Abstract
Analyses of apoptosis and of the apoptosis regulatory proteins Bcl-2, Bax, Bcl-X, and Bad were done in 95 nontumorous and neoplastic pituitary tissues by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL), immunohistochemistry, and Western blotting. The apoptotic index was relatively low in all groups but was at least fourfold higher in pituitary carcinomas compared with any other groups. Pituitaries from pregnant and postpartum women had a fivefold higher apoptotic index compared with matched controls from nonpregnant females. Preoperative treatment of adenomas with octreotide or dopamine agonists did not change the apoptotic index significantly. The lowest levels of Bcl-2, Bax, and Bcl-X expression were in pituitary carcinomas as detected by immunostaining. An immortalized human pituitary adenoma cell line, HP75, developed in our laboratory using a replication-defective recombinant human adenovirus with an early large T-antigen, had a much higher level of apoptosis than nontumorous and neoplastic pituitaries. Treatment with transforming growth factor (TGF)-β1 and protein kinase C (PKC) inhibitors increased apoptosis in this cell line. Analysis of the Bcl-2 family of proteins after treatment with TGF-β1 and PKC inhibitors showed a 20% to 30% decrease in Bcl-X in the treated groups compared with controls. These results, which represent the first study of apoptosis in pituitaries from pregnant and postpartum cases and in pituitary carcinomas, indicate that 1) the apoptotic rate is low in nontumorous and neoplastic pituitary tissues but is relatively higher in pituitary carcinomas, 2) there are alterations in the expression of the Bcl-2 family of proteins in pituitary neoplasms with a decrease in Bcl-2 expression in pituitary carcinomas that may contribute to pituitary tumor pathogenesis and/or proliferation, and 3) cultured pituitary tumor cells respond to TGF-β1 and PKC inhibitors by undergoing apoptotic cell death.
Programmed cell death, or apoptosis, is frequently observed in hormone-dependent cells, usually after hormonal withdrawal. 1-3 Various pituitary models of apoptosis have been reported in experimental animals. 4-6 Studies with animal models have provided some insights into the effects of hormonal manipulation on apoptosis in pituitary cells. However, the few reported studies of apoptosis in human pituitaries have simply described the detection of apoptotic cells in various types of pituitary adenomas 7,8 or in primary cultures of human pituitary adenoma cells. 9 The effects of specific drugs that block pituitary tumor hormone secretion and of physiological alterations in the pituitary, such as pregnancy, the postpartum period, and lactation, on apoptosis have not been examined in humans.
The Bcl-2 family of proteins regulate various steps in apoptosis. 10,11 Some of the members of this gene family, including Bcl-2, Bcl-XL, and Mcl-1, block cell death whereas others, such as Bax, Bad, and Bcl-Xs, promote programmed cell death. 10,11 These proteins often interact with each other as homo- and heterodimers in which the relative proportions of the anti-apoptotic and pro-apoptotic members determine the ultimate susceptibility of cells to apoptosis. A recent study has shown overexpression of Bcl-2 reported in nine (30%) of the pituitary adenomas examined. 12 However, expression of other pro-apoptotic and anti-apoptotic proteins have not been previously examined in human pituitary adenomas. Overexpression of Bcl-2 provides protection against various apoptotic stimuli, including growth factor deprivation, oncogenes such as c-myc, tumor suppressor genes such as p53, radiation, and chemotherapeutic drugs. 1-3 In this report, we examined a large series of pituitaries, including normal pituitaries, pituitaries from pregnant and postpartum patients, pituitary adenomas with and without drug therapy, and pituitary carcinomas for evidence of apoptosis and for expression of the Bcl-2-related pro-apoptotic and anti-apoptotic proteins to determine the role of physiological alterations and neoplastic development and progression on apoptosis in pituitary cells. An immortalized human pituitary cell line, developed in our laboratory, was also studied to analyze regulation of expression of the Bcl-2 family of proteins in pituitary cells during induction of apoptosis.
Materials and Methods
Pituitary Tissues
Formalin-fixed and paraffin-embedded human pituitary tissues (n = 95) retrieved from the files of the Mayo Clinic and the St. Michael’s Hospital were used for these studies. These included postmortem pituitaries from women who died during pregnancy (n = 5) and women who died during the postpartum period (n = 20). Ten postmortem pituitary tissues from young women were used as a control for pituitaries from pregnant and postpartum women. In addition, resected pituitary adenomas (n = 35) from untreated patients, growth hormone (GH)-producing adenomas from patients treated with octreotide before surgery (n = 9), prolactin (PRL)-producing tumors from patients treated with dopamine agonist before surgery (n = 8), and pituitary carcinomas with proven metastases (n = 8) were also studied. All pituitary adenomas and carcinomas were classified by immunohistochemistry (IHC). Electron microscopy was performed on some adenomas to analyze for apoptotic bodies and for other ultrastructural changes associated with apoptosis.
TUNEL Reaction
Apoptotic activity on paraffin sections of the pituitary tissue was analyzed by terminal deoxynucleotide transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) with a commercial kit (Boehringer Mannheim, Indianapolis, IN), following the manufacturer’s instructions. Briefly, pituitary sections were treated with proteinase K (25 μg/ml in phosphate-buffered saline (PBS), Boehringer Mannheim) for 15 minutes at 37°C before the TUNEL reaction. After incubation with anti-digoxigenin alkaline phosphatase, the sections were developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate for 5 to 10 minutes. Apoptotic cells had blue nuclear staining. Omission of the TdT enzyme in the TUNEL reaction was used as a negative control and resulted in no staining. Tonsil tissue was used as a positive control. Apoptosis was evaluated by randomly counting 3000 cells from each section with a microscope containing a 1 × 1 cm 2 grid in the ocular, and the results were expressed as an apoptotic index (AI = number of apoptotic cells per 100 cells). Apoptotic bodies were also detected on H&E-stained sections.
Immunohistochemistry
The expression of various apoptotic regulatory proteins was analyzed on paraffin sections of pituitaries from all groups after IHC. The anti-human antibodies used in this study included Bax (used at a 1/1000 dilution; from Pharmingen, San Diego, CA), Bcl-X (1/250) and Bad (1/100; both from Transduction Laboratories, Lexington, KY), and Bcl-2 (1/25; from Dako, Carpinteria, CA). Sections were treated with antigen retrieval by incubating for 15 minutes in a 700-W microwave oven in 10 mmol/L citric acid, pH 6.0, to increase the sensitivity of antigen detection. All immunostains were performed with the avidin biotin peroxidase method as previously reported. 13 Substitution of normal serum for primary antibodies, as a negative control, resulted in no staining of the cells. Spleen tissue was used as a positive control. The specificity of each antibody was also checked by Western blotting. Immunostaining results were graded as follows: 0, negative; 1+, weakly positive; 2+, moderately positive; 3+, strongly positive.
IHC for pituitary hormones was done as previously described. 13 Immunostaining for Ki67 with the MIB-1 antibody (AMAC, Westbrook, ME) at a 1:50 dilution was done as previously described. 14
Combined immunostaining for S100 protein (Sigma Chemical Co., St. Louis, MO) and Bcl-X was done to co-localize Bcl-X in folliculo-stellate cells. The antibody co-localizations were visualized with diaminobenzidine and then with alkaline phosphatase after sequential staining.
Apoptotic Activity in Vitro
To analyze the apoptotic activity of pituitary tumors in vitro, a cell culture study was performed using six pituitary adenomas (three gonadotroph (GTH) and three null cell adenomas) and the HP75 cell line. The immortalized HP75 cell line, originally derived from a human nonfunctional pituitary adenoma, was developed in our laboratory using a replication-defective recombinant human adenovirus that contains an SV40 early large T antigen. 15 The HP75 cells were grown in serum-free Dulbecco’s modified essential medium (DMEM; Life Technologies, Grand Island, NY) for 1 to 3 days, at 37°C in an atmosphere of 5% CO2 and 95% air. At the start of the experiment the HP75 cells were treated with the protein kinase C (PKC) inhibitor hypericin 16,17 (2 to 20 μmol/L) or chelerythrine chloride (1 to 5 μmol/L) 18 and transforming growth factor (TGF)-β1 (10−9 mol/L; R&D Systems, Minneapolis, MN). After culture, the tumor cells were harvested and used for protein extraction. Cytocentrifuge preparations were also made for IHC and TUNEL staining.
Immunoblot Analysis
Cultured HP75 pituitary cells and spleen tissue were used for protein extraction. One-dimensional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed with a 12% gel using the discontinuous buffer system of Laemmli (Bio-Rad Laboratories, Richmond, CA), as previously reported. 14 The electrophoresed proteins were transferred to a polyvinylidene difluoride membrane and subjected to immunoblot analysis with anti Bcl-X (used at a 1/500 dilution), Bax (1/4000), Bcl-2 (1/200), and Bad (1/400). The reaction was detected with enhanced chemiluminescence (Amersham Life Science, Arlington Heights, IL). The membranes were reblotted with a β-actin antibody (1/2500; Sigma) after washing to check for equal loading of the gel. Spleen tissue was used as a positive control.
Statistical Analysis
Results were expressed as the mean ± SEM. Student’s t-test was used to evaluate statistical significance.
Results
Cases
The cases studied are summarized in Table 1 ▶ . The 10 control pituitaries were obtained from premenopausal women obtained at autopsy from patients without endocrine diseases. The five pituitaries from pregnant women who died during the last trimester of pregnancy showed prolactin cell hyperplasia. The 20 pituitaries from postpartum women included glands from patients who died between 28 days and 399 days after delivery. It was not known how many of these patients were nursing at the time of death. The 35 adenomas from patients who had surgery without previous treatment included 6 GH, 6 PRL, 6 ACTH, 3 TSH, 6 GTH, and 8 null cell adenomas. Nine cases of GH adenomas treated with octreotide and eight cases of prolactinomas treated with bromocriptine or parlodel before surgery were studied. The eight carcinomas with proven metastatic disease included five PRL and three ACTH tumors.
Table 1.
Detection of Apoptotic Cells and Bcl-2 Protein Family Expression in Human Pituitaries by Immunohistochemistry
| Group | n | Number of cases with apoptosis (%) | Positive cases (%) | |||
|---|---|---|---|---|---|---|
| Bcl-2 | Bcl-X | Bax | Bad | |||
| Normal pituitaries, female | 10 | 3 (30%) | 8 (80) (2+) | 10 (100) (3+) | 10 (100) (2+) | 10 (100) (2+) |
| Pituitaries, pregnancy | 5 | 2 (40%) | 5 (100) (2+) | 5 (100) (2+) | 5 (100) (2+) | 5 (100) (2+) |
| Pituitaries, postpartum | 20 | 8 (40%) | 19 (95) (2+) | 18 (90) (2+) | 20 (100) (2+) | 19 (95) (2+) |
| Adenomas, untreated | 35 | 5 (14%) | 24 (68) (2+) | 35 (100) (3+) | 35 (100) (2+) | 33 (94) (2+) |
| GH adenomas, octreotide | 9 | 1 (11%) | 8 (89) (2+) | 9 (100) (2+) | 9 (100) (2+) | 8 (89) (1+) |
| PRL adenoma, dopamine agonist | 8 | 0 (0%) | 6 (75) (2+) | 8 (100) (2+) | 8 (100) (2+) | 8 (100) (1+) |
| Carcinomas | 8 | 7 (88%) | 2 (25) (1+) | 8 (100) (1+) | 8 (100) (1+) | 6 (75) (1+) |
Staining intensity was evaluated as follows: 1+, weak staining; 2+, moderate staining; 3+, strong staining; f, focal staining in less than 25% of the tumor cells. n, number of cases.
Apoptosis in Vivo
Ultrastructural studies of two pituitary adenomas (ACTH and GTH) that had apoptotic cells detected with the TUNEL assay showed condensation of the nuclear chromatin and occasional apoptotic bodies (Figure 1) ▶ .
Figure 1.
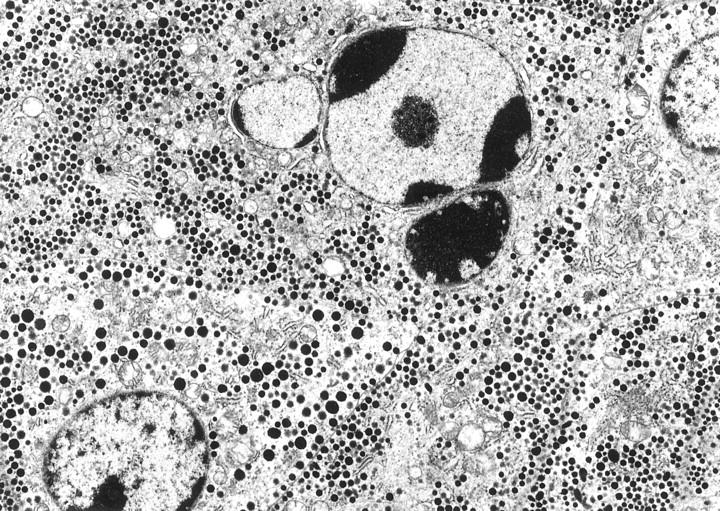
ACTH adenoma undergoing apoptosis with prominent condensed chromatin and apoptotic body formation in portion of the nucleus. Magnification, ×7500.
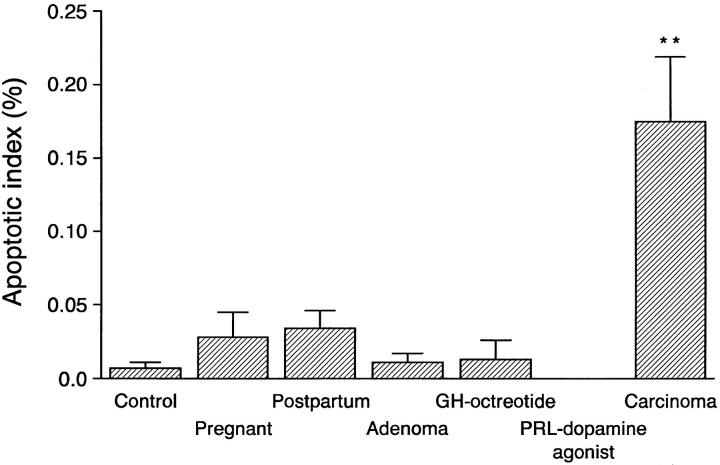
The percentage of cells undergoing apoptosis was very low in all groups. Apoptosis, detected by the TUNEL reaction, was most frequent in pituitary carcinomas and was significantly greater than in adenomas (Figure 2) ▶ . Apoptotic cells were identified in most carcinomas (7/8 cases), but only in 40% or less of the cases in all other groups (Table 1) ▶ . Relatively few apoptotic cells were present in the normal pituitaries or in adenomas. Quantitation of the AI showed that pituitaries from pregnant and postpartum women had four- to fivefold higher AI compared with control nonpregnant women (Figure 3) ▶ . There were no significant differences in the AI in functioning (PRL, GH, ACTH, and TSH) adenomas compared with the nonfunctioning (GTH and null cell) adenomas. There was no difference in the AI of GH-secreting adenomas or the octreotide-treated GH adenomas. Prolactin adenomas treated with bromocriptine did not contain any detectable apoptotic cells.
Figure 2.
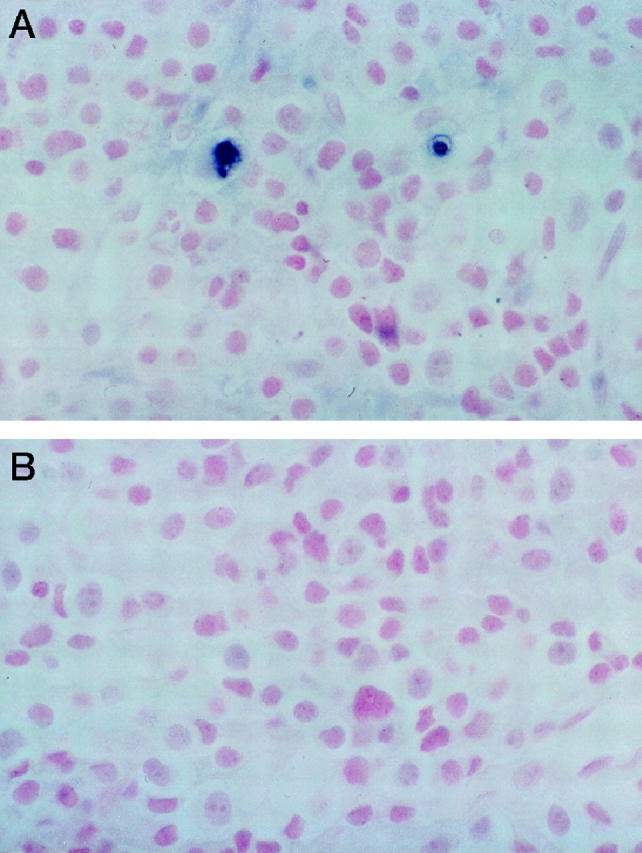
A: Pituitary carcinoma showing two cells with apoptosis in the field shown by dark blue nuclear staining with TUNEL staining. B: The control section in which the terminal deoxynucleotide transferase was omitted was negative for apoptotic cells. Magnification, ×250.
Figure 3.
Analysis of the apoptotic index after TUNEL staining in normal and neoplastic pituitaries. Pituitary carcinomas had a significantly higher apoptotic index than all other groups of tumors and non-neoplastic pituitaries. Apoptotic cells were not detected in prolactinomas treated with dopamine agonist. **P < 0.01 compared with adenomas.
Bcl-2 Family of Proteins
Immunostaining of the pituitary tissues in paraffin sections showed moderate (2+) Bcl-2 staining in most groups, except for the carcinomas in which weak staining (1+) was present in only 2/8 (25%) cases (Table 1) ▶ . Two of four null cell adenomas and six of nine GTH adenomas had weak staining for Bcl-2, and the remaining null cell and GTH tumors were Bcl-2 negative. Other adenomas had strong to moderate staining for Bcl-2. Immunostaining for Bcl-X, Bax, and Bad were not very different among the various groups except that Bax and Bcl-X stained more weakly in carcinomas (Figure 4) ▶ . Bcl-X staining was very strong in ∼10% of the S100-positive folliculo-stellate cells in normal pituitaries and in pituitaries from pregnant and postpartum women (Figure 4) ▶ . Co-localization studies confirmed that some of these Bcl-X-positive cells were angular folliculo-stellate cells with positive nuclear stains for S100 protein and brown cytoplasmic staining for Bcl-X (Figure 4D ▶ , inset).
Figure 4.
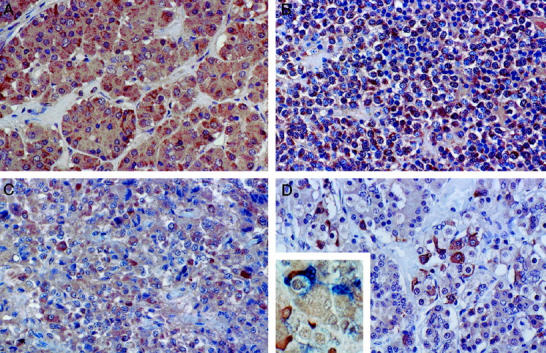
Immunostaining for Bcl-2 family of proteins in pituitary. A: Normal pituitary showing moderate (2+) diffuse staining for Bax in most anterior pituitary cells. B: GH adenoma with strong staining for Bax in all of the tumor cells. C: ACTH pituitary carcinoma with weak (1+) to moderate (2+) staining for Bax. D: Pituitary for pregnant woman stained for Bcl-X. The folliculate stellate cells that have slender cytoplasmic processes stain strongly for Bcl-X, whereas many of the secretory anterior pituitary cells are staining weakly (1+). The inset shows co-localization of Bcl-x with brown cytoplasmic staining with some S100-protein-positive folliculo-stellate cells with blue nuclear staining. Magnification, ×300 (A), ×250 (B–D), and ×350 (inset).
Apoptosis in Vitro
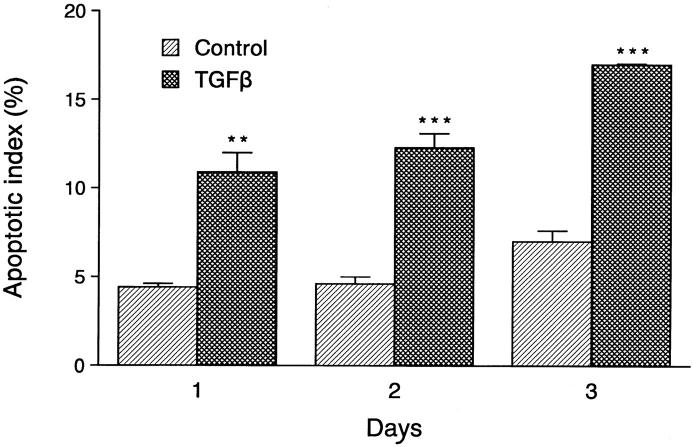
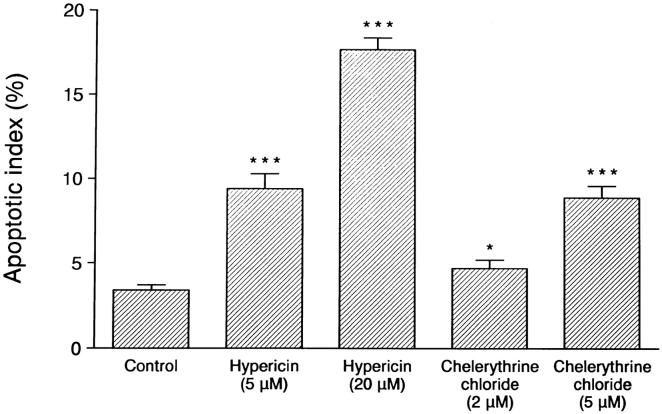
Analysis of the HP75 cell line cultured in serum-free medium showed an AI of 3% to 5% by TUNEL analysis, whereas pituitary adenoma cells in primary cultures consisting of three null cells and three GTH adenomas had AI values of 0.17 ± 0.09% and 0.10 ± 0.06%, respectively. Apoptotic bodies were readily identified by light and electron microscopic analysis (Figure 5) ▶ . Treatment of the cells with 10−9 mol/L TGF-β1 for 1 to 3 days produced an increase in the number of apoptotic cells, with an AI of ∼10% on day 1 to 15% on day 3 (Figure 6) ▶ . The protein kinase inhibitor hypericin stimulated apoptosis in the HP75 cells (Figure 7) ▶ . Because hypericin is not a specific inhibitor of PKC, the experiments were repeated with chelerythrine chloride, which also stimulated apoptosis in the HP75 cells but at a much lower concentration of the drug. Cells treated with chelerythrine chloride had a lower AI than with hypericin but significantly higher than controls (Figure 7) ▶ .
Figure 5.
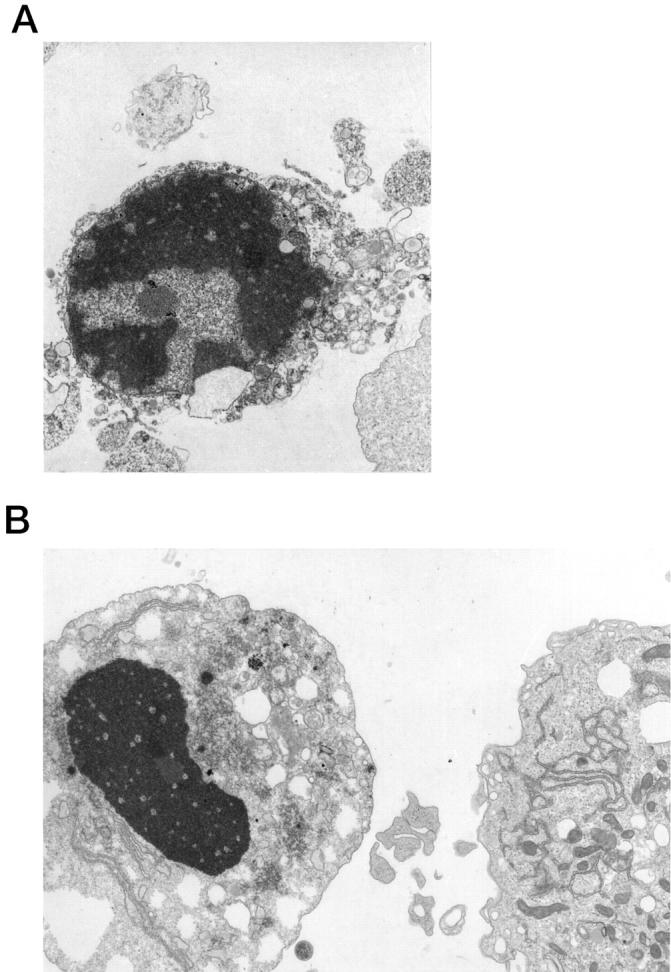
Ultrastructural examination of cultured HP75 cells. A: An apoptotic cell with nuclear fragmentation and compact cytoplasm is present. B: An apoptotic body is present in the cell on the left. Magnification, ×5000 (A) and ×6000 (B).
Figure 6.
Effects of TGF-β1 treatment on the apoptotic index (AI) in cultured HP75 cells. The AI increased with duration of treatment between 1 and 3 days using 10−9 mol/L TGF-β1. Data are from three experiments. **P < 0.01; ***P < 0.001.
Figure 7.
Analysis of the effects of hypericin and chelerythrine chloride treatment on the AI in HP75 cells. Both drugs increased the AI in cultured HP75 cells. Data are from three experiments. *P < 0.05; ***P < 0.001.
Analysis of the changes in the Bcl-2 family of proteins by Western blotting after induction of apoptosis in HP75 cells showed that TGF-β1, and hypericin-treated cells had a 30% decrease in Bcl-X compared with controls. Cells treated with chelerythrine chloride had a 20% decrease in Bcl-X when analyzed by densitometry. The other Bcl-2 family members examined, including Bax, Bcl-2, and Bad, were similar in the control and treated groups (Figure 8) ▶ .
Figure 8.
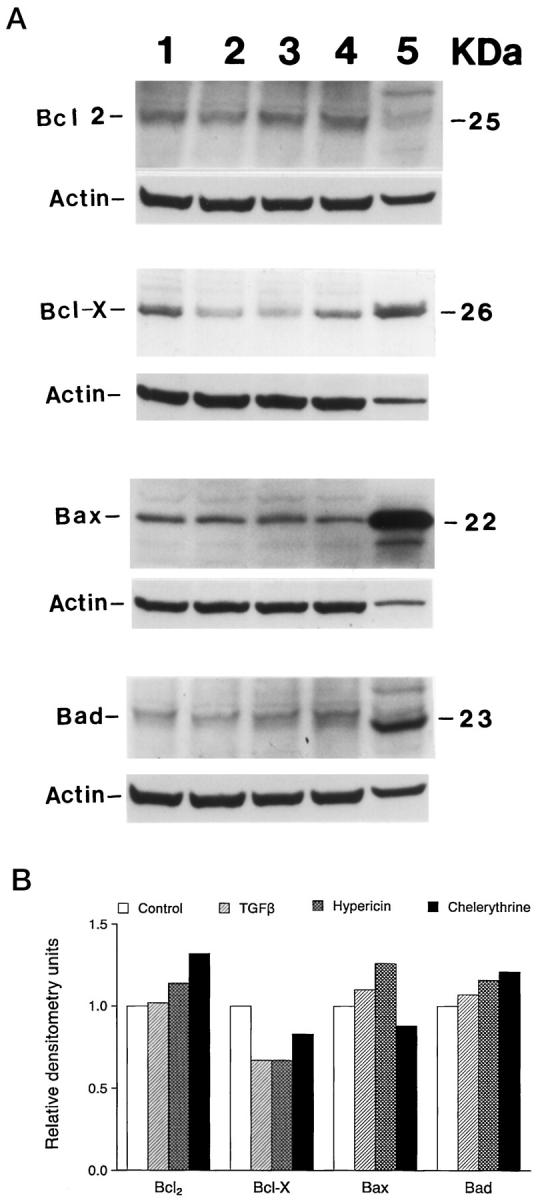
Immunoblot analysis of various Bcl-2 protein family members on HP75 cells after TGF-β1, hypericin, and chelerythrine chloride treatment. Cells were cultured in the presence of the chemicals for 24 hours, and the proteins were extracted and analyzed for specific proteins as indicated. A representative result from two experiments is shown. A: Western blot analysis after enhanced chemiluminescence. Lane 1, control; lane 2, TGF-β1; lane 3, hypericin; lane 4, chelerythrine choloride; lane 5, spleen control tissue. B: Densitometric analysis showing changes in Bcl-2 family of proteins relative to controls.
Discussion
Analysis of apoptosis in a wide range of human pituitaries showed a relatively low level of programmed cell death; however, the highest apoptotic index was present in pituitary carcinomas. Pituitaries from pregnant and postpartum women had apoptotic indices that were four- to fivefold higher than control pituitaries, indicating increased programmed cell death in these pituitaries. The pituitary increases 80% to 100% in weight during pregnancy secondary to prolactin cell hyperplasia. 19 During the postpartum period discontinuation of lactation leads to an increase in apoptosis, which is well documented in experimental animals. 20,21 These later findings are similar to estrogen treatment and withdrawal in the rat model of pituitary hyperplasia and apoptosis. 4 In the rat model, apoptotic cells and other cells containing phagocytized apoptotic bodies increased during a period of 44 hours after estrogen withdrawal. 4
The apoptotic indices in this study and others 8,22 were significantly lower than those observed in a recent report of 85 human pituitary adenomas. 7 This discrepancy probably reflects technical differences in detecting and quantifying apoptotic cells with the TUNEL reaction. We also used the presence of apoptotic bodies and ultrastructural evidence of apoptosis to support the light microscopic observations. 23 Comparison of functioning and nonfunctioning adenomas in this study showed no significant difference in apoptotic indices between the two groups of tumors in contrast to a previous report. 7 Functioning somatotropinomas in the study of Saitoh et al22 and Green et al 8 did not have increased apoptotic indices, which agrees with our observations. GH-producing adenomas treated with octreotide had a similar rate of apoptosis as untreated adenomas, whereas apoptotic cells were not detected in prolactin cell adenomas after treatment with dopamine agonist. This observation is in agreement with the study of Kontogeorgos et al. 7 Similarly, Saitoh et al 22 found no significant change in apoptosis in octreotide-treated somatotropinomas when compared with untreated tumors. However, they reported an increased apoptotic index in bromocriptine-treated prolactinomas. Divergent findings about the effects of bromocriptine on apoptosis in prolactin cells have also been reported in animal model studies 4 in which hyperplasia was induced by high doses of estrogen. Withdrawal of estrogen induced cell death, which was further increased by application of bromocriptine. In the mouse AtT20 corticotroph cell line, bromocriptine stimulated apoptosis in up to 40% of the cells after 3 days of treatment. 5 In vitro studies using SMS-201-995, a drug similar to octreotide, showed induction of apoptosis in AtT20 cells from 8% to 41% after 24 hours. 6 The effect of SMS-201-995 on MCF-7 breast cancer cells was similar and caused an increase in the percentage of apoptotic cells from 8% to 34% after 24 hours. 24 The contradictory reports about the effects of dopamine and somatostatin analogues on pituitary cell apoptosis may result from different doses and duration of treatment in particular groups of patients and in animal and/or in vitro experiments.
In this report we studied several members of the Bcl-2 family of apoptosis-regulating genes in normal and neoplastic human pituitary tissues. Bcl-2 expression has been analyzed in pituitary adenomas 12 but not in physiological stages such as pregnancy and lactation and in pituitary carcinomas. In our study, Bcl-2 was moderately expressed in all groups of tumors and normal pituitary and was much weaker in pituitary carcinomas (Table 1) ▶ , which correlates with the increased rate of apoptosis. Bax, Bcl-X, and Bad were expressed in most pituitary tissues with less intensive staining in carcinomas, indicating that both pro-apoptotic and anti-apoptotic proteins are expressed concurrently in the same cells. The Bcl-X antibody recognizes the anti-apoptotic Bcl-XL and the pro-apoptotic Bcl-Xs forms of the protein. However, the predominant form of Bcl-X seen in most tissues to date is Bcl-XL, and Bcl-Xs is usually absent or present in very low amounts, 25 so Bcl-X may be acting as an anti-apoptotic protein in these tissues.
The expression of the Bcl-2 family of proteins has been studied in several neoplastic tissues, indicating its importance in tumor development. 26 A recent model of a transgenic mouse in which p53-mediated apoptosis modified brain tumor growth showed that p53-dependent expression of Bax was induced in slow-growing tumors. 27 In Bax-deficient mice, tumor growth was accelerated and apoptosis decreased by 50%, suggesting that Bax acts as a tumor suppressor gene. 27 In primary colorectal adenocarcinomas, 28 Bcl-2 immunostaining was weaker than in adjacent normal epithelium, which is similar to our observations in pituitary carcinomas. In the study of colonic adenocarcinomas, fewer cells were Bcl-2 positive in carcinomas than in adenomas. Expression of Bax was not significantly altered in colorectal carcinomas whereas the intensity of Bcl-X immunostaining was increased. Bcl-2 was expressed in ∼70% of pituitary adenomas in our study, which is significantly more than in previous report. 12 Two of four null cell adenomas in our study of resected pituitary adenomas stained weakly (1+) for Bcl-2 protein. This is in contrast to a previously published report in which all null cell adenomas were Bcl-2 negative. 12 These differences may be related to the sensitivity of the assay techniques and the Bcl-2 antibodies used. We used microwave antigen retrieval in our studies, which increases the sensitivity of the assay. Recent studies have described markers of the Bcl-2 family of proteins in other endocrine tumors, including thyroid tumors. 29 Both Bcl-2 and Bax were weakly expressed in undifferentiated thyroid tumors, which is similar to our observations in pituitary carcinomas.
The immortalized human pituitary cell line HP75 was derived from a nonfunctional adenoma 15 by overexpression of SV40 T antigen. Analysis of the Bcl-2 family of proteins in this cell line revealed stronger expression of Bcl-X and Bax and decreased levels of Bcl-2 and Bad. Interestingly, induction of apoptosis in the HP75 cells by TGF-β or PKC inhibitors correlated with significantly decreased Bcl-X expression whereas Bcl-2 levels remained unchanged. It suggests that Bcl-X may function as a protective protein in this cell line.
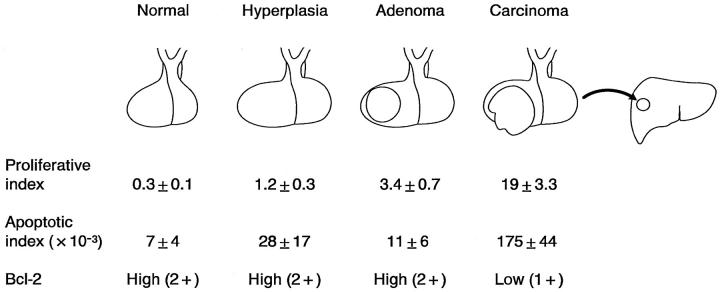
Relatively few TUNEL-positive cells were observed in our study and in other in vivo studies of normal pituitaries and in pituitary adenomas. 8,22 Hyperplastic pituitaries in pregnant women showed intermediate values of proliferation rates and cell death compared with pituitary adenomas (Figure 9) ▶ . There may be several reasons for these low apoptotic indices in pituitary and other tissues; low values of apoptotic indices in vivo may result from very rapid kinetics of induction of apoptosis and elimination of dead cells by phagocytosis. 30 Active phagocytosis of apoptotic cells in rat pituitary by folliculo-stellate cells has been reported. 4 In the rat liver, histologically detectable apoptosis lasts only ∼3 hours. 31 Thus, the rapid clearance of apoptotic cells in this study may result in low numbers of measured apoptotic indices in vivo, despite the dramatic changes in the size of the pituitary. 4,19
Figure 9.
Diagramatic summary of proliferative index, apoptotic index, and Bcl-2 expression in normal, hyperplastic, and neoplastic human pituitaries. Both proliferative and apoptotic indices are relatively low in normal, hyperplastic, and adenomatous pituitaries whereas Bcl-2 protein expression is high in these pituitaries. (The data for the proliferative indices for normal pituitary, pituitary adenoma, and carcinomas are from Ref. 14 .) The proliferative index in pituitaries from pregnant women (n = 5) was analyzed in the present study by MIB-1 immunostaining. Pituitary carcinomas, defined by metastatic disease, have much higher proliferative and apoptotic indices and decreased Bcl-2 protein expression indicating that with tumor progression there is increased proliferation as well as increased apoptosis.
Dissociated pituitary adenoma cells in primary culture showed increased numbers of apoptotic cells in vivo compared with surgically excised adenomas. The disruption of cell-cell and cell-matrix contact 32 and the absence of phagocytosis probably account for increased apoptotic indices in primary cultures.
Pituitary carcinomas, in contrast to adenomas, have been shown to have a higher proliferation rate and to overexpress p53 protein. 33-35 Carcinonomas also had an increased rate of apoptosis compared with adenomas in this study. The levels of Bcl-2 protein are significantly decreased in carcinomas compared with adenomas. Comparison of normal and hyperplastic pituitaries, pituitary adenomas, and carcinomas in vivo indicates that an increased proliferative index and lower Bcl-2 expression correlate with a higher rate of apoptosis in pituitary tissues (Figure 9) ▶ . In a recent review on the evaluation of apoptosis in various tumor tissues, 36 the authors reported a positive correlation between the grade of tumor, proliferation rates, and apoptotic indices in hormone-dependent epithelial tumors, which agrees with our findings.
Immortalized HP75 pituitary cell lines have a higher rate of apoptosis as well as proliferation than that observed in primary cultures of pituitary adenomas. However, positive correlation between the rate of proliferation and cell death was not observed by us in the mouse pituitary gonadotroph cell lines αT3-1 and LβT2 (unpublished data). These observations suggest that established cell lines cannot be directly compared with primary tumors, although they may serve as a very useful tool for studies of the molecular mechanisms of apoptosis.
TGF-β is known to stimulate apoptosis in various tissues, 37 and this growth factor also stimulated apoptosis in the HP75 cell line, which has been shown to have the three principal TGF-β receptors. 14 Recent studies indicate that TGF-β activates multiple signaling pathways, including the caspase family of protease leading to apoptosis in various cell types. 38 The observation that hypericin and the more specific PKC inhibitor chelerythrine chloride stimulated apoptosis in pituitary tumor cells in vitro agrees with a recent study 17,18 and suggests that these molecules may have potential utility in the therapy of pituitary adenomas.
In summary, apoptosis is relatively infrequent in normal and neoplastic human pituitaries, but the AI is higher in pituitary carcinomas than in adenomas. Apoptosis is stimulated by TGF-β1 and PKC inhibitors in the HP75 cell lines, implicating these molecules in the regulation of programmed cell death in the pituitary.
Acknowledgments
We thank the National Pituitary Hormone Distribution Agency for the antibodies for pituitary hormones and Shuya Zhang for technical assistance.
Footnotes
Address reprint requests to Dr. Ricardo V. Lloyd, Department of Laboratory Medicine and Pathology, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905. E-mail: lloyd.ricardo@mayo.edu.
Supported in part by NIH grant CA42951.
References
- 1.Schwartzman RA, Cidlowski JA: Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev 1993, 14:133-151 [DOI] [PubMed] [Google Scholar]
- 2.Vaux DL, Strasser A: The molecular biology of apoptosis. Proc Natl Acad Sci USA 1996, 93:2239-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson EB: Apoptosis and steroid hormones. Mol Endocrinol 1994, 8:665-673 [DOI] [PubMed] [Google Scholar]
- 4.Drewett N, Jacobi JM, Willgoss DA, Lloyd HM: Apoptosis in the anterior pituitary gland of the rat: studies with estrogen and bromocriptine. Neuroendocrinology 1993, 57:89-95 [DOI] [PubMed] [Google Scholar]
- 5.Yin D, Kondo S, Takeuchi J, Morimura T: Induction of apoptosis in murine ACTH-secreting pituitary adenoma cells by bromocriptine. FEBS Lett 1994, 339:73-75 [DOI] [PubMed] [Google Scholar]
- 6.Srikant CB: Cell cycle dependent induction of apoptosis by somatostatin analog SMS 201-995 in AtT-20 mouse pituitary cells. Biochem Biophys Res Commun 1995, 209:400-406 [DOI] [PubMed] [Google Scholar]
- 7.Kontogeorgos G, Sambaziotis D, Piaditis G, Karameris A: Apoptosis in human pituitary adenomas: a morphologic and in situ end-labeling study. Mod Pathol 1997, 10:921-926 [PubMed] [Google Scholar]
- 8.Green VL, White MC, Hipkin LJ, Jeffreys RV, Foy PM, Atkin SL: Apoptosis and p53 suppressor gene protein expression in human anterior pituitary adenomas. Eur J Endocrinol 1997, 136:382-387 [DOI] [PubMed] [Google Scholar]
- 9.Hamilton HB, Hinton DR, Law RE, Gopalakrishna R, Su YZ, Chen ZH, Weiss MH, Couldwell WT: Inhibition of cellular growth and induction of apoptosis in pituitary adenoma cell lines by the protein kinase C inhibitor hypericin: potential therapeutic application. J Neurosurg 1996, 85:329-334 [DOI] [PubMed] [Google Scholar]
- 10.Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994, 124:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang E, Korsmeyer SJ: Molecular thanatopsis: a discourse on the Bcl-2 family and cell death. Blood 1996, 88:386-401 [PubMed] [Google Scholar]
- 12.Wang D-G, Johnston CF, Atkinson AB, Heaney AP, Mirakhur M, Buchanan KD: Expression of bcl-2 oncoprotein in pituitary tumors: comparison with c-myc. J Clin Pathol 1996, 49:795-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd RV, Jin L, Qian X, Scheithauer BW, Young WF, Jr, Davis DH: Analysis of the chromogranin A post-translational cleavage product pancreastatin and the prohormone convertases PC2 and PC3 in normal and neoplastic human pituitaries. Am J Pathol 1995, 146:1188-1198 [PMC free article] [PubMed] [Google Scholar]
- 14.Jin L, Qian X, Kulig E, Sanno N, Scheithauer BW, Kovacs K, Young WF, Jr, Lloyd RV: Transforming growth factor-β, transforming growth factor-β receptor II, and p27KIP1 expression in nontumorous and neoplastic human pituitaries. Am J Pathol 1997, 151:509-519 [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L, Kulig E, Qian X, Scheithauer BW, Eberhardt NL, Lloyd RV: A human pituitary adenoma cell line proliferates and maintains some differentiated functions following expression of SV40 Large T antigen. Endocr Pathol 1998, 9:169-184 [Google Scholar]
- 16.Couldwell WT, Law RE, Hinton DR, Gopalakrishna R, Yong VW, Weiss MH: Protein kinase C and growth regulation of pituitary adenomas. Acta Neurochir 1996, 65:22-26 [DOI] [PubMed] [Google Scholar]
- 17.Takahashi I, Nakanishi S, Kobayashi E, Nakano H, Suzuki K, Tamaoki T: Hypericin and pseudohypericin specifically inhibit protein kinase C: possible relation to their antiretroviral activity. Biochem Biophys Res Commun 1989, 165:1207-1212 [DOI] [PubMed] [Google Scholar]
- 18.Jarvis WD, Turner AJ, Povirk LF, Traylor RS, Grant S: Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Res 1994, 54:1707-1714 [PubMed] [Google Scholar]
- 19.Stefaneanu L, Kovacs K, Lloyd RV, Scheithauer BW, Young WF, Sano T, Jin L: Pituitary lactotrophs and somatotrophs in pregnancy: a correlative in situ hybridization and immunocytochemical study. Virchows Arch Pathol 1992, 62:291-296 [DOI] [PubMed] [Google Scholar]
- 20.Haggi ES, Torres AI, Maldonado CA, Aoki A: Regression of redundant lactotrophs in rat pituitary gland after cessation of lactation. Endocrinology 1986, 111:367-373 [DOI] [PubMed] [Google Scholar]
- 21.Ahlbom E, Grandison L, Zhivotovsky B, Ceccatelli S: Termination of lactation induces apoptosis and alters the expression of the Bcl-2 family members in the rat anterior pituitary. Endocrinology 1998, 139:2465-2471 [DOI] [PubMed] [Google Scholar]
- 22.Saitoh Y, Arita N, Ohnishi T, Ekramullah S, Takemura K, Hayakawa T: Absence of apoptosis in somatotropinomas treated with octreotide. Acta Neurochir 1997, 139:851-856 [DOI] [PubMed] [Google Scholar]
- 23.Kerr JF, Wyllie AH, Currie AR: Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972, 26:239-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagliacci MC, Tognellini R, Grignani F, Nicoletti L: Inhibition of human breast cancer cell (MCF-7) growth in vitro by the somatostatin analog SMS 201-995: effects on cell cycle parameters and apoptotic cell death. Endocrinology 1991, 129:2555-2562 [DOI] [PubMed] [Google Scholar]
- 25.Krajewski S, Krajewska M, Shabaik A, Wang HG, Irie S, Fong L, Reed JC: Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res 1994, 54:5501-5507 [PubMed] [Google Scholar]
- 26.Rinkenberg JL, Korsmeyer SJ: Errors of homeostasis and deregulation of apoptosis. Curr Opin Genet Dev 1977, 7:589-596 [DOI] [PubMed] [Google Scholar]
- 27.Yin C, Knudson M, Korsmeyer SJ, Van Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 1997, 385:637-640 [DOI] [PubMed] [Google Scholar]
- 28.Krajewska M, Moss SF, Krajewski S, Song K, Hold PR, Reed JC: Elevated levels of Bcl-X and reduced Bax in primary colorectal adenocarcinomas. Cancer Res 1996, 56:2422-2427 [PubMed] [Google Scholar]
- 29.Branet F, Brousset P, Krajewski S, Schlaifer D, Selves J, Reed JC, Caron P: Expression of the cell death-inducing gene bax in carcinomas developed from the follicular cells of the thyroid glands. J Clin Endocrinol Metab 1996, 81:2726-2730 [DOI] [PubMed] [Google Scholar]
- 30.Potten CS: What is an apoptotic index measuring? A commentary. Br J Cancer 1996, 74:1743-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bursch W, Paffe S, Putz B, Barthel G, Schulte-Herman NR: Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci in rats. Carcinogenesis 1990, 11:847-853 [DOI] [PubMed] [Google Scholar]
- 32.Olie RA, Boersma AW, Dekker MC, Nooter K, Looijenga LHJ, Oosterhuis JW: Apoptosis of human seminoma cells upon disruption of their microenvironment. Br J Cancer 1996, 73:1031-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER, Jr: p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery 1996, 38:763-770 [PubMed] [Google Scholar]
- 34.Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PI, Murray D, Laws ER, Jr: Proliferative activity and invasiveness among pituitary adenomas and carcinoma: an analysis using the MIB-1 antibody. Neurosurgery 1996, 38:99-107 [DOI] [PubMed] [Google Scholar]
- 35.Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young WF, Jr, Lloyd RV, Davis DH, Guthrie BL, Schoene WC: Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer 1997, 79:804-812 [DOI] [PubMed] [Google Scholar]
- 36.Soini Y, Paakko P, Lehto V-P: Histopathological evaluation of apoptosis in cancer. Am J Pathol 1998, 153:1041-1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alevizopoulos A, Mermod N: Transforming growth factor beta: the breaking open of a black box. Bioessays 1997, 19:581-591 [DOI] [PubMed] [Google Scholar]
- 38.Chen RH, Chang TY: Involvement of caspase family proteases in transforming growth factor-beta-induced apoptosis. Cell Growth Differ 1997, 8:821-827 [PubMed] [Google Scholar]