Abstract
The goal of this study was to determine the efficacy of local IL-1Ra gene therapy by intra-articular plasmid injections on structural changes in the meniscectomy rabbit model of osteoarthritis. A partial meniscectomy of the right knee was performed on the rabbits through a medial parapatellar incision. The rabbits were then divided into four experimental groups. Group 1 received no treatment. Group 2 received three consecutive intra-articular injections at 24-hour intervals of 0.9% saline containing a lipid, γAP-DLRIE/DOPE, and a DNA plasmid, VR1012. Group 3 received three consecutive injections of saline containing 1000 μg of canine IL-1Ra plasmid and lipid. The injections were given starting 4 weeks post-surgery. Rabbits from Group 1 were killed 4 weeks post-surgery, and all other rabbits 8 weeks post-surgery. The severity of macroscopic and microscopic changes on cartilage on the medial and femoral condyles and tibial plateaus and synovium were graded separately. Specimens were also processed for immunohistochemical staining using a rabbit polyclonal antibody against canine IL-1Ra. The level of canine IL-1Ra in synovial fluid was determined using enzyme-linked immunosorbent assay. The presence of the DNA plasmid in the synovium was tested by polymerase chain reaction. A significant reduction in the width of osteophytes and size of macroscopic lesions (P < 0.04) was observed, and was dependent on the amount of IL-1Ra plasmid injected. A significant reduction was also noted in the severity of histologic cartilage lesions (P < 0.01) in the group that received the highest dosage (1000 μg) of IL-1Ra plasmid. IL-1Ra was detected in synovial fluid by enzyme-linked immunosorbent assay and by immunohistochemical staining in the synovium and cartilage of rabbits that received injections containing the IL-1Ra plasmid. Polymerase chain reaction analysis of synovial DNA revealed the presence of the cloned cDNA dog IL-1Ra up to 4 weeks after the first intra-articular injection. This study demonstrates that direct in vivo transfer of the IL-1Ra gene into osteoarthritis knee cells using intra-articular injections of a plasmid vector and lipids can significantly reduce the progression of experimental osteoarthritis. This avenue may therefore represent a promising future treatment for osteoarthritis.
Morphological changes observed in osteoarthritis (OA) include cartilage erosion as well as a variable degree of synovial inflammation. 1,2 Current research attributes these changes to a complex network of biochemical factors, including proteolytic enzymes, that lead to a breakdown of the cartilage macromolecules. 1 Proinflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor α (TNF-α), locally produced by the inflamed synovium, also likely contribute to these alterations. 2,3 Moreover, in OA synovium, a relative deficit in the production of natural IL-1 receptor antagonists (IL-1Ra) has been demonstrated, and could be related to an excess production of nitric oxide in OA tissues. 4,5 This, coupled with an up-regulation in the receptor level, has been shown to be an additional enhancer of the catabolic effect of IL-1 in this disease. 6,7 These findings, therefore, strongly support the rationale for developing anti-IL-1 therapeutic strategies for the treatment of OA.
Several studies illustrate the potential importance of modulating IL-1 activity as a means to reduce the progression of the structural changes in OA. Several in vitro studies have demonstrated that the use of IL-1Ra can reduce the degradation of cartilage induced by IL-1. 8-10 An in vivo study has shown that intra-articular injections of IL-1Ra can retard the progression of experimental OA. 11 More recently, the development of gene therapy has provided several new methods to control the activity of IL-1. The IL-1Ra gene has been transduced in vitro in synovial cells using a retrovirus, MFG. 12 This gene has also been successfully transduced to articular chondrocytes using an adenovirus, rendering the cartilage resistant to IL-1-induced degradation. 13 In the experimental dog model of OA, we have demonstrated that in vivo intraarticular injections of autologous synovial cells transduced with the human IL-Ra gene using the MFG retrovirus, or injecting synovial cells transduced ex vivo with the human IL-1Ra coding sequence of the gene, 14 can prevent the progression of structural changes in OA.
In the near future, gene therapy in OA may become the vehicle for intra-articular protein delivery. Traditional methods of drug delivery have many pitfalls: targeting difficulty, side effects, short-lasting efficacy, need for frequent administration, and, most importantly, unsuitability of delivering proteins as drugs. 15-17 Gene therapy, on the other hand, presents no targeting difficulties once the gene is in place and has minimal potential side effects and long-lasting therapeutic effects. However, use of a viral vector for the transfer of genes in a chronic nonfatal disease such as OA has obvious limitations. 15 The development of a direct in vivo method that uses a nonviral vector would present many advantages.
Our goal was to determine the efficacy of in vivo IL-1Ra gene therapy through the direct delivery of a lipoplex (plasmid complexed to a lipid) in a rabbit model of OA, as a new therapeutic approach to control the progression of the disease.
Materials and Methods
Surgery
Twenty-eight white New Zealand rabbits weighing 2.5 to 3 kg each were used in this study. Partial medial meniscectomy of the right knee was performed through a medial parapatellar incision on all rabbits as previously described. 18-20 Briefly, rabbits were anesthetized with ketamine (30 mg/kg, Wyeth-Ayerst, Montréal, QC, Canada), zylazine (5 mg/kg, Bayer, Etobicoke, ON, Canada) and acepromazine maleate (1 mg im; Wyeth-Ayerst). Under sterile conditions, an anteromedial incision of the right knee was done. The subcutaneous tissue and retinaculum were incised and retracted, along with the articular capsule. The medial compartment was visualized and the peripheral capsular insertion of the medial meniscus was dissected. The anterior half of the meniscus was resected and the capsule, the medial retinaculum, and the skin were sutured. All rabbits were housed in regular individual cages and exercised and fed ad libitum.
Experimental Groups
The rabbits were divided into four experimental groups. Rabbits in Group 1 (n = 8) received no treatment. Those in Group 2 (n = 8) received three intra-articular injections at 24-hour intervals of 0.9% saline (0.5 ml), lipid (GAP-DLRIE/DOPE, a gift of Dr. Carl Wheeler, Vical, San Diego, CA) and VR1012 plasmid (a gift of Dr. Peter Hobart, Vical). Rabbits in Group 3 (n = 8) received three injections at 24-hour intervals of 0.5 ml saline containing 500 μg canine IL-1Ra in VR1012 plasmid DNA and lipid. Rabbits in Group 4 (n = 4) received three injections at 24-hour intervals of 0.5 ml saline containing 1000 μg canine IL-1Ra plasmid and lipid. All injections were given beginning four weeks postsurgery.
Macroscopic Grading
Immediately after sacrifice, the right knees of the rabbits were dissected on ice and cartilage was examined for gross morphologic lesions as previously described. 11,21 The cartilage changes on the medial and lateral femoral condyles and tibial plateaus were graded separately under a dissecting microscope (Stereozoom, Bausch & Lomb, Rochester, NY). The depth of erosion was graded on a scale of 0 to 4 as follows: grade 0 = normal surface; grade 1 = minimal fibrillation or a slight yellowish discoloration of the surface; grade 2 = erosion extending into the superficial or middle layers only; grade 3 = erosion extending into the deep layers; grade 4 = erosion extending to subchondral bone. The surface area changes were measured and expressed in mm2. The severity of OA lesions was graded through independent double-blind observation (JF, JPP).
Histologic Grading
Histologic evaluation was performed on sagittal sections of cartilage from the lesional areas of each medial femoral condyle and tibial plateau. 11,21 Following dissection, specimens were fixed in 10% buffered formalin and embedded in paraffin. Serial sections (5 μm) were prepared and stained with safranin-O. The severity of OA lesions was graded on a scale of 0–14 by through double-blind observation (JF, JPP), using the histologic-histochemical scale of Mankin et al. 22 This scale evaluates the severity of OA lesions based on loss of safranin-O staining (scale, 0–4), cellular changes (scale, 0–3), invasions of tidemark by blood vessels (scale, 0–1), and structural changes (scale, 0–6, where 0 = normal cartilage structure and 6 = cartilage erosion down to the subchondral bone). The scoring system is based on the most severe histologic changes in the multiple sections.
Representative specimens of synovial membrane were dissected from underlying tissues. The specimens were fixed, embedded, sectioned (5 μm) as above, and stained with hematoxylin and eosin. Two synovial membrane specimens were examined for scoring purposes and the higher score from each was retained. The average was calculated and considered as a unit for the whole knee. The severity of synovitis was graded on a scale of 0 to 10 23 through double-blind observation (JF, JPP) adding the scores of three histologic criteria: synovial lining cell hyperplasia (scale, 0–2), villous hyperplasia (scale, 0–3), and degree of cellular infiltration by mononuclear and polymorphonuclear cells (scale, 0–5), with 0 indicating normal structure.
IL-1Ra ELISA
The level of dog IL-1Ra in synovial fluid was determined as previously described 14 using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Quantikine, R&D Systems, Minneapolis, MN). For the synovial fluid, 200 μl was tested according to the aforementioned instructions. The limit of detection of the assay is 10 pg/ml, and the one used in this study does not recognize rabbit IL-1Ra.
Immunohistochemical Analysis
Cartilage and synovial membrane specimens were processed for immunohistochemistry as previously described 24,25 with modifications. Sections (5 μm) of paraffin-embedded specimens were placed on slides, deparaffinized in benzene, hydrated in a graded series of ethanol, rinsed in phosphate-buffered saline (PBS; 10 mmol/L sodium phosphate, pH 7.5, 0.9% saline), incubated with chondroitinase ABC (0.25 U/ml in PBS; Sigma Chemical, St. Louis, MO) for 1 hour at 37°C, and washed in PBS. Incubation with 0.3% Triton X-100 (30 minutes) was followed by a quenching of the peroxidases with 2% H2O2 in PBS for 30 minutes at room temperature. Slides were further incubated with 3% bovine serum albumin BSA-PBS (Gibco BRL, Life Technologies, Gaithersburg, MD) and 0.3% Triton X-1000 for 3 hours at room temperature, then blotted and overlaid with the following primary polyclonal goat IgG antibodies (mAb) against canine IL-1Ra (R&D Systems). The specificity of the antibody toward the dog protein was verified by Western immunoblot and the characteristic bands were found. Moreover, no cross-reactivity of the antibody with the rabbit IL-1Ra was found (personal observation).
Slides were incubated in a humidified chamber for 18 hours at 4°C with 5 μg/ml for IL-1Ra, stained using an IgG antigoat antibody (dilution 1:1500; R & D Systems), then washed twice in PBS, and the color developed by 3-3′ diaminobenzidine tetrahydrochloride (DAB; Dako, Inc., Mississauga, ON, Canada). Slides were mounted using Gel/Mount (Fisher Scientific, Montréal, QC, Canada) and each section was examined under light microscopy (Leitz Diaplan, Leitz Canada, Ville St. Laurent, QC, Canada). Photographs were taken with Kodak Ecktachrome ASA 60 film.
Control and verification of the specificity of the immune reaction were done as follows: 24,25 (i) omission of the primary antibody; (ii) substitution of the primary antibody with preimmune goat IgG, following the same experimental protocol; and (iii) absorption of the immune serum with rhIL-1Ra (3 hours at 22°C) with a 20× equivalent of the purified antigen.
DNA Extraction from Synovial Membranes
The total DNA was extracted from the synovial membranes of rabbits in Groups 1 (n = 3) and 4 (n = 4) by first mincing the tissues with sterile razor blades. Each sample was processed as previously described. 26 Basically, the minced tissues were incubated overnight at 65°C in a solution containing 0.5 mg/ml proteinase K (Gibco BRL, Life Technologies) dissolved in 50 mmol/L Tris, pH 8.0, 100 mmol/L EDTA, 100 mmol/L NaCl, 1% sodium dodecyl sulfate. The samples were extracted three times with buffered phenol and then chloroform. Total DNA was precipitated with an equal volume of isopropanol at room temperature and centrifuged. DNA pellets were resuspended in sterile water and quantified spectrophotometrically.
DNA Construct of Dog IL-1Ra
The plasmid was constructed by cloning a polymerase chain reaction (PCR)-generated dog IL-1Ra fragment into the vector VR1012 (Vical). The PCR fragment contained only the open reading frame of the gene, and was generated with the following two primers: 5′-ATCGATGATATCCCACCATGGAAACCTGCAGGTGTCCT-3′ (sense primer) and 5′-TCAAGTAGATCTCTATTATTCCTTCTGGAAGTA-3′ (antisense primer). The sense primer contained an EcoRV recognition site and the antisense primer a BglII recognition site at their respective 5′- ends to facilitate the cloning of the fragment. The sequence of the primers was derived from the sequence of the whole canine cDNA clone (provided by Dr. G. Fuller, University of Alabama, Birmingham, AL).
The PCR fragment was digested with EcoRV and BglII, and cloned into the vector VR1012 predigested with EcoRV and BglII. This construct was transformed into Escherichia coli. Plasmid DNA was extracted and sequenced to confirm that the IL-1Ra open reading frame was cloned in its entirety and properly oriented.
PCR Assays
The PCR assays were carried out in the DNA thermal cycler 480 (Perkin Elmer, Foster City, CA), using 1 μg of total DNA as template in a reaction mixture consisting of IX PCR buffer (Perkin Elmer), 2 mmol/L MgCl2, 0.2 mmol/L of each dNTPs, 2.5 units of Taq DNA polymerase (Gibco BRL, Life Technologies), 0.2 μmol/L of sense and antisense primers in a total volume of 100 μl. The reactions were amplified for 35 cycles, each consisting of a denaturation step at 94°C for 1 minute and an annealing/denaturing step at 60°C for 1 1/2 minutes; in the last cycle, the elongation step was extended to 7 minutes.
The sequences of the primers (5′-TGCAAGCCTTCAGAATCTGG-3′ sense, 127–146 bp, and 5′-TCGTCCTCCTGGAAGTAGAA-3′ antisense, 540–553 bp) were derived from the IL-1Ra cDNA sequence 27 and the size of the specific amplified fragment was 427 bp. The primers are conserved between the dog and human genes and span introns, so that amplification from the genomic DNA instead of the transfected cloned cDNA would be detected. The amplified DNA were electrophoresed in 1.2% agarose gels and stained with ethidium bromide. The identity of the fragments amplified from the tissues was tested by digestion with enzymes with known sites in the amplified fragment.
Statistical Analysis
Mean values and SE were calculated and statistics were analyzed using Student’s t-test with P < 0.05 considered significant.
Results
Macroscopy
Small osteophytes, along with femoral condylar ridges, were present in all rabbit specimens (Figure 1) ▶ . The osteophytes were much larger in the 8-week as compared to the 4-week OA rabbits (Table 1) ▶ . The width of the osteophytes in the rabbits treated with the IL-1Ra plasmid was smaller. The grade and size of cartilage macroscopic lesions showed more severe lesions on the 8-week as compared to the 4-week rabbits. The progression of lesions during this period was more pronounced on the condyles (Table 2 ▶ , Figure 2 ▶ ); however, by 8 weeks post-surgery, the lesions were of similar grade and size on medial condyles and plateaus. In rabbits treated with intraarticular injections of lipoplex containing IL-1Ra plasmid, there was a visible trend for macroscopic lesions to be less severe. The reduction in severity of lesions was dose-dependent, with the most marked and significant (P < 0.04) decrease observed on the femoral condyles of rabbits treated with the highest dosage of the plasmid (1000 μg; Table 2 ▶ ).
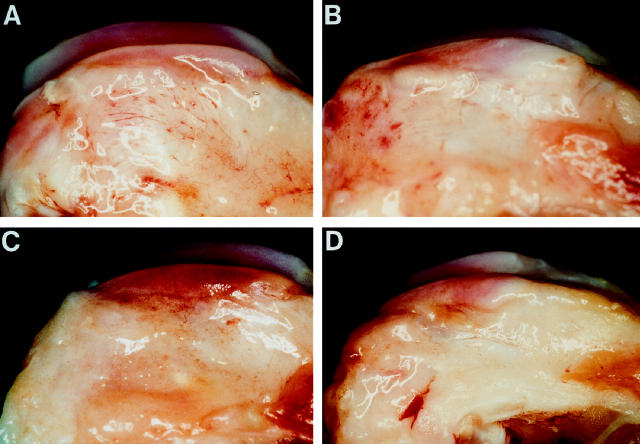
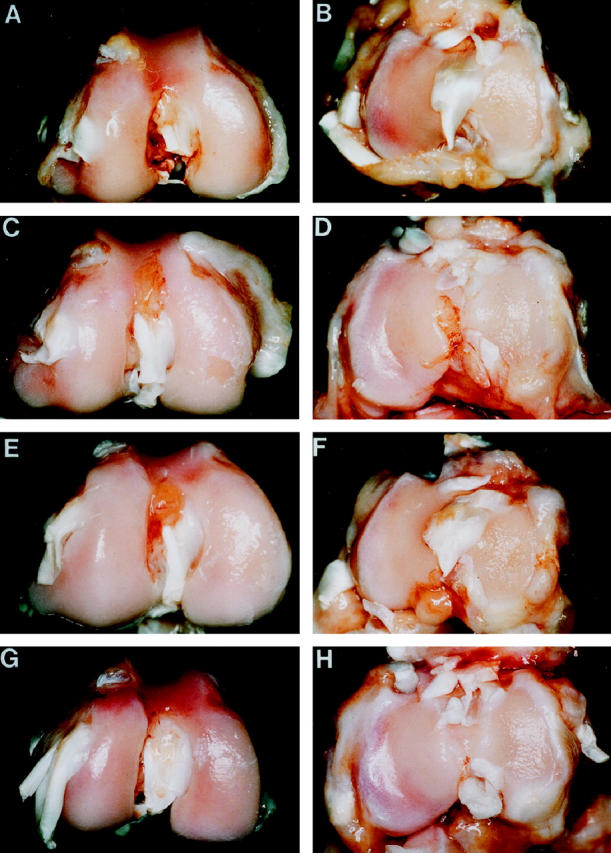
Figure 1.
Femoral condyles from (A) a 4-week OA rabbit; (B) an 8-week OA rabbit injected with the control plasmid; (C) an 8-week OA rabbit treated with 500 μg of IL-1Ra plasmid; (D) an 8-week OA rabbit treated with 1000 μg of IL-1Ra plasmid. Note the presence of osteophyte formations along the condylar ridge of OA rabbits killed at either 4 weeks or 8 weeks postsurgery. The osteophytes were smaller in rabbits injected with the IL-1Ra plasmid.
Table 1.
Osteophyte Incidence on Femoral Condyles in Rabbits
| Group | No. of animals | Osteophyte Size (mm) |
|---|---|---|
| 1, OA* 4 weeks | 8 | 0.16 ± 0.08 |
| 2, OA† 8 weeks | 8 | 0.63 ± 0.24 |
| 3, IL-1Ra 500 μg‡ 8 weeks | 4 | 0.25 ± 0.16 |
| 4, IL-1Ra 1000 μg§ 8 weeks | 8 | 0.41 ± 0.17 |
*Rabbits were killed and tissue examined at 4 weeks post-medial meniscectomy. No treatment was given to these rabbits.
†Rabbits received intra-articular injections for 3 consecutive days beginning 4 weeks post-medial meniscectomy. The preparation contained 1000 μg of the vector VR1012 complexed to the lipid gAP-DLRIE-DOPE in 700 μl of 0.9% sterile saline as vehicle. Rabbits were killed and tissue examined 8 weeks after medial meniscectomy.
‡Rabbits received intraarticular injections for 3 consecutive days beginning at 4 weeks post-medial meniscectomy. The preparation contained 500 μg of plasmid DNA (Vector VR1012) containing the coding sequence of the canine IL-1Ra gene complexed to the lipid gAP-DLRIE-DOPE (500 μg) in 700 μl sterile 0.9% saline. Rabbits were killed and tissue examined 8 weeks after surgery.
§Rabbits received intra-articular injections for 3 consecutive days beginning at 4 weeks post-medial meniscectomy. The preparation contained 1000 μg of plasmid DNA (Vector VR1012) containing the coding sequence of the canine IL-1Ra gene complexed to the lipid gAP-DLRIE-DOPE (1000 μg) in 700 μl sterile 0.9% saline as vehicle. Rabbits were killed and tissue examined 8 weeks after surgery.
Table 2.
Cartilage Macroscopic Lesions on Femoral Condyles and Tibial Plateaus in Rabbits
| Group | No. of animals | Medial Femoral Condyles | Medial Tibial Plateaus | ||
|---|---|---|---|---|---|
| Size (mm2) | Grade (0–4 scale) | Size (mm2) | Grade (0–4 scale) | ||
| 1, OA* 4 weeks | 8 | 7.88 ± 1.51 | 1.12 ± 0.13 | 16.88 ± 1.83 | 1.12 ± 0.13 |
| 2, OA† 8 weeks | 8 | 17.88 ± 3.48 | 1.62 ± 0.26 | 19.88 ± 2.61 | 1.75 ± 0.25 |
| 3, IL-1Ra 500 μg‡ 8 weeks | 4 | 11.50 ± 2.90 | 2.00 ± 0.41 | 17.50 ± 4.06 | 1.50 ± 0.29 |
| 4, IL-1Ra 1000 μg§ 8 weeks | 8 | 9.25 ± 1.75 (P < 0.04)¶ | 1.44 ± 0.18 | 14.62 ± 2.19 | 1.31 ± 0.16 |
*Rabbits were killed and tissue examined at 4 weeks post-medial meniscectomy. No treatment was given to these rabbits.
†Rabbits received intra-articular injections for 3 consecutive days beginning 4 weeks after surgery. The preparation contained 1000 μg of the vector VR1012 complexed to the lipid gAP-DLRIE-DOPE in 700 μl of 0.9% sterile saline as vehicle. Rabbits were killed and tissue examined at 8 weeks post medial meniscectomy.
‡Rabbits received intra-articular injections for 3 consecutive days beginning 4 weeks post medial meniscectomy. The preparation contained 500 μg of plasmid DNA (Vector VR1012) containing the coding sequence of the canine IL-1Ra gene complexed to the lipid gAP-DLRIE-DOPE (500 μg) in 700 μl of sterile 0.9% saline. Rabbits were killed and tissue examined 8 weeks after surgery.
§Rabbits received intra-articular injections for 3 consecutive days beginning at 4 weeks post medial meniscectomy. The preparation contained 1000 μg of plasmid DNA (Vector VR1012) containing the coding sequence of the canine IL-1Ra gene complexed to the lipid gAP-DLRIE-DOPE (1000 μg) in 700 μl of sterile 0.9% saline as vehicle. Rabbits were killed and tissue examined 8 weeks after surgery.
¶Statistics were analyzed using the Mann-Whitney U-test; comparison was made with the 8-week OA group. P values < 0.05 were considered significant.
Figure 2.
Macroscopic appearance of cartilage from femoral condyles (left panels) and tibial plateaus (right panels) of (A,B) a 4-week OA rabbit showing pitting of the central, weight-bearing region of the medial condyles and plateaus; (C,D) an 8-week OA rabbit injected with the control plasmid showing erosion and pitting of the medial condyle and plateau; (E,F) an 8-week OA rabbit treated with 500 μg of IL-1Ra plasmid showing pitted areas of cartilage on medial condyle and plateau; (G,H) an 8-week rabbit treated with 1000 μg of IL-1Ra plasmid showing pitted areas of cartilage on the medial condyle and plateau resembling those of a 4-week OA rabbit.
Microscopy
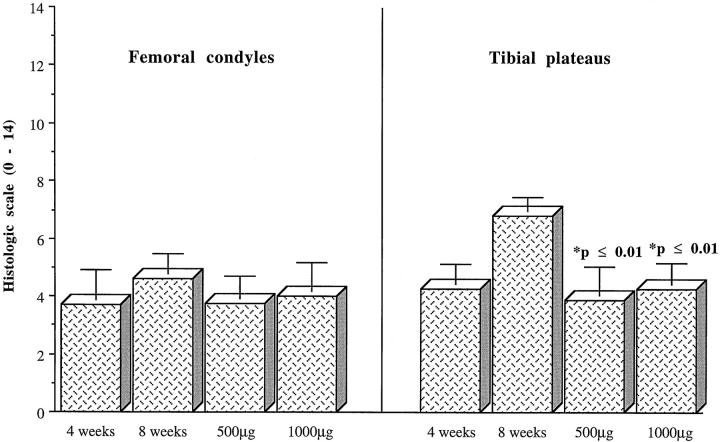
The severity of histologic lesions was greater in the 8-week as compared to the 4-week rabbits, the lesions being slightly more severe on the medial plateaus (Figures 3 and 4) ▶ ▶ . There was a noticeable decrease in the severity of lesions on both the medial condyles and plateaus in rabbits treated with IL-1Ra plasmid, a reduction found to be statistically significant on the medial plateaus of rabbits treated with the highest dosage of IL-1Ra (Table 2) ▶ .
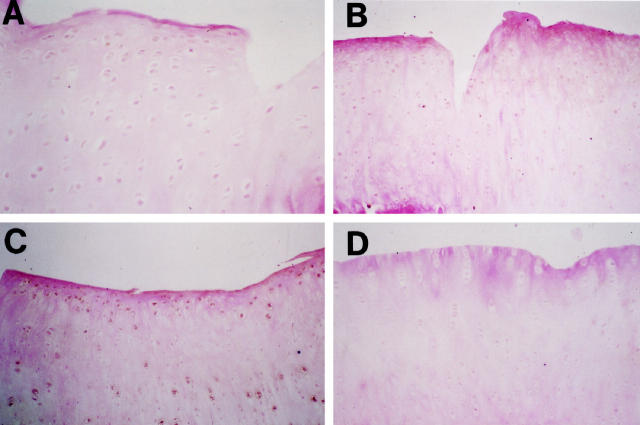
Figure 3.
Representative sections of articular cartilage from a medial femoral condyle (left panels) and tibial plateau (right panels) of (A,B) a 4-week OA rabbit; (C,D) an 8-week OA rabbit injected with the control plasmid; (E,F) an 8-week OA rabbit treated with 500 μg IL-1Ra plasmid; (G,H) an 8-week rabbit treated with 1000 μg IL-1Ra plasmid. safranin-O staining. Original magnification, ×250 (A, C, F), ×100 (B, D, E, G, H).
Figure 4.
Histologic grading of cartilage from femoral condyles and tibial plateaus of OA dogs. Values are the mean (± SE) total score (Mankin scale) of lesions from 4-week OA rabbits; 8-week OA rabbits injected with the control plasmid; 8-week OA rabbits treated with 500 μg of IL-1Ra plasmid; 8-week OA rabbits treated with 1000 μg of IL-1Ra plasmid. P values were calculated using the two-tailed Student’s t-test and compared to the 8-week OA group.
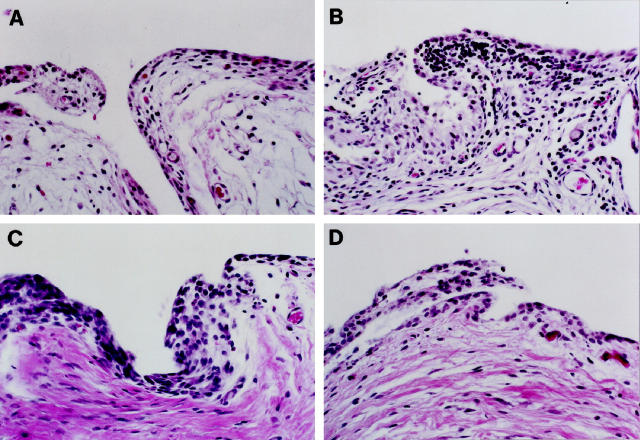
Histological scores for severity of synovial inflammation were similar in all four groups of rabbits with a moderate degree of inflammatory reaction seen in all specimens (Figure 5) ▶ . The reaction was characterized by synovial hyperplasia and hyperthrophia with a mixed mononuclear cell infiltration in the sublining tissue. No invading neutrophils were observed.
Figure 5.
Representative sections of synovium from (A) a 4-week OA rabbit and (B) an 8-week OA rabbit injected with the control plasmid, (C) an 8-week OA rabbit treated with 500 μg of IL-1Ra plasmid and, (D) an 8-week OA rabbit treated with 1000 μg of IL-1Ra plasmid. Hematoxylin and eosin staining; original magnification, ×250.
Synovial Fluid IL-Ra Levels
A detectable level of dog IL-1Ra was found in only two of the eight synovial fluid samples obtained from rabbits treated with the highest dosage of IL-1Ra plasmid (1000 μg); the level was 35.7 ± 20.7 pg/ml (mean ± SE).
Immunohistochemistry
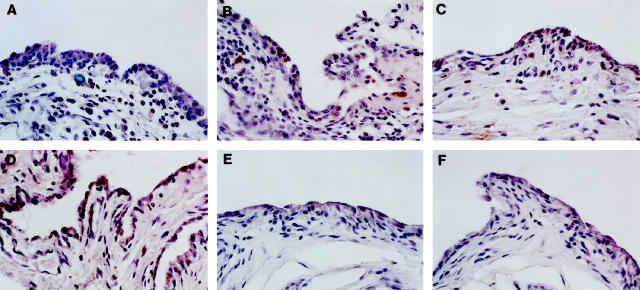
Immunohistochemistry demonstrated the presence of dog IL-1Ra protein only in the synovial membrane specimens of the rabbits given IL-1Ra plasmid (Figure 6) ▶ . Canine IL-Ra could not be detected in the specimens of rabbits transfected with the control vector or in the untreated OA rabbits. Positive staining was noted in 7 of the 8 rabbits injected with 1000 μg of IL-1Ra plasmid, and in 2 of 4 specimens injected with 500 μg of IL-1Ra plasmid. The specific staining was observed mostly in the synovial lining and mononuclear cells.
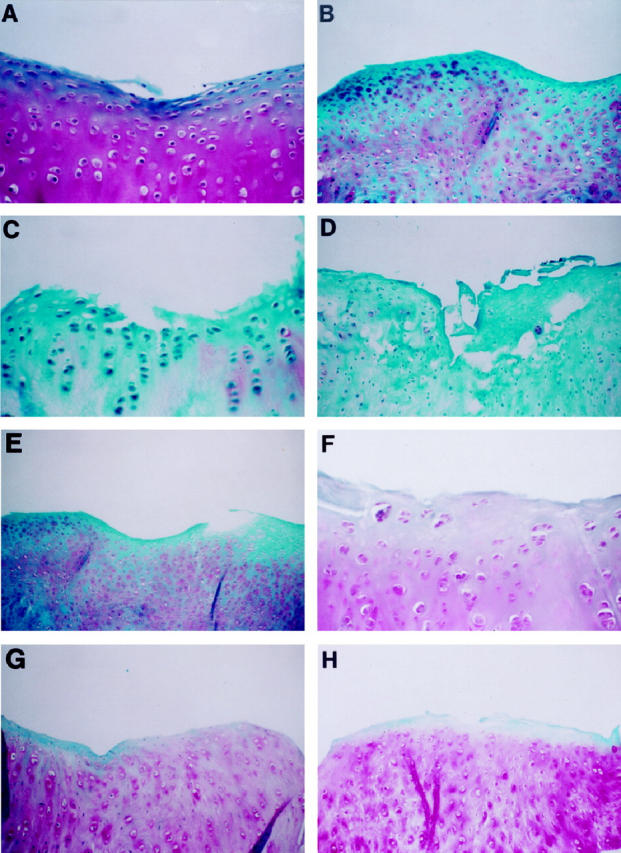
Figure 6.
Immunohistochemical detection of canine IL-1Ra in synovium of rabbit OA knees. A: A 4-week OA rabbit. B: An 8-week OA rabbit injected with the control plasmid showing no specific staining for canine IL-1Ra. C: An 8-week OA rabbit treated with 500 μg of IL-1Ra plasmid. D: An 8-week OA rabbit treated with 1000 μg of IL-1Ra plasmid showing some staining in chondrocytes mainly in the superficial layer. Controls using specimens from 8-week old rabbits treated with 500 μg (E) and 1000 μg (F) of IL-1Ra plasmid performed with an immune absorbed serum using a 20-fold mass equivalent to the purified antigen showed no specific staining, Original magnification, ×250.
The immunohistochemistry of cartilage specimens from the medial condyles and plateaus was negative for dog IL-1Ra, with the exception of approximately half the specimens from the rabbits injected with IL-1Ra plasmid. In these specimens, a small group of chondrocytes in a few areas of the superficial layer were found to stain positive for dog IL-1Ra (Figure 7) ▶ .
Figure 7.
Immunohistochemical detection of canine IL-1Ra in cartilage of rabbit OA knees. A: An 8-week OA rabbit injected with the control plasmid showing no specific staining for canine IL-1Ra. B: An 8-week OA rabbit treated with 500 μg of IL-1Ra plasmid. C: An 8-week OA rabbit treated with 1000 μg of IL-1Ra plasmid showing intense specific staining for IL-1Ra, mostly at the synovial lining cell level. D: Control using a specimen from an 8-week old rabbit treated with 1000 μg of IL-1Ra plasmid performed with an immune absorbed serum using a 20-fold mass equivalent to the purified antigen showed no specific staining. Original magnification, ×100.
PCR Assays
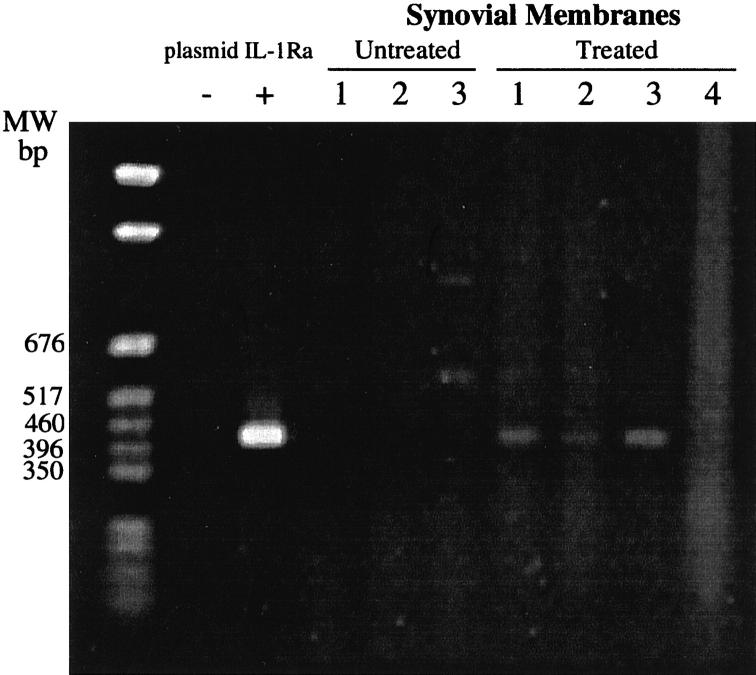
PCR assays of DNA extracted from the synovial membranes from untreated rabbits failed to generate the specific IL-1Ra-amplified DNA fragment of 427 bp (Figure 8) ▶ . In contrast, specific bands were obtained from the amplification of DNA extracted from 3 of 4 IL-1Ra-transfected synovia tested (Figure 8) ▶ . Although these PCR assays are not quantitative, they suggest that some plasmid remained in the synovial membranes and may still have expressed the IL-1Ra protein.
Figure 8.
PCR analysis of rabbit synovial membranes for the presence of the dog IL-1Ra gene. Plasmids were injected into the knee joints of rabbits as described in Materials and Methods. DNA was extracted from the synovial membranes of untreated (Group 1; n = 3) and treated (Group 4; n = 4) rabbits and subjected to PCR analysis to detect the cloned cDNA dog IL-1Ra. MW, molecular weight markers (pGEM markers, Promega, Madison, WI); −, negative control (reaction mixture without template DNA); +, positive control (plasmid IL-1Ra used as DNA substrate). The size of the specific amplified DNA fragment is 427 bp.
Discussion
Despite an extensive armamentarium and a number of surgical options, OA remains incurable. An improved approach in the treatment of this disease is imperative. Drug delivery, the chief vehicle for existing antiarthritic therapies, has major drawbacks. This study demonstrates that the direct in vivo transfer of the IL-1Ra gene into the OA cells by means of a plasmid vector can successfully induce the production of IL-1Ra in situ and can significantly reduce the progression of experimental OA. This protective effect seems to be dose-dependent and likely reflects the achievement of a higher level of transfection and, secondarily, IL-1Ra production.
Local delivery of anti-inflammatory cytokines or the induction in vivo of their expression using gene transfer may provide a novel approach for the treatment of OA. 17,28-30 The transfer of genes into joint cells can be done using both in vivo and ex vivo methods. 14,28,31-34 The indirect ex vivo approach is complex and involves several steps including the removal of synovium from the joint, transfection of the cells in vitro by a viral or nonviral gene transfer method, and reintroduction of the transfected cells into the joint. 29 Adenoviral vectors are considered best suited to in vivo gene transfection of synoviocytes, as these cells normally have a low rate of turnover. 35,36 In fact, adenovirus has been demonstrated to transduce synovial cells efficiently in vivo. 34 However, achieving a similar level of gene transfer using a nonviral vector would clearly be more desirable in the context of clinical studies in a chronic nonfatal disease. Several modes of gene transfer that target therapy for the treatment of joint disease have been attempted, although to date few genes have been successfully transfected into articular cartilage or synovium without viral vectors. 35-38 In fact, using technology that could target both articular cartilage and synovial membrane may represent the ideal strategy for OA modulation. Recent studies have shown that the use of cationic lipid DNA complex with improved expression vector and new cationic lipid could greatly optimize gene transfer into cells. 39-41 A new cationic lipid, GAP-DLRIE/DOPE, has been shown to provide a very high level of gene transfection into artery and skeletal muscle cells using plasmid DNA in vivo. Moreover, the plasmid DNA vector VR1012 has been proven in former experiments to provide a higher level of expression compared to many other commonly available expression vectors. 40
The present study shows that the IL-1Ra gene can be transduced directly in vivo using a nonviral plasmid technology and incorporated into the synovial cells and chondrocytes to produce the IL-1Ra protein. The immunohistochemical study has confirmed that transfer of the IL-1Ra gene was much more efficient in OA synovial cells as compared to chondrocytes. This could be explained by several hypotheses, one being taught that it may be related to the higher replicating rate of synovial cells. On the other hand, the diffusion rate of the lipoplex into the cartilage matrix may be another factor responsible for the much lower level of cell transfection. In vitro experiments in cell culture have also demonstrated that the transfection of the IL-1Ra gene in chondrocytes using lipoplex was less efficient than in synovial cells (Pelletier, Tardif, and Dupuis, personal observation). 35 In light of the present study, however, the lipoplex alone seemed less effective in inducing the synthesis of IL-1Ra than the technology used in our previous study, in which we employed a viral vector. 14 For instance, the level of IL-1Ra in the synovial fluid was lower and could be detected only in a limited number of rabbits that received the IL-1Ra gene. One must, however, use caution when interpreting the data from the ELISA, because neutralizing antibodies that could be present in the synovial fluid may have induced a falsely low reading from these assays. Studies that may allow us to overcome this difficulty are currently underway in our laboratory.
This study demonstrates that, using a direct in vivo technology for gene transfer, it is possible to ensure a detectable level of expression of the IL-1Ra with persistence of the plasmid for at least 4 weeks following the lipoplex injections. These results are most encouraging and further studies are needed to explore the length of time for the plasmid and gene expression to last. The level of IL-1Ra produced was sufficient to block the biological activity of the IL-1 as judged by the effect of such therapy on the progression of OA structural changes. The strong gene expression observed in synovial cells is also noteworthy. The gene was, in fact, transfected not only in lining cells but also in mononuclear cells of the sublining tissue, indicating that the gene could be transfected in different cell types as previously reported. 38 In contrast to our previous study using a retroviral vector, no increase in the level of synovial inflammation was noted, even in the presence of an active secretion of canine IL-1Ra. Under the actual experimental conditions, the foreign protein seemed to have been well tolerated by the rabbits, though one cannot exclude the presence of neutralizing antibodies. 42
Reduction in the growth of osteophytes in rabbits that received the IL-1Ra plasmid was very marked. From previous studies, it has become evident that the growth of these cartilage-bone structures are, at least in part, related to the action of IL-1. For instance, in the experimental dog model of OA, intra-articular injections of the IL-1Ra proved very effective at reducing the progression of osteophytes. 11 Recent reports, however, have also demonstrated the involvement of transforming growth factor-β in the formation of osteophytes, indicating that these structural changes may have a multifactorial origin. 43 The exact link between IL-1 and transforming growth factor-β in the formation of osteophytes remains to be determined.
Direct transfection of the IL-1Ra gene and its in vivo expression was also found to effectively reduce, in a manner dependent on the amount of plasmid injected, the progression of cartilage lesions. The severity of macroscopic lesions was reduced on both condyles and plateaus, with a reduction in the severity of histologic lesions on plateaus. The pathophysiology of OA lesions, both in the model and in the natural disease, has been shown to be induced by proteolytic enzymes, the synthesis of which is up-regulated by proinflammatory cytokines such as IL-1. 1,2 Therefore, the actual findings with regard to cartilage lesions are most likely explained by the inhibitory action of increased local production of IL-1Ra on IL-1 action. The extent of the effect, which was dependent on the amount of the IL-1Ra plasmid injected, correlated well with the number of chondrocytes synthesizing IL-1Ra as shown by immunohistochemistry. The staining for IL-1Ra was observed almost exclusively in the rabbits that received the higher dosage of the IL-1Ra lipoplex. The absence of staining in rabbits that received the lower dosage of plasmid could possibly be explained by a lower level of production of IL-1Ra not detectable by immunohistochemistry, or by the fact that production of IL-1Ra in these rabbits may have stopped before they were sacrificed, as suggested in literature. 35,44
In summary, this study demonstrates that the use of DNA plasmid represents a promising method for the direct transfer of therapeutic gene(s) in vivo. This technology presents several obvious advantages, including safety and efficacy. The use of the IL-1Ra gene also seems to hold interesting future prospects for the treatment of OA. This conclusion is based on the results of multiple studies, including ours, which have shown that the use of this gene under several experimental ex vivo and in vivo conditions could reduce cartilage degradation and retard the progression of structural changes in OA.
Acknowledgments
The authors thank Colleen Byrne and Jeannine Amyot for their assistance in manuscript preparation and Dr. G. Fuller for providing the canine DNA.
Footnotes
Address reprint requests to Jean-Pierre Pelletier, M.D., Director, Unité des Maladies Rhumatismales, Centre Hospitalier de l’Université de Montréal, Campus Notre-Dame, 1560 rue Sherbrooke Est, Montréal, Québec, Canada H2L 4M1.
Supported in part by a grant from Pfizer Central Research, Groton, CT.
References
- 1.Pelletier JP, Martel-Pelletier J, Howell DS: Etiopathogenesis of osteoarthritis. Ed. 13 Koopman WJ eds. Arthritis and Allied Conditions: A Textbook of Rheumatology. 1997, :pp 1969-1984 Williams & Wilkins, Baltimore [Google Scholar]
- 2.Pelletier JP, Di Battista JA, Roughley PJ, McCollum R, Martel-Pelletier J: Cytokines and inflammation in cartilage degradation. Osteoarthritis. Edition of Rheumatic Disease Clinics of North America. Edited by RW Moskowitz. Philadelphia, W.B. Saunders Company, 1993, pp 545–568 [PubMed]
- 3.Pelletier JP, McCollum R, Cloutier JM, Martel-Pelletier J: Synthesis of metalloproteases and interleukin 6 (IL-6) in human osteoarthritic synovial membrane is an IL-1 mediated process. J Rheumatol 1995, 22:109-114 [PubMed] [Google Scholar]
- 4.Firestein GS, Berger AE, Tracey DE, Chosay JG, Chapman DL, Paine MM, Yu C, Zvaifler NJ: IL-1 receptor antagonist protein production and gene expression in rheumatoid arthritis and osteoarthritis synovium. J Immunol 1992, 149:1054-1062 [PubMed] [Google Scholar]
- 5.Pelletier JP, Mineau F, Ranger P, Tardif G, Martel-Pelletier J: The increased synthesis of inducible nitric oxide inhibits IL-1Ra synthesis by human articular chondrocytes: possible role in osteoarthritic cartilage degradation. Osteoarthritis Cartilage 1996, 4:77-84 [DOI] [PubMed] [Google Scholar]
- 6.Sadouk M, Pelletier JP, Tardif G, Kiansa K, Cloutier JM, Martel-Pelletier J: Human synovial fibroblasts coexpress interleukin-1 receptor type I and type II mRNA: the increased level of the interleukin-1 receptor in osteoarthritic cells is related to an increased level of the type 1 receptor. Lab Invest 1995, 73:347-355 [PubMed] [Google Scholar]
- 7.Martel-Pelletier J, McCollum R, Di Battista JA, Faure MP, Chin JA, Fournier S, Sarfati M, Pelletier JP: The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes: identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum 1992, 35:530-540 [DOI] [PubMed] [Google Scholar]
- 8.Arend WP, Dayer JM: Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum 1995, 38:151-160 [DOI] [PubMed] [Google Scholar]
- 9.Arner EC, Harris RR, DiMeo TM, Collins RC, Galbraith W: Interleukin-1 receptor antagonist inhibits proteoglycan breakdown in antigen induced but not polycation induced arthritis in the rabbit. J Rheumatol 1995, 22:1338-1346 [PubMed] [Google Scholar]
- 10.Arend WP: Interleukin-1 receptor antagonist [review]. Adv Immunol 1993, 54:167-227 [DOI] [PubMed] [Google Scholar]
- 11.Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, Pelletier JP: Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis: suppression of collagenase-1 expression. Arthritis Rheum 1996, 39:1535-1544 [DOI] [PubMed] [Google Scholar]
- 12.Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, Hung GL, Glorioso JC, Robbins PD, Evans CH: Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci USA 1993, 90:10764-10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baragi VM, Renkiewicz RR, Jordan H, Bonadio J, Harman JW, Roessler BJ: Transplantation of transduced chondrocytes protects articular cartilage from interleukin 1-induced extracellular matrix degradation. J Clin Invest 1995, 96:2454-2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelletier JP, Caron JP, Evans CH, Robbins PD, Georgescu HI, Jovanovic D, Fernandes JC, Martel-Pelletier J: In vivo suppression of early experimental osteoarthritis by IL-Ra using gene therapy. Arthritis Rheum 1997, 40:1012-1019 [DOI] [PubMed] [Google Scholar]
- 15.Chernajovsky Y, Annenkov A, Herman C, Triantaphyllopoulos K, Gould D, Dreja H, Moyes SP, Croxford JL, Mageed RA, Podhajcer OL, Baker D: Gene therapy for rheumatoid arthritis. Theoretical considerations. Drugs Aging 1998, 12:29-41 [DOI] [PubMed] [Google Scholar]
- 16.Campion GV, Lebsack ME, Lookabaugh J, Gordon G, Catalano M: Dose-range and dose-frequency study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis. The IL-1Ra Arthritis Study Group. Arthritis Rheum 1996, 39:1092-1101 [DOI] [PubMed] [Google Scholar]
- 17.Evans CH, Robbins PD: Pathways to gene therapy in rheumatoid arthritis. Curr Opin Rheumaol 1996, 8:230-234 [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz RW, Davis W, Sammarco J, Martens M, Baker J, Mayor M, Burstein AH, Frankel VH: Experimentally induced degenerative joint lesions following partial meniscectomy in the rabbit. Arthritis Rheum 1973, 16:397-405 [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz RW: Experimental models of osteoarthritis. Osteoarthritis. Ed. 2 Moskowitz RW Howell DS Goldberg VM Mankin HJ eds. Diagnosis and Medical/Surgical Management. 1992, :pp 213-232 W.B. Saunders Company, Philadelphia [Google Scholar]
- 20.Moskowitz RW, Goldberg VM: Studies of osteophyte pathogenesis in experimentally induced osteoarthritis. J Rheumatol 1987, 14:311-320 [PubMed] [Google Scholar]
- 21.Pelletier JP, Mineau F, Raynauld JP, Woessner JF, Jr, Gunja-Smith Z, Martel-Pelletier J: Intraarticular injections with methylprednisolone acetate reduce osteoarthritic lesions in parallel with chondrocyte stromelysin synthesis in experimental osteoarthritis. Arthritis Rheum 1994, 37:414-423 [DOI] [PubMed] [Google Scholar]
- 22.Mankin HJ, Dorfman H, Lippiello L, Zarins A: Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 1971, 53:523-537 [PubMed] [Google Scholar]
- 23.Pelletier JP, Martel-Pelletier J, Ghandur-Mnaymneh L, Howell DS, Woessner JF, Jr: Role of synovial membrane inflammation in cartilage matrix breakdown in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum 1985, 28:554-561 [DOI] [PubMed] [Google Scholar]
- 24.Moldovan F, Pelletier JP, Hambor J, Cloutier JM, Martel-Pelletier J: Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor β. Arthritis Rheum 1997, 40:1653-1661 [DOI] [PubMed] [Google Scholar]
- 25.Pelletier JP, Faure MP, Di Battista JA, Wilhelm S, Visco D, Martel-Pelletier J: Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis: an immunohistochemical study. Am J Pathol 1993, 142:95-105 [PMC free article] [PubMed] [Google Scholar]
- 26.Parker SE, Khatibi S, Margalith M, Anderson D, Yankauckas M, Gromkowski SH, Latimer T, Lew D, Marquet M, Manthorpe M, Hobart P, Hersh E, Stopeck AT, Norman J: Plasmid DNA gene therapy: studies with the human interleukin-2 gene in tumor cells in vitro and in the murine B16 melanoma model in vivo. Cancer Gene Ther 1996, 3:175-185 [PubMed] [Google Scholar]
- 27.Eisenberg SP, Evares RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, Thompson RC: Primary structure and functional expression of complimentary DNA of a human interleukin-1 receptor antagonist. Nature 1990, 343:341-346 [DOI] [PubMed] [Google Scholar]
- 28.Evans CH, Robbins PD: Gene therapy for arthritis. Wolff JA eds. Gene Therapeutics: Methods and Applications of Direct Gene Transfer. 1994, :pp 320-343 Birkhauser, Boston [Google Scholar]
- 29.Evans C, Robbins PD: Prospects for treating arthritis by gene therapy [editorial]. J Rheumatol 1994, 21:779-782 [PubMed] [Google Scholar]
- 30.Bandara G, Robbins PD, Georgescu HI, Mueller GM, Glorioso JC, Evans CH: Gene transfer to synoviocytes: prospects for gene treatment for arthritis. DNA Cell Biol 1992, 11:227-231 [DOI] [PubMed] [Google Scholar]
- 31.Otani K, Nita IM, Macaulay W, Georgescu HI, Robbins PD, Evans CH: Suppression of antigen-induced arthritis in rabbits by ex vivo gene therapy. J Immunol 1996, 156:3558-3562 [PubMed] [Google Scholar]
- 32.Makarov SS, Olsen JC, Johnston WN, Schwab JH, Anderle SK, Brown RR, Haskill JS: Retrovirus mediated in vivo gene transfer to synovium in bacterial cell wall-induced arthritis in rats. Gene Ther 1995, 2:424-428 [PubMed] [Google Scholar]
- 33.Hung GL, Galea-Lauri J, Mueller GM, Georgescu HI, Larkin LA, Suchanek MK, Tindal MH, Robbins PD, Evans CH: Suppression of intraarticular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther 1994, 1:64-69 [PubMed] [Google Scholar]
- 34.Roessler BJ, Allen ED, Wilson JM, Hartman JW, Davidson BL: Adenoviral-mediated gene transfer to rabbit synovium in vivo. J Clin Invest 1993, 92:1085-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nita IM, Ghivizzani SC, Galea-Lauri J, Bandara G, Georgescu HI, Robbins PD, Evans CH: Direct gene delivery to synovium. An evaluation of potential vectors in vitro and in vivo. Arthritis Rheum 1996, 39:820-828 [DOI] [PubMed] [Google Scholar]
- 36.Tomita N, Morishita R, Higaki J, Tomita S, Aoki M, Ogihara T, Kaneda Y: In vivo gene transfer to insulin gene into neonatal rats by the HVJ-liposome method resulted in sustained transgene expression. Gene Ther 1996, 3:477-482 [PubMed] [Google Scholar]
- 37.Tomita T, Hashimoto H, Tomita N, Morishita R, Lee SB, Hayashida K, Nakamura N, Yonenobu K, Kaneda Y, Ochi T: In vivo direct gene transfer into articular cartilage by intraarticular injection mediated by HVJ (sendai virus) and liposomes. Arthritis Rheum 1997, 40(5):901-906 [DOI] [PubMed] [Google Scholar]
- 38.Yovandich J, O’Malley B, Sikes M, Ledley FD: Gene transfer to synovial cells by intra-articular administration of plasmid DNA. Hum Gene Ther 1995, 6:603-610 [DOI] [PubMed] [Google Scholar]
- 39.Budker V, Zhang G, Danko I, Williams P, Wolff J: The efficient expression of intravascularly delivered DNA in rat muscle. Gene Ther 1998, 5:272-276 [DOI] [PubMed] [Google Scholar]
- 40.Hartikka I, Sawdey M, Cornefert-Jensen F, Margalith M, Barnhart K, Nolasco M, Vahlsing HL, Meek J, Marquet M, Hobart P, Norman J, Manthorpe M: An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum Gene Ther 1996, 7:1205-1217 [DOI] [PubMed] [Google Scholar]
- 41.Stephan DJ, Yang ZY, San H, Simari RD, Wheeler CJ, Felgner PL, Gordon D, Nabel GJ, Nabel EG: A new cationic liposome DNA complex enhances the efficiency of arterial gene transfer in vivo. Hum Gene Ther 1996, 7:1803-1812 [DOI] [PubMed] [Google Scholar]
- 42.Tripathy SK, Black HB, Goldwasser E, Leiden JM: Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirum vectors. Nat Med 1996, 2:545-550 [DOI] [PubMed] [Google Scholar]
- 43.Glansbeek HL, Van Beuningen HM, Vitters EL, Van der Kraan PM, van den Berg WB: Stimulation of articular cartilage repair in established arthritis by local administration of transforming growth factor-β into murine knee joints. Lab Invest 1998, 78:133-142 [PubMed] [Google Scholar]
- 44.Palmer TD, Rosman GJ, Osborne WR, Miller AD: Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci USA 1996, 88:1330-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]