Abstract
Our group had previously shown that transfer of the mouse interferon (IFN)-α1 gene into the metastasizing TS/A mammary adenocarcinoma resulted in T-cell-mediated tumor rejection and development of antitumor immunity. Moreover, we had shown that the metastatic ability of TS/A tumor cells producing IFN-α was strongly impaired, whereas IFN-γ expression did not influence or augmented metastasis formation by TS/A cells. In this study, we have analyzed the in vitro and in vivo behavior of various TS/A tumor cell clones isolated after the transduction with a recombinant retroviral vector carrying the mouse IFN-β gene. We have also compared the tumorigenicity of these clones with that of TS/A cells expressing IFN-α1. BALB/c mice were inoculated subcutaneously with parental TS/A cells, transduction control TS/A cells, or TS/A cells producing IFN-α or IFN-β. Tumor growth was evaluated by the measurement of tumor masses and analysis of survival. The features of tumor growth and rejection were examined by histological and immunohistochemical analyses. The metastatic ability of parental TS/A cells, transduction control TS/A cells, or TS/A cells producing IFN-α, IFN-β, or IFN-γ was evaluated after intravenous injection of the tumor cells into BALB/c mice by counting of the lung metastatic nodules and analysis of survival. A strong inhibition of tumorigenicity and development of tumor immunity were observed upon subcutaneous injection of syngeneic mice with TS/A tumor cells producing high amounts of IFN-β, but not with clones expressing low levels of the cytokine, as observed for cells expressing IFN-α. IFN-α secretion by TS/A cells at the site of tumor growth induced a stronger inflammatory response as compared with IFN-β, which appeared to be more active in the inhibition of tumor-induced angiogenesis. Notably, the metastatic ability of IFN-β-producing TS/A cells after intravenous injection was either not affected or only slightly impaired as compared with parental TS/A tumor cells. In contrast, even cells producing low levels of IFN-α proved to be poorly metastatic. These findings represent the first comparison of the effectiveness of IFN-α versus IFN-β produced by genetically modified cells on their tumorigenic behavior and suggest the existence of some notable differences in the capabilities of these two cytokines to induce a host antitumor reactivity in mice.
Tumor-cell-targeted cytokine gene therapy has recently received particular consideration as a potential alternative to systemic administration of cytokines in the therapy of cancer. 1 The possibility of obtaining appropriate cytokine production at the tumor cell site has opened the way to new strategies for defining more physiological and selective antitumor therapies with cytokines. It is becoming evident that the antitumor activity of any given cytokine constitutively expressed by tumor cells and the mechanisms elicited in the host are a function not only of the intrinsic biological activities of the cytokine itself but also of the characteristics of the specific tumor model. Consequently, it is particularly important to conduct systematic studies on the effects exerted by different cytokines expressed in the same tumor. In recent years, some groups have joined their efforts for studying the effects of the transduction of several cytokine genes in one selected tumor model, represented by a poorly immunogenic mammary carcinoma (TS/A) metastasizing to the lung after injection into syngeneic BALB/c mice. The use of this model allowed a careful comparison of the effectiveness of many cytokines on tumor behavior in immunocompetent animals. 2 In this model, we had previously shown that transfer of the mouse IFN-α1 gene resulted in a loss of tumorigenicity and an acquired capacity to induce a long-lasting antitumor immunity after injection into syngeneic mice. 3 Similar results were more recently obtained by Tüting and colleagues. 4 Moreover, we had compared the metastatic ability of IFN-α- versus IFN-γ-secreting clones and showed that the expression of IFN-α into TS/A tumor cells resulted in a potent inhibition of metastasis formation, whereas IFN-γ expression either did not affect or even enhanced the metastatic behavior of TS/A cells. 3 Notably, despite the many articles published on TS/A clones expressing various cytokines (reviewed in Ref. 2 ), studies on TS/A cells producing IFN-β had not been performed so far. This reflects a more general lack of information on the effects of IFN-β gene transfer into tumor cells. In fact, whereas several reports have shown that IFN-α gene transfer into tumor cells resulted in a marked inhibition of tumor growth and induction of an antitumor immune response in different mouse metastatic tumor systems 3-8 and in the suppression of the growth of human tumors implanted into nude mice, 9,10 only two studies describing the tumorigenic behavior of mouse 11 or human 12 tumor cells expressing IFN-β have been performed so far. This is probably due to the general assumption that the in vivo properties of IFN-β gene-modified tumor cells are similar to those exhibited by cells producing IFN-α.
In this study, we describe the isolation and the in vitro and in vivo properties of TS/A cells transduced with a recombinant retroviral vector carrying the mouse IFN-β gene. Our results indicate that the subcutaneous (s.c.) injection of IFN-β-secreting TS/A cells into immunocompetent mice results in the induction of a host antitumor response and tumor rejection, the extent of which is dependent on the amount of IFN-β secreted and is inferior to that generated by IFN-α-producing TS/A cells. The metastatic behavior of IFN-β- versus IFN-α-producing TS/A after intravenous (i.v.) injection was also compared. The overall results describe important differences in the ability of these two cytokines of affecting the tumorigenic behavior and the host response to the tumor.
Materials and Methods
Mice
BALB/c mice, 6 to 7 weeks old, were obtained from Charles River Breeding Laboratories (Italia Calco, Italy) and were treated in accordance with the European Community guidelines.
Cell Lines
TS/A is a tumor cell line established from the first in vivo transplant of a moderately differentiated mammary adenocarcinoma that arose spontaneously in a 20-month-old multiparous BALB/c mouse. 13 The TS/A parental cell line used here was reestablished in vitro from a tumor induced in a BALB/c mouse with eight-passage TS/A cells. TS/A tumor cells are highly metastatic to the lung after s.c. or i.v. injection into syngeneic mice; 4 × 10 4 cells s.c. is approximately the minimal 100% TS/A parental cell tumor-inducing dose in BALB/c mice. These cells have been shown to be poorly immunogenic in syngeneic BALB/c mice. 13 TS/A-IFN-γ 14 and TS/A-IFN-α1 3 cells have been previously isolated and characterized. 3,14 TS/A parental cells were cultivated in Dulbecco’s medium supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin (BioWhittaker, Verviers, Belgium), and 10% FCS (Sebam, Berlin, Germany). TS/A parental cells exhibited a phenotype of resistance to the antiproliferative effect of IFN-α/β, 3 as assessed by cultivating them in the presence of IFN-α/β. 15 The TS/A-IFN-α1 and the TS/A-IFN-γ cells were cultivated in the same medium containing 400 μg/ml G418 (calculated to give 100% antibiotic activity; Geneticin, Life Technologies, Grand Island, NY). Confluent monolayers were trypsinized and used for in vitro and in vivo experiments. GP+E-86 ecotropic 16 and GP+envAm12 amphotropic 17 packaging cell lines were cultivated in HXM medium as described elsewhere. 17 GP+envAm12 cells cultivated in the presence of 100 IU/ml mouse IFN-α/β for several passages were used for production of the LMuIFN-βSN retroviral vector. These GP+envAm12 cells exhibited a phenotype of resistance to the antiviral activity of IFN-α/β, as assessed by titering the viral yield after infection with vesicular stomatitis virus. 18
Retroviral Vector Production and Transduction of TS/A Tumor Cells
The LXSN retroviral vector, 19 containing the neomycin resistance gene under the control of the SV40 promoter, was obtained from A.D. Miller (Seattle, WA). The LMuIFN-βSN retroviral vector was constructed by insertion of a 680-bp murine IFN-β PstI complementary DNA fragment into the unique PstI site of the pSP72 plasmid (Promega, Madison, WI). The XhoI-EcoRI fragment was excised from this construct and inserted into the corresponding restriction sites of the LXSN retroviral vector. In the resulting construct, the IFN complementary DNA is under the transcriptional control of the 5′ retroviral LTR. Both LXSN and LMuIFN-βSN retroviral vectors were transfected in their plasmidic form into co-cultures of GP+E-86 plus GP+envAm12 packaging cells (1:1 ratio) by a standard calcium-phosphate DNA precipitation procedure. Supernatants collected 7 days and thereafter after transfection were assayed for IFN and for recombinant retrovirus (assayed by infecting NIH 3T3 cells with dilutions of the same supernatants in the presence of 4 μg/ml polybrene and determining the number of G418-resistant colonies). The supernatants exhibiting the highest titers of recombinant retrovirus and expression of IFN were then used for transduction of GP+envAm12 cells. The supernatants of individual clones of transduced GP+envAm12 cells, isolated by selection into G418-containing medium, were assayed for IFN and retrovirus production. The supernatants of the clones exhibiting the highest titer of LXSN and LMuIFN-βSN retroviruses were used for transduction of TS/A parental cells. Briefly, 10 6 exponentially growing TS/A cells were infected for 12 hours with undiluted supernatants containing 8 μg/ml polybrene, grown for 48 hours, and then selected in 0.8 mg/ml G418. Individual clones were isolated, expanded into cell lines, and subjected to further analysis.
IFN Titration
IFN was titrated on murine L929 cells as described previously. 18 Briefly, L929 cells were seeded at 2 × 10 4 cells/100 μl/well in 96-well plates in Dulbecco 2% FCS. After a 24-hour incubation at 37°C in 5% CO2 atmosphere, 100 μl of test supernatants or of a standard mouse IFN-α/β preparation were added in duplicate to the first well of each row and serially twofold diluted. An equal volume of Dulbecco 2% FCS was added to two series of control wells. After 20 to 24 hours of incubation at 37°C in 5% CO2 atmosphere, the medium was aspirated from each well, and 100 μl of a vesicular stomatitis virus suspension (multiplicity of infection of 0.05 PFU/cell) in Dulbecco 2% FCS were added to each well, except for the wells serving as uninfected cell controls. After a 1-hour incubation at 37°C in 5% CO2 atmosphere, the medium was removed and 100 μl of fresh Dulbecco 2% FCS were added to each well. The cytopathic effect was observed under a light microscope after 24 and 48 hours of incubation at 37°C in 5% CO2 atmosphere. One unit of IFN was defined as the amount necessary to inhibit 50% of the cytopathic effect. IFN titers are expressed as IU.
Expression of Surface Antigens
The membrane expression of the various proteins was determined by flow cytometric analysis as described elsewhere. 20 Mouse monoclonal antibodies (MAbs) recognizing H-2Kd (clone 31-3-4S) and H-2Dd (clone 34-5-8S) were purchased from Cedarlane (Hornby, Ontario, Canada); rat MAb against Ly-6A/E (clone D7) was kindly donated by Dr. E. Shevach (National Institute of Allergy and Infectious Diseases, Bethesda, MD). Fluorescein-isothiocyanate-conjugated antibodies against mouse and rat Ig were purchased from KpL (Gaithersburg, MD).
Morphological Analysis
Groups of three mice were killed 4, 7, and 10 days after challenge. For histological evaluation, tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin or Giemsa. For immunohistochemistry, acetone-fixed cryostat sections were incubated for 30 minutes with anti-CD4 and anti-CD8 (from Sera-Lab, Crawley Down, UK); anti-Mac-1 (anti CD11b/CD18), anti-Mac-3, and anti-Ia (all from Boehringer Mannheim, Milan, Italy); anti-polymorphonuclear leukocytes (RB6–8C5; provided by Dr. R. L. Coffman, DNAX, Palo Alto, CA); anti-IL-1β (Genzyme, Cambridge, MA); anti-TNF-α (Immuno Kontact, Frankfurt, Germany); anti-IFN-γ (provided by Dr. S. Landolfo, Turin University, Turin, Italy); anti-IL-6 (Pharmingen, San Diego, CA); anti-iNOS (Transduction Laboratories, Lexington, Ky); anti-CD31 and anti-ELAM-1 (provided by Dr. A. Vecchi, Negri Nord Institute, Milan, Italy); anti-VEGF, anti-bFGF, and anti-ICAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA); and anti-VCAM-1 (Pharmingen) antibodies (Abs). After washing, they were overlaid with biotinylated goat anti-rat, anti-hamster, or anti-rabbit or horse anti-goat Ig (Vector Laboratories, Burlingame, CA) for 30 minutes. Unbound Igs were removed by washing, and the slides were incubated with ABC complex/AP (Dako, Glostrup, Denmark). Quantitative studies of immunohistochemically stained sections were performed independently by three pathologists in a blind fashion. At least three samples (one sample per tumor growth area) and 10 randomly chosen fields in each sample were evaluated for each point determination. For microvessel and cell counts, individual microvessels and cells were counted under a microscope ×400 field (40× objective and 10× ocular lens; 0.180 mm 2 per field). The expression of adhesion molecules, cytokines, and mediators was defined as absent (−) or scarcely (±), moderately (+), or frequently (++) present on cryostat sections tested with the corresponding Abs.
Statistical Analyses
The significance of the differences in the mean day of death was determined by Student’s t-test, those in the number of lung metastases by Wilcoxon’s rank sum test.
Results
Isolation of IFN-β-Producing TS/A Cell Clones and Their in Vitro Characterization
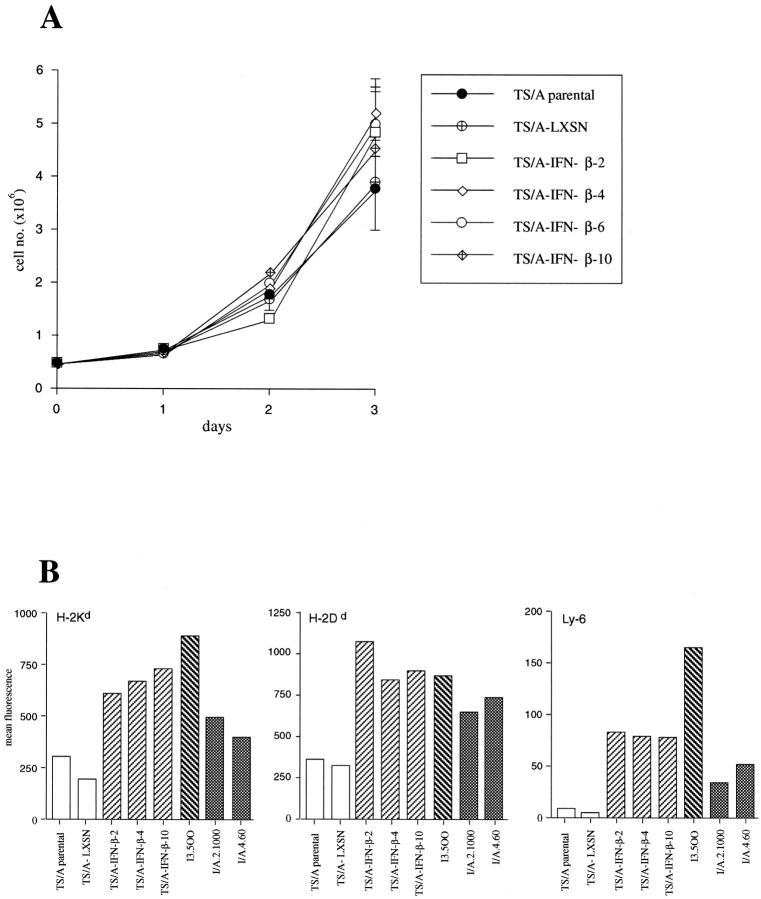
TS/A cells were transduced with the retroviral vectors LMuIFN-βSN and LXSN, whose genomic structure is schematically depicted in Figure 1 ▶ . Several individual clones producing variable amounts of biologically active IFN-β were isolated, and those clones chosen for further characterization are indicated in Table 1 ▶ . Stable levels of IFN-β secretion were maintained by the different clones over time, as assessed by titrating the cytokine produced in the culture supernatant before each in vitro and in vivo experiment, similarly to what previously observed for IFN-α-producing TS/A clones. 3 TS/A-LXSN represents a clone transduced with the LXSN retrovirus and used as a control for potential effects of the vector sequences. The in vitro proliferation rate of the TS/A-IFN-β cell clones did not differ substantially from that of parental TS/A cells (Figure 2A) ▶ , indicating that the constitutive production of IFN-β did not result in any antiproliferative activity, as previously shown for IFN-α-producing TS/A clones. 3 The expression of some IFN-inducible surface antigens, such as class I major histocompatibility complex and Ly-6 molecules, was augmented in the IFN-β-producing TS/A cell clones as compared with parental TS/A and TS/A-LXSN cells (Figure 2B) ▶ , as it was in the previously characterized IFN-α- and IFN-γ-producing TS/A cell clones (I/A.4.60, I/A.2.1000, and I.3.500, respectively). 3,14 These results indicate that the IFN-β gene transduction and constitutive expression had not led to the selection of IFN-resistant variants.
Figure 1.
Structures of the retroviral vectors used for transducing TS/A tumor cells. For details see Materials and Methods.
Table 1.
In Vitro Production of IFN-β by Transduced TS/A Clones
| Cell clone | Transduced retroviral vector | IFN production (IU/ml) |
|---|---|---|
| TS/A parental | None | <2 |
| TS/A-LXSN | LXSN | <2 |
| TS/A-IFN-β-2 | LMuIFN-βSN | 64 |
| TS/A-IFN-β-4 | LMuIFN-βSN | 128 |
| TS/A-IFN-β-12 | LMuIFN-βSN | 512 |
| TS/A-IFN-β-8 | LMuIFN-βSN | 1024 |
| TS/A-IFN-β-6 | LMuIFN-βSN | 2048 |
| TS/A-IFN-β-10 | LMuIFN-βSN | 2048 |
Supernatants were harvested and titrated for IFN-β production as described in Materials and Methods.
Figure 2.
In vitro growth properties (A) and expression of IFN-inducible membrane glycoproteins (B) in TS/A-IFN-β clones. A: A total of 5 × 10 5 cells of the different clones were seeded in 35-mm-diameter petri dishes. On the day indicated, the number of viable cells was determined by trypan blue exclusion. Each point represents the mean ± SE value of a triplicate. B: Flow cytometric analysis of the expression of class I H-2 and of Ly-6 glycoproteins in TS/A-β clones was performed as described in Materials and Methods.
In Vivo Growth Characteristics of IFN-β-Producing TS/A Tumor Cells after s.c. Injection into Immunocompetent Mice
Figure 3 ▶ illustrates the results of a representative experiment in which the in vivo behavior of individual IFN-β-producing TS/A clones was compared with that of parental or transduction control TS/A-LXSN cells, on s.c. injection of 10 5 tumor cells into syngeneic BALB/c mice. At this dose, both parental and control TS/A-LXSN tumor cells formed progressively growing s.c. tumors (Figure 3A) ▶ in the totality of the injected mice, which died in approximately 50 to 60 days (Figure 3B) ▶ . In contrast, a varying degree of tumor rejection occurred in the mice receiving a similar inoculum of IFN-β-producing TS/A tumor cells, apparently proportional to the amount of secreted IFN. In fact, whereas the low-producer TS/A-IFN-β-2 cells (64 IU/ml) provoked tumor formation and death in all of the injected mice, the intermediate-producer TS/A-IFN-β-4 and TS/A-IFN-β-12 cells (256 to 512 IU/ml) and the high-producer TS/A-IFN-β-6, TS/A-IFN-β-8, and TS/A-IFN-β-10 cells (1024 to 2048 IU/ml) were completely rejected in 30% to 40% and 60% to 90% of the animals, respectively (Figure 3, A and B) ▶ . A notable increase of the survival time was clearly observed in the groups of mice inoculated with the high-IFN-β-producer tumor cells (Figure 3B) ▶ . A high proportion (50% to 80%) of the mice surviving the initial inoculum with IFN-β-producing TS/A cells rejected a subsequent challenge of parental TS/A tumor cells, indicating that these animals had developed a long-lasting antitumor immune response (data not shown).
Figure 3.
Tumorigenicity of TS/A-IFN-β clones after s.c. injection into BALB/c mice. Six mice were challenged s.c. with 10 5 cells of the different clones, as indicated. A: Mean diameter of the tumor at different times after tumor cell injection. Lines represent data for individual mice. B: Percentage of surviving mice at different times after tumor cell injection. On day 120, all of the surviving mice were sacrificed and found to be tumor free.
Comparative in Vivo Studies with IFN-β- and IFN-α-Producing TS/A Clones: Tumorigenicity of IFN-β- versus IFN-α-Secreting TS/A Tumor Cells after s.c. Injection
We then compared the tumorigenic characteristics of some TS/A-IFN-β clones versus those of the IFN-α-producing I/A.4.60 TS/A clone (secreting approximately 60 IU/ml of IFN-α) on s.c. injection into BALB/c mice. The results shown in Table 2 ▶ indicate that the increase in survival time occurring in mice injected with I/A.4.60 tumor cells was comparable to that observed on injection of TS/A tumor cells producing high amounts of IFN-β. The percentage of mice undergoing complete tumor rejection increased proportionally with the amount of IFN-β secreted, being maximal in the groups of animals injected with the TS/A cells producing approximately 2000 IU/ml IFN (Table 2) ▶ .
Table 2.
Comparison of Tumorigenicity of TS/A-IFN-β and TS/A-IFN-α Cells When Injected s.c. in BALB/c Mice
| Challenging TS/A cells | IFN produced in vitro (IU/ml) | Number of mice with s.c. tumor/total number of mice (%) | Mean day of death ± SE* |
|---|---|---|---|
| TS/A parental | None | 6 /6 (100) | 63.8 ± 4.1 |
| TS/A-IFN-β-2 | β (64) | 5 /6 (83) | 65.2 ± 5.1 |
| TS/A-IFN-β-4 | β (256) | 4 /6 (67) | 78.7 ± 7.7 |
| TS/A-IFN-β-6 | β (2048) | 1 /6 (16) | >110 |
| TS/A-IFN-β-10 | β (2048) | 1 /6 (16) | >86 |
| I/A.4.60 | α (64) | 1 /6 (16) | >86 |
Mice were challenged s.c. with 105 cells as indicated.
*Calculated for dead mice only.
Histology and Immunohistochemistry of TS/A-IFN-β and TS/A-IFN-α s.c. Tumors
Four days after challenge, parental TS/A cells had already formed a small solid tumor exhibiting several mitotic figures, whereas TS/A-IFN-β and TS/A-IFN-α cells were not well organized in a tumor mass but were aggregated in several clusters intermingled with host reactive cells (data not shown). An evident fibroblastic reaction was present in the stromal tissue surrounding the IFN-producing tumor cells (data not shown). By day 7, the tumor mass formed by parental TS/A cells (Figure 4A) ▶ had invaded the fibroadipous tissue and epidermis. Reactive cells mainly consisted of macrophages and a few granulocytes, mostly located on the outskirts of the tumor. By day 10, few areas of ischemic coagulative necrosis formed of cell debris were present in the center of the tumor, despite the evenly distributed vascularization. The tumor then grew progressively with no evident morphological changes. In contrast, the tumor mass formed by TS/A-IFN-β and TS/A-IFN-α cells 7 days after injection exhibited a large central area of ischemic coagulative necrosis bordered by numerous host-infiltrating cells (macrophages, lymphocytes, and granulocytes) and some residual tumor cell aggregates. The tumor growth area was circumscribed by an impressive fibroblastic reaction resulting in a fibrous wall (Figure 4, B and C) ▶ . Progressively enlarging necrotic areas containing residual tumor cells were subsequently replaced by granulation tissue (data not shown). Table 3 ▶ summarizes the results of the immunohistochemical analysis performed 7 days after tumor challenge. Host-infiltrating cells were significantly more numerous in TS/A-IFN-α and TS/A-IFN-β tumors than in parental TS/A tumors and were mainly represented by macrophages, granulocytes, and lymphocytes, with CD8+ T cells significantly exceeding CD4+ T cells. The macrophage and granulocyte contents of TS/A-IFN-β tumor were significantly lower than that of TS/A-IFN-α tumor although higher than that observed in TS/A parental tumor. Blood vessels were less numerous in TS/A-IFN-α or TS/A-IFN-β tumors as compared with parental TS/A tumors, as revealed by staining with the anti-endothelial cell MAb (anti-CD31) (Table 3 ▶ and Figure 4, D–F ▶ ). The vasculature network substaining TS/A-IFN-β tumor growth was scarcer than that of TS/A-IFN-α tumors. Moreover, in both cases the blood vessels were especially represented at the edge of the tumor mass, being almost absent in the central area, and were frequently interrupted and obstructed by thrombi (Figure 4, E and F) ▶ . No appreciable differences in the expression of angiogenic factors (VEGF and bFGF) were found between parental TS/A and TS/A-IFN-α or TS/A-IFN-β tumors (Table 3) ▶ , consistent with the results of the immunocytochemical analysis of the expression of bFGF and VEGF in vitro (data not shown). The endothelial cells in the IFN-producing tumors were stained by anti-VCAM-1 and anti-ELAM-1 antibodies, in contrast to that observed in parental TS/A tumors (Table 3) ▶ . Expression of pro-inflammatory cytokines such as interleukin-1β, IFN-γ, and tumor necrosis factor-α (Figure 4, G–I) ▶ was higher in the host cells infiltrating the TS/A-IFN-α tumors as compared with the TS/A-IFN-β counterparts and virtually absent in the parental TS/A tumor cellular infiltrates. The levels of cytokine expression paralleled those of macrophage iNOS (Table 3 ▶ and Figure 4, J–L ▶ ).
Figure 4.
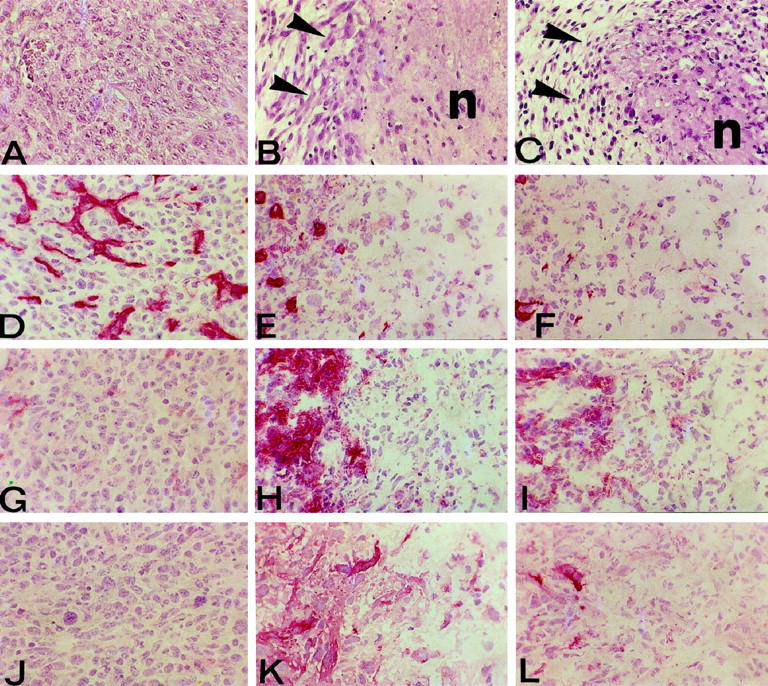
Histological and immunohistochemical features of the tumor growth area 7 days after the s.c. injection of 10 6 parental TS/A cells (left panels), TS/A-IFN-α cells (middle panels), or TS/A-IFN-β cells (right panels). The growth area of TS/A-IFN-α (clone I/A.4.60) (B) and TS/A-IFN-β (clone β-10) (C) tumors show a large area of ischemic coagulative necrosis (n) circumscribed by an impressive fibroblastic reaction (arrowheads). By contrast, parental TS/A cells (A) form an invasive tumor mass composed of round to polygonal cells. Tumor cryostat section immunostaining (red color) with anti-endothelial cell MAb shows that parental TS/A tumor blood vessels are well represented and evenly distributed (D). By contrast, TS/A-IFN-α (E) and TS/A-IFN-β (F) tumor blood vessels are few and especially represented at the edge of the tumor mass being almost absent in the central necrotic area. The expression of TNF-α (G, H, and I) and iNOS (J, K, and L), almost absent in parental TS/A tumors (G and J), is evident in reactive cells infiltrating TS/A-IFN-α (H and K) and TS/A-IFN-β (I and L) tumors, being higher in the former (H and K). Magnification, ×360.
Table 3.
Analysis of the Host-Infiltrating Cells and Microvessel Content of the Expression of Angiogenic Factors, Cytokines, and Adhesion Molecules at 7 Days after the s.c. Injection of 106 Parental TS/A, TS/A-IFN-α, or TS/A-IFN-β Cells in BALB/c Mice
| TS/A parental | TS/A-IFN-α (clone I/A.4.60) | TS/A-IFN-β (clone β-10) | |
|---|---|---|---|
| Macrophages | 35.0 ± 6.3* | 94.5 ± 11.6† | 63.4 ± 9.3† |
| Granulocytes | 7.4 ± 3.1 | 51.3 ± 7.9† | 25.1 ± 6.3† |
| CD8+ | 5.2 ± 1.3 | 57.5 ± 6.3† | 62.8 ± 9.1† |
| CD4+ | 1.1 ± 0.6 | 38.7 ± 5.2† | 30.6 ± 4.7† |
| CD31 | 20.4 ± 4.1‡ | 13.4 ± 2.5§ | 8.0 ± 1.6§ |
| bFGF | +¶ | + | + |
| VEGF | + | + | + |
| IL-1β | − | + | ± |
| TNF-α | ± | ++ | + |
| IFN-γ | − | + | ± |
| IL-6 | + | ++ | + |
| iNOS | − | + | ± |
| ICAM-1 | ± | + | + |
| VCAM-1 | − | + | + |
| ELAM-1 | − | + | ± |
*Cell counts were determined at ×400 in a 0.180-mm2 field. At least 10 fields per tissue sample were evaluated. Values are expressed as mean ± SD.
†Value significantly higher (P < 0.001) than that observed in TS/A parental tumor.
‡Microvessel count was performed in at least 10 ×400 fields (0.180 mm2 per field) on cryostat sections tested with anti-endothelial (CD31) MAb.
§Value significantly lower (P < 0.001) than observed in TS/A parental tumor. The TS/A-IFN-β tumor value was significantly lower (P < 0.005) than that observed in TS/A-IFN-α tumor.
¶The expression of adhesion molecules, cytokines, and mediators was defined as absent (−) or scarcely (±), moderately (+), or frequently (++) present on cryostat sections tested with the corresponding antibodies.
Comparative in Vivo Studies with IFN-β- and IFN-α-Producing TS/A Clones: Tumorigenicity of IFN-β- versus IFN-α-Secreting TS/A tumor Cells after i.v. Injection
We had previously reported that the expression of IFN-α into TS/A tumor cells resulted in a potent inhibition of metastases formation on i.v. injection, whereas IFN-γ expression either did not affect or even enhanced the metastatic behavior of TS/A cells. 3 As the studies summarized above had revealed some dissimilarities in the patterns of tumor growth/rejection in mice injected s.c. with TS/A-IFN-β versus TS/A-IFN-α cells, it was of interest to extend this comparison by using the i.v. route of injection, including in the same set of experiments TS/A cells expressing IFN-γ. Table 4 ▶ shows the results of two experiments in which BALB/c mice were injected i.v. with parental TS/A cells or different cloned TS/A-IFN-α, -IFN-β, or -IFN-γ cells. In agreement with our previous results, the TS/A-pc or TS/A-IFN-γ cell-injected mice died within similar periods of time, whereas a three- to fourfold increase in survival time was observed in the groups of mice receiving the TS/A-IFN-α cells, independently from the amount of secreted IFN. The TS/A-IFN-β cells exhibited different behaviors on i.v. injection, apparently dependent on the dose of IFN produced by the various clones. In fact, the survival time of the mice receiving TS/A-IFN-β cells producing low to intermediate amounts of IFN (TS/A-IFN-β-2 and TS/A-IFN-β-4) was similar to that of TS/A-IFN-γ cell-injected mice, whereas a significant delay in the mean day of death occurred in those mice inoculated with the high-producer cells (TS/A-IFN-β-6 and TS/A-IFN-β-10). However, in no case was the prolongation of survival time comparable to that observed in TS/A-IFN-α cell-injected mice. Quantitative evaluation of lung metastases also indicated that the metastatic ability of TS/A cells was not impaired by production of low amounts of IFN-β, whereas a clear reduction in the number of lung metastatic nodules was observed, at the time of sacrifice, in mice injected i.v. with TS/A cells secreting high amounts of the cytokine (Table 5) ▶ .
Table 4.
Comparative Evaluation of the Tumorigenicity of TS/A-IFN-α, TS/A-IFN-β, and TS/A-IFN-γ Cells upon i.v. Injection into Immunocompetent Mice
| Experiment | Challenging TS/A cells (IFN) | Mean day of death (±SE)* |
|---|---|---|
| 1 | TS/A parental (none) | 17.0 ± 0.4 |
| TS/A-LXSN (none) | 22.2 ± 0.6 | |
| I/A.4.60 (α) | 73.3 ± 6.1 | |
| I/A.2.1000 (α) | 66.3 ± 3.5 | |
| TS/A-IFN-β-2 (β) | 27.8 ± 6.5†‡ | |
| TS/A-IFN-β-4 (β) | 35.1 ± 8.4†‡ | |
| TS/A-IFN-β-6 (β) | 38.3 ± 3.4†§ | |
| TS/A-IFN-β-10 (β) | 40.3 ± 2.3†§ | |
| I.2.4 (γ) | 28.5 ± 3.9 | |
| I.3.500 (γ) | 22.8 ± 0.7 | |
| 2 | TS/A parental (none) | 20.1 ± 0.9 |
| TS/A-LXSN (none) | 17.0 ± 0.5 | |
| I/A.4.60 (α) | 73.0 ± 6.7 | |
| TS/A-IFN-β-2 (β) | 21.8 ± 0.7†‡ | |
| TS/A-IFN-β-4 (β) | 22.3 ± 1.5†‡ | |
| TS/A-IFN-β-6 (β) | 34.1 ± 2.8†§ | |
| TS/A-IFN-β-10 (β) | 39.8 ± 4.3†§ | |
| I.3.500 (γ) | 20.6 ± 1.7 |
Six- to seven-week-old BALB/c mice were injected i.v. with 5 × 104 cells of the indicated cell line. There were six mice per group in each experiment.
*All of the injected mice in each experimental group died. The mean survival times of the mice injected with TS/A-IFN-α cells are underlined.
†P < 0.005 versus I/A.4.60 or I/A.2.1000 cell-injected mice.
‡Not significant versus I.2.4 or I.3.500 cell-injected mice.
§P < 0.002 versus I.2.4 or I.3.500 cell-injected mice.
Table 5.
Metastatic Potential of TS/A-IFN-α, TS/A-IFN-β, and TS/A-IFN-γ Cells Injected i.v.
| Challenging TS/A cells (IFN) | Lung colonies | ||
|---|---|---|---|
| Incidence | Median | Range | |
| TS/A parental (none) | 5 /5 | 34 | 26–51 |
| I/A.4.60 (α) | 5 /5 | 13† | 4–23 |
| I/A.2.1000 (α) | 5 /5 | 5† | 1–8 |
| TS/A-IFN-β-4 (β) | 5 /5 | 38‡ | 3–76 |
| TS/A-IFN-β-6 (β) | 5 /5 | 12† | 8–26 |
| TS/A-IFN-β-10 (β) | 5 /5 | 6† | 3–11 |
| I.3.500 (γ) | 5 /5 | 117† | 41–168 |
Six- to seven-week-old BALB/c mice were injected i.v. with 5 × 104 cells of the indicated cell lines. After 20 days, all of the mice in each group were sacrificed for evaluation of lung metastases, which was performed as described elsewhere. 11 The lung metastatic nodules formed by both TS/A-IFN-β and TS/A-IFN-α tumor cells appeared, at a gross examination, smaller in size and flatter as compared with the metastases developed in mice injected with parental or IFN-γ-producing TS/A tumor cells.
†P < 0.05 versus TS/A parental cell-injected mice.
‡Not significant versus TS/A parental cell-injected mice.
Discussion
Type I IFNs are a family of cytokines that elicit diverse biological activities, including antiviral, antiproliferative, and immunomodulatory effects. 21,22 Although IFN-α and IFN-β have been considered to share many of their biological activities, mainly because of the binding to the same receptor, evidence exists indicating possible differences in their in vitro and in vivo effects, in terms of antiproliferative, 23-29 antiviral, 30,31 signal transduction, 32-35 immunomodulatory, 36-39 anti-invasive, 40,41 and anti-angiogenic properties. 42,43 Although IFN-β as a single agent has been successfully used in the therapy of chronic hepatitis C, 44 some benign proliferative diseases, 45 and most recently in relapsing multiple sclerosis, 46 IFN-α is the most widely used cytokine in the therapy of certain human malignancies, 21 and its antitumor activity is generally assumed to be superior to that of IFN-β, even though comparative clinical evaluations in cancer patients have not been performed.
Most of the preclinical studies in animal tumor models have been done with preparations containing both IFN-α and IFN-β, in contrast to the human situation where single subtypes are used. Several reports have indicated that IFN-β is superior to IFN-α in inhibiting the in vitro proliferation of different tumor cell types of human origin 24-28 and in suppressing in vivo the growth of human tumors transplanted into immunodeficient mice. 26 However, the in vivo antitumor activity of type I IFNs cannot be predicted on the basis of the in vitro sensitivity of tumor cells to the antiproliferative effect of these cytokines, as the antitumor response induced by type I IFNs is largely mediated by host immune mechanisms (reviewed in Ref. 22 ). To our knowledge, no comparative studies of the antitumor effects of IFN-α versus IFN-β have been performed so far in immunocompetent host-tumor systems. The results reported in this article represent the first example of a comparative study of genetically modified mouse tumor cells expressing IFN-β with homologous tumor cells expressing IFN-α (or IFN-γ). We found that TS/A cells transduced with the mouse IFN-β gene exhibit similar or dissimilar tumorigenic behaviors as compared with IFN-α1-producing TS/A cells, strictly dependent on the site of tumor cell injection. In particular, whereas both IFN-α1- and IFN-β-producing TS/A tumor cells were able to induce an effective antitumor response after s.c. inoculation, only the TS/A tumor cells secreting IFN-α1 were significantly inhibited in their metastatic ability when injected i.v. Although the i.v. route of injection revealed the most striking difference in the in vivo behavior of IFN-β- versus IFN-α-producing TS/A cells, some distinct effects were also noticed in tumor growth and elicitation of host response after s.c. administration. The degree of tumor rejection occurring in mice receiving a s.c. injection of IFN-β-producing TS/A cells was apparently dependent on the amount of cytokine released, and only the high-producer cell clones were completely rejected in the vast majority of the injected mice (Figure 3) ▶ . This extent of tumor growth inhibition was obtained in mice injected s.c. with TS/A cells producing low amounts (60 IU/ml) of IFN-α1 (Table 2) ▶ . A recent study reported that murine fibrosarcoma cells genetically modified for the production of IFN-β were significantly suppressed in their tumorigenicity when injected s.c. or i.v. into immunocompetent mice and that this inhibition was dependent, in part, on the induction of host-mediated mechanisms. 11 Interestingly, large amounts of IFN-β were secreted by the modified tumor cells. 11 Our observations suggested that IFN-α is more potent in inducing an antitumor response as compared with IFN-β, at least in the TS/A tumor model. This concept is supported by the comparative histological and immunohistochemical analyses of the tumor area in mice injected s.c. with low-producer TS/A-IFN-α cells or high-producer TS/A-IFN-β cells. In fact, despite the presence of extensive necrosis and fibroblastic reaction in both IFN-α- and IFN-β-TS/A tumors (Figure 4) ▶ , the macrophage and granulocyte content of the host cell infiltrates as well as the level of expression of pro-inflammatory cytokines was higher in the area of TS/A-IFN-α tumor growth as compared with TS/A-IFN-β tumors (Table 3) ▶ . These observations suggest that IFN-β may exert anti-inflammatory effects. In this regard, it is worth noting that the therapeutic effects of IFN-β in patients with multiple sclerosis have been suggested to depend on the inhibition of the inflammatory response and cytotoxic function. 46
Whereas IFN-α acted as a stronger inducer of an inflammatory response, inhibition of tumor-induced angiogenesis appeared to be a major component of IFN-β-mediated TS/A tumor rejection. Neoformation of blood vessels was more strongly impaired in the TS/A-IFN-β-10 tumor areas as compared with I/A.4.60 tumors (Figure 4 ▶ and Table 3 ▶ ). The inhibition of tumor-induced angiogenesis and induction of extensive ischemic necrosis in the tumor area by type I IFNs are well documented phenomena in animal models. 47,48 Moreover, IFN-α was the first of the known inhibitors of angiogenesis to be evaluated clinically and to show its therapeutic potential in angiogenic diseases, such as childhood hemangiomas and Kaposi’s sarcoma. 49,50 Initial studies by Sidky and Borden 51 indicated that the inhibition of tumor-induced angiogenesis mediated by type I IFNs was independent of their antiproliferative activity and tumor species specific, suggesting that these cytokines could modulate angiogenic signals provided by the tumor cells. Notably, recent reports by Fidler’s group indicate that endogenous IFN-β may act physiologically as a potent inhibitor of tumor-induced angiogenesis in the subcutis, 52 where its production by fibroblasts or epithelial cells might modulate the expression of basic fibroblast growth factor (bFGF), a potent inducer of angiogenesis. More recently, this group showed that IFN-β is able to induce a stronger down-regulation of bFGF expression in vitro in a variety of human tumor cell lines as compared with IFN-α. 42 As for the data presented in this article, it might appear that, consistently with data from other groups, 42,52 IFN-β is superior to IFN-α in terms of anti-angiogenic effects (Table 3) ▶ . However, careful studies by comparing several clones producing comparable amounts of IFNs are necessary to address this issue. On the other hand, the factors/mechanisms involved in the anti-angiogenic effects of IFNs in this system remain unknown. Notably, the immunohistochemical analysis showed that in both IFN-α- and IFN-β-producing TS/A tumors the expression of bFGF and vascular endothelial growth factor was not diminished as compared with parental TS/A tumors, indicating that, at least in this tumor model, the IFN-mediated inhibition of tumor angiogenesis is not dependent on the down-regulation of the expression of these growth factors, considered to be major positive regulators of angiogenesis in vivo.
The most notable difference between TS/A-IFN-β and TS/A-IFN-α tumor cells concerned their relative metastatic ability after i.v. injection. In fact, TS/A tumor cells producing low levels of IFN-β exhibited a metastatic behavior similar to parental TS/A cells, and only the cell clones secreting high amounts of the cytokine were slightly inhibited, as indicated by the survival time of the injected mice (Table 4) ▶ . In contrast, IFN-α-secreting TS/A cells were strongly impaired in their ability of forming lung metastases. 3 It should be noted that the mice injected with the high-producer TS/A-IFN-β cells presented, at the time of sacrifice, a number of lung metastases comparable to that of the mice injected with the low-producer TS/A-IFN-α cells (Table 5) ▶ . Nevertheless, mice injected with the low-producer TS/A-IFN-α cells survived much longer than animals injected with the high-producer TS/A-IFN-β cells (Table 4) ▶ , suggesting that even low levels of IFN-α may be superior in maintaining an immunological control of metastatic growth. In the light of several findings describing different signal transduction pathways elicited by IFN-α versus IFN-β in some cell systems, 32-35 we can envisage that IFN-α can induce, more efficiently than IFN-β, certain signals leading to the activation and/or differentiation of specific subsets of host T cells relevant for the generation of a long-lasting cell-mediated antitumor immune response. Alternatively, we may assume that the poor effectiveness of IFN-β in suppressing the metastatic growth of TS/A cells can be simply due to a more rapid catabolism and clearance of the locally produced IFN-β as compared with IFN-α. This hypothesis is supported by early studies on the distribution, catabolism, and pharmacokinetics of IFNs (for a review see Ref. 53 ), showing that IFN-β differs from IFN-α in its higher lipophilia and greater tissue affinity.
In conclusion, the results presented herein represent the first evidence that IFN-α and IFN-β can exert different effects on the in vivo behavior of the same tumor cells and suggest that IFN-α may act as a stronger adjuvant in the generation of an effective antitumor immune response. Our study underlines the importance of comparative studies for a better understanding of the different responses observed in patients and for the identification of novel and more selective interventions in the therapy of cancer.
Acknowledgments
We thank Dr. Arthur Bank (New York, NY) for providing the GP+envAm12 packaging cell line. The generation of GP+envAm12 cells was through the support of the Virology, 167:400, 1988 contract. We thank Genetics Pharmaceuticals (Tarrytown, NY) for providing the GP+E-86 packaging cell line. We are grateful to Cinzia Gasparrini for her excellent secretarial assistance.
Footnotes
Address reprint requests to Dr. Maria Ferrantini, Laboratory of Virology, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy. E-mail: ferrant@virusl.net.iss.it.
Supported by grants from the “Associazione Italiana Ricerca sul Cancro” (Milan) and from the Italy-USA Special Project on “Therapy of Tumors”.
References
- 1.Hussein AM: The potential application of gene transfer in the treatment of patients with cancer: a concise review. Cancer Invest 1996, 14:343-352 [DOI] [PubMed] [Google Scholar]
- 2.Musiani P, Modesti A, Giovarelli M, Cavallo F, Colombo MP, Lollini PL, Forni G: Cytokines, tumor-cell death, and immunogenicity: a question of choice. Immunol Today 1997, 18:32-36 [DOI] [PubMed] [Google Scholar]
- 3.Ferrantini M, Giovarelli M, Modesti A, Musiani P, Modica A, Venditti M, Peretti E., Lollini PL, Nanni P, Forni G, Belardelli F: IFN-α1 gene expression into a metastatic murine adenocarcinoma (TS/A) results in CD8+ T cell-mediated tumor rejection and development of antitumor immunity: comparative studies with IFN-γ producing TS/A cells. J Immunol 1994, 153:4604-4615 [PubMed] [Google Scholar]
- 4.Tüting T, Gambotto A, Baar J, Storkus WJ, Zavodny PJ, Narula S, Tahara H, Robbins PD, Lotze MT: Interferon-α gene therapy for cancer: retroviral transduction of fibroblasts and particle-mediated transfection of tumor cells are both effective strategies for gene delivery in murine tumor models. Gene Ther 1997, 4:1053-1060 [DOI] [PubMed] [Google Scholar]
- 5.Ferrantini M, Proietti E, Santodonato L, Gabriele L, Peretti M, Plavec I, Meyer F, Kaido T, Gresser I, Belardelli F: Alpha1-interferon gene transfer into metastatic Friend leukemia cells abrogated tumorigenicity in immunocompetent mice: antitumor therapy by means of interferon-producing cells. Cancer Res 1993, 53:1107-1112 [PubMed] [Google Scholar]
- 6.Kaido T, Bandu MT, Maury C, Ferrantini M, Belardelli F, Gresser I: IFN-α1 gene transfection completely abolishes the tumorigenicity of murine B16 melanoma cells in allogeneic DBA/2 mice and decreases their tumorigenicity in syngeneic C57BL/6 mice. Int J Cancer 1995, 60:1-9 [DOI] [PubMed] [Google Scholar]
- 7.Gabriele L, Kaido T, Woodrow D, Moss J, Ferrantini M, Proietti E, Santodonato L, Rozera C, Maury C, Belardelli F, Gresser I: The local and systemic response of mice to interferon-α1 transfected Friend leukemia cells. Am J Pathol 1995, 147:445-460 [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar S, Flores I, De Rosa C, Ozzello L, Ron Y, Pestka S: Injection of irradiated melanoma genetically modified to secrete IFN-α causes regression of an established tumor. Int J Oncol 1995, 7:17-24 [DOI] [PubMed] [Google Scholar]
- 9.Belldegrun A, Tso CL, Sakata T, Duckett T, Brunda MJ, Barsky SH, Chai J, Kaboo R, Lavey RS, McBride WH, deKernion JB: Human renal carcinoma line transfected with interleukin-2 and/or interferon α gene(s): implications for live cancer vaccines. J Natl Cancer Inst 1993, 85:207-216 [DOI] [PubMed] [Google Scholar]
- 10.Zhang JF, Hu C, Geng Y, Blatt LM, Taylor MW: Gene therapy with an adeno-associated virus carrying an interferon gene results in tumor growth suppression and regression. Cancer Gene Ther 1996, 3:31-38 [PubMed] [Google Scholar]
- 11.Dong Z, Juang SH, Kumar R, Eue I, Xie K, Bielenberg D, Lu W, Bucana C, Yang X, Fidler I: Suppression of tumorigenicity and metastasis in murine UV-2237 fibrosarcoma cells by infection with a retroviral vector harboring the interferon-β gene. Cancer Immunol Immunother 1998, 46:137-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin XQ, Tao N, Dergay A, Moy P, Fawell S, Davis A, Wilson JM, Barsoum J: Interferon-β gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proc Natl Acad Sci USA 1998, 95:14411-14416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanni P, De Giovanni C, Lollini PL, Nicoletti G, Prodi G: TS/A: a new metastasizing cell line from a BALB/c spontaneous mammary adenocarcinoma. Clin Exp Metastasis 1983, 1:373-380 [DOI] [PubMed] [Google Scholar]
- 14.Lollini PL, Bosco MC, Cavallo F, De Giovanni C, Giovarelli M, Landuzzi L, Musiani P, Modesti A, Nicoletti G, Palmieri G, Santoni A, Young HA, Forni G, Nanni P: Inhibition of tumor growth and enhancement of metastasis after transfection of the γ-interferon gene. Int J Cancer 1993, 55:320-329 [DOI] [PubMed] [Google Scholar]
- 15.Belardelli F, Gresser I, Maury C, Maunoury MT: Antitumor effects of interferon in mice injected with interferon-sensitive and interferon-resistant Friend leukemia cells. Int J Cancer 1982, 30:813-820 [DOI] [PubMed] [Google Scholar]
- 16.Markowitz D, Goff S, Bank A: A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol 1988, 62:1120-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markowitz D, Goff S, Bank A: Construction and use of a safe and efficient amphotropic packaging cell line. Virology 1988, 167:400-406 [PubMed] [Google Scholar]
- 18.Belardelli F, Gessani S, Proietti E, Locardi C, Borghi P, Watanabe Y, Kawade Y, Gresser I: Studies on the expression of spontaneous and induced interferons in mouse peritoneal macrophages by means of monoclonal antibodies to mouse interferons. J Gen Virol 1987, 68:2203-2212 [DOI] [PubMed] [Google Scholar]
- 19.Miller AD, Rosman GJ: Improved retroviral vectors for gene transfer and expression. BioTechniques 1989, 7:980-984 [PMC free article] [PubMed] [Google Scholar]
- 20.De Giovanni C, Palmieri G, Nicoletti G, Landuzzi L, Scotlandi K, Bontadini A, Tazzari PL, Sensi M, Santoni A, Nanni P, Lollini PL: Immunological and nonimmunological influence of H-2Kb gene transfection on the metastatic ability of B16 melanoma cells. Int J Cancer 1991, 48:270-276 [DOI] [PubMed] [Google Scholar]
- 21.Gutterman JU: Cytokine therapeutics: lessons from interferon α. Proc Natl Acad Sci USA 1994, 91:1198-2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belardelli F, Gresser I: The neglected role of type I interferon in the T-cell response: implications for its clinical use. Immunol Today 1996, 17:369-372 [DOI] [PubMed] [Google Scholar]
- 23.Tahara H, Kamada K, Sato E, Tsuyama N, Kim JK, Hara E, Oda K, Ide T: Increase in expression levels of interferon-inducible genes in senescent human diploid fibroblasts and in SV40-transformed human fibroblasts with extended lifespan. Oncogene 1995, 11:1125-1132 [PubMed] [Google Scholar]
- 24.Horikoshi T, Fukuzawa K, Hanada N, Ezoe K, Eguchi H, Hamaoka S, Tsujiya H, Tsukamoto T: In vitro comparative study of the antitumor effects of human interferon-α, β, and γ on the growth and invasive potential of human melanoma cells. J Dermatol 1995, 22:631-636 [DOI] [PubMed] [Google Scholar]
- 25.Garbe C, Krasagakis K: Effects of interferons and cytokines on melanoma cells. J Invest Dermatol 1993, 100(Suppl 2):239S-244S [PubMed] [Google Scholar]
- 26.Johns TG, Mackay IR, Callister KA, Hertzog PJ, Devenish RJ, Linnane AW: Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon β. J Natl Cancer Inst 1992, 84:1185-1190 [DOI] [PubMed] [Google Scholar]
- 27.Rosenblum MG, Yung WK, Kelleher PJ, Ruzicka F, Steck PA, Borden EC: Growth inhibitory effects of interferon-β but not interferon-α on human glioma cells: correlation of receptor binding, 2′,5′-oligoadenylate synthetase and protein kinase activity. J Interferon Res 1990, 10:141-151 [DOI] [PubMed] [Google Scholar]
- 28.Hubbell HR, Liu RS, Maxwell BL: Independent sensitivity of human tumor cell lines to interferon and double-stranded RNA. Cancer Res 1984, 44:3252-3257 [PubMed] [Google Scholar]
- 29.Willson JK, Bittner G, Borden EC: Antiproliferative activity of human interferons against ovarian cancer cells grown in human tumor stem cell assay. J Interferon Res 1984, 4:441-447 [DOI] [PubMed] [Google Scholar]
- 30.De Marco F, Giannoni F, Marcante ML: Interferon-β strong cytopathic effect on human papillomavirus type 16-immortalized HPK-IA cell line, unexpectedly not shared by interferon-α. J Gen Virol 1995, 76:445-450 [DOI] [PubMed] [Google Scholar]
- 31.De Marco F, Marcante ML: Cellular and molecular analyses of interferon β cytopathic effect on HPV-16 in vitro transformed human keratinocytes (HPK-IA). J Biol Omeost Agents 1995, 9:24-30 [PubMed] [Google Scholar]
- 32.Rani MRS, Foster GR, Leung S, Leaman D, Stark GR, Ransohoff RM: Characterization of β-R1, a gene that is selectively induced by interferon β (IFN-β) compared with IFN-α. J Biol Chem 1996, 271:22878-22884 [DOI] [PubMed] [Google Scholar]
- 33.Platanias LC, Uddin S, Colamonici OR: Tyrosine phosphorylation of the α and β subunits of the type I interferon receptor. Interferon-β selectively induces tyrosine phosphorylation of an α subunit-associated protein. J Biol Chem 1994, 269:17761-17764 [PubMed] [Google Scholar]
- 34.Miscia S, Baldassarre AD, Rana RA, Cataldi A: Phospholipase C γ 1 overexpression and activation induced by interferon β in human T lymphocytes: an ISGF3-independent response. Cytokine 1997, 9:660-665 [DOI] [PubMed] [Google Scholar]
- 35.Domanski P, Nadeau OW, Platanias LC, Fish E, Kellum M, Pitha P, Colamonici OR: Differential use of the βL subunit of the type I interferon (IFN) receptor determines signaling specificity for IFNα2 and IFNβ. J Biol Chem 1998, 273:3144-3147 [DOI] [PubMed] [Google Scholar]
- 36.Soilu-Hanninen M, Salmi A, Salonen R: Interferon-β downregulates expression of VLA-4 antigen and antagonizes interferon-γ-induced expression of HLA-DQ on human peripheral blood monocytes. J Neuroimmunol 1995, 60:99-106 [DOI] [PubMed] [Google Scholar]
- 37.Pette M, Pette DF, Muraro PA, Farnon E, Martin R, McFarland HF: Interferon-β interferes with the proliferation but not with the cytokine secretion of myelin basic protein-specific, T-helper type 1 lymphocytes. Neurology 1997, 49:385-392 [DOI] [PubMed] [Google Scholar]
- 38.Genc K, Dona DL, Reder AT: Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon β-1b therapy. J Clin Invest 1997, 99:2664-2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McRae BL, Picker LJ, van Seventer GA: Human recombinant interferon-β influences T helper subset differentiation by regulating cytokine secretion pattern and expression of homing receptors. Eur J Immunol 1997, 27:2650-2656 [DOI] [PubMed] [Google Scholar]
- 40.Fabra A, Nakajima M, Bucana CD, Fidler IJ: Modulation of the invasive phenotype of human colon carcinoma cells by organ specific fibroblasts of nude mice. Differentiation 1992, 52:101-110 [DOI] [PubMed] [Google Scholar]
- 41.Gohji K, Fidler IJ, Tsan R, Radinsky R, von Eschenbach AC, Tsuruo T, Nakajima M: Human recombinant interferons-β and -γ decrease gelatinase production and invasion by human KG-2 renal-carcinoma cells. Int J Cancer 1994, 58:380-384 [DOI] [PubMed] [Google Scholar]
- 42.Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ: Interferons α and β down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci USA 1995, 92:4562-4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato N, Nawa A, Tamakoshi K, Kikkawa F, Suganuma N, Okamoto T, Goto S, Tomoda Y, Hamaguchi M, Nakajima M: Suppression of gelatinase production with decreased invasiveness of choriocarcinoma cells by human recombinant interferon β. Am J Obstet Gynecol 1995, 172:601-606 [DOI] [PubMed] [Google Scholar]
- 44.Omata M, Yokosuka O, Takano S, Kato N, Hosoda K, Imazeki F, Tada M, Ito Y, Ohto M: Resolution of acute hepatitis C after therapy with natural β interferon. Lancet 1991, 338:914-915 [DOI] [PubMed] [Google Scholar]
- 45.Dinsmore W, Jordan J, O’Mahony C, Harris JR, McMillan A, Radcliffe KW, Engrand P, Jackson BW, Galazka AR, Abdul-Ahad AK, Illingworth JM: Recombinant human interferon-β in the treatment of condylomata acuminata. Int J STD AIDS 1997, 8:622-628 [DOI] [PubMed] [Google Scholar]
- 46.Hall GL, Compston A, Scolding NJ: Beta-interferon and multiple sclerosis. Trends Neurosci 1997, 20:63-67 [DOI] [PubMed] [Google Scholar]
- 47.Dvorak HF, Gresser I: Microvascular injury in pathogenesis of IFN-induced necrosis of subcutaneous tumors in mice. J Natl Cancer Inst 1989, 81:497-502 [DOI] [PubMed] [Google Scholar]
- 48.Thomas H, Balkwill FR: Effects of interferons and other cytokines on tumors in animals: a review. Pharmacol Ther 1991, 52:307-330 [DOI] [PubMed] [Google Scholar]
- 49.Ezekowitz RAB, Mulliken JB, Folkman J: Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med 1992, 326:1456-1463 [DOI] [PubMed] [Google Scholar]
- 50.Volm MD, von Roenn JH: Treatment strategies for epidemic Kaposi’s sarcoma. Curr Opin Oncol 1995, 7:429-436 [DOI] [PubMed] [Google Scholar]
- 51.Sidky YA, Borden EC: Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer Res 1987, 47:5155-5161 [PubMed] [Google Scholar]
- 52.Singh RK, Bucana CD, Gutman M, Fan D, Wilson MR, Fidler IJ: Organ site-dependent expression of basic fibroblast growth factor in human renal cell carcinoma cells. Am J Pathol 1994, 145:365-374 [PMC free article] [PubMed] [Google Scholar]
- 53.Bocci V: Distribution, catabolism, and pharmacokinetics of interferons. Finter NB Oldham RK eds. Interferon: In Vivo and Clinical Studies, 1985, vol 4.:pp 47-72 Elsevier Science Publishers, Amsterdam [Google Scholar]