | (40) | (8) | (48) | (48) | (4) | |
| >4.0 | no. | 3 | 8 | 9 | 7 | 11 | 2 | 9 | 7 | 9 | 9 | 7 | 9 | 7 | 8 | 10 |
| (%) | (15) | (40) | (45) | (35) | (55) | (10) | (36) | (28) | (36) | (36) | (28) | (36) | (28) | (32) | (40) |
+, positive (>75%); +/−, heterogeneous (25–75%); −, negative (<25%)
LMP, TAP, and HLA Class I antigen expression in primary lesions displayed a relationship with stage of the disease (Table 4) ▶ . Specifically, a higher percentage of lesions from patients in Stages 1 and 2 than from patients in Stages 3 and 4 was stained by anti-LMP2, anti-LMP7, anti-TAP1, anti-TAP2, and anti-HLA Class I mAb. However, the difference was statistically significant only for LMP2 (P = 0.04), LMP7 (P = 0.017), and HLA Class I antigens (P = 0.004).
Table 4.
Relationship of LMP2, LMP7, TAP1, TAP2, and HLA Class I Antigen Expression in Primary Melanoma Lesions with Stage of Disease
| Stage of disease* | Score | LMP2 | LMP7 | TAP1 | TAP2 | HLA Class I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | % | no. | % | no. | % | no. | % | no. | % | ||
| 1 and 2 | + | 12 | (50) | 18 | (75) | 15 | (58) | 13 | (50) | 15 | (58) |
| +/− | 10 | (42) | 6 | (25) | 9 | (35) | 11 | (42) | 11 | (42) | |
| − | 2 | (8) | 0 | (0) | 2 | (8) | 2 | (8) | 0 | (0) | |
| 3 and 4 | + | 6 | (24) | 9 | (36) | 14 | (42) | 13 | (38) | 10 | (30) |
| +/− | 10 | (40) | 14 | (56) | 10 | (29) | 12 | (35) | 13 | (38) | |
| − | 9 | (36) | 2 | (8) | 10 | (29) | 9 | (27) | 11 | (32) |
+, positive (>75%); +/−, heterogeneous (25–75%); −, negative (<25%)
*TNM staging classification.
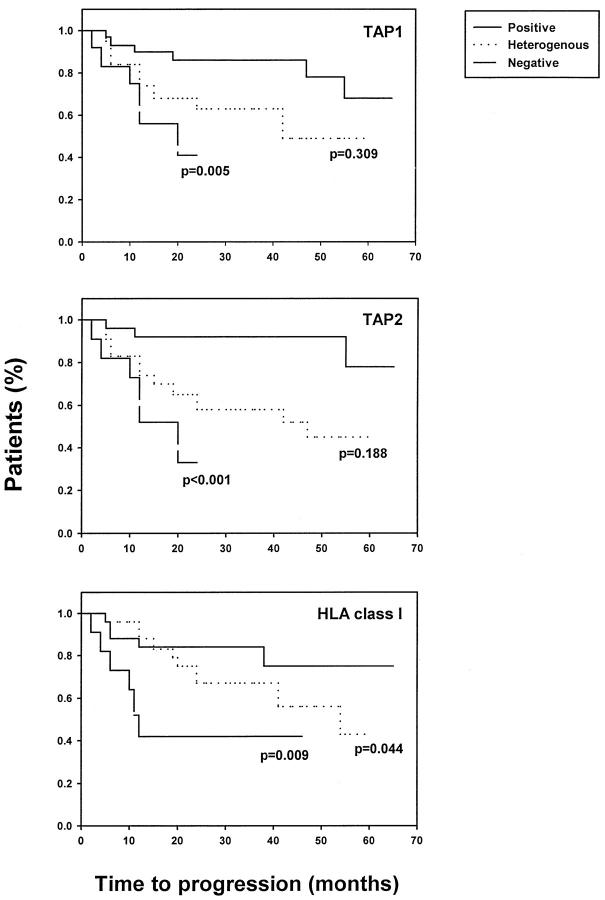
Expression of the components of the antigen processing machinery and of HLA Class I antigens in primary lesions was associated with clinical parameters of the disease. Specifically, the time to disease progression and the survival time of the patients whose primary lesions were stained by anti-TAP1, anti-TAP2, and anti-HLA Class I antibodies with a positive score were significantly longer than those of patients whose lesions were stained with a negative score (Figures 3 and 4) ▶ ▶ . Furthermore, both the time to disease progression and the survival of patients whose lesions were stained by anti-TAP1, anti-TAP2 and anti-HLA Class I antibodies with a positive score were longer than those of patients whose lesions were stained with a heterogeneous score. However, the only statistically significant difference was that of the association between heterogeneous staining for HLA Class I antigens and time to disease progression (P = 0.044).
Figure 3.
Association of TAP1, TAP2, and HLA Class I antigen down-regulation in primary melanoma lesions with patient time to disease progression. Serial sections of formalin-fixed, paraffin-embedded primary lesions were stained with anti-TAP1, anti-TAP2, or anti-HLA Class I antibodies. The time to disease progression was compared among patients with lesions scored as positive, heterogeneous, or negative.
Figure 4.
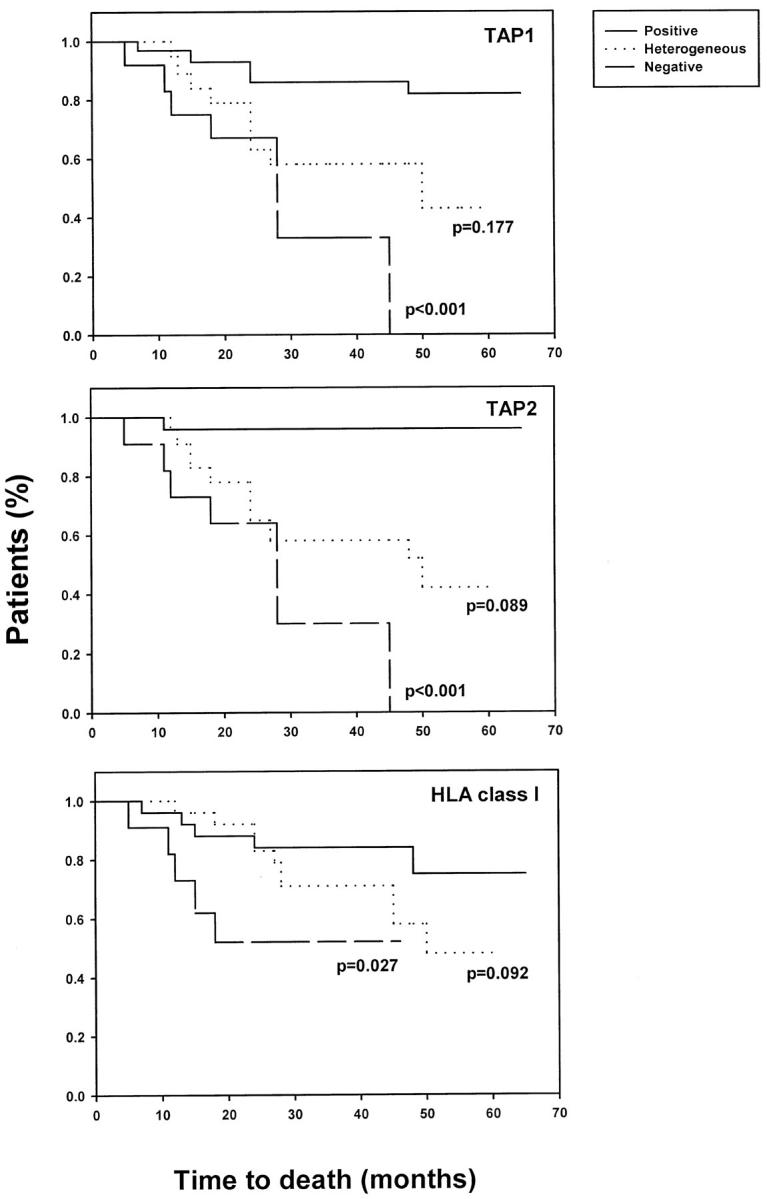
Association of TAP1, TAP2, and HLA Class I antigen down-regulation in primary melanoma lesions with patient survival. Serial sections of formal-fixed, paraffin-embedded primary lesions were stained with anti-TAP1, anti-TAP2, or anti-HLA Class I antibodies. The survival of patients with lesions scored as positive, heterogeneous, or negative was compared by Kaplan-Meier analysis.
Discussion
In the present study, LMP2, LMP7, TAP1, TAP2, and HLA Class I antigen expression in melanoma lesions was evaluated by immunohistochemical staining and correlated with histopathological features of the lesions and with the clinical course of the disease. The results of the present study demonstrate for the first time that: (1) LMP2 is less frequently expressed in melanoma lesions than in nevi, while LMP7 is similarly expressed in benign and malignant lesions of melanocytic origin; (2) TAP1, TAP2, and HLA Class I antigen expression is frequently down-regulated in melanoma lesions; (3) TAP subunit down-regulation increases with disease progression, because these abnormalities are markedly less frequent in nevi and in primary lesions than in metastatic lesions; (4) TAP subunit and HLA Class I antigen down-regulation is synchronous in many of the lesions; (5) TAP1, TAP2, and HLA Class I antigen down-regulation in primary lesions is inversely correlated with their thickness; and (6) TAP1, TAP2, and HLA Class I antigen down-regulation in primary lesions is significantly associated with patients’ time to disease progression and survival.
The constitutive expression of LMP2 and the down-regulation of TAP1 and TAP2 we found in the surgically removed melanoma lesions parallel similar results we and others have obtained by analyzing LMP and/or TAP subunit expression at the mRNA and protein level in a panel of melanoma cell lines. 20,21 These findings are at variance with those of Thor-Stratten et al, 22 who detected no abnormalities in the expression of LMP and TAP subunits at the mRNA level in a panel of melanoma cell lines. If not caused by technical reasons, the conflicting results may reflect differences in the characteristics of the cell lines analyzed. Whatever the reason, the abnormalities in LMP and TAP subunit expression, including their noncoordinate expression at the mRNA level we have identified in some melanoma cell lines, parallel the results we have obtained by analyzing melanoma lesions in the present study. These findings argue against the possibility that the defects in melanoma cell lines are an in vitro artifact or that the differential LMP and TAP subunit expression we have found in some melanoma lesions reflect differences in the characteristics of the antibodies used for immunostaining.
Melanocytes in culture constitutively express TAP1, TAP2, and, to a lesser extent, LMP7. LMP2 is not constitutively expressed in melanocytes but is readily induced by IFN-γ (D.J. Hicklin and S. Ferrone, unpublished results). Therefore, if the phenotype we have identified in melanocytes in culture reflects that of melanocytes in vivo, LMP2 expression in nevi and some of the melanoma lesions analyzed represents an up-regulation of this molecule in these tissues. This phenotype in nevi may be due to changes in gene regulation or induction by endogenous factors from infiltrating cells or surrounding stroma. Interestingly, LMP2 expression was found less frequently in primary and metastatic melanoma lesions, suggesting that the mechanisms responsible for LMP2 up-regulation in nevi were altered in melanoma lesions.
The importance of TAP and LMP expression for MHC Class I-dependent antigen processing has been directly demonstrated in experimental animal models. TAP1 knockout mice are defective in the stable assembly of MHC Class I molecules and show extremely reduced cell surface MHC Class I antigen expression. 23 Furthermore, cells of TAP1 knockout mice are defective in processing cytosolic antigens for recognition by CTL and lack CD4−8+ T cell subpopulations. 23 Similarly, LMP2 and LMP7 knockout mice display reduced MHC Class I antigen cell surface expression and process certain endogenous antigens inefficiently. 24,25 However, these defects in MHC Class I antigen expression and presentation are less dramatic than in TAP1-deficient mice. Furthermore, no abnormalities in CD8+ T cell populations were found in LMP2 or LMP7 knockout mice. TAP subunit down-regulation in melanoma lesions, combined with the role of these molecules in proper MHC Class I antigen presentation, suggests that recognition of melanoma cells by the host immune system is impaired. Additionally, the increased frequency of abnormalities in TAP expression in advanced disease suggests that immune recognition of melanoma cells decreases over time with disease progression.
There is little information regarding the role of TAP in the presentation of MAA to CTL. It is likely that the LMP and/or TAP down-regulation we have found in melanoma cell lines 20 and melanoma lesions (this study) impairs the proper processing and presentation of at least some MAA. White et al 21 recently demonstrated loss of antigen processing function in a panel of melanoma cell lines that was due to TAP1 and/or TAP2 and HLA Class I antigen down-regulation. These melanoma cell lines are defective in antigen processing and poorly recognized by antigen-specific CTL. Endogenous processing function and CTL recognition of these melanoma cell lines was restored following transduction with TAP and HLA Class I genes. These studies provide evidence for antigen processing component down-regulation in melanoma cell lines that results in loss of CTL recognition. However, there is no direct evidence to date in patients demonstrating that TAP defects result in loss of CTL recognition of MAA.
TAP1 down-regulation has been described in various types of surgically removed carcinoma lesions. 26-31 The frequency of TAP1 down-regulation we have found in melanoma lesions is similar to that described in breast carcinoma, non-small-cell lung carcinoma, and cervical carcinoma lesions and higher than that found in colon carcinoma lesions. Consistent with previous findings in breast, non-small-cell lung, colon, and cervical carcinoma 26-31 synchronous TAP1 and HLA Class I antigen down-regulation was found in melanoma lesions. Furthermore, this synchronous loss of expression was expanded in the present study to include TAP2 and HLA Class I antigens. Although it is possible that genetic mutations in TAP1, TAP2, and HLA Class I heavy chain genes account for the combined loss of expression in some lesions, it is unlikely to be the predominant underlying molecular mechanism. A more plausible explanation for these findings is that one or more common regulatory mechanisms result in reduced expression of these genes in melanoma lesions.
The clinical significance of TAP and HLA Class I antigen down-regulation in melanoma lesions is suggested by its correlation with the histopathological characteristics of the lesions and with the clinical status of the patients from whom the lesions originated. TAP1, TAP2, and HLA Class I antigen down-regulation in primary lesions was associated with their thickness. This finding may reflect the selection of tumor cells that have acquired resistance to T cell recognition and outgrowth of these cells in advanced lesions of increased thickness. In addition, the association between TAP and HLA class I down-regulation and lesion thickness may account for the correlation between lesion thickness and poor prognosis in patients. The clinical significance of TAP and HLA class I antigen down-regulation is also demonstrated by the higher frequency of this defect in metastases than in primary lesions. This is not a general phenomenon, because a higher TAP1, TAP2, and HLA Class I antigen expression was found in some metastases when compared to autologous primary lesions. In most cases, these findings were related to increased thickness of the primary lesion. These findings illustrate the need to consider multiple histopathological characteristics of tumors that may contribute to the TAP and HLA class I antigen phenotype. TAP and HLA class I antigen expression in primary lesions was also associated with stage of disease, time to disease progression, and survival. If not fortuitous, this association provides evidence for a role in recognition of melanoma cells by MAA-specific, HLA Class I-restricted CTL in the clinical course of the disease. It is noteworthy that the significantly higher frequency of TAP1 down-regulation in metastases than in primary lesions we have found in malignant melanoma has been found also in breast and cervical carcinoma. 26,29 Furthermore, we recently reported an association between tumor grade and TAP down-regulation in breast carcinoma. 31 However, the relationship of TAP1 down-regulation with the clinical parameters of the disease appears to be restricted to malignant melanoma. The latter association may reflect the role that immunological events may play in the pathogenesis and clinical course of malignant melanoma. In view of the growing interest in applying T cell-based immunotherapy for the treatment of malignant melanoma, it is noteworthy that abnormalities in the antigen processing machinery in melanoma cells are likely to have a negative impact on the outcome of this type of immunotherapy. Although such a conclusion is necessarily speculative at this time, analysis of the antigen processing machinery in melanoma lesions warrants consideration when establishing patient selection criteria for T cell-based immunotherapy.
Acknowledgments
The authors thank Ms. Donna D. James, Ms. Harriett V. Harrison, Ms. Derina McDonald, and Ms. Elba Osorio for excellent secretarial assistance.
Footnotes
Address reprint requests to Soldano Ferrone, M.D., Department of Microbiology and Immunology, New York Medical College, Valhalla, NY 10595. E-mail: soldano_ferrone@nymc.edu.
Supported by United States Public Health Service grants CA51814 and CA67988 awarded by the National Cancer Institute.
References
- 1.Germain RN: MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell 1994, 76:287-299 [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL, Rock KL: Proteolysis, proteasomes, and antigen presentation. Nature 1992, 357:375-379 [DOI] [PubMed] [Google Scholar]
- 3.Townsend A, Trowsdale J: The transporters associated with antigen presentation. Semin Cell Biol 1993, 4:53-61 [DOI] [PubMed] [Google Scholar]
- 4.Beck S, Kelly A, Radley E, Khurshid F, Alderton RP, Trowsdale J: DNA sequence analysis of 66kb of the human MHC class II region encoding a cluster of genes for antigen processing. J Mol Biol 1992, 228:433-441 [DOI] [PubMed] [Google Scholar]
- 5.Spies T, DeMars R: Restored expression of major histocompatibility Class I molecules by gene transfer of a putative peptide transporter. Nature 1991, 351:323-324 [DOI] [PubMed] [Google Scholar]
- 6.Powis SJ, Townsend ARM, Deverson EV, Bastin J, Butcher GW, Howard JC: Restoration of antigen presentation in the mutant cell line RMA-S by an MHC-linked transporter. Nature 1991, 354:528-531 [DOI] [PubMed] [Google Scholar]
- 7.Attaya M, Jameson S, Martinez CK, Hermel E, Aldrich C, Forman J, Lindahl KF, Bevan MJ, Monaco JJ: Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature 1992, 355:647-649 [DOI] [PubMed] [Google Scholar]
- 8.Glynne R, Powis SH, Beck S, Kelly A, Kerr LA, Trowsdale J: A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature 1991, 353:357-360 [DOI] [PubMed] [Google Scholar]
- 9.Kelly A, Powis SH, Glynne R, Radley E, Beck S, Trowsdale J: Second proteasome-related gene in the human MHC class II region. Nature 1991, 353:667-668 [DOI] [PubMed] [Google Scholar]
- 10.Gaczynska M, Rock KL, Spies T, Goldberg AL: Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proc Natl Acad Sci USA 1994, 91:9213-9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuckelkorn U, Frentzel S, Kraft R, Kostka S, Groettrup M, Kloetzel PM: Incorporation of major histocompatibility complex-encoded subunits LMP2 and LMP7 changes the quality of the 20S proteasome polypeptide processing products independent of interferon-γ. Eur J Immunol 1995, 25:2605-2611 [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Kawakami Y, Robbins PF, Wang R: Identification of the genes encoding cancer antigens: implications for cancer immunotherapy. Adv Cancer Res 1996, 70:145-177 [DOI] [PubMed] [Google Scholar]
- 13.Ferrone S, Marincola FM: Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today 1995, 16:487-494 [DOI] [PubMed] [Google Scholar]
- 14.van Duinen SG, Ruiter DJ, Broecker EB, van der Velde EA, Sorg C, Welvaart K, Ferrone S: Level of HLA antigens in locoregional metastases and clinical course of the disease in patients with melanoma. Cancer Res 1988, 48:1019-1025 [PubMed] [Google Scholar]
- 15.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli YR, Rosenberg SA: Loss of functional β2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst 1996, 88:100-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellaratta DV, Hicklin DJ, Kishore R, Kageshita T, Ferrone S: Characterization of rabbit antisera elicited with human LMP2- and LMP7-specific peptides and recombinant proteins. Tissue Antigens 1997, 50:567-575 [DOI] [PubMed] [Google Scholar]
- 17.Hicklin DJ, Kageshita T, Ferrone S: Development and characterization of rabbit antisera to human MHC-linked transporters associated with antigen processing. Tissue Antigens 1996, 48:38-46 [DOI] [PubMed] [Google Scholar]
- 18.Stam NJ, Spits H, Ploegh HL: Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol 1986, 137:2299-2306 [PubMed] [Google Scholar]
- 19.Kageshita T, Nakamura M, Yamada, Kuriya N, Arao T, Ferrone S: Differential expression of melanoma associated antigens in acral lentiginous melanoma and in nodular melanoma lesions. Cancer Res 1991, 51:1726-1732 [PubMed] [Google Scholar]
- 20.Hicklin DJ, Kageshita T, Dellaratta D, Ferrone S: Transporter associated with antigen processing (TAP) downregulation in human melanoma cells. In HLA. Genetic Diversity of HLA. Functional and Medical Implication. Proceedings of the Twelfth International Histocompatibility Workshop and Conference. Vol. II. Edited by D Charron. Sevres, France, EDK, 1997, pp 742–744
- 21.White CA, Thomson SA, Cooper L, van Endert PM, Tampe R, Coupar B, Qiu L, Parsons PG, Moss DJ, Khanna R: Constitutive transduction of peptide transporter and HLA genes restores antigen processing function and cytotoxic T cell-mediated immune recognition of human melanoma cells. Int J Cancer 1998, 75:590-595 [DOI] [PubMed] [Google Scholar]
- 22.Thor Straten P, Kirkin AF, Seremet T, Zeuthen J: Expression of transporter associated with antigen processing 1 and 2 (TAP1/2) in malignant melanoma cell lines. Int J Cancer 1997, 70:582-586 [DOI] [PubMed] [Google Scholar]
- 23.Van Kaer L, Ashton Rickardt PG, Ploegh HL, Tonegawa S: TAP1 mutant mice are deficient in antigen presentation,surface class I molecules, and CD4−8+ T cells. Cell 1992, 71:1205-1214 [DOI] [PubMed] [Google Scholar]
- 24.Van Kaer L, Ashton Rickardt PG, Eichelberger M, Gaczynska M, Nagashima K, Rock KL, Goldberg AL, Doherty PC, Tonegawa S: Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity 1994, 1:533-541 [DOI] [PubMed] [Google Scholar]
- 25.Fehling HJ, Swat W, Laplace C, Kuhn R, Rajewsky K, Muller U, von Boehmer H: MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 1994, 265:1234-1237 [DOI] [PubMed] [Google Scholar]
- 26.Cromme FV, van Bommel PF, Walboomers JM, Gallee MP, Stern PL, Kenemans P, Helmerhorst TJ, Stukart MJ, Meijer CJ: Differences in MHC and TAP-1 expression in cervical cancer lymph node metastases as compared with the primary tumours. Br J Cancer 1994, 69:1176-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cromme FV, Airey J, Heemels M-T, Ploegh HL, Keating PJ, Stern PL, Meijer CJLM, Walboomers JMM: Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med 1994, 179:335-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaklamanis L, Townsend A, Doussis-Anagnostopoulou IA, Mortensen N, Harris AL, Gatter KC: Loss of major histocompatibility complex-encoded transporter associated with antigen presentation (TAP) in colorectal cancer. Am J Pathol 1994, 145:505-509 [PMC free article] [PubMed] [Google Scholar]
- 29.Kaklamanis L, Leek R, Koukourakis M, Gatter KC, Harris AL: Loss of transporter in antigen processing 1 transport protein and major histocompatibility complex class I molecules in metastatic versus primary breast cancer. Cancer Res 1995, 55:5191-5194 [PubMed] [Google Scholar]
- 30.Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC: Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer 1996, 73:148-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitale M, Rezzani R, Rodella L, Zauli G, Grigolato P, Cadei M, Hicklin DJ, Ferrone S: HLA Class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res 1998, 58:737-742 [PubMed] [Google Scholar]