Abstract
Focal segmental glomerulosclerosis is a steroid-resistant glomerular disease characterized by foot process flattening and heavy proteinuria. A similar disease was found to occur spontaneously in mice in which the Mpv17 gene was inactivated by retroviral insertion (Mpv17−/− mice). Here evidence is provided that glomerular damage in this murine model is due to overproduction of oxygen radicals and accumulation of lipid peroxidation adducts that were found in isolated glomeruli of Mpv17−/− mice. The development of glomerular disease in Mpv17−/− mice was inhibited by scavengers of oxygen radicals (dithiomethylurea) and lipid peroxidation (probucol), but not by steroid treatment. Although the glomerular polyanion was greatly reduced in proteinuric Mpv17−/− mice, it was preserved by antioxidative therapy. These results indicate that the glomerular disease in Mpv17−/− mice qualifies as a model of steroid-resistant focal segmental glomerulosclerosis and that experimental therapies with scavengers of oxygen radicals and lipid peroxidation efficiently ameliorate glomerular damage.
Minimal change nephrosis (MCN) and focal segmental glomerular sclerosis (FSGS) are relatively frequent glomerular diseases that cause heavy proteinuria and syndrome. The characteristic morphological lesion in both nephrotic diseases is extensive flattening of podocyte foot processes, suggesting a primary defect in these cells. 1 The precise distinction between MCN and FSGS is sometimes difficult to make and there is an argument that both diseases are actually two variants of a single entity. 2 MCN is usually encountered in children and responds to treatment with steroids, whereas FSGS is more frequently diagnosed in adults, is insensitive to steroids, and shows a tendency to progress to end stage renal failure and rapid recurrence in transplants. A putative circulating proteinuria-inducing factor is currently under investigation. 3,4
There are several instructive experimental animal models for MCN/FSGS that show features similar to the human disease. One well studied example relies on intoxication of rats with puromycin aminonucleoside. 5 Podocytes are very sensitive to this toxic antibiotic for unknown reasons and respond promptly with foot process retraction 6 and proteinuria at the nephrotic level. In this and several other experimental glomerular diseases overproduction of reactive oxygen species (ROS) and local accumulation of lipid peroxidation (LPO) adducts were identified as prime causes for glomerular damage 7 and interventional therapies with specific scavengers drastically reduced foot process flattening and proteinuria. 8 Although these model diseases require exogenous intoxication and cause complex reactions of podocytes, similar symptoms of disease were found to occur spontaneously in mice in which the gene Mpv17 was inactivated by retroviral insertion (Mpv17−/− mice). 9 Here we have examined the possibility that in this system foot process flattening and proteinuria are also caused by excessive production of ROS and LPO adducts in glomeruli.
Materials and Methods
Materials
Hanks’ balanced salt solution, luminol (5-amino-2,3-dihydro-1,4-pthalaiznedione), probucol, and dimethylthiourea (DMTU) were obtained from Sigma Chemical Corp. (St. Louis, MO) and methylprednisolon from Upjohn Corp. (Kalamazoo, MI). Rabbit anti-Mpv17 IgG was prepared and characterized as previously described. 10 Tetramethylrhodamine isothiocyanate-labeled goat anti-rabbit IgG was from DAKO (Copenhagen, Denmark).
Mpv17−/− Mice
Homozygous Mpv17−/− mice were generated by retroviral insertion as described elsewhere. 9 As these original Mpv17−/− mice showed reduced breeding capacity and died from renal failure within a few months after birth, in this study we used a strain selected for extended survival (Weiher, unpublished observations). This strain showed proteinuria and histopathological features of FSGS, as did the original Mpv17−/− mice, but failed to develop interstitial fibrosis. Development of glomerular disease was documented by routine histology in paraffin sections and by standard electron microscopy. As controls, age-matched BALB/c mice were used. Use of experimental mice was authorized by the Austrian Ministry of Science and Traffic.
Scavenger Treatment
One group of Mpv17−/− mice was treated with DMTU administered by osmotic pumps 11 (Alzet 2002, 200 μl; 0.5 μl/hour, Alza Corp., Palo Alto, CA) filled with 312 mg/ml DMTU in phosphate-buffered saline (PBS), 8 or with PBS alone in controls. Pumps were implanted subcutaneously into the back between the scapulae of anesthetized mice at postnatal day 29 and were active for exactly 14 days. The pump’s weights were determined before implantation and after explantation to calculate the exact amounts of DMTU delivered. The average dose of DMTU administered was 15 μg per kilogram of body weight per day. Treated and control animals were sacrificed as indicated in the respective figures.
Probucol was administered to Mpv17−/− mice by feeding standard chow pellets containing probucol (1% w/w, Altromin, Lage, Germany). Experimental animals were exposed to probucol from early in their mothers’ pregnancy until their sacrifice. Five Mpv17−/− mice were injected intraperitoneally with methylprednisolon (30 mg/kg) at postnatal day 57 and proteinuria was recorded for 10 days.
Proteinuria
Urine was collected from Mpv17−/− and control BALB/c mice and proteinuria was determined by Pierce Coomassie Blue and albumin assays conducted according to the manufacturer’s instructions.
Quantitative Electron Microscopy
Development of podocytes was recorded by quantitative electron microscopy, as described. 12 Kidney cortex samples were fixed in 2.5% glutaraldehyde/4% paraformaldehyde in 100 mmol/L Na cacodylate buffer, followed by OsO4 in the same buffer. After rinsing in distilled water, the samples were incubated in 0.5% uranyl acetate and embedded in epoxy resin. The number of foot processes per micrometer of glomerular basement membrane was determined from electron micrographs of capillary loops of at least 3 different glomeruli per kidney.
Histochemistry and Immunofluorescence Microscopy
The glomerular polyanion was visualized on paraffin sections of treated and untreated Mpv17−/− and BALB/c control kidneys by a colloidal iron procedure, followed by a Prussian blue reaction. 13 Cryostat sections (3 μm) of unfixed Mpv17−/− mouse kidneys were incubated with rabbit anti-Mpv17 IgG (1 μg/ml), followed by tetramethylrhodamine isothiocyanate-conjugated goat anti-rabbit IgG.
Detection of Oxygen Radicals by the Luminol Method
Forty-day-old proteinuric Mpv17−/− and control BALB/c mice were ether anesthetized and perfused with Hanks’ buffered salt solution via the left cardiac ventricle. Isolation of glomeruli was performed by sequential sieving and differential centrifugation. The purity of the fractions was >90% as determined by direct microscopy. Isolated glomeruli were incubated in Hanks’ buffered salt solution for 15 minutes at 37°C, and the production of oxygen radicals was determined by measuring the peak of chemiluminiscence induced with 5-amino-2,3-dihydro-1,4-pthalaiznedione (luminol) at a final concentration of 3 × 10−5 M in a Beckman LS6500 liquid scintillation counter (Beckman Instruments, Fullerton, CA) at 25°C. 14
Detection of Lipid Peroxidation Adducts
Kidneys of Mpv17−/− and of control BALB/c mice were perfused via the left cardiac ventricle with ice-cold PBS. After decapsulation kidneys were weighted, snap frozen in liquid N2, and homogenized in a microdismembrator. Tris-HCl buffer (pH 7.4) was added to the homogenates (10 ml/g) and the tissue suspension was centrifuged at 3000 × g for 10 minutes. The concentrations of malondialdehyde (MDA) and 4-hydroxynonenal per milligram of kidney protein were determined by the LPO test kit (Bioxytech LPO-586, Oxis International, Portland, OR). In this test different substrates were used to indicate the concentration of MDA alone or of MDA plus 4-hydroxynonenal combined according to the manufacturer’s instructions.
Statistical Methods
Data are expressed as mean and standard deviations (SD) of the respective groups or time points, as indicated in the figure legends. Differences between control and Mpv17−/− mice as well as the effects of treatment on proteinuria and the number of foot processes were analyzed by the variance test. Differences between control and Mpv17−/− mice with respect to the generation of oxygen radical formation and lipid peroxidation products in isolated glomeruli and kidney homogenates, respectively, were analyzed by the unpaired t-test. A P value <0.05 was regarded as statistically significant.
Results
Mpv17−/− Mice Develop FSGS
Histopathological changes of glomeruli of Mpv17−/− mice were examined 50 and 180 days after birth, and several morphological features were found that closely resemble human FSGS. 1 At 50 days, glomeruli showed slight enlargement and segmental mesangial sclerosis (Figure 1A) ▶ , and at 180 days hyaline and scarred segmental lesions were also observed (Figure 1, B–D) ▶ . Interstitial inflammation and fibrosis were not present. By electron microscopy almost complete flattening of foot processes was found, as shown previously. 9
Figure 1.
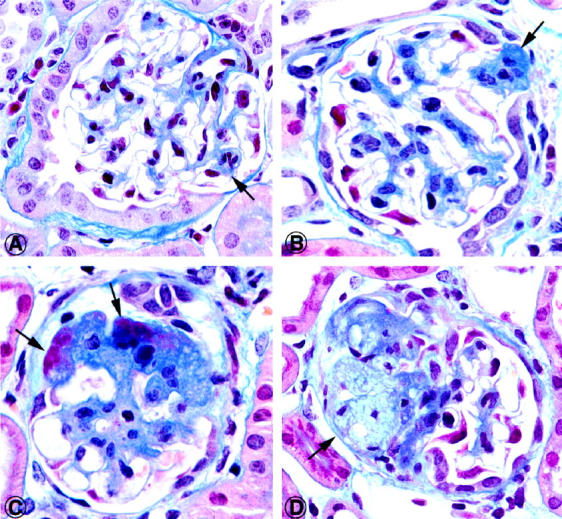
Gallery of histopathological lesions of glomeruli of Mpv17−/− mice 50 days after birth. In early stages there is mesangial thickening by moderate cell proliferation and sclerosis (A, B, arrows). In advanced lesions, hyaline changes of glomerular segments and sclerotic scars are found. The lesion in D contains a cluster of foam cells embedded in a sclerotic lesion (arrow). Mallory trichrome stain; original magnification, ×750.
The Mpv17 Antigen Is Expressed in Glomerular Podocytes
Diffuse to fine granular staining of podocytes was observed on cryostat sections of normal mouse kidney (Figure 2A) ▶ by indirect immunofluorescence with affinity purified antibody raised against the recombinant Mpv17 protein. In proximal tubular epithelial cells a coarse granular pattern was obtained, presumably corresponding to peroxisomes (Figure 2C) ▶ . By contrast, both glomerular and tubular staining were abolished in the Mpv17−/− mouse (Figure 2, B and D) ▶ . These data are in agreement with previous biochemical data, confirming lack of the Mpv17 gene expression in Mpv17−/− mice. 9,10
Figure 2.

Localization of the 20-kd Mpv17 gene product (A-C), and the glomerular polyanion (E and F). A: Indirect immunofluorescence was carried out on cryostat sections of unfixed mouse kidneys with affinity purified rabbit antibody specific for the 20-kd protein that corresponds to the Mpv17 gene product. The antibody labels podocytes of BALB/c control mice (arrowheads) in a coarse granular or diffuse cytoplasmic pattern. B: The same antibody fails to label any glomerular structure in Mpv17−/− mice. C: In proximal tubule epithelial cells the anti-20-kd protein antibody specifically labels cytoplasmic granules that are presumably peroxisomes (arrowheads). D: In Mpv17−/− mice no labeling is observed. E: Staining of the glomerular polyanion by the colloidal iron method on paraffin sections of Mpv17−/− mice during infusion of the oxygen radical scavenger DMTU. In these animals proteinuria was abolished as long as the minipump used for infusion was active. The dense blue reaction product outlines the capillary loops. F: By contrast, glomerular staining of Mpv17−/− mice was reduced to a few granular deposits of the colloidal iron reagent. CL, capillary lumen; N, nucleus; L, lumen. Original magnification, ×800.
The Glomerular Polyanion Is Reduced in Mpv17−/− Mice
When the overall amount of negatively charged groups in glomeruli was determined by cationic colloidal iron cytochemistry, strong labeling was observed in controls and Mpv17−/− mice treated with probucol or DMTU (Figure 2E) ▶ , but only traces were detected in proteinuric Mpv17−/− mice (Figure 2F) ▶ . Labeling of endothelia of interstitial blood vessels was similar in all groups.
Proteinuria in Mpv17−/− Mice Is Selective
Total proteinuria was determined by the Coomassie blue assay, and albumiuria with a mouse albumin-specific assay. It was observed that < 95% of the proteinuria was accounted for by urinary albumin excretion (data not shown).
Isolated Glomeruli of Mpv17−/− Mice Produce Excessive ROS and Contain LPO Adducts
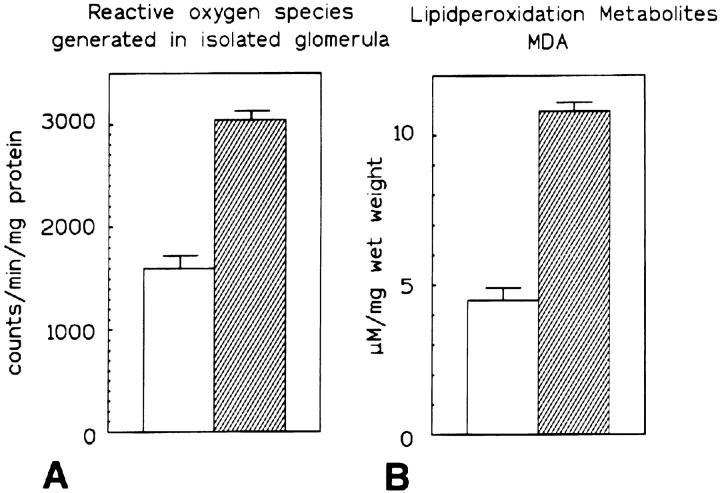
ROS production by equal numbers of isolated glomeruli of BALB/c-controls and Mpv17−/− mice was measured by the luminol assay that provides an overall estimate on a wide variety of ROS. A statistically significant twofold increase in ROS production/glomerulus over controls was observed in Mpv17−/− mice (Figure 3A) ▶ .
Figure 3.
Production of reactive oxygen species and lipid peroxidation metabolites in normal versus Mpv17−/−glomeruli. A: Production of reactive oxygen species by equal numbers of isolated glomeruli of control BALB/c and Mpv17−/− mice, as determined by the luminol method. Glomeruli of Mpv17−/− mice produce approximately the double amount of oxygen radicals of controls. Production of radicals is expressed as counts (determined in the scintillation counter) per milligram of glomerular protein. B: The amount of lipid peroxidation adducts was determined by the dithiobarbituric assay specific for MDA adducts. Mpv17−/− kidney cortex contains more than twice the amount of MDA adducts when compared to normal controls. The heights of the columns represent the mean values of 5 experiments and the bars indicate standard deviations. In both assays the differences were statistically significant (unpaired t-test).
LPO adducts are rapidly formed by ROS and polyunsaturated fatty acids and were previously shown to mediate glomerular damage. 15 Comparison of renal LPO adduct concentrations in Mpv17−/− and control mice revealed ∼2.5 times higher levels of MDA (11 mmol/L per mg wet tissue) than in normal BALB/c mice (4.5 mmol/L per mg wet tissue, Figure 3B ▶ ). Similar results were obtained for MDA plus 4-hydroxy-2(E)-nonenal, another LPO adduct (data not shown).
Podocyte Damage and Proteinuria Develop in Mpv17−/− Mice after Maturation of Glomeruli
For the targeting of a pharmacological intervention it was necessary to determine the optimal time point for treatment at the onset of development of the glomerular lesions. Therefore the maturation of podocytes was recorded by quantitation from conversion of flattened podocytes at birth to full development of the arborized foot processes by quantitative electron microscopy. Subcapsular glomeruli were analyzed to minimize the sampling error. In normal BALB/c mice, full maturation of podocytes was observed stereotypically at days 30–32 postpartum. The same time schedule of podocyte maturation was also observed for Mpv17−/− mice. However, immediately after reaching full expansion, their foot processes started to flatten again until neonatal levels of spreading were restored on days 33–35 postpartum (Figure 4) ▶ that remained for the rest of the animal’s life.
Figure 4.
Time course of the development of podocyte foot processes in Mpv17−/− and BALB/c control mice. The number of foot processes per 10 μm of GBM was determined by quantitative electron microscopy in subcapsular glomeruli of mice of different ages, as indicated in the x axis. Podocytes were flat at birth and developed to maturity with extensive foot processes around days 30–35. Although the number of foot processes per 10 μm of GBM remained constant in controls, it sharply dropped at days 33–35 in Mpv17−/− mice. Data in this graph represent mean values and standard deviations of 12 normal and 24 Mpv17−/− mice, respectively. The differences were statistically significant (analysis of variance).
Proteinuria increased in parallel with flattening of foot processes after day 30 postpartum and reached a plateau around day 33 for the rest of the animal’s life (Figure 4) ▶ .
Oxygen Radical Scavengers Prevent Glomerular Damage in Mpv17−/− Mice
The potent oxygen radical scavenger DMTU was infused continuously at a constant rate via an implanted minipump 11 for 2 weeks. The start of the infusion was set at day 29 postpartum to cover the entire period of foot process flattening after initial maturation. The major finding was that the mature foot process morphology was preserved, precisely as long as the pump was active and the level of DMTU was maintained (Figure 5) ▶ . Immediately after exhaustion of the DMTU reservoir the foot processes rapidly converted into flat epithelial sheets in 1–3 days (Figure 6A) ▶ . Within the follow-up of 2 weeks, proteinuria redeveloped only slowly (Figure 6B) ▶ . The results obtained in Mpv17−/− mice were controlled by a group of Mpv17−/− mice with a minipump containing PBS. In these controls foot process flattening developed identically to that in unmanipulated mice (Figure 5) ▶ .
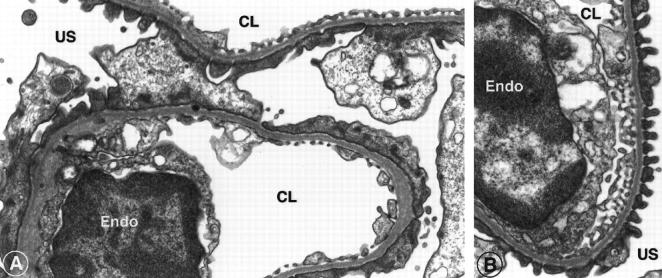
Figure 5.
Comparison of foot process architecture by electron microscopy of PBS-infused control Mpv17−/− mice (A), and of animals infused with DMTU via an implanted minipump for 14 days (B). Tissue samples were collected on day 14 of the infusion from the subcortical area. Untreated Mpv17−/− mice show extensive flattening of the foot processes, whereas the DMTU-treated group consistently showed normal podocyte foot processes and slit diaphragms. E, endothelium; CL, capillary lumen; US, urinary space. Original magnification, ×28,000.
Figure 6.
Treatment of Mpv17−/− mice with the oxygen radical scavenger DMTU prevents proteinuria and flattening of the podocytes. A: DMTU was delivered subcutaneously for 2 weeks by an Alzet minipump in three Mpv17−/− mice. Treatment was started on day 28, ie, close to the time point when a dichotomy of the development of normal and Mpv17−/− glomerular podocytes occurs. Although in the Mpv17−/− control group treated with PBS (n = 3) the same development of proteinuria occurred as in completely untreated animals (data not shown), the infusion of DMTU (shaded area) efficiently prevented the development of proteinuria, which was only slightly regained after the pump was empty on day 43. Data represent mean values and standard deviations. B: Flattening of podocytes was also prevented in Mpv17−/− mice as long as the minipump delivered DMTU into the animal (shaded area). Following this period the number of foot processes sharply dropped and reached the same extent as Mpv17−/− mice. The values given represent mean values and standard deviations for 9 DMTU-treated and 24 control animals. The differences between treated and untreated mice were statistically significant (analysis of variance).
Cortisol Treatment of Mpv17−/− Mice Fails to Prevent Glomerular Damage
Proteinuric Mpv17−/− mice treated with a single dose of methylprednisolon at postnatal day 57 failed to show any effect on proteinuria within 10 days of follow-up and podocytes remained flat (data not shown).
The Lipid Peroxidation Scavenger Probucol Prevents Glomerular Damage in Mpv17−/− Mice
To saturate experimental animals with the lipophilic drug probucol, breeding pairs of Mpv17−/− mice were kept on a diet containing probucol for 2 months, and their offspring were also fed chow containing probucol after birth. Development of foot processes was followed by quantitative electron microscopy and urine protein concentration was monitored. When compared to control BALB/c mice, no differences in the time course of postnatal development of foot processes were found and foot process morphology was completely preserved, even at time points when their architecture was completely lost in untreated Mpv17−/− mice (Figure 7A) ▶ . Proteinuria was completely absent (Figure 7B) ▶ .
Figure 7.
Treatment with probucol prevented flattening of foot processes and development of proteinuria. A: Mpv17−/− mice were fed probucol-containing chow from early pregnancy, and their offspring were also kept on a probucol diet. This treatment efficiently prevented the development of proteinuria, whereas untreated Mpv17−/− mice developed proteinuria in the same time course, as shown in Figure 3 ▶ . Data represent mean values and standard deviations of three animals in each group. B: In probucol-treated animals the development of podocytes is normal. The number of foot processes per 10 μm of GBM in BALB/c-controls and probucol-treated mice remained the same. By contrast, Mpv17−/− mice receiving standard chow followed the same pattern of podocyte flattening as shown in Figure 4 ▶ . The values represent mean values and standard deviations for 9 probucol-treated and 24 control animals. The differences between treated and untreated mice were statistically significant (analysis of variance).
Discussion
Flattening of podocyte foot processes and selective proteinuria are hallmarks of steroid-insensitive FSGS. 1 A novel murine model for this disease was generated by retroviral insertion into one preferred genomic integration site, Mpv17, resulting in inactivation of the Mpv17 gene. 9 Mice homozygous for this integration developed the pathological phenotype of progressive glomerulosclerosis and proteinuria at a young age and died from renal failure after 9–12 months. A human homologue of the Mpv17 gene was identified and localized to chromosome 2p23-p21, 16 and insertion of this human gene into the genome of transgenic mice rescued their renal function, thus clearly linking the Mpv17 gene to glomerular disease. 17 The Mpv17 gene product was identified as a 20-kd membrane protein in membranes of peroxisomes, and it was hypothesized that it plays a major role in the peroxisomal metabolism of ROS. 10 However, it was recently discovered that the gene of the peptide hormone urocortin is topographically closely associated with the Mpv17 gene. Because it cannot be excluded that this gene was also affected by the retroviral insertion, it remains to be determined whether urocortin could also contribute to the Mpv17−/− phenotype. 18
ROS were recently found to mediate glomerular damage in several experimental models of renal disease 7 and we have therefore investigated in this study their role in the glomerular disease of homozygous mice lacking functional Mpv17 genes. The results of this study provide evidence that increased production and/or reduced degradation of extracellularly released ROS within glomeruli of Mpv17−/− mice is causally related to the glomerular disease observed in these animals. This interpretation rests primarily on direct demonstration of enhanced ROS production by isolated glomeruli of Mpv17−/− mice, and on interventional therapies with specific ROS scavenging drugs.
The precise biochemical mechanisms of increased glomerular ROS production in MPV17−/− mice are unclear. Using hyroethidine that is converted into a fluorescent dye by superoxide radicals and other ROS species, we previously reported that overexpression of the Mpv17 protein resulted in accumulation of ROS within tissue culture cells and consequently proposed that knockout of this protein should reduce ROS production. 10 However, the use of fluorescent or luminescent compounds of low molecular weight to detect ROS is not unanimously accepted 19 and scavengers of high molecular weight, such as superoxide dismutase and catalase, pose the problem of access to ROS in glomeruli in vitro and in vivo. One major point of criticism is that ROS could be self-generated by the detecting dyes 19 but, by contrast, the specificity of luminol for detection of ROS was recently validated. 20 To clarify this controversial issue of detectors biasing ROS determinations in Mpv17−/− cells, we have performed in vitro electron spin resonance experiments that directly revealed a significantly increased release of superoxide anion into the culture media by Mpv17-deficient cells when compared with Mpv17-proficient cells (Wagner and Weiher, manuscript in preparation). This finding is compatible with the concept of increased release and extracellular accumulation of ROS in glomeruli of Mpv17−/− mice, but it remains to be determined why pathology develops preferentially in glomeruli and is not more widespread.
In vitro studies have provided evidence that ROS cause direct, profound effects on the integrity and composition of basal membrane matrix proteins. 21 ROS produced by the Fenton reaction were shown to disintegrate and fragment EHS sarcoma matrix in vitro in a dose-dependent fashion, with entactin and laminin being most sensitive to detachment and degradation and type IV collagen most resistant. It remains to be determined whether similar events also occur in vivo in Mpv17−/− mice. Heparan sulfate proteoglycan extracted from glomerular basement membrane (GBM) was incubated in vitro with ROS generated by xanthine-xanthine oxidase, 22 resulting in specific degradation of glucosaminoglycan side chains and reduced charge density of the proteoglycan. Thus, ROS-mediated destruction of glucosaminoglycans could contribute to loss of the “glomerular polyanion” and selective proteinuria observed in Mpv17−/− mice, because reduction of electronegatively charged groups is known to increase GBM permeability selectively for anionic molecules such as albumin. 23
There is increasing evidence that flattening of podocytes could be not only a consequence but also the cause of increased glomerular permeability. Multiple and diverse stimuli act on podocytes to induce shape changes. They include toxic reactions, such as puromycin aminonucleoside and adriamycine, decrease in podocyte surface charge by neuraminidase digestion, 24 and compensation with the polycations protamine sulfate and l-polylysine. 12 Other means leading to the same effect are modification of the GBM composition, eg, by genetic knockout of s-laminin, 25 or in situ immune complex formation with epitope-specific antibodies to podocyte membrane proteins such as podoplanin, 26,27 aminopeptidase A, 28 and p51, 29 and with the antigen of Heymann nephritis, gp330/megalin. 30 However, at present one can only speculate about mechanisms of ROS-induced foot process deformation in the Mpv17−/− mouse. It is possible that chemical ROS-induced modifications of matrix or adhesion molecules play a role, but actions of ROS-activated transcription factors cannot be excluded. Intriguingly, postnatal development of podocytes follows the same pattern of maturation in Mpv17−/− mice as in controls. Assuming that innate overexpression of ROS was established at birth, it is possible that scavengers yet unidentified are produced within glomeruli or resorbed from the mother’s milk, or that antioxidative compounds are expressed only during the postnatal period and decrease later.
In addition to the glomerulosclerotic phenotype Mpv17−/− mice showed pathological changes in their inner ear, reminiscent of Alport’s syndrome. 31 Indeed, as in Alport’s syndrome, both the glomerular and the cochlear basement membranes are affected and recent results indicate that the matrix metalloprotease II (MMP-2) plays a key role in progression of this cochlear disease. 18 It is also possible that increased release of MMP-2 is involved in glomerular damage; however, the results of this study clearly indicate that this could be only a consequence of ROS-induced damage, as selective ROS scavenging globally reduced glomerular lesions.
Molecular mechanisms of GBM damage and proteinuria are relatively well understood in Heymann nephritis, a model of human membranous nephropathy. 15 A detailed analysis of its pathogenesis of proteinuria revealed a chain of events that include intraglomerular overproduction of ROS, generation of lipid peroxidation adducts in the GBM, and crosslinking of Type IV collagen via its NC-1 domains. Consequently, therapy with the lipid peroxidation scavenger probucol efficiently reduced proteinuria, 15 and probucol was recently identified as effective antiproteinuric agent in human patients with membranous nephropathy. (Meyer G, Haas M, Wirnsberger G, Holzer H, Ratschek M, Neyer U, Neuweiler R, Kramar R, Schneider B, Breiteneder S, Regele H, Hörl W, Kerjaschki D, unpublished manuscript). Intraglomerular LPO is also clearly involved in the glomerular disease of Mpv17−/− mice, because LPO adducts were found in high concentrations in isolated glomeruli of Mpv17−/− mice and probucol completely prevented the disease when it was fed to Mpv17−/− mice from birth. The possibility that in this setting the major LPO product MDA 32 was generated as a byproduct of increased glomerular prostaglandin synthesis rather than as a consequence of LPO is unlikely, because its formation was reduced by probucol, which has no influence on prostaglandin synthesis. It remains to be shown whether probucol is also effective in interventional therapy and reverses the glomerular changes in advanced stages of Mpv17−/− disease.
Collectively, the results of this study provide evidence for the central involvement of ROS and LPO in the pathogenesis of the Mpv17−/− glomerulopathy. This mouse disease resembles human FSGS not only in its morphology and proteinuria, but also in its resistance to a bolus therapy with a high dose of steroids. Thus, it will be of some interest to determine the pathogenic role of glomerular ROS production and the effect of antioxidative therapy in this human disease.
Footnotes
Address reprint requests to Dontscho Kerjaschki, M.D., Department of Clinical Pathology, University of Vienna, AKH, Währinger Gürtel 18–20, A-1090 Vienna, Austria.
Supported by Sonderforschungsbereich 05, Project 007 from the Österreichischen Fonds zur Förderung der Wissenschaftlichen Forschung and the EC Concerted Action Contract no. BMH4–98-3631 (to D.K.).
Dr. Binder’s current address: Division of Endocrinology and Metabolism, Department of Internal Medicine, University of California, San Diego, San Diego, California.
References
- 1.Olson JL: The nephrotic syndrome. 4th edition. Heptinstall RH eds. Pathology of the Kidney, 1992, :pp 779-869 Little Brown, Boston [Google Scholar]
- 2.Fogo A, Hawkins EP, Berry PL, Glick AD, Chiang ML, MacDonell RC, Ichikawa I: Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int 1990, 38:115-121 [DOI] [PubMed] [Google Scholar]
- 3.Dantal J, Bigot E, Bogers W, Testa A, Kria F, Jacques Y, Hurault de Ligny B, Niaudet P, Charpentier B, Soulillou JP: Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. New Engl J Med 1994, 330:7-14 [DOI] [PubMed] [Google Scholar]
- 4.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F: Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerular sclerosis. New Engl J Med 1996, 334:878-883 [DOI] [PubMed] [Google Scholar]
- 5.Vernier RL, Papermaster RW, Good RA: Aminonucleoside nephrosis. I. Electron microscope study of the renal lesions in rat. J Exp Med 1959, 109:115-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inokuchi S, Sakai T, Shirato I, Tomino Y, Koide H: Ultrastructural changes in glomerular epithelial cell in acute puromycin aminonucleoside nephrosis: a study by high-resolution scanning electron microscopy. Virchows Arch A 1993, 423:111-119 [DOI] [PubMed] [Google Scholar]
- 7.Johnson DR, Lovett D, Lehrer RI, Couser WG, Klebanoff SJ: Role of oxidants and proteases in glomerular injury. Kidney Int 1994, 45:352-359 [DOI] [PubMed] [Google Scholar]
- 8.Thakur V, Walker PD, Shah SV: Evidence suggesting a role for hydroxyl radical in puromycin aminonucleoside-induced proteinuria. Kidney Int 1988, 34:494-499 [DOI] [PubMed] [Google Scholar]
- 9.Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R: Transgenic mouse model of kidney disease: insertional inactivartion of ubiquituously expressed gene leads to nephrotic syndrome. Cell 1990, 62:425-434 [DOI] [PubMed] [Google Scholar]
- 10.Zwaka RM, Reuter A, Pfaff E, Moll J, Gorgas K, Karasawa M, Weiher H: The glomerulosclerosis gene Mpv17 encodes a peroxisomal protein producing reactive oxygen species. EMBO J 1994, 13:5129-5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricardo SD, Bertram JF, Ryan GB: Antioxidants protect podocyte foot processes in puromycin aminonucleoside-treated rat. J Am Soc Nephrol 1986, 12:1974-1981 [DOI] [PubMed] [Google Scholar]
- 12.Kerjaschki D: Polycation induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli. Lab Invest 1978, 39:430-440 [PubMed] [Google Scholar]
- 13.Michael AF, Blau E, Vernier RL: Glomerular polyanion: Alteration in aminonucleoside nephrosis. Lab Invest 1970, 23:649-657 [PubMed] [Google Scholar]
- 14.Basci A, Shah SV: Trypsin and chymotrypsin induced chemiluminiscence by isolated rat glomeruli. Am J Physiol 1987, 252:C611-617 [DOI] [PubMed] [Google Scholar]
- 15.Kerjaschki D, Neale JT: Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J Am Soc Nephrol 1996, 7:2518-2526 [DOI] [PubMed] [Google Scholar]
- 16.Karasawa M, Zwacka RM, Reuter A, Fink T, Hsieh CL, Lichter P, Francke U, Weiher H: The human homolog of the glomerulosclerosis gene Mpv17: structural and genomic organization. Hum Mol Genom 1993, 11:1829-1834 [DOI] [PubMed] [Google Scholar]
- 17.Schenkel J, Zawka RM, Rutenberg C, Reuter A, Waldherr R, Weiher H: Functional rescue of the glomerulosclerosis phenotype in Mpv17 mice by transgenesis with the human Mpv17 homologue. Kidney Int 1995, 48:80-84 [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Donaldson CJ, Smith GW, Vale WW: The structure of the mouse and human urocortin genes (Ucn and UCN). Genomics 1998, 50:23-33 [DOI] [PubMed] [Google Scholar]
- 19.Liochev SI, Fridovich I: Lucigenin luminescence as a measure of intracellular superoxide dismutase activity in Escherichia coli. Proc Natl Acad Sci USA 1997, 94:2891-2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA: Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem 1998, 273:2015-2021 [DOI] [PubMed] [Google Scholar]
- 21.Riedle B, Kerjaschki D: Reactive oxygen species cause direct damage of Engelbrecht-Holm-Swarm marix. Am J Pathol 1997, 151:215-231 [PMC free article] [PubMed] [Google Scholar]
- 22.Raats CJI, Bakker MAH, van den Born J, Beerden JHM: Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem 1997, 272:26734-26741 [DOI] [PubMed] [Google Scholar]
- 23.Bohrer MP, Baylis C, Humes HD, Glassock RJ, Robertson CR, Brenner BM: Permselectivity of the glomerular capillary wall: facilitated filtration of circulating polycations. J Clin Invest 1978, 61:72-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelber H, Healy L, Whiteley H, Miller LA, Vimr E: In vivo enzymatic removal of α2-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest 1996, 74:907-920 [PubMed] [Google Scholar]
- 25.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP: The renal glomerulus of mice lacking s-laminin/laminin β2: nephrosis despite molecular compensation by laminin β1. Nat Genet 1995, 10:400-406 [DOI] [PubMed] [Google Scholar]
- 26.Breiteneder S, Matsui Y, Schaffner G, Kerjaschki D: Podoplanin, a novel 43 kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 1997, 151:1141-1152 [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui K, Breiteneder-Geleff S, Kerjaschki D: Epitope specific antibodies to the 43 kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J Am Soc Nephrol 1998, 9:2013-2026 [DOI] [PubMed] [Google Scholar]
- 28.Assmann KJM, van Son JPHF, Dijkman, HBP M, Koene RAP: A nephritogenic rat monoclonal antibody to mouse aminopeptidase A: induction of massive albuminuria after a single intravenous injection. J Exp Med 1992, 175:623–635 [DOI] [PMC free article] [PubMed]
- 29.Narisawa M, Kawachi H, Oite T, Shimizu F: Divalency of the monoclonal antibody 5–1-6 is required for induction of proteinuria in rat. Clin Exp Immunol 1993, 92:522-526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerjaschki D: Molecular pathogenesis of membranous nephropathy. Kidney Int 1992, 41:1090-1105 [DOI] [PubMed] [Google Scholar]
- 31.Meyer zum Gottesberge AM, Reuter A, Weiher H: Inner ear defect similar to Alport’s syndrome in the glomerulosclerosis mouse model Mpv17. Eur Arch Oto-Rhalyngol 1996, 253:470-474 [DOI] [PubMed] [Google Scholar]
- 32.Houghlum K, Filip M, Witztum JL, Choijker M: Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest 1998, 86:1991. [DOI] [PMC free article] [PubMed] [Google Scholar]