Abstract
The genotypic features of mature ovarian teratomas (MOTs) are controversial. Early studies detected a homozygous genotype in MOTs suggesting that these tumors are composed of germ cells that have undergone meiosis I. Other studies, however, revealed a heterozygous genotype in a substantial proportion of MOTs suggesting an origin either from premeiotic germ cells or from a somatic cell line. In view of the complex morphology of MOTs and to increase the sensitivity of teratoma genotyping, we applied tissue microdissection before genetic analysis of teratomatous tissue. This approach allowed selective analysis of different heterotopic tissue elements as well as the lymphoid tissues within MOTs the origin of which is unknown. After DNA extraction, the tissue samples were polymerase chain reaction amplified using a random panel of highly informative genetic markers for different chromosomes to evaluate heterozygosity versus homozygosity. In all seven cases that were analyzed, heterotopic tissues consistently revealed a homozygous genotype with several markers; in two cases, heterozygosity was detected with a single marker, indicating a meiotic recombination event. Lymphoid aggregates within MOTs were heterozygous and derived from host tissue rather than from teratomatous growth. However, well differentiated thymic tissue was consistently homozygous, suggesting lymphoid differentiation capability of MOTs. We conclude that potential pitfalls in genotyping of teratomas including meiotic recombination and host cell participation can be avoided by a microdissection-based approach in combination with a panel of genetic markers.
Teratomas are tumors that are composed of a variety of tissue elements derived from two or more of the embryonic germ layers. 1,2 Classic theories of the origin of teratomas include incomplete twinning, neoplastic proliferation of sequestered totipotent blastomeres or primordial germ cells, derepression of totipotent genetic information in the nuclei of somatic cells, and parthenogenetic development of germ cells. 1,2 Due to the frequent occurrence of teratomas in the ovary, a germ cell origin was postulated decades ago. Utilizing enzyme polymorphisms and chromosome banding studies, Linder and co-workers demonstrated that teratomas are homozygous for chromosomal polymorphisms whereas nonteratomatous host tissue is heterozygous. 3-6 These findings not only proved a fundamentally different genetic composition of the teratomatous tissue as compared with normal host tissue but also strongly suggested that the teratomatous genotype was acquired secondary to meiotic cell division. The data were subsequently confirmed by larger studies 7 and experimental mouse models 8 that continued to support a germ cell origin of ovarian teratomas.
Subsequent studies, however, failed to consistently detect a homozygous genetic composition of teratomas. 9-13 Instead, heterozygous centromeric markers and other chromosomal heteromorphisms were reported in a subset of tumors, raising the possibility of either postmeiotic or premeiotic origin of these tumors.
Compared with other tumors, teratomas exhibit unique histological features being composed of a variety of architecturally and cytologically mature tissues rather than a proliferating pool of neoplastic cells. Histologically, teratomas are composed of heterotopic tissues, including tissues such as epidermis, central nervous system tissue, or mature cartilage. 1,2 However, teratomas also contain nonspecific tissue types, eg, lymphoid tissue or fibrous stroma. Whereas the teratomatous nature of specific heterotopic tissues is evident and even mandatory for diagnosis, it is unknown to what extent immunological cell elements within heterotopic parenchyma represent either pluripotent differentiation capability of teratomatous tissue or host cell reactivity. Better clarification of this question may reveal the full differentiation potential of teratomatous growth that may include not only heterotopic parenchymal tissue but also lymphoid cells. As development of specific tissue is dependent on specific signaling pathways, more selective analysis of the histopathological features of teratomas may explain the enormous intratumor and intertumor diversity of these neoplasms. In addition, we may obtain closer insight into host-tumor interactions that are represented by different cellular systems with fundamental genetic diversity, yet full immunological compatibility.
Despite the ongoing controversy about the genotype of ovarian teratoma, and our ignorance about reactivity versus neoplasticity of various teratomatous elements, selective analysis of different histological components has never been attempted. In the present study, we performed selective genetic analysis of seven teratomas by microdissection of a wide diversity of histological features.
Materials and Methods
Patients and Tumors
Seven solitary mature ovarian teratomas were retrieved from the files of the Armed Forces Institute of Pathology. The patients’ ages at surgery were between 10 and 20 years. All cases showed a variety of areas with different histological differentiation (Table 1 ▶ and Figure 1 ▶ ). From each case, between 8 and 18 different histological areas were selectively dissected as described previously. 14,15 The following tissues were selectively dissected from the seven cases (Table 1) ▶ : 1) areas of specific histological differentiation, eg, respiratory epithelium and salivary gland; contamination with cells that are not mandatory constituents of the targeted area, eg, cells from other tissue types or blood-borne cells, was avoided or minimized; 2) areas in which a few lymphocytes were admixed with various tissue elements; and 3) pure lymphoid aggregates that were observed in association with different types of histological differentiation; the lymphoid aggregates consisted of clusters of lymphoid cells that did not contain germinal centers. Whenever feasible, tissues were removed in duplicate or triplicate.
Table 1.
Seven Mature Ovarian Teratomas: Histological Components Identified and Analyzed
| Specific types of tissues | Specific types of tissues with lymphoid cells | Lymphoid aggregates |
|---|---|---|
| 1a. Normal ovarian stroma/Fallopian tube | 2a. Squamous epithelium with lymphoid cells | 3a. Lymphoid aggregate in squamous epithelium |
| 1b. Squamous epithelium | 2b. Gastrointestinal epithelium with lymphoid cells | 3b. Lymphoid aggregate in respiratory epithelium |
| 1c. Gingival epithelium | 2c. Sebaceous glands with lymphoid cells | 3c. Lymphoid aggregate in gastrointestinal epithelium |
| 1d. Respiratory epithelium | 2d. Neuroglial tissue with lymphoid cells | 3d. Lymphoid aggregate in gingival epithelium |
| 1e. Gastrointestinal epithelium | ||
| 1f. Sebaceous gland | ||
| 1g. Salivary gland | ||
| 1h. Pilar follicle | ||
| 1i. Neuroglia | ||
| 1j. Cerebellum | ||
| 1k. Cartilage | ||
| 1l. Dental bud | ||
| 1m. Thymus | ||
| 1n. Smooth muscle |
Types of tissue dissected were as follows: case 1, 1a, 1b, 1d, 1f, 1i, 1k, 1l, 3a, and 3b; case 2, 1a, 1b, 1d, 1f, 1i, 1k, 2a, 2c, 2d, and 3b; case 3, 1a, 1b, 1d, 1e, 1i, 1k, and 1m; case 4, 1a, 1b, 1e, 1f, 1h, 1i, 1j, 1n, 3a, and 3c; case 5, 1a, 1b, 1d, 1e, 1f, 1i, 1k, 2c, and 3a; case 6, 1a, 1b, 1d, 1f, 1k, and 3b; case 7, 1a, 1b, 1c, 1f, 1g, 1i, 3a, and 3d.
Figure 1.
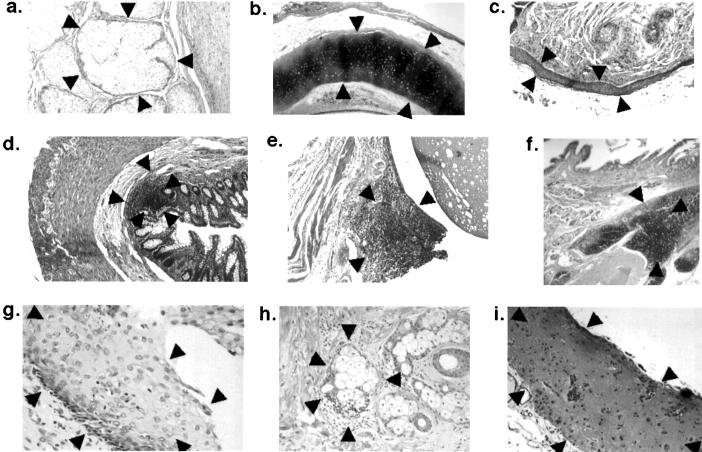
Representative histological areas from different mature ovarian teratomas that were selectively procured and genetically analyzed; the microdissected areas are surrounded by arrowheads. a–c: Areas of specific histological differentiation: a, sebaceous glands; b, cartilage; c, squamous epithelium. d–f: Areas with lymphoid tissue: d, lymphoid aggregate in gastrointestinal epithelium; e, lymphoid aggregate associated with respiratory epithelium; f, thymic tissue. g–i: Areas in which a few lymphocytes were admixed with various tissue elements: g, lymphocytes in squamous epithelium: h, lymphocytes associated with sebaceous gland; i, lymphocytes in neuroglial tissue.
Microdissection
Unstained 5-μm sections on glass slides were deparaffinized with xylene, rinsed in ethanol from 100% to 80%, briefly stained with hematoxylin and eosin, and rinsed in 10% glycerol in TE buffer. Tissue microdissection was performed as described previously. 14,15
DNA Extraction
Produced cells were immediately resuspended in 30 μl of buffer containing Tris/HCl, pH 8.0, 10 mmol/L ethylenediamine tetraacetic acid, pH 8.0, 1% Tween 20, and 0.5 mg/ml proteinase K and were incubated at 37°C overnight. The mixture was boiled for 10 minutes to inactivate the proteinase K, and 1.5 μl of this solution was used for polymerase chain reaction (PCR) amplification of the DNA.
Genetic Analysis
Tissue samples were analyzed with different microsatellite markers, including D1S1646 (1p), Int-2 (11q13), Ank-1 (8p), D9S303 (9q), D9S171 (9p), IFNA (9p), D5S346 (5q), and D3S2452 (3p14–21). Each PCR sample contained 1.5 μl of template DNA as described above, 10 pmol of each primer, 20 nmol each of dATP, dCTP, dGTP, and dTTP, 15 mmol/L MgCl2, 0.1 U of Taq DNA polymerase, 0.05 ml of [32P]dCTP (6000 Ci/mmol), and 1 μl of 10X buffer in a total volume of 10 μl. PCR was performed with 35 cycles: denaturing at 95°C for 1 minute, annealing at 55°C to 60°C for 1 minute, and extending at 72°C for 90 seconds. The final extension was continued for 10 minutes. Labeled amplified DNA was mixed with an equal volume of formamide loading dye (95% formamide, 20 mmol/L EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol). Samples were then denatured for 5 minutes at 95%, loaded onto a gel consisting of 6% acrylamide (acrylamide:bisacrylamide 49:1), and electrophoresed at 1800 V for 90 minutes. After electrophoresis, the gels were transferred to 3-mm Whatman paper and dried. Autoradiography was performed with Kodak X-OMAT film (Eastman Kodak, Rochester, NY).
Results and Discussion
In all seven cases, host tissue genotype and teratoma were analyzed by microdissecting and analyzing normal ovarian/Fallopian tube tissue and different areas of teratomatous tissue with a series of markers, including D1S1646 (1p), Int-2 (11q), Ank-1 (8p), D9S303 (9q), D9S171, (9p), IFNA (9p), D5S346 (5q), and D3S2452 (3p). In each individual case, the majority of markers revealed a heterozygous and therefore informative genotype in normal ovarian tissue. With the same informative markers, selectively procured teratomatous tissue was genetically homozygous whenever the dissected tissue was strictly parenchymal, ie, selectively dissected without potential contamination with nonconstitutive cells (Figure 2A) ▶ .
Figure 2.
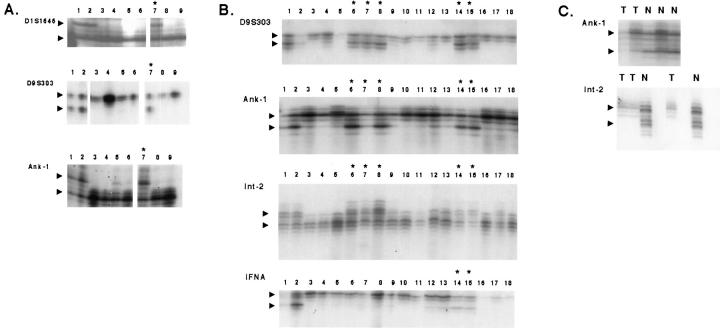
Representative findings after genetic analysis of various histological components from different mature ovarian teratomas. After PCR, the amplification products were separated using 8% polyacrylamide gels; each lane depicted below represents a separately procured tissue sample. A: Case 1, various histological components being consistently homozygous with markers Int-2, D1S1646, and D3S2452; normal host ovarian stroma is heterozygous. lane 1, normal ovarian stroma; lane 2, squamous epithelium; lane 3, neuroglial tissue; lane 4, sebaceous gland; lane 5, striated muscle; lane 6, neuroglial tissue; lane 7, sebaceous gland; lane 8, neuroglial tissue; lane 9 , sebaceous gland; lane 10, squamous epithelium; lane 11, squamous epithelium; lane 12, neuroglial tissue; lane 13, squamous epithelium) B: Case 7, evidence of genetic recombination. Lane 1, Normal ovarian tissue; lane 2, normal ovarian tissue; lane 3, squamous epithelium; lane 4, lymphoid aggregate in squamous epithelium. Teratomatous squamous epithelium is homozygous with Ank-1 but heterozygous with D9S303; in contrast, normal ovarian tissue and lymphoid aggregate are heterozygous with both markers.
In two cases, tumor areas were homozygous with all markers except one marker that indicated heterozygosity (Figure 2B ▶ , marker D9S303). This finding was interpreted as a recombination event during meiosis I involving the respective marker locus resulting in preservation of heterozygosity.
Vice versa, aggregates of lymphoid tissue that were usually associated with different epithelial structures were consistently heterozygous (Figure 2B ▶ , lane 4; Figure 3A ▶ , lane 7; Figure 3B ▶ , lanes 14 and 15). Although these findings could indicate heterogeneity of different tumor compartments, we interpret these findings in most cases as a contaminating effect caused by infiltration of host lymphoid cells. This interpretation is based on our observations on teratomatous tissues in which the number of lymphoid cells did not exceed that of strictly ectopic parenchymal cells (Figure 3B ▶ , lanes 6 to 8). These samples demonstrated variable intensities of the second allele with different markers; eg, in case 2, teratoma tissues containing lymphocytes showed similar allelic intensities with D9S303 and Ank-1 and variable intensities with Int-2, whereas no second allele was observed with IFNA (Figure 3B ▶ , lanes 6 to 8). This observation indicates allelic imbalance secondary to the presence of heterozygous cells that contribute a second allele of variable intensity; it also points out that different genetic markers may show different degrees of amplification sensitivity of heterogeneous cell populations. The interpretation of cellular contamination rather than true heterogeneity is further corroborated by the observation that selective procurement of lymphoid aggregates demonstrated two alleles of equal intensity (Figure 2B ▶ , lane 4; Figure 3A ▶ , lane 7; Figure 3B ▶ , lanes 13 and 14). In contrast, thymic tissue from case 3 was genetically homozygous (Figure 3C) ▶ . It therefore appears that the differentiation potential of mature ovarian teratoma does include lymphoid tissue.
Figure 3.
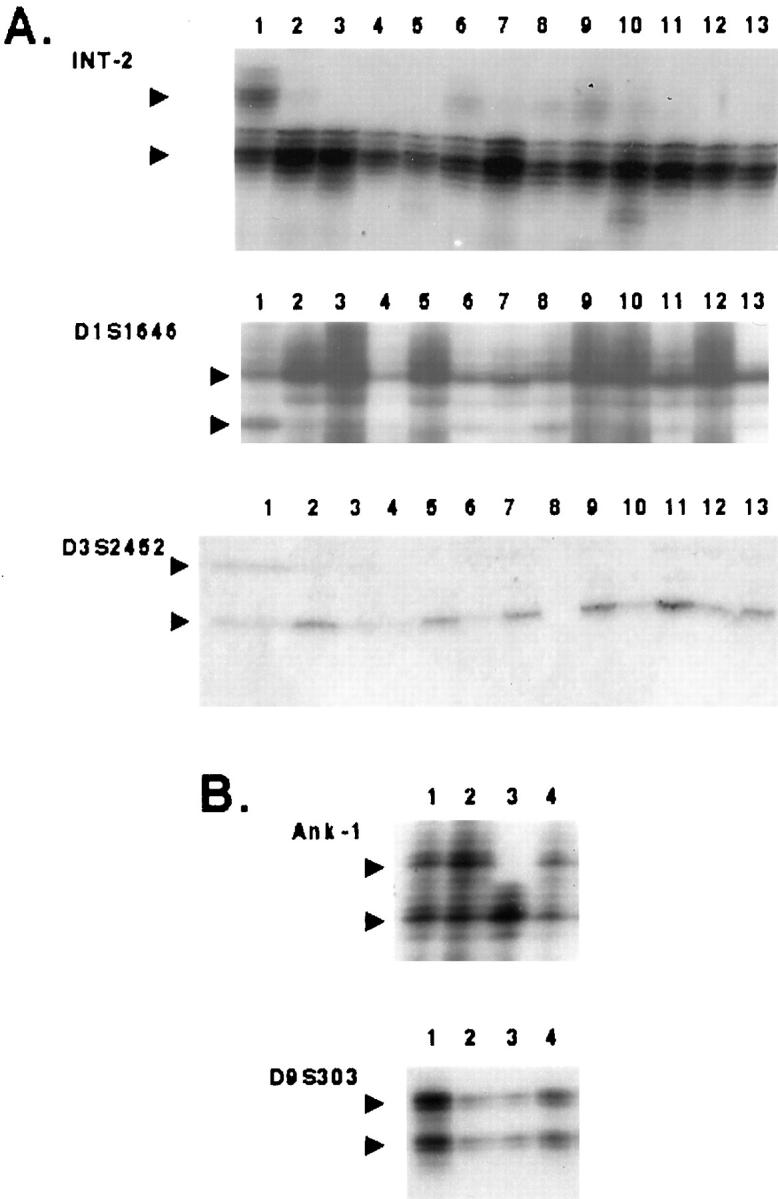
A: Case 4, analysis of lymphoid aggregate (lane 7, marked by asterisk) reveals two (heterozygous) alleles with markers D1S1646, D9S303, and Ank-1, whereas microdissected teratomatous tissues of variable heterotopic differentiation are consistently homozygous.lane 1, normal ovarian stroma; lane 2, normal ovarian stroma; lane 3, cerebellum; lane 4, cerebellum; lane 5, squamous epithelium; lane 6, neuroglial tissue; lane 7, lymphoid aggregate in squamous epithelium; lane 8, sebaceous glands; lane 9, pilar follicles. B: Case 2, various histological components being consistently homozygous with markers D9S303, Ank-1, Int-2, and IFNA. Normal host ovarian stroma is heterozygous; however, a second allele of variable intensity can be observed in samples 6, 7, and 8 (marked by asterisks) that represent different teratomatous tissues with lymphoid cells. Samples 14 and 15 (marked by asterisks) represent selectively procured lymphoid aggregates with two alleles of equal intensity. Lane 1, normal ovarian stroma; lane 2, normal ovarian stroma; lane 3, sebaceous gland; lane 4; sebaceous gland; lane 5, squamous epithelium; lane 6, neuroglial tissue; lane 7, squamous epithelium with lymphoid cells; lane 8, sebaceous glands with lymphoid cells; lane 9, neuroglial tissue; lane 10, cartilage; lane 11, sebaceous gland; lane 12, respiratory epithelium; lane 13, sebaceous glands; lane 14, lymphocytes associated with respiratory epithelium; lane 15, lymphocytes associated with respiratory epithelium; lane 16, sebaceous glands; lane 17, respiratory epithelium; lane 18, respiratory epithelium. C: Case 3. Thymic tissue obtained from case 3 reveals homozygosity with markers Ank-1 and Int-2. Normal host ovarian stroma is heterozygous T, thymus; N, normal ovarian tissue.
As postulated more than 100 years ago, the germ cell line differentiates during early embryonic life and is responsible for species preservation by developing along the Keimbahn or germ track. 2 In contrast to the somatic cell line, germ cells physiologically undergo a series of unique and fundamental genetic modifications. In particular, it is the unique event of meiotic division that distinguishes germ cells from all other somatic cells by producing an offspring of cells with fundamentally modified genetic composition. In contrast to regular mitotic division, division I of meiosis (meiosis I) creates cells in which both of the two DNA copies of each chromosome are derived from only one of the two homologous chromosomes present in the original cell. 16,17 In other words, during meiosis I, the heterozygous premeiotic germ cell (oogonium) divides into two homozygous postmeiotic cells. In addition, parts of homologous chromosomes are exchanged by genetic recombination during the long prophase of meiosis I. On average, one or two crossover events occur on each pair of homologs, 16 allowing for occasional detection of allelic heterozygosity in postmeiotic cells. In two of the analyzed cases, heterozygosity was retained with one and two markers, respectively. However, as the same samples were consistently homozygous with other chromosomal markers, we interpret these findings as evidence for genetic recombination rather than intratumor heterogeneity.
Analysis of heterozygosity versus homozygosity has been of fundamental importance to establish germ cell derivation of ovarian teratomas. Original studies with enzyme polymorphisms 3 were subsequently confirmed cytogenetically. 5,7 However, several studies identified a subgroup of teratomas that was genetically heterozygous, 9-13 challenging the concept of a consistently postmeiotic germ cell origin of these tumors. Analysis of purely heterotopic teratomatous areas in our series of seven cases revealed consistent homozygosity of the tumor tissue with all or the majority of genetic markers.
For analysis of genetic polymorphoisms, it is desirable or even mandatory to separate and selectively procure the neoplastic cells. 14,15 Vice versa, PCR-based identification of genetic monomorphism in tissue DNA extracted from a heterozygous host may indicate that the genetic changes affect all cellular constituents. With this study, we performed a tissue-specific genetic analysis of seven ovarian teratomas using tissue microdissection and PCR analysis with a panel of polymorphic markers. Analyzed tissues were selectively procured, guided by histopathological appearance.
In conclusion, the results of this study indicate that 1) ovarian teratomas reveal genetic homozygosity in the procured tissues strongly supporting Linder’s original hypothesis of a germ cell origin; with a few markers, heterozygosity was observed in some tumors indicative of genetic recombination. 2) Within teratomas with homozygous genotype, lymphoid aggregates reveal allelic heterozygosity indicative of host cell contamination. In one case, well differentiated thymic tissue revealed a homozygous genotype emphasizing the pluripotent capability of teratoma cells to differentiate along hematopoetic cell lines. 3) Tissue microdissection may be the most sensitive method to evaluate the genotype of individual teratomas.
Footnotes
Address reprint requests to Dr. Zhengping Zhuang, Laboratory of Pathology, Building 10 Room 2A33, National Cancer Institute/NIH, 9000 Rockville Pike, Bethesda, MD 20892.
A.O.V. and M.D.-S. contributed equally to this study.
References
- 1.Scully RE: Tumors of the ovary and maldeveloped gonads. Atlas of Tumor Pathology. 1979, Armed Forces Institute of Pathology, Washington, DC,
- 2.Gonzales-Crussi F: Extragonadal teratomas. Atlas of Tumor Pathology. 1982, Armed Forces Institute of Pathology, Washington, DC,
- 3.Linder D: Gene loss in human teratomas. Proc Natl Acad Sci USA 1969, 63:699-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linder D, Power J: Further evidence for post-meiotic origin of teratomas in the human female. Ann Hum Genet 1970, 34:21-30 [DOI] [PubMed] [Google Scholar]
- 5.Linder D, Hecht F, McCaw BK, Campbell JR: Origin of extragonadal teratomas and endodermal sinus tumors. Nature 1975, 254:597-598 [DOI] [PubMed] [Google Scholar]
- 6.Kaiser-McCaw BK, Hecht F, Linder D, Lovrien EW, Wyandt H, Bacon D, Clark B, Lea N: Ovarian teratomas: cytologic data. Cytogenet Cell Genet 1976, 16:391-395 [DOI] [PubMed] [Google Scholar]
- 7.Patil SR, Kaiser-McCaw B, Hecht F, Linder D, Lovrien EW: Human benign ovarian teratomas: chromosomal and electrophoretic enzyme studies. Birth Defects Original Article Ser 1978, 14:297-301 [PubMed] [Google Scholar]
- 8.Eppig JJ, Kozak LP, Eicher EM, Stevens LC: Ovarian teratomas in mice are derived from oocytes that have completed the first meiotic division. Nature 1977, 269:517-518 [DOI] [PubMed] [Google Scholar]
- 9.Carritt B, Parrington JM, Welch HM, Povey S: Diverse origins of multiple ovarian teratoma in a single individual. Proc Natl Acad Sci USA 1982, 79:7400-7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parrington JM, West LF, Povey S: The origin of ovarian teratomas. J Med Genet 1984, 21:4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surti U, Hoffner L, Chakravarti A, Ferrell RE: Genetics and biology of human ovarian teratomas. I. Cytogenetic analysis and mechanism of origin. Am J Hum Genet 1990, 47:635-643 [PMC free article] [PubMed] [Google Scholar]
- 12.Deka R, Chakravarti A, Surti U, Hauselman E, Reefer J, P. MP, Ferrell RE: Genetics and biology of human ovarian teratomas. II. Molecular analysis of origin of nondisjunction and gene-centromere mapping of chromosome I markers. Am J Hum Genet 1990, 47:644–655 [PMC free article] [PubMed]
- 13.Dahl N, H. GK, Rune C, Gustavsson I, Pettersson U: Benign ovarian teratomas: an analysis of their cellular origin. Cancer Genet Cytogenet 1990, 46:115–123 [DOI] [PubMed]
- 14.Zhuang Z, Bertheau P, Emmert-Buck MR, Liotta LA, Gnarra J, Linehan WM, Lubensky IA: A microdissection technique for archival DNA analysis of specific cell populations in lesions <1 mm in size. Am J Pathol 1995, 146:620-625 [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang Z, Vortmeyer AO: Applications of tissue microdissection in cancer genetics. Cell Vis 1998, 5:43-48 [PubMed] [Google Scholar]
- 16.Kleckner N: Meiosis. How could it work? Proc Natl Acad Sci USA 1996, 93:8167-8174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bascom-Slack CA, Ross LO, Dawson DS: Chiasmata, crossovers, and meiotic chromosome segregation. Adv Genet 1997, 35:253-284 [DOI] [PubMed] [Google Scholar]