Abstract
Tissue injury initiates a temporally ordered sequence of local cellular and metabolic responses presumably necessary for successful repair. Previous investigations demonstrated that metabolic evidence for nitric oxide synthase (NOS) activity is detectable in wounds only during the initial 48 to 72 hours of the repair process. Present results identify the cell types contributing inducible NOS (iNOS) to experimental wounds in rats. iNOS antigen was expressed in most macrophages present in wounds 6 to 24 hours after injury, and these cells exhibited NAPDH diaphorase and NOS activity. Polymorphonuclear leukocytes contained little iNOS antigen and no NADPH diaphorase activity and were minimally able to convert l-arginine to l-citrulline. The frequency of iNOS-positive macrophages declined on days 3 and 5 after wounding. By day 10, most macrophages in the wound were negative for iNOS. These cells, however, acquired iNOS antigen and activity in culture. Wound fluids, but not normal rat serum, suppressed the induction of iNOS during culture. Findings indicate that the expression of iNOS in healing wounds is restricted to macrophages present during the early phases of repair and that components of wound fluid suppress the induction of iNOS in macrophages in late wounds. Polymorphonuclear leukocytes contribute little iNOS activity to the healing wound.
Previous work described a temporally restricted pattern of activity for two distinct enzymes of l-arginine metabolism, arginase and the inducible form of nitric oxide synthase (iNOS), in healing wounds. 1,2 Arguing from the accumulation of specific l-arginine catabolites in extracellular fluid obtained from experimental wounds and in cultures of whole-wound explants, it was proposed from this laboratory that the expression of iNOS in acute wounds was restricted to the period of polymorphonuclear leukocyte (PMN) infiltration, which encompasses the initial 24 to 72 hours after injury. Results also indicated that macrophage-derived arginase was the preponderant, and probably the only, high-flux enzyme of arginine catabolism in the wounds thereafter. Additional support to the latter conclusion and an enzymatic basis for the virtual disappearance of l-arginine from the extracellular space of late wounds were given by the accumulation of arginase in extracellular fluids obtained from wounds of increasing maturity. 1,2 Additional studies identified other wound-associated microenvironmental factors, such as hypoxia, as decidedly preferential in inducing l-arginine metabolism in macrophages through arginase rather than iNOS. 3,4
The relevance of the product of iNOS, NO, and its putative derivatives to inflammatory processes in general and more specifically to wound healing has recently been highlighted. 5 It has been proposed, in this regard, that the sustained expression of NOS in healing wounds is critical to the accumulation of collagen and the acquisition of mechanical strength in wounds. 5 These results were, interestingly, obtained in part using the same wound model used in previous reports from this laboratory. Because they differed so substantially from observations contrary to the significant expression of NOS in the wound past the initial 24 to 72 hours after wounding, experiments were performed to better define the temporal pattern of iNOS expression and identify the cells expressing this enzyme in healing wounds. Results presented herein further support a redundant system of NOS regulation in healing wounds that includes, along with the degradation of extracellular l-arginine by arginase in late wounds, 1,2 the restricted expression of iNOS to macrophages in the early phases of repair and the suppression of iNOS induction in macrophages from late wounds by factors present in the wound’s extracellular fluid.
Materials and Methods
Wound Model: Cell Harvesting and Culture
Male Fischer rats (150 to 200 g; VAF-Plus, Charles River Breeding Laboratories, Wilmington, MA) were used in all wounding experiments. VAF-Plus animals are certified free of common rat pathogens by the supplier and housed in an isolation environment on their arrival at the laboratory. The animals were monitored by Brown University/Rhode Island Hospital veterinary personnel. Sterile circular polyvinyl alcohol sponges (Unipoint Industries, High Point, NC) measuring approximately 1 cm in diameter and 0.4 cm in thickness were implanted subcutaneously through a 7-cm midline incision in the dorsum of each animal (10 sponges per animal) under anesthesia (Pentobarbital, Abbott Laboratories, North Chicago, IL; 5 mg/100 g body weight). 1,2
At designated times after sponge implantation, the animals were sacrificed with CO2, and cells contained in the sponges were harvested exactly as described previously. 1,2 That cellular recovery from the sponges is virtually complete was demonstrated experimentally by the lack of detectable DNA in the sponges after cell extraction (not shown). Total cell yields were used for differential counts and for the immunoblot detection of iNOS. Wound-derived macrophages were isolated from the wound cell preparation by adherence to plastic and recovered with ice-cold Ca- and Mg-free Hanks’ balanced salt solution. Purity was greater than 90% as determined by Wright-Giemsa staining and by immunofluorescence using an anti-rat macrophage antibody (Ab). 6 Viability at the time of harvest was >95% by trypan blue exclusion. Viability after various periods of incubation in culture medium (RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 1% bovine serum albumin, 10 mmol/L 3-(N-morpholino)-propanesulfonic acid, and antibiotics) was determined by lactic dehydrogenase release into supernatants and was >85% at 24 hours. Cell-free wound fluid was obtained by centrifugation of whole sponges in the cold as described previously. 2
Peritoneal exudate cells were obtained by peritoneal lavage 6 hours after the intraperitoneal injection of oyster glycogen (Sigma Chemical Co., St. Louis, MO). These cells were >90% PMNs by staining with a specific mouse anti-rat PMN monoclonal (M)Ab. 7
Immunoblot Analysis of iNOS Expression
Postnuclear supernatants of cell lysates obtained from freshly harvested or cultured cells were size fractionated in 7.5% SDS-polyacrylamide gel electrophoresis loaded with 15 μg of cell protein per lane. Proteins were transferred to a nitrocellulose membrane and incubated overnight in blocking buffer containing 10 mmol/L Tris/HCl, pH 7.5, 1% bovine serum albumin, 100 mmol/L NaCl, and 0.1% Tween-20. Blots were probed with a mouse MAb against macrophage iNOS (Transduction Laboratories, Lexington, KY) diluted 1:500 in blocking buffer for 1 hour at room temperature followed by washing for 30 minutes in blocking buffer. Antibody was detected using alkaline-phosphatase-conjugated goat anti-mouse immunoglobulin (Sigma) and visualized with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Bio-Rad Laboratories, Hercules, CA). A contour plot of each band was generated, and optical density of the area was determined using a PDI desktop densitometer and Quantity One one-dimensional gel analysis software (PDI, Huntington Station, NY).
Immunofluorescence Analysis
Cells were either cytospun onto glass slides when immunostaining was to be performed on freshly isolated preparations or adhered in Permanox eight-well chambered slides (Nunc, Naperville, IL) in experiments warranting an incubation period. Fixation was accomplished in acetone at −20°C for 5 minutes. Cells were blocked in PBS containing 1% normal goat serum and incubated with primary Ab in PBS plus 1% normal goat serum for 45 minutes at room temperature. Primary antibodies included rabbit anti-iNOS (1:50; Transduction Laboratories) and ascitic fluids containing either mouse anti-rat macrophage IgG (KU-1) at 1:200 dilution 6 or mouse anti-rat neutrophil IgG (RP3) at 1:200 dilution. 7 Slides were washed and incubated with fluorescein-conjugated goat anti-mouse IgG (1:100; Sigma) and Texas-Red-conjugated sheep anti-rabbit IgG (1:500; Biosource, Camarillo, CA) for 30 minutes at room temperature. Cells were observed using a Nikon Microphot-FXA, and images were captured using a SenSys CCD fitted with PVCAM acquisition software (Photometrics, Tucson, AZ) and analyzed using IPLab Spectrum 3.1 (Signal Analytics Co., Vienna, VA).
l-Arginine Metabolism by Wound-Derived Cells
Cells freshly harvested from 1-day-old wounds were enriched for macrophages or PMNs using NIM.2 density gradients (Cardinal Associates, Santa Fe, NM). Enriched populations were cultured in medium containing 1 mmol/L l-arginine and 0.1 μCi of [guanido-14C]l-arginine (DuPont NEN, Cambridge, MA) for 1, 3, or 5 hours at a density of 10 6 cells/ml. At each time point the appearance of radiolabeled l-citrulline in culture supernatant was determined using a Bio-LC amino acid analyzer (Dionex, Sunnyvale, CA) and on-line peak detection. Flux of l-arginine through iNOS is reported as cpm in its product, [ureido-14C]l-citrulline. Similar metabolic experiments were performed using freshly harvested or overnight cultured 10 day wound-derived macrophages. In this case, cultures with radiolabeled l-arginine were conducted for 2 hours. Flux of l-arginine through iNOS was calculated from the radioactivity in l-citrulline and the initial l-arginine-specific radioactivity in the medium at the beginning of culture.
NADPH Diaphorase Activity
Wound-derived cells were fixed for 5 minutes with 4% paraformaldehyde and rinsed with PBS. NADPH diaphorase activity was demonstrated by incubating cells at 37°C for 45 minutes with 0.5 ml of NADPH (1.25 mg/ml), nitroblue tetrazolium (100 μg/ml), and 0.2% Triton X-100 in 0.1 mol/L phosphate buffer (pH 8.0) and an additional 1 ml of PBS. The cells were washed with PBS and stained with nuclear fast red (1%) for 3 minutes to chelate ammonium ions.
Results
Evidence that iNOS Antigen Is Predominantly Detectable in Healing Wounds during the Initial 72 Hours after Wounding: iNOS Immunoreactivity Is Mostly Associated with Macrophages and Not with PMNs in Early Wounds
Figure 1 ▶ displays immunoblots for iNOS in lysates of unfractionated cell preparations harvested from wounds 1 through 10 days after injury. As can be seen, iNOS immunoreactivity was prominently present in lysates from freshly harvested wound-derived cells on days 1 and 3 and declined thereafter. Rat blood monocytes lacked immunodetectable iNOS protein (not shown).
Figure 1.
Immunoblot analysis of iNOS in freshly isolated wound cells. Wound cells harvested 1, 3, 5, or 10 days after wounding were isolated, lysed, and immunoblotted for iNOS antigen as described in Materials and Methods.
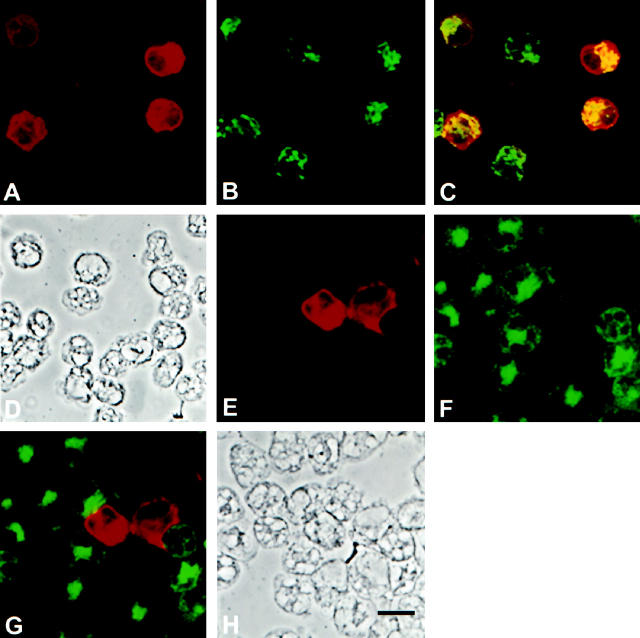
Differential counts of cells isolated from the wounds over time are shown in Table 1 ▶ . PMNs constitute the bulk of the inflammatory infiltrate during the initial 48 hours after wounding and are progressively replaced by macrophages. By 10 days after injury, practically all cells isolated from the wounds are macrophages. Because of the high proportion of PMNs contained in early wounds and their reported ability to express iNOS, 8 it was proposed from this laboratory that it was these cells that contained and expressed iNOS in the early wounds. 2 Adding support to this hypothesis, Miles, et al reported that lysates of peritoneal cells isolated shortly after the intraperitoneal injection of oyster glycogen, a technique for the enrichment of peritoneal exudate for PMNs, strongly immunoreacted to an Ab against iNOS. 9 Immunofluorescent examination of cells isolated from 1-day-old wounds (Figure 2) ▶ , however, demonstrated that PMNs provide only low-level signal for iNOS antigen and indicated that iNOS antigen present in wound cells at that time is predominantly associated with the macrophages found in the preparation. Evidence in this regard includes the coincident staining of macrophages with an anti-rat macrophage-specific Ab and the anti-iNOS Ab (Figure 2C) ▶ as well as the mutual exclusion of iNOS staining and that obtained with an Ab specific for rat PMNs (Figure 2G) ▶ . Almost identical findings resulted from the examination of wounds harvested 6 hours after wounding, with a predominance of iNOS-negative PMNs in the preparations and the presence of occasional macrophages that were, for the most part, iNOS positive (not shown).
Table 1.
Changing Cellularity of the Healing Wound
| Post-wound day | PMNs (×106/animal) | Macrophages (×106/animal) |
|---|---|---|
| 1 | 33.2 ± 1.4 | 4.8 ± 0.4 |
| 2 | 15.0 ± 1.1 | 7.9 ± 1.9 |
| 3 | 11.3 ± 0.6 | 19.6 ± 0.6 |
| 5 | 1.4 ± 0.5 | 21.7 ± 3.2 |
| 10 | 0.3 ± 0.03 | 11.0 ± 0.3 |
Differential counts of cells isolated from sponge wounds are expressed as total recovered cells per animal. Data are mean ± SEM of cell counts from three to nine experiments per harvest day. Day 10 wound preparations also contained 8.4 ± 2.4 × 105 lymphocytes and 8.7 ± 2.8 × 105 fibroblasts.
Figure 2.
Immunofluorescent analysis of wound-derived cells harvested 24 hours after injury demonstrating strong iNOS antigen expression among infiltrating macrophages and undetectable or weak staining of PMNs. A: anti-iNOS polyclonal Ab; B: Anti-rat macrophage Ab; C: Dual staining demonstrating iNOS expression in macrophages; D: Phase contrast of cells shown in A to C; E: Anti-iNOS polyclonal Ab; F: Anti-rat PMN Ab; G: Dual staining demonstrating minimal iNOS expression in PMNs; H: Phase contrast of cells shown in E to G. Bar, 10 μm.
To quantify iNOS expression in wound macrophages and PMNs, all cells displaying any specific staining for iNOS were scored as positive and counted, and their relative fluorescence was quantified using image analysis (Table 2) ▶ . Applying this criterion to results from six independent experiments, the majority of day 1 wound macrophages were positive for iNOS, whereas approximately one-half of PMNs in these wounds contained detectable iNOS antigen. Although there were, indeed, iNOS-positive PMNs to be found in these preparations, there was an order of magnitude difference in the intensity of iNOS-specific staining between macrophages and PMNs (Table 2) ▶ . Moreover, as will be shown later, PMNs found by immunofluorescent staining to contain iNOS antigen may not actually express iNOS activity.
Table 2.
Immunodetection of iNOS in Day 1 Wound-Derived PMNs and Macrophages
| Cells | % iNOS-positive cells | Relative fluorescent intensity |
|---|---|---|
| PMNs | 40–65 | 1.16 × 105 |
| Macrophages | 70–85 | 1.12 × 106* |
Data are the range of the percentage of iNOS-positive PMNs or macrophages in six independent experiments and the relative immunofluorescent staining intensity in these cells determined as described in Materials and Methods.
*P = 0.0001 versus PMN (Mann-Whitney U test).
The frequency of iNOS-positive macrophages in the wound declined after day 1. On days 3 and 5 after wounding, 26.7% and 10% of macrophages contained iNOS by immunofluorescent staining, respectively (not shown). By day 10, 5% to 7% of macrophages, which as stated previously constitute >95% of the cells harvested from the wounds at this time, showed immunofluorescent evidence for iNOS antigen (Figure 3) ▶ .
Figure 3.
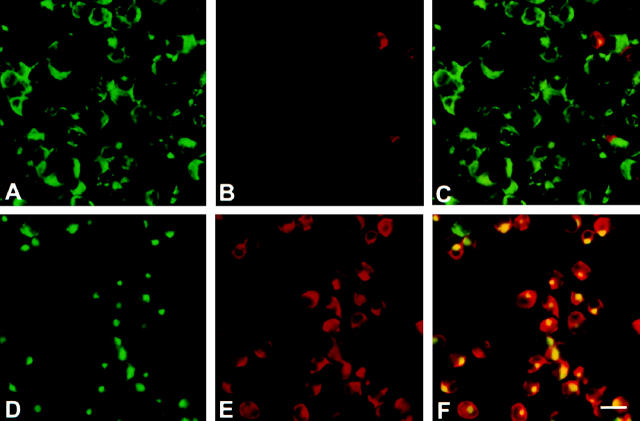
Immunofluorescent analysis of iNOS expression in wound macrophages harvested 10 days after injury demonstrating infrequent iNOS expression in freshly harvested cells and its induction after overnight culture. Macrophages were stained on harvest (A to C) or after overnight culture (D to F). A and D: Anti-rat macrophage Ab; B and E: Anti-iNOS polyclonal Ab; C and F: Dual staining. Bar, 20 μm.
Acquisition of iNOS Antigen by Late Wound Macrophages in Culture
Previous findings from this laboratory demonstrated that overnight culture of wound-derived macrophages obtained 10 days after injury resulted in increased cellular content of iNOS antigen. 3 In agreement with these findings, immunofluorescent staining demonstrated that 5% to 7% of the wound day 10 cells expressed iNOS immediately on harvest, increasing to 29% to 49% iNOS-positive cells after overnight culture (Figure 3) ▶ . This variability represents the range found in data collected from six separate experiments. iNOS was found to be present in a subset of macrophages rather than being uniformly distributed among the entire population both in freshly harvested and overnight cultured cells. No quantitative differences were detected in the intensity of iNOS immunofluorescent staining between iNOS-positive macrophages stained immediately on harvest and those examined after overnight culture. A redistribution of the antigen recognized by the anti-rat macrophage Ab KU-1 may be noted after overnight culture (Figure 3, A and D) ▶ . This is a reproducible, albeit incidental, finding in the context of this report as the Ab is used here solely as a phenotypic marker. The nature of the epitope recognized by this Ab remains uncharacterized although preliminary data suggest it might be the rat equivalent of the human CD68 antigen (unpublished observations).
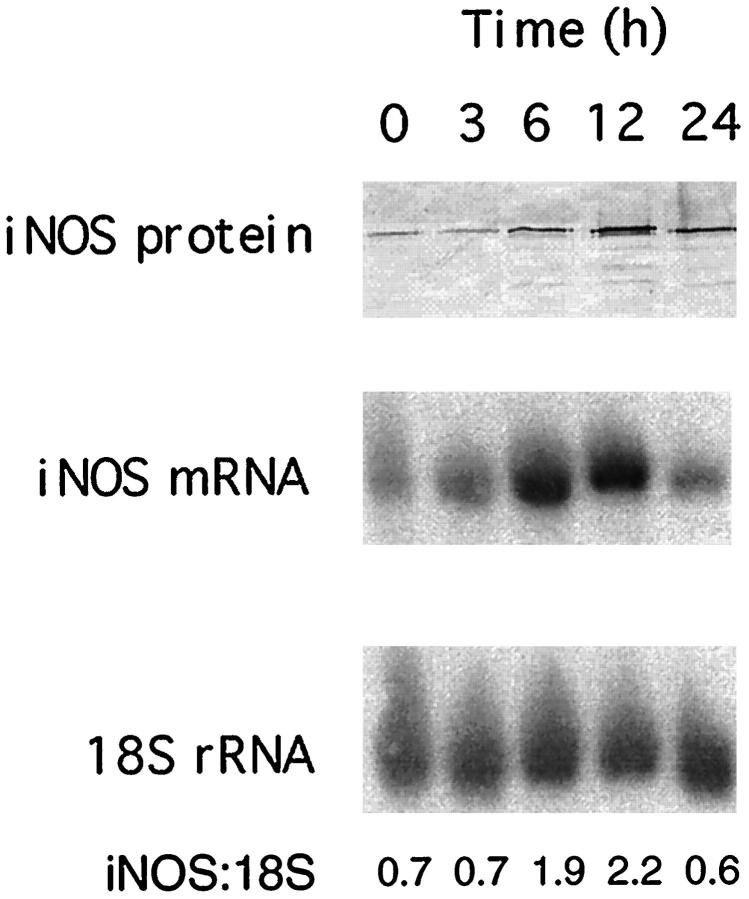
More closely examining the acquisition of iNOS by macrophages harvested from 10-day-old wounds in culture, results shown in Figure 4 ▶ illustrate the time course of the appearance of iNOS antigen in these cells during an overnight incubation as well as evidence that iNOS mRNA accumulates in the cells before the appearance of iNOS protein. That the expression of iNOS in cultured wound macrophages did not result from cell adherence in culture was demonstrated in experiments where cells were incubated in Teflon bags. Cells so cultured also acquired iNOS protein during overnight incubation (not shown). Differentiation into wound macrophages is apparently necessary for the induction of iNOS in culture as blood monocytes did not express iNOS after overnight culture (not shown).
Figure 4.
Immunoblot and Northern blot analysis of iNOS in macrophages harvested from wounds 10 days after injury and cultured for the times indicated.
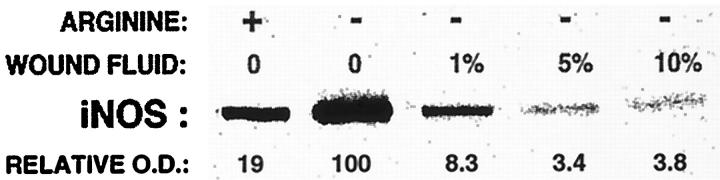
As demonstrated in Figure 4 ▶ , removal of macrophages from the wound and their culture results in increased expression of iNOS in the cells. Although this phenomenon could result from the effects of any of multiple media components, it could also be explained by the presence of inhibitors or suppressors of iNOS induction in the extracellular space of the wound. To explore this possibility, wound macrophages harvested 10 days after wounding were cultured overnight in the presence of varying concentrations of cell-free wound fluid obtained from the same animals (1% to 10% v/v) or normal rat serum up to 20% by volume. Figure 5 ▶ illustrates results from these experiments. Data shown there demonstrate, first, that the addition of 1 mmol/L l-arginine to culture media suppresses the induction of iNOS relative to results obtained when l-arginine was added at 6 μmol/L, a concentration closely resembling that actually found in the extracellular space of wounds 10 days after wounding. 2 This finding supports the conclusion made previously, on the basis of experiments where NO production by wound macrophages was modulated through the regulation of O2 availability, that NO down-regulates iNOS expression. 3 In regard to the potential presence of an inhibitor of iNOS expression in wound fluids, addition of such fluids to 6 μmol/L l-arginine medium resulted in a dose-related suppression of iNOS appearance. Rat serum was without effect and is not shown.
Figure 5.
Evidence that day 10 wound macrophage culture in media containing l-arginine (1 mmol/L) or wound fluid harvested from the same wounds suppresses the induction of iNOS. Wound macrophages harvested 10 days after wounding were cultured overnight in media containing 1 mmol/L (+) or 6 μmol/L (−) l-arginine, with or without the addition of cutaneous wound fluid at 1, 5, or 15% by volume. The addition of normal rat serum up to 20% by volume did not suppress iNOS induction (not shown).
Correlation between Immunodetectable iNOS and iNOS Activity
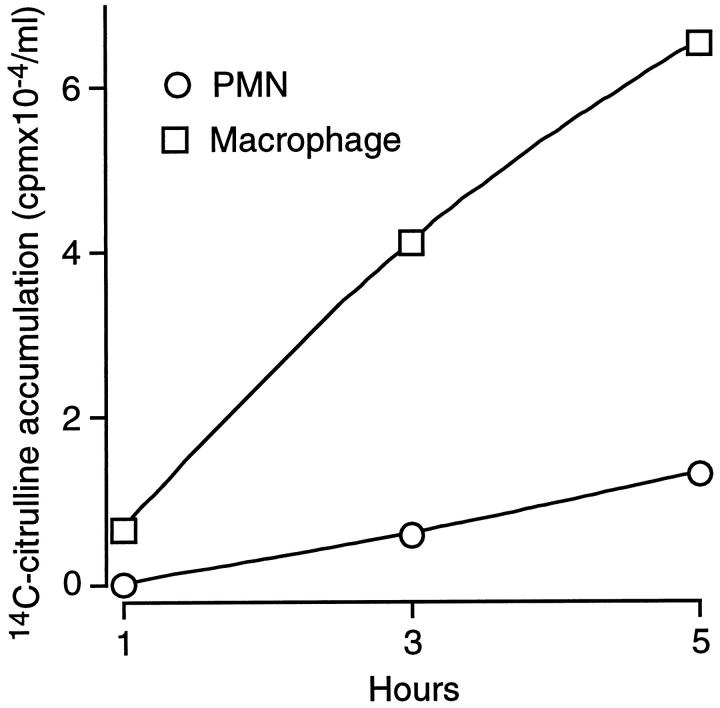
To further define the activity of iNOS in freshly harvested day 1 and day 10 wound cells, these cells were analyzed for their ability to metabolize l-arginine to l-citrulline in culture and for NADPH diaphorase activity. 10 Figure 6 ▶ illustrates results of experiments where wound cells isolated 1 day after wounding were partially enriched for PMNs or macrophages and cultured for up to 5 hours in the presence of [guanido-14C]l-arginine. Results in Figure 6 ▶ indicate that most l-arginine catabolism through NOS to [ureido-14C]l-citrulline was conducted by the macrophage-enriched population, with little flux through this enzyme in the PMN-enriched fraction. Moreover, Figure 7A ▶ demonstrates that only macrophages in day 1 wound-cell preparation expressed NADPH diaphorase activity.
Figure 6.
NOS activity in day 1 wound cells enriched for macrophages or PMNs. Cells isolated from day 1 wounds were enriched for macrophages or PMNs as described in Materials and Methods. Unfractionated cell preparations contained 14.5% macrophages and 83.7% PMNs. The macrophage-enriched population contained 41.8% macrophages and 56.9% PMNs. The PMN-enriched population contained 3.3% macrophages and 96.3% PMNs. Cells were cultured for 1, 3, or 5 hours in the presence of [guanido-14C]l-arginine (1 μCi/ml). Culture supernatants were assayed for the appearance of [ureido-14C]l-citrulline by HPLC. Error bars were smaller than the symbols indicating the values of the means.
Figure 7.
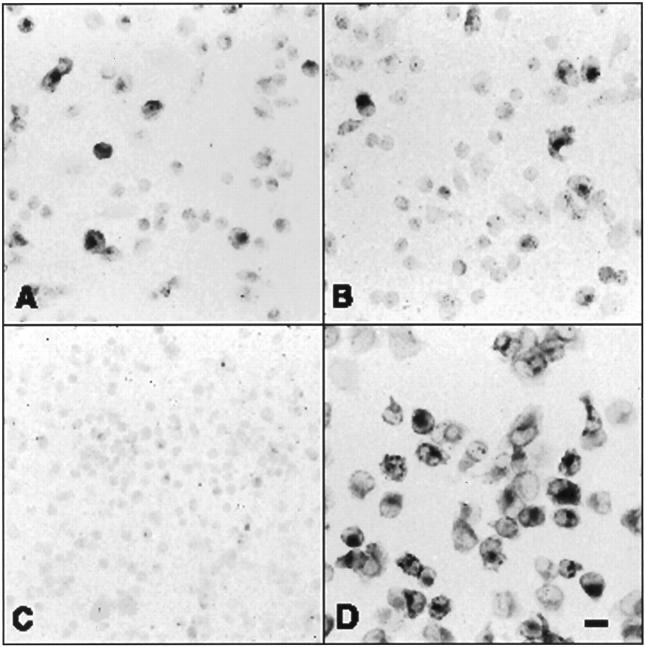
Histochemical detection of NADPH diaphorase activity in freshly harvested day 1 wound cells (A and B), freshly harvested day 10 wound cells (C), and overnight cultured day 10 wound cells (D).
Similar metabolic experiments were performed using day 10 wound macrophages. Results from these experiments are shown in Table 3 ▶ , demonstrating that freshly harvested macrophages produced little radiolabeled l-citrulline from radiolabeled l-arginine. Results shown in Figure 7C ▶ , in addition, demonstrate that no NADPH diaphorase activity was detectable in these cells. Overnight cultured day 10 macrophages produced substantially more labeled l-citrulline from l-arginine (Table 3) ▶ and stained strongly for NAPDH diaphorase activity (Figure 7D) ▶ . These results suggest that iNOS present in freshly harvested day 10 wound-derived macrophages, in contrast to that found in day 1 cells and in overnight cultured day 10 macrophages, has limited to nil functional activity.
Table 3.
l-Arginine Metabolism by Freshly Harvested and Overnight Cultured Day 10 Wound-Derived Macrophages
| Macrophage | Citrulline production (nmol/h/106 cells) |
|---|---|
| Freshly harvested | 0.18 ± 0.07 |
| Overnight cultured | 4.01 ± 0.33 |
Wound-derived macrophages obtained 10 days after injury were cultured in the presence of 1 mmol/L l-arginine and 0.1 μCi/ml [guanido 14C]l-arginine for 2 hours either immediately after harvest or after overnight culture. The appearance of radiolabel in l-citrulline, an indicator of iNOS activity, in culture supernatants was determined as indicated in Materials and Methods.
Discussion
The present results confirm and extend previous reports from this laboratory indicating a temporally segregated pattern of expression of enzymes of l-arginine catabolism in experimental wounds. 1,2 According to data reported here and elsewhere, 2 the inducible form of NOS is significantly and actively expressed in wound cells only during the initial 72 hours after injury.
Additional results identified the cellular population expressing iNOS in early wounds as macrophages and not PMNs. Although multiple cell types, including endothelial cells and platelets, could contribute to the production of NO in the early phases of inflammation, it was of interest to note that blood monocytes lacked iNOS and that wound macrophages expressed this enzyme as early as 6 hours after wounding. PMNs present in wounds harvested at that time, although theoretically capable of expressing iNOS, were mostly silent for immunodetectable iNOS, consumed little l-arginine in culture, and showed no histochemical evidence for NADPH diaphorase activity.
As mentioned previously, a report by Miles et al 9 concluded that PMNs present in the peritoneal cavity of rats injected with oyster glycogen expressed iNOS. This conclusion was based on findings in immunoblots performed on lysates of unfractionated peritoneal exudate cells. It appeared unlikely that two methods for PMN elicitation, wounding or glycogen injection, would give discordant results in terms of iNOS detection. Immunodetection experiments were performed, therefore, using glycogen-elicited peritoneal PMNs. Just as described by Miles et al, immunoblotting of lysates prepared from these cells provided a strong signal for iNOS (not shown). However, immunofluorescent staining of the cells indicated that, just as found in wound cells, it is the macrophages present in the preparation that account for the positive identification of iNOS (not shown).
Macrophages proved to be, on a cell-by-cell basis, the predominant cell in the expression of iNOS in early wounds. It must be kept in mind, however, that over 85% of all cells isolated from those wounds are PMNs, so that low-level iNOS expression by these cells could result in substantial aggregate NO production. This does not seem to be the case because these cells were not capable of metabolizing significant amounts of l-arginine to l-citrulline in culture and because NADPH diaphorase staining of early wound cells demonstrated no enzyme activity in the PMNs. It may be that iNOS protein that is immunodetected in these cells is functionally inert.
iNOS expression in wound cells declined as the wound aged. The markedly reduced immunodetectable iNOS in macrophages freshly harvested from day 10 wounds, their limited capacity to metabolize l-arginine to l-citrulline when assayed immediately after harvesting, and results of NADPH diaphorase staining experiments are in line with previous observations indicating the inability of late wound explants to process l-arginine to l-citrulline in culture. 2
As demonstrated in this communication, freshly harvested day 10 wound macrophages contain little immunoreactive iNOS and iNOS mRNA. iNOS-specific mRNA appears in the cells within 3 hours of culture, and iNOS protein follows at approximately 12 hours. The suppression of iNOS appearance in the cells by culture with wound fluids and not with normal rat serum suggests the presence of a fluid-phase inhibitor of iNOS induction in late wounds. Preliminary results argue against transforming growth factor (TGF)-β as the cytokine responsible for the suppressive effects of wound fluids because the inclusion of a neutralizing anti-TGF-β antibody in cultures of wound macrophages with wound fluid failed to allow the appearance of iNOS protein in the cells. The potential roles of other compounds known to be able to inhibit the induction of iNOS in macrophages (interleukin-10, interleukin-4, or corticosterone) are under current investigation. Incidentally, the appearance of iNOS protein and activity in day 10 wound-derived macrophages during culture explains the finding of high concentrations of NO2−, a degradation product of NO, in supernatants of overnight incubations of these cells that was reported by others. 11
The temporal restriction in the expression of iNOS in wounds shown here may not precisely apply to other species or wound types. A recent report describes the delayed expression of iNOS, with peak message found in the wound 4 days after injury, in cutaneous wounds in mice. 12 Moreover, that report indicates that wound closure proceeds slower in iNOS knock-out mice than in wild-type animals and that delayed repair in iNOS-deficient animals could be reversed by the local application of an adenoviral vector containing human iNOS cDNA at the time of injury. It must be kept in mind that knock-out animals did, after all, close their wounds. Thus, although potentially relevant in determining the rate of repair, early iNOS expression does not appear to be essential to wound healing.
It can be proposed that the suppression of iNOS in late wounds may be important to the process of repair. In this connection, evidence reported here and elsewhere indicates that the late wound environment is particularly adverse to the expression of iNOS. First because, as shown here, wound fluids suppress the expression of iNOS in wound macrophages. Second, because wound fluids harvested from late wounds contain a remarkably high arginase activity, which results in the almost complete disappearance of l-arginine from the free amino acid pool of the wound extracellular space. 1,2 The wound environment, then, both suppresses iNOS expression and decreases substrate availability to this enzyme. As judged from the amino acid composition of wound fluid 1,2 and the lack of l-citrulline production by cultured whole wounds previously reported from this laboratory, 2 this double hit effectively suppresses iNOS activity in late wounds. The antiproliferative effects of NO or its derivatives, 13,14 their ability to suppress protein synthesis, 15 and their capacity to inhibit prolyl hydroxylase, 16 an enzyme that is rate limiting for the processing of nascent collagen, may explain the need to suppress the expression of iNOS in wounds after the phase of early inflammation by multiple and overlapping mechanisms.
Acknowledgments
The authors thank Jill Rose for her assistance in manuscript preparation.
Footnotes
Address reprint requests to Dr. Jonathan S. Reichner, Surgical Research, Rhode Island Hospital, 593 Eddy Street, Providence, RI 02903. E-mail: Jonathan_Reichner@brown.edu.
Supported in part by National Institutes of Health grants GM-42859 (J.E. Albina) and GM-51493 (J.S. Reichner). A.J. Meszaros and C.A. Louis are supported in part by NIEHS Pathobiology Graduate Program training grant T32 ES07272. Additional funding was provided by the Anita Allard Memorial Fund and by Rhode Island Hospital.
References
- 1.Albina JE, Mills CD, Barbul A, Thirkill CE, Henry WL, Jr, Mastrofrancesco B, Caldwell MD: Arginine metabolism in wounds. Am J Physiol 1988, 254:E459-E467 [DOI] [PubMed] [Google Scholar]
- 2.Albina JE, Mills CD, Henry WL, Jr, Caldwell MD: Temporal expression of different pathways of l-arginine metabolism in healing wounds. J Immunol 1990, 144:3877-3880 [PubMed] [Google Scholar]
- 3.Albina JE, Henry WL, Jr, Mastrofrancesco B, Martin B-A, Reichner JS: Macrophage activation by culture in an anoxic environment. J Immunol 1995, 155:4391-43967594599 [Google Scholar]
- 4.Louis CA, Reichner JS, Henry WL, Jr, Mastrofrancesco B, Gotoh T, Mori M, Albina JE: Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. Am J Physiol 1998, 274:R775-R782 [DOI] [PubMed] [Google Scholar]
- 5.Schäffer MR, Tantry U, Gross SS, Wasserkrug HL, Barbul A: Nitric oxide regulates wound healing. J Surg Res 1996, 63:237-240 [DOI] [PubMed] [Google Scholar]
- 6.Bodenheimer HC, Faris RA, Charland C, Hixson DC: Characterization of a new monoclonal antibody to rat macrophages and Kupffer cells. Hepatology 1988, 8:1667-1672 [DOI] [PubMed] [Google Scholar]
- 7.Sekiya S, Gotoh S, Yamashita T, Watanabe T, Saitoh S, Sendo F: Selective depletion of rat neutrophils by in vivo administration of a monoclonal antibody. J Leukoc Biol 1990, 46:96-102 [DOI] [PubMed] [Google Scholar]
- 8.Albina JE, Reichner JS: Nitric oxide in inflammation and immunity. New Horizons 1995, 3:46-64 [PubMed] [Google Scholar]
- 9.Miles AM, Owens MW, Milligan S, Johnson GG, Fields JZ, Ing TS, Kottapalli V, Keshavarzian A, Grisham MB: Nitric oxide synthase in circulating versus extravasated polymorphonuclear leukocytes. J Leukoc Biol 1995, 58:616-622 [DOI] [PubMed] [Google Scholar]
- 10.Hon WM, Chhatwal VJS, Khoo HE, Moochhala SM: Histochemical method for detecting nitric oxide synthase activity in cell cultures. Biotech Histochem 1997, 72:29-32 [DOI] [PubMed] [Google Scholar]
- 11.Schäffer MR, Tantry U, Van Wesep RA, Barbul A: Nitric oxide metabolism in wounds. J Surg Res 1997, 71:25-31 [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki K, Edington HDJ, McClosky C, Tzeng E, Lizonova A, Kovesdl I, Steed DL, Billiar TR: Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest 1998, 101:967-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albina JE, Henry WL, Jr: Suppression of lymphocyte proliferation through the nitric oxide synthesizing pathway. J Surg Res 1991, 50:403-409 [DOI] [PubMed] [Google Scholar]
- 14.Albina JE, Abate JA, Henry WL, Jr: Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. J Immunol 1991, 147:144-148 [PubMed] [Google Scholar]
- 15.Kolpakov V, Gordon D, Kulik TJ: Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res 1995, 76:305-309 [DOI] [PubMed] [Google Scholar]
- 16.Cao M, Westerhausen-Larson A, Niyibizi C, Kavalkovich K, Georgescu HI, Rizzo CF, Hebda PA, Stefanovic-Racic M, Evans CH: Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J 1997, 324:305-310 [DOI] [PMC free article] [PubMed] [Google Scholar]