Abstract
Rheumatoid arthritis is characterized by hyperplasia of the synovial lining and invasion of cartilage and bone by a subset of resident synovial cells named fibroblast-like synoviocytes. They are characterized by elevated expression of the vascular cell adhesion molecule-1 (VCAM-1). The intensity of VCAM-1 expression correlates with the degree of inflammation of the synovial joint. Differential VCAM-1 expression may determine inflammatory cell accumulation through its interaction with leukocytes that express the counterreceptor integrins α4β1 and α4β7. Elevated levels of VCAM-1 expression are thought to be a consequence of the presence of inflammatory mediators, in particular IL-1β and TNF-α. Fibroblast-like synoviocytes rapidly up-regulate VCAM-1 expression in response to IL-1β and TNF-α, but also to IL-4. However, we now show that the response to IL-1β or TNF-α is of a brief transient nature, even when applied continuously over a period of 12 days, whereas the response to IL-4 or IL-13 is sustained. Great synergy is obtained by combining either IL-4 or IL-13 with TNF-α, which results in a highly elevated but also sustained expression of VCAM-1. The mechanism by which IL-4 or IL-13 prolongs VCAM-1 expression can be explained by a dramatic increase in the half-life of VCAM-1 mRNA.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory condition which is characterized in part by hyperplasia of the synovial lining. 1 Hyperplasia of the synovial lining results from proliferation of resident fibroblast-like synoviocytes (FLS) together with invasion of blood-borne inflammatory cells. These inflammatory cells migrate through the synovial high endothelial venules and partition themselves within the synovium and synovial fluid. The recruitment and retention of these cells is mediated by a family of cell surface receptors known as the cell adhesion molecules. 2 Adhesion molecules are now believed to play a crucial role in the pathogenesis of RA due to their up-regulation in response to certain pro-inflammatory cytokines and their ability to act as costimulatory receptors in the activation of T cells. 3
One adhesion molecule that shows prominent up-regulation in RA synovium is vascular cell adhesion molecule-1 (VCAM-1), a member of the immunoglobulin (Ig) superfamily of adhesion molecules. 4-6 VCAM-1 was originally cloned from pro-inflammatory cytokine-treated human umbilical vein endothelial cells (HUVECs). 7,8 VCAM-1 is expressed on fibroblast-like cells within the intimal layer of normal, osteoarthritis, and RA synovium. 5 Moreover, the intensity of VCAM-1 expression correlates with the degree of synovial inflammation. 4 Differential VCAM-1 expression may determine inflammatory cell accumulation: adhesive interactions between VCAM-1 and its ligands, the integrins α4β1 and α4β7, may explain the accumulation of α4β1-positive mononuclear cells and α4β7-positive lymphocytes within the synovium, and the loss of α4β1-negative neutrophils to the synovial fluid. 4,9 RA FLS are also able to induce the release of a number of pro-inflammatory cytokines and metalloproteinases from both T cells and monocytes following their co-culture. 10-12 A specific role for VCAM-1 in the induction of metalloproteinase expression was clearly demonstrated in a study where activated endothelial cells were combined with lymphocytes. 13 These results, together with those recently published by Müller-Ladner and co-workers (1996) demonstrating that VCAM-1-positive FLS possess an invasive and destructive phenotype, suggests that FLS are involved in the pathogenesis and perpetuation of RA, with VCAM-1 possibly playing a central role. 13,14
Whereas the mechanisms of VCAM-1 transcriptional control have been extensively studied in HUVECs, 15-19 it is not known whether similar mechanisms control VCAM-1 expression in RA FLS. In contrast to HUVECs, which demonstrate no basal expression of VCAM-1, FLS were shown to have some form of constitutive or sustained VCAM-1 expression. 4 During isolation and subsequent culture of synovial fibroblasts we found that VCAM-1 expression was initially very high, but declined with time to low basal levels. The initially high level could be explained by the presence of inflammatory cytokines present in the joint. 20 The subsequent absence of these cytokines in culture would explain the decline. For instance, it has been demonstrated that TNF-α rapidly induces VCAM-1 expression in FLS at the levels of mRNA and protein. 4,21 However, the long-term consequences of the presence of inflammatory cytokines on VCAM-1 expression has not yet been addressed. We have set out in a series of experiments to study the time course of VCAM-1 expression in cultured FLS after the addition of various cytokines (IL-1β, TNF-α, IL-4, and IL-13). In this report we show that chronic administration of TNF-α over a period of 12 days can prolong the transient expression of VCAM-1 observed with a single TNF-α treatment from 3 to 6 days. The combination of TNF-α with either IL-4 or IL-13 results in sustained elevated levels of VCAM-1 expression. At the molecular level, we demonstrate that IL-4 and IL-13 mediate these effects by greatly reducing degradation of VCAM-1 mRNA transcripts. We conclude that the presence of either TNF-α and IL-1β alone is not sufficient to achieve sustained elevated expression of VCAM-1 on FLS in the RA synovial joint, and that IL-4-like cytokines are needed.
Materials and Methods
Reagents
Recombinant human IL-1β, IL-4, IL-13, and TNF-α were purchased from R&D Systems (Abingdon, UK) and used at a final concentration as indicated in the figure legends. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin were obtained from Life Technologies (Paisley, UK). Mouse anti-human VCAM-1 (BBA-5) was purchased from R&D Systems. Phycoerythrin (PE)-conjugated Fab2 anti-mouse IgG, tri-reagent and actinomycin D were purchased from Sigma-Aldrich Chemical Company (Poole, UK). Bovine serum albumin (BSA), fraction V, was obtained from ICN Biomedicals (Thame, UK). Collagenase type II (204 U/mg) was obtained from Worthington Biochemical (Lakewood, NJ). Ready-To-Go first strand cDNA-synthesis kit was from Amersham-Pharmacia Biotech (St. Albans, UK).
Cell Culture
FLS were isolated by enzymatic dispersion of synovial tissue collected from patients suffering from osteoarthritis and rheumatoid arthritis at the time of total hip or knee joint replacement surgery. All tissues were processed within 1–2 hours after surgery. In brief, synovium was dissected away from the surrounding joint capsule and washed extensively with phosphate buffered saline (PBS). Tissue was minced and digested in serum-free DMEM containing 2 mg/ml collagenase and penicillin-streptomycin (1% v/v) for 1 hour at 37°C in a shaking incubator. The cell suspension was sheared by repeatedly passing through a sterile syringe, filtered through sterile nylon gauze (100 μm) and washed repeatedly with PBS containing 1% w/v BSA. The cell pellet obtained following the last wash was resuspended in DMEM containing FBS (10% v/v) and penicillin-streptomycin (1% v/v) and cultured for 18 hours in a humidified 5% CO2 atmosphere at 37°C. Nonadherent cells were removed by extensive washing with PBS. Adherent cells were cultured in DMEM containing FBS (10% v/v) and penicillin-streptomycin (1% v/v). Cells were passaged when reaching confluence and replated at a ratio of 1:3. In the case where VCAM-1 expression was measured over an 8-week culture, cells were not passaged but medium was replaced every 5 days. For cytokine studies FLS were used between passages 3 and 10, corresponding to 3–12 weeks.
Flow Cytometry
Following the required time in culture, cells were washed twice with ice-cold PBS. FLS were harvested by incubation with PBS-4 mmol/L EDTA (20 minutes; 4°C). Most of the cells rounded up following this treatment and could be removed by gentle agitation. Any cells which failed to detach were removed with gentle scraping. Cells were incubated (20 minutes at 4°C) in an appropriate volume of 3% v/v human serum (Type AB) and stained with either control anti-IgG1 antibody or anti-VCAM-1 antibody (40 minutes at 4°C). Cells were washed with PBS-BSA (1% w/v) and incubated with PE-conjugated Fab2 anti-mouse IgG. Flow cytometry was performed using a FACScan flow cytometer (Becton Dickinson, Cowley, UK). Cells were gated using forward versus side scatter to remove any dead cells and cellular debris and thus give a uniform population of FLS. For each sample 5000 cells were analyzed. Results are expressed as the corrected mean fluorescence intensity (MFI) following subtraction of nonspecific fluorescence of IgG control.
Immunohistochemistry
Cells were cultured on 4-well chamber slides, washed in PBS twice, and fixed, in ice-cold methanol (4 minutes), followed by an incubation in ice-cold acetone (1 minute). Fixed cells were then incubated in FBS (10% v/v in PBS). All the following procedures were performed at room temperature. After washing 4 times in PBS, cells were incubated with a mouse anti-human-VCAM-1 monoclonal antibody (60 minutes). Endogeneous peroxidase activity was quenched with 3% hydrogen peroxide in methanol (5 minutes). After washing in PBS, a biotinylated goat-anti-mouse-IgG antibody was added for 30 minutes. Antibody binding was visualized by the addition of a streptavidin-coupled peroxidase complex in the presence of 3,3-amino-9-ethylcarbazole for 10 minutes. The reaction was stopped by washing the slides in H2O and nuclei were counterstained with hematoxylin.
RNA Isolation and Semiquantitative RT-PCR
Total cellular RNA was isolated using tri-reagent according to the manufacturer’s instructions. Total RNA (2 μg) was reverse transcribed using a Ready-To-Go first strand cDNA synthesis kit for 60 minutes at 37°C. This kit utilizes the Moloney murine leukemia virus (M-MuLv) reverse transcriptase and an oligo (dT) primer to generate first strand cDNA. The completed first-strand reaction mix was heated (5 minutes at 90°C) to inactivate the reverse transcriptase and stored at −20°C.
Semiquantitative PCR was carried out on cDNA (equivalent to 10 ng of RNA) using oligonucleotide primers specific for the VCAM-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes. For each set of PCR primers the reaction conditions were optimized by varying cDNA dilutions, primer concentrations, annealing temperatures, and number of cycles. By optimizing the cycle number for each set of primers we ensured that amplification was in the linear range. VCAM-1 (sense, CAA GTC TAC ATA TCA CCC AAG (nucleotides 751–771), and antisense, GGA ACC TTG CAG CTT ACA GTG (nucleotides 1361–1382)) were modified from those previously published by Hession et al (1991). 8 This primer pair straddle the domain-4 insertion site and amplify both the 6-domain (355-bp product) and 7-domain (631-bp product) forms of VCAM-1. GAPDH primers (362-bp product), (sense, AAG GTG AAG GTC GGA GTC AAC (nucleotides 67–88), and antisense, GGC AGA GAT GAT GAC CCT TTT GGC (nucleotides 406–429)), were modified from those published by Dall et al (1995). 22 Each PCR reaction contained amplitaq polymerase (1.25 units), 1 × PCR buffer (10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.001% (w/v) gelatin), 200 μmol/L deoxynucleotide triphosphates (dNTPs), [α32P] dATP (2.5 μCi), 0.2 μmol/L of sense primer, 0.2 μmol/L of anti-sense primer, and cDNA (or water control) in a total volume of 25 μl. For each primer pair a master mix containing all of the above components except cDNA was made up and aliquots were placed in micro-PCR tubes containing cDNA to minimize contamination and variations between tubes. Negative controls were also performed to exclude the possibility of reagent contamination and the amplification of genomic DNA. Hot start PCR was carried out using a GeneAmp PCR system 9600 thermal cycler (Perkin Elmer) for varying cycles of amplification (26 and 30 cycles for GAPDH and VCAM-1, respectively). Following the initial denaturing step (94°C for 5 minutes), each cycle of amplification consisted of 30 seconds at 94°C, 30 seconds at an appropriate annealing temperature (60°C and 64°C for GAPDH and VCAM-1, respectively), and 1 minute at 72°C. PCR reactions were finished off with a final extension period at 72°C for 10 minutes. Primer-specific PCR products incorporating [α32P] dATP were electrophoresed through 8% polyacrylamide gel and quantified using a Storm 840 phosphorimager (Molecular Dynamics, Sunnyvale, CA) and ImageQuant software.
Statistical Analysis
All values given are the means ± standard error (SE) of the corrected MFI for the number (n) of experiments performed. Statistical analyses were performed using analysis of variance (ANOVA). P values <0.05 were considered significant. Where statistical significance was found, Student’s paired t-tests were performed to identify differences between groups.
Results
Gradual Loss of Highly Elevated Levels of VCAM-1 during Culture of FLS
Upon removal from the rheumatoid synovium, FLS demonstrate high levels of cell surface VCAM-1 expression for a period of 2 weeks. With increasing time in culture this level gradually decreases to a low but constitutive level of expression (Figure 1A) ▶ . With respect to the mRNA, a similar pattern is observed. High levels of both the 6- and 7-domain forms of VCAM-1 mRNA are observed in the first week, with a gradual loss over the following weeks (Figure 1B) ▶ . A basal or constitutive level of 7-domain VCAM-1 mRNA is observed during long-term culture. We took these data to mean that upon removal of the FLS from the rheumatoid synovial membrane, a number of inflammatory cytokines that control elevated VCAM-1 expression are lost.
Figure 1.
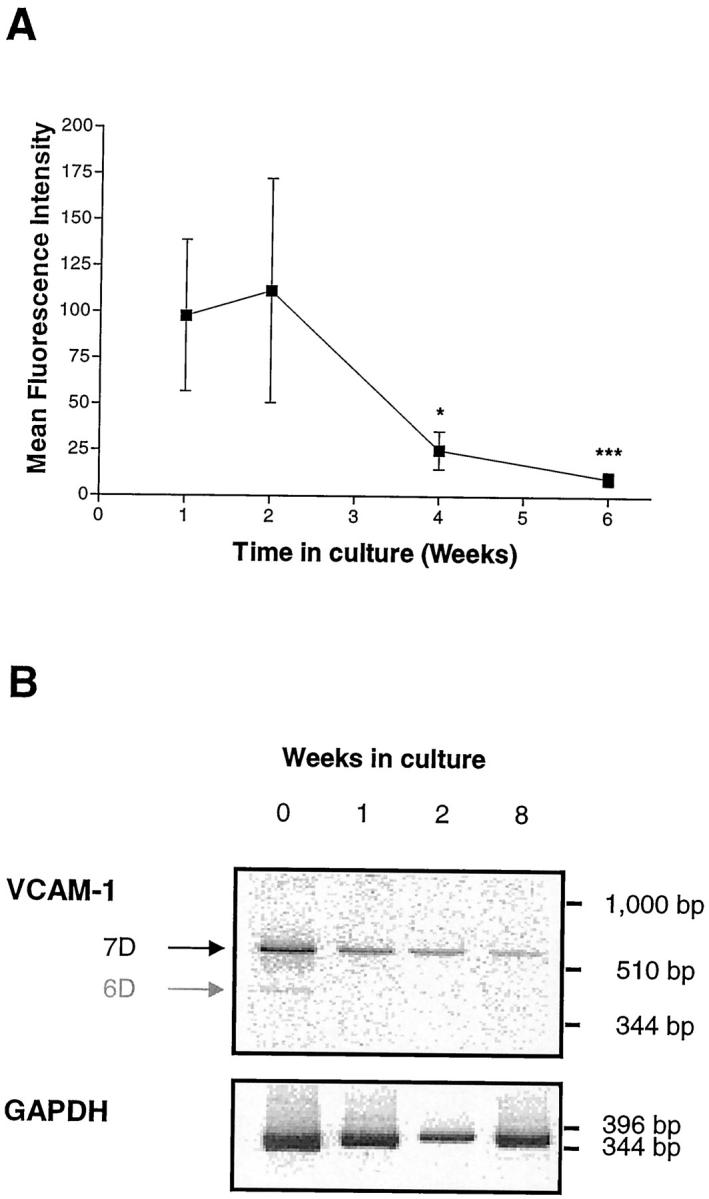
Elevated expression of VCAM-1 cell surface protein (A) and mRNA (B) by freshly isolated rheumatoid fibroblast-like synoviocytes declines with increasing time in culture. FLSs were isolated as described in Methods and cultured with 10% FBS for various periods of time. Medium was replenished every 5 days. A: cell surface VCAM-1 expression was analyzed after 1, 2, 4, and 6 weeks by flow cytometry using mAb BBA-5. Samples were analyzed using a FACScan flow cytometer and the results expressed as corrected mean fluorescence intensity. Data are presented as the mean ± SE of four experiments. * indicates p < 0.05, and *** p < 0.001 (compared with the results at week 1). B: VCAM-1 mRNA levels after 0 (ie, from freshly isolated synovial cells), 1, 2, and 8 weeks. Total RNA was isolated and semiquantitative RT-PCR performed using VCAM-1-specific and GAPDH-specific primers (see Methods). PCR products were visualized after electrophoresis on an 8% polyacrylamide gel and exposure to a phosphorimager. Note the presence of both the 6D and 7D mRNA transcripts in freshly isolated synovial cells.
Chronic Treatment of Primary FLS with IL-1β or TNF-α Induces Only Transient Expression of VCAM-1
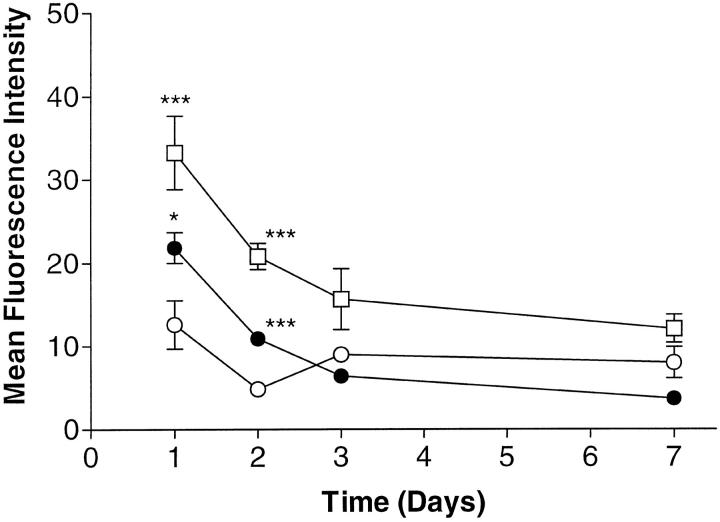
Two prominent inflammatory cytokines present in the inflamed joint, TNF-α and IL-1β, were found to be absent in our cell culture 12 and we therefore reasoned that re-addition of these cytokines could possibly restore constitutively elevated levels of expression of VCAM-1, as observed following removal from the RA synovium. At first, we tested the time course of VCAM-1 expression over a period of 7 days following a single addition of either IL-1β (10 ng/ml) or TNF-α (10 ng/ml). Elevated cell surface levels of VCAM-1 were obtained at 24 hours but the level returned to control levels by 72 hours, confirming previously published data (Figure 2) ▶ . 4 TNF-α was found to be more potent than IL-1β at inducing VCAM-1 expression.
Figure 2.
IL-1β and TNF-α induce transient VCAM-1 expression on fibroblast-like synoviocytes. Cells were treated with either medium only (open circles) , or medium plus IL-1β (10 ng/ml) (closed circles), or medium plus TNF-α (10 ng/ml) (open squares) for 1, 2, 3, or 7 days. VCAM-1 cell-surface expression was determined by flow cytometry using mAb BBA-5 as primary antibody. Samples were analyzed using a FACScan and the results expressed as corrected MFI. Data are presented as the mean ± SE of six experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with medium-only at the respective time point).
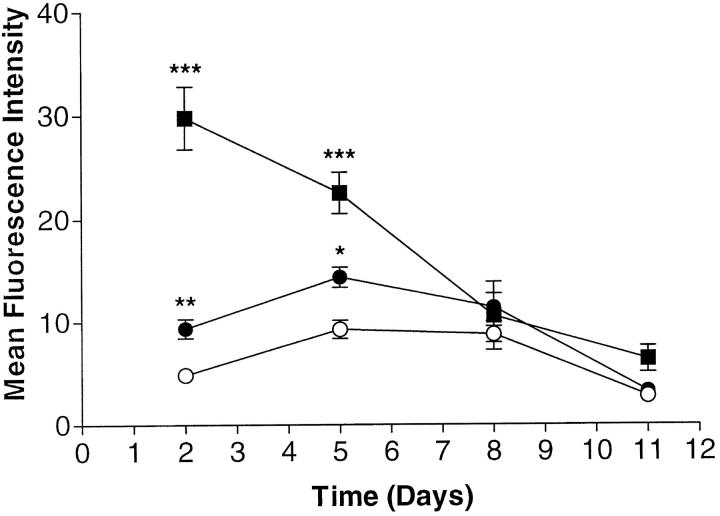
We next assessed the effect of repeated additions of IL-1β or TNF-α over a long-term incubation where cytokines were added at day 0 and re-added after each third-day change of medium. Cell surface expression of VCAM-1 was determined on days 2, 5, 8, and 11. Surprisingly, as can be seen from Figure 3 ▶ , VCAM-1 expression was maximal at day 2 but then declined and returned to baseline at day 8. Once VCAM-1 expression has reached baseline it remains at this level throughout the remaining time in culture (see also following sections). We took these data to mean that the addition of TNF-α alone could not explain prolonged elevated levels of VCAM-1, nor could it explain the slow decline that we observed after removal of cells from the synovial membrane. Another cytokine or other cytokines had to be implicated.
Figure 3.
Chronic-cytokine treatment with TNF-α and IL-1β prolongs transient VCAM-1 expression on fibroblast-like synoviocytes. Medium plus IL-1β (10 ng/ml) (closed circles), medium plus TNF-α (10 ng/ml), or medium alone (open circles) were added at day 0 and re-added at days 3, 6, and 9, over a 12-day period. Cell surface VCAM-1 expression was determined 48 hours after each cytokine addition using flow cytometry and mAb BBA-5 as primary antibody. Samples were analyzed using a FACScan and the results expressed as corrected MFI. Data are presented as the mean ± SE of six experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with medium-only control at the respective time point).
A Synergistic Increase in VCAM-1 Expression Is Observed with TNF-α in Combination with IL-4
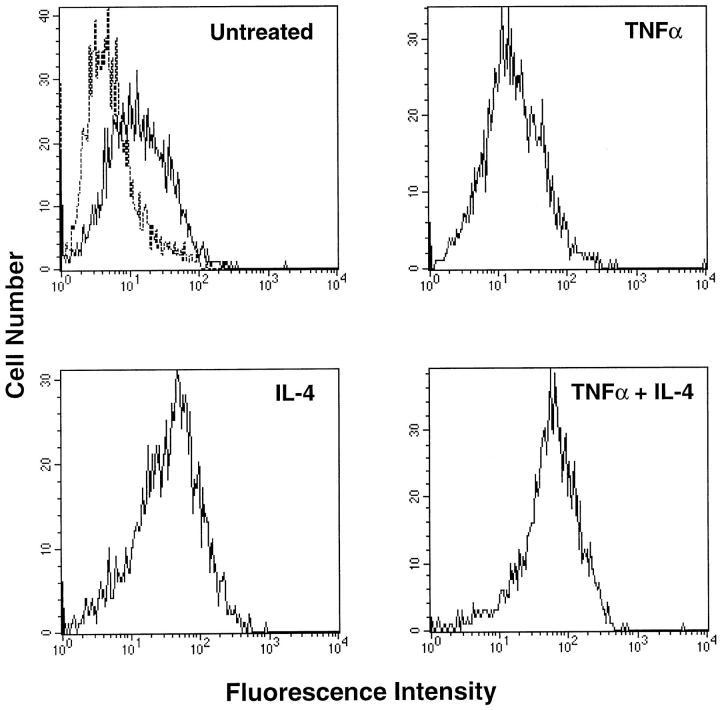
IL-4 had previously been described as a stimulus for the induction of VCAM-1 expression in FLS. 4 It was also shown that in HUVECs and smooth muscle cells that IL-4 can combine with TNF-α to give a synergistic increase in VCAM-1 levels. 16,23 We therefore tested the effect of IL-4 on the expression of VCAM-1 alone and in combination with TNF-α. We first tested the effects of this cytokine in a single-addition experiment and measured VCAM-1 levels after 72 hours. Treatment with IL-4 alone could induce VCAM-1 expression (MFI of 37.5) whereas TNF-α-induced expression had returned to baseline levels (MFI of 14.99). However, the combination of IL-4 with TNF-α resulted in a synergistic increase in cell surface VCAM-1 expression (MFI of 67.77) (Figure 4) ▶ . Treatment with higher doses of IL-4 (50 ng/ml) either alone or in combination with TNF-α could not further increase VCAM-1 expression (data not shown).
Figure 4.
Flow-cytometric analysis of VCAM-1 on RA fibroblast-like synoviocytes treated with TNF-α and IL-4. Cells were cultured with either medium only (untreated), TNF-α (10 ng/ml), IL-4 (10 ng/ml), or TNF-α plus IL-4 for 3 days. Flow cytometry for cell surface VCAM-1 was carried out using mAb BBA-5 as primary antibody. Samples were analyzed using a FACScan flow cytometer. The dashed histogram (in untreated cells) represents staining with PE-conjugated anti-mouse IgG only. Solid line histograms represent cells stained with anti-VCAM-1 and PE-conjugated anti-mouse IgG. Results shown are representative of four experiments.
TNF-α in Combination with IL-4 or IL-13 Can Greatly Prolong the Expression of VCAM-1 by FLS
IL-13 has a number of similar biological actions, which are mediated via its binding to the IL-4 receptor. 24-26 In addition, highly elevated levels of IL-13 were found in the synovial joint. 27 We therefore next tested whether the presence of IL-4 or IL-13 affected the time course of VCAM-1 expression as induced by TNF-α. We tested the effects of IL-4 or IL-13 in two experimental setups, a single cytokine application and multiple cytokine applications (long-term treatment). Addition of a single dose of IL-4 alone resulted in an immediate elevated expression that peaked at day 3 and returned to basal levels at day 7 (Figure 5A) ▶ . The combination of IL-4 and TNF-α, however, gave a synergistic increase in VCAM-1 expression, which peaked at day 7 and returned to baseline by day 14. Similar data were obtained with IL-13, with the difference that VCAM-1 expression levels, when added either alone or in combination with TNF-α, were lower compared to IL-4 (Figure 5B) ▶ .
Figure 5.
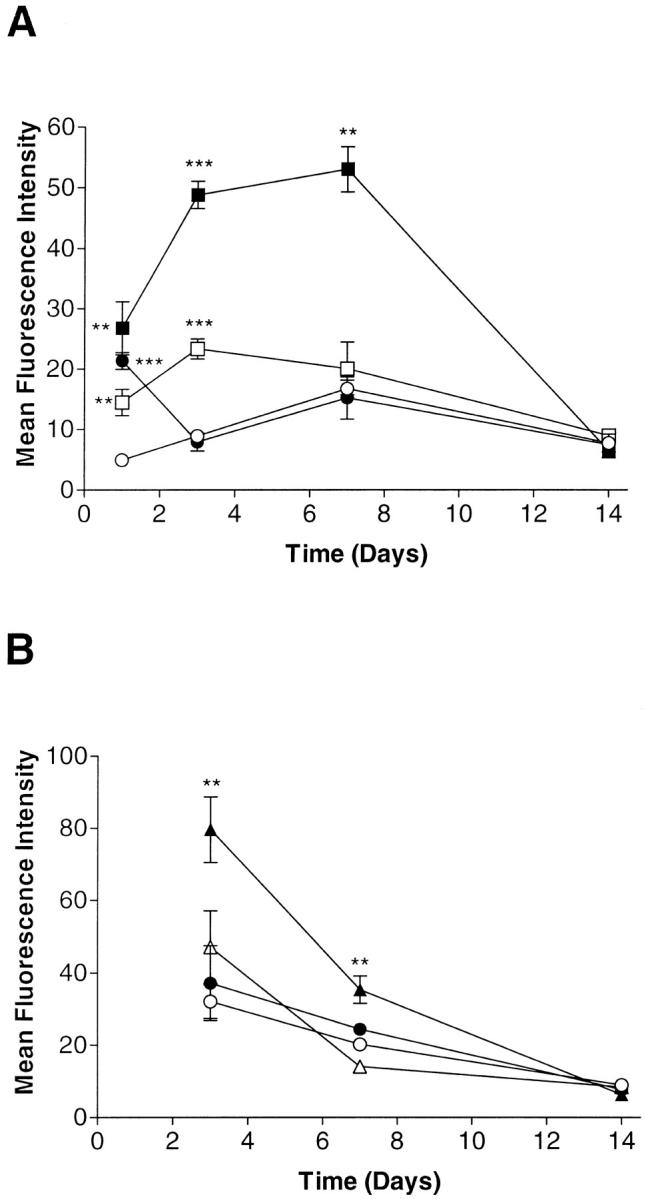
Time course of VCAM-1 expression in fibroblast-like synoviocytes treated with a single application of IL-4 (A) and IL-13 (B). Cells were cultured with either medium only (open circles), TNF-α (10 ng/ml) (closed circles), IL-4 (10 ng/ml) (open squares), IL-4 plus TNF-α (both at 10 ng/ml) (closed squares) , IL-13 (10 ng/ml) (open triangles), or IL-13 plus TNF-α (both at 10 ng/ml) (closed triangles). Cell surface VCAM-1 expression was analyzed at days 1, 3, 7, and 14 by flow cytometry using mAb BBA-5. Samples were analyzed using a FACScan and the results expressed as corrected MFI. Data are presented as the mean ± SE of four or five experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with medium-only control at the respective time point).
We next assessed the effect of chronic treatment with TNF-α (10 ng/ml) in combination with IL-4 or IL-13 (each at 10 ng/ml) over a long-term incubation. Cytokines were added at day 0 and re-added after each fourth-day medium change. Cell surface expression of VCAM-1 was determined on days 4, 8, 16, and 20. As shown in Figure 6 ▶ , chronic treatment with TNF-α was unable to prolong the transient expression of VCAM-1. Either IL-4 or IL-13 alone gave a significant up-regulation compared to the control and, moreover, induced an apparently higher level of expression than TNF-α alone. The combination of either IL-4 or IL-13 with TNF-α not only resulted in a synergistic increase in VCAM-1 expression, but also prolonged elevated expression for over 16 days. Again, IL-4 was more effective than IL-13 at similar concentrations.
Figure 6.
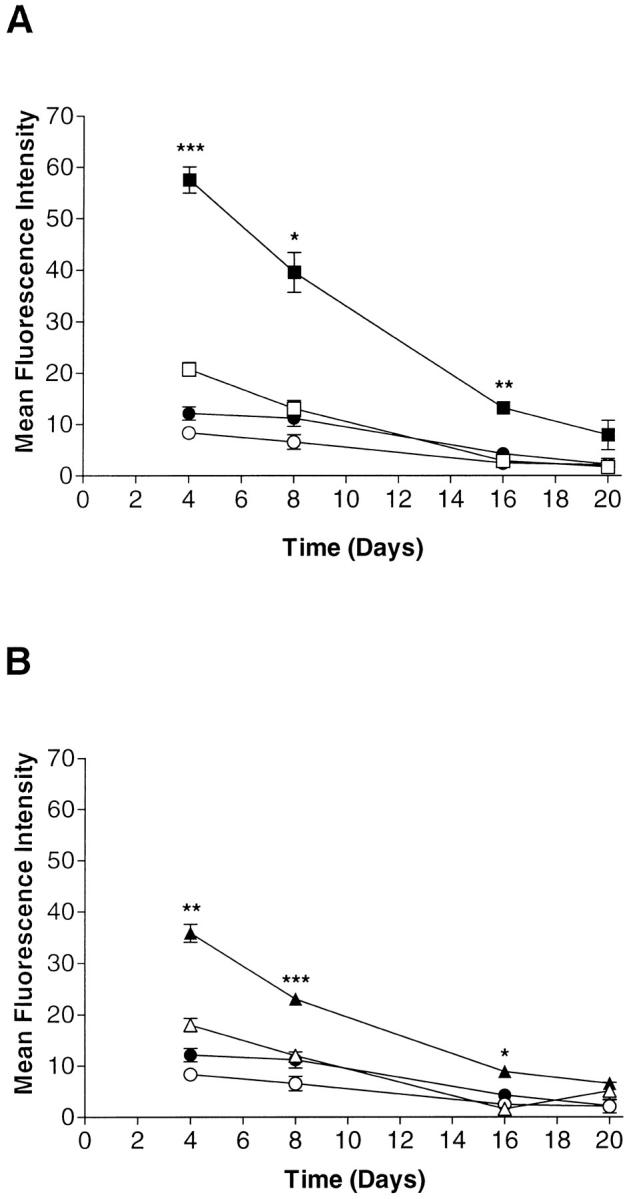
Chronic treatment with TNF-α in combination with either IL-4 (A) or IL-13 (B) can maintain elevated VCAM-1 expression on fibroblast-like synoviocytes for up to 16 days. Cells were treated with either medium only (open circles), TNF-α (10 ng/ml) (closed circles), IL-4 (10 ng/ml) (open squares) , or IL-4 plus TNF-α (both at 10 ng/ml) (closed squares), IL-13 (10 ng/ml) (open triangles), or IL-13 plus TNF-α (both at 10 ng/ml) (closed triangles) at day 0. Cytokines were re-added at days 4, 8, 12, and 16 over a 20-day period. Cell surface VCAM-1 expression was measured 4 days after cytokine application in a flow cytometry protocol with the aid of mAb BBA-5. Results are expressed as corrected MFI. Data are presented as the mean ± SE of four experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (In the top panel comparison is between IL-4 alone and IL-4 plus TNF-α and in the bottom panel IL-13 alone is compared with IL-13 plus TNF-α). In addition, for both IL-4 and IL-13 alone, VCAM-1 expression was significantly different from medium-only control (P < 0.05) at days 4 and 8.
Elevated Levels of Expression as Detected by Immunohistochemistry Can Be Achieved by Pathophysiological Concentrations of IL-13 in Combination with TNF-α
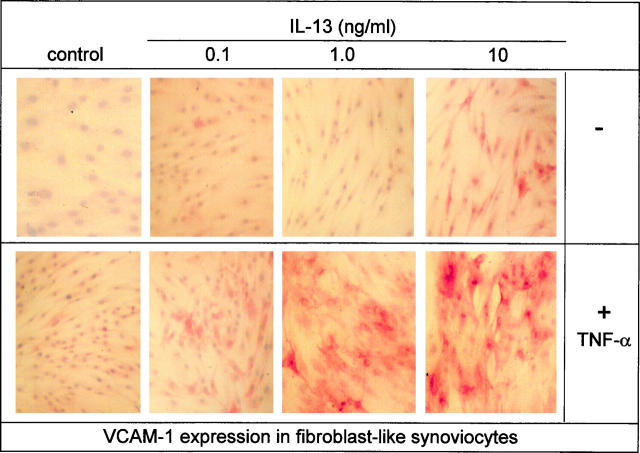
VCAM-1 expression levels in situ have been determined predominantly by the use of immunohistochemistry. Therefore, using this detection method we screened VCAM-1 expression levels induced by different concentrations of IL-13 in the presence of TNF-α (10 ng/ml). Highly elevated levels could be observed with as little as 1.0 ng/ml of IL-13 and 10 ng/ml of TNF-α, which is within the range of concentrations detected in the diseased joint (Figure 7) ▶ . We therefore concluded that under pathophysiological conditions IL-13 could be instrumental in maintaining high VCAM-1 expression in the synovial joint.
Figure 7.
Immunohistochemical staining demonstrating that pathophysiological concentrations of IL-13 can synergize with TNF-α to enhance VCAM-1 expression on fibroblast-like synoviocytes. Cultured FLS were treated with IL-13 at 0.1, 1, and 10 ng/ml as indicated and TNF-α (10 ng/ml) for 4 days. FLSs were fixed with methanol and acetone and then stained with mouse anti-human VCAM-1 mAb (BBA-5) and visualized using the streptavidin-biotin-peroxidase method. The results shown are representative of four separate experiments.
Induction and Prolonged Expression of VCAM-1 mRNA by TNF-α in Combination with Either IL-4 or IL-13
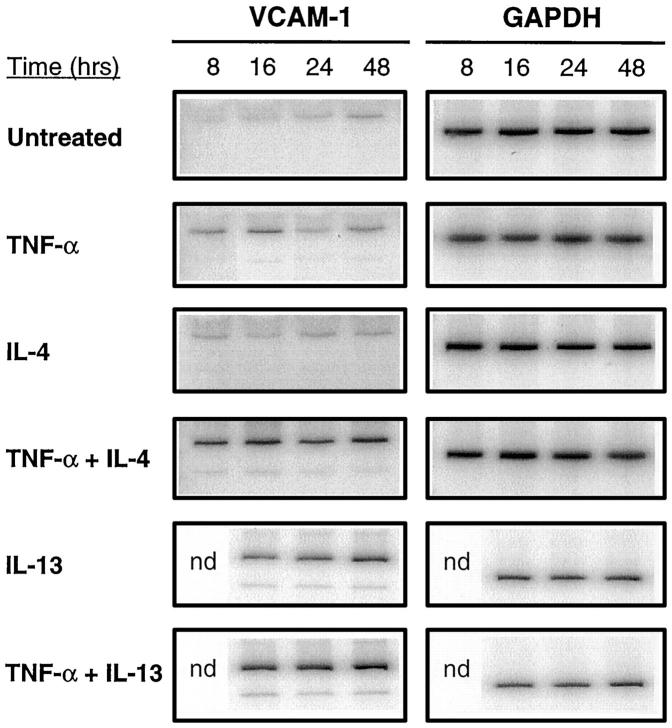
In order to study further the possible mechanism of action of IL-4 and IL-13 we studied expression of mRNA for VCAM-1 using a semiquantitative RT-PCR technique. VCAM-1 mRNA transcripts were found to be expressed at low levels in untreated cells. The addition of TNF-α transiently up-regulated VCAM-1 mRNA, with expression reaching a peak at 16 hours. IL-4 and IL-13 were both able to elevate VCAM-1 mRNA expression, however, with an apparently slower onset. The combination of IL-4 with TNF-α resulted in a dramatic increase in both the level and the duration of expression of VCAM-1 (Figure 8) ▶ . Only moderate synergy was found with IL-13 and TNF-α. These findings reflect the results obtained with measurements of cell surface expression of VCAM-1.
Figure 8.
Time course of induction of VCAM-1 mRNA transcripts in fibroblast-like synoviocytes by combinations of cytokines. Cells were cultured with either medium only (untreated), IL-4 (10 ng/ml), IL-13 (10 ng/ml), or TNF-α (10 ng/ml) either alone or in combination with IL-4 or IL-13. Total RNA was isolated at the indicated time periods and semiquantitative RT-PCR performed using VCAM-1-specific and GAPDH-specific primers (see Materials and Methods). PCR products were electrophoresed on an 8% polyacrylamide gel and visualized using a phosphorimager. Similar amounts of amplified GAPDH PCR product indicate similar levels of GAPDH mRNA in each sample. The results shown above are representative of three separate experiments.
IL-4 and IL-13 Stabilize VCAM-1 mRNA Transcripts
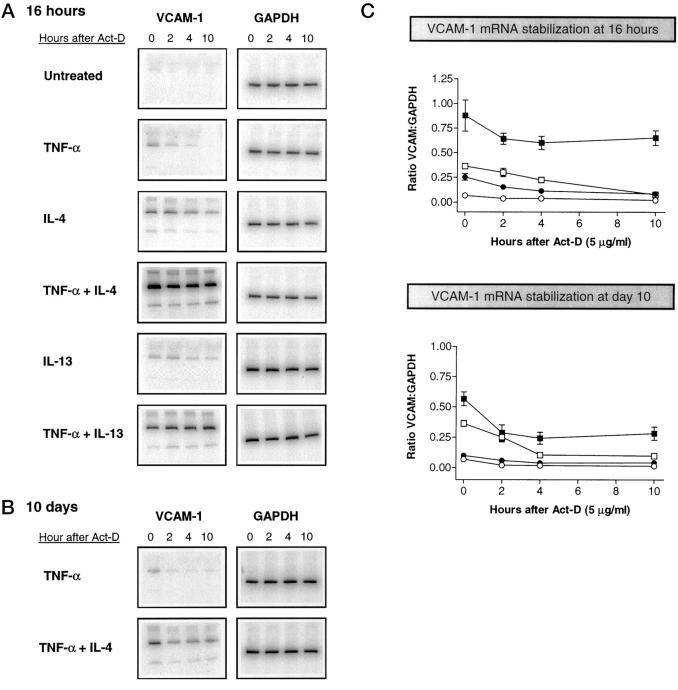
To determine the mechanism that controls prolonged VCAM-1 mRNA up-regulation in response to combination treatment with IL-4 or IL-13 and TNF-α we studied the time course of mRNA degradation in the presence of actinomycin D, an inhibitor of transcription. FLS were cultured with IL-4, IL-13, or TNF-α, alone or in combination, for 16 hours. Actinomycin D (5 μg/ml) was then added and total RNA isolated thereafter at 0, 2, 4, and 10 hours. Semiquantitative RT-PCR was carried out (Figure 9A) ▶ . VCAM-1 mRNA transcripts induced by TNF-α and IL-4 exhibited half-lives of approximately 2 and 6 hours, respectively. When TNF-α and IL-4 were combined the half-life extended to over 10 hours, ie, we could not detect any reduction in the level of VCAM-1 mRNA expression during that period. Similar results were seen with IL-13. We next assessed whether or not transcript stability was altered after 10 days of cell culture in the presence of similar combinations of cytokines. Elevated levels of VCAM-1 mRNA were observed in cells treated with the combination of IL-4 and TNF-α but not with TNF-α alone. Stability of the transcripts was still increased at day 10 in cells treated with the combination of IL-4 and TNF-α (Figure 9B) ▶ , albeit at a lower level (Figure 9, B and C) ▶ . We conclude that IL-4 and IL-13 may play a prominent part in maintaining a sustained cell surface expression of VCAM-1 as a consequence of stabilization of VCAM-1 mRNA transcripts.
Figure 9.
VCAM-1 mRNA stability at 16 hours and 10 days after treatment of fibroblast-like synoviocytes with various combinations of cytokines. Cells were cultured with medium-only (untreated), TNF-α (10 ng/ml), IL-4 (10 ng/ml), or IL-13 (10 ng/ml) and, in addition, were treated with TNF-α (10 ng/ml) in combination with IL-4 or IL-13. Cytokines were re-added at days 5 and 10. At 16 hours (A) or 10 days (B) after the start of the culture, Actinomycin-D (5 μg/ml) was added and mRNA was isolated at 0, 2, 4, or 10 hours thereafter. To measure the amount of mRNA transcripts, semiquantitative RT-PCR was performed with VCAM-1-specific and GAPDH-specific primers. C: Normalized results of three separate PCR experiments. Normalized data are expressed as the ratio of each VCAM-1 band intensity to the respective GAPDH band intensity. Medium-only (open circles); TNF-α (closed circles); IL-4 (open squares); TNF-α and IL-4 (closed squares).
Discussion
Elevated expression of VCAM-1 by synovial fibroblasts is understood to be mediated by the cytokine-rich milieu present in the RA synovium. 4 In particular, emphasis has been placed on the putative role of IL-1β and TNF-α because it was shown that expression of VCAM-1 on RA FLS can be transiently restored by the addition of these cytokines. 4,21 In addition, treatment with anti-TNF-α antibody appears to decrease VCAM-1 expression in synovial biopsies taken from RA patients. 20 We now demonstrate that sustained elevated expression of VCAM-1 on cultured FLS cannot be obtained by the presence of TNF-α or IL-1β alone. Even prolonged treatment, where cytokines are added every 3 or 4 days, results only in an initial transient expression that, once returned to baseline, remains low throughout the remaining time in culture (up to 3 weeks). This pattern of regulation greatly resembles that observed in vascular endothelial cells, where it was found that transient VCAM-1 expression results from rapid transcriptional repression of NFκB by its inhibitor IκBα. 28,29 It is argued that transient expression is essential not only in the initiation but also in the subsequent termination of the local inflammatory response. With respect to TNF-α or IL-1β, we feel that a similar mechanism of action may apply to FLS.
Interestingly treatment of FLS with IL-4 alone also results in a rapid up-regulation of VCAM-1 and this was apparently more effective than TNF-α, certainly when measured over a 2- to 3-week period. The combination of IL-4 and TNF-α was most effective not only in increasing the level of VCAM-1 to levels that we measured on cells in their first week of culture, but also in maintaining high levels of expression for 20 days. This study further highlights the differing mechanisms of transcriptional control of the VCAM-1 gene in different cell types. 16,23 VCAM-1 regulation in FLS is different from that in HUVECs or smooth muscle cells for the following reasons. First, FLS transiently up-regulate VCAM-1 mRNA expression in response to TNF-α (as with HUVECs, but in contrast to smooth muscle cells). Second, IL-4 rapidly induces VCAM-1 mRNA followed by cell surface expression (as with smooth muscle cells but in contrast to HUVECs). What they have in common, however, is that all three cell types show a synergistic increase in VCAM-1 expression following treatment with IL-4 in combination with TNF-α. 23
We furthermore show that the contribution of IL-4 and IL-13 to the sustained elevated levels could in part be explained by their capacity to prolong dramatically the half-life of VCAM-1 mRNA. Stable transcripts were still observed at day 10 of incubation, albeit at a lower level of protection. This finding indicates that, indeed, the preservation of mRNA may be one mechanism by which elevated levels of VCAM-1 protein expression can be achieved in FLSs. Post-transcriptional mechanisms that prevent the degradation of mRNA transcripts are also used by other inflammatory genes. For instance, the up-regulation of cyclooxygenase-2 (COX-2) by proinflammatory cytokines is a consequence of both transcriptional activation and increased COX-2 mRNA stability. 30,31 Our studies, however, have not addressed the possibility that IL-4 or IL-13 can also activate transcription of VCAM-1. Recently, IL-4 has been shown to transcriptionally activate the VCAM-1 gene in smooth muscle cells. 23 Moreover, other studies have shown that both these cytokines are able to activate transcription through the signal transducer and activator of transcription pathway. 32 We have attempted to study this aspect in a nuclear run-on assay but could not achieve the number of cells required for these studies. This aspect therefore remains to be studied.
A recent study by Isomäki and colleagues (1996) identified the presence of IL-4 and IL-13 in the synovial fluid and synovium of RA patients. 27 Our observation that pathophysiological concentrations augment VCAM-1 expression, particularly in the presence of TNF-α, indicates that these cytokines may be instrumental in the regulation of VCAM-1 expression in the joint. This possibility is perhaps best illustrated by our finding that VCAM-1 expression (both mRNA and cell surface protein) by FLS slowly declines when these cells are removed from the RA synovial joint and placed in culture. If expression of VCAM-1 in the synovial joint was solely regulated by elevated levels of TNF-α and IL-1β, then, when cells are placed in culture, we would expect a rather rapid decline in the levels of expression.
In conclusion, our results have shown that IL-4 or IL-13, either alone or in combination with TNF-α, results in elevated levels of VCAM-1 expression in fibroblast-like synoviocytes. Moreover, the chronic administration of these cytokine combinations can achieve a sustained elevated level of VCAM-1 expression. This sustained expression can be explained in part by the capacity of IL-4 or IL-13 to stabilize VCAM-1 mRNA. Thus, VCAM-1 mRNA stability appears to be a major factor in the sustained up-regulation of VCAM-1 expression in fibroblast-like synoviocytes.
Acknowledgments
We thank the Novartis Institute for Medical Sciences for the generous use of their facilities and Derek Davies (Imperial Cancer Research Fund Laboratories, Lincoln’s Inn Fields, London) for expert assistance with flow cytometry.
Footnotes
Address reprint requests to Dr. IJsbrand M. Kramer, Department of Pharmacology, University College London, Gower Street, London WC1E 6BT, UK. E-mail: i.kramer@ucl.ac.uk.
Supported by the Middlesex Hospital Clinical Research and Development Committee, the University of London Central Research Fund, and the Biotechnology and Biological Sciences Research Council.
References
- 1.Gay S, Gay RE, Koopman WJ: Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: Two cellular mechanisms explain joint destruction? Ann Rheum Dis 1993, 52:S39-S47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haskard DO: Cell adhesion molecules in rheumatoid arthritis. Curr Opin Rheumatol 1995, 7:229-234 [DOI] [PubMed] [Google Scholar]
- 3.Liao H-X, Haynes BF: Role of adhesion molecules in the pathogenesis of rheumatoid arthritis. Rheum Dis Clin N Am 1995, 21:715-740 [PubMed] [Google Scholar]
- 4.Morales-Ducret J, Wayner E, Elices MJ, Alvaro-Gracia JM, Zvaifler NJ, Firestein GS: α4/β1 integrin (VLA-4) ligands in arthritis: vascular cell adhesion molecule-1 in synovium and on fibroblast-like synoviocytes. J Immunol 1992, 149:1424-1431 [PubMed] [Google Scholar]
- 5.Wilkinson LS, Edwards JC, Poston RN, Haskard DO: Expression of vascular cell adhesion molecule-1 in normal and inflammed synovium. Lab Invest 1993, 68:82-88 [PubMed] [Google Scholar]
- 6.Kriegsmann J, Keyszer GM, Geiler T, Brauer R, Gay RE, Gay S: Expression of vascular cell adhesion molecule-1 mRNA and protein in rheumatoid synovium demonstrated by in situ hybridization and immunohistochemistry. Lab Invest 1995, 72:209-214 [PubMed] [Google Scholar]
- 7.Osborn L, Hession C, Tizzard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R: Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989, 59:1203-1211 [DOI] [PubMed] [Google Scholar]
- 8.Hession C, Tizard R, Vassallo C, Schiffer SB, Goff D, Moy P, Chi Rosso G, Luhowskyj S, Lobb R, Osborn L: Cloning of an alternative form of vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem 1991, 266:6682-6685 [PubMed] [Google Scholar]
- 9.Lazarovits AI, Karsh J: Differential expression in rheumatoid synovium and synovial fluid of α4β7 integrin: A novel receptor for fibronectin and vascular cell adhesion molecule-1. J Immunol 1993, 151:6482-6489 [PubMed] [Google Scholar]
- 10.Bombara MP, Webb DL, Conrad P, Marlor CW, Sarr T, Ranges GE, Aune TM, Greve JM, Blue M-L: Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J Leukoc Biol 1993, 54:399-406 [DOI] [PubMed] [Google Scholar]
- 11.Blue M-L, Conrad P, Webb DL, Sarr T, Macaro M: Interacting monocytes and synoviocytes induce adhesion molecules by a cytokine-regulated process. Lymphokine Cytokine Res 1993, 12:213-218 [PubMed] [Google Scholar]
- 12.Chen V, Croft D, Purkis P, Kramer IM: Co-culture of synovial fibroblasts and differentiated U937 cells is sufficient for high interleukin-6 but not interleukin-1β or tumour necrosis factor-α release. Br J Rheumatol 1998, 37:148-156 [DOI] [PubMed] [Google Scholar]
- 13.Romanic AM, Madri JA: The induction of a 72-kD gelatinase in T-cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol 1994, 125:1165-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, Gay S: Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol 1996, 149:1607-1615 [PMC free article] [PubMed] [Google Scholar]
- 15.Iademarco MF, McQuillan JJ, Rosen GD, Dean DC: Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem 1992, 267:16323-16329 [PubMed] [Google Scholar]
- 16.Iademarco MF, Barks JL, Dean DC: Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-α in cultured endothelial cells. J Clin Invest 1995, 95:264-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty JM, Yee EK, Deisher TA, Harlan JM, Kaushansky K: Interleukin-4 induces endothelial vascular cell adhesion molecule-1 (VCAM-1) by an NFκ-B-independent mechanism. FEBS Lett 1995, 372:194-198 [DOI] [PubMed] [Google Scholar]
- 18.Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T: Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med 1992, 176:1583-1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neish AS, Read MA, Thanos D, Pine R, Maniatis T, Collins T: Endothelial interferon regulatory factor 1 cooperates with NF-κB as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol 1995, 15:2558-2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmann M, Brennan F, Maini RN: Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 1996, 14:397-440 [DOI] [PubMed] [Google Scholar]
- 21.Marlor CW, Webb DL, Bombara MP, Greve JM, Blue M-L: Expression of vascular cell adhesion molecule-1 in fibroblast-like synoviocytes after stimulation with tumor necrosis factor. Am J Pathol 1992, 140:1055-1060 [PMC free article] [PubMed] [Google Scholar]
- 22.Dall P, Heider KH, Sinn HP, Skroch-Angel P, Adolf G, Kaufmann M, Herrlich P, Ponta H: Comparison of immunohistochemistry and RT-PCR for detection of CD44v-expression, a new prognostic factor in human breast cancer. Int J Cancer 1995, 60:471-477 [DOI] [PubMed] [Google Scholar]
- 23.Barks JL, McQuillan JJ, Iademarco MF: TNF-α and IL-4 synergistically increase vascular cell adhesion molecule-1 expression I cultured vascular smooth muscle cells. J Immunol 1997, 159:4532-4538 [PubMed] [Google Scholar]
- 24.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Scleimer RP: IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol 1995, 154:799-803 [PubMed] [Google Scholar]
- 25.Jahnsen FL, Brandtzaeg P, Haye R, Haraldsen G: Expression of functional VCAM-1 by cultured nasal polyp-derived microvascular endothelium. Am J Pathol 1997, 150:2113-2123 [PMC free article] [PubMed] [Google Scholar]
- 26.Kotowicz K, Callard RE, Friedrich K, Matthews DJ, Klein N: Biological activity of IL-4 and IL-13 on human endothelial cell: functional evidence that both cytokines act through the same receptor. Int Immunology 1996, 8:1915-1925 [DOI] [PubMed] [Google Scholar]
- 27.Isomäki P, Luukkainen R, Toivanen P, Punnonen J: The presence of interleukin-13 in rheumatoid synovium and its antiinflammatory effects on synovial fluid macrophages from patients with rheumatoid arthritis. Arthritis Rheum 1996, 39:1693-1702 [DOI] [PubMed] [Google Scholar]
- 28.Read MA, Whitley MZ, Williams AJ, Collins T: NF-κB and IκBα: An inducible regulatory system in endothelial activation. J Exp Med 1994, 179:503-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read MA, Neish AS, Gerritsen ME, Collins T: Postinduction transcriptional repression of E-selectin and vascular cell adhesion molecule-1. J Immunol 1996, 157:3472-3479 [PubMed] [Google Scholar]
- 30.Crofford LJ, Tan B, McCarthy CJ, Hla T: Involvement of nuclear factor κB in the regulation of Cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum 1996, 40:226-286 [DOI] [PubMed] [Google Scholar]
- 31.Ristimäki H, Sano H, Remmers EF, Epps HR, Hla T: Induction of cyclooxygenase-2 by interleukin-1α: Evidence for post-transcriptional regulation. J Biol Chem 1994, 269:11769-11775 [PubMed] [Google Scholar]
- 32.Lugli SM, Feng N, Heim MH, Adam M, Schnyder B, Etter H, Yamage M, Eugster H-P, Lutz RA, Zurawski G, Moser R: Tumor necrosis factor α enhances the expression of the interleukin (IL)-4 receptor α-chain on endothelial cells increasing IL-4 or IL-13-induced Stat6 activation. J Biol Chem 1997, 272:5487-5494 [DOI] [PubMed] [Google Scholar]