Abstract
LAT (linker for activation of T cells) is an integral membrane protein of 36–38 kd that plays an important role in T cell activation. Using a rabbit polyclonal antibody generated against the cytosolic portion of LAT, we investigated the immunohistochemical expression of LAT in normal and pathological hematolymphoid tissues. LAT reacts with human T cells in paraffin sections, including decalcified bone marrow trephines. LAT appears early in T cells at the thymocyte stage and before TdT expression in embryos, and is expressed in peripheral lymphoid tissues, without restriction to any T cell subpopulations. In addition to T cells, natural killer (NK) cells (evaluated with flow cytometry), megakaryocytes and mast cells are also LAT-positive, whereas B cells and other myeloid and monocytic derived cells are negative. Tested on a total of 264 paraffin-embedded tissue biopsies, LAT reacted with the great majority (96.8%) of T/NK-cell neoplasms, covering the full range of T cell maturation. Although antibodies to both LAT and CD3 had a similarly high sensitivity in the staining of T/NK-cell lymphomas, when used in conjunction, they successfully identified a higher number of cases (98.4%). Atypical megakaryocytes from different hematological disorders, as well as mast cells in mastocytosis were also LAT-positive, but all neoplasms of B cell origin, Hodgkin’s lymphomas, and several nonlymphoid malignancies were negative. These data indicate that the anti-LAT antibody may be of value to diagnostic histopathologists for the identification of T cell neoplasms.
Stimulation of the T cell antigen receptor (TCR) results in activation of several protein tyrosine kinases (PTKs) associated with the TCR. These activated PTKs form phosphorylate tyrosine residues on multiple protein substrates. Such phosphorylation results in the activation of enzymes such as phospholipase Cγ (PLC-γ1) or creates sites of binding for proteins involved in the activation cascade. 1-3 Linker for activation of T cells (LAT) is an integral membrane protein of 36–38 kd that plays an important role in linking engagement of the TCR to the biochemical events of T cell activation. 4,5 It is one of the most prominent tyrosine-phosphorylated proteins after TCR engagement. 4,6 LAT is a substrate of activated ZAP-70 and Syk PTKs and, on tyrosine phosphorylation, it binds Grb2, PLC-γ1, the p85 subunit of PI3K, and other critical signaling molecules, thereby recruiting these molecules to the plasma membrane. 7-10 Localization of these signaling molecules to the membrane has several consequences. Phosphorylation of tyrosine residues required for enzymatic activation is enhanced and formation of protein complexes occurs. 4 By analysis of RNA, LAT also was shown to be expressed in NK and mast cells. 4 Much less is known about its function in these cell types.
A rabbit polyclonal antibody, which was generated against the cytosolic portion of LAT, 4 was used in this study to evaluate the immunohistochemical expression of LAT in normal and pathological hematolymphoid tissues. We also assessed the specificity of anti-LAT antibodies for the identification of T cells, tested its usefulness as an immunohistochemical reagent, and investigated its possible role in the study of lymphoid neoplasms.
Materials and Methods
Normal Tissues and Cell Populations
Paraffin-embedded normal lymphoid tissues included lymph nodes showing various forms of reactive changes (n = 10 cases), thymuses obtained during cardiac surgery or surrounding thymomas (n = 3), spleens removed after trauma or because of immune thrombocytopenia (n = 4), bone marrows (n = 6), and small intestine (n = 2). In addition, hematopoietic tissues from three embryos aged 11–12 weeks of gestation were analyzed. Freshly frozen samples of reactive lymph nodes (n = 3), spleen (n = 1), and thymus (n = 1) were also used.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood after Ficoll-Hypaque gradient centrifugation and depleted of plastic adherent cells. For purification of polyclonal natural killer (NK) or T cell populations, PBMC were incubated with anti-CD3 monoclonal antibody (JT3A, gift of Dr. A. Moretta, University of Genova, Italy) for 30 minutes at 4°C, followed by treatment with goat anti-mouse-coated dynabeads (Dynal, Oslo, Norway) for 30 minutes at 4°C. The resulting CD3-negative lymphocyte populations, containing approximately 1% CD3+ cells, 20–30% HLADR+ cells, and 70–80% CD16+CD56+ cells, were cultured in rIL-2 (Cetus Corp., Emeryville, CA). To obtain polyclonally activated T cell-enriched lymphocyte populations, PBMC were stimulated with 0.1% (v/v) PHA (Gibco, Paisley, UK) for 24 hours and then cultured in rIL-2.
Neoplastic Tissues
Two-hundred and sixty-four cases of nodal and extranodal hematolymphoid neoplasms were gathered from different institutions. All neoplasms had been previously characterized immunophenotypically on paraffin sections, and in many cases on frozen sections as well (Tables 1–4) ▶ ▶ ▶ . All lymphomas were classified according to the International Lymphoma Study Group Classification 11 and included all major subtypes of Hodgkin’s and non-Hodgkin’s lymphomas.
Table 1.
Details of Negative LAT Staining Hodgkin’s Lymphomas and Malignant B Cell Lymphomas
| Tissue type | No. of cases |
|---|---|
| Hodgkin’s lymphomas | |
| Lymphocyte predominance | 2 |
| Nodular sclerosis | 10 |
| Mixed cellularity | 2 |
| Total | 15 |
| B-cell lymphomas | |
| I. Precursor B-lymphoblastic leukemia/lymphoma | 8 |
| II. Peripheral B-cell neoplasms | |
| B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma | 14* |
| Lymphoplasmacytoid lymphoma | 3 |
| Mantle cell lymphoma | 11† |
| Follicle center cell lymphoma, follicular | 16 |
| Marginal zone B-cell lymphoma | |
| nodal | 1 |
| extranodal | 4 |
| Hairy cell leukemia | 6 |
| Myeloma | 8 |
| Diffuse large B-cell lymphoma | 25‡ |
| Burkitt’s lymphoma | 4 |
| Total | 100 |
*One with immunoblastic transformation.
†One “blastoid” variant.
‡Included four T-cell-rich B large cell lymphomas and three CD30+/ALK1− anaplastic large cell lymphomas.
Table 2.
Details of LAT and CD3 Staining in Malignant T Cell and NK/T Cell Lymphomas (Excluding ALCL)
| Diagnosis | No. of cases | LAT-positive cases | CD3-positive cases | T-cell antigen loss (other than CD3)§ |
|---|---|---|---|---|
| I. Precursor T-lymphoblastic lymphoma/leukemia | 8 | 8 | 8 | |
| II. Peripheral T-cell and NK neoplasms | ||||
| T-cell chronic lymphocytic leukemia | 1 | 1 | 1 | |
| Large granular lymphocytic leukemia, T-cell type | 1 | 1 | 1 | |
| Mycosis fungoides* | 16 | 16 | 14 | 4# |
| Peripheral T-cell lymphomas, unspecified | 11 | 11 | 11 | |
| Peripheral T-cell lymphomas, mixed medium and large cells | 3 | 3 | 2 | 1¶ |
| Peripheral T-cell lymphomas, large cells | 3 | 2 | 2 | 1∥ |
| Hepatosplenic γδ T-cell† | 4 | 4 | 4 | |
| Angioimmunoblastic T-cell lymphoma (AILD) | 2 | 2 | 2 | |
| Nasal and nasal type NK/T lymphoma (angiocentric)‡ | 12 | 11 | 12 | |
| Intestinal T-cell lymphoma | 2 | 2 | 2 | |
| Total | 63 | 61 | 59 | 6 |
*13 stage I micosis fungoides, 2 stage II, one stage III
†three primary cutaneous, one hepatosplenic
‡10 nasal, one cutaneous and one subcutaneous
§Extensive frozen-tissue phenotyping available in 30 cases.
#Lack of CD7 and TCR-β (2 cases), CD5 and CD7 (2 cases);
¶Lack of CD5 alone;
∥Lack of CD5, CD43, and TCR-β
Table 3.
Details of LAT, CD3 and ALK1 Staining in Anaplastic Large Cell Lymphomas (ALCL)
| T-cell type | Null-cell type | |||||||
|---|---|---|---|---|---|---|---|---|
| LAT+ CD3+ | LAT+ CD3− | LAT− CD3− | LAT− CD3+ | LAT+ CD3+ | LAT+ CD3− | LAT− CD3− | LAT− CD3+ | |
| ALK1-positive | 3 | 3 | 1 | — | — | — | 3 | — |
| ALK1-negative | 4 | — | 4 | 8 | — | 4 | 12 | — |
| ALK1 n.a.* | — | — | 1 | 1 | — | — | — | — |
| Total | 7 | 3 | 6 | 9 | 0 | 4 | 15 | 0 |
*ALK1 was not assessable because only B5-fixed specimens were available and there is poor reactivity of anti-ALK1 on this fixative.
Fixatives and Tissue and Cell Processing
Tissue samples had been fixed in various fixatives, including buffered formalin, B5, Bouin, and Hollande, and embedded in paraffin. Bone marrow biopsies were fixed in B5 for 3 hours and decalcified in 0.1 mol/L EDTA disodium salt aqueous solution for 2–8 hours. Fresh tissues were immediately frozen after biopsy in liquid nitrogen and stored at −80°C until used.
CD3+CD16−CD56− T cell and CD3−CD16+CD56+ NK cell populations were washed 3 times in 0.9% NaCl solution, resuspended at a concentration of 5 × 10 6 cells/ml, and used for cytospin preparations (100 μl/each slide). Slides were air-dried for 24 hours, then fixed in −20°C absolute ethanol for 30 minutes, dried, and used for immunocytochemical staining.
Immunostaining
Details on the production and characterization of the rabbit anti-LAT antibody are reported elsewhere. 4 Immunostaining for LAT was performed on paraffin sections after antigen retrieval in microwave (3 boiling cycles, 5 minutes each, at 750 W, with an interval of 1 minute between cycles) in citrate buffer, pH 6.0. The polyclonal antibody anti-LAT was applied at a dilution of 1:800 in Tris-HCl buffer, pH 7.2–7.4, for 45 minutes and was followed by biotinylated anti-rabbit antibody (30 minutes) and peroxidase-conjugated streptavidin-biotin complex (30 minutes) (Bio-S.P.A., Milan, Italy). On cryostat sections, the procedure was similar but the microwave heating was avoided. Sections were air-dried for 18 hours and LAT was applied at a dilution of 1:100. On cytospins, the sample was subjected to microwave heating once.
In all cases of T-cell lymphomas and anaplastic large cell lymphomas (ALCL), serial paraffin sections were also stained with a polyclonal antibody anti-CD3 (Dako, Milan) (1:200; microwave antigen retrieval in 1 mmol/L EDTA buffer, pH 8.0, 2× 5′ cycles). Furthermore, the cases of ALCL were also evaluated for their reactivity with the monoclonal antibody ALK1 (Dako) (1:10; microwave antigen retrieval in citrate buffer, 3× 5′ cycles), which recognizes a formalin-resistant epitope in the nucleophosmin-anaplastic lymphoma kinase chimeric protein. 12 Both CD3 and ALK1 immunostaining were performed using the same indirect immunoperoxidase technique adopted for LAT. Thymuses from embryos were also stained with polyclonal antibodies anti-CD3 and anti-TdT (Dako) (1:200; microwave antigen retrieval in 1 mmol/L EDTA buffer, pH 8.0, 3× 5′ cycles, overnight incubation of the primary antibody).
Negative controls for both tissue and cytospin immunostainings were performed using either the preimmune serum or an irrelevant polyclonal antibody (anti-Hepatitis B virus core antigen, Dako) instead of anti-LAT.
Analysis of the distribution of LAT on PMBC was performed using two-color fluorescence cytofluorometric analysis (FACS) (Ortho Cytoron Absolute) as previously described. 13 For FACS analysis of LAT in combination with monoclonal antibodies JT3A (IgG2a, anti-CD3), kd1 (IgG2a, anti-CD16), and C218 (IgG1, anti-CD56) (all antibodies provided by Dr. A. Moretta), membranes were permeabilized with 0.2% saponin (Sigma) in phosphate buffered saline (PBS), pH 7.6, for 5 minutes after fixation with 4% paraformaldehyde in PBS. As second reagents, fluorescein isothiocyanate-conjugated swine anti-rabbit antibody (Dako) and phycoerythrin-conjugated isotype-specific goat anti-mouse antibodies (Southern Biotechnology Associates, Birmingham, AL) were used.
Results
In both cryostat and paraffin-embedded tissues, positive staining for LAT was crisp and localized mainly on the cell membrane and the sub-plasmalemmal area. Variable numbers of cells also showed diffuse cytoplasmic reactivity and, on occasion, dot-like positivity in the Golgi region. On paraffin sections, optimal staining was observed in tissues fixed in both buffered formalin and B5, whereas occasionally some background staining was observed for tissues fixed in Bouin or Hollande. Decalcification of bone marrow specimens in EDTA did not affect LAT immunoreactivity. No staining was found on negative controls (data not shown).
Normal Tissues and Cells
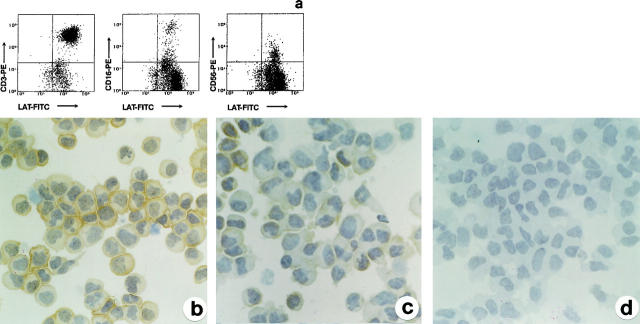
In the thymus, expression of LAT was identifiable throughout all stages of thymocyte differentiation, including the large cortical blasts (Figure 1, a and b) ▶ . In embryos of 10–12 weeks’ gestation, thymuses already showing a lobular architecture contained lymphoid cells that expressed LAT (Figure 1c) ▶ and CD3, but were negative for TdT (data not shown). In peripheral lymphoid tissues, LAT-positive lymphocytes were located in the known T-cell areas in lymph nodes and spleen (Figure 1d) ▶ and the immunoreactivity paralleled that obtained with anti-CD3 in both frozen and paraffin sections. In the small intestine, intraepithelial T-cells were also positive for LAT (Figure 1e) ▶ . In bone marrow, LAT was expressed by the sparse T lymphocytes present in interstitial spaces, and also by platelets and megakaryocytes (Figure 1g) ▶ that exhibited a strong reactivity in the cytoplasm; all other hematopoietic cells were negative. Reactivity for LAT was also noticed on tissue mast cells (Figure 1h) ▶ in the form of delicate plasma membrane and granular cytoplasmic labeling.
Figure 1.
Expression of LAT in normal hematolymphoid tissues. LAT is expressed on cortical and medullar thymocytes (including subcortical large blasts, see arrow in b) from adult thymus (a, b) and on embryonal thymocytes (c). Mature T cells in the paracortex and associated with B-follicle in a reactive lymph node are also LAT-positive, whereas B cells are totally negative (d). Positivity is also evident on intraepithelial T cells in the small intestine (e). f: In the paracortical area from a reactive lymph node there is a large cluster of plasmacytoid monocytes unreactive for LAT. Cytoplasmic reactivity for LAT is evident on bone marrow megakaryocytes (g) and on a tissue mast cell (h); note the negativity of myeloid cells (g). Indirect immunoperoxidase technique for LAT with light hematoxylin counterstain. Original magnifications, ×64 (a), ×375 (b, e, g, h), ×16 (c) and ×125 (d, f).
In all tissues, non-T cell components, including B cells, macrophages, plasmacytoid monocytes (Figure 1f) ▶ , epithelioid histiocytes, and dendritic cells were completely negative for LAT. Similarly, LAT was not expressed on endothelial cells and epithelia, including thymic epithelium and Hassal bodies.
On PBMC, LAT was expressed, in addition to CD3+ T cells, on resting NK cells CD16+ and CD56+ (Figure 2a) ▶ . As shown in Figure 2, b and c ▶ , LAT staining was also found on T and NK cells in culture with rIL-2; on CD3−CD16+CD56+ NK cells purified from PBMC, LAT was obviously expressed, although the intensity of reactivity was much weaker than that recognized on purified CD3+ T cells. Cell labeling was completely absent on cytospins stained with negative control antibodies (Figure 2d) ▶ .
Figure 2.
Expression of LAT in peripheral blood mononuclear T and NK cells. a: FACS analysis of LAT in combination with anti-CD3 and CD16 and anti-CD56 on PBMC shows that LAT is expressed on CD3+ T cells, as well as on CD16+CD56+ NK cells. b and c: Immunohistochemical expression of LAT on purified and cultured CD3+ T cells (b) and CD16+CD56+ NK cells (c). NK cells express LAT at a lower level than T cells. d: NK cells stained with preimmune serum show no reactivity. a: FACS analysis; LAT developed with fluorescein isothiocyanate-conjugated secondary antibody; CD3, CD16, and CD56 developed with phycoerythrin-conjugated secondary antibody. b and c: Indirect immunoperoxidase technique for LAT. d: Negative control immunostaining using preimmune serum. Original magnifications, ×375 (b, c, d).
Neoplastic Tissues
Hodgkin’s and non-Hodgkin’s Lymphomas
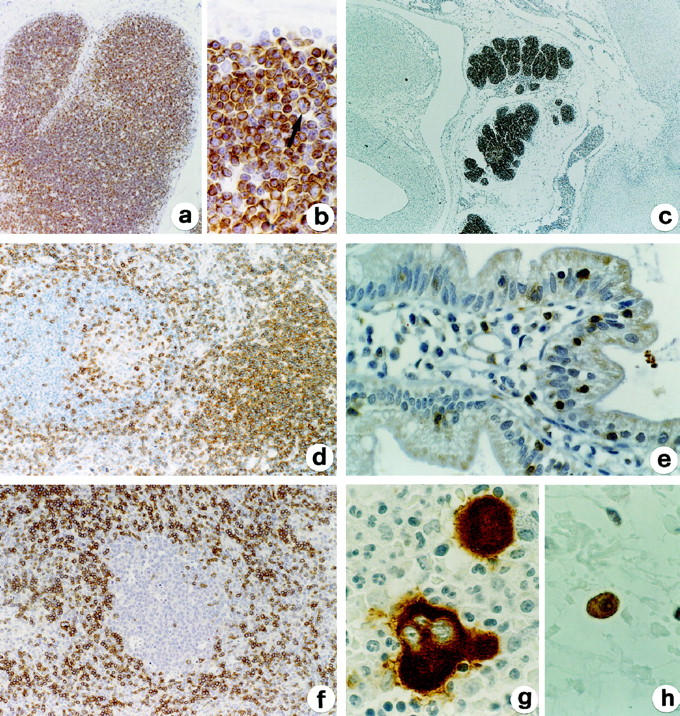
Neoplastic cells in all cases of Hodgkin’s (n = 15) and B-cell non-Hodgkin’s lymphomas (n = 100) (Table 1) ▶ were negative for LAT, but contained variable amounts of LAT-reactive nonneoplastic T cells (Figure 3, a and b) ▶ .
Figure 3.
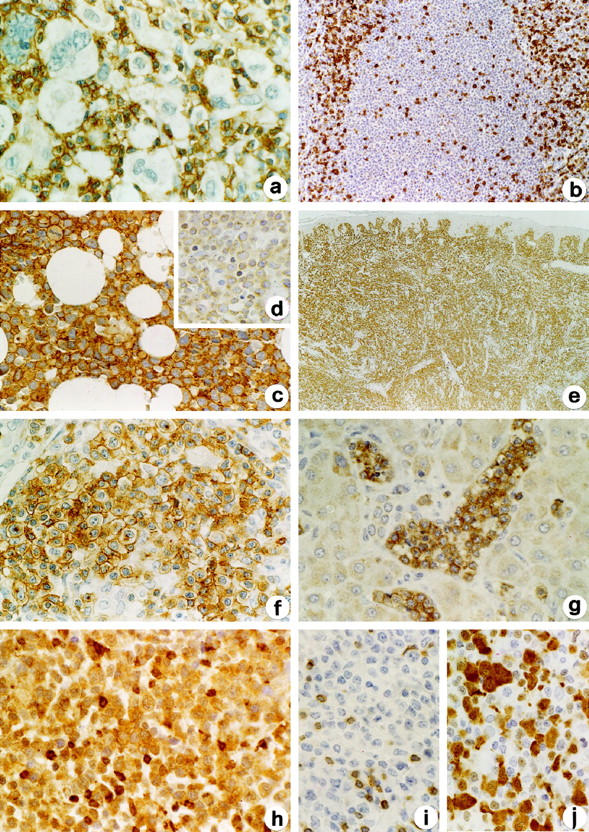
Expression of LAT in lymphoid neoplasms. Examples of negativity of LAT in Hodgkin’s lymphomas and B-cell lymphomas are illustrated in a and b from a case of nodular sclerosis Hodgkin’s lymphoma and one of mantle cell lymphoma, respectively. Note the strong reactivity of LAT on associated nontumoral T cells. Examples of positivity of LAT in T-cell lymphomas are shown in c, e, f, and g, represented by cases of lymphoblastic lymphoma involving the bone marrow (c), cutaneous epidermotropic T-cell lymphoma (e), nodal AILD-type peripheral T-cell lymphoma (f), and liver localization of hepatosplenic γδ T-cell lymphoma (g). Compare the strong reactivity for LAT of T lymphoblasts (c) and their weak labeling with anti-CD3 (d). h-j: A case of ALCL-null type expressing LAT (h) and ALK-1 (j) but unreactive for CD3 (i). Indirect immunoperoxidase technique for LAT (a-c, e-h), CD3 (d, i), and ALK-1 (j). Original magnifications, ×64 (e), ×375 (all other parts).
In T-cell lymphomas (excluding ALCL), LAT stained 8/8 lymphoblastic lymphomas, 42/43 peripheral T-cell lymphomas, and 11/12 NK/T-cell lymphomas (Figure 3, c, e, f, and g) ▶ . The intensity of reactivity and the percentage of labeled cells varied among different types of T-cell lymphomas. In lymphoblastic lymphomas all neoplastic cells were strongly labeled by anti-LAT; in contrast, in peripheral T-cell and NK/T-cell lymphomas the reactivity was intense in 28/53 cases (52.8%) and recognizable in the vast majority of cells in 50/53 (94.3%). LAT expression showed no substantial differences in peripheral T-cell lymphomas with relationship to primary location, cell morphology, and immunophenotype, with the exception of NK/T-cell lymphomas, which were more frequently characterized by weaker and a lower number of labeled cells.
The ALCL included 39 extracutaneous and 5 purely cutaneous cases. On the basis of the expression of at least one T-cell marker (CD3, CD5, CD8, CD43, CD45R0, TCR-β) and no B-cell markers (CD20 or CD79a), 25 cases were classified as T-cell and 19 as null-cell phenotype. Fourteen cases (31.8%) reacted with LAT; all were represented by extracutaneous lymphomas. Ten of them were of T-cell phenotype and four were null-cell. LAT-positive ALCL showed intense reactivity in 5/14 cases (35.7%) and it was recognizable in the vast majority of cells in 10/14 (71.4%) (Figure 3h) ▶ .
Comparison of LAT and CD3 Expression in T-Cell and NK/T-Cell Lymphomas Excluding ALCL
In T-lymphoblastic lymphoma, LAT positivity was more intense and diffuse than CD3 (Figure 3, c and d) ▶ . Among peripheral T-cell lymphomas, only two LAT-negative cases were observed. One was a large cell lymphoma presenting an aberrant phenotype with loss of CD3,CD5, CD43, and TCR-β. The other LAT-negative case was a NK/T cell lymphoma that strongly expressed cytoplasmic CD3. Three cases of LAT-positive cutaneous T-cell lymphomas did not express CD3 (two also lacked CD5), but were positive for CD2, CD4, and TCR-β.
Comparison of LAT, CD3, and ALK1 Expression in ALCL
The distribution of LAT, CD3, and ALK1 in ALCL is reported in Table 3 ▶ . Seven ALCL cases were LAT+CD3+, 7 were LAT+CD3−, 9 were LAT−CD3+, and 21 were LAT−CD3−. Among ALCL T-cell type (25 cases), CD3 labeled a higher number of cases (19/25; 76%) than LAT (10/25; 40%). Interestingly, LAT and CD3 expression in ALCL T-cell type was discordant in a significantly higher number of cases than in other peripheral T-cell lymphomas (12/25 versus 4/55, respectively; Fisher’s exact test: P < 0.001) (Figure 3, h and i) ▶ . Immunostaining for ALK1 was available in 42 cases, 10 of which showed nuclear ± cytoplasmic positivity. Although the majority of ALK1-positive cases were LAT-positive (6/10), in comparison with ALK1-negative cases (8/32) this difference was not statistically significant (Fisher’s exact test: P = 0.059) (Figure 3j) ▶ .
Hematopoietic Nonlymphoid Neoplasms
In chronic myeloproliferative disorders and myelodysplastic syndromes, cytoplasmic LAT expression was consistently identified in normal and atypical megakaryocytes, including the micromegakaryocytes typically found in chronic myeloid leukemia and myelodysplasia (Figure 4, a and c) ▶ .
Figure 4.
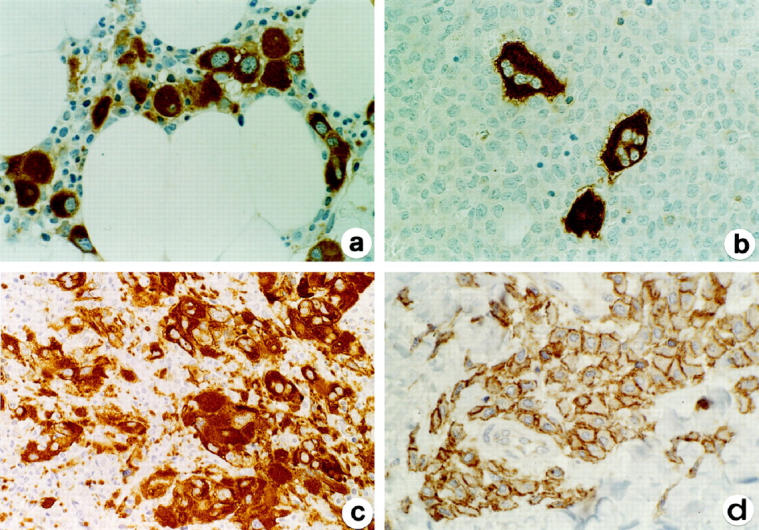
Expression of LAT in nonlymphoid hematological neoplasms. LAT is strongly expressed in the cytoplasm of micromegakaryocytes from a case of myelodysplasia (a) and by the atypical megakaryocytes in the large aggregates from a case of idiopathic myelofibrosis (c). b: Residual LAT-positive megakaryocytes are surrounded by LAT-negative leukemic cells from a case of acute monocytic leukemia. d: Cell membrane and delicate cytoplasmic LAT positivity in case of cutaneous mastocytosis. Indirect immunoperoxidase technique for LAT. Original magnifications, ×125 (a, c), ×375 (b, d).
Among 28 cases of acute nonlymphoid leukemia (Figure 4b) ▶ , the only positive cases were two megakaryocytic leukemias, where the blast cells were labeled in their cytoplasm. An additional case of chronic myeloid leukemia in blastic transformation contained two cell populations, one formed by myeloid blasts (myeloperoxidase and CD34+) and one by megakaryocytic blasts, expressing LAT, Factor VIII-related antigen, and CD34.
In all pathological conditions, reactivity of LAT on megakaryocytes was strong and particularly helpful in the identification of abnormal forms; moreover, it was frequently stronger than that of other megakaryocytic markers on paraffin sections, such as Factor VIII-related antigen and CD61 (data not shown).
Four of five cases of mastocytosis showed LAT positivity, including 3/3 cases of cutaneous mastocytosis and 1/2 cases of systemic mastocytosis (Figure 4d) ▶ . As with normal mast cells, LAT expression in neoplastic mast cells was finely granular in the cytoplasm and delicate on the cell membrane, a pattern of staining that clearly differed from that on T cells.
Discussion
It has previously been shown that LAT mRNA is expressed in human peripheral blood lymphocytes, thymus, and spleen, as well as in Jurkat/T cell, YT/NK-like cell, and RBL/rat mast cell lines, but not on Raji and Jiyoye/B-cell and THP1/monocyte cell lines. 4 The present study shows that LAT can be successfully identified using in situ immunohistochemistry on frozen and paraffin-embedded human tissue specimens. Using this technique, we have confirmed and extended the earlier analysis of LAT distribution by showing LAT protein expression in human T-lymphocytes, NK cells, and mast cells, but not in B-cells or macrophages. LAT immunoreactivity has been documented in stage I cortical thymocytes, represented by the large blasts localized to the sub-capsular area of the thymus. The observation that LAT reactivity can be detected in TdT negative thymocytes from 10- to 12-week-old embryos 14,15 indicates that this protein is expressed early in T cell development and suggests that LAT might play an important role in the mechanisms of T-cell regulation, even before external antigen stimulation occurs. In accordance with the idea that LAT is critical to TCR-mediated activation, we found that in normal lymphoid tissues, LAT expression fully overlaps CD3 expression. LAT is not restricted to any T-cell subpopulations. Furthermore, LAT protein is widely recognizable in T cells from all peripheral tissues, including the intraepithelial lymphocytes of the small intestine, containing large numbers of TCR-δ-positive cells. 16,17
According to our findings, CD16+ and CD56+ NK cells from peripheral blood express LAT; the same result was obtained on cytospins from CD3−CD16+CD56+ NK cells and CD3+CD16−CD56− T cells in culture with r-IL2, where it was also obvious that NK cells express less LAT than T cells. These observations confirm the data obtained on YT-NK-like cells. 4 Because NK cells express TCR-ζ 18 together with both Syk and ZAP-70, 19,20 and in view of what is known about these molecules in T cells, 21,22 it is very possible that LAT is also involved along the TCR-ζ-Syk/ZAP-70 pathways in NK cells.
Expression of LAT in normal human mast cells was also shown. The fine granular positivity in the cytoplasm and on the cell membrane on mast cells is a distinctive cellular pattern of distribution, as compared to T cells. Signaling pathways coupled to the Fcε receptor in mast cells are quite similar to those involved in TCR-mediated signaling in T cells. 23 The Syk PTK is recruited to the receptor and a number of molecules such as Grb2 and PLC-γ1 are involved in intracellular pathways. 4 It is likely that LAT has a central function in these cells as well.
The strong reactivity of anti-LAT with human megakaryocytes and platelets is a novel finding. Mice genetically engineered to lack the adapter protein SLP-76, a protein known to bind LAT, 24,25 develop a profound block in thymocyte development and demonstrate severe bleeding associated with thrombocytopenia. 26 Moreover, it is well recognized that megakaryocytes express Syk and it has been shown that Syk−/− mice show severe hemorrhagic manifestations in utero and die shortly after birth, though they do not have a defect in platelet number. 27,28 The detection of LAT in platelets and its known interactions with SLP-76 and Syk suggest that LAT may have a critical function in these cells.
The general pattern of reactivity observed in normal human tissues have been largely reproduced by studies in neoplastic counterparts. Among hematolymphoid neoplasms, anti-LAT stained the great majority of T-cell neoplasms (excluding ALCL), but staining was negative in all cases of B-cell lymphomas and Hodgkin’s lymphomas. Anti-LAT did not label B-lymphoblastic lymphomas but strongly labeled T-lymphoblastic lymphomas. The reagent thus represents a valuable immunohistochemical marker for lineage assignment in lymphoblastic neoplasms. Existing antibodies reactive with antigens in paraffin samples have not been optimal for this purpose. For example, as also shown in this study the T-cell marker CD3 can be weak and focal, 29,30 and UCHL1/CD45R0 is frequently negative; 31,32 moreover, L26/CD20 is not uncommonly negative in B-cell lymphoblastic lymphomas 33 and CD79a has recently been shown to stain a proportion of T-lymphoblastic tumors. 34 Thus, LAT should help in determining the presence of a T-cell phenotype in lymphoblastic lymphomas. The observed expression of LAT on thymocytes at the TdT-negative stage suggests that it may represent a more reliable T-cell marker detectable in paraffin-embedded specimens in lymphomas of early precursors of T cells. Anti-LAT antibody showed a high sensitivity among mature T-cell and NK/T-cell neoplasms, identifying 96.3% of all cases. Two T-cell lymphomas were LAT-negative; one of them showed loss of CD3 and CD5 as well as CD43 and TCR-β. The other, in contrast, was a NK/T-cell lymphoma strongly expressing cytoplasmic CD3. Interestingly, the three CD3-negative T-cell lymphomas that expressed LAT were positive for TCR-β, but two of them lacked CD5. Discordant expression of TCR-β and CD3 is not uncommon in T-cell lymphomas; 35 together with the loss of other T-cell antigens 35-37 this process has been called “aberrant antigen profile” 35 and is considered to represent abnormal gene expression in these malignancies, rather than clonal expansion of a normal T-cell subset. 35 The results obtained in the present study indicate that LAT may be helpful in the identification of T-cell lymphomas that display antigenic loss. In addition, although both LAT and CD3 were similarly high sensitive in the staining of T-cell lymphomas (96.8% and 93.6%, respectively), when used in conjunction, they successfully identified a higher number of cases (98.4%). The use of CD3 and LAT together may represent the optimal method of immunohistochemical diagnosis of T-cell neoplasms in paraffin sections.
In contrast with other T-cell lymphomas, in ALCL T-cell type, anti-CD3 labeled a higher number of cases (19/25) than anti-LAT (10/25); however, seven cases showed the LAT+CD3− phenotype, and, the use of both markers had the highest sensitivity in the identification of T-cell ALCL (22/25). Because loss of T-cell antigens is frequently found in T-cell-type ALCL, 38 our results indicate that LAT is definitely of value in the phenotypic study of ALCL. Interestingly, a discordant expression of the two antigens was more frequently observed in ALCL than in other peripheral T-cell lymphomas (48.0% versus 7.2%, respectively) and this difference was statistically significant. This observation might reflect a more severe phenotypic aberration observed in these tumors. 38,39 Furthermore, we also correlated the expression of LAT in ALCL with their positivity for ALK-1, and found that the majority of ALK1-positive cases (6/10) were LAT-positive, unlike ALK1-negative cases (8/32). Whether these differences indicate a functional relationship between LAT and NPM/ALK remains unknown. In addition, further clinicopathological studies are necessary to establish whether LAT expression in ALK-positive ALCL may define a subset of tumors and correlate with prognosis. 12,40,41
In contrast to CD3, currently the most specific T-cell marker for paraffin sections, 30,33,42 LAT shows reactivity with other hematopoietic cell types, in both normal and neoplastic conditions. LAT stained mast cells and labeled 4/5 cases of mastocytosis. It should be noted that the pattern of reactivity in mast cells differs from that shown by T lymphocytes, making distinction between these cell types easier. Finally, LAT was strongly expressed in the cytoplasm of megakaryocytes, both in normal bone marrow and in various hematological neoplasms characterized by the occurrence of atypical forms of megakaryocytes and megakaryoblasts. Because few paraffin markers for megakaryocytes are currently available and some of them, such as anti-CD61, react poorly in B5-fixed biopsies (personal observation), we conclude that anti-LAT has potential use for demonstrating megakaryocytic components in leukemias.
In conclusion, this study has confirmed the previous data on LAT mRNA expression in normal tissues by demonstration of LAT protein. The study adds new information on LAT distribution in T and NK cells and on its expression in platelets and megakaryocytes. The anti-LAT antibody has a high specificity and sensitivity for immunostaining T cells and T- and NK/T-cell lymphomas. From these results, it is evident that anti-LAT represents a valuable addition to the panel of immunohistochemical markers that can be used for lymphoma phenotyping in routinely processed tissues. Finally, anti-LAT is a promising reagent for the screening of patients with primary defects of T cell and/or platelet functions to identify anomalies of LAT gene expression.
Note added in proof: In keeping with our findings, after this paper was submitted for publication, two studies have provided evidence that LAT is expressed in platelets and plays a role during platelet activation.
Sarkar S: Tyrosine phosphorylation and translocation of LAT in platelets. FEBS Lett 1998, 441:357–360.
Gibbins JM, Briddon S, Shutes A, van Vugt MJ, van de Winkel JGJ, Saito T, Watson SP: The p85 subunit of phosphatidylinositol 3-kinase associate with the Fc receptor γ-chain and linker for activation of T cells (LAT) in platelets stimulated by collagen and convulxin. J Biol Chem 1998, 273:34437–34443.
Table 4.
Nonlymphoid Hematopoietic Neoplasms Included in This Study
| Tissue type | No. of cases/No. of positive cases |
|---|---|
| Chronic myeloproliferative disorders | |
| Essential thrombocythemia | 2* |
| Chronic myeloid leukemia | 2* |
| Idiopathic myelofibrosis | 1* |
| Myelodysplastic syndromes | 4* |
| Acute non-lymphoid leukemia (F.A.B. classification) | |
| M1 | 3 /0 |
| M2 | 6 /0 |
| M3 | 4 /0 |
| M4 | 7 /0 |
| M5 | 2 /0 |
| M6 | 2 /0 |
| M7 | 2 /2 |
| Unclassified† | 2 /1 |
| Mastocytosis | 5 /4 |
*In all cases, positivity only on typical and atypical megakaryocytes.
†One case represented by blastic transformation of chronic myeloid leukemia with development of myeloid and megakaryocytic blasts.
Acknowledgments
We thank Dr. A. Moretta for his generous gift of monoclonal antibodies JT3A, kd1, C218, and for helpful suggestions, and O. Alebardi, L. Salvi, L. Breda, M. Gattamelata, and C. Solfrini for their technical assistance.
Footnotes
Address reprint requests to Fabio Facchetti, MD, PhD, Department of Pathology, University of Brescia, Spedali Civili Brescia, 25124 Brescia, Italy. E-mail: facchett@master.cci.unibs.it.
Supported in part by Associazione Italiana per la Ricerca sul Cancro (to M.C.) and by Biomed contract CT98-3007 (to F.F. and L.D.L.).
References
- 1.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T: Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J 1995, 14:2499-2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wange RL, Guitian R, Isakov N, Watts JD, Aebersold R, Samelson LE: Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem 1995, 270:18730-18733 [DOI] [PubMed] [Google Scholar]
- 3.Pawson T: Protein modules and signaling networks. Nature 1995, 373:573-580 [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE: LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 1998, 92:83-92 [DOI] [PubMed] [Google Scholar]
- 5.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A: LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity 1998, 9:617-626 [DOI] [PubMed] [Google Scholar]
- 6.June CH, Fletcher MC, Ledbetter JA, Samelson LE: Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol 1990, 144:1591-1599 [PubMed] [Google Scholar]
- 7.Gilliland LK, Schieven GL, Norris NA, Kanner SB, Aruffo A, Ledbetter JA: Lymphocyte lineage-restricted tyrosine-phosphorylated proteins that bind PLCγ1 SH2 domains. J Biol Chem 1992, 267:13610-13616 [PubMed] [Google Scholar]
- 8.Buday L, Egan SE, Rodriguez Viciana P, Cantrell DA, Downward J: A complex of Grb2 adaptor protein, Sos exchange factor, and a 36-kd membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells. J Biol Chem 1994, 269:9019-9023 [PubMed] [Google Scholar]
- 9.Sieh M, Batzer A, Schlessinger J, Weiss A: GRB2 and phospholipase C-γ 1 associate with a 36- to 38-kilodalton phosphotyrosine protein after T-cell receptor stimulation. Mol Cell Biol 1998, 14:4435-4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukazawa T, Reedquist KA, Panchamoorthy G, Soltoff S, Trub T, Druker B, Cantley L, Shoelson SE, Band H: T cell activation-dependent association between the p85 subunit of the phosphatidylinositol 3-kinase and Grb2/phospholipase C-γ 1-binding phosphotyrosyl protein pp36/38. J Biol Chem 1998, 270:20177-20182 [DOI] [PubMed] [Google Scholar]
- 11.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller Hermelink H, K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361–1392 [PubMed]
- 12.Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, Delsol G, Mason DY: Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997, 89:1394-1404 [PubMed] [Google Scholar]
- 13.Moretta A, Tambussi G, Bottino C, Tripodi G, Merli A, Ciccone E, Pantaleo G, Moretta L: A novel surface antigen expressed by a subset of human CD3-CD16+ natural killer cells: role in cell activation and regulation of cytolytic function. J Exp Med 1990, 171:695-714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deibel MR, Jr., Riley LK, Coleman MS, Cibull ML, Fuller SA, Todd E: Expression of terminal deoxynucleotidyl transferase in human thymus during ontogeny and development. J Immunol 1983, 131:195-200 [PubMed] [Google Scholar]
- 15.Bodger MP, Janossy G, Bollum FJ, Burford GD, Hoffbrand AV: The ontogeny of terminal deoxynucleotidyl transferase positive cells in the human fetus. Blood 1983, 61:1125-1131 [PubMed] [Google Scholar]
- 16.Vroom TM, Scholte G, Ossendorp F, Borst J: Tissue distribution of human γ delta T cells: no evidence for general epithelial tropism. J Clin Pathol 1991, 44:1012-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell GJ, Winter HS, Fox VL, Bhan AK: Lymphocytes bearing the γ delta T-cell receptor in normal human intestine and celiac disease. Hum Pathol 1991, 22:690-694 [DOI] [PubMed] [Google Scholar]
- 18.Anderson P, Caligiuri M, Ritz J, Schlossman SF: CD3-negative natural killer cells express zeta TCR as part of a novel molecular complex. Nature 1989, 341:159-162 [DOI] [PubMed] [Google Scholar]
- 19.Ting AT, Dick CJ, Schoon RA, Karnitz LM, Abraham RT, Leibson PJ: Interaction between lck and syk family tyrosine kinases in Fc γ receptor-initiated activation of natural killer cells. J Biol Chem 1995, 270:16415-16421 [DOI] [PubMed] [Google Scholar]
- 20.Viver E, da Silva AJ, Ackerly M, Levine H, Rudd CE, Anderson P: Association of a 70-kd tyrosine phosphoprotein with the CD16ζγ complex expressed in human natural killer cells. Eur J Immunol 1993, 23:1872-1876 [DOI] [PubMed] [Google Scholar]
- 21.Nel AE, Gupta S, Lee L, Ledbetter JA, Kanner SB: Ligation of the T-cell antigen receptor (TCR) induces association of hSos1, ZAP-70, phospholipase C-γ 1, and other phosphoproteins with GRb2 and the ζ-chain of the TCR. J Biol Chem 1995, 270:18428-18436 [DOI] [PubMed] [Google Scholar]
- 22.Jin YJ, Friedman J, Burakoff SJ: Regulation of tyrosine phosphorylation in isolated T cell membrane by inhibition of protein tyrosine phosphatases. J Immunol 1998, 161:1743-1750 [PubMed] [Google Scholar]
- 23.Beaven MA, Baumgartner RA: Downstream signals initiated in mast cells by Fcε RI and other receptors. Curr Opin Immunol 1996, 8:766-772 [DOI] [PubMed] [Google Scholar]
- 24.Yablonski D, Kuhne MR, Kadlecek T, Weiss A: Uncoupling of nonreceptor tyrosine kinases from PLCγ1 in an SLP-76-deficient T cell. Science 1998, 281:413-416 [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Trible RP, Samelson LE: LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 1998, 9:239-246 [DOI] [PubMed] [Google Scholar]
- 26.Pivniouk V, Tsitsikiv E, Swinton P, Rathbun G, Alt FW, Geha RS: Impaired viability and profound block in thymocyte development in mice lacking the adapter protein SLP-76. Cell 1998, 94:229-238 [DOI] [PubMed] [Google Scholar]
- 27.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL: Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature 1995, 378:298-302 [DOI] [PubMed] [Google Scholar]
- 28.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T: Syk tyrosine kinase required for mouse viability and B-cell development. Nature 1995, 378:303-306 [DOI] [PubMed] [Google Scholar]
- 29.Wood KM, Pallesen G, Ralfkiaer E, Warnke R, Gatter KC, Mason DY: Heterogeneity of CD3 antigen expression in T-cell lymphoma. Histopathology 1993, 22:311-317 [DOI] [PubMed] [Google Scholar]
- 30.Cabecadas JM, Isaacson PG: Phenotyping of T-cell lymphomas in paraffin sections–which antibodies? Histopathology 1991, 19:419-424 [DOI] [PubMed] [Google Scholar]
- 31.Davey FR, Elghetany MT, Kurec AS: Immunophenotyping of hematologic neoplasms in paraffin-embedded tissue sections. Am J Clin Pathol 1990, 93:S17-26 [PubMed] [Google Scholar]
- 32.Linder J, Ye YL, Harrington DS, Armitage JO, Weisenburger DD: Monoclonal antibodies marking T lymphocytes in paraffin-embedded tissue. Am J Pathol 1987, 127:1-8 [PMC free article] [PubMed] [Google Scholar]
- 33.Chadburn A, Knowles DM: Paraffin-resistant antigens detectable by antibodies L26 and polyclonal CD3 predict the B- or T-cell lineage of 95% of diffuse aggressive non-Hodgkin’s lymphomas. Am J Clin Pathol 1994, 102:284-291 [DOI] [PubMed] [Google Scholar]
- 34.Pilozzi E, Pulford K, Jones M: Coexpression of CD79a (JCB117) and CD3 by lymphoblastic lymphoma. J Pathol 1998, 186:140-143 [DOI] [PubMed] [Google Scholar]
- 35.Picker LJ, Brenner MB, Weiss LM, Smith SD, Warnke RA: Discordant expression of CD3 and T-cell receptor β-chain antigens in T-lineage lymphomas. Am J Pathol 1987, 129:434-440 [PMC free article] [PubMed] [Google Scholar]
- 36.Picker LJ, Weiss LM, Medeiros LJ, Wood GS, Warnke RA: Immunophenotypic criteria for the diagnosis of non-Hodgkin’s lymphoma. Am J Pathol 1987, 128:181-201 [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss LM, Crabtree GS, Rouse RV, Warnke RA: Morphologic and immunologic characterization of 50 peripheral T-cell lymphomas. Am J Pathol 1985, 118:316-324 [PMC free article] [PubMed] [Google Scholar]
- 38.Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugieres L, Terrier-Lacombe MJ, Haralambieva E, Pulford K, Pileri S, Morris SW, Mason DY, Delsol G: ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 1998, 91:2076-2084 [PubMed] [Google Scholar]
- 39.Beljaards RC, Kaudewitz P, Berti E, Gianotti R, Neumann C, Rosso R, Paulli M, Meijer CJ, Willemze R: Primary cutaneous CD30-positive large cell lymphoma: definition of a new type of cutaneous lymphoma with a favorable prognosis. A European Multicenter Study of 47 patients. Cancer 1993, 71:2097-2104 [DOI] [PubMed] [Google Scholar]
- 40.Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, Mori N, Fujimoto J, Miyauchi J, Mikata A, Nanba K, Takami T, Yamabe H, Takano Y, Izumo T, Nagatani T, Mohri N, Nasu K, Satoh H, Katano H, Yamamoto T, Mori S: Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood 1995, 86:1954-1960 [PubMed] [Google Scholar]
- 41.Bitter MA, Franklin WA, Larson RA, McKeithan TW, Rubin CM, Le Beau MM, Stephens JK, Vardiman JW: Morphology in Ki-1(CD30)-positive non-Hodgkin’s lymphoma is correlated with clinical features and the presence of a unique chromosomal abnormality, t(2;5)(p23;q35). Am J Surg Pathol 1990, 14:305-316 [DOI] [PubMed] [Google Scholar]
- 42.Mason DY, Krissansen GW, Davey FR, Crumpton MJ, Gatter KC: Antisera against epitopes resistant to denaturation on T3 (CD3) antigen can detect reactive and neoplastic T cells in paraffin embedded tissue biopsy specimens. J Clin Pathol 1988, 41:121-127 [DOI] [PMC free article] [PubMed] [Google Scholar]