Abstract
The stratum corneum of the skin serves as an effective barrier for maintenance of the internal milieu against the external environment. At the cell periphery of the stratum corneum is the cell envelope, a highly insoluble membranous structure composed of precursor proteins cross-linked by ɛ-(γ-glutamyl)lysine bonds. Transglutaminase 1 (TGase 1; keratinocyte TGase), a membrane-bound isozyme of the TGase family, has been proposed to catalyze this process of assembly. Deficient cross-linking of the cell envelope in some patients with the autosomal recessive skin disorder lamellar ichthyosis (LI) and several mutations of the TGase 1 gene that have been identified in families with LI suggest the importance of this gene in production of the cell envelope. In this study, we generated mice lacking the TGase 1 gene, and we report that they have erythrodermic skin with abnormal keratinization. In their stratum corneum, degradation of nuclei and keratohyalin F-granules was incomplete and cell envelope assembly was defective. The skin barrier function of TGase 1-null mice was markedly impaired, and these mice died within 4–5 h after birth. These results clearly demonstrate that the TGase 1 gene is essential to the development and maturation of the stratum corneum and to adaptation to the environment after birth. Thus, these TGase 1 knockout mice may be a useful model for severe cases of LI.
Keywords: gene targeting, cell envelope, keratinization, skin barrier function
The outermost surface of the body is covered by the stratum corneum, which is an effective barrier essential to protect the internal milieu of the organism from the external environment (1). This structure is made of layered cornified cells, produced by terminal differentiation of keratinocytes in the stratified squamous epithelium (2). This process of keratinization proceeds according to a complex and elaborately controlled differentiation program with the coordinated expression of genes encoding specialized components, enzymes, and regulatory molecules (3).
The dead cells of the stratum corneum contain a keratin filament–matrix composite in the cytoplasm (4). The periphery of the cells is lined with a 15-nm-thick membranous structure, termed either the marginal band ultrastructurally (5, 6) or the cornified cell envelope (7, 8). The cell envelope is a highly insoluble structure, which is composed of ɛ-(γ-glutamyl)lysine cross-linked proteins, including involucrin, cystatin-α/keratolinin, loricrin, elafin/SKALP, cornifins/small proline-rich proteins, keratin intermediate filaments, filaggrin, annexin I, and various other proteins (9, 10).
Transglutaminases (EC 2.3.2.13) are Ca2+-dependent enzymes that catalyze ɛ-(γ-glutamyl)lysine cross-linking reactions in a wide variety of biological processes to produce stable structures composed of polymerized proteins and thereby maintain cell and tissue integrity (11). Transglutaminase 1 (TGase 1; keratinocyte TGase) has been identified as a membrane-bound TGase isozyme (12), the activation of which is coordinated with formation of cross-linked cell envelope in cultured epidermal keratinocytes (13, 14). TGase 1 is expressed in the stratified squamous epithelium, predominantly in the upper spinous and granular layers beneath the stratum corneum (15, 16).
The human TGase 1 gene (TGM1), which encodes a 92-kDa protein consisting of 816 amino acid residues, is located on chromosome 14q11.2 (17, 18). Several mutations of TGM1 have been found in some families with an autosomal recessive skin disorder termed lamellar ichthyosis (LI) (19–21), a clinically heterogeneous disease (22), and linkages to chromosome 2q33–q35 (23) and another unknown locus have been reported (21, 24). LI is frequently evident at birth as a “collodion baby” because of a collodion-like translucent membrane encasing the body, sometimes accompanied by erythroderma (1, 22). After the membrane is shed, the skin develops severe ichthyosis with large, thick, dark-brown hyperkeratotic scales. Reports of deficient cell envelopes in some cases of LI (25, 26) suggest that TGase 1 is important in the formation of the cell envelope.
In this study, to elucidate the function of TGase 1 in epidermal development and keratinization, to characterize its contribution to skin barrier function in vivo, and to produce a murine model for LI, we generated mice lacking the TGase 1 gene by homologous recombination in embryonic stem (ES) cells.
MATERIALS AND METHODS
Materials.
pPNT (27) and ES cell line R1 were kindly provided by R. Mulligan (Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology, Cambridge, MA) and by A. Nagy (Mount Sinai Hospital, Toronto), respectively.
Construction of the Targeting Vector.
A mouse TGase 1 genomic DNA was isolated from a 129/SVJ genomic library (Stratagene). The 5′ flanking region and a 3′ portion of exon 3, which correspond to 5.5-kb XbaI–BamHI and 2.5-kb XbaI–XhoI fragments from the TGase 1 genomic DNA, were subcloned in pBluescript II KS(+) (Stratagene), then inserted into the XbaI–KpnI and XhoI–NotI sites of the targeting vector pPNT, respectively, to produce pPNTmtg1 for gene targeting of TGase 1.
Selection of Targeted ES Cell Clones.
Ten million ES cells (line R1) were transfected with 25 μg of a NotI-linearized pPNTmtg1 by electroporation. After positive selection with 150 μg/ml G418 and negative selection with 0.51 μg/ml ganciclovir, homologous recombination of ES cell colonies was analyzed by PCR using the primers P1 (5′-GCGCATGCTCCAGACTGCCTTGGGAAAAGC-3′) and P2 (5′-GCCCATTCCCTGTACTACCTGTGGTGGTCA-3′), and by Southern hybridization of BamHI- and HindIII-digested DNA.
Generation of TGase 1−/− Mice and Genotyping.
Two clones, in which one allele of the TGase 1 gene was disrupted, were expanded and injected into C57BL/6 blastocysts. Male chimeras derived from the clones were bred with C57BL/6 female germ-line mice carrying the targeted allele, and F2 offspring generated by their interbreeding were identified by Southern hybridization and PCR analysis of tail DNA by using three primers, P3 (5′-GGTTGNGGGGGCGGGCGGGTGACTCTTCTA-3′), P4 (5′-GCGNAGGTTAGGTGTGTCCGTTGTTCTTAG-3′), and P5 (5′-CCAAAGGCCTACCCGCTTCCATTGCTCAGC-3′), (N = A, G, C, or T). These primers were designed to distinguish the 0.7-kb targeted allele band (P3, P5) from the 0.5-kb wild-type (P4, P5) band (Fig. 1A).
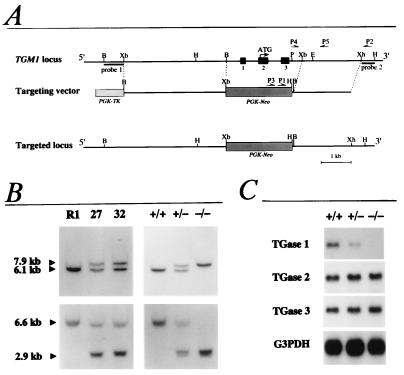
Figure 1.
Targeted disruption of the mouse TGase 1 gene. (A) Strategy of TGase 1 gene targeting. PGK, the phosphoglycerate kinase I promoter; Neo, the neomycin phosphotransferase gene; TK, the thymidine kinase gene; B, BamHI; Xb, XbaI; H, HindIII; P, PstI; Xh, XhoI; E, EcoRI. (B) Southern blot analysis of genomic DNAs from ES cells (Left) and mouse tails (Right). DNA was digested with BamHI (Upper) or HindIII (Lower) and hybridized with probe 1 or probe 2, respectively. R1, parental ES cells; 27 and 32, mutant ES cell clones; +/+, wild type; +/−, heterozygous mutant; −/−, homozygous mutant. (C) Northern blot analysis of RNAs from 18.5 days post coitum (dpc) fetal skin hybridized with TGase 1, TGase 2, TGase 3, or glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNA. +/+, wild type; +/−, heterozygous mutant; −/−, homozygous mutant.
Northern Blot Hybridization.
For Northern blot hybridization, 5 μg of poly(A)+ RNA partially purified from fetal skin (18.5 dpc) by Oligotex dT30 (Takara Shuzo, Kyoto) were used. A 1.1-kb cDNA for mouse TGase 1, extending from exon 2 to exon 8, was amplified by reverse transcriptase (RT)-PCR using the primers U205 (5′-CCTTCTGGGCTCGCTGTGG-3′) and L1353 (5′-CAGAATCATGGTTCAGGTGCTC-3′), which were designed on the basis of the homology between human and rat TGase 1 cDNA sequences (18, 28, 29). The TGase 1 cDNA and RT-PCR products of TGase 2, TGase 3, and G3PDH, the sequences of which were compatible with each cDNA, were subcloned and used as probes for hybridization. Primers used were as follows: TGase 2, U1861 (5′-TTTCCGATCCCCTGTATGACTGC-3′) and L2322 (5′-TGCTGGTGATGGCTCTCCTCTTA-3′), TGase 3, U1509 (5′-GAAGTTCAAGGTCACGGGGATAC-3′), and L2063 (5′-GCAGGAAAAGTCAGCGAGCAGTT-3′), and G3PDH, primer R (5′-TCCACCACCCTGTTGCTGTA-3′), and primer F (5′-ACCACAGTCCATGCCATCAC-3′).
Histological Analysis.
Tissues processed into 4-μm paraffin sections were subjected to staining with hematoxylin and eosin and to in situ hybridization. The procedures for electron microscopy and immunoelectron microscopy were as described previously (30). Ultrathin sections were incubated with rabbit anti-mouse loricrin antibody (Babco, Richmond, CA) and then with AuroProbe EM GAR G10 (10 nm in size; Amersham). To test the resistance of the cell envelope against protease digestion, ultrathin sections were incubated prior to immunolabeling either with 0.25% trypsin in phosphate-buffered saline (pH 7.4) at 37°C for 1 h or with 0.4 mg/ml proteinase K in 50 mM Tris⋅HCl (pH 7.4) at room temperature for 5 min.
In Situ Hybridization.
In situ hybridization was essentially as described previously (31). Digoxigenin-labeled antisense complementary RNA (cRNA) probes for in situ hybridization were prepared by using the mouse TGase 1 cDNA used for Northern hybridization and keratin 14 cDNA (32).
Measurement of Transepidermal Water Loss (TEWL).
TEWL from the dorsal skins of neonatal mice was examined under normal conditions, by using a Courage and Khazaka Tewameter TM210, as described previously (33). The instrument is an open chamber system with two humidity and temperature sensors which measure the water evaporation gradient at the surface of the skin. Parameters were determined by using the software of the apparatus according to the manufacturer’s instructions.
In Vitro Diffusion Experiment.
Mouse dorsal skins were excised, mounted in side-by-side diffusion chambers (effective surface area: 0.126 cm2), and allowed to stabilize at 37°C with Ringer’s solution (pH 7.4). Donor and receiver fluid volumes were 1.5 ml. After 1 h of preincubation, [3H]mannitol (NEN) was added to the epidermal donor fluid to yield a final concentration of 10 μCi/ml (1 μCi = 37 kBq). Five hundred μl of receiver fluid was taken at the times noted in the legend of Fig. 5 for measurement of radioactivity and was immediately replaced with an equal volume of fresh Ringer’s solution.
Figure 5.
Defects in the skin barrier function of TGase 1−/− mice. (A) TEWL was measured in wild-type (+/+), TGase 1+/− (+/−), and TGase 1−/− (−/−) neonatal mice. Error bars represent SEM of ten different measurements. (B) In vitro diffusion of mannitol across neonatal mouse skins; transport of [3H]mannitol across skins excised from control (○) and TGase 1−/− (•) neonatal mice was assayed. The steady-state flux normalized to the initial donor concentration was 1.09 ± 0.09 × 10−1 cm/h in TGase 1−/− skin, which was more than 1,000 times higher than in normal skin (0.818 ± 0.233 × 10−5 cm/h). Each data point is expressed as the mean ± SEM of three observations. (C and D) In vivo diffusion of Lucifer yellow in neonatal mice. The fluorescent micrographs show the distribution of Lucifer yellow in the skin of control (C) and TGase 1−/− (D) mice. Sections were counterstained with propidium iodide. Arrowheads, basement membrane. (Bar, 30 μm.)
In Vivo Transdermal Absorption of Fluorescent Dye.
Neonatal mice were restrained in Petri dishes with their backs in contact with 1 mM Lucifer yellow in Ringer’s solution (pH 7.4) at 37°C. After 1 h of incubation, mice were sacrificed, then frozen and dorsoventrally sliced at a thickness of 5 μm. The sections were counterstained with 5 μg/ml propidium iodide and then analyzed by fluorescence microscopy.
RESULTS
Targeted disruption of the TGase 1 gene was achieved by replacing exons 1–3 with a Neo cassette by using homologous recombination in R1 ES cells (Fig. 1A). These exons encode the translation initiation codon and an N-terminal stretch unique to TGase 1 that is required for membrane anchoring (34). Screening of 144 ES cell clones by PCR and Southern blot analysis revealed 20 mutant ES clones carrying the targeted allele. Of these, 2 clones (nos. 27 and 32) were used to generate chimeric animals (Fig. 1B), and a TGase 1+/− strain heterozygous for the mutation was established from chimeric males generated from ES clone 27. TGase 1+/− mice were intercrossed to generate homozygous TGase 1−/− progeny, the targeted locus of which was confirmed by Southern hybridization (Fig. 1B). Northern blot analysis revealed that the level of TGase 1 mRNA in the TGase 1+/− skin was about half that present in the wild-type skin, and no TGase 1 mRNA was detectable in the TGase 1−/− skin (Fig. 1C). The mRNA level of TGase 2 and TGase 3, the other TGases expressed in epidermal keratinocytes (35, 36), was not different between wild-type, TGase 1+/−, and TGase 1−/− skin (Fig. 1C).
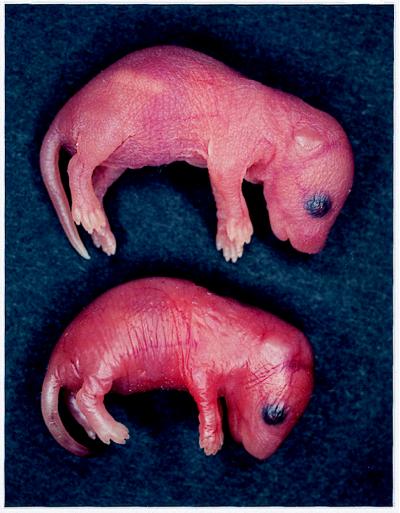
TGase 1+/− mice were indistinguishable phenotypically from wild-type mice, but TGase 1−/− progeny were born with marked abnormal appearance (Fig. 2); TGase 1−/− neonates were smaller, had a lower body weight, and were less active than control littermates. At birth, TGase 1−/− skin was erythematous and shiny, characteristic of a taut surface with coarse wrinkles. Resembling the appearance of a collodion baby (37), TGase 1−/− animals were often covered with an inelastic, translucent membrane. The neonates did not feed, and became progressively dehydrated in the tail and extremities, eventually becoming waxy or shriveled; all TGase 1−/− progeny died within 4–5 h after birth. Genotype analysis of more than 100 F2 offspring from matings of F1 mice showed the normal Mendelian frequency, indicating no embryonic lethality of the TGase 1−/− mice.
Figure 2.
Gross morphology of TGase 1−/− neonates. (Upper) Control (wild-type) newborn mouse. (Lower) Newborn TGase 1−/− mouse. TGase 1−/− neonates were smaller, and their skin was erythematous and taut, with coarse wrinkles.
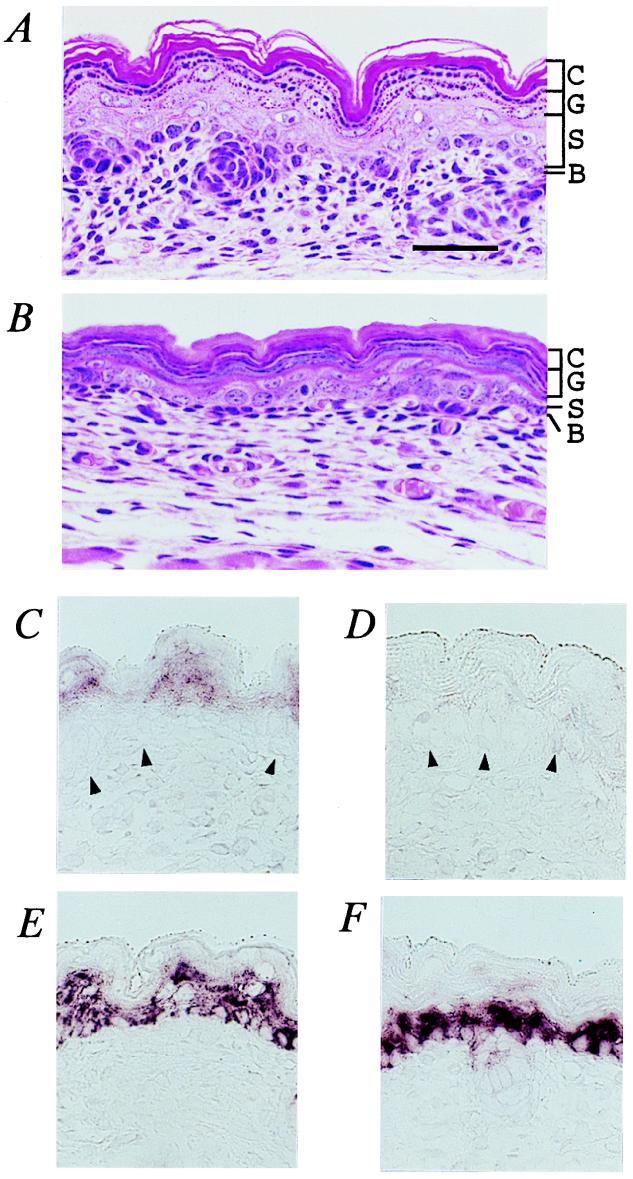
In contrast with the ridged and laminated appearance of normal stratum corneum (Fig. 3A), the epidermis of TGase 1−/− progeny was extended and characterized by a thickened stratum corneum in which flattened keratohyalin granules were prominent (Fig. 3B). Hence, the boundary between the stratum corneum and stratum granulosum was often unclear in TGase 1−/− mice. During embryonic development of mice, maturation of the keratinized stratified epidermis begins at 15 dpc (38, 39). It was not until 16.5 or 17.5 dpc that the unique appearance and these histological features of the TGase 1−/− pups became evident. Hence, late developmental maturation of the epidermis seemed to be compromised in TGase 1−/− mice. In situ hybridization revealed that TGase1 mRNA was localized in the upper spinous and granular layers of the normal epidermis (Fig. 3C), but it was undetectable in TGase1−/− epidermis (Fig. 3D). Keratin 14 mRNA, used as a control, was evident by in situ hybridization in a few epidermal layers, including the basal layer, of TGase 1−/− pups as well as controls (Fig. 3 E and F).
Figure 3.
Histological analysis of TGase 1−/− skin. (A and B) Neonatal dorsal skin stained with hematoxylin and eosin: control epidermis (A) and TGase 1−/− epidermis (B). C, stratum corneum; G, stratum granulosum; S, stratum spinosum; B, stratum basale. (C–F) In situ hybridization of TGase 1 (C and D) and keratin 14 (E and F) in 18.5 dpc fetal mouse skin: wild-type skin (C and E) and TGase 1−/− skin (D and F). Arrowheads, basement membrane. (Bar, 30 μm in A–F.)
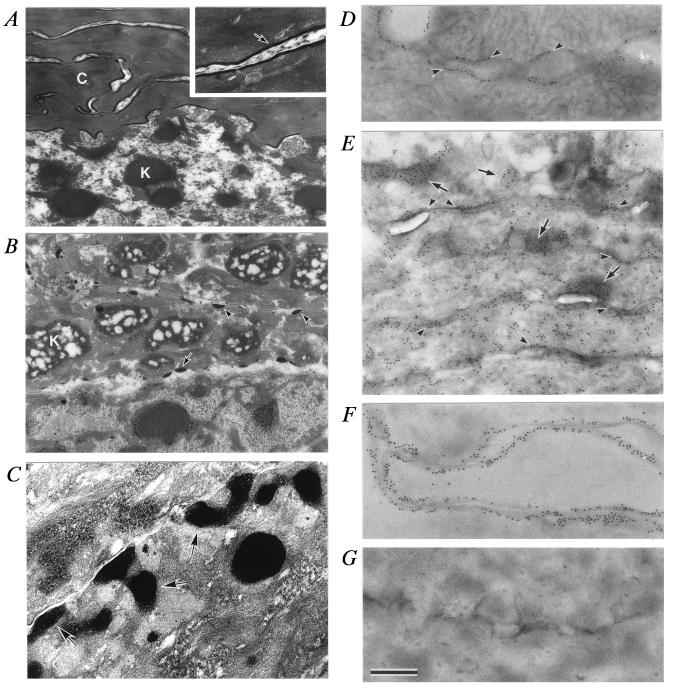
The cornified cells of the stratum corneum in the wild-type and TGase 1+/− epidermis consisted of a compact, electron-dense cytoplasm with condensed tonofibrils and a cell envelope (marginal band) at the periphery (Fig. 4A). Nuclei and cytoplasmic organelles, including keratohyalin granules, were absent there. In the TGase 1−/− progeny, the stratum corneum was entirely swollen and the lower cornified cells were filled with remnants of keratohyalin F-granules (40) surrounded by loosely packed tonofibril bundles (Fig. 4B). After birth of TGase 1−/− pups, the stratum corneum became more compact, possibly by drying, but nuclei and keratohyalin F-granules were still present in the lower stratum corneum. In TGase 1−/− mice, the cell envelope was virtually lost, and instead, electron-dense aggregates of various sizes were adherent or close to the plasma membrane (Fig. 4 B and C). Thus, the TGase 1−/− mutation causes not only causes defects in cell envelope formation but also damages the process of degradation of nuclei and keratohyalin granules.
Figure 4.
Ultrastructure of TGase 1−/− epidermis. Sections of normal (A, D, and F) and TGase1−/− (B, C, E, and G) mouse epidermis (18.5 dpc). C in A, cornified cell; arrow in the Inset of A, cell envelope; K in A, keratohyalin F-granule in the stratum granulosum; K in B, a swollen keratohyalin F-granule; arrows in B and C), abnormal electron-dense aggregates adherent or close to the plasma membrane. (D–G) Section of stratum corneum labeled with anti-loricrin antibody and 10 nm gold. Arrowheads in D, cell envelope; arrows in E, abnormal electron-dense aggregates; arrowheads in E, cell membrane. (F and G) Sections digested with proteases. (×12,200 in A and B; ×28,400 in the Inset of A and in C; ×19,500 in D–G. Bar, 0.8 μm in A and B; 0.34 μm in the Inset of A and in C; 0.5 μm in D–G.)
Loricrin is a major protein component of the cell envelope that is polymerized by ɛ-(γ-glutamyl)lysine cross-linking by epidermal TGases (41, 42). The localization of loricrin in the epidermis was examined by using immunoelectron microscopy. Loricrin labeling was detectable in the cytoplasm, in keratohyalin L-granules (40), and in the nuclei of the stratum granulosum of skins from TGase 1−/− and control mice. In the stratum corneum, loricrin labeling was present along the cell envelope in control epidermis (Fig. 4D). However, in the TGase 1−/− epidermis, it was distributed diffusely in the cytoplasm and along the plasma membrane, and accumulated within novel electron-dense aggregates, indicating that these aggregates correspond to abnormal keratohyalin L-granules (Fig. 4E). After protease digestion of ultrathin sections prior to immunolabeling, loricrin labeling was still retained along the cell envelope in control epidermis (Fig. 4F), but it was completely lost in TGase 1−/− epidermis (Fig. 4G). Therefore, both translocation of loricrin from L-granules to the plasma membrane and polymerization of the precursor proteins may be damaged in TGase 1−/− mice.
As has been described for LI neonates (43), the severe dehydration of TGase 1−/− pups might be due to impairment of the skin’s barrier function. To examine this, the transepidermal water loss (TEWL) of the TGase 1−/− skin was assessed with an evaporimeter. As shown in Fig. 5A, TEWL was very low in wild-type and TGase 1+/− mice, but was increased more than 100-fold in TGase 1−/− mice. As an alternative approach to evaluate skin barrier function, percutaneous absorption was examined by using [3H]mannitol transport across full-thickness, excised skin (Fig. 5B). The steady-state flux in the TGase 1−/− skin was more than 1,000 times higher than in normal skin. Further, when diffusion of the fluorescent dye Lucifer yellow in the skin was examined, the dye was found to be retained in the upper layers of the stratum corneum in the control neonatal mice (Fig. 5C). In contrast, in TGase 1−/− pups, the dye was not only distributed throughout the stratum corneum but was even detectable in the dermis (Fig. 5D).
DISCUSSION
This paper reports the successful targeted disruption of the TGase 1 gene, demonstrating the definitive role of the gene in the construction of the stratum corneum. In contrast to TGase 2, which is expressed in many tissues and is functional in a variety of biological events (44), such as apoptosis, signal transduction, cytokine modulation, and neurodegeneration, the gene expression of TGase 1 and its regulation are confined to the late stage of keratinization in the stratified squamous epithelium (29). Indeed, the major apparent phenotype of TGase 1−/− mice was the abnormality in the epidermis. The null mutation of the TGase 1 gene in mice was confirmed by a lack of expression of TGase 1 mRNA in the skin of TGase 1−/− mice by using Northern blotting and in situ hybridization. Kim et al. (36) have suggested that TGase 1 and TGase 3, excluding TGase 2, which is expressed only in the basal keratinocyte fraction, are involved in epidermal keratinization. Because the TGase 2 and TGase 3 mRNA in TGase 1−/− mice was at levels similar to those in control mice, it is unlikely that TGase 2 or 3 is compensating for the phenotypes of the TGase 1−/− skin. Therefore, the phenotype of TGase 1−/− skin seems to reflect well the function of TGase 1 in vivo.
As TGase 1−/− mice were smaller than control mice, we examined the skeleton of neonatal TGase 1−/− mice by using Alcian blue and alizarin red S staining (45). No anomaly of cartilage or bones in the skeleton was observed, except that skeletal growth was slightly retarded in these mice. Fetal growth retardation is uncommon in LI patients and the reason for this phenotype in TGase 1−/− mice is unknown; it may be secondary to abnormal skin development in these mice.
Neonatal TGase 1−/− mice were frequently encased with a transparent membrane, just like collodion babies with LI. Although the nature of the collodion-like membrane in LI has not been fully delineated, the membrane is composed of thickened stratum corneum in some cases (37, 46). Ultrastructurally, a sheet of cells with characteristics of periderm was seen on the surface of the neonatal stratum corneum in TGase 1−/− mice (data not shown). The periderm, which should normally detach from the epidermis before birth in mice (39), may be a component of the translucent collodion-like membrane in TGase 1−/− mice, possibly because the underlying abnormal stratum corneum affects the detachment of periderm in TGase 1−/− mice.
Severe ichthyosis develops with hyperkeratotic dark scales in LI, after neonatal periods (22). The erythrodermic skin of neonatal TGase 1−/− mice became dried and more inelastic with time, and these mice died within several hours of birth. We could not follow further changes in the skin because of that early neonatal lethality. However, grafts of 18.5 dpc TGase 1−/− skin to nude mice developed thickened scales within 2 weeks after transplantation, and the epidermis became hyperplastic with hyperkeratosis (data not shown), similar to histological findings of LI (22). Analysis of those skin grafts to nude mice is now under way to help further elucidate abnormalities in TGase 1−/− skin, including skin appendages, after the neonatal period.
In the stratum corneum of TGase 1−/− mice, the cell envelope was defective, loricrin accumulated in the novel electron-dense aggregates (abnormal L-granules) which were seen along or near the plasma membrane, and moreover, loricrin immunoreactivity in the stratum corneum was lost with protease digestion. These findings show that TGase 1 is of primary importance in the formation of the cell envelope, especially in the distribution of cell envelope precursors extensively onto the plasma membrane and in firmly cross-linking them together in the cell periphery. Interestingly, Candi et al. (47) have shown that loricrin is cross-linked by TGase 1 and TGase 3, and they suggest that TGase 3 primarily catalyzes intrachain cross-linking and small oligomer formation of the precursor, whereas TGase 1 forms very large oligomeric complexes by interchain cross-links. Because TGase 3 seems unable to compensate for the cell envelope defect elicited in TGase 1 knockout mice, it is likely that the functions of TGase 1 and TGase 3 in cell envelope formation are different from each other. On the basis of that study (47) and our results, we suggest that TGase 3 oligomerizes loricrin in the cytoplasm and TGase 1 then polymerizes these small oligomers together by interchain cross-linking at the cell periphery, which stabilizes them firmly into the cell envelope.
In TGase 1−/− mice, keratohyalin F-granules remained in the lower stratum corneum, indicating that TGase 1 is involved in the degradation of those granules. Filaggrin, a major component of F-granules, not only is associated with the densely packed keratin intermediate filament bundles in the cytoplasm but also is cross-linked with cell envelope precursors (9). F-granules that remain in the lower stratum corneum of TGase 1−/− mice may be due, at least in part, to defective incorporation of filaggrin into the cell envelope, although it is unclear how TGase 1 and cross-linking reactions contribute to the condensation of tonofibrils with filaggrin in the cytoplasm of the stratum corneum. It is also noteworthy that the disappearance of nuclei as well as of the granules was also impaired in the stratum corneum of TGase 1−/− mice. Polakowska et al. (48) have proposed that keratinization is a specialized form of apoptosis. DNA fragmentation in some granular cells is detectable by using in situ labeling (49), and it appears to colocalize with epidermal TGase (48), but the mechanism of the apoptotic process at the final stage of keratinization has not been well defined. From the results of these TGase 1 knockout mice, we suggest that TGase 1 also plays a critical role in the programmed cell death during keratinization.
The drastic increase in the skin permeability of TGase 1−/− mice with immature stratum corneum agrees with the earlier suggestion that the barrier is located in the stratum corneum (50). The stratum corneum of TGase 1−/− mice at 18.5 dpc was swollen, and after birth, it became compact. This is possibly due to invasion of amniotic fluid during the embryonic stage and to dryness after birth because the stratum corneum of TGase 1−/− mice is impaired for barrier function. Considering the nature of the cell envelope and its strong resistance to treatment with such chemicals and denaturants as diluted alkali or concentrated urea (7, 8), a marked increase in TEWL and in permeance of mannitol or Lucifer yellow in TGase 1−/− skin might be due to deficiency of the cell envelope. Thus, the TGase 1 knockout mice represent a model with defects in the cell envelope, which has not been previously available and which may be useful for evaluating effects of cosmetics and drugs on improvement of skin barrier function.
In conclusion, this knockout study has clearly demonstrated that the TGase 1 gene is essential for the formation of the cell envelope and for maturation of the stratum corneum. Early neonatal death in TGase 1−/− mice, which is accompanied by impairment of skin barrier function, shows that the TGase 1 gene is important for the survival of animals and their adaptation to the environment after birth. The phenotype of TGase 1−/− mice suggests that null mutations in the human TGase 1 gene might cause severe cases of LI associated with neonatal death. Thus, these TGase 1 knockout mice, with their characteristic epidermal defects, may prove to be a useful model for the investigation of therapy, including gene therapy, for keratinization disorders such as LI.
Acknowledgments
We thank Prof. Yasuo Kitajima (Department of Dermatology, Gifu University School of Medicine, Gifu, Japan) for helpful discussion. This work was supported in part by a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan, by a grant for basic dermatological research from Shiseido Co., Ltd. and by a grant from the Cosmetology Research Foundation, Tokyo.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TGase, transglutaminase; LI, lamellar ichthyosis; ES cells, embryonic stem cells; dpc, days post coitum; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; TEWL, transepidermal water loss.
References
- 1.Roop D R. Science. 1995;267:474–475. doi: 10.1126/science.7529942. [DOI] [PubMed] [Google Scholar]
- 2.Blumenberg M, Tomic-Canic M. EXS. 1997;78:1–29. doi: 10.1007/978-3-0348-9223-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Eckert R L, Welter J F. Mol Biol Rep. 1996;23:59–70. doi: 10.1007/BF00357073. [DOI] [PubMed] [Google Scholar]
- 4.Dale B A, Ling S-Y. J Invest Dermatol. 1979;72:257–261. doi: 10.1111/1523-1747.ep12531715. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto K. Arch Klin Exp Derm. 1969;235:374–385. [Google Scholar]
- 6.Brody I. Acta Derm Venereol. 1969;49:128–138. [PubMed] [Google Scholar]
- 7.Matoltsy A G, Balsamo C A. J Biophys Biochem Cytol. 1955;1:339–360. doi: 10.1083/jcb.1.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matoltsy A G, Matoltsy M N. J Invest Dermatol. 1966;46:127–129. [PubMed] [Google Scholar]
- 9.Steinert P M, Marekov L N. J Biol Chem. 1995;270:17702–17711. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- 10.Robinson N A, Lapic S, Welter J F, Eckert R L. J Biol Chem. 1997;272:12035–12046. doi: 10.1074/jbc.272.18.12035. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg C S, Birckbichler P J, Rice R H. FASEB J. 1991;5:3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 12.Thacher S M, Rice R H. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- 13.Rubin A L, Rice R H. Cancer Res. 1986;46:2356–2361. [PubMed] [Google Scholar]
- 14.Lichti U, Yuspa S H. Cancer Res. 1988;48:74–81. [PubMed] [Google Scholar]
- 15.Parenteau N L, Pilato A, Rice R H. Differentiation. 1986;33:130–141. doi: 10.1111/j.1432-0436.1986.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim S-Y, Chung S-I, Yoneda K, Steinert P M. J Invest Dermatol. 1995;104:211–217. doi: 10.1111/1523-1747.ep12612769. [DOI] [PubMed] [Google Scholar]
- 17.Kim I-G, McBride O W, Wang M, Kim S-Y, Idler W W, Steinert P M. J Biol Chem. 1992;267:7710–7717. [PubMed] [Google Scholar]
- 18.Yamanishi K, Inazawa J, Liew F-M, Nonomura K, Ariyama T, Yasuno H, Abe T, Doi H, Hirano J, Fukushima S. J Biol Chem. 1992;267:17858–17863. [PubMed] [Google Scholar]
- 19.Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen S P M, Ponec M, Bon A, Lautenschlager S, Schorderet D F, Hohl D. Science. 1995;267:525–528. doi: 10.1126/science.7824952. [DOI] [PubMed] [Google Scholar]
- 20.Russell L J, DiGiovanna J J, Rogers G R, Steinert P M, Hashem N, Compton J G, Bale S J. Nat Genet. 1995;9:279–283. doi: 10.1038/ng0395-279. [DOI] [PubMed] [Google Scholar]
- 21.Parmentier L, Blanchet-Bardon C, Nguyen S, Prud’homme J-F, Dubertret L, Weissenbach J. Hum Mol Genet. 1995;4:1391–1395. doi: 10.1093/hmg/4.8.1391. [DOI] [PubMed] [Google Scholar]
- 22.Williams M L, Elias P M. Arch Dermatol. 1985;121:477–488. doi: 10.1001/archderm.121.4.477. [DOI] [PubMed] [Google Scholar]
- 23.Parmentier L, Lakhdar H, Blanchet-Bardon C, Marchand S, Dubertret L, Weissenbach J. Hum Mol Genet. 1996;5:555–559. doi: 10.1093/hmg/5.4.555. [DOI] [PubMed] [Google Scholar]
- 24.Huber M, Rettler I, Bernasconi K, Wyss M, Hohl D. J Invest Dermatol. 1995;105:653–654. doi: 10.1111/1523-1747.ep12324122. [DOI] [PubMed] [Google Scholar]
- 25.Niemi K-M, Kanerva L, Kuokkanen K. Arch Dermatol Res. 1991;283:211–218. doi: 10.1007/BF01106104. [DOI] [PubMed] [Google Scholar]
- 26.Hohl D, Huber M, Frenk E. Arch Dermatol. 1993;129:618–624. [PubMed] [Google Scholar]
- 27.Tybulewicz V L J, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1992;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 28.Phillips M A, Stewart B E, Qin Q, Chakravarty R, Floyd E E, Jetten A M, Rice R H. Proc Natl Acad Sci USA. 1990;87:9333–9337. doi: 10.1073/pnas.87.23.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanishi K, Liew F-M, Konishi K, Yasuno H, Doi H, Hirano J, Fukushima S. Biochem Biophys Res Commun. 1991;175:906–913. doi: 10.1016/0006-291x(91)91651-r. [DOI] [PubMed] [Google Scholar]
- 30.Ishida-Yamamoto A, Eady R A J, Watt F M, Roop D R, Hohl D, Iizuka H. J Histochem Cytochem. 1996;44:167–175. doi: 10.1177/44.2.8609373. [DOI] [PubMed] [Google Scholar]
- 31.Yamada K, Matsuki M, Morishima Y, Ueda E, Tabata K, Yasuno H, Suzuki M, Yamanishi K. Hum Mol Genet. 1997;6:2223–2231. doi: 10.1093/hmg/6.13.2223. [DOI] [PubMed] [Google Scholar]
- 32.Konishi K, Morishima Y, Ueda E, Kibe Y, Nonomura K, Yamanishi K, Yasuno H. Biochem Biophys Res Commun. 1994;202:976–983. doi: 10.1006/bbrc.1994.2025. [DOI] [PubMed] [Google Scholar]
- 33.Barel A O, Clarys P. Skin Pharmacol. 1995;8:186–195. doi: 10.1159/000211345. [DOI] [PubMed] [Google Scholar]
- 34.Phillips M A, Qin Q, Mehrpouyan M, Rice R H. Biochemistry. 1993;32:11057–11063. doi: 10.1021/bi00092a015. [DOI] [PubMed] [Google Scholar]
- 35.Kim I-G, Gorman J J, Park S-C, Chung S-I, Steinert P M. J Biol Chem. 1993;268:12682–12690. [PubMed] [Google Scholar]
- 36.Kim S-Y, Chung S-I, Steinert P M. J Biol Chem. 1995;270:18026–18035. doi: 10.1074/jbc.270.30.18026. [DOI] [PubMed] [Google Scholar]
- 37.Lentz C L, Altman J. Arch Dermatol. 1968;97:3–13. doi: 10.1001/archderm.97.1.3. [DOI] [PubMed] [Google Scholar]
- 38.Byrne C, Tainsky M, Fuchs E. Development (Cambridge, UK) 1994;120:2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- 39.Bickenbach J R, Greer J M, Bundman D S, Rothnagel J A, Roop D R. J Invest Dermatol. 1995;104:405–410. doi: 10.1111/1523-1747.ep12665896. [DOI] [PubMed] [Google Scholar]
- 40.Steven A C, Bisher M E, Roop D R, Steinert P M. J Struct Biol. 1990;104:150–162. doi: 10.1016/1047-8477(90)90071-j. [DOI] [PubMed] [Google Scholar]
- 41.Mehrel T, Hohl D, Rothnagel J A, Longley M A, Bundman D, Cheng C, Lichti U, Bisher M E, Steven A C, Steinert P M, Yuspa S H, Roop D R. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 42.Hohl D, Mehrel T, Lichti U, Turner M L, Roop D R, Steinert P M. J Biol Chem. 1991;266:6626–6636. [PubMed] [Google Scholar]
- 43.Buyse L, Graves C, Wijeyesekera K, Alfaham M, Finlay A. Br J Dermatol. 1993;129:86–88. doi: 10.1111/j.1365-2133.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 44.Fesus L, Madi A, Balajthy Z, Nemes Z, Szondy Z. Experientia. 1996;52:942–949. doi: 10.1007/BF01920102. [DOI] [PubMed] [Google Scholar]
- 45.Inouye M. Congenital Anomalies. 1976;16:171–173. [Google Scholar]
- 46.Frenck E, Mevorah B. J Cutan Pathol. 1977;4:329–337. doi: 10.1111/j.1600-0560.1977.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 47.Candi E, Melino G, Mei G, Tarcsa E, Chung S I, Marekov L N, Steinert P M. J Biol Chem. 1995;270:26382–26390. doi: 10.1074/jbc.270.44.26382. [DOI] [PubMed] [Google Scholar]
- 48.Polakowska R R, Piacentini M, Bartlett R, Goldsmith L A, Haake A R. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- 49.Tamada Y, Takama H, Kitamura T, Yokochi K, Nitta Y, Ikeya T, Matsumoto Y. Br J Dermatol. 1994;131:521–524. doi: 10.1111/j.1365-2133.1994.tb08553.x. [DOI] [PubMed] [Google Scholar]
- 50.Blank I H. J Invest Dermatol. 1965;45:249–256. doi: 10.1038/jid.1965.125. [DOI] [PubMed] [Google Scholar]