Abstract
Granzyme B (GrB) and T-cell-restricted intracellular antigen (TIA-1) are cytotoxic proteins that are specifically expressed by cytotoxic CD4 or CD8 positive T cells and natural killer cells. Recent studies demonstrated frequent expression of GrB and TIA-1 by neoplastic cells in primary cutaneous CD30+ large T-cell lymphomas and lymphomatoid papulosis but not in CD30− large T-cell lymphomas. In the present study, 74 biopsies from 54 patients with mycosis fungoides (MF) were investigated for the expression of GrB and TIA-1 using immunohistochemistry on paraffin sections. Staining of more than 10% of the neoplastic T cells for GrB or TIA-1 was considered positive. All but two follow-up biopsies had been obtained from patients without extracutaneous disease at the time of biopsy. Expression of TIA-1 and GrB was found in 33 (45%) and 14 (19%) of 74 MF biopsies, respectively. Comparison of biopsies from T3NoMo-stage MF (n = 27) and T2NoMo-stage MF (n = 45) showed increased expression of TIA-1 (55 versus 37%) and GrB (33 versus 9%) in T3NoMo-stage MF. Evaluation of multiple sequential biopsies from successive stages of MF also revealed an increase in the GrB/TIA-1 expression with tumor progression in five of eight cases. A clearcut relation between the expression of TIA-1 and/or GrB and the type of skin lesion biopsied was found. Considering all 74 biopsies, expression of TIA-1 and GrB was found in 18 of 50 (35%) and 5 of 50 (10%) patches or plaques, 9 of 16 (55%) and 3 of 16 (20%) tumors without blastic transformation, and 6 of 8 (75%) and 6 of 8 (75%) tumors with blastic transformation (defined as >50% blast cells). Correlation between GrB/TIA-1 expression in first diagnostic biopsies from patches or plaques from 40 patients with T2NoMo-stage MF and clinical follow-up data did not reveal differences in clinical behavior and survival between patients with (n = 14) or without (n = 26) expression of cytotoxic proteins, indicating that MF expressing cytotoxic proteins should not be considered as a separate group.
Recently, monoclonal antibodies against components of the cytotoxic granules present in the cytoplasm of cytotoxic granules present in the cytoplasm of cytotoxic T-lymphocytes (CTL) and natural killer (NK) cells have become available. These cytotoxic molecules include perforin, 1 the serine proteases granzyme A and B, 2,3 and the T-cell restricted intracellular antigen (TIA-1). 4,5
After target cell recognition, cytotoxic lymphocytes reorient their cytoplasmic granules to the area of the target cell followed by vectorial exocytosis of granule contents into a tightly apposed, synaptosomal-like space between the cells. After interaction with specific binding sites, granzymes are endocytozed with adjacent sublytic perforin pores. The role of perforin lies in the delivery of granzymes to the cytosol by compromising the integrity of the endocytosed vesicle. 6-8 Once in the cytosol, granzymes can initiate apoptosis by activation of caspases and induction of intranuclear cyclin A/kinase complexes. 9-11
Initial studies on the components of cytotoxic granules were focused primarily on the expression of these molecules by CTL and NK cells considered to play an important role in immunesurveillance and antitumor responses. However, studies in malignant lymphomas demonstrated that not only reactive cytotoxic leukocytes but also the neoplastic cells in various types of malignant lymphomas expressed these cytotoxic proteins. 12-14 Apart from rare NK/T-cell lymphomas, 15,16 expression of granzyme B (GrB), TIA-1, and perforin was noted in 70 to 90% of noncutaneous CD30+ anaplastic large cell lymphomas (ALCL) of T- or null-cell phenotype and in sporadic cases of Hodgkin’s disease. 17-19 In primary cutaneous lymphomas, recent studies demonstrated expression of GrB and TIA-1 proteins in variable proportions of neoplastic cells in most cases of lymphomatoid papulosis and primary cutaneous CD30+ ALCL, but not or rarely in CD30− primary cutaneous large T-cell lymphomas. 20 Because these primary cutaneous CD30+ lymphoproliferations may coexist or progress from typical mycosis fungoides (MF), the most common type of cutaneous T-cell lymphoma (CTCL), we wondered if the neoplastic cells in MF also express TIA-1 and GrB. Therefore, immunohistochemical stainings for GrB and TIA-1 were performed on paraffin sections from 74 biopsies of 54 MF patients. The results were correlated with clinical stage according to the TNM classification and with the type of skin lesion biopsied. To establish whether cases of MF expressing TIA-1 and/or GrB should be regarded as a distinct subgroup of MF, expression of cytotoxic proteins was correlated with clinical behavior and survival.
Material and Methods
Patients
Seventy-four paraffin-embedded skin specimens from 54 patients with MF were selected from the tissue bank of the Dutch Cutaneous Lymphoma Working Group. The diagnosis was based on a combination of clinical, histological, and immunophenotypical data as described previously. 21 Biopsies had been obtained from untreated skin lesions at the time of diagnosis (57 biopsies) or at the time of relapse or disease progression (17 biopsies). For all 74 biopsies, both the clinical stage at the time of biopsy, which was assessed according to the staging criteria proposed by the National Cancer Institute workshop on cutaneous T-cell lymphomas (TNM classification), 22 as well as the type of skin lesion from which the biopsy was taken, are presented in Table 1 ▶ . With respect to the clinical stage, it should be noted that at the time of biopsy only two patients (Table 1 ▶ ; patients 44 and 54) had concurrent lymph node involvement (T3N3Mo). Because all but these two follow-up biopsies had been obtained from patients without evidence of extracutaneous disease at the time of biopsy the clinical stage was determined only by the type of skin lesions, defined as the presence of patches and plaques covering more than 10% of the skin surface (T2) or the presence of tumors (T3). Because previous studies demonstrated unequivocally that skin tumors showing blastic transformation have a significantly worse prognosis than tumors without blastic transformation 23,24 within the T3 category, distinction was made between tumors with and tumors without blastic transformation, defined by the presence of more than 50% blast cells. Therefore, we will refer primarily to the type of skin lesion (patches/plaques, tumors without, and tumors with blastic transformation). Whenever appropriate, the TNM categories will be indicated as well.
Table 1.
Stage of Disease (TNM Classification) 22 and Type of Skin Lesion of 74 Biopsies in 54 Patients with Mycosis Fungoides
| Patient | Biopsy at time of diagnosis | Biopsy at time of relapse/progression | ||||||
|---|---|---|---|---|---|---|---|---|
| TNM | Skin lesions | TNM | Skin lesions | |||||
| PL | T− | T+ | PL | T− | T+ | |||
| 1–29 | T2N0M0 | + | ||||||
| 30–34 | T2N0M0 | + | T2N0M0 | + | ||||
| 35–37 | T2N0M0 | + | T3N0M0 | + | ||||
| 38 | T2N0M0 | + | T3N0M0 | +* | ||||
| 39 | T2N0M0 | + | T3N0M0 | + | ||||
| 40 | T2N0M0 | + | T3N0M0 | + | + | |||
| 41 | T3N0M0 | + | + | |||||
| 42–43 | T3N0M0 | + | + | |||||
| 44 | T3N0M0 | + | + | T3N3M0 | + | |||
| 45–50 | T3N0M0 | + | ||||||
| 51 | T3N0M0 | + | T3N0M0 | + | ||||
| 52 | T3N0M0 | + | T3N0M0 | + | ||||
| 53 | T3N0M0 | + | ||||||
| 54 | T3N3M0 | + | ||||||
| Total number of skin biopsies | 44 | 10 | 3 | 6 | 6 | 5 |
Definitions of TNM classification: T1, patches/plaques covering <10% of skin surface; T2, patches/plaques covering 10% or more of skin surface; T3, tumor(s); T4, erythroderma; N0, no clinically enlarged peripheral lymph nodes, histology (if performed) negative; N1, clinically abnormal peripheral lymph nodes, pathology negative for CTCL; N2, clinically nonpalpable, pathologically positive lymph nodes; N3, clinically abnormal, pathology positive for CTCL; M0, no visceral involvement; M1, visceral involvement (biopsy documented); PL, plaque; T−, tumor without transformation; T+, tumor with blastic transformation, defined by the presence of >50% blast cells.
*Two tumors biopsied 2 years apart.
Skin biopsies had been obtained from typical patches (cases 1 through 4; Table 1 ▶ ), plaques (n = 46), and tumors without (n = 16) or tumors with blastic transformation (n = 8) (see Table 1 ▶ ). Multiple biopsies were available in 17 of 54 patients. In five cases (patients 30 to 34) biopsies from two different plaques of patients with T2NoMo stage disease were studied. The time interval between the first and second biopsy ranged between 6 and 45 months (median, 21 months). In 10 patients biopsies from both plaques and tumors obtained at the same time at diagnosis (patients 41 to 44) and/or during follow-up (patients 35 to 40 and 44) were studied. In three patients (patients 44, 51, and 52) skin biopsies from two tumorous lesions, biopsied 12 to 15 months apart, were available for examination.
All cases had been immunophenotyped previously as part of routine diagnostic procedures both on paraffin material and fresh material, the latter either on another part of the same biopsy or on another biopsy from a similar lesion concurrently taken. Sixty-seven of 74 biopsies had a CD3+, CD4+, and CD8− immunophenotype, 6 of 74 had a CD3+, CD4−, and CD8− immunophenotype, and one biopsy had a CD3+, CD4−, and CD8+ immunophenotype. This latter patient was included because both the clinical presentation, the histological appearance, and the clinical course were all characteristic of MF. In none of the cases the neoplastic T cells expressed CD56 or CD57.
Immunohistochemistry
Immunostaining on formalin-fixed, paraffin-embedded sections with monoclonal antibodies against granzyme B (GrB7) 25,26 and TIA-1 (Coulter Immunology, Hialeah, FL) 4 was performed using a streptavidin-biotin-peroxidase technique following antigen retrieval with microwave heating, as described previously. 18 In addition, in all cases serial sections were stained with monoclonal antibodies against CD4 (Novocastra, Newcastle upon Tyne, UK), CD8 (144B; a gift from Dr. Mason, Oxford, UK), Ber-H2/CD30 (DAKO, Glostrup, Denmark), and polyclonal antibodies against CD3 (DAKO).
Interpretation of Immunohistochemical Staining
Identification of neoplastic T cells, including medium-sized to large cerebriform cells and blast cells, was based on a combination of morphology and phenotype. In the early plaque lesions the neoplastic T-cells could be recognized rather easily by their cerebriform nuclear morphology, their haloed appearance and their typical lining-up at the dermal-epidermal junction. In addition, in all cases, examination of serial sections stained with monoclonal antibodies against CD3, CD4, and CD8 were used as an additional tool to differentiate the neoplastic T cells from reactive CD8+ T cells and dendritic cells/macrophages (Figure 1) ▶ . Because interpretation of cases showing only occasional GrB and/or TIA-1+, neoplastic T cells may be difficult in particular in cases containing many admixed GrB+, TIA-1+, and CD8+ T cells; cases with less than 10% GrB+ or TIA-1+ neoplastic cells were considered negative. The percentage of neoplastic cells positive for GrB and TIA-1 were scored as follows: −, no or occasional (<10%) tumor cell stained; +, 10 to 50%; and ++, >50% positive tumor cells.
Figure 1.
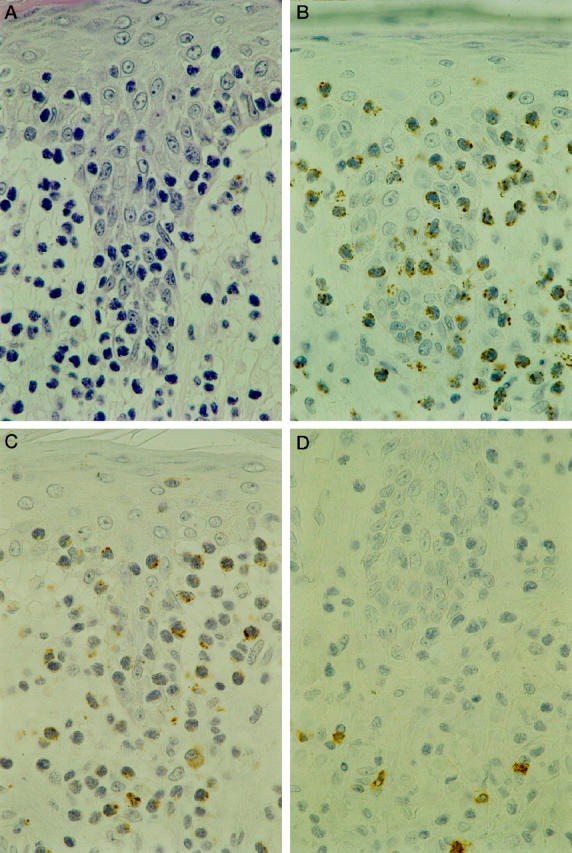
Mycosis fungoides, plaque. Characteristic infiltration of the epidermis by neoplastic cells (epidermotropism) A: Immunohistochemistry on serial sections demonstrates the expression of TIA-1 (B) and Granzyme B (C) by the neoplastic cells. Staining with CD8 antibody demonstrates reactive CD8+ T cells in the dermis but not in the epidermis (D). Streptavidin-biotin-peroxidase technique; hematoxylin counterstain; original magnification, ×630.
Statistical Analysis
The relationship between the expression of TIA-1 and GrB and TNM-stage was evaluated with Fisher’s exact test by comparing all biopsies from stage T2NoMo and T3NoMo, irrespective of the type of skin lesion biopsied. The increase of expression of TIA-1 and GrB in relation to the type of skin lesion biopsied was evaluated with the nonparametric Spearman rank correlation test. Disease-related survival probabilities for T2NoMo-stage MF were estimated using Kaplan-Meier survival analysis. 27 Survival of patients without TIA-1 and GrB expression was compared with cases expressing TIA-1 or expressing TIA-1 and GrB with the log rank test. Observations were considered statistically significant if P < 0.05.
Results
In 33 of 74 biopsies, more than 10% of the neoplastic cells showed a clear granular cytoplasmic staining for GrB and/or TIA-1 (Figures 1 and 2) ▶ ▶ . Tumor cells expressing TIA-1 and/or GrB included both neoplastic T cells with cerebriform nuclei and blast cells. Expression of TIA-1 was more frequent (33 of 74) than GrB (14 of 74). Whereas 14 of 33 TIA-1+ biopsies also expressed GrB in more than 10% of the neoplastic cells, biopsies showing tumor cells reactive with GrB but negative for TIA-1 were not observed. Moreover, in most biopsies TIA-1+ neoplastic T cells outnumbered GrB+ tumor cells. In 21 and 17 of 74 biopsies occasional tumor cells (<10%) expressing TIA-1 or GrB, respectively, were observed. In 20 biopsies, staining of neoplastic T cells for both TIA-1 and GrB was completely negative. In these biopsies, the reactive T lymphocytes expressing GrB and TIA-1 acted as a positive control. Correlation between the expression of cytotoxic proteins and the phenotype of the neoplastic cells showed expression of GrB and/or TIA-1 by more than 10% of the neoplastic cells in 28 of 67 (40%) of CD3+, CD4+, and CD8− MF, in four of six (60%) of CD3−, CD4−, and CD8− MF, and in the single case with a CD3+, CD4−, and CD8+ immunophenotype.
Figure 2.
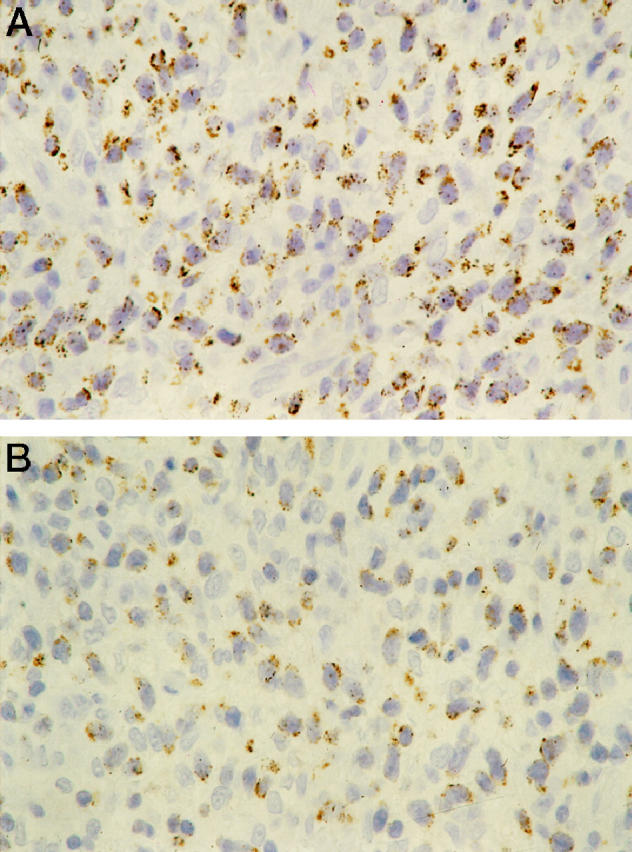
Mycosis fungoides, tumor. Diffuse proliferation of neoplastic cells that show a diffuse granular cytoplasmic staining for both TIA-1 (A) and GrB (B). Streptavidin-biotin-peroxidase technique; hematoxylin counterstain; original magnification, ×400.
Increased Expression of GrB and TIA-1 with Progression from Plaque to Tumor Stage MF
The results of TIA− and GrB staining in the different clinical stages and in the different types of skin lesions are presented in Table 2 ▶ . Comparison between the 45 biopsies obtained from patients with T2NoMo-stage MF with the 27 biopsies obtained from patients with T3NoMo-stage MF showed expression of TIA-1 and GrB by the neoplastic cells in 17 of 45 (37%) and 4 of 45 (9%) skin lesions in T2NoMo-stage MF, and in 15 of 27 (55%) and 9 of 27 (33%) skin lesions in T3NoMo-stage MF, respectively. Thus, the expression of cytotoxic proteins was higher in T3NoMo-stage MF than in T2NoMo-stage MF, and this increased expression reached statistical significance for GrB (P = 0.01) but not for TIA-1. Within T3NoMo-stage MF, a clearcut relation between the expression of TIA-1 and GrB and the type of skin lesion biopsied was found. TIA-1 and GrB were expressed by one in five (20%) plaques, by 10 in 16 (62%), and 3 in 16 (18%) tumors without transformation, respectively, and by 5 in 6 (80%) tumors with blastic transformation. Thus, no significant differences in the expression of TIA-1 and GrB were found between patches/plaques in patients with (T3NoMo) or without (T2NoMo) concurrent tumors. Consistently, evaluation of four patients presenting with T3NoMo-stage MF (patients 41 to 44), in whom both plaques and tumors could be investigated, both TIA-1 and GrB were expressed by 20% of the neoplastic cells in one of four plaques, whereas both proteins were expressed by more than 75% of the neoplastic cells in three of four concurrent tumors. In one of these four cases both plaque and tumor were completely negative for TIA-1 and GrB. In another eight cases, in which multiple biopsies from successive stages of MF were available, an increase in the percentages of neoplastic cells expressing GrB and/or TIA-1 was observed in five of the eight (Table 3) ▶ . In all five cases, in which two plaques biopsied 6 to 45 months apart were studied, no major differences in GrB and TIA-1 expression between the first and the second biopsy were found.
Table 2.
Expression of Cytotoxic Molecules in Relation to Different Stages of Mycosis Fungoides (TNM Classification) and the Type of Biopsied Skin Lesion
| TNM classification at time of biopsy | Type of skin lesion | n | TIA-1* | Granzyme B* | ||||
|---|---|---|---|---|---|---|---|---|
| − | + | ++ | − | + | ++ | |||
| T2N0M0 | Patch/plaque | 45 | 28 (62%) | 12 (27%) | 5 (11%) | 41 (91%) | 2 (4%) | 2 (4%) |
| T3N0M0 | Plaque | 5 | 4 (80%) | 1 (20%) | 0 | 4 (80%) | 1 (20%) | 0 |
| Tumor | 22 | 8 (36%) | 5 (23%) | 9 (41%) | 14 (64%) | 6 (27%) | 2 (9%) | |
| T3N3M0 | Tumor | 2 | 1 (50%) | 0 | 1 (50%) | 1 (50%) | 0 | 1 (50%) |
| Type of skin lesion† | Patch/plaque | 50 | 32 (64%) | 13 (26%) | 5 (10%) | 45 (90%) | 3 (6%) | 2 (4%) |
| Tumor without transformation | 16 | 7 (44%) | 5 (31%) | 4 (25%) | 13 (81%) | 2 (13%) | 1 (6%) | |
| Tumor with transformation‡ | 8 | 2 (25%) | 0 | 6 (75%) | 2 (25%) | 4 (50%) | 2 (25%) |
*−, no positive cells or <10% positive neoplastic cells; +, 10–50% positive neoplastic cells; ++, >50% positive neoplastic cells;
†Statistical analysis with Spearman rank correlation coefficient is 0.35 (P = 0.002) for TIA-1 and 0.39 (P = 0.0006) for GrB.
‡Transformation is defined by the presence of >50% blast cells.
Table 3.
Expression of Cytotoxic Proteins in Sequential Biopsies from Successive Stages of Mycosis Fungoides
| Number (patient number) | Biopsy at time of diagnosis | Biopsy after disease progression | Expression of GrB/TIA-1† | ||||
|---|---|---|---|---|---|---|---|
| TNM | Skin lesion | TIA-1/GrB* | TNM | Skin lesion | TIA-1/GrB | ||
| 1 (35) | T2N0M0 | Plaque | −/++ | T3N0M0 | Tumor | +/++ | ↑ |
| 2 (36) | T2N0M0 | Plaque | −/− | T3N0M0 | Tumor | −/− | ≈ |
| 3 (37) | T2N0M0 | Plaque | −/− | T3N0M0 | Tumor | −/+ | ↑ |
| 4 (38) | T2N0M0 | Plaque | −/− | T3N0M0 | Tumor | −/−‡ | ≈ |
| 5 (39) | T2N0M0 | Plaque | −/− | T3N0M0 | Tumor (+Tr) | +/++ | ↑ |
| 6 (40) | T2N0M0 | Plaque | −/− | T3N0M0 | Tumor (+Tr) | −/− | ≈ |
| 7 (44) | T3N0M0 | Plaque | +/+ | T3N3M0 | Tumor (+Tr) | ++/++ | ↑ |
| Tumor | +/+ | ||||||
| 8 (52) | T3N0M0 | Tumor | −/+ | T3N0M0 | Tumor (+Tr) | +/++ | ↑ |
Abbreviations: *−, No positive cells or <10% positive neoplastic cells; +, 10 to 50% positive neoplastic cells; ++, >50% positive neoplastic cells.
†Result over sequential biopsies.
‡Confirmed on an additional biopsy two years later.
Tr, transformation, defined as >50% blast cells.
Taken together, these results demonstrate an increase in the expression of TIA-1 and GrB with progression from plaque stage (T2N0M0) to tumor stage (T3N0M0) MF. In addition, a clearcut relationship between the expression of cytotoxic molecules and the type of skin lesion biopsied was observed. Evaluation of all 74 biopsies confirmed that the increase in TIA-1/GrB expression strongly correlated with the development of tumors (P = 0.03 for TIA-1 and P = 0.007 for GrB). Thus, TIA-1 expression by more than 10% of the neoplastic cells was detected in 18 of 50 (35%) patches or plaques, in 9 of 16 (55%) tumors without transformation, and in six of eight (75%) tumors with blastic transformation. Similarly, the expression of GrB in more than 10% of neoplastic cells increased from 5 in 50 (10%) of patches or plaques and 3 of 16 (20%) tumors without transformation to six of eight (75%) tumors with transformation. In the nonparametric Spearman rank correlation test, this increase in the expression of TIA-1 and GrB was statistically significant with a correlation coefficient of 0.35 (P = 0.002) and 0.39 (P = 0.0006), respectively (Table 2) ▶ .
Correlation of GrB/TIA-1 Expression with Clinical Behavior and Outcome
To study whether cases of MF expressing cytotoxic molecules have a different clinical behavior and prognosis compared with GrB−/TIA-1− cases, the results of staining for GrB/TIA-1 in the first diagnostic biopsy of 40 patients with T2N0M0-stage MF (patients 1 to 40; Table 1 ▶ ) were correlated with the clinical follow-up data, including the development of tumors, the development of nodal or visceral involvement, and survival. In 16 of 40 biopsies more than 10% of the neoplastic cells expressed both TIA-1 and GrB (four patients) or only TIA-1 but not GrB (12 patients), whereas in 24 of 40 biopsies no or occasional tumor cells stained for GrB and/or TIA-1. The follow-up data did not show major differences in disease progression or prognosis between these three groups, neither between the first two positive groups together compared with the negative group (Table 4) ▶ . Also the four cases, in which more than 50% of the neoplastic cells expressed cytotoxic molecules, did not show another clinical behavior as compared with the GrB−/TIA-1− cases (data not shown). Thus, no significant differences in disease progression and survival were found between patients with and without expression of cytotoxic molecules in T2NoMo-stage MF.
Table 4.
Disease Progression and Disease-Related Survival in 40 Patients with T2N0M0-Stage Mycosis Fungoides Stratified for the Expression of Cytotoxic Proteins in the First Diagnostic Biopsy of Patches or Plaques
| Parameters | TIA-1+/GrB+ | TIA-1+/GrB− | TIA-1−/GrB− | P value |
|---|---|---|---|---|
| Number of patients | 4 | 12 | 24 | |
| Progression to tumor stage MF | 1 | 2 | 7 | ns |
| Development of involved lymph nodes | 0 | 1 | 6 | ns |
| Died of lymphoma | 0 | 1 | 5 | ns |
| Median follow-up (months) | 31 | 71 | 62 | |
| Range of follow-up (months) | 12–49 | 10–128 | 12–161 | |
| 5 years survival (%) | (100) | 100 | 90 | ns |
−, No positive cells or <10% positive neoplastic cells; +, >10% positive neoplastic cells; ns, not significant.
P values were estimated using the Fisher’s exact test.
Discussion
Whereas previous studies already described expression of cytotoxic proteins by the neoplastic cells of primary cutaneous CD30+ lymphoproliferations, this is the first study demonstrating that also the neoplastic cells in MF may express cytotoxic molecules. Expression of GrB and TIA-1 by at least 10% of the neoplastic cells was observed in 14 (19%) and 33 (45%) of 74 biopsies, respectively. The observation that TIA-1 is expressed more frequently and by larger numbers of neoplastic T cells than GrB may relate to the functional state of these cells because TIA-1 is expressed by both resting and activated CTL, whereas expression of GrB and perforin are only expressed after activation. 28,29
Expression of TIA-1 and GrB was much higher in skin biopsies obtained from patients with T3NoMo-stage MF than from patients with T2NoMo-stage MF. Also in five of eight cases, in which multiple biopsies from successive stages of MF were available, an increase in the percentages of neoplastic cells expressing GrB and/or TIA-1 was observed (Table 3) ▶ . Further evaluation demonstrated that this increase in TIA-1/GrB expression is determined by the development of skin tumors, in particular the development of tumors showing blastic transformation. Evaluation of all 74 biopsies showed a clearcut relationship between TIA-1/GrB expression and the type of skin lesion biopsied with expression of GrB and/or TIA-1 in 35% of patches or plaques, 55% of tumors without histological transformation, and in 75% of tumors showing transformation into a diffuse large T-cell lymphoma.
The increase in the expression of cytotoxic proteins with progression from plaque stage (T2NoMo) to tumor stage (T3NoMo) MF is consistent with the results of Asadullah et al., 30 who reported a stage-dependent increase in granzyme A mRNA in 19 patients with MF, using a competitive reverse transcriptase-polymerase chain reaction technique. The authors concluded that high levels of granzyme A expression in MF represents a poor prognostic sign. They hypothesized that if the neoplastic T cells would be the source of this increased granzyme A expression, these cells might have the capacity to induce apoptosis in antitumor immune cells and in this way escape from immune control. This hypothesis might explain the gradual decrease in the numbers of CD8+ T cells and the worsening prognosis observed during disease progression in MF. 31 However, recent studies demonstrate that CTL themselves are resistant to perforin/granzyme B-mediated lysis. Several mechanisms may be involved in producing this resistance. There is evidence for the existence of as yet unidentified proteins on the surface of CTL and NK cells protecting these cells against perforin-mediated lysis. 32 Other studies demonstrated that CTL can escape from GrB-mediated apoptosis by expressing intracellular serine protease inhibitors (serpins). 33 One could speculate that similar mechanisms are operative not only in CTL and NK cells but also in tumor cells expressing these cytotoxic proteins. This would imply that such tumor cells might be rather resistant against perforin/granzyme B-mediated, eg. CTL-mediated apoptosis, and in view of the fact that different apoptosis inducing signals share one of more common final lethal pathways, perhaps also against therapy-induced apoptosis. Also in this scenario, one would expect that GrB+/TIA-1+ MF would have a more unfavorable course than cases of MF not expressing these cytotoxic proteins.
Because of these theoretical considerations, the relationship between clinical behavior and outcome and GrB/TIA-1 expression was investigated in the present study. However, examination of first diagnostic biopsies from patches or plaques in 40 patients with T2NoMo-stage MF did not reveal differences in disease progression and prognosis between GrB+ and/or TIA-1+ and GrB− and/or TIA-1− cases. These findings indicate that cases of MF expressing GrB/TIA-1 do not have a more aggressive clinical behavior or a worse prognosis compared with cases of MF, which do not express these cytotoxic molecules. In addition, cases of MF expressing cytotoxic molecules did not have a different clinical appearance, and all but few cases had a CD3+, CD4+, and CD8− phenotype. Taken together, these observations indicate that cases of MF expressing cytotoxic molecules do not differ clinically from MF not expressing these cytotoxic molecules and should not be considered as a separate group.
The mechanisms underlying the stage-dependent increase in the expression of GrB and TIA-1 in MF are unknown. The observation that the neoplastic T cells in tumorous skin lesions but not in concurrent patches or plaque express these cytotoxic proteins may result from intrinsic differences between the neoplastic T cells in these plaques and tumors, eg, additional genetic alterations or differences in the state of activation. Alternatively, one might speculate that the differences in the expression of GrB and TIA-1 between the neoplastic cells in patches or plaques and tumors may be attributed to differences in the local microenvironment. In vitro studies have demonstrated chronic stimulation can induce the expression of cytotoxic proteins on CD4+ T cells. 34,35 Recent studies demonstrated that murine CD4+ T-cell lines develop a perforin-dependent cytotoxicity only in the absence of activated CD8+ T cells. 36 The authors suggested that CD8+ T cells may regulate the functional phenotype of these CD4+ T cells, either by direct cell contact or by an as yet unidentified soluble factor. The decreasing number of CD8+ T cells in successive stages of MF, as has been demonstrated in several publications, 31,37 fits well in this hypothesis. However, evidence for a regulatory role of these CD8+ T cells in the acquisition of a cytotoxic phenotype by the neoplastic T cells in MF is lacking.
In conclusion, in the present study we have shown that the neoplastic T cells in a proportion of MF patients have the phenotype of cytotoxic CD4+ T cells. Expression of GrB and TIA-1 increases with the development of tumors, in particular tumors showing blastic transformation. However, within the group of patients with T2NoMo-stage MF, GrB+ and/or TIA-1+ cases do not differ in clinical behavior and prognosis from cases of MF not expressing these cytotoxic proteins and should therefore not be considered as a separate group.
Acknowledgments
The authors thank Carolien C.W. Klaver for statistical analysis and Els de Vries for excellent technical assistance.
Footnotes
Address reprint requests to Dr. M.H. Vermeer, Department of Dermatology, Free University Hospital, De Boelelaan 1117, 1081 HV Amsterdam. E-mail: mh.vermeer@azvu.nl.
References
- 1.Lichtenheld MG, Olsen KJ, Lu P, Lowrey DM, Hameed A, Hengartner H, Podack ER: Structure and function of human perforin. Nature 1988, 335:448-451 [DOI] [PubMed] [Google Scholar]
- 2.Krahenbuhl O, Rey C, Jenne D, Lanzavecchia A, Groscurth P, Carrel S, Tschopp J: Characterization of granzymes A and B isolated from granules of cloned human cytotoxic T lymphocytes. J Immunol 1988, 141:3471-3477 [PubMed] [Google Scholar]
- 3.Griffiths GM, Mueller C: Expression of perforin and granzymes in vivo: potential diagnostic markers for activated cytotoxic cells. Immunol Today 1991, 12:415-419 [DOI] [PubMed] [Google Scholar]
- 4.Anderson P, Nagler-Anderson C, O’Brien C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF: A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol 1990, 144:574-582 [PubMed] [Google Scholar]
- 5.Anderson P: TIA-1: structural and functional studies on a new class of cytolytic effector molecule. Curr Top Microbiol Immunol 1995, 198:131-143 [DOI] [PubMed] [Google Scholar]
- 6.Froelich CJ, Hanna WL, Poirier GG, Duriez PJ, Damours D, Salvesen GS, Alnemri ES, Earnshaw WC, Shah GM: Granzyme B perforin-mediated apoptosis of Jurkat cells results in cleavage of poly (ADP-ribose) polymerase to the 89-kDa apoptotic fragment and less abundant 64-kDa fragment. Biochem Biophys Res Commun 1996, 227:658-665 [DOI] [PubMed] [Google Scholar]
- 7.Froelich CJ, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, Shah GM, Bleackley RC, Dixit VM, Hanna W: New paradigm for lymphocyte granule-mediated cytotoxicity: target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem 1996, 271:29073-29079 [DOI] [PubMed] [Google Scholar]
- 8.Froelich CJ, Dixit VM, Yang XH: Lymphocyte granule-mediated apoptosis: matters of viral mimicry and deadly proteases. Immunol Today 1998, 19:30-36 [DOI] [PubMed] [Google Scholar]
- 9.Shi LF, Chen G, He DL, Bosc DG, Litchfield DW, Greenberg AH: Granzyme B induces apoptosis and cyclin A-associated cyclin-dependent kinase activity in all stages of the cell cycle. J Immunol 1996, 157:2381-2385 [PubMed] [Google Scholar]
- 10.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan JY: Human ICE/CED-3 protease nomenclature. Cell 1996, 87:171. [DOI] [PubMed] [Google Scholar]
- 11.Zou H, Henzel WJ, Liu XS, Lutschg A, Wang XD: APAF-1, a human protein homologous to c-elegans CED-4, participates in cytochrome C-dependent activation of caspase-3. Cell 1997, 90:405-413 [DOI] [PubMed] [Google Scholar]
- 12.De Bruin PC, Kummer JA, Van der Valk P, Van Heerde P, Kluin PM, Willemze R, Ossenkoppele GJ, Radaszkiewicz T, Meijer CJ: Granzyme B-expressing peripheral T-cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa-associated lymphoid tissue localization. Blood 1994, 84:3785-3791 [PubMed] [Google Scholar]
- 13.De Bruin PC, Connolly CE, Oudejans JJ, Kummer JA, Jansen W, McCarthy CF, Meijer CM: Enteropathy-associated T-cell lymphomas have a cytotoxic T-cell phenotype. Histopathology 1997, 31:313-317 [DOI] [PubMed] [Google Scholar]
- 14.Daum S, Foss HD, Anagnostopoulos I, Dederke B, Demel G, Araujo I, Riecken EO, Stein H: Expression of cytotoxic molecules in intestinal T-cell lymphomas: the German Study Group on intestinal non-Hodgkin lymphoma. J Pathol 1997, 182:311-317 [DOI] [PubMed] [Google Scholar]
- 15.Van Gorp J, De Bruin PC, Siego DS, Van Heerde P, Ossenkoppele GJ, Rademakers LM, Meijer CM, Vandentweel JG: Nasal T-cell lymphoma: a clinicopathological and immunophenotypic analysis of 13 cases. Histopathology 1995, 27:139-148 [DOI] [PubMed] [Google Scholar]
- 16.Ohshima K, Suzumiya J, Shimazaki K, Kato A, Tanaka T, Kanda M, Kikuchi M: Nasal T/NK cell lymphomas commonly express perforin and Fas ligand: important mediators of tissue damage. Histopathology 1997, 31:444-450 [DOI] [PubMed] [Google Scholar]
- 17.Foss HD, Anagnostopoulos I, Araujo I, Assaf C, Demel G, Kummer JA, Hummel M, Stein H: Anaplastic large-cell lymphomas of T-cell and null-cell phenotype express cytotoxic molecules. Blood 1996, 88:4005-4011 [PubMed] [Google Scholar]
- 18.Oudejans JJ, Kummer JA, Jiwa M, Van der Valk P, Ossenkoppele GJ, Kluin PM, Kluin-Nelemans JC, Meijer CM: Granzyme B expression in Reed-Sternberg cells of Hodgkins disease. Am J Pathol 1996, 148:233-240 [PMC free article] [PubMed] [Google Scholar]
- 19.Krenacs L, Wellmann A, Sorbara L, Himmelmann AW, Bagdi E, Jaffe ES, Raffeld M: Cytotoxic cell antigen expression in anaplastic large cell lymphomas of T- and null-cell type and Hodgkin’s disease: evidence for distinct cellular origin. Blood 1997, 89:980-989 [PubMed] [Google Scholar]
- 20.Kummer JA, Vermeer MH, Dukers D, Meijer CJ, Willemze R: Most primary cutaneous CD30-positive lymphoproliferative disorders have a CD4-positive cytotoxic T-cell phenotype. J Invest Dermatol 1997, 109:636-640 [DOI] [PubMed] [Google Scholar]
- 21.Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, Diazperez JL, Geerts ML, Goos M, Knobler R, Ralfkiaer E, Santucci M, Smith N, Wechsler J, Van Vloten WA, Meijer CM: EORTC classification for primary cutaneous lymphomas: a proposal from the cutaneous lymphoma study group of the European organization for research and treatment of cancer. Blood 1997, 90:354-371 [PubMed] [Google Scholar]
- 22.Diamandidou E, Cohen PR, Kurzrock R: Mycosis fungoides and Sezary syndrome. Blood 1996, 88:2385-2409 [PubMed] [Google Scholar]
- 23.Dmitrovsky E, Matthews MJ, Bunn PA, Schechter GP, Makuch RW, Winkler CF, Eddy J, Sausville EA, Ihde DC: Cytologic transformation in cutaneous T cell lymphoma: a clinicopathologic entity associated with poor prognosis. J Clin Oncol 1987, 5:208-215 [DOI] [PubMed] [Google Scholar]
- 24.Greer JP, Salhany KE, Cousar JB, Fields JP, King LE, Graber SE, Flexner JM, Stein RS, Collins RD: Clinical features associated with transformation of ceribriform T-cell lymphoma to a large cell process. Hematol Oncol 1990, 8:215-227 [DOI] [PubMed] [Google Scholar]
- 25.Kummer JA, Kamp AM, Van Katwijk M, Brakenhoff JP, Radosevic K, van Leeuwen AM, Borst J, Verweij CL, Hack CE: Production and characterization of monoclonal antibodies raised against recombinant human granzymes A and B and showing cross reactions with the natural proteins. J Immunol Methods 1993, 163:77-83 [DOI] [PubMed] [Google Scholar]
- 26.Kummer JA, Kamp AM, Tadema TM, Vos W, Meijer CJ, Hack CE: Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin Exp Immunol 1995, 100:164-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958, 53:457-481 [Google Scholar]
- 28.Hanson RD, Sclar GM, Kanagawa O, Ley TJ: The 5′-flanking region of the human CGL-1/granzyme B gene targets expression of a reporter gene to activated T-lymphocytes in transgenic mice. J Biol Chem 1991, 266:24433-24438 [PubMed] [Google Scholar]
- 29.Russell GJ, Nagler-Anderson C, Anderson P, Bhan AK: Cytotoxic potential of intraepithelial lymphocytes (IELs): presence of TIA-1, the cytolytic granule-associated protein, in human IELs in normal and diseased intestine. Am J Pathol 1993, 143:350-354 [PMC free article] [PubMed] [Google Scholar]
- 30.Asadullah K, Friderich M, Haeussler A, Sterry W, Docke WD, Volk HD: Granzyme A mRNA expression in mycosis fungoides progression. Blood 1997, 90:3810-3811 [PubMed] [Google Scholar]
- 31.Hoppe RT, Medeiros LJ, Warnke RA, Wood GS: CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol 1995, 32:448-453 [DOI] [PubMed] [Google Scholar]
- 32.Muller C, Tschopp J: Resistance of CTL to perforin-mediated lysis: evidence for a lymphocyte membrane protein interacting with perforin. J Immunol 1994, 153:2470-2478 [PubMed] [Google Scholar]
- 33.Sun JR, Bird CH, Sutton V, Mcdonald L, Coughlin PB, Dejong TA, Trapani JA, Bird PI: A Cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier a is present in cytotoxic lymphocytes. J Biol Chem 1996, 271:27802-27809 [DOI] [PubMed] [Google Scholar]
- 34.Fleischer B: Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature 1984, 308:365-367 [DOI] [PubMed] [Google Scholar]
- 35.Susskind B, Shornick MD, Iannotti MR, Duffy B, Mehrotra PT, Siegel JP, Mohanakumar T: Cytolytic effector mechanisms of human CD4+ cytotoxic T lymphocytes. Hum Immunol 1996, 45:64-75 [DOI] [PubMed] [Google Scholar]
- 36.Williams NS, Engelhard VH: Perforin-dependent cytotoxic activity and lymphokine secretion by CD4+ T cells are regulated by CD8+ T cells. J Immunol 1997, 159:2091-2099 [PubMed] [Google Scholar]
- 37.Kim YH, Bishop K, Varghese A, Hoppe RT: Prognostic factors in erythrodermic mycosis fungoides and the Sezary syndrome. Arch Dermatol 1995, 131:1003-1008 [PubMed] [Google Scholar]


