Abstract
The basidiomycete fungus Cryptococcus neoformans is an opportunistic pathogen of worldwide importance that causes meningitis, leading to death in immunocompromised individuals. Unlike many basidiomycete fungi, C. neoformans is thermotolerant, and its ability to grow at 37°C is considered to be a virulence factor. We used serial analysis of gene expression (SAGE) to characterize the transcriptomes of C. neoformans strains that represent two varieties with different polysaccharide capsule serotypes. These include a serotype D strain of the C. neoformans variety neoformans and a serotype A strain of variety grubii. In this report, we describe the construction and characterization of SAGE libraries from each strain grown at 25°C and 37°C. The SAGE data reveal transcriptome differences between the two strains, even at this early stage of analysis, and identify sets of genes with higher transcript levels at 25°C or 37°C. Notably, growth at the lower temperature increased transcript levels for histone genes, indicating a general influence of temperature on chromatin structure. At 37°C, we noted elevated transcript levels for several genes encoding heat shock proteins and translation machinery. Some of these genes may play a role in temperature-regulated phenotypes in C. neoformans, such as the adaptation of the fungus to growth in the host and the dimorphic transition between budding and filamentous growth. Overall, this work provides the most comprehensive gene expression data available for C. neoformans; this information will be a critical resource both for gene discovery and genome annotation in this pathogen.
[This paper is dedicated to the memory of Michael Smith, founding director of the Biotechnology Laboratory and the British Columbia Genome Sciences Centre. The following individuals kindly provided reagents, samples, or unpublished information as indicated in the paper: Brendan Loftus, Claire Fraser, Richard Hyman, Eula Fung, Don Rowley, Ron Davis , Bruce A. Roe, Doris Kupfer, Jennifer Lewis, Sola Yu, Kent Buchanan, Dave Dyer, and Juneann Murphy.]
Cryptococcus neoformans has received considerable attention recently because of the high incidence of infections caused by this fungus in immunocompromised individuals (Casadevall and Perfect 1998; Harrison 2000). C. neoformans causes life-threatening infections in AIDS patients and people receiving immunosuppressive therapy. Cryptococcal meningitis is recognized as an AIDS-related infection, and C. neoformans is also capable of causing disease in immunocompetent individuals (Harrison 2000). Documented virulence factors include the production of a polysaccharide capsule, the formation of melanin, and the ability to grow at 37°C (Casadevall and Perfect 1998). Capsule-defective mutants of C. neoformans have reduced virulence compared with that of wild-type strains (Chang and Kwon-Chung 1998). Similarly, mutants defective in their ability to produce melanin on media containing phenolic compounds and mutants defective in their ability to grow at 37°C also show reduced virulence (Kwon-Chung and Rhodes 1986; Wang et al. 1995; Odom et al. 1997; Nosanchuk et al. 2000). The tolerance of C. neoformans to elevated temperatures has not been explored in detail, although it is known that mutations in RAS1 and in CNA1 (encoding calcineurin) cause growth defects at elevated temperature (Odom et al. 1997; Alspaugh et al. 2000). There is also an intriguing connection between mating and virulence in C. neoformans; strains of mating-type MATα have been shown to be more virulent than strains of the MATa mating type, and the majority of clinical isolates are MATα (Kwon-Chung et al. 1992). One explanation for this prevalence is that only strains of the MATα mating type form the filamentous cell type that produces the small spores believed to serve as infectious propagules (Wickes et al. 1996).
C. neoformans is a dimorphic fungus that displays a yeast morphology in the haploid phase of the life cycle and a filamentous, dikaryotic cell morphology on mating between compatible haploid strains (Kwon-Chung and Bennett 1992; Casadevall and Perfect 1998). C. neoformans grows primarily by budding during infection, although filamentous growth is sometimes observed in the host (Bemis et al. 2000). Haploid strains of the MATα mating type can also show filamentous growth in culture in response to nitrogen starvation (Wickes et al. 1996). This filamentous growth (termed haploid fruiting) is associated with the formation of small asexual spores, which may serve as infectious agents via inhalation. Recently, it has been shown that stable diploid strains of C. neoformans can be obtained from crosses of compatible haploid mating partners (Sia et al. 2000). These diploid strains are thermally dimorphic in that they grow as yeast at 37°C and have a filamentous morphology at 24°C. At the lower temperature, the filaments formed by the diploid strains sporulate to produce haploid, meiotic progeny. Temperature regulation of the morphological switch in C. neoformans is reminiscent of the situation in other fungal pathogens of humans, including Histoplasma capsulatum, Blastomyces dermatitidis, and Paracoccidioides brasiliensis (Medoff et al. 1987; Maresca et al. 1994).
An international consortium has been established to determine the genomic sequence of C. neoformans (Heitman et al. 1999b). Initially, the MATα strain JEC21 was chosen for sequencing because this strain and a congenic MATa isolate (JEC20) have been developed as genetically useful experimental strains (Heitman et al. 1999a). These strains represent the neoformans variety of C. neoformans defined in part by the D serotype of the polysaccharide capsule. In addition, there is considerable interest in obtaining the genomic sequence of other varieties of C. neoformans, including the clinical isolate H99 of the serotype A group of C. neoformans (variety grubii). A genomic shotgun sequencing effort is underway at Stanford University and at The Institute for Genomic Research (TIGR) for serotype D strain JEC21 and a related (progenitor) strain, B3501 (Heitman et al. 1999a). Expressed sequence tag (EST) projects for strains JEC21 and H99 are ongoing at the University of Oklahoma's Advanced Center for Genome Technology. In addition, limited shotgun sequencing has been performed for H99 at the Duke University Center for Genome Technology. To contribute to sequencing efforts, we have constructed physical maps of the genomes of strains JEC21 and H99 by bacterial artificial chromosome (BAC) fingerprinting, and we have performed BAC end sequencing to contribute to assembly of the genomic sequences (J. Schein et al. 2002).
In this report, we describe the use of serial analysis of gene expression (SAGE) to examine the transcriptome of C. neoformans as a function of temperature. SAGE involves generating short sequence (nine to 13 bp) tags that represent individual transcripts and using large-scale sequencing to establish the frequency of occurrence of these tags as a measure of transcript levels (Velculescu et al. 1995). SAGE has been used to define the transcriptome for Saccharomyces cerevisiae (Velculescu et al. 1997) and to explore transcription in normal and tumor cells (see Zhang et al. 1997). We chose SAGE instead of microarrays for defining the C. neoformans transcriptome because the small collections of available ESTs precluded the use of microarrays. In addition, SAGE data are digital and provide the opportunity for robust statistical analysis (Audic and Claverie 1997). Furthermore, when used in conjunction with genomic sequence data, SAGE results have been useful in all stages of genome annotation and, in particular, for gene identification (see Jones et al. 2001). Our experiments show the utility of SAGE for the genome-wide analysis of transcription in fungi and represent the first application of this technique to a human pathogen. Our SAGE analysis for C. neoformans revealed substantial differences in the transcriptomes of different serotypes and allowed the identification of sets of genes whose transcript levels vary with temperature. The characterization of the latter genes provides insight into the ability of C. neoformans to grow at 37°C in the human host.
RESULTS AND DISCUSSION
Temperature Regulation of Transcript Levels in C. neoformans
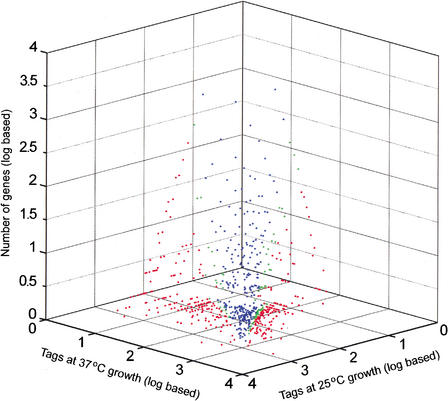
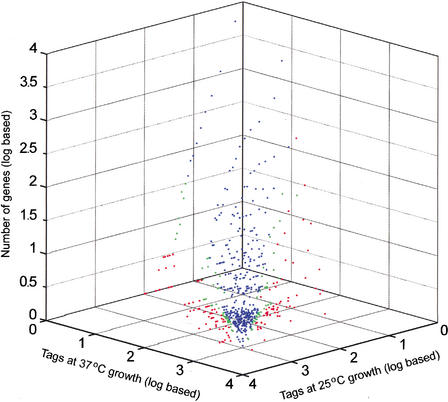
Four SAGE libraries were constructed and sequenced to generate RNA expression data for C. neoformans strains B3501 and H99, each grown at 25°C and 37°C. A summary of the collection of tags for each library is presented in Table 1. The collection and processing of the tag data included the use of Phred scores for the sequence traces to establish a statistical level of confidence in the sequence of each tag (see Methods). The data shown in Table 1 reflect Phred scores that provide a 99% probability that each tag sequence is correct. The collection of SAGE tags at two different temperatures provided a means to assess genome-wide changes in expression for two strains. Figure 1 presents the expression profile at the two temperatures for the serotype A strain H99. Of 12,056 tag species analyzed, 12.5% (1507 tag species) showed a significant difference (P ≤ 0.05) between the two temperatures. A tag species is defined as the unique sequence identifier of a particular tag. Figure 2 presents the expression profile at the two temperatures for the serotype D strain B3501. For this strain, a total of 13,615 tag species were analyzed, and 4.9% (664 tag species) showed a significant difference (P ≤ 0.05) between the two temperatures. For comparison, a recent analysis of the influence of temperature on global gene expression in group A Streptococcus revealed that 9% of the genes were differentially transcribed at 29°C versus 37°C (Smoot et al. 2001).
Table 1.
Analysis of SAGE libraries
| H99 | B3501 | |||
|---|---|---|---|---|
| 25°C | 37°C | 25°C | 37°C | |
| Sequence reads | 1815 | 2213 | 4126 | 2165 |
| Total tags* | 30,181 | 37,467 | 65,399 | 15,363 |
| Tag families | ||||
| Singletons | 4406 (14.1%) | 4196 (10.6%) | 5967 (8.8%) | 3693 (23.8%) |
| 2 to 10 | 2703 (8.7%) | 2821 (6.9%) | 4468 (6.6%) | 2029 (13.0%) |
| 11 to 100 | 441 (1.4%) | 564 (1.4%) | 1082 (1.6%) | 201 (1.3%) |
| >100 | 25 (0.09%) | 43 (0.13%) | 65 (0.11%) | 3 (0.02%) |
| Total | 7575 | 7624 | 11,582 | 5926 |
Ninety-nine percent probability that each tag sequence is correct.
Figure 1.
Expression profile comparing relative transcript levels at 25°C and 37°C in strain H99. Singleton tags were excluded. Blue dots indicate tags that do not show a significant expression difference; green dots, tags with a difference that is significant at 95% to 99% confidence; and red dots, tags with a significance of >99% confidence.
Figure 2.
Expression profile comparing relative transcript levels at 25°C and 37°C in strain B3501. Singleton tags were excluded. Blue dots indicate tags that do not show a significant expression difference; green dots, tags with a difference that is significant at 95% to 99% confidence; and red dots, tags with a significance of >99% confidence.
Identification of Genes for the Most Highly Expressed Tags for the Serotype A and D Strains
Although an annotated genomic sequence is not available for any strain of C. neoformans, we were able to make preliminary tag assignments to specific predicted genes with the partial genomic and EST sequence data for both strains. For strain H99, we have made preliminary gene assignments for 19 and 29 of the top 50 most abundant tags from the 25°C and 37°C libraries, respectively (Table 2A,B). In this strain, 20 tags were found to be identical in the top 50 of both libraries. A total of 70 unique tag species were studied, and 42 of these were associated with an EST sequence; 38 of the EST sequences gave significant BLASTP results, leading to putative gene assignments. Within the top 50 tags, we identified genes for three ribosomal proteins at 25°C and 12 ribosomal proteins at 37°C. Furthermore, the top 50 tags (for both libraries) identified genes for proteins that are generally considered to be abundant in other organisms. These include GAPDH, translation elongation factor, pyruvate decarboxylase, malate dehydrogenase, and fructose-bisphosphate aldolase. Interestingly, a tag representing the transcript of a zinc transport protein was the most highly expressed tag at 25°C but was not seen in the top 50 tags for the 37°C library. As well, the tag representing cyclophilin A (CPA1 and CPA2) was identified in the top 50 of both libraries but was expressed 1.47 times higher at 37°C. The genes encoding cyclophilin A have been characterized in C. neoformans, and Cpa1 is required for growth at elevated temperature and for virulence (Wang et al. 2001). Two of the abundant tags at 37°C identified transcripts for a thioredoxin peroxidase (0.74%) and a superoxide dismutase (0.35%). These tags were approximately fourfold higher at 37°C relative to 25°C. Lee and Park (1998) have shown that a thioredoxin peroxidase contributes to thermotolerance in S. cerevisiae, presumably by acting as an antioxidant. Superoxide dismutase plays a well-characterized role in antioxidant defense, and the production of the enzyme is known to be higher at 37°C than at 25°C in C. neoformans (Jacobson et al. 1994). The expression of this protein is also known to be influenced by temperature in other pathogens such as group A Streptococcus (Smoot et al. 2001). In general, these results indicate that growth at 37°C may induce the expression of genes involved in a stress response in C. neoformans.
The availability of more genomic sequence information for the serotype D strains JEC21 and B3501 (relative to strain H99) allowed us to make preliminary gene assignments for 33 and 34 of the top 50 most abundant tags prepared with cells grown at 25°C and 37°C, respectively (Table 3A,B). In this strain, substantially more tags (33) were found to be identical in the top 50 of both libraries compared with the H99 libraries. This finding is consistent with the lower percentage of differentially expressed genes for B3501 (Fig. 2). In total, 141 unique tag species were studied for strain B3501, 75 of which were given putative gene assignments based on a significant BLASTP result. Only eight tags did not associate with an EST or a genomic sequence contig. Of those tags that did not result in a putative gene assignment, 20 tags were ambiguous because they hit more than one sequence contig.
As in the H99 libraries, the top 50 tags represented genes for four (25°C) and 11 (37°C) ribosomal proteins, as well as genes for proteins that are expected to be abundant such as translation elongation factor, pyruvate decarboxylase, and GAPDH. The tag for the cyclophilin A transcript was seen at both 25°C and 37°C for B3501, although the tag was differentially expressed in an opposite manner (approximately twofold higher at 25°C in B3501) compared with the results for the H99 libraries. Also in contrast to the H99 libraries, the 50 most abundant tags in both B3501 libraries did not include a tag representing fructose-bisphosphate aldolase. For B3501, the list of abundant tags also revealed high transcript levels for the genes predicted to encode a ubiquitin RPS27A fusion protein, a ubiquitin conjugating enzyme, an iron permease, and a serine-threonine protein kinase that may be involved in pre-mRNA splicing (similar to Prp4p of Schizosaccharomyces pombe; Schwelnus et al. 2001). These genes were not identified in the top 50 tags from the H99 libraries at either temperature. In addition, the most abundantly expressed genes from both the 25°C and 37°C libraries of B3501 contained a zinc transporter that was seen only in the 25°C library from H99.
The B3501 37°C library revealed tags representing several proteins not seen in the 25°C library. These included the ER chaperone BiP (approximately twofold higher at 37°C), a peripheral benzodiazepine receptor homolog (discussed below), and several ribosomal proteins. Interestingly, the thioredoxin peroxidase tag that was found only in the top 50 tags of the 37°C library from H99 was identified in both the 25°C and 37°C top 50 tags of strain B3501. Overall, these results indicate that there are several differences in the response of H99 and B3501 to elevated temperature. A more extensive comparison will be possible when more tags can be matched with genes on completion and annotation of the genomic sequences of both strains.
The SAGE analysis of the most highly expressed genes in C. neoformans is comparable to that of S. cerevisiae (Velculescu et al. 1997). In yeast, the proteins encoded by the top 30 highly expressed genes included GAPDH, translation elongation factor-α, alcohol dehydrogenase, fructose-bisphosphate aldolase, pyruvate decarboxylase, and 18 ribosomal proteins. On the other hand, a comparison of our results with the changes in transcript levels observed for S. cerevisiae genes at 25°C and 37°C (as measured by microarray analysis; Gasch et al. 2000) indicates that temperature influences the transcription of a relatively greater number of genes in C. neoformans.
Tags With Higher Levels at 25°C
To begin to determine differences in transcript levels at the two temperatures, we made preliminary gene assignments for a selected group of 100 tags that showed the most statistically significant different expression levels between the two temperatures. All of these tags have a value of P < 0.05 as the minimum level of significance for concluding that a given tag showed differential expression. The fold difference for the tag levels was determined by normalizing the total tag numbers to represent libraries of equal sizes. We note that the calculation of fold-difference is less accurate in this analysis when the number of tags is small, although the P value calculation is unaffected. We focused our analysis on the data for strain B3501 because, as noted earlier, there is substantially more genomic sequence information available for this strain compared with H99.
The analysis of 50 tags with higher levels at 25°C revealed several patterns of transcription that may reflect general features of temperature adaptation in C. neoformans (Table 4). First, the tags representing transcripts for histones H1, H3, and H4 were all elevated at 25°C compared with 37°C (approximately two- to sevenfold). Assuming that these changes in transcript levels reflect changes in the abundance of histone proteins, our results indicate that growth temperature may exert a general influence on chromatin structure in C. neoformans. This was corroborated by the fact that at 37°C H4 was expressed 10-fold more than H1, whereas at 25°C, H4 was expressed only threefold more than H1. These observations indicate that growth temperature causes a change in the relative expression of histone gene families. In turn, this may reflect a broad shift in gene expression for this pathogen as a function of temperature. This conclusion is supported by results from S. cerevisiae in which the examination of changes in histone abundance (e.g., by depletion of histone H4) revealed changes in the expression of ∼25% of all of the genes (Wyrick et al. 1999).
Table 4.
B3501 Tags More Highly Expressed at 25°C
| SAGE tag | B3501 25 | 25 normalized | B3501 37 | FOLD difference | Preliminary gene designation | E-value | Accession no.of BLASTx | |
|---|---|---|---|---|---|---|---|---|
| Genomic BLASTx | EST BLASTx | |||||||
| caagtaattt | 293 | 69 | 11 | 6.3 | NO HITSb | |||
| gaacgatgct | 607 | 143 | 56 | 2.6 | NO HITSb | |||
| aaagcgcgtt | 151 | 35 | 3 | 11.7 | Inositol 1-phosphate synthase | 1.00E-144 | Pichia pastorisAF078915 | |
| catttacata | 546 | 128 | 57 | 2.2 | NO HITSb | |||
| gacgatatat | 204 | 48 | 9 | 5.3 | C-4 methyl sterol oxidase | 2.00E-84 | 2.00E-48 | Schizosaccharomyces pombeAL109832 |
| gctctccagg | 250 | 59 | 15 | 3.9 | Histone H3 | 2.00E-48 | 9.00E-64 | Mortierella alpinaAJ249812 |
| cgagtcgtat | 539 | 127 | 62 | 2.0 | Iron permease | 2.00E-23 | S. pombeZ67998 | |
| cagagatgtg | 197 | 46 | 14 | 3.3 | Nonhistone protein | 1.00E-06 | 7.00E-09 | Saccharomyces cerevisiaeZ94864 |
| tatctgaaag | 93 | 22 | 2 | 11.0 | Delayed-type hypersensitivity antigen | 1.00E-103 | Cryptococcus neoformansAF246128 | |
| cattcggttt | 60 | 14 | 0 | unique to 25 | NO HITSb | |||
| gtattgaccc | 430 | 101 | 52 | 1.9 | Phosphoketolase | 1.00E-165 | 1.00E-106 | Lactococcus lactisAE006381 |
| tgatgggaag | 57 | 13 | 0 | unique to 25 | Sterol C-5-desaturase | 1.00E-56 | Rattus norvegicusAB052846 | |
| tcgagaatgg | 218 | 51 | 19 | 2.7 | NO HITSb | |||
| aattcgcttt | 133 | 31 | 8 | 3.9 | 14-3-3-Protein | 5.00E-84 | 1.00E-124 | Schizophyllum communeAY029473 |
| tatatgtgta | 79 | 19 | 2 | 9.5 | Heat shock protein 12 | 7.50E-02 | 4.00E-12 | S. cerevisiaeX55785 |
| ataaaaaaaa | 159 | 37 | 12 | 3.1 | NO HITSa | |||
| tgaaaatata | 62 | 15 | 1 | 15.0 | Δ9 fatty acid desaturase | 1.00E-136 | 1.00E-111 | Mortierella alpinaY18553 |
| tacttttttt | 108 | 25 | 6 | 4.2 | NO HITSa | |||
| tatcccacca | 99 | 23 | 5 | 4.6 | NO HITSb | |||
| atgatttgag | 77 | 18 | 3 | 6.0 | NO HITSb | |||
| cctcaacggc | 101 | 24 | 6 | 4.0 | NO HITSb | |||
| gaactggcgg-3′ | 37 | 9 | 0 | unique to 25 | Adenosyl homocysteinase | 0.00E + 00 | 2.00E-40 | S. pombeAB004537 |
| agtgctgctg | 63 | 15 | 2 | 7.5 | Histone H1 | 1.10E-02 | S. cerevisiaeU43703 | |
| caaaaaggat | 63 | 15 | 2 | 7.5 | NO HITSb | |||
| tcaaagaaga | 62 | 15 | 2 | 7.5 | NO HITSb | |||
| ctgaggctga | 50 | 12 | 1 | 12.0 | NO HITSb | |||
| ggcttgacca | 50 | 12 | 1 | 12.0 | High-affinity monosaccharide transporter | 9.00E-30 | 8.00E-48 | Amanita muscariaZ83828 |
| ccggctaatg | 117 | 27 | 9 | 3.0 | NO HITSb | |||
| ctgtatgtcc | 34 | 8 | 0 | unique to 25 | NO HITSb | |||
| accttgatgg | 78 | 18 | 4 | 4.5 | NO HITSb | |||
| gacttttgac | 94 | 22 | 6 | 3.7 | NO HITSb | |||
| tctggtcgag | 187 | 44 | 20 | 2.2 | Histone H4 | 7.00E-19 | 2.00E-38 | Agaris bisporusP35058 |
| ggcatttagt | 32 | 8 | 0 | unique to 25 | NO HITSb | |||
| attggtttga | 32 | 8 | 0 | unique to 25 | NO HITSb | |||
| gctaacgctg | 238 | 56 | 29 | 1.9 | Cyclophilin A | 5.00E-76 | 2.00E-91 | C. neoformansAF333996 |
| ttcgcgctaa | 66 | 16 | 3 | 5.3 | NO HITSb | |||
| caagcagata | 45 | 11 | 1 | 11.0 | Fatty acid synthase α-chain | 1.00E-68 | S. pombeD83412 | |
| tatccgggtc | 72 | 17 | 4 | 4.3 | Aspartate aminotransferase | 6.00E-89 | Homo sapiensM22632 | |
| tctaacccta | 28 | 7 | 0 | unique to 25 | Hmp1 of U. maydis | 1.00E-14 | 1.00E-22 | Ustilago maydisU39049 |
| cacattgata | 70 | 16 | 4 | 4.0 | NO HITSb | |||
| cctgcgagac | 27 | 6 | 0 | unique to 25 | NO HITSb | |||
| tcataaagca | 26 | 6 | 0 | unique to 25 | NO HITSb | |||
| tatatccatt | 26 | 6 | 0 | unique to 25 | NO HITSb | 2.00E-71 | ||
| tatcatccgt | 26 | 6 | 0 | unique to 25 | Myo-inositol transporter A | 9.00E-59 | N. crassaAL390218 | |
| tatgatgttt | 26 | 6 | 0 | unique to 25 | NO HITSb | |||
| catctattcc | 286 | 67 | 40 | 1.7 | NO HITSa | |||
| tatttgttgt | 25 | 6 | 0 | unique to 25 | DNA-directed RNA polymerase II | 1.00E-91 | S. pombeD13337 | |
| ttagcgacag | 25 | 6 | 0 | unique to 25 | NO HITSb | |||
| gtttaatcaa | 24 | 6 | 0 | unique to 25 | COPII-coated vesicle component | 3.00E-18 | 7.00E-19 | S. pombeAL109831 |
| aatgactttt | 427 | 100 | 68 | 1.5 | NO HITSb | |||
| tctttgatgt-5′ | 328 | 77 | 49 | 1.6 | ADP, ATP carrier protein | 1.00E-133 | 2.00E-72 | Gossypium hirsutumAF006489 |
| tgtcataaaa | 23 | 5 | 0 | unique to 25 | NO HITSb | |||
3′ or 5′ denotes that a second serial analysis of gene expression (SAGE) tag was found and that the tag is either the 3′ most tag or more 5′. Tags in this table are differentially expressed with a statistic significance of P < 0.05.
Tag did not have a corresponding contig at Stanford and did not have a corresponding expressed sequence tag (EST) at Oklahoma.
No significant BLAST hit results for the genomic or EST sequence associated with the SAGE tag.
A second notable group of tags that were up-regulated at 25°C represented genes for sterol and lipid metabolism. The expression pattern for these genes is consistent with observations in other organisms in which adjustments in membrane composition are correlated with growth temperature (Steels et al. 1994; Los et al. 1997; Aguilar et al. 1998). In general, cells adapt to a lower temperature by an increase in the production of desaturase, resulting in unsaturated fatty acids in membrane phospholipids to maintain proper fluidity. That is, we would expect the SAGE data to reveal changes in transcript levels for desaturase genes as a function of temperature, and we did identify a tag for the transcript of a Δ9 fatty acid desaturase that was elevated 14.56-fold at 25°C. Other tags that were elevated at 25°C included those representing genes for sterol synthesis (sterol C-5 desaturase and C-4 methyl sterol oxidase) and fatty acid synthesis (fatty acid synthases). Sterol content in C. neoformans is known to change in response to passage of the fungus through an animal host (Currie et al. 1995). Changes in membrane composition have also been correlated with morphogenesis and thermotolerance in other fungal pathogens. For example, a Δ9 fatty acid desaturase is regulated by temperature and cAMP signaling during the dimorphic transition in Histoplasma capsulatum (Storlazzi et al. 1999). These observations may be relevant for C. neoformans because signaling via a cAMP pathway is known to play an important role in the virulence (Alspaugh et al. 1997, 2001; D'Souza et al. 2001).
An additional general observation for the tags with higher levels at 25°C is that many represent genes for transport functions. These included a gene involved in iron transport, as well as glucose and inositol transporters. Inositol metabolism has been examined in C. neoformans and is proposed to be important for pathogenesis (Luberto et al. 2001). This may be relevant for virulence because of the preference of C. neoformans for growth in the central nervous system, a location known to be rich in inositol (Vincent and Klig 1995). We also found that the tag for a putative inositol synthase gene was up-regulated at 25°C, further indicating a connection between inositol metabolism and growth temperature.
In addition to our analysis of 50 differentially expressed tags (Table 4), we also found that the tag for a C. neoformans translation elongation factor-3 (TEF3; ATGTATATAC) was 6.10-fold more abundant at 25°C. TEF3 is a fungal-specific elongation factor, and transcript levels for this gene are known to change in C. albicans as a function of temperature. That is, changes in transcript levels have been observed during growth at different temperatures, although these changes do not seem to be associated with temperature-regulated dimorphism in this fungus. As well, there is evidence to support the idea that reduced transcription of TEF3 in C. albicans results in decreased virulence in a mouse model of infection (Nakayama et al. 2000).
Tags With Higher Levels at 37°C
We also made preliminary gene assignments for 50 tags that showed statistically significant elevated levels at 37°C (Table 5). The tag with the greatest difference was approximately 47-fold higher at 37°C but represented a transcript from a putative open reading frame on sequence contig cneo010512.Contig5001 with no similarity to known genes. For the other tags, a number of categories of expression were noted that could reflect the adaptation of C. neoformans to growth at 37°C. This adaptation could include changes in the rate of protein synthesis because up-regulated tags matched transcripts for translation elongation factor-1α, a translation initiation factor, and three ribosomal proteins. As well, a change in protein synthesis correlated with the earlier observation that 12 and 11 ribosomal proteins were found in the 37°C libraries for both H99 and B3501, relative to three and four ribosomal proteins at 25°C for H99 and B3501, respectively.
Table 5.
B3501 Tags More Highly Expressed at 37°C
| SAGE tag | B3501 37 | B3501 25 | 25 normalized | FOLD difference | Preliminary gene designation | E-value | Accession no.of BLASTx | |
|---|---|---|---|---|---|---|---|---|
| Genome BLASTx | EST BLASTx | |||||||
| atatgaaaga | 55 | 5 | 1 | 55.0 | NO HITSb | |||
| aggaagagaa | 125 | 186 | 44 | 2.8 | Hypothetical protein | 4.00E-22 | Agaris bisporusAJ271701 | |
| acgtaccttt | 41 | 21 | 5 | 8.2 | NO HITSb | |||
| cgacagaccg | 207 | 529 | 124 | 1.7 | Translation elongation factor 1α | 0.00E + 00 | 0.00E + 00 | Cryptococcus neoformansU81804 |
| ggaatttgct | 24 | 17 | 4 | 6.0 | NO HITSb | |||
| tagacagact | 15 | 6 | 1 | 15.0 | Carboxypeptidase D | 1.00E-121 | P. janthinellumAAB35195.1 | |
| accgacgtga | 22 | 19 | 4 | 5.5 | Aconitate hydratase | 1.00E-165 | Piromyces spY16747 | |
| cggaaaaaac | 7 | 0 | 0 | unique to 37 | Hypothetical protein | 1.00E-11 | Arabidopsis thalianaAC002294 | |
| cctgttctcg | 36 | 51 | 12 | 3.0 | NO HITSb | |||
| gccgcttctg | 13 | 6 | 1 | 13.0 | Ubiquinol-cytochrome C reductase iron-sulfur | 1.00E-84 | Neurospora crassaX02472 | |
| aaccagcggt | 8 | 1 | 0 | 34.1 | Salicylate hydroxylase | 3.00E-12 | Streptomyces coelicolorAL035707 | |
| aagacatcgt | 9 | 2 | 0 | 19.2 | NO HITSb | |||
| attttagaaa | 9 | 2 | 0 | 19.2 | NADH-ubiquinone oxidoreductase subunit | 6.00E-07 | N. crassaX60829 | |
| gtccataagg | 13 | 7 | 2 | 6.5 | NO HITSb | |||
| taactcgcat | 6 | 0 | 0 | unique to 37 | NO HITSb | |||
| tctaagtata | 6 | 0 | 0 | unique to 37 | NO HITSb | |||
| aacgtctgcc | 45 | 80 | 19 | 2.4 | NO HITSa | |||
| cgcgcgatgc | 16 | 14 | 3 | 5.3 | NO HITSb | |||
| gcattggcgt | 39 | 70 | 16 | 2.4 | ER chaperone BiP | 0.00E + 00 | 5.00E-20 | Aspergillus oryzaeAB030231 |
| catctggatg | 5 | 0 | 0 | unique to 37 | NO HITSb | |||
| tgttatcggt | 16 | 15 | 4 | 4.0 | Heat shock protein 80 | 1.00E-135 | N. crassaAL513463 | |
| gcattttggg | 18 | 20 | 5 | 3.6 | Ubiquinol-cytochrome C | 1.00E-34 | N. crassaY08841 | |
| aaccgcacca | 29 | 46 | 11 | 2.6 | Reductase core protein peripheral benzodiazepine receptor—human | 6.00E-16 | Homo sapiensJE0149 | |
| tgtagtatct | 13 | 11 | 3 | 4.3 | NO HITSb | |||
| tcgagtttca | 11 | 8 | 2 | 5.5 | NO HITSb | |||
| attgagatgg | 44 | 91 | 21 | 2.1 | NO HITSb | |||
| ctaggttatg | 4 | 0 | 0 | unique to 37 | 3′ to 5′ DNA/RNA helicase | 1.00E-165 | Schizosaccharomyces pombeAL590902 | |
| ccgcctgccg | 4 | 0 | 0 | unique to 37 | NO HITSb | |||
| gctgcaagcg | 4 | 0 | 0 | unique to 37 | Hypothetical protein | 2.00E-43 | N. crassaAL513463 | |
| ttcgcggtag | 4 | 0 | 0 | unique to 37 | NO HITSb | |||
| gtgatggtgg | 4 | 0 | 0 | unique to 37 | NO HITSb | |||
| ccctacgaga | 4 | 0 | 0 | unique to 37 | NO HITSa | |||
| atcgcgatgt | 4 | 0 | 0 | unique to 37 | Putative protein | 4.00E-05 | Mus musculusNM_025872 | |
| gggagccata | 4 | 0 | 0 | unique to 37 | NO HITSb | |||
| atcctttgtc | 4 | 0 | 0 | unique to 37 | NO HITSb | |||
| actcaaccgt | 10 | 7 | 2 | 5.0 | NO HITSb | |||
| catagttggt | 27 | 47 | 11 | 2.5 | Heat shock protein 70 family | 0.00E + 00 | Malassezia sympodialisAJ428052 | |
| ctcaagaagg | 17 | 22 | 5 | 3.4 | Subtilisin-like serine protease | 4.00E-91 | Penicillium citrinumAF098517 | |
| tcagaaccgt | 6 | 2 | 0 | 12.8 | NO HITSb | |||
| cagaacaaag | 5 | 1 | 0 | 21.3 | protein with similarity to GAPDH | 3.00E-28 | Mesorhizobium lotiAP003004 | |
| tatggctgga | 5 | 1 | 0 | 21.3 | NO HITSb | |||
| gaagtccgga | 5 | 1 | 0 | 21.3 | NO HITSb | |||
| tacactgtcg | 12 | 12 | 3 | 4.0 | NO HITSb | |||
| gtttatggaa | 11 | 11 | 3 | 3.7 | Heat shock protein 60 | 0.00E + 00 | Coccidioides immitisU81786 | |
| aacgtaaagc | 7 | 4 | 1 | 7.0 | NO HITSb | |||
| gtgtggggca | 7 | 4 | 1 | 7.0 | NO HITSb | |||
| caacgtagaa | 7 | 4 | 1 | 7.0 | NO HITSb | |||
| tcatcaccat | 7 | 4 | 1 | 7.0 | Translation initiation factor 3 | 6.00E-15 | Myxococcus xanthusAF261103 | |
| aactcgtgaa | 15 | 20 | 5 | 3.0 | Hypothetical protein | 2.00E-22 | 5.00E-05 | A. bisporusAJ271701 |
| gcggtgggat | 14 | 18 | 4 | 3.5 | NO HITSa | |||
3′ or 5′ denotes that a second serial analysis of gene expression (SAGE) tag was found and that the tag is either the 3′ most tag or more 5′. Tags in this table are differentially expressed with a statistic significance of P < 0.05.
Tag did not have a corresponding contig at Stanford and did not have a corresponding expressed sequence tag (EST) at Oklahoma.
No significant BLAST hit results for the genomic or EST sequence associated with the SAGE tag.
We identified tags representing several heat shock proteins (HSP60, HSP70, HSP80) that had higher transcript levels at 37°C. This observation is particularly interesting in light of observations that heat shock proteins 60 and 70 have been identified as prominent antigens in animals and humans infected with C. neoformans (Kakeya et al. 1997, 1999). The expression of heat shock proteins appears to be a feature of growth in an animal host, and the in vitro growth conditions that we used for the SAGE libraries reflect the host conditions in this regard. The correlation between heat shock gene transcription and growth at 37°C is not absolute because we also observed one protein from the heat shock protein 12 family (HSP12) to be up-regulated at 25°C (Table 4). Interestingly, one of the highest BLASTP results for this putative C. neoformans Hsp12 showed 60% similarity with Wh11p from C. albicans; the expression of the gene for this protein is not regulated by temperature (Soll 1997). The influence of growth at 37°C on both translation elongation machinery and heat shock proteins is consistent with observations in E. coli. Farewell and Neidhardt (1998) have shown that the polypeptide elongation rate increases as a function of temperature and that the rate of elongation appears to be linked mechanistically to the heat shock response. An association between the expression of heat shock proteins and thermotolerance has also been noted in other fungal pathogens such as H. capsulatum (Caruso et al. 1987).
We also found that the collection of tags up-regulated at 37°C included genes for two proteases (carboxypeptidase D, serine protease) and a hydroxylase that may be involved in phenolic metabolism (putative salicylate hydroxylase). Our investigation of other tags not included in Table 5 also revealed that transcripts for enzymes involved in phenolic metabolism (aryl-alcohol dehydrogenase and cinnomoyl CoA reductase) were higher at 37°C (data not shown). These results indicate a relationship between growth temperature and the metabolism of phenolic compounds in C. neoformans. This may be related to the well-characterized ability of this fungus to convert diphenolic compounds into melanin (Salas et al. 1996; Casadevall and Perfect 1998).
Our results revealed that some genes predicted to encode proteins with iron as a cofactor (aconitase, ubiquinol-cytochrome C reductase) have higher transcript levels at 37°C (Table 5). In this regard, Perfect et al. (1998) found that the C. neoformans COX1 gene encoding cytochrome C oxidase subunit 1 is up-regulated in a rabbit model of infection and during a temperature shift from 30°C to 37°C. This indicates an important role for mitochondrial function in the stress response of C. neoformans, and our observations indicate a general influence of temperature on respiration and iron homeostasis in C. neoformans. In further support of an influence on iron homeostasis, we observed a tag for a predicted iron permease that was elevated at 25°C. A similar theme regarding iron homeostasis has emerged from the global analysis of the influence of temperature on transcription in group A Streptococcus (Smoot et al. 2001). As indicated above, the parallels between the responses of group A Streptococcus and C. neoformans to elevated temperature also extended to the expression of the antioxidant protein superoxide dismutase. Our examination of the influence of temperature on gene expression in C. neoformans, although at a relatively early stage, indicates that striking parallels may exist with the response of group A Streptococcus to elevated temperature.
The 37°C B3501 library also contained a putative ortholog of a peripheral benzodiazepine receptor (2.68-fold higher at 37°C). The peripheral-type benzodiazepine receptor is localized to the outer mitochondrial membrane and is important for the regulation of cholesterol transport into the mitochondria, a rate-determining step in steroid biosynthesis (Li et al. 2001). Amino acid alignments showed conservation of the cholesterol-binding motif in the cytoplasmic C-terminal domain predicted from the C. neoformans sequence (data not shown). In this context, the elevated tag level for this gene might reflect an adaptation at 37°C that involves steroid metabolism; this observation is intriguing because of the elevated transcript levels that we observed at 25°C for genes involved in sterol biosynthesis.
Tags Representing Putative Regulatory Proteins
As indicated in Figures 1 and 2, many more tags than those analyzed so far are known to be present at different levels between the two temperatures. As part of our ongoing analysis of the SAGE tags for strain B3501, we performed an initial scan for tags that may represent genes for regulatory proteins in an additional 50 tags at each temperature. Although a complete analysis is not yet possible, we did match tags with genes for several putative proteins of interest. For example, we found a tag (elevated at 37°C) for a gene with similarity to an engrailed-related gene from insects (AATGGATTAA) that functions in development (Marie and Bacon 2000). We also found tags that were elevated at 37°C for two WD repeat proteins, one of which showed similarity to the Tup1p global repressor of S. cerevisiae (CAGACGCTGT) and the other to the Pop1p protein of S. pombe (Kominami et al. 1998). The possibility that a TUP1-like gene is regulated by temperature in C. neoformans is intriguing in light of the role of a TUP1 ortholog in the filamentous growth of the fungal pathogen C. albicans (Braun and Johnson 1997). We should note, however, that a BLAST search of the C. neoformans genomic database with the Tup1p sequence of C. albicans revealed a gene with a greater level of sequence similarity than the one identified by our SAGE tag. The possibility of temperature control of a global regulator like Tup1p is interesting, however, because it has recently been shown that diploid strains of C. neoformans shows a temperature-dependent shift between budding (37°C) and filamentous growth (24°C; Sia et al. 2000). As we identify additional temperature regulated genes in our SAGE analysis, it will be possible to screen for C. neoformans orthologs of genes known to regulated by Tup1p in S. cerevisiae and C. albicans (Braun et al. 2000; Wu et al. 2001).
Confirmation of SAGE Results by RNA Blot Analysis
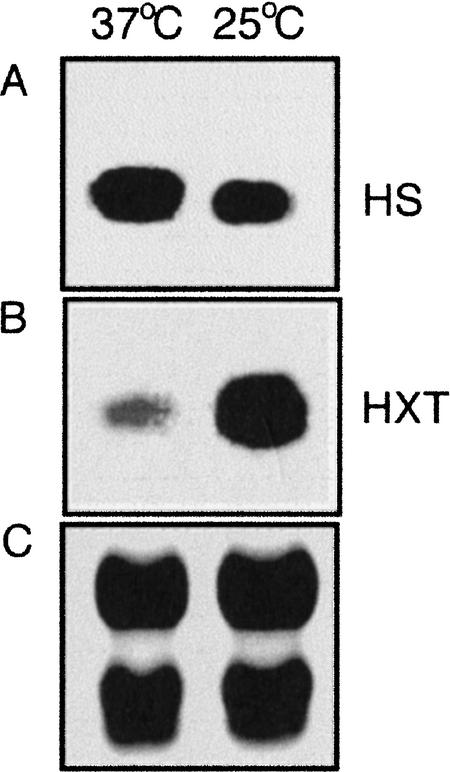
RNA blot analysis was used to confirm that the observed differences in tag levels reflected differences in transcript levels. As shown in Figure 3A, the transcript level for a putative heat shock 70 protein was found to be elevated at 37°C compared with 25°C; this result was predicted by the SAGE data, which indicated an approximately twofold higher RNA expression at 37°C. Similarly, the RNA level detected for a predicted monosaccharide transporter gene from B3501 was found to be higher at 25°C compared with 37°C, as predicted by the SAGE results (∼12-fold higher; Fig. 3B). The differential RNA levels indicated by the SAGE results were also confirmed by RNA blot analysis for eight additional genes, and all hybridization experiments were performed with two independent preparations of RNA from cells grown at the two temperatures (data not shown). Overall, the hybridization results support the conclusion that SAGE accurately identified genes with transcript levels that are influenced by temperature.
Figure 3.
RNA blot analysis of two representative temperature-regulated genes in strain B3501. The RNA was isolated from cells grown at 25°C or 37°C. (A) Hybridization with a polymerase chain reaction (PCR) amplicon from a gene for a heat shock protein 70 (tag, CATAGTTGGT) with a higher transcript level at 37°C (HS). (B) Hybridization with a PCR amplicon from a gene for a high-affinity monosaccharide transporter (tag, GGCTTGACCA) with a higher transcript level at 25°C (HXT). (C) Ribosomal RNA (18S and 28S) bands as a loading control.
Summary
This report describes the first genome-wide analysis of the temperature-regulated transcriptome of C. neoformans. The results indicate that the transcript levels for a large number of genes are influenced by growth temperature in this fungal pathogen and that differences exist in the response of different varieties. Our data indicate that the fungus may respond to temperature with a change in chromatin packaging, as indicated by the differential transcript levels for histone genes. At 37°C, the fungus responds by elevating transcript levels for heat shock proteins, translation machinery components, mitochrondrial proteins, and stress proteins such as superoxide dismutase. These results indicate that elevated temperature is a stressful condition for this fungus. It will be interesting to examine whether this pattern is reinforced by a more detailed analysis of the H99 strain because isolates of this serotype (A) are more commonly associated with infections in North America, and strains of this serotype are generally more heat tolerant (Martinez et al. 2001). The completion and annotation of the genomic sequence for C. neoformans will allow a more detailed exploration of the generalities of the differential expression described above, and allow the identification of new patterns of temperature-regulated gene expression. Finally, even at this level of analysis at which the genomes of strains H99 and B3501 are only partially characterized, we noticed significant differences between the two strains and intriguing similarities with expression patterns for group A Streptococcus in terms of connections between temperature, iron homeostasis, and the stress response. These observations may reflect a general response of pathogens to growth at host temperature. Of course, the in vitro conditions used here do not adequately mimic the host environment, and transcriptional changes that reflect the pathogen response to the host immune system and host nutritional conditions may not be identified. To address this limitation, additional SAGE experiments are underway with C. neoformans cells isolated from infected animals or grown under iron limiting conditions. Finally, the SAGE tags generated in this study will be useful for the annotation of the Cryptococcus genome, particularly in the identification of transcribed regions.
METHODS
Strains and Growth Conditions
C. neoformans serotype A, MATα strain H99 and serotype D, MATα strain B3501 were supplied by J. Heitman (Duke University) and J. Kwon-Chung (National Institutes of Health), respectively. For SAGE library construction, 2-mL cultures of yeast extract, peptone, dextrose broth were inoculated with single colonies and grown overnight at 30°C in a gyratory shaker (250 rpm). The cells from 1 mL of the culture were collected by centrifugation, washed twice with yeast nitrogen base broth, and resuspended in 1 mL of YNB buffered with 50 mM 3-[N-morpholino] propanesulfonic acid (pH 7.0). One hundred microliters of washed cells were used to inoculate 50 mL of the same medium in a sterilized 1-L Erlenmeyer flask. Cultures were grown at either 25°C or 37°C in a gyratory shaker until early log phase (OD600 ≅ 14.0). The cells for mRNA isolation were in the exponential phase of growth, and the growth rate was similar at both temperatures (data not shown). Cells were harvested by centrifugation and immediately flash frozen in a dry ice–ethanol bath.
RNA Isolation and Analysis
Frozen cell pellets were lyophilized overnight at −20°C until dry and resuspended in 15 mL of TRIZOL extraction buffer (GIBCO BRL). Total RNA was isolated according to the manufacturer's recommendations with the addition of an overnight LiCl precipitation at 4°C following the standard ethanol precipitation step. PolyA+ RNA was isolated using the MessageMaker kit (GIBCO BRL). RNA blot preparation and hybridization was performed as described (Sambrook et al. 1989). A hybridization probe was prepared for a gene encoding high-affinity monosaccharide transporter (tag, CATGGGCTTGACCA) using the primers 5′-AAGATAAGGAG TAATGACGGGCGA-3′ and 5′-CTATTGGTGAAATTTTCCCA-3′ (107-bp amplicon). The primers for the heat shock gene were 5′-ATGGTTCACCGACGTCCAGA-3′ and 5-‘GCCACC GAAATGCCTGTCAT-3′ (262-bp amplicon). These DNAs were labeled with an Oligolabeling kit (Amersham Pharmacia Biotech Inc.).
SAGE Analysis
SAGE was performed as described by Velculescu et al. (1995) using the protocol available at www.sagenet.org. Poly-A RNA was converted to double-stranded cDNA using the GIBCO BRL synthesis kit and biotinylated oligo-dT18. Briefly, the cDNA was cleaved with NlaIII, the 3′-terminal cDNA fragments were bound to streptavidin beads (Dynal), and oligonucleotide linkers containing BsmFI restriction sites were ligated to the 5′ ends. The linkered cDNA was released from the streptavidin bead by BsmFI digestion, and tags were ligated to one another, polymerase chain reaction (PCR) amplified, concatemerized, and cloned into the SphI site of pZERO 1.0 (Invitrogen). Twenty-eight PCR cycles were used to amplify ditags during library construction. Colonies were screened by PCR (M13F and M13R primers) to assess the average clone insert size and percentage of nonrecombinants. Tags were obtained by BigDye primer cycle sequencing and analysis on an ABI PRISM 3700 DNA analyzer. Sequence chromatograms were processed using Phred (Ewing and Green 1998; Ewing et al. 1998) and vector sequence detected using CROSS_MATCH (Gordon et al. 1998). Fourteen-bp tags were extracted from the vector clipped sequence, and an overall quality score for each tag was derived based on the cumulative Phred score. Duplicate di-tags and linker sequences were removed as decribed (Velculescu et al. 1995). Only tags with a predicted accuracy of ≥99% were used in this study. Statistical differences between tag abundance in different libraries was determined using the G-test (Sokal and Rohlf 1991) and the methods of Audic and Claverie (1997).
Tag Identification
To make preliminary assignments of tags to genes, we used the shotgun sequence data from the C. neoformans Genome Project (assemblies 010512 and 011005), Stanford Genome Technology Center (http://www-sequence.stanford.edu; funded by the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health under cooperative agreement AI47087) and at TIGR (http://www.tigr.org/tdb/edb2/crypt/htmls/index.shtml). A limited amount of genomic shotgun sequence data is also available for strain H99 from our BAC clone end sequencing (see accompanying paper by Schein et al. in this issue) and at the Duke University Center for Genome Technology (http://cgt.genetics.duke.edu/data/index.html). In addition, limited EST databases are available for strains JEC21 and H99 at the University of Oklahoma’s Advanced Center for Genome Technology (http://www.genome.ou.edu/cneo.html, funded under the cooperative agreement UO1 AI 485 94-01). We restricted our analysis to those genes for which an unambiguous tag assignment could be obtained either by annotation of the Stanford genomic data for JEC21 (assembly) or by analysis of ESTs from JEC21 or H99. BLASTx (basic local alignment search tool) results were recorded for those genes that had significant similarity with other proteins in the nonredundant database and National Center for Biotechnology Information (NCBI). Expect values and tentative gene assignments were recorded for those tags that were found to correspond to the 3′ most NlaIII site within the putative open reading frame or within a 3′ untranslated region. In addition, the BLASTx results were inspected individually. In some cases, we found a high Expect value when the alignment of the protein from the nonredundant database and the C. neoformans sequence showed significant identity such that the Expect value did not reflect the extent of similarity. This occurred most frequently with small proteins. Because of the presence of introns in the genomic sequence and the length of the contigs, the Expect values recorded here are much lower than those that would be found if introns were removed, sequences were translated, and BLASTp analysis was performed. For the preliminary identification of ribosomal proteins, our nomenclature followed the outlined standards for S. cerevisiae (Mager et al. 1997). It should be noted that C. neoformans genes typically have an average of 5.6 introns per gene, and this complicates unambiguous identification of the 3′ end of genes. We did note that tags were often near a putative polyadenylation signal that corresponded with the consensus sequence AAC/GAAA similar to what has been observed previously (Chaturvedi et al. 2001).
WEB SITE REFERENCES
http://cgt.genetics.duke.edu/data/index.html; genomic shotgun sequence data, Duke University Center for Genome Technology.
http://mgm.duke.edu; Duke University Department of Molecular Genetics & Microbiology
http://www.genome.ou.edu/cneo.html; EST databases for strains JEC21 and H99, University of Oklahoma's Advanced Center for Genome Technology.
http://www.ncbi.nlm.nih.gov; National Center for Biotechnology Information
http://www.sagenet.org; protocol for performing serial analysis of gene expression (SAGE).
http://www-sequence.stanford.edu; Shotgun sequence data from the C. neoformans Genome Project (assemblies 010512 and 011005), Stanford Genome Technology Center.
http://www.tigr.org/tdb/edb2/crypt/htmls/index.shtml; The Institute for Genomic Research.
Table 2A.
Top 50 Tags Expressed at 25°C for Strain H99
| SAGE tag | Frequency (30,181 total) | % Abundance | Prelimary gene designation | E-value of BLASTx | Accession no.of BLASTx |
|---|---|---|---|---|---|
| ttcagcaggc | 430 | 1.42% | Zinc transport protein | 7.00E-15 | Saccharomyces cerevisiae Z72777 |
| ctcagcgatg | 352 | 1.17% | NO HITa | ||
| cattcgcata | 309 | 1.02% | NO HITa | ||
| cgacagaccg | 222 | 0.74% | Translation elongation factor 1α | 0.00E + 00 | Cryptococcus neoformans U81804 |
| aaaaaaaaaa | 211 | 0.70% | NO HITa | ||
| atatgacata | 210 | 0.70% | NO HITa | ||
| gccaacgccg | 203 | 0.67% | Cyclophilin A | 2E-72c | C. neoformansU81804 |
| gctctccagg | 171 | 0.57% | NO HITa | ||
| catctgttcc | 171 | 0.57% | NO HITa | ||
| cgcggaaagg | 162 | 0.54% | NO HITa | ||
| tagcgatcac | 153 | 0.51% | NO HITa | ||
| tagccgcgaa | 153 | 0.51% | NO HITb | ||
| ataagctttc | 148 | 0.49% | Mannitol 1-phosphate dehydrogenase | 3.00E-18 | C. neoformansAF175685 |
| gtttccgctg | 147 | 0.49% | NO HITa | ||
| ttcggcaagg | 132 | 0.44% | ADP, ATP carrier protein | 1.40E-131 | Neurospora crassaX00363 |
| gtcggtggta | 130 | 0.43% | ATP synthase β-chain | 8.00E-59 | Kluyveromyces lactisU37764 |
| gtggacacga | 129 | 0.43% | Nucleoside diphosphate-sugar hydrolase | 4.00E-26 | S. cerevisiaeCAA85068 |
| aatgaatctt | 122 | 0.40% | NO HITa | ||
| tctggtcgag | 121 | 0.40% | Histone H4 | 2.90E-36 | Agaris bisporusP35058 |
| tcagaagttg | 121 | 0.40% | Thioredoxin | 9.00E-25 | Coprinus comatus AJ242791 |
| agcgagcact | 120 | 0.40% | NO HITa | ||
| gtattgaccc | 113 | 0.37% | Hypothetical protein | 4E-69c | Streptomyces coelicolor AL132991 |
| atgatcgggc | 108 | 0.36% | NO HITa | ||
| aaaaacgcgt | 107 | 0.35% | Myo-inositol-1-phosphate synthase | 1.00E-66 | Drosophila melanogaster AF071103 |
| catcactctt | 103 | 0.34% | Pyruvate decarboxylase | 8.0E-34c | Saccharomyces kluyveri AF193853 |
| ccgcgaccgt | 98 | 0.32% | NO HITa | ||
| gctgcctaca | 93 | 0.31% | ATP synthase—γ-chain | 5.00E-21 | N. crassaAL355930 |
| acggtggcaa | 92 | 0.30% | NO HITa | ||
| acacgtctgg | 91 | 0.30% | NO HITa | ||
| ggttacgccg | 91 | 0.30% | Malate dehydrogenase | 2.00E-35 | S. cerevisiaeJ02841 |
| gcgttctcgg | 86 | 0.28% | Transaldolase | 1.00E-102 | Schizosaccharomyces pombe AL023518 |
| actcaggttg | 83 | 0.28% | Fructose 1,6-bisphosphate aldolase | 2.00E-46 | N. crassaL42380 |
| gaatagtggg | 81 | 0.27% | NO HITa | ||
| ggccgacctg | 80 | 0.27% | 60S ribosomal protein RPL11 | 3.00E-81 | S. pombeZ69240 |
| atgcatttcg | 80 | 0.27% | NO HITa | ||
| gctcgcgacg | 77 | 0.26% | 60S ribosomal protein RPL2 | 1.00E-103 | Xenopus laevisU00920 |
| atatgtatcg | 75 | 0.25% | NO HITa | ||
| aacgtctgcc | 74 | 0.25% | NO HITa | ||
| accgtcgttg | 74 | 0.25% | NO HITa | ||
| tgcaaacgcg | 74 | 0.25% | Peroxisomal membrane protein | 6.00E-12 | S. pombeAJ002536 |
| gcgccgctta | 72 | 0.24% | NO HITa | ||
| aagcgcatttt | 71 | 0.24% | NO HITa | ||
| tagtgtcccg | 70 | 0.23% | NO HITa | ||
| aagggtggtg | 68 | 0.23% | NO HITa | ||
| aagcctgacg | 67 | 0.22% | NO HITb | ||
| aaatggtttg | 66 | 0.22% | NO HITa | ||
| catcacgctt | 64 | 0.21% | 60s ribosomal protein RPL5 | 3.00E-26 | S. pombeAL031528 |
| agcaaggagg | 63 | 0.21% | NO HITa | ||
| taacgcataa | 63 | 0.21% | NO HITb | ||
| agcaaggagg | 63 | 0.21% | NO HITa |
Serial analysis of gene expression (SAGE) tag does not have an associated expressed sequence tag (EST) at http://www.genome.ou.edu/cneo.html.
Identified EST does not have a significant BLASTx result at http://www.ncbi.nlm.nih.gov/.
BLASTx results for a contig identified at http://mgm.duke.edu.
Table 2B.
Top 50 Tags Expressed at 37°C for Strain H99
| SAGE tag | Frequency (37,467 total) | % Abundance | EST hit | E-value | Accession no.of BLASTx |
|---|---|---|---|---|---|
| cgacagaccg | 931 | 2.48% | Translation elongation factor 1α | 0.00E + 00 | C. neoformansU81804 |
| ggcctcggtt | 387 | 1.03% | NO HITSa | ||
| tccccgtaca | 330 | 0.88% | NO HITSa | ||
| gccaacgccg | 300 | 0.80% | Cyclophilin A | 2E-72c | C. neoformansU81804 |
| cacgttcacg | 276 | 0.74% | Thioredoxin peroxidase | 9.00E-64 | S. pombeAL031798 |
| aacgtctgcc | 272 | 0.73% | NO HITSa | ||
| cgcggaaagg | 264 | 0.70% | NO HITSa | ||
| gctcgcgacg | 259 | 0.69% | 60S ribosomal protein RPL2 | 1E-103 | X. laevisU00920 |
| ggccgacctg | 256 | 0.68% | 60S ribosomal protein RPL11 | 3E-81 | S. pombeZ69240 |
| gtcggtggta | 228 | 0.61% | ATP synthase β-chain | 8E-59 | Kluyveromyces lactisU37764 |
| gtttccgctg | 223 | 0.60% | NO HITSa | ||
| aagggtggtg | 204 | 0.54% | NO HITSa | ||
| aagcccgttg | 194 | 0.52% | NO HITSa | ||
| tctgtcgagg | 183 | 0.49% | 40S ribosomal protein RPS12 | 3E-41 | SusscrofaX79417 |
| gagaagcgtg | 174 | 0.46% | 60S ribosomal protein RPL21A | 4.10E-51 | S. cerevisiaeM86408 |
| ctcagcgatg | 173 | 0.46% | NO HITSb | ||
| cacggcgcat | 164 | 0.44% | 60S ribosomal protein RPL41 | 2.00E-58 | Xanthophyllomyces dendrorhous AF004672 |
| taggccgtct | 158 | 0.42% | NO HITSa | ||
| aaggactctc | 158 | 0.42% | 40S ribosomal protein RPS15 | 2.60E-42 | Podospora anserinaZ23267 |
| gctctccagg | 155 | 0.41% | NO HITSa | ||
| tctggtcgag | 152 | 0.41% | Histone H4 | 2.9E-36 | Agaris bisporusP35058 |
| tccctattaa | 151 | 0.40% | NO HITSa | ||
| cagaaccccg | 147 | 0.39% | 40s ribosomal protein RPS18 | 6.9E + 45 | S. pombeAL034564 |
| acggccgtta | 139 | 0.37% | NO HITSa | ||
| aaaaaaaaaa | 135 | 0.36% | NO HITSa | ||
| ctcttcccct | 135 | 0.36% | 60S ribosomal protein RPL33B | 9E-31 | S. cerevisiaeL23923 |
| tctttccgag | 135 | 0.36% | GAPDH | 2.80E-59 | C. neoformansAF106950 |
| gtattgaccc | 131 | 0.35% | Hypothetical protein | 4.0E-69c | Streptomyces coelicolorAL132991 |
| cacgtccacg | 131 | 0.35% | Cu,Zn superoxide dismutase | 5.5E-51 | Aspergillus fumigatusAF128886 |
| gctgcctaca | 130 | 0.35% | ATP synthase—γ-chain | 2.00E-33 | N. crassaAL355930 |
| gccgtccgaa | 130 | 0.35% | 40S ribosomal protein RPS5 | 4.40E-71 | Mus musculuU78085 |
| gctcctctta | 128 | 0.34% | ATP synthase α-chain | 1.90E-01 | N. crassaM84191 |
| tctttgatgt | 125 | 0.33% | ADP, ATP carrier protein | 1.4E-122 | N. crassaX00363 |
| tccatccgat | 123 | 0.33% | 60S ribosomal protein RPL10 | 8.90E-83 | S. cerevisiaeU06952 |
| atgatcgggc | 123 | 0.33% | NO HITSa | ||
| gctttgctgc | 122 | 0.33% | Hypothetical protein (Schizosaccharomyces pombe) | 1.80E-11 | S. pombeZ97992 |
| atgggctccc | 119 | 0.32% | ATP synthase—γ-chain | 2.9E-30 | S. pombeAL031856 |
| gacgactcta | 116 | 0.31% | NO HITSa | ||
| gagttgttga | 115 | 0.31% | 60S ribosomal protein RPL36 | 3.6E-13 | S. pombeD88771 |
| actcaggttg | 114 | 0.30% | Fructose 1,6-bisphosphate aldolase | 2.0E-46c | Aspergillus oryzaeAB032272 |
| ccgcgaccgt | 113 | 0.30% | NO HITSa | ||
| gcttttgccc | 110 | 0.29% | NO HITSa | ||
| ttcggcaagg | 107 | 0.29% | ADP, ATP carrier protein | 1.4E-131 | N. crassaX00363 |
| tcggtcgtgt | 104 | 0.28% | Suppressor protein STM1 | 0.05 | S. cerevisiaeD26183 |
| cctcttcctg | 102 | 0.27% | NO HITSa | ||
| ggttacgccg | 98 | 0.26% | Malate dehydrogenase | 2E-35 | S. cerevisiaeZ28085 |
| gcgttctcgg | 95 | 0.25% | Transaldolase | 3.30E-102 | S. pombe AL023518 |
| cgtgtcaagc | 95 | 0.25% | NO HITSa | ||
| gtcaagaagc | 95 | 0.25% | NO HITSa | ||
| ggtatcctcg | 95 | 0.25% | Putative 40S ribosomal protein | 5E-50 | S. pombe NC_003424 |
Serial analysis of gene expression (SAGE) tag does not have an associated expressed sequence tag (EST) at http://www.genome.ou.edu/cneo.html.
Identified EST does not have a significant BLASTx result at http://www.ncbi.nlm.nih.gov/.
BLASTx results for a contig identified at http://mgm.duke.edu
Table 3A.
Top 50 Tags Expressed at 25°C for Strain B3501
| SAGE tag | Frequency (65,399 total) | Percentage | Preliminary gene designation | E-value of top BLASTx result | Accession no.of BLASTx |
|---|---|---|---|---|---|
| gaacgatgct | 607 | 0.93% | NO HITSb | ||
| catttacata | 546 | 0.83% | NO HITSb | ||
| cgagtcgtat | 539 | 0.82% | Iron permease | 2E-23 | Schizosaccharomyces pombeZ67998 |
| cgacagaccg | 529 | 0.81% | Translation elongation factor 1 | 0.0/0.0c | Cryptococcus neoformansU81804 |
| aaaaaaaaaa | 452 | 0.69% | NO HITSb | ||
| gtattgaccc | 430 | 0.66% | Phosphoketolase | 1.00E-165/1.00E-106c | Lactococcus lactis AE006381 |
| aatgactttt | 427 | 0.65% | NO HITSb | ||
| gcgttacttg | 348 | 0.53% | Zinc transporter | 2E-27 | Saccharomyces cerevisiaeZ72777 |
| tctttgatgt-3′ | 328 | 0.50% | ADP, ATP carrier protein | 1.00E-110/2.00E-72c | Gossypium hirsutumAF006489 |
| gtcgtagagt | 327 | 0.50% | Enolase | 1E-131 | S. cerevisiaeJ01322 |
| atatgacata | 305 | 0.47% | Glycine dehydrogenase | 0 | S. pombeZ54308 |
| caagtaattt | 293 | 0.45% | NO HITSb | ||
| catctattcc | 286 | 0.44% | NO HITSa | ||
| ccagaagttg | 267 | 0.41% | Mitochondrial thioredoxin | 2E-39/2.00E-54c | S. cerevisiaeX59720 |
| ttcggcaagg-5′ | 264 | 0.40% | ADP, ATP carrier protein | 1.00E-115/1.00E-132c | G. hirsutumAF006489 |
| ctccgccgag | 261 | 0.40% | Pyruvate decarboxylase | 1.00E-72/3.00E-40c | Pichia stipitisU75310 |
| gctctccagg | 250 | 0.38% | Histone H3 | 1.00E-48/9.00E-64c | Mortierella alpinaAJ249812 |
| gctaacgctg | 238 | 0.36% | Cyclophilin A | 5.00E-76/2.00E-91c | C. neoformansAF333996 |
| gtcggtggta | 230 | 0.35% | ATP synthase—β-chain | 0.0/3.00E-43c | Kluyveromyces lactis U37764 |
| tcgagaatgg | 218 | 0.33% | NO HITSb | ||
| gacgatatat | 204 | 0.31% | C-4 methyl sterol oxidase | 2E-84/2.00E-48c | S. pombeAL109832 |
| cagagatgtg | 197 | 0.30% | Nonhistone protein | 1.00E-6/7.00E-9c | S. cerevisiaeZ94864 |
| tctggtcgag | 187 | 0.29% | Histone H4 | 7.00E-19/2.00E-38 | Phanerochaete chrysosporiumZ15134 |
| aggaagagaa | 186 | 0.28% | Hypothetical protein | 2.00E-22/5.00E-05c | Agaricus bisporusAJ271701 |
| cgcggaaagg | 184 | 0.28% | NO HITSa | ||
| aaatggtttg | 183 | 0.28% | NO HITSb | ||
| tagccgggaa | 182 | 0.28% | NO HITSb | ||
| tccttccgag | 179 | 0.27% | GAPDH | 1.00E-112/0.0c | C. neoformansAF106950 |
| atttccgccg | 178 | 0.27% | Serine-threonine protein kinase | 7E-65 | Mus musculusU48737 |
| cacgttcacg | 168 | 0.26% | Thioredoxin peroxidase | 2.00E-39/2.00E-64c | S. pombeAL031798 |
| ataaaaaaaa | 159 | 0.24% | NO HITSa | ||
| catattgaat | 157 | 0.24% | Uracil ribosyl transferase | 3.00E-10 | S. pombeZ98598 |
| gcagatcgat | 154 | 0.24% | 60S ribosomal protein RPL39 | 3.00E-09/1.00E-13c | K. marxianusS53434 |
| gctcctctta | 152 | 0.23% | ATP synthase—α-chain | 2.00E-58/9.00E-46c | S. pombeM57955 |
| aaagcgcgtt | 151 | 0.23% | Inositol 1-phosphate synthase | 1E-144 | Pichia pastorisAF078915 |
| agtcctcttc | 150 | 0.23% | 60S ribosomal protein RPP2 | 1.00E-15 | Alternaria alternataU87806 |
| actaccttct | 149 | 0.23% | Ribosomal protein RPP1 | 1E-13 | C. elegansAF003139 |
| ccatatgttt | 149 | 0.23% | Glycogen phosphorylase | 6.00E-95/2.00E-40c | Dictyostelium discoideumM77492 |
| actatcgcct | 142 | 0.22% | Ubiquitin conjugating enzyme | 8.00E-45/2.00E-75c | Glomerella cingulataAF030296 |
| cagcagttta | 139 | 0.21% | NO HITSb | ||
| agtggcagtt | 138 | 0.21% | Opsin | 0.004/3.00E-21c | Leptosphaeria maculansAF290180 |
| cattcgttca | 137 | 0.21% | NO HITSb | ||
| aattcgcttt | 133 | 0.20% | 14-3-3 Protein | 5.00E-84/1.00E-124c | Schizophyllum communeAY029473 |
| tagcctttcg | 127 | 0.19% | NO HITSb | ||
| cgtgaggctg | 125 | 0.19% | 6-Phosphogluconate dehydrogenase | 1.00E-170/0.0c | |
| catacaggtc | 122 | 0.19% | Glutamine synthase | 1.00E-133/1.00E-163c | A. bisporusY12704 |
| ggttacgctg | 121 | 0.19% | Mitochondrial malate dehydrogenase | 1.00E-115c | S. cerevisiaeJ02841 |
| taacgcataa | 117 | 0.18% | NO HITSb | ||
| ccggctaatg | 117 | 0.18% | NO HITSb | ||
| acatcgatct | 117 | 0.18% | 60S ribosomal protein RPL31 | 3E-25 | Cyanophora paradoxaAJ005204 |
Serial analysis of gene expression (SAGE) tag does not have an associated genomic contig at Stanford or expressed sequence tag (EST) at http://www.genome.ou.edu/cneo.html.
Identified EST or contig does not have a significant BLASTx result at http://www.ncbi.nlm.nih.gov/.
EST BLASTx result.
Table 3B.
Top 50 Tags Expressed at 37°C for Strain B3501
| SAGE tag | Frequency (15,363 total) | Percentage | Preliminary gene designation | E-value of top BLASTx result | Accession no. of BLASTx |
|---|---|---|---|---|---|
| cgacagaccg | 207 | 1.35% | Translation elongation factor 1 | 0.0/0.0c | C. neoformansU81804 |
| aggaagagaa | 125 | 0.81% | Hypothetical protein (Agaricus bisporus) | 2.00E-22/5.00E-05c | A. bisporusAJ271701 |
| aaaaaaaaaa | 119 | 0.77% | NO HITSb | ||
| gcgttacttg | 85 | 0.55% | Zinc transporter | 2.00E-27 | S. cerevisiaeZ72777 |
| ctccgccgag | 85 | 0.55% | Pyruvate decarboxylase | 1.00E-72/3.00E-40c | Pichia stipitisU75310 |
| gtcgtagagt | 82 | 0.53% | Enolase | 1E-131 | S. cerevisiaeJ01322 |
| gtcggtggta | 76 | 0.49% | ATP synthase—β chain | 0.0/3.00E-43c | K. lactisU37764 |
| ccagaagttg | 68 | 0.44% | Mitochondrial thioredoxin | 2.00E-39/2.00E-72c | S. cerevisiaeX59720 |
| aatgactttt | 68 | 0.44% | NO HITSb | ||
| atatgacata | 67 | 0.44% | Glycine dehydrogenase | 0 | S. pombeZ54308 |
| cgagtcgtat | 62 | 0.40% | Iron permease | 2E-23 | S. pombeZ67998 |
| catttacata | 57 | 0.37% | NO HITSb | ||
| ttcggcaagg-5′ | 57 | 0.37% | ADP, ATP carrier protein | 1E-115/1.00E-132c | G. hirsutumAF006489 |
| gaacgatgct | 56 | 0.36% | NO HITSb | ||
| atatgaaaga | 55 | 0.36% | NO HITSb | ||
| cgcggaaagg | 54 | 0.35% | NO HITSa | ||
| gtattgaccc | 52 | 0.34% | Phosphoketolase | 1.00E-165/1.00E-106c | Lactococcus lactisAE006381 |
| tctttgatgt-3′ | 49 | 0.32% | ADP, ATP carrier protein | 1.00E-110/2.00E-72c | Gossypium hirsutum AF006489 |
| aacgtctgcc | 45 | 0.29% | NO HITSa | ||
| attgagatgg | 44 | 0.29% | NO HITSb | ||
| atttccgccg | 43 | 0.28% | Serine-threonine protein kinase | 7E-65 | M. musculusU48737 |
| actaccttct | 42 | 0.27% | Ribosomal protein RPP1 | 1.00E-13 | C. elegansAF003139 |
| acgtaccttt | 41 | 0.27% | NO HITSb | ||
| cacaatcctt | 41 | 0.27% | Ubiquitin/ribosomal protein RPS27A fusion protein | 6.00E-36/7.00E-40c | N. crassaU01220 |
| ggccgacctg | 41 | 0.27% | Ribosomal protein RPL11 | 2.00E-56/5.00E-73c | S. pombeZ69240 |
| catctattcc | 40 | 0.26% | NO HITSa | ||
| cacgttcacg | 40 | 0.26% | Thioredoxin peroxidase | 2.00E-39/2.00E-64c | S. pombeAL031798 |
| gcattggcgt | 39 | 0.25% | ER chaperone BiP | 0.0/5.00E-20 | Aspergillus oryzaeAB030231 |
| actatcgcct | 38 | 0.25% | Ubiquitin conjugating enzyme | 8.00E-45/2.00E-75c | Glomerella cingulataAF030296 |
| gctcgcgacg | 36 | 0.23% | 60S ribosomal protein RPL2 | 2.00E-72 | D. melanogasterAF098520 |
| tccttccgag | 36 | 0.23% | Glyceraldehyde-3-phosphate dehydrogenase | 1.00E-112/0.0c | C. neoformansAF106950 |
| cctgttctcg | 36 | 0.23% | NO HITb | ||
| tctgtcgagg | 35 | 0.23% | 40S ribosomal protein RPS12 | 6.00E-36/7.00E-42c | S. pombeAL031154 |
| cattcgttca | 35 | 0.23% | NO HITb | ||
| tagcctttcg | 34 | 0.22% | NO HITb | ||
| atgggctccc | 34 | 0.22% | ATP synthase—γ-chain | 6.00E-44/9.00E-66c | S. pombeAL031856 |
| gctcctctta | 33 | 0.21% | ATP synthase—α-chain | 2.00E-58/9.00E-46c | S. pombeM57955 |
| acatcgatct | 32 | 0.21% | 60S ribosomal protein RPL31 | 3E-25 | Cyanophora paradoxaAJ005204 |
| gcagatcgat | 32 | 0.21% | 60S ribosomal protein RPL39 | 3.00E-09/1.00E-13c | K. marxianusS53434 |
| gatgcttttt | 30 | 0.20% | 60S ribosomal protein RPL19 | 9.00E-31 | S. pombeAB010048 |
| ggttacgctg | 30 | 0.20% | Mitochondrial malate dehydrogenase | 1.00E-115c | S. cerevisiaeJ02841 |
| cggtgcctgc | 30 | 0.20% | 60S ribosomal protein RPL15 | 3.00E-47/4.00E-88c | Quercus suberAJ001346 |
| aaatggtttg | 29 | 0.19% | NO HITb | ||
| gctaacgctg | 29 | 0.19% | Cyclophilin A | 5.00E-76/2.00E-91c | C. neoformansAF333996 |
| aaccgcacca | 29 | 0.19% | Peripheral benzodiazepine receptor | 6.00E-16c | Homo sapiensJE0149 |
| agtcctcttc | 28 | 0.18% | 60S ribosomal protein RPP2 | 1E-15 | Alternaria alternataU87806 |
| cagcagttta | 28 | 0.18% | NO HITSb | ||
| cacggcgcat | 27 | 0.18% | 60S ribosomal protein RPL41 | 2E-39 | C. neoformansAF118148 |
| tagccgggaa | 27 | 0.18% | NO HITSb | ||
| catagttggt | 27 | 0.18% | Heat shock protein 70 family | 0 | Malassezia sympodialisAJ428052 |
Serial analysis of gene expression (SAGE) tag does not have an associated contig at Stanford or expressed sequence tag (EST) at http://www.genome.ou.edu/cneo.html.
Identified EST does not have a significant BLASTx result at http://www.ncbi.nlm.nih.gov/.
EST BLASTx result.
Acknowledgments
We thank Jacquie Schein and Duane Smailus for contributing support for this work along with the GSC sequencing team (S. Chan, R. Guin, M. Krzywinski, R. Kutsche, C. Mathewson, P. Pandoh, A. Prabhu, J. Stott, M. Tsai, and G. Yang). We thank Jennifer Gorlach, John Perfect and Dena Toffaletti for advice on RNA isolation. We gratefully acknowledge Richard Hyman, Eula Fung, Don Rowley, and Ron Davis at the Stanford Genome Technology Center, funded by the cooperative agreement U01 AI47087; Brendan Loftus and Claire Fraser at The Institute for Genomic Research, funded by the NIAID/National Institutes of Health under cooperative agreement U01 AI48594 for access to the Cryptococcus Genome Project data; Bruce A. Roe, Doris Kupfer, Jennifer Lewis, Sola Yu, Kent Buchanan, Dave Dyer, and Juneann Murphy at the University of Oklahoma for access to the Cryptococcus neoformans cDNA Sequencing Project (strains JEC21 and H99; National Insitutes of Health-NIAID grant number AI147079); and Fred Dietrich at the Duke Centre for Genome Technology for access to the Duke University database (strain H99). This work was supported by grants from the Canadian Institutes of Health Research (to J.W.K.) and the Natural Sciences and Engineering Research Council of Canada (NSERC) Genomics Program (to S.J., J.W.K. and M.M.) and by a Scholar Award in pathogenic mycology from the Burroughs Wellcome Fund (to J.W.K.). M.M. is a Michael Smith Foundation for Health Research Biomedical Scholar.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL kronstad@interchange.ubc.ca; FAX (604) 822-6097.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.80202.
REFERENCES
- Aguilar PS, Cronan JE, Jr, de Mendoza D. A Bacillus subtilisgene induced by cold shock encodes a membrane phospholipid desaturase. J Bacteriol. 1998;180:2194–2200. doi: 10.1128/jb.180.8.2194-2200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformansmating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh AJ, Cavallo LM, Perfect JR, Heitman J. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol Microbiol. 2000;36:352–365. doi: 10.1046/j.1365-2958.2000.01852.x. [DOI] [PubMed] [Google Scholar]
- Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, Perfect JR, Kronstad JW, Heitman J. Adenylyl cyclase functions downstream of the Gα protein GPA1 and controls mating and pathogenicity in Cryptococcus neoformans. Euk Cell. 2002;1:75–84. doi: 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audic S, Claverie J-M. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- Bemis DA, Krahwinkel DJ, Bowman LA, Mondon P, Kwon-Chung KJ. Temperature-sensitive strain of Cryptococcus neoformansproducing hyphal elements in a feline nasal granuloma. J Clin Microbiol. 2000;38:926–928. doi: 10.1128/jcm.38.2.926-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- Braun BR, Head SW, Wang MX, Johnson AD. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics. 2000;156:31–44. doi: 10.1093/genetics/156.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso M, Sacco M, Medoff G, Maresca B. Heat shock 70 gene is differentially expressed in Histoplasma capsulatumstrains with different levels of thermotolerance and pathogenicity. Mol Microbiol. 1987;1:151–158. doi: 10.1111/j.1365-2958.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- Chang YC, Kwon-Chung KJ. Isolation of a third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect Immun. 1998;66:2230–2236. doi: 10.1128/iai.66.5.2230-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, Hamilton AJ, Hobby P, Zhu G, Lowry CV, Chaturvedi V. Molecular cloning, phylogenetic analysis and three-dimensional modeling of Cu, Zn superoxide dismutase (CnSOD1) from three varieties of Cryptococcus neoformans. Gene. 2001;268:41–51. doi: 10.1016/s0378-1119(01)00408-5. [DOI] [PubMed] [Google Scholar]
- Currie B, Sanati H, Ibrahim AS, Edwards JE, Casadevall A, Ghannoum MA. Sterol compositions and susceptibilities to amphotericin B of environmental Cryptococcus neoformansisolates are changed by murine passage. Antimicrob Agents Chemother. 1995;39:1934–1937. doi: 10.1128/aac.39.9.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, Heitman J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing B, Green P. Base-calling of automated sequencer traces using phred, II: Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred, I: Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- Farewell A, Neidhardt FC. Effect of temperature on in vivo protein synthetic capacity in Escherichia coli. J Bacteriol. 1998;180:4704–4710. doi: 10.1128/jb.180.17.4704-4710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botsein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Abajian C, Green P. Consed: A graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- Harrison TS. Cryptococcus neoformansand Cryptococcosis. J Infect. 2000;41:12–17. doi: 10.1053/jinf.2000.0695. [DOI] [PubMed] [Google Scholar]
- Heitman J, Allen B, Alspaugh JA, Kwon-Chung KJ. On the origins of congenic MATα and MATa strains of the pathogenic yeast Cryptococcus neoformans. Fungal Genet Biol. 1999a;28:1–5. doi: 10.1006/fgbi.1999.1155. [DOI] [PubMed] [Google Scholar]
- Heitman J, Casadevall A, Lodge JK, Perfect JR. The Cryptococcus neoformansgenome sequencing project. Mycopathologia. 1999b;148:1–7. doi: 10.1023/a:1007136602930. [DOI] [PubMed] [Google Scholar]
- Jacobson ES, Jenkins ND, Todd JM. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infect Immun. 1994;62:4085–4086. doi: 10.1128/iai.62.9.4085-4086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SJ, Riddle DL, Pouzyrev AT, Velculescu VE, Hillier L, Eddy SR, Stricklin SL, Baillie DL, Waterston R, Marra M,A. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Genome Res. 2001;11:1346–1352. doi: 10.1101/gr.184401. [DOI] [PubMed] [Google Scholar]
- Kakeya H, Udono H, Ikuno N, Yamamoto Y, Mitsutake K, Miyazaki T, Tomono K, Koga H, Tashiro T, Nakayama E, et al. A 77-kilodalton protein of Cryptococcus neoformans, a member of the heat shock protein 70 family, is a major antigen detected in the sera of mice with pulmonary cryptococcosis. Infect Immun. 1997;65:1653–1658. doi: 10.1128/iai.65.5.1653-1658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeya H, Udono H, Maesaki S, Sasaki E, Kawamura S, Hossain MA, Yamamoto Y, Sawai T, Fukuda M, Mitsutake K, et al. Heat shock protein 70 (hsp70), as a major target of the antibody response in patients with pulmonary cryptococcosis. Clin Exp Immunol. 1999;115:485–490. doi: 10.1046/j.1365-2249.1999.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami K, Ochotorena I, Toda T. Two F-box/WD-repeat proteins Pop1 and Pop2 form hetero- and homo-complexes together with cullin-1 in the fission yeast SCF (Skp1-Cullin-1-F-box) ubiquitin ligase. Genes Cells. 1998;3:721–735. doi: 10.1046/j.1365-2443.1998.00225.x. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung KJ, Bennett JE. Cryptococcosis. In: In: Kwong-Chung KJ, Bennett JE, editors. Medical mycology. Malvern, PA: Lea & Febiger; 1992. pp. 397–446. [Google Scholar]
- Kwon-Chung KJ, Rhodes JC. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung KJ, Edman JC, Wickes BL. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun. 1992;60:602–605. doi: 10.1128/iai.60.2.602-605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Park JW. Thermosensitive phenotype of yeast mutant lacking thioredoxin peroxidase. Arch Biochem Biophys. 1998;359:99–106. doi: 10.1006/abbi.1998.0896. [DOI] [PubMed] [Google Scholar]
- Li H, Yao Z-X, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los DA, Ray MK, Murata N. Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis sp. PCC6803. Mol Microbiol. 1997;25:1167–1175. doi: 10.1046/j.1365-2958.1997.5641912.x. [DOI] [PubMed] [Google Scholar]
- Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casadevall A, Perfect JR, Hannun YA, Del Poeta MM. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 2001;15:201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager WH, Planta RJ, Ballesta J-PG, Lee JC, Mizuta K, Suzuki K, Warner JR, Woolford J. A new nomenclature for the cytoplasmic ribosomal proteins of Saccharomyces cerevisiae. Nucl Acids Res. 1997;25:4872–4875. doi: 10.1093/nar/25.24.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca BL, Carratu L, Kobayashi GS. Morphological transition in the human fungal pathogen Histoplasma capsulatum. Trends Microbiol. 1994;2:110–114. doi: 10.1016/0966-842x(94)90596-7. [DOI] [PubMed] [Google Scholar]
- Marie B, Bacon JP. Two engrailed-related genes in cockroach: Cloning, phylogenetic analysis, expression and isolation of splice variants. Dev Genes Evol. 2000;210:436–448. doi: 10.1007/s004270000082. [DOI] [PubMed] [Google Scholar]
- Martinez LR, Garcia-Rivera J, Casadevall A. Cryptococcus neoformans var. neoformans (serotype D) strains are more susceptible to heat than C. neoformans var. grubii(serotype A) strains. J Clin Microbiol. 2001;39:3365–3367. doi: 10.1128/JCM.39.9.3365-3367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G, Painter A, Kobayashi GS. Mycelial to yeast phase transitions of the dimorphic fungi Blastomyces dermatitidis and Paracoccidiodes brasiliensis. J Bacteriol. 1987;169:4055–4060. doi: 10.1128/jb.169.9.4055-4060.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Mio T, Nagahashi S, Kokado M, Arisawa M, Aoki Y. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect Immun. 2000;68:6712–6719. doi: 10.1128/iai.68.12.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk JD, Rosas AL, Lee C, Casadevall A. Melanization of Cryptococcus neoformansin human brain tissue. Lancet. 2000;355:2049–2050. doi: 10.1016/S0140-6736(00)02356-4. [DOI] [PubMed] [Google Scholar]
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR, Wong B, Chang Y, Kwon-Chung KJ, Williamson PR. Cryptococcus neoformans: Virulence and host defenses. Med Mycol. 1998;36:79–86. [PubMed] [Google Scholar]
- Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schein JE, Tangen KL, Chiu R, Shin H, Lengeler KB, MacDonald WK, Bosdet I, Heitman J, Jones SJM, Marra MA, et al. Physical maps for genome analysis of serotype A and D strains of the fungal pathogen Cryptococcus neoformans. Genome Res. 2002;12:1445–1453. doi: 10.1101/gr.81002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwelnus W, Richert K, Opitz F, Gross T, Habara Y, Tani T, Kaufer NF. Fission yeast Prp4p kinase regulates pre-mRNA splicing by phosphorylating a non–SR-splicing factor. EMBO Rep. 2001;2:35–41. doi: 10.1093/embo-reports/kve009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia RA, Lengeler KB, Heitman J. Diploid strains of the pathogenic basidiomycete Cryptococcus neoformansare thermally dimorphic. Fungal Genet Biol. 2000;29:153–163. doi: 10.1006/fgbi.2000.1192. [DOI] [PubMed] [Google Scholar]
- Smoot LM, Smoot JC, Graham MR, Somerville GA, Sturdevant DE, Lux Migliaccio CA, Sylva GL, Musser JC. Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc Natl Acad Sci. 2001;98:10416–10421. doi: 10.1073/pnas.191267598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York, NY: W.H. Freeman; 1981. [Google Scholar]
- Soll DR. Gene regulation during high-frequency switching in Candida albicans. Microbiology. 1997;143:279–288. doi: 10.1099/00221287-143-2-279. [DOI] [PubMed] [Google Scholar]
- Steels EL, Learmonth RP, Watson K. Stress tolerance and membrane lipid unsaturation in Saccaromyces cerevisiaegrown aerobically or anaerobically. Microbiology. 1994;140:569–576. doi: 10.1099/00221287-140-3-569. [DOI] [PubMed] [Google Scholar]
- Storlazzi A, Maresca B, Gargano S. cAMP is involved in transcriptional regulation of Δ9-desaturase during Histoplasma capsulatummorphogenesis. Mol Cell Biol Res Commun. 1999;2:172–177. doi: 10.1006/mcbr.1999.0169. [DOI] [PubMed] [Google Scholar]
- Velculescu VE, Zhang L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- Velculescu VE, Zhang L, Zhou W, Vogelstein J, Basrai MA, Bassett DE, Jr, Hieter P, Vogelstein B, Kinzler KW. Characterization of the yeast transcriptome. Cell. 1997;88:243–251. doi: 10.1016/s0092-8674(00)81845-0. [DOI] [PubMed] [Google Scholar]
- Vincent VL, Klig LS. Unusual effect of myo-inositol on phospholipid biosynthesis in Cryptococcus neoformans. Microbiology. 1995;141:1829–1837. doi: 10.1099/13500872-141-8-1829. [DOI] [PubMed] [Google Scholar]
- Wang Y, Aisen P, Casadevall A. Cryptococcus neoformansmelanin and virulence: Mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Cardenas ME, Cox GM, Perfect JR, Heitman J. Two cyclophilin A homologs with shared and distinct functions important for growth and virulence of Cryptococcus neoformans. EMBO Rep. 2001;2:511–518. doi: 10.1093/embo-reports/kve109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickes BL, Mayorga ME, Edman U, Edman JC. Dimorphism and haploid fruiting in Cryptococcus neoformans: Association with the α-mating type. Proc Natl Acad Sci. 1996;93:7327–7331. doi: 10.1073/pnas.93.14.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Suka N, Carlson M, Grunstein M. TUP1 utilizes histone H3/H2B-specific HDA1 deacetylase to repress gene activity in yeast. Mol Cell. 2001;7:117–126. doi: 10.1016/s1097-2765(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Wyrick JJ, Holstege FCP, Jennings EG, Causton HC, Shore D, Grunstein M, Lander ES, Young RA. Chromosomal landscape of nucleosome-dependent gene expression and silencing in yeast. Nature. 1999;402:418–421. doi: 10.1038/46567. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]