Abstract
Chemokines may be important in the control of leukocytosis in inflammatory disorders of the central nervous system. We studied cerebral chemokine expression during the evolution of diverse neuroinflammatory disorders in transgenic mice with astrocyte glial fibrillary acidic protein-targeted expression of the cytokines IL-3, IL-6, or IFN-α and in mice with experimental autoimmune encephalomyelitis. Distinct chemokine gene expression patterns were observed in the different central nervous system inflammatory models that may determine the phenotype and perhaps the functions of the leukocytes that traffic into the brain. Notably, high expression of C10 and C10-related genes was found in the cerebellum and spinal cord of GFAP-IL3 mice with inflammatory demyelinating disease and in mice with experimental autoimmune encephalomyelitis. In both these neuroinflammatory models, C10 RNA and protein expressing cells were predominantly macrophage/microglia and foamy macrophages present within demyelinating lesions as well as in perivascular infiltrates and meninges. Intracerebroventricular injection of recombinant C10 protein promoted the recruitment of large numbers of Mac-1+ cells and, to a much lesser extent, CD4+ lymphocytes into the meninges, choroid plexus, ventricles, and parenchyma of the brain. Thus, C10 is a prominent chemokine expressed in the central nervous system in experimental inflammatory demyelinating disease that, we show, also acts as a potent chemotactic factor for the migration of these leukocytes to the brain.
Leukocyte recruitment to and infiltration of the central nervous system (CNS) is a central feature in the pathogenesis of diverse inflammatory neurological disorders ranging from bacterial and viral meningoencephalitis to multiple sclerosis, HIV encephalopathy, cerebral malaria, and cerebral ischemia. Understanding the mechanisms responsible for regulating the CNS recruitment and trafficking of these cells has been the subject of much interest, which has been focused recently on the possible involvement of chemoattractant cytokines, also called chemokines. 1,2 The chemokines are a broad family of mediators that can be separated into four distinct subfamilies: α, β, γ, and the recently identified δ subfamily. 3,4 Members of each subfamily possess a variation of a conserved cysteine structural motif: CXC in the α subfamily, CC in the β subfamily, C in the γ subfamily, and CX3C in the δ subfamily, where X is the intervening amino acid residue. In general, within each chemokine subfamily the members show considerable homology in their amino acid sequence and often possess overlapping chemoattractant specificity. The α chemokines include MIP-2, crg-2/IP-10, IL-8, and GRO-α-γ and are known to be chemotactic mainly for polymorphonuclear cells. The β chemokines include C10, MCP-3, MIP-1β, TCA-3, MCP-1, MIP-1α, and RANTES and are chemotactic principally for monocytes and lymphocytes. The γ and δ chemokine subfamilies are each currently represented by only a single member, lymphotactin 5 and human fractalkine 3 or mouse neurotactin, 4 respectively. Induction or up-regulation in the CNS expression of a number of chemokines belonging to the α and β subfamilies has been reported in viral 6-8 and bacterial meningoencephalitis, 9 in experimental autoimmune encephalomyelitis (EAE), 10-13 cerebral ischemia, 14,15 and trauma. 16
Clearly, the chemokine gene expression profile in different pathological states may influence the nature of the leukocytes that traffic into the brain during inflammation. Therefore, the objective of the current study was to compare the regulation of chemokine gene expression in a number of different models for CNS inflammation which included EAE and recently developed transgenic mice with astrocyte-targeted expression of the proinflammatory cytokines IL-3, 17 IL-6, 18 or IFNα. 19 These transgenic mice develop distinct neuroinflammatory disorders 20 and are therefore useful tools for better understanding the role of chemokines in leukocyte migration to the CNS. The GFAP-IL6 mice exhibit diffuse inflammation in the brain and have some perivascular mononuclear cell accumulation, mainly with B lymphocytes, but little or no parenchymal leukocyte accumulation. These animals develop progressive neurodegeneration and learning impairment. 18,21 The GFAP-IL3 mice develop, from approximately 5 months of age, a progressive motor disorder due to a macrophage/microglial-mediated demyelinating disease affecting predominantly the cerebellar and brain stem regions. 17 Finally, the GFAP-IFNα mice are characterized by a diffuse inflammatory neurodegeneration with meningitis and a lymphocytic encephalitis. 19
Materials and Methods
Transgenic Animals
The development and characterization of the different transgenic mice were described in detail previously. 17-19 For the GFAP-IL6 mice, 4- to 6-month-old homozygous animals of the G167 line were used that, at this age, exhibit in the brain perivascular mononuclear cell accumulation with mainly B lymphocytes. For the GFAP-IL3 mice, animals of the G3C2 line were used. 17 To determine the degree of motor impairment, GFAP-IL3 or control mice were inspected visually by two individuals and scored according to the following scale: 0, no abnormality; 1, slight gait disturbance; 2, slight gait disturbance and tremor; 3, moderate gait disturbance, tremor, and mild loss of balance; 4, severe gait disturbance, tremor, and mild loss of balance; 5, severe gait disturbance, tremor, and severe loss of balance. For the GFAP-IFNα mice, 4- to 6-month-old animals of the GIFN39 line were used. They are characterized by a diffuse inflammatory neurodegeneration with meningitis and a lymphocytic encephalitis. 19 Transgenic and nontransgenic wild-type control mice used in this study were maintained under specific pathogen-free conditions in the closed breeding colony of the Scripps Research Institute.
Induction of EAE
For the induction of EAE, adjuvant immunization was performed with either bovine myelin basic protein (MBP) or synthetic myelin oligodendrocyte glycoprotein (MOG) peptides. For MBP-EAE, female SWR/J mice were obtained from Jackson Laboratory (Bar Harbor, ME) and used at 8–12 weeks of age. On day 0, each mouse received subcutaneous injections of immunizing emulsion (200 μl) at two sites on the back over the hindlimbs. The immunizing emulsion consisted of 200 μg of bovine MBP (4 mg/ml in saline; Sigma, St. Louis, MO) and 500 mg heat-killed mycobacterium tuberculosis (10 mg/ml in saline, H37RA, Difco, Detroit, MI) which were emulsified in an equal volume (100 μl) incomplete Freund’s adjuvant (Difco). On days 0 and 3, mice were also injected intravenously with phosphate buffered saline (PBS) (100 μl) containing 200 ng pertussis toxin (Sigma). For MOG-EAE, female C57BL6/J X SJL/J F1 mice were obtained from the rodent breeding colony of the Scripps Research Institute and used at 8–12 weeks of age. The immunization schedule was the same as for MBP-EAE, with the exception that the MBP was replaced by 300 μg of MOG peptides containing 150 μg of MOG35–56 (MEVGWYRSPFSRVVHLYRNGK) and 150 μg of MOG92–106 (DEGGGVTTCFFRDHSYQ). MOG35–56 produces active EAE in mice of the H-2b MHC background, 22 whereas MOG92–106 is effective in the mice of the H-2s MHC background. 23 On days 0 and 2, mice also received 500 ng pertussis toxin intraperitoneally.
The severity of EAE symptoms was evaluated each day and graded as follows: 1, tail weakness; 2, mild paraparesis and/or ataxia of the hind limbs; 3, severe paraparesis of the hind limbs; 4, moribund; 5, death due to EAE. Approximately 50% of MBP-immunized animals developed a monophasic disease course with EAE symptoms appearing 14–20 days postimmunization. In MOG-immunized animals, approximately 90% developed a chronic relapsing-remitting disease course, with onset of disease occurring at day 12–16 postimmunization.
RNA Isolation
Mice were killed and organs were immediately removed, snap-frozen in liquid nitrogen, and stored at −80°C until RNA preparation. Poly (A+) RNA was prepared according to a previously described method. 24 Total RNA was isolated using Trizol reagent (Gibco, Gaithersburg, MD) according to the manufacturer’s instructions.
RNase Protection Assay
For the RNase protection assay (RPA), the development and characterization of the multiprobe set used to detect the simultaneous expression of multiple chemokine RNA transcripts was described previously. 6 Briefly, the RPA probe set included probes against the CXC chemokines MIP-2 and crg-2/IP-10, the CC chemokines C10, TCA-3, MCP-1, MCP-3, MIP-1α, MIP-1β, and RANTES, and the C chemokine lymphotactin. For quantification, autoradiographs were scanned (Scanjet 4C/T, Hewlett Packard, San Jose, CA), individual band densities were measured with NIH Image 1.57 software, and the raw data were analyzed using Microsoft Excel.
In Situ Hybridization
Anesthetized mice were perfused transcardially with ice-cold saline followed by 4% paraformaldehyde in PBS (pH 7.4). Brains and spinal cords were removed, postfixed in the same fixative overnight at 4°C, processed, and embedded in paraffin. Sagittal sections (10 μm) were cut onto polylysine-coated slides and used for in situ hybridization as described previously. 25
The C10 riboprobe (315 bp) used for in situ hybridization was synthesized using the same ribovector used in the chemokine RPA described above. In addition to sections from control brain, the specificity of the probes was confirmed by comparison with sections hybridized to corresponding C10 sense probe. After the final PBS wash and before dehydration in graded alcohol, some hybridized sections were immunostained for glial fibrillary acidic protein (GFAP) diluted 1:2000 (DAKO, Carpinteria, CA) to identify astrocytes or labeled with the lectin Ricinus communis agglutinin-I (RCA-I) diluted 1:500 (Sigma) to identify cells of the macrophage lineage. 26 Briefly, after the final posthybridization wash, slides were transferred to PBS containing 2% goat serum (for GFAP antibody) or 10% goat serum, 0.1% Triton-X100, 0.1% bovine serum albumin (for the RCA-I lectin) for 1 hour at room temperature to block nonspecific binding. Sections were incubated overnight with the primary antibody or lectin and then, after extensive washing, sections were incubated with either anti-rabbit avidin-biotinylated horseradish peroxidase complex (ABC Kit, Vector, Burlingame, CA) used according to the manufacturer’s instructions or with streptavidin-peroxidase complex (Pierce, Rockford, IL) diluted 1:500 in PBS. Staining reactions were performed with 3,3 diaminobenzidine (Sigma) as substrate. After dehydration through graded alcohol and air drying, slides were dipped in Kodak NTB-2 emulsion, dried, and stored in the dark for 2 weeks, after which time the slides were developed, counterstained with Mayer’s hematoxylin, and examined by dark- and bright-field microscopy.
Immunocytochemical Staining for C10
Mice were anesthetized and the brain and spinal cord were removed and fixed overnight in Bouin’s solution. Brains were processed, embedded in paraffin, and 10-μm sagittal sections were prepared. For C10 protein detection, sections were deparaffinized, rehydrated in graded alcohol, and rinsed in PBS. Endogenous peroxidase was blocked for 20 minutes at room temperature in methanol containing 3% H2O2. After a PBS wash, sections were blocked for 1 hour at room temperature in PBS containing 10% rabbit serum and 0.05% Tween 20. The sections were next incubated overnight at 4°C with a goat polyclonal anti-murine C10 antibody (R&D Systems, Minneapolis, MN) that had been diluted 1:200 in blocking buffer. The sections were then washed in PBS and incubated with biotinylated anti-goat and avidin horseradish peroxidase complex (ABC Kit, Vector, Burlingame, CA) used according to the manufacturer’s instructions. Staining reactions were done with 3,3 diaminobenzidine (Sigma) as substrate, the sections were finally counterstained with Mayer’s hematoxylin, dehydrated through graded alcohol, and air-dried. After coverslipping, the slides were examined by bright-field microscopy. Controls for specificity of the immunostaining included incubation of the sections with a nonimmune goat serum as well as omission of the primary antibody.
Intracerebroventricular (ICV) Injections and Cellular Phenotypic Analysis
Adult C57BL6/J × SJL/J mice were anesthetized with sodium pentobarbital (50 mg/kg) and placed in a stereotaxic apparatus. Body temperature was monitored and maintained at 37.0 ± 0.1°C by a feedback-regulated heating pad. A small incision was made in the scalp, the skull was exposed, and a 2 mm diameter trephine hole was drilled in the cranium overlying the right lateral ventricle. The dura was opened to prevent breakage of a micropipette (tip broken to 50 μm) that was subsequently lowered into the right lateral ventricle (coordinates relative to bregma: 0.5 mm P, 1.7 mm L, 2.0 mm V). One microliter of recombinant C10 protein (0.5 μg/μl, R&D Systems) diluted in PBS, 0.1% bovine serum albumin was injected into the right lateral ventricle over a 1-minute period with a PPM-2 Pneumatic Pump (Medical Systems Corp., Greenvale, NY) set at a 1–3 p.s.i. pressure head. In parallel, control mice were injected with 1 μl of vehicle (PBS, 0.1% bovine serum albumin) alone. Five minutes after injection of C10 or vehicle, the micropipette was slowly removed, the hole in the cranium was filled with bone wax, and the incision in the scalp was sutured closed. The animal was removed from the stereotaxic device and allowed to recover from anesthesia under a warming lamp. After 24 hours, ICV-injected mice were again anesthetized and perfused transcardially with ice-cold saline. Brains were immediately removed, embedded in tissue-Tek O.C.T. compound (Fisher, Plainfield, NJ), and snap-frozen. Coronal sections (14 μm) were cut on a cryomicrotome, air-dried, and stored at −70°C pending immunophenotyping of the infiltrating cells.
For immunophenotyping, sections were brought to room temperature, fixed in cold (−20°C) methanol:acetone (1:1) solution for 45 seconds, and blocked for 30 minutes at room temperature in PBS containing 2% rabbit and goat serum. Sections were then incubated for 1 hour at room temperature with rat monoclonal antibody to Mac-1 (ATCC, TIB126, Rockville, MD), CD4 (L3T4, Pharmingen, San Diego, CA), or CD8a (Ly-2, Pharmingen) diluted in the blocking solution. After extensive washing, sections were incubated with a biotinylated anti-rat antibody followed by avidin-labeled horseradish peroxidase (Sigma) both for 1 hour at room temperature, then stained with 3,3 diaminobenzidine as substrate. Before mounting, sections were counterstained in Mayer’s hematoxylin.
Results
Cerebral Chemokine Gene Expression in GFAP-Cytokine Transgenic Mice
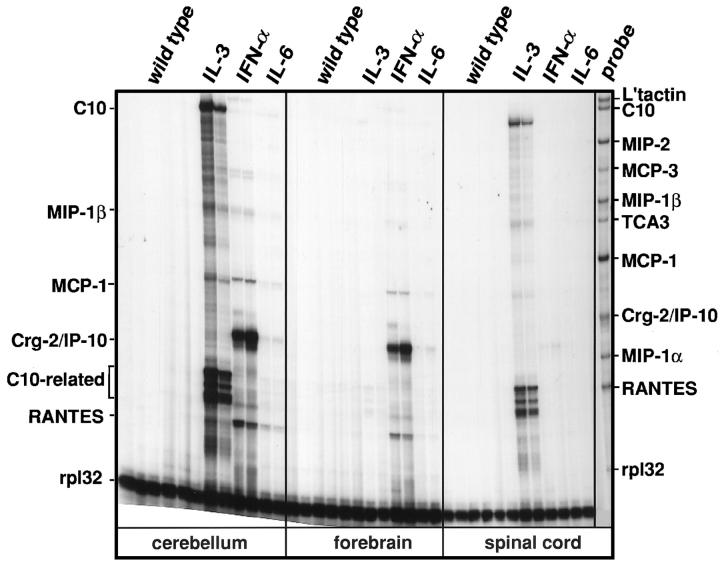
Chemokine gene expression was initially examined in various regions of the CNS in the different GFAP-cytokine transgenic mice. In wildtype mice no detectable chemokine RNA expression was found in any CNS region (Figure 1) ▶ . In contrast, a specific pattern of chemokine gene expression was observed in the various GFAP-cytokine transgenic mice and in the different regions of the CNS. In the cerebellum and spinal cord of GFAP-IL3 mice, high levels of C10 and lower levels of MIP-1β and MCP-1 mRNAs were expressed. In addition to these chemokines, high levels of multiple mRNA species at 90–100 bp were also present. Subsequent analysis (not shown) using single RPA probes showed these multiple species were protected fragments from the C10 probe and may have corresponded to the reported 27 highly homologous C10-like gene-encoded transcripts or alternatively spliced transcripts of the C10 gene. In the forebrain of the GFAP-IL3 mice no detectable chemokine gene expression was observed.
Figure 1.
Chemokine mRNA expression in GFAP-cytokine transgenic mice. In this representative experiment, poly (A+) RNA was isolated from cerebellum, forebrain, and spinal cord of wild-type or transgenic mice and 2 μg analyzed by RNase protection assay as outlined in Materials and Methods.
In the GFAP-IFNα mice, the chemokine gene expression pattern was quite different from that of the GFAP-IL3 mice (Figure 1) ▶ . In the cerebellum and forebrain of GFAP-IFNα mice, high levels of crg-2/IP-10 and much lower expression of RANTES and MCP-1 mRNA transcripts were detectable. No detectable chemokine gene expression was observed in the spinal cord of GFAP-IFNα mice. In the GFAP-IL6 mice, the chemokine gene expression was observed predominantly in the cerebellum but, in contrast to the GFAP-IL3 and GFAP-IFNα mice, only very low expression of crg-2 and RANTES transcripts were detectable.
Temporal and Disease-Associated Expression of Chemokine Genes in the Cerebellum of GFAP-IL3 Mice
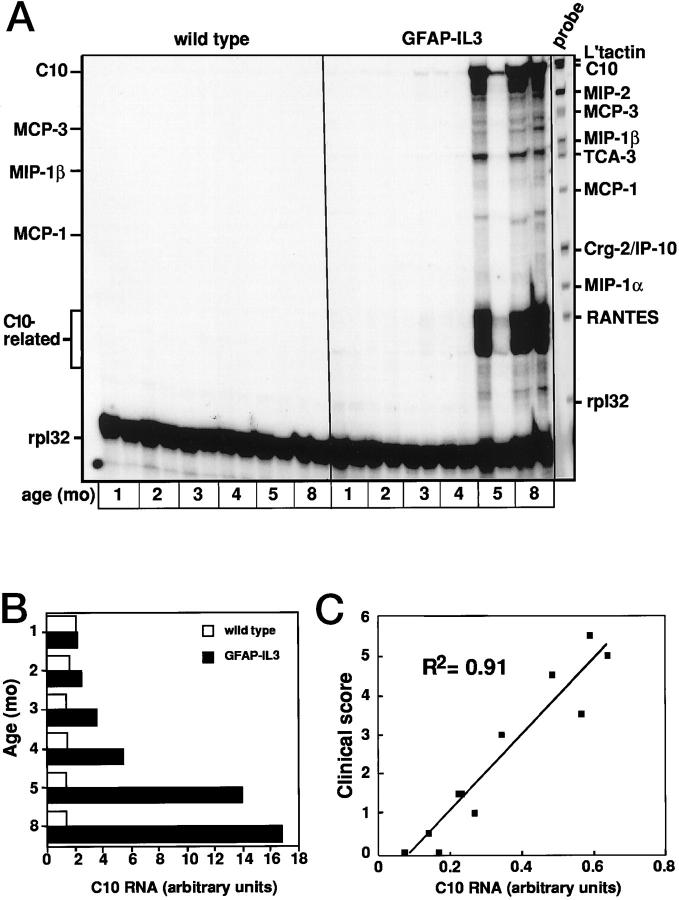
Expression of the chemokine C10 in vivo has not been reported previously. To further determine the relationship of the expression of this chemokine gene to the development of the neurological disease in the GFAP-IL3 mice, we examined the cerebellum from mice of different ages and with different levels of motor impairment (Figure 2) ▶ . Similar to the findings described above, high expression of C10 and C10-related mRNA transcripts was found at 5 months of age, when the GFAP-IL3 mice presented with motor symptoms (Figure 2A) ▶ . In addition to C10, the other chemokine genes, MIP-1β and MCP-1, were also expressed at higher levels in the brain of transgenic mice at 5 months of age. Densitometric quantitation revealed increased levels of C10 mRNA transcripts were also detectable very early in presymptomatic mice of 1–2 months age before increasing progressively at 5–8 months of age (Figure 2B) ▶ . The relationship between the expression of the C10 gene and the severity of motor disease was assessed (Figure 2C) ▶ . This analysis revealed a significant correlation between the clinical score and the level of C10 RNA found in the cerebellum of the GFAP-IL3 mice.
Figure 2.
Temporal and disease-associated expression of the C10 mRNA in cerebellum of GFAP-IL3 transgenic mice. Brains were removed from transgenic mice at the ages shown and poly (A+) RNA isolated from the cerebellum and analyzed by RPA as outlined in Materials and Methods (A). Quantitative analysis of C10 RNA levels shown in A revealed an age-dependent progressive increase in the C10 gene expression (B). For the comparison of C10 RNA levels with disease severity (C), linear regression analysis was performed using Microsoft Excel software.
Chemokine Gene Expression in EAE
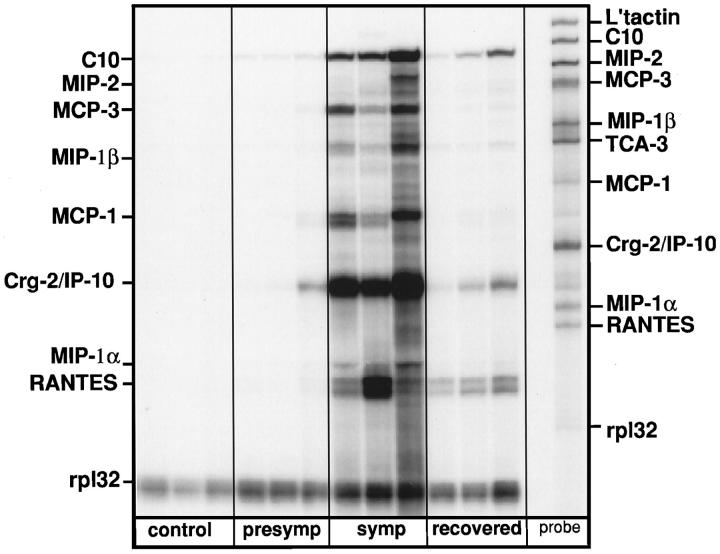
The finding of C10/C10-related gene expression in the cerebellum of the GFAP-IL3 mice but not in the GFAP-IFNα or GFAP-IL6 mice suggested a possible association between these chemokines and inflammatory demyelination. To further establish the credibility of this notion, we next examined chemokine gene expression in the EAE model (Figure 3) ▶ . In control animals little or no significant expression of any chemokine gene was observed in the spinal cord. In contrast, in the spinal cord of MOG-EAE symptomatic mice there was overlapping expression of several chemokine mRNA transcripts, including C10. Other chemokine transcripts that were also expressed included MIP-2, MCP-3, MIP-1β, MCP-1, Crg-2, MIP-1α, and RANTES. Expression of the C10, MCP-1, MCP-3, and Crg-2/IP-10 mRNAs was somewhat higher than for the other chemokines. The chemokine gene expression profile was also examined in spinal cord from presymptomatic mice and mice in the recovery phase of EAE. In presymptomatic animals at the time point examined (day 10 postimmunization), a small but consistent increase in transcripts corresponding to C10 and Crg-2/IP-10 was observed. In mice in the recovery phase the chemokine gene expression pattern was qualitatively similar to that seen in the symptomatic phase, but was significantly reduced. In symptomatic mice with MBP-EAE, the pattern of chemokine gene expression was both qualitatively and quantitatively similar to that observed in mice with MOG-EAE (not shown).
Figure 3.
Chemokine mRNA expression in the brain of mice with MOG-EAE. After adjuvant immunization with bovine MOG peptides, mice were killed at day 10 (presymptomatic), between days 14 and 20 (symptomatic), or after recovery (recovered). Total RNA was isolated from spinal cord of these animals as well as controls and analyzed by RPA as outlined in Materials and Methods.
Anatomical and Cellular Localization of C10 Gene Expression
The findings above indicated that expression of the chemokine C10 and related genes was most prominent in the cerebellum and spinal cord of GFAP-IL3 and EAE mice. We next examined by in situ hybridization the relationship between the expression of C10 RNA to sites of pathology in the brain and spinal cord. Using a C10 sense probe, no hybridization was detected in the CNS of control, presymptomatic, or symptomatic GFAP-IL3 mice or in mice with MBP-EAE (data not shown). With a C10 antisense probe, no hybridization was detected in brain or spinal cord from control mice (Figure 4, A and B ▶ , top brain sections). However, hybridization to C10 RNA was observed in the cerebellum and brain stem of symptomatic GFAP-IL3 mice (Figure 4A ▶ , arrows) and to focal areas in the brain stem and spinal cord of mice with EAE (Figure 4B ▶ , arrows).
Figure 4.
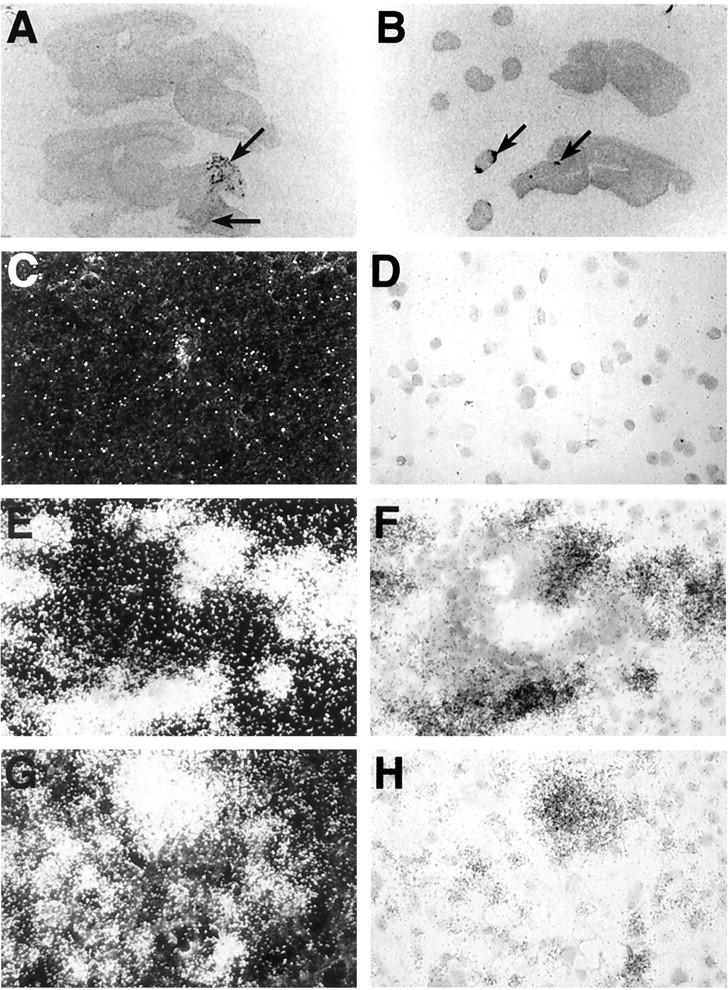
Anatomical and microanatomical localization of C10 RNA in brain from GFAP-IL3 mice or brain and spinal cord from mice with MBP-EAE. Images from Cronex film (5 day exposure) of sagittal sections (10 μm) from GFAP-IL3 transgenic mice (A) or mice with EAE (B). The sections located at the top in A and B represent tissue from wild-type control mice. Sections were hybridized with 35S-labeled antisense RNA probe to C10 RNA as outlined in Materials and Methods. Areas of probe hybridization are indicated by the arrows. For microanatomical analysis, sections hybridized with the 35S-labeled antisense probe to C10 RNA were coated with photographic emulsion, developed after 1 week, and visualized using dark-field (C, E, G) or bright-field (D, F, H) microscopy. No hybridization was detected in brain or spinal cord from control mice (C, D). In cerebellum from GFAP-IL3 mice (E, F), intense hybridization signal is shown surrounding a vascular lymphocytic cuff. In EAE (G, H), hybridization signal was closely associated with the infiltrating mononuclear cell population in the spinal cord.
Microautoradiographic analysis of the hybridized sections revealed background level hybridization to specimens from wild type mice (Figure 4, C and D) ▶ . In contrast, in the GFAP-IL3 transgenic mice, high expression of C10 RNA was observed in infiltrating mononuclear cells in the white matter lesions in the cerebellum and in scattered cells in the brain stem (data not shown). Expression of C10 RNA transcripts was also seen in cells associated with perivascular infiltrates, which were located at the periphery surrounding the lymphocytes (Figure 4, E and F) ▶ . Similarly, in MBP-EAE, cells expressing C10 RNA were identified as being predominantly infiltrating mononuclear cells present in inflammatory foci in the spinal cord and brain stem (Figure 4, G and H) ▶ .
Double labeling experiments were performed to identify the specific cells responsible for C10 RNA expression (Figure 5) ▶ . In both MBP-EAE (Figure 5C) ▶ and GFAP-IL3 (Figure 5E) ▶ specimens, a significant number of RCA-1-positive cells (arrows) were observed that coexpressed C10 RNA and accounted for most of the detectable C10 RNA expression. In contrast, in MBP-EAE (Figure 5D) ▶ and GFAP-IL3 (Figure 5F) ▶ specimens, GFAP-positive cells were negative for C10 RNA expression. However, in these same sections, hybridization of the C10 probe to non-GFAP-labeled cells was conspicuous (arrows). These experiments therefore revealed that macrophages and possibly microglial cells were the major cell type expressing the C10/C10-related RNA in the GFAP-IL3 transgenic mice and in EAE.
Figure 5.
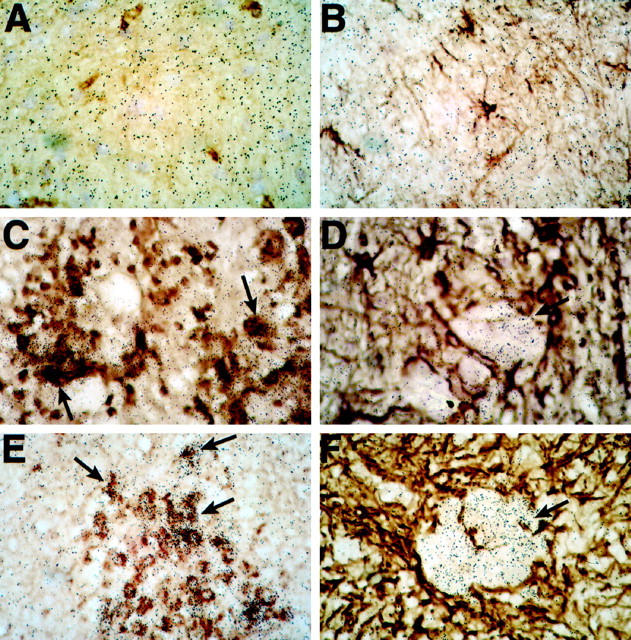
Cellular localization of C10 RNA expression in the spinal cord of control mice (A, B) or mice with MBP-EAE (C, D) or in the brain of GFAP-IL3 mice (E, F). Double-labeling experiments used antisense riboprobe for C10 RNA and immunostaining for GFAP (to label astrocytes) or binding of RCA-1 lectin (to label macrophage/microglia). In the GFAP-IL3 and EAE specimens numerous cells positive for C10 (arrows) colabeled with the RCA-1 lectin (C and E). In contrast, no GFAP-positive cells (D and F) were observed that were also positive for C10. Hybridization of the C10 probe to non-GFAP-labeled cells was conspicuous (arrows).
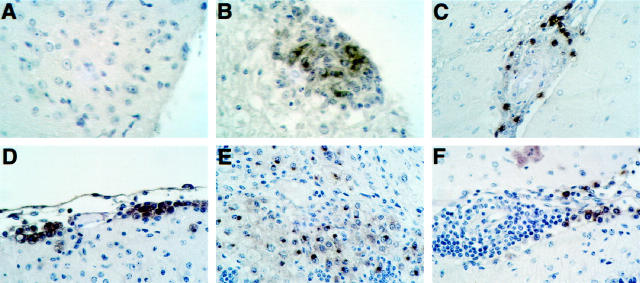
To determine whether C10 was expressed at the protein level we performed immunostaining experiments using a polyclonal antibody against C10 (Figure 6) ▶ . No immunostaining for C10 protein was detectable on sections of brain or spinal cord (Figure 6A) ▶ from control mice. However, in mice with EAE, numerous C10-positive cells were identified associated with inflammatory lesions (Figure 6B) ▶ . Similarly, in older (4 months) presymptomatic GFAP-IL3 mice without white matter lesions, the C10 antibody stained a small number of cells present in the early perivascular infiltrates (Figure 6C) ▶ . In symptomatic GFAP-IL3 mice, numerous cells positive for C10 immunostaining were observed located at the edges of perivascular cuffs (Figure 6F) ▶ , at the brain surfaces such as the meninges (Figure 6D) ▶ , and lining the cerebellar sulci. In addition, C10-immunopositive cells corresponding to foamy macrophages were numerous in the inflammatory lesions in the white matter (Figure 6E) ▶ . The localization of the C10-immunopositive cells thus showed a distribution similar to that of cells expressing the C10-associated RNA detected by in situ hybridization (see above).
Figure 6.
Expression of C10 protein detected by immunohistochemistry. Sections from Bouin’s fixed tissues were immunostained using a goat polyclonal antibody against the murine C10 protein as described in Materials and Methods. No staining was evident in spinal cord from control mice (A); however, numerous immunostained cells are evident in an inflammatory lesion in spinal cord from a mouse with EAE (B). In presymptomatic GFAP-IL3 mice, cells immunostained for C10 were localized to perivascular infiltrates (C). In symptomatic GFAP-IL3 mice, numerous cells positive for C10 were observed surrounding perivascular lymphocytic cuffs (F) in the meninges (D) and within parenchymal white matter lesions (E).
ICV Injection of C10 Promotes the CNS Recruitment of Mac-1+ Cells
To ascertain the functionality of C10 and particularly whether this chemokine was a leukocyte chemoattractant factor in the CNS, mice were injected ICV with recombinant C10 protein. In vehicle-injected control mice, apart from the area of the cannula track injury in the cortex, few or no Mac-1+ cells were observed in the ventricles, choroid plexus, or meninges (Figure 7, A, C, and E) ▶ . In contrast, in mice injected with C10, large numbers of Mac-1+ cells were observed in the meninges (Figure 7B) ▶ , ventricular areas and in the CNS parenchyma adjacent to these areas (Figure 7D) ▶ , and in the choroid plexus (Figure 7F) ▶ . Although less in number, there were also CD4+ T lymphocytes and a few CD8+ T lymphocytes in the meninges and in the choroid plexus (not shown). These T lymphocytes were rarely if at all observed in brain from vehicle-injected mice.
Figure 7.
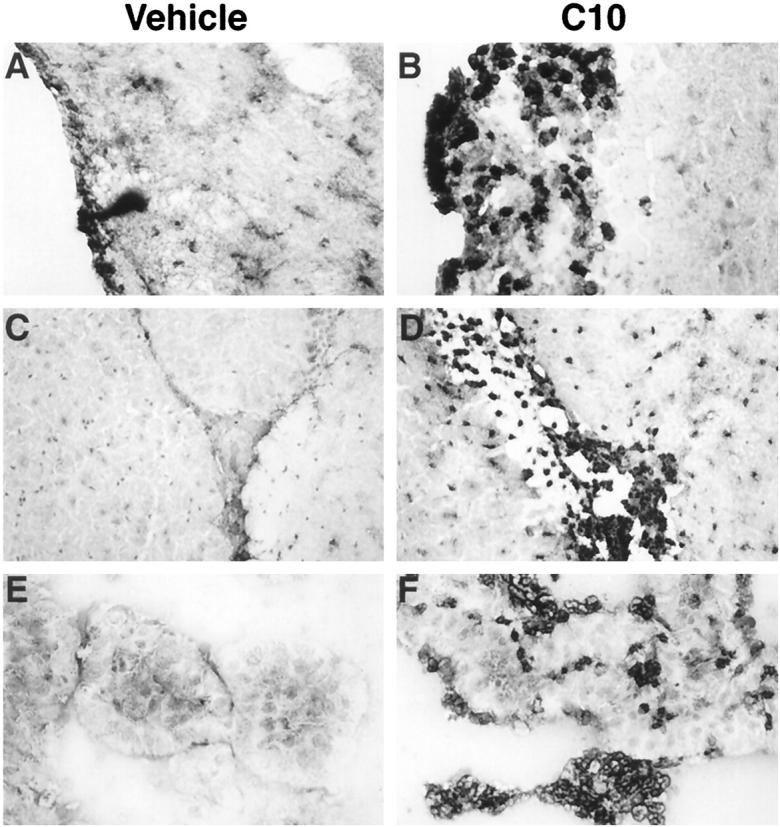
Mac-1-immunostained cryostat sections of brain from mice injected ICV with either vehicle (A, C, E) or C10 (B, D, F). Pronounced infiltration of the meninges (B), subependymal plate and surrounding parenchyma (D), and the choroid plexus and lateral ventricle (F) was observed 24 hours after injection of C10.
Discussion
The chemokines are a broad family of host-derived, soluble mediators that are assumed to play a key role in recruit and traffic of leukocytes to the CNS during inflammatory responses in a variety of disease processes ranging from viral 6-8 and bacterial infection 9 to autoimmune disorders. 10-13 In support of this, different chemokines elicit cerebral leukocytosis following either their injection into the brain 28 or their chronic expression in the brain of transgenic mice. 29,30 Moreover, the central role of chemokines in the pathogenesis of EAE was further clarified with the demonstration that neutralization of different chemokines such as MIP-1α 31 or Crg-2/IP-10 32 produced a marked attenuation in disease. Other than providing a chemotactic signal for the recruitment of leukocytes to the CNS, there is evidence 2 that the expression of different chemokine genes may vary in different neuroinflammatory states and may thereby directly determine the nature of the leukocytes that traffic into the CNS during inflammation. Here we showed that there are distinct disease-related cerebral chemokine gene expression profiles in a number of different models for CNS inflammation, including recently developed transgenic mice with astrocyte-targeted expression of the proinflammatory cytokines IL-3, 17 IL-6, 18 or IFN-α 19 and the murine model for autoimmune demyelinating disease, EAE. In the symptomatic transgenic mice that exhibit distinct neurological disorders, 20 striking differences in the cerebral chemokine gene expression were observed with dominance of specific chemokine genes. Thus, in GFAP-IL3 mice, C10 and the C10-related chemokine gene expression was most prominent. Our data here, as discussed below, support a key role for C10 in the recruitment of macrophage lineage cells to the CNS in these mice. On the other hand, in the GFAP-IFNα mice, the Crg-2/IP-10 gene was expressed at highest levels, with more modest expression of the RANTES chemokine gene. CNS-infiltrating cells in these transgenic mice consist of predominantly CD4+ and CD8+ lymphocytes and both these cell populations are known to respond to chemotactic stimulation by Crg-2/IP-10 33,34 or RANTES. 35 Finally, in GFAP-IL6 mice, which exhibit minimal mononuclear cell infiltration in the brain, there was only very low levels of expression of the Crg-2/IP-10 and RANTES genes. At the other extreme from these transgenic models, EAE which is characterized by the presence in the CNS of mixed infiltrates of immunoinflammatory cells, 36 was associated with the overlapping expression of multiple chemokine genes. These findings in EAE confirmed previous reports 10-13 documenting expression in the CNS of a number of chemokine genes belonging to both the α and β chemokine families. In all, our findings make the important point that there are significant differences in the patterns and levels of chemokine gene expression in different inflammatory states that may ultimately determine the type and function of the leukocytes that migrate to the CNS.
The novel finding of prominent C10 gene expression in the CNS in the GFAP-IL3 transgenic model and in EAE highlights a possible role for this chemokine in the pathogenesis of CNS leukocytosis in inflammatory demyelinating disease processes. C10 belongs to the β chemokine family and is closely related to the MIP-1 subfamily based on amino acid sequence similarity. 37 A β chemokine gene cloned from macrophages, named MRP-1 (MIP-related protein-1), is identical to C10. 27 A high degree of homology also exists between C10 and MRP-2 27 or CCF18 38 and MIP-1γ. 39 C10 and MRP-2 contain two more cysteine residues than the other members of the β chemokine family, suggesting that the tertiary structure and function of these two chemokines may be distinct. C10 was originally identified as a product of bone marrow cells stimulated with either GM-CSF or IL-3. 37 More recent studies of the regulation of C10 gene expression show that this differs somewhat from that of MIP-1α in being strongly induced in mouse monocytes and neutrophils by the cytokines IL-3, IL-4, and GM-CSF, but not by LPS or the proinflammatory cytokines IFN-γ, IL-1α, and TNF-α. 40 Based on these findings it has been proposed that C10 may be important in the development of humoral and allergic responses. 40 Our findings here document for the first time the significant expression of the C10 and C10-related genes in vivo in two different models of inflammatory demyelination and suggest a role for this chemokine in the pathogenesis of these disorders. Consistent with this, in the GFAP-IL3 transgenic mice there was a significant correlation between the levels of C10 mRNA present in the cerebellum and the severity of motor disease. In EAE and in the GFAP-IL3 transgenic mice, C10 mRNA and protein expression were localized to white matter lesions. In the GFAP-IL3 mice, increased expression of the C10 and C10-related mRNAs were detectable in the brain of young mice well before the development of the inflammatory lesions, whereas in presymptomatic mice C10 protein could be detected in perivascular cells. Activation of perivascular cells with increased expression of MHC class II molecules has been reported previously in presymptomatic GFAP-IL3 mice. 17 Although our data do not permit us to conclude that the same perivascular cells express both the MHC class II molecules and C10, it is clear that cells located in this CNS compartment may play an important signaling role for the subsequent recruitment of monocytes to the brain in these animals.
CNS C10 expression in symptomatic mice from both the GFAP-IL3 and EAE models was found to be closely associated with infiltrating mononuclear cells present at the brain surfaces (eg, meninges), in perivascular infiltrates, and within the parenchymal white matter lesions. Further dual labeling experiments in situ indicated that cells of the macrophage lineage were primarily responsible for C10 expression. The finding that cells of this lineage are the major source of C10 expression in vivo in these experimental models is consistent with in vitro studies showing that macrophages are a prodigious source of C10 following treatment with GM-CSF, IL-3, or IL-4. 40 In recent experiments we have found that recombinant murine IL-3 stimulates C10 gene expression in cultured microglia but not astrocytes (Asensio and Campbell, unpublished data). Therefore, in the GFAP-IL3 transgenic mice the induction of C10 gene expression is likely due to the direct action of the transgene encoded IL-3. On the other hand, in EAE, it is less clear what signal(s) are responsible for inducing C10 gene expression. One candidate might be IL-4, expression of which has been documented in the spinal cord during active EAE. 41
Our finding of significant expression of C10 in the inflammatory white matter lesions associated with the development of EAE contrasts with those of Godiska et al, who failed to detect C10 RNA transcripts in CNS tissue of mice with active EAE. 12 The explanation for these disparate findings is unknown but could reflect differences in the EAE models used. Godiska and colleagues used a passive transfer model in which MBP-reactive encephalitogenic T cells were injected into mice. This approach differs considerably from the adjuvant-immunization protocol reported here. It is therefore conceivable that differences also exist in the nature of the inflammatory processes associated with the development of EAE after different disease-inducing protocols. Our studies demonstrate a role for C10 in the recruitment of macrophages to the CNS (see below). In view of the absence of significant C10 expression in the passive transfer EAE model, it would be of interest to determine the relative number and contribution of macrophage/microglial cells in the demyelinating disease process in these animals.
The current observations raise the issue of the function of C10, which at the time of our studies remained an enigma. Its homology to other members of the MIP-1 family suggests it may have similar chemoattractant properties. In support of this, C10 has been shown to promote the chemotaxis of human peripheral blood mononuclear cells and peritoneal exudate cells in vitro, 42 although the precise identity of the responding cells in these studies was not defined. The finding that C10 was expressed as the dominant chemokine in the brain of the GFAP-IL3 mouse, in which the lesions are composed almost entirely of macrophage/microglial cells, led us to surmise that C10 may have a central role in the recruitment and possibly in the function of these cells. The ICV injection of recombinant C10 protein allowed a direct approach to study this possibility and provided convincing evidence that C10 can indeed effectively promote the recruitment of cells of monocytic lineage to the CNS. This action was quite potent with infiltrating cells not only being disseminated throughout the ventricular and meningeal compartments but also apparently migrating into the parenchyma. In view of the histological difficulty to distinguish between macrophage and microglia, it is also possible that some of the Mac-1-positive cells accumulating at these sites may have been microglia that migrated from the parenchyma in response to the C10. The findings suggest that C10, in common with some other chemokines such as MCP-1, 28 can, when injected into the brain, overcome the putative intrinsic resistance of the brain parenchyma to leukocyte migration. Our ICV injection studies also make another point: that C10 was chemotactic primarily for Mac-1+ cells. Although CD4+ T lymphocytes were also present, their numbers were considerably lower, suggesting their recruitment may be the result of secondary influences. Further studies will be necessary to establish the precise chemotactic signature of C10 as well as to define whether this factor has functions other than chemoattraction. However, our findings here indicate that C10 is not only produced by macrophages but also acts on these cells and may therefore serve as an autocrine regulator for macrophage chemotaxis.
In conclusion, the current study demonstrated distinct chemokine gene expression profiles in various experimental CNS disorders that display different immunopathological features. The expression of specific chemokines in these different inflammatory states may represent a defining event in controlling the type of leukocyte that is ultimately recruited to the brain. Our paper reports on a number of novel observations that identify the chemokine C10 as a potentially significant factor for the migration of macrophages to sites of tissue inflammation. Specifically, these findings (1) provide the first demonstration of the in vivo expression of the chemokine C10 and its prominent involvement with inflammatory demyelinating disease of the CNS, (2) identify the macrophage microglia as a major cellular source for C10 expression in vivo, (3) demonstrate that C10 is expressed at the RNA and protein level, and (4) document C10 functionality in vivo, showing that this chemokine is not only produced by macrophages but also acts as a potent chemoattractant for these cells to the CNS. Human β chemokines with close homology to the murine C10 termed MIP-5 43 and leukotactin-1, 44 were recently reported and it will be of interest to determine whether these or other related chemokines are expressed in the brain in human inflammatory demyelinating disorders. In any case, macrophage/microglial cells have increasingly assumed a central role as key mediators of inflammatory demyelination in human neurological disorders such as multiple sclerosis. 45 Therefore, macrophage acting chemokines such as C10 offer a potential therapeutic target for the treatment of such disorders, which could be tested in the animal models.
Acknowledgments
We thank Carrie Kincaid and Megan Benedict for their technical assistance. This is manuscript number 11414-NP from the Scripps Research Institute.
Footnotes
Address reprint requests to Dr. Iain L. Campbell, Department of Neuropharmacology, CVN-9, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037. E-mail: icamp@scripps.edu.
Supported by U. S. Public Health Service grants MH 47680 and MH 50426 (to I. L. C.). V.C.A. was supported by Fondation Singer Polignac (Paris) and is currently a postdoctoral fellow of the National Multiple Sclerosis Society. S. L. is supported by a NATO Postdoctoral Fellowship. A. P. was a postdoctoral fellow of the Deutsche Forschungsgmeinschaft (grant Pa 602/1–1).
Axel Pagenstecher’s current address: Department of Neuropathology, University of Freiburg, Freiburg, Germany.
References
- 1.Glabinski AR, Tani M, Aras S, Stoler MH, Tuohy VK, Ransohoff RM: Regulation and function of central nervous system chemokines. Int J Dev Neurosci 1995, 13:153-165 [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff RM: Chemokines in neurological disease models: correlation between chemokine expression patterns and inflammatory pathology. J Leukoc Biol 1997, 62:645-652 [DOI] [PubMed] [Google Scholar]
- 3.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ: A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385:640-644 [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D: Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387:611-617 [DOI] [PubMed] [Google Scholar]
- 5.Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, Copeland NG, Bazan JF, Moore KW, Schall TJ, Zlotnik A: Lymphotactin: a cytokine that represents a new class of chemokine. Science 1994, 266:1395-1399 [DOI] [PubMed] [Google Scholar]
- 6.Asensio VC, Campbell IL: Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J Virol 1997, 71:7832-7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane TE, Asensio VC, Yu N, Paoletti A, Campbell IL, Buchmeier MJ: Dynamic regulation of α-and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol 1998, 160:970-978 [PubMed] [Google Scholar]
- 8.Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA: Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol 1996, 149:1459-1467 [PMC free article] [PubMed] [Google Scholar]
- 9.Spanaus KS, Nadal D, Pfister HW, Seebach J, Widmer U, Frei K, Gloor S, Fontana A: C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis, and mediate chemotactic activity on peripheral blood-derived polymorphonuclear, and mononuclear cells in vitro. J Immunol 1997, 158:1956-1964 [PubMed] [Google Scholar]
- 10.Ransohoff RM, Hamilton TA, Tani M: Stole rMH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK: Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J 1993, 7:592-600 [DOI] [PubMed] [Google Scholar]
- 11.Hulkower K, Brosnan CF, Aquino DA, Cammer W, Kulshrestha S, Guida MP, Rapoport DA, Berman JW: Expression of CSF-1, c-fms, and MCP-1 in the central nervous system of rats with experimental allergic encephalomyelitis. J Immunol 1993, 150:2525-2533 [PubMed] [Google Scholar]
- 12.Godiska R, Chantry D, Dietsch GN, Gray PW: Chemokine expression in murine experimental allergic encephalomyelitis. J Neuroimmunol 1995, 58:167-176 [DOI] [PubMed] [Google Scholar]
- 13.Glabinski AR, Tani M, Tuohy VK, Tuthill RJ, Ransohoff RM: Central nervous system chemokine mRNA accumulation follows initial leukocyte entry at the onset of acute murine experimental autoimmune encephalomyelitis. Brain Behav Immun 1995, 9:315-330 [DOI] [PubMed] [Google Scholar]
- 14.Gourmala NG, Buttini M, Limonta S, Sauter A, Boddeke HW: Differential and time-dependent expression of monocyte chemoattractant protein-1 mRNA by astrocytes and macrophages in rat brain: effects of ischemia and peripheral lipopolysaccharide administration. J Neuroimmunol 1997, 74:35-44 [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Young PR, McDonnell PC, White RF, Barone FC, Feuerstein GZ: Cytokine-induced neutrophil chemoattractant mRNA expressed in cerebral ischemia. Neurosci Lett 1993, 164:125-128 [DOI] [PubMed] [Google Scholar]
- 16.Berman JW, Guida MP, Warren J, Amat J, Brosnan CF: Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol 1996, 156:3017-3023 [PubMed] [Google Scholar]
- 17.Chiang CS, Powell HC, Gold LH, Samimi A, Campbell IL: Macrophage/microglial-mediated primary demyelination, and motor disease induced by the central nervous system production of interleukin-3 in transgenic mice J Clin Invest 1996, 97:1512-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MBA, Mucke L: Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin-6. Proc Natl Acad Sci USA 1993, 90:10061-10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akwa Y, Hassett DE, Eloranta M-L, Sandberg K, Masliah E, Powell H, Whitton JL, Bloom FEB, Campbell IL: Transgenic expression of interferon-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol 1998, 161:5016-5026 [PubMed] [Google Scholar]
- 20.Campbell IL, Stalder AK, Chiang CS, Bellinger R, Heyser CJ, Steffensen S, Masliah E, Powell HC, Gold LH, Henriksen SJ, Siggins GR: Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Mol Psychiatry 1997, 2:125-129 [DOI] [PubMed] [Google Scholar]
- 21.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH: Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci USA 1997, 94:1500-1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendel I, de Rosbo NK, Ben-Nun A: A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor Vβ expression of encephalitogenic T cells. Eur J Immunol 1995, 25:1951–1959 [DOI] [PubMed]
- 23.Amor S, Groome N, Linington C, Morris MM, Dornmair K, Gardinier MV, Matthieu J-M, Baker D: Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of eperimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol 1994, 153:4349-4356 [PubMed] [Google Scholar]
- 24.Badley JE, Bishop GA, St. John T, Frelinger JA: A simple, rapid method for the purification of poly A+ RNA. Biotechniques 1988, 6:114-116 [PubMed] [Google Scholar]
- 25.Campbell IL, Hobbs MV, Kemper P, Oldstone MBA: Cerebral expression of multiple cytokine genes in mice with lymphocytic choriomeningitis. J Immunol 1994, 152:716-723 [PubMed] [Google Scholar]
- 26.Hauke C, Korr H: RCA-I lectin histochemistry after trypsinisation enables the identification of microglial cells in thin paraffin sections of the mouse brain J Neurosci Meth 1993, 50:273-277 [DOI] [PubMed] [Google Scholar]
- 27.Youn BS, Jang IK, Broxmeyer HE, Cooper S, Jenkins NA, Gilbert DJ, Copeland NG, Elick TA, Fraser MJ, Jr., Kwon BS: A novel chemokine, macrophage inflammatory protein-related protein-2, inhibits colony formation of bone marrow myeloid progenitors. J Immunol 1995, 155:2661-2667 [PubMed] [Google Scholar]
- 28.Bell MD, Taub DD, Perry VH: Overriding the brain’s intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience 1996, 74:283-292 [DOI] [PubMed] [Google Scholar]
- 29.Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA: Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol 1995, 155:5769-5776 [PubMed] [Google Scholar]
- 30.Tani M, Fuentes ME, Peterson JW, Trapp BD, Durham SK, Loy JK, Bravo R, Ransohoff RM, Lira SA: Neutrophil infiltration, glial reaction, and neurological disease in transgenic mice expressing the chemokine N51/KC in oligodendrocytes. J Clin Invest 1996, 98:529-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD: An important role for the chemokine macrophage inflammatory protein-1-α in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol 1995, 155:5003-5010 [PubMed] [Google Scholar]
- 32.Wojcik WJ, Swoveland P, Zhang X, Vanguri P: Chronic intrathecal infusion of phosphorothioate or phosphodiester antisense oligonucleotides against cytokine responsive gene-2/IP-10 in experimental allergic encephalomyelitis of lewis rat. J Pharmacol Exp Ther 1996, 278:404-410 [PubMed] [Google Scholar]
- 33.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ: Recombinant human-interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med 1993, 177:1809-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biddison WE, Cruikshank WW, Center DM, Pelfrey CM, Taub DD, Turner RV: CD8+ myelin peptide-specific T cells can chemoattract CD4+ myelin peptide-specific T cells: importance of IFN-inducible protein 10. J Immunol 1998, 160:444-448 [PubMed] [Google Scholar]
- 35.Schall TJ, Bacon K, Toy KJ, Goeddel DV: Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990, 347:669-671 [DOI] [PubMed] [Google Scholar]
- 36.Martin R, McFarland HF: Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci 1995, 32:121-182 [DOI] [PubMed] [Google Scholar]
- 37.Orlofsky A, Berger MS, Prystowsky MB: Novel expression pattern of a new member of the MIP-1 family of cytokine-like genes. Cell Regul 1991, 2:403-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara T, Bacon KB, Cho LC, Yoshimura A, Morikawa Y, Copeland NG, Gilbert DJ, Jenkins NA, Schall TJ, Miyajima A: Molecular cloning and functional characterization of a novel member of the C-C chemokine family. J Immunol 1995, 155:5352-5358 [PubMed] [Google Scholar]
- 39.Poltorak AN, Bazzoni F, Smirnova II, Alejos E, Thompson P, Luheshi G, Rothwell N, Beutler B: Mip-1 γ: molecular cloning, expression, and biological activities of a novel CC chemokine that is constitutively secreted in vivo. J Inflamm 1995, 45:207-219 [PubMed] [Google Scholar]
- 40.Orlofsky A, Lin EY, Prystowsky MB: Selective induction of the β chemokine C10 by IL-4 in mouse macrophages. J Immunol 1994, 152:5084-5091 [PubMed] [Google Scholar]
- 41.Kennedy MK, Torrance DS, Picha KS, Mohler KM: Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol 1992, 149:2496-2505 [PubMed] [Google Scholar]
- 42.Berger MS, Taub DD, Orlofsky A, Kleyman TR, Coupayegerard B, Eisner D, Cohen SA: The chemokine C10: immunological and functional analysis of the sequence encoded by the novel second exon. Cytokine 1996, 8:439-447 [DOI] [PubMed] [Google Scholar]
- 43.Coulin F, Power CA, Alouani S, Peitsch MC, Schroeder JM, Moshizuki M, Clark-Lewis I, Wells TNC: Characterisation of macrophage inflammatory protein-5/human CC cytokine-2, a member of the macro-phage-inflammatory-protein family of chemokines. Eur J Biochem 1997, 248:507-515 [DOI] [PubMed] [Google Scholar]
- 44.Youn B-S, Zhang SM, Lee EK, Park DH, Broxmeyer HE, Murphy PM, Locati M, Pease JE, Kim KK, Antol K, Kwon BS: Molecular cloning of leukotactin-1: a novel human β-chemokine, a chemoattractant for neutrophils, monocytes, and lymphocytes, and a potent agonist at CC chemokine receptors 1 and 3. J Immunol 1997, 159:5201-5205 [PubMed] [Google Scholar]
- 45.Sriram S, Rodriguez M: Indictment of the microglia as the villain in multiple sclerosis. Neurology 1997, 48:464-470 [DOI] [PubMed] [Google Scholar]