Abstract
During early human pregnancy extravillous cytotrophoblasts invade the uterus and also migrate up the spiral arteries, transforming them into large vessels of low resistance. Failure of transformation has been described in pre-eclampsia, fetal growth restriction, and miscarriage. Recent evidence suggests that some maternal vessels undergo structural changes without interaction with cytotrophoblasts. The possibility arises that local vasoactive mediators such as nitric oxide result in spiral artery dilatation before their invasion. In support of this, a recent histological study in the guinea pig suggested that cytotrophoblasts expressed nitric oxide synthase (NOS) as they surrounded vessels. This study tested the hypothesis that invading cytotrophoblasts express NOS and therefore have the potential to induce vasodilatation by releasing nitric oxide. The expression of NOS on extravillous cytotrophoblasts was studied in placental bed biopsies, obtained, using a transcervical sampling technique, from normal human pregnancies between 8 to 19 weeks of gestation and in the third trimester. Whereas eNOS was expressed by syncytiotrophoblast, neither eNOS or iNOS was expressed by extravillous cytotrophoblasts at any time during invasion. The mechanisms controlling spiral artery transformation are pivotal to understanding normal and abnormal placentation. These results suggest that trophoblast-derived nitric oxide is unlikely to contribute to spiral artery dilatation.
During early human pregnancy, extravillous cytotrophoblast (CTB) invade the uterine decidualized endometrium and myometrium (interstitial trophoblast) and migrate in a retrograde direction up the spiral arteries (endovascular trophoblast), transforming them into large bore tortuous vessels of low resistance. 1 This physiological transformation is characterized by a gradual loss of the normal musculoelastic structure of the arterial wall and replacement by amorphous fibrinoid material in which trophoblast cells are embedded. 2 These physiological changes are required for a successful pregnancy and failure of spiral artery transformation has been well documented in pre-eclampsia, fetal growth restriction without maternal hypertension, 3-8 and miscarriage. 3,9-11 Despite the importance of spiral artery transformation, the mechanisms that control these processes are poorly understood.
Nitric oxide (NO) is a small molecular weight mediator with diverse functions that include vasodilatation, inhibition of platelet aggregation, 12 and vascular remodeling. 13 NO results from the enzymatic action of nitric oxide synthase (NOS) which converts l-arginine, in the presence of oxygen, to l-citrulline and NO. 14 Three NOS enzymes have been cloned and sequenced: bNOS (type I NOS), 15 iNOS (type II NOS), 16 and eNOS (type III NOS). 17 Human placental syncytiotrophoblast express eNOS but not iNOS. 18-24 eNOS is also expressed on villous endothelial cells and NO produced from these cells is believed to be an important vasodilator within the placental vasculature. 25-28
Spiral artery transformation is thought to result from the loss of normal musculoelastic structure by CTB invasion. 2,8,29-32 However, vascular changes have been reported as early as 8 to 10 weeks of gestation before endovascular CTB invasion has occurred. 1,33 Pijnenborg et al 1,29 have related these vascular changes to the presence of interstitial CTB, suggesting these cells may produce vasoactive mediators. Furthermore in the guinea pig, in which maternal arterial vasodilatation also preceeds endovascular trophoblast invasion, 34 interstitial trophoblast has been shown to express eNOS and iNOS. Thus, local production of NO by invading CTB may be an important mediator of spiral artery transformation.
This study tests the hypothesis that in the human placental bed, invading CTB express eNOS or iNOS and therefore have the potential to directly influence spiral artery transformation. This hypothesis was tested in placental bed biopsies obtained from normal pregnancies between 8 to 19 weeks of gestation and from the third trimester.
Materials and Methods
Sample Collection
First and second trimester samples were obtained from women undergoing termination of apparently normal pregnancy at the Royal Victoria Infirmary, Newcastle-upon-Tyne. An initial ultrasound scan was performed to confirm fetal viability and to determine gestational age and placental position. After evacuation of the uterine contents, three placental bed biopsies were taken under ultrasound guidance using biopsy forceps (Wolf, UK) introduced through the cervix. Third trimester samples were obtained from women with normal pregnancies undergoing elective Cesarean section at term at the Royal Victoria Infirmary, Newcastle-upon-Tyne. After delivery of the infant, the position of the placenta was determined by manual palpation. Six placental bed biopsies were then taken under direct vision using the same biopsy forceps. Placental samples were collected from all cases. The Joint Ethics Committee of Newcastle-on-Tyne Health Authority and the University of Newcastle approved the study.
All samples were collected directly into liquid nitrogen-cooled isopentane and stored sealed at −70°C until required. Each specimen was stained with hematoxylin and eosin for histological analysis. In addition, sections were immunostained with antibodies to cytokeratin to detect trophoblast, smooth muscle actin to detect muscle, and CD34 to detect endothelium. Placental bed biopsies were included in this study if they contained decidual and/or myometrial spiral arteries with perivascular interstitial trophoblast.
Antibodies
Cytokeratin (LP34), CD34, and smooth muscle mouse monoclonal actin antibodies were obtained from Novocastra Laboratories (Newcastle-upon-Tyne, UK). The eNOS/type III mouse monoclonal antibody was purchased from Transduction Laboratories from a UK supplier (Affiniti, Exeter, UK). A 20.4-kd protein fragment corresponding to amino acids 1030–1209 of human eNOS was used as an immunogen. Two separate iNOS antibodies were used. The first was a gift from Prof. F.Y. Liew, University of Glasgow, Scotland. This anti-peptide polyclonal antibody (NOS 53) was raised in rabbits 35 against a sequence corresponding to the 7 COOH-terminal residues of human iNOS (Ser-Leu-Glu-Met-Ser-Ala-Leu-COOH). This sequence is not represented elsewhere in the currently available protein databases and is absent from the bNOS and eNOS. This antibody has been extremely well characterized, is specific for human iNOS, and has been used to demonstrate iNOS in macrophages of patients with tuberculosis 35 and fibroblasts and macrophages of rheumatoid and osteoarthritis patients. 36 The other iNOS antibody, a rabbit polyclonal antibody (C-19/SC649) raised against amino acids 1135–1153 mapping at the carboxy terminus of human iNOS, was obtained from Santa Cruz Biotechnology, Santa Cruz, CA.
Immunohistochemistry
Immunohistochemistry was performed using the Vectastain Universal kit (Vector Laboratories, Peterborough, UK). Cryostat sections (5 μm) were mounted onto silane-coated slides, air dried overnight, wrapped in foil, and stored at −70°C until required. Sections were then fixed in acetone for 5 minutes, followed by immersion in ethanol for 5 minutes and then in distilled water for 5 minutes. The protocol for the eNOS (1:200), LP34 (1:100), CD34 (1:50), and smooth muscle actin antibodies (1:250) was as follows. Sections were blocked with the blocker supplied with the kit for 30 minutes at 37°C, washed in phosphate-buffered saline (PBS) 2× 5 minutes, and then the primary antibody (diluted in blocking buffer) was added for 90 minutes at 37°C. Following 2× 5-minute PBS washes the secondary antibody was added for 30 minutes at 37°C. Two more PBS washes were performed and then endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in methanol for 15 minutes at room temperature. The remaining steps were performed according to the instructions supplied with the kit. The reaction was developed with Fast diaminobenzidine tablets (Sigma, Poole, Dorset, UK). Sections were counterstained in Harris’s hematoxylin (BDH, Poole, UK) and mounted in synthetic resin. Omission of primary antibody or substitution of nonimmune serum for the primary antibody were both included as controls and resulted in no immunostaining.
For the iNOS antibodies the above protocol was followed with the following modifications. For NOS 53, sections were blocked in PBS containing 10% normal goat serum, 10% horse serum, and 10% human serum. The primary antibody (1:1000) was diluted in blocking buffer, and all procedures were performed at room temperature. The PBS also contained 0.1% Tween 20. For iNOS (C-19), sections were blocked in PBS containing 10% horse serum and 10% human serum in PBS. The primary antibody was diluted (1:250) in blocking buffer.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blots
Western blotting analysis was performed to confirm the specificity of the NOS antibodies. Positive control for iNOS antibodies were obtained as follows: A549 cells (a human lung airway epithelial cell line) treated with a cytokine cocktail, which selectively induces iNOS, were a gift from Dr. Simon Bartlett, King’s College, London. The cells were plated in 9-cm dishes and grown to confluence in Dulbecco’s Modified Eagle Medium containing 10% fetal calf serum. The cells were serum deprived overnight before addition of the cytokine cocktail; 100 μmol/L lipopolysaccharide, 10 ng/ml interferon-γ, 10 ng/ml tumor necrosis factor-α, 10 ng/ml interleukin-1β. Cells were incubated for 48 hours with cytokines, were washed twice with ice-cold PBS, and were lysed in 0.5 ml of lysis buffer (50 mmol/L HEPES, pH 7.5, 2 mmol/L EDTA, 0.2% CHAPS, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 5 μg/ml chymostatin, 100 μg/ml antipain, 100 μg/ml soy bean trypsin inhibitor). The cells then underwent two freeze-thaw cycles and were spun at 13,000 rpm for 5 minutes at 4°C. The supernatant was removed and 30 μl of expanded adenosine 5′-diphosphate-sepharose (Pharmacia) were added and mixed continuously for 45 minutes at 4°C. The beads were spun briefly in a microcentrifuge, the supernatant discarded, and the beads were washed with 250 μl of lysis buffer. This was repeated once more, then the beads were washed 3× in lysis buffer containing 0.5 mol/L NaCl, and were followed twice more with standard lysis buffer. Finally, 200 μl of loading buffer was added to the pelleted beads, the sample was boiled for 3 minutes, and stored at −20°C until sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blots were performed.
Normal term placental villous tissue and myometrium (obtained from a hysterectomy performed for benign disease) were used to validate the eNOS antibody. Tissues were snap frozen in liquid nitrogen and stored at −70°C until required. Because, in contrast to iNOS, eNOS is membrane bound, membranes were prepared as follows. Tissue samples were ground to a fine powder with a mortar and pestle in liquid nitrogen and added to 4 volumes of cold lysis buffer (25 mmol/L Tris/0.25 mol/L sucrose/1 mmol/L EDTA, pH 7.6), which also contained a protease inhibitor cocktail (1 ml per 20 g tissue weight) for mammalian cell extracts (Sigma). Using a Polytron homogenizer at setting 10, the sample containers were surrounded by ice and homogenized 3× for 10-second intervals. The homogenate was spun at 5000 × g for 10 minutes at 4°C to remove debris and the resultant supernatant spun again at 50,000 × g for 20 minutes at 4°C to pellet the membranes. The supernatant containing the cytosolic fraction was aliquoted and stored at −70°C. The membrane pellet was resuspended in 25 mmol/L Tris, pH 7.6, and spun again at 50,000 × g for 20 minutes at 4°C. The supernatant was removed, and the membrane pellet resuspended in 25 mmol/L Tris, pH 7.6, and stored at −70°C. Protein concentrations were determined by the method of Lowry et al 37 using bovine serum albumin as a standard.
In preparation for electrophoresis, samples were mixed 1:1 with loading buffer (1.2 ml/1 mol/L Tris, pH 6.8, 2 ml of glycerol, 4 ml of 10% sodium dodecyl sulfate, 2 ml of 1 mol/L dithiothreitol, 0.8 ml of distilled water) with bromophenol blue added to give a deep blue color and boiled for 5 minutes before loading. Samples were separated on 7.5% sodium dodecyl sulfate-polyacrylamide resolving gels 38 with a 4% stacking gel at 20 mA through the stacking gel and at 30 mA through the resolving gel. Forty μg of placental villous or myometrium membranes were loaded onto the gel. Molecular weight markers (Sigma, UK SDS-7B prestained 33- to 205-kd range) were loaded beside the samples. The contents of each vial of markers were dissolved in 0.5 ml of 0.8 mol/L urea solution. 0.5 ml of sample buffer were added, mixed, and then aliquoted and stored at −20°C. Protein was transferred overnight in buffer containing 25 mmol/L Tris, 19 mmol/L glycine, and 20% methanol at a constant 30 V to BioBlot NC nitrocellulose membranes (Costar, Corning Inc., Canada).
For detection of eNOS, the filters were blocked for 1 hour at room temperature in PBS/0.1% Tween 20/5% Marvel (Premier Beverages, Stafford, UK) and then washed 1× 1-minute and then 2 × 5 minutes in PBS/0.1% Tween 20. The eNOS antibody was added at a dilution of 1:500 in PBS/0.1% Tween 20/1% Marvel for 1.5 hours at room temperature. The filters were washed 1 × 1 minute, 1 × 15 minutes, then 2 × 5 minutes in PBS/0.1% Tween 20 and were then incubated with horseradish peroxidase-conjugated sheep anti-mouse IgG (SAPU, Carluke, UK) diluted 1:2000 in PBS/0.1% Tween 20/1% Marvel for 1 hour at room temperature. Blots were then washed 1 × 1 minute, 1 × 15 minutes, then 2 × 5 minutes in PBS/0.1% Tween 20 followed by 1 × 5 minutes in distilled water. Proteins were detected using the Amersham ECLTM detection system, and filters were exposed to HyperfilmTM ECLTM (Amersham, Buckinghamshire, UK).
For detection of iNOS with the C-19 antibody, the above procedure was followed except the blocking buffer was Tris-buffered saline (TBS) (20 mmol/L Tris, pH7.5, 0.5 mol/L NaCl)/5% donkey serum/0.4% Tween 20/0.25% bovine serum albumin, and the wash buffer was TBS/0.4% Tween 20/0.25% bovine serum albumin. The antibody (1:500) was first preabsorbed for 1 hour at room temperature in TBS/0.4% Tween 20/0.25% bovine serum albumin/5% normal human serum and then directly hybridized with the filters at room temperature for 1.5 hour. The secondary antibody was donkey anti-rabbit IgG-HRP (SAPU) diluted 1:2000 in wash buffer. For NOS 53 the same procedure as for the C-19 antibody was followed except that the blocking buffer contained 5% Marvel rather than donkey serum and the primary antibody was added at 1:1500.
Results
Western Blots
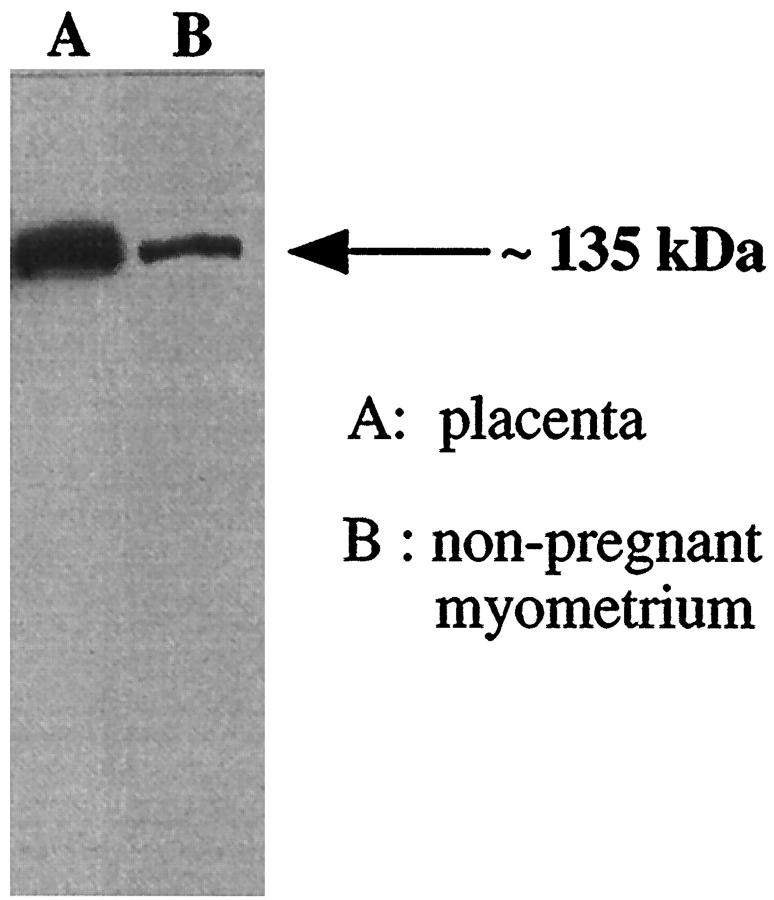
The Western blot with the eNOS antibody (Figure 1) ▶ showed an eNOS immunoreactive band at approximately 135 kd in membrane preparations of both term placental villous and non-pregnant myometrium. The eNOS band in placenta reflects the eNOS found in endothelium and syncytiotrophoblast whereas the less abundant eNOS band in the myometrial sample reflects the eNOS in endothelium alone since subsequent immunostaining results showed eNOS was not identified in myometrial muscle. This proved the specificity of the eNOS antibody.
Figure 1.
Western blot showing an eNOS immunoreactive band at approximately 135 kd in a membrane preparation of term placental villous tissue (A) and a membrane preparation of nonpregnant myometrium (B). Forty-μg membrane protein was loaded in each lane.
The Western blots with the iNOS antibodies are shown in Figure 2 ▶ . Both SC-649/C-19 and NOS 53 antibodies produced an iNOS immunoreactive band at approximately 130 kd in cytokine-stimulated cells only. Thus the specificity of SC-649/C-19 and NOS 53 were confirmed for detection of human iNOS in human tissues. Both antibodies were used in the immunohistochemistry experiments with similar results and the data shown is for SC-649/C-19.
Figure 2.
Western blot with the iNOS antibodies. A in both blots is ADP-sepharose extracted proteins from unstimulated A549 cells and no band was evident. B in both blots is the cytokine cocktail stimulated cells (100 μmol/L lipopolysaccaride, 10 ng/ml interferon-γ, 10 ng/ml tumor necrosis factor-α, 10 ng/ml interleukin-1β). An iNOS immunoreactive band at approximately 130 kd is clearly identifiable with both antibodies. Twenty μl of reaction mix was loaded in each lane.
Biopsies
Twenty-six placentas (with cell columns) and early placental bed biopsies were examined; 12 from pregnancies between 8 to 13 weeks of gestation and 14 from pregnancies between 14 to 19 weeks of gestation. Nine placentas and placental bed biopsies from term pregnancies were also included for comparison. Consistent results were found between samples.
eNOS/Cytokeratin within the Placenta
The photomicrographs in Figure 3 ▶ show the expression of eNOS and cytokeratin on extravillous trophoblast cells in cytotrophoblast cell columns or islands across the gestational range covered. Syncytiotrophoblast of floating villi is eNOS positive, whereas the trophoblast cells that have broken through the syncytiotrophoblast and formed cytotrophoblast cell columns are eNOS negative. These cells were all cytokeratin positive. Similarly whereas extravillous trophohoblast of cytotrophoblast cell islands were cytokeratin positive, these cells were eNOS negative.
Figure 3.

Expression of cytokeratin (A, E, G) and eNOS (B, C, D, F, H) on placental cytotrophoblast columns/islands at 8 weeks of gestation (A and B, cell island), 11 weeks of gestation (C, cytotrophoblast cell column), 14 weeks of gestation (D, cytotrophoblast cell column), 16 weeks of gestation (E and F, cytotrophoblast cell column), and 19 weeks of gestation (cytotrophoblast cell island, G and H). Cytotrophoblast cell islands are indicated by double arrows and cell columns by single arrows. Cytokeratin staining indicates presence of trophoblasts in syncytiotrophoblast, cell islands, cell columns, and decidua. No eNOS was detected on any of these cells. Scale bar, 200 μm (A and B), 100 μm (C, D, E, F, G, H).
eNOS/Cytokeratin Immunostaining within the Placental Bed
8 to 13 Weeks of Gestation
Histological examination of specimens showed the presence of interstitial CTB in both decidua and myometrium in all specimens between 8 and 13 weeks of gestation. The number of CTBs in the myometrium increased with gestational age. Endovascular invasion of decidual maternal vessels was down to the decidual/myometrial junction, but there was no evidence of myometrial vessel invasion. Figure 4 ▶ (A-H) shows representative photomicrographs of the eNOS and cytokeratin immunostaining. Positive eNOS staining was found on the vessel endothelium, but cytokeratin positive interstitial CTB never showed eNOS immunostaining (Figure 4, A-H ▶ ). Other cells that were eNOS positive were also CD34 positive and thus were probably small capillaries. An example of cytokeratin-positive trophoblast cells both within the muscle and also within the lumen of the vessel is also shown (Figure 4G) ▶ . Some of these cells show the spider-like appearance (indicated by the arrow) typical of these cells when embedded in muscle. None of the trophoblast cells within the vessel or in the section as a whole expressed eNOS.
Figure 4.

Expression of cytokeratin (A, C, E, G) and eNOS (B, D, F, H) on placental bed biopsies at 8 weeks of gestation (A and B), 10 weeks of gestation (C and D), 12 weeks of gestation (E and F), and 13 weeks of gestation (G and H). Cytokeratin reactivity indicates presence of trophoblasts. Whereas eNOS staining is present on blood vessels, none was identified on trophoblast. Scale bar, 50 μm (A and B), 100 μm (C, D, E, F, G, H).
14 to 18 Weeks of Gestation and Term
Histological examination of specimens from 14 to 18 weeks of gestation revealed extensive interstitial CTB invasion both within decidua and myometrium. Intravascular invasion was apparent in myometrial as well as decidual vessels. Figure 5 ▶ (A-H) show representative photomicrographs of eNOS and cytokeratin immunostaining. As in earlier gestation sections, CTB surrounding vessels were cytokeratin positive but eNOS negative. Vascular endothelium continued to show positive eNOS immunostaining. More examples of the characteristic spider-like appearance of the trophoblast within the muscle wall can be seen in Figure 5A ▶ . As for the other cases, no interstitial trophoblasts or giant cells within the sample were eNOS positive. The placental bed findings were similar during the third trimester; none of the cytokeratin-positive CTB stained positive for eNOS (Figure 5, G and H) ▶ . Trophoblast cells within superficial decidua, presumably remnants of the cytotrophoblastic shell, were also eNOS negative (not shown).
Figure 5.

Expression of cytokeratin (A, C, E, G) and eNOS (B, D, F, H) on placental bed biopsies at 14 weeks of gestation (A and B), 16 weeks of gestation (C and D ), 18 weeks of gestation (E and F), and 35 weeks of gestation (G and H). Cyokeratin staining indicates presence of trophoblasts. Whereas eNOS staining is present on blood vessels, none was identified on trophoblast. Scale bar, 100 μm.
iNOS/Cytokeratin Immunostaining within the Placenta and Placental Bed
iNOS was not expressed either on trophoblasts within cytotrophoblast cell columns, in cytotrophoblast cell islands, interstitially, or as they surrounded blood vessels. One example at 16 weeks of gestation has been selected to show the absence of iNOS immunostaining on cell columns and within the placental bed (Figure 6) ▶ .
Figure 6.

Expression of cytokeratin (C and E), iNOS (B and D), and eNOS (A) on placental bed biopsies (A, B, C) and placental columns (D and E) at 16 weeks of gestation. Cytokeratin staining indicates presence of trophoblasts. Within the placental bed, eNOS is present on blood vessels, and these vessels were surrounded by trophoblasts. None of the cells were positive for iNOS. Similarly, none of the cells within the cell column expressed iNOS. Scale bar, 200 μm (A, B, C ), 100 μm (D and E).
Discussion
In this study we have found no evidence that invading interstitial CTB express eNOS or iNOS. These findings strongly suggest that CTB-derived NO is not responsible for dilatation of the spiral arteries during human placentation.
It has been reported that in the guinea pig 34 invading CTB switch on expression of both iNOS and eNOS as they surround maternal arteries. It was suggested that NO released from these cells may dilate the vessels before physiological destruction. Direct comparison with our results is difficult because human placentation differs from the guinea pig, and cell columns do not exist in rodents. Furthermore, the NOS isoforms expressed in the guinea pig differ from humans. Zarlingo et al 39 used the same eNOS antibodies as in the present study to perform a comparative localization of nitric oxide synthase isoforms on hemochorial and epitheliochorial placentae. The study found neither eNOS or iNOS in trophoblasts of term guinea pig placenta. This compares with the abundant eNOS expression in human syncytiotrophoblast. The study by Zarlingo et al 39 confirmed previous studies that the syncytiotrophoblast does not express iNOS. Therefore, studies in guinea pig may be of limited relevance to human placentation.
One recent human study reported eNOS staining on CTB columns and on the interstitial CTB of first- (6 to 12 weeks) and early second- (13 to 15 weeks) trimester placentas. 40 This group used a polyclonal eNOS antibody (Transduction Laboratories) but on formalin-fixed paraffin-embedded sections. Our only explanation for these differences is that we have found that application of the monoclonal eNOS antibody used in the present study on paraffin-embedded sections resulted in nonspecific reactivity throughout myometrium and with trophoblast. We have confirmed with Western blotting (unpublished data), as have others, 41 that human myometrium does not express eNOS. In agreement with our study, Eis et al 23 detected eNOS immunostaining in syncytiotrophoblast and endothelial cells of term placenta but no eNOS positive immunostaining in either extravillous trophoblast or other cell populations of the basal plate. This study also reported that extravillous trophoblast had nicotinamide adenine dinucleotide phosphate diaphorase activity that was not related to eNOS; it was suggested that diaphorase activity may be related to some other flavoprotein.
Studies of NOS expression by cultured CTB have also reported conflicting results. The findings of this study are consistent with our previous findings that purified CTB cells in culture do not express eNOS or iNOS and that it is only after the cells have fused to form a syncytium that eNOS, but not iNOS, expression is switched on. 24 This study used primary cultures of CTB that had been well characterized both as CTB and through differentiation to syncytiotrophoblast. 42 Incubation of confluent monolayers of a trophoblast cell line with vascular endothelial growth factor has been shown to result in the release of nitric oxide into the medium, 43 suggesting the presence of a constitutively expressed NOS. Confirmation that these cells expressed eNOS mRNA was performed by reverse transcriptase-polymerase chain reaction; however, whereas eNOS mRNA was present in placental tissue (as expected from the eNOS in syncytiotrophoblast and endothelial cells), the amount of eNOS mRNA in the cultured cells was extremely small. The authors speculated that vascular endothelial growth factor might be an autocrine regulator of NO biosynthesis by aiding trophoblast penetration into spiral arteries, however these results must be interpreted with caution. Although the cells used in that particular study are a useful model to answer particular questions regarding regulation pathways in trophoblast, they are not CTB. The cells (ED27) are an immortalized cell line derived from human chorionic villous samples. 44 They express β-hCG and also express increased eNOS with increasing degrees of confluence. Thus the cells used in this study are trophoblast, but not CTB, and are probably a form of trophoblast between cytotrophoblast and syncytiotrophoblast (Doug Kniss, personal communication). In contrast, we have previously shown that highly purified CTB cells in culture do not express iNOS and only express eNOS after syncytium formation. 24 These culture findings support the in situ results of the present study.
In this study we have shown that eNOS was only present on endothelium of blood vessels within the myometrium at all stages of pregnancy examined. No eNOS or iNOS was detectable on myometrial muscle throughout gestation (8 weeks to term). These data are in keeping with our unpublished observations (using Western blotting for iNOS) that both nonpregnant and pregnant myometrium do not express eNOS or iNOS. This is also in agreement with the findings of a recently published abstract 41 in which both eNOS and iNOS was undetectable in pregnant myometrium at term.
Finally, we have used immunohistochemistry to detect NOS. Whereas arginine to citrulline conversion assays are also useful indicators for measuring NOS activity, we did not feel these were appropriate in this setting for the following reasons. First, we have previously shown in cultured CTB that NOS enzyme activity parallels protein expression. 24 CTB do not express NOS or have enzyme activity, whereas syncytiotrophoblasts do express NOS, and this is paralleled by expression of NOS. Thus, because the CTB in this study did not express NOS protein, it is unlikely they would have NOS activity. Second, because NOS assays are usually performed on homogenates of tissues, any enzyme activity on CTB would be masked by NOS activity on endothelial cells.
In summary, understanding the control of trophoblast invasion is an area of major physiological importance with potential implications for failed pregnancy. This study has shown that extravillous CTB do not express NOS and therefore are incapable of producing NO. These findings do not support the hypothesis that NO produced by invading trophoblast is the mechanism of vasodilatation of spiral arteries and even in the absence of NOS in CTB normal physiological changes in the vessels still occur. Additional studies are required to identify the mechanisms underlying these processes.
Acknowledgments
We thank Claire Gilfillan, Barbara Innes, and Helen Glass for technical support and Dr. Simon Bartlett for assistance with the iNOS positive control samples.
Footnotes
Address reprint requests to Dr. Fiona Lyall, Maternal and Fetal Medicine Section, Institute of Medical Genetics, Yorkhill, Glasgow, G3 8SJ, United Kingdom. E-mail: f.lyall@udcf.gla.ac.uk.
Supported in part by the British Heart Foundation and Action Research.
References
- 1.Pijnenborg R, Bland JM, Robertson WB, Brosens I: Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983, 4:397-414 [DOI] [PubMed] [Google Scholar]
- 2.Brosens I, Robertson WB, Dixon HG: The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol 1967, 93:569-579 [DOI] [PubMed] [Google Scholar]
- 3.Khong TY, Liddell HS, Robertson WB: Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol 1987, 94:649-655 [DOI] [PubMed] [Google Scholar]
- 4.Khong TY: Growth: Fetus and Neonate. Edited by M Hanson, J Spencer, C Rodeck. Cambridge, Cambridge University Press, 1995
- 5.McFadyen IR, Price AB, Geirsson RT: The relation of birth-weight to histological appearances in vessels of the placental bed. Br J Obstet Gynaecol 1986, 93:476-481 [PubMed] [Google Scholar]
- 6.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, Van Assche FA: Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991, 98:648-655 [DOI] [PubMed] [Google Scholar]
- 7.Sheppard BL, Bonnar J: An ultrastructural study of utero placental arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol 1981, 88:695-705 [DOI] [PubMed] [Google Scholar]
- 8.Sheppard BL, Bonnar J: The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. J Obstet Gynaecol Br Commonw 1976, 83:948-959 [DOI] [PubMed] [Google Scholar]
- 9.Michel MZ, Khong TY, Clark DA, Beard RW: A morphological and immunological study of human placental bed biopsies in miscarriage. Br J Obstet Gynaecol 1990, 97 [DOI] [PubMed]
- 10.Hustin J, Jauniaux E, Schaaps JP: Histological study of the materno-embryonic interface in spontaneous abortion. Placenta 1990, 11:477-486 [DOI] [PubMed] [Google Scholar]
- 11.Jauniaux E, Zaidi J, Jurkovic D, Campbell S, Hustin J: Comparison of colour Doppler features and pathological findings in complicated early pregnancy. Reprod 1994, 9:2432-2437 [DOI] [PubMed] [Google Scholar]
- 12.Knowles RG, Moncada S: Nitric oxide synthases in mammals. Biochem J 1994, 298:249-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakaki T, Kato R: Nitric oxide in vascular remodelling. J Invest Surg 1996, 9:431-445 [DOI] [PubMed] [Google Scholar]
- 14.Palmer RMJ, Moncada S: A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun 1989, 59:348-352 [DOI] [PubMed] [Google Scholar]
- 15.Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH: Cloned and expressed nitric oxide synthase structurally resembles cytochrome p-450 reductase. Nature 1991, 351:714-718 [DOI] [PubMed] [Google Scholar]
- 16.Lowenstein CJ, Glatt CS, Bredt DS, Snyder SH: Cloned and expressed macrophage NOS contrast with the brain enzyme. Proc Natl Acad Sci USA 1992, 89:6711-6715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsden PA, Schapport KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, Lamas S, Michel T: Molecular cloning and characterization of human endothelial NOS. FEBS Lett 1992, 307:287-293 [DOI] [PubMed] [Google Scholar]
- 18.Myatt L, Brockman DE, Langdon G, Pollock JS: Constitutive calcium dependent isoform of nitric oxide synthase in the human placental villous vascular tree. Placenta 1993, 14:373-383 [DOI] [PubMed] [Google Scholar]
- 19.Myatt L, Brockman DE, Eis ALW, Pollock JS: Immunohistochemical localization of nitric oxide synthase in the human placenta. Placenta 1993, 14:487-495 [DOI] [PubMed] [Google Scholar]
- 20.Conrad KD, Davis AK: Nitric oxide synthase activity in placentae from women with pre-eclampsia. Placenta 1995, 16:691-699 [DOI] [PubMed] [Google Scholar]
- 21.Conrad KP, Viii M, McGuire PG, Dail WG, Davis AK: Expression of nitric oxide synthase by syncytiotrophoblast in human placental villi. FASEB J 1993, 7:1269-1276 [DOI] [PubMed] [Google Scholar]
- 22.Buttery LDK, McCarthy A, Springall DR, Sullivan MHF, Elder MG, Michel T, Pollock JM: Endothelial nitric oxide synthase in the human placenta: regional distribution and proposed regulatory role at the feto-maternal interface. Placenta 1994, 15:257-265 [DOI] [PubMed] [Google Scholar]
- 23.Eis ALW, Brockman DE, Pollock JS, Myatt L: Immunohistochemical localization of endothelial nitric oxide synthase in human villous and extravillous trophoblast populations and expression during syncytiotrophoblast formation in vitro. Placenta 1995, 16:113-126 [DOI] [PubMed] [Google Scholar]
- 24.Lyall F, Jablonka-Shariff A, Johnson RD, Olson LM, Nelson DM: Gene expression of nitric oxide synthase in cultured human term placental trophoblast during in vitro differentiation. Placenta 1998, 17:253-260 [DOI] [PubMed] [Google Scholar]
- 25.Myatt L, Brewer A, Langdon G, Brockman DE: Attenuation of the vasoconstrictor effects of thromboxane and endothelin by nitric oxide in the human fetal-placental circulation. Am J Obstet Gynecol 1992, 166:224-230 [DOI] [PubMed] [Google Scholar]
- 26.Myatt L, Brewer AS, Brickman DE: The action of nitric oxide on the perfused fetal-placental circulation. Am J Obstet Gynecol 1991, 164:687-692 [DOI] [PubMed] [Google Scholar]
- 27.Boura ALA, Walters WAW, Read MA, Leitch IM: Autocoids and control of human placental blood flow. Clin Exp Pharmacol Physiol 1994, 21:737-748 [DOI] [PubMed] [Google Scholar]
- 28.Gude NM, King RG, Brennecke SP: Role of endothelium-derived nitric oxide in maintenance of low fetal vascular resistance in placenta. Lancet 1990, 336:1589-1590 [DOI] [PubMed] [Google Scholar]
- 29.Pijnenborg R, Dixon G, Robertson WB, Brosens I: Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1:3-19 [DOI] [PubMed] [Google Scholar]
- 30.De Wolf F, De Wolf-Peeters C, Brosens I: Ultrastructure of the spiral arteries in human placental bed at the end of normal pregnancy. Am J Obstet Gynecol 1973, 117:833-848 [DOI] [PubMed] [Google Scholar]
- 31.Khong TY, De Wolf F, Robertson WB, Brosens I: Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986, 93:1049-1059 [DOI] [PubMed] [Google Scholar]
- 32.Blankenship TN, Enders AC, B.F. K: Trophoblastic invasion and modification of uterine veins during placental development macaques. Cell Tissue Res 1993, 274:135-244 [DOI] [PubMed] [Google Scholar]
- 33.Craven CM, Morgan T, Ward K: Decidal spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 1998, 19:241-252 [DOI] [PubMed] [Google Scholar]
- 34.Nanaev AK, Chwalisz K, Frank-HG, Kohnen G, Hegele-Hartung C, Kaufmann P: Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase of extravillous trophoblast. Cell Tissue Res 1995, 282:407–421 [DOI] [PubMed]
- 35.Nicholson S, Bonecinialmeida MDG, Silva JRLE, Nathan C, Xie QW, Mumford R, Weider JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho J: Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med 1996, 183:2293-2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McInnes IB, Leung BP, Field M, Wei XQ, Huang F-P, Sturrock RD, Kinninmonth A, Weider J, Mumford R, Liew FY: Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med 1996, 184:1519-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. Biol Chem 1951, 193:265-275 [PubMed] [Google Scholar]
- 38.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 39.Zarlingo TJ, Eis ALW, Brockman DE, Kossenjans W, Myatt L: Comparative localization of endothelial and inducible nitric oxide synthase isoforms in haemochorial and epitheliochorial placenta. Placenta 1997, 18:511-520 [DOI] [PubMed] [Google Scholar]
- 40.Ilana A, Hochberg A, Shochina M: Endothelial nitric oxide synthase immunoreactivity in early gestation and in trophoblastic disease. J Clin Pathol 1998, 51:427-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campa JS, Poston R, Poston L: Nitric oxide synthase (eNOS) is localized to the vascular endothelium of myometrium from pregnant women at term. J Physiol 1998, 507:69P [Google Scholar]
- 42.Farmer DR, Nelson DM: A fibrin matrix modulates the proliferation, hormone secretion and morphologic differentiation of cultured human placental trophoblast. Placenta 1992, 13:163-177 [DOI] [PubMed] [Google Scholar]
- 43.Ahmed A, Dunk C, Kniss D, Wilkes M: Role of VEGF receptor (Flt-1) in mediating calcium-dependent nitric oxide release and limiting DNA synthesis in human trophoblast cells. Lab Invest 1997, 76:779-791 [PubMed] [Google Scholar]
- 44.Diss EM, Moore J, Gabbe SG, Kniss DA: Study of thromboxane and prostacyclin metabolism in an in vitro model of first-trimester human trophoblasts. Am J Obstet Gynecol 1992, 167:1046-1052 [DOI] [PubMed] [Google Scholar]