Abstract
It has been demonstrated that α-catenin is frequently lost in diffuse type adenocarcinomas. We have isolated α-catenin-deficient mouse teratocarcinoma F9 cells by gene targeting. Wild-type F9 cell aggregates cultured in the presence of retinoic acid differentiated into embryoid bodies with an outer layer of epithelial cells. In contrast, cell aggregates of α-catenin-deficient cells did not develop outer layers under the same conditions. The outer surface cells of α-catenin-deficient cell aggregates, however, differentiated into epithelial cells as determined by their expression of epithelial marker proteins. These differentiated cells scattered from aggregates and showed signet ring cell morphology, which is frequently observed in diffuse type adenocarcinomas. We have provided clear evidence that a single mutation in the α-catenin gene may be a direct cause not only of the scattered properties of cells but also of signet ring cell formation in diffuse type adenocarcinoma.
α-Catenin associates with the carboxy-terminal region of the cadherin cytoplasmic domain via β-catenin to form a functional cadherin-catenin cell adhesion complex. 1 It has been demonstrated that α-catenin is frequently lost in diffuse type adenocarcinomas. 2-5 Loss of α-catenin expression has also been observed in some human adenocarcinoma cell lines. 6-11 The epithelial morphology and rigid cell adhesion activity were lost in these α-catenin-deficient carcinoma cells but restored by the exogenous expression of α-catenin. 11-14 These observations strongly suggest that loss of α-catenin function is involved in the scattered phenotype of diffuse type adenocarcinoma cells. 15
Signet ring cell carcinomas have been described in most but not all series of diffuse type gastric carcinomas. 16 Frequent loss of α-catenin expression was observed in gastric carcinomas with scattered cell growth. 5 Loss of α-catenin expression was also observed in a signet ring cell gastric carcinoma cell line. 7, 17 However, the loss of α-catenin expression in colon cancer cell lines did not cause a morphological change of cells to signet ring cells. 10 Thus, it is not clear whether loss of α-catenin expression is involved in signet ring cell formation.
F9 is a clonal line of mouse teratocarcinoma-derived embryonal carcinoma cells that shows no epithelial cell morphology under standard culture conditions. However, F9 cultures treated with retinoic acid when they are in the form of small aggregates differentiate epithelial cells on the outer surface of aggregates. 18 Due to these properties, F9 cells are regarded as a good in vitro model system for the study of epithelium formation mechanisms. The present study was performed to explore the effects of loss of α-catenin expression on epithelial cell morphogenesis using α-catenin-deficient F9 cells.
Materials and Methods
Targeting and Expression Vectors
The 17-kb mouse α-catenin genomic clone (p16-2) was isolated from a 129/Sv mouse genomic library. For construction of the targeting vector, the PSIBP trap-selection cassette replaced the SmaI-XhoI fragment of the p16-2 clone, which contains the 5′ half of an exon encoding β catenin binding site. In the PSIBP trap-selection cassette, splicing acceptor, 19 IRES, 20 and β-geo 21 sequences are tandemly connected and inserted between two lox P sequences. 22 For negative selection, the DTA gene replaced the 4.5-kb fragment following unique BstPI site of the p16-2 clone. 23
For construction of the α-catenin expression vector (pEFαFL), the 3.7-kb full-length α-catenin cDNA 24 replaced the XbaI-XbaI stuffer sequence of pEF-MC1neo. 25
Gene Targeting
Mouse F9 EC cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal calf serum in gelatin-coated (0.2%) culture dishes. Cell aggregates, initially consisting of 2.5 × 10 3 cells per aggregate, were formed by the hanging drop culture method. 26 For induction of visceral endoderm differentiation, 5 × 10−8 M retinoic acid were added to the medium from the beginning of aggregate formation.
The targeting vector (25 μg) was linearized with SalI and electroporated at 250 V and 960 μF using a gene pulser into 2 × 10 7 F9 cells in 0.4 ml of HEPES-buffered saline. Cells were subjected to G418 selection at 370 μg/ml for 1 week and at 100 μg/ml for another 12 days. For isolation of F9Dα(−/−) cells, the heterozygous clone F9Sα(+/−) (clone 13) was re-electroporated with the targeting vector as described above and then subjected to G418 selection at 650 μg/ml for 4 days, 360 μg/ml for 5 days, and 150 μg/ml for 5 days. To obtain αF9Dα(−/−) cells, 20 μg of pEFαFL expression vector were electroporated into F9Dα(−/−) as described above, then subjected to G418 selection at 900 μg/ml for 14 days. Resistant clones were picked after selection. DNA was isolated from cultured cells as described 27 and tested for integration at the targeted locus by Southern blotting of HindIII digests. The probe was a 1-kb BstPI-HindIII fragment located immediately downstream of the region of homology of the targeting vector (see Figure 1 ▶ ).
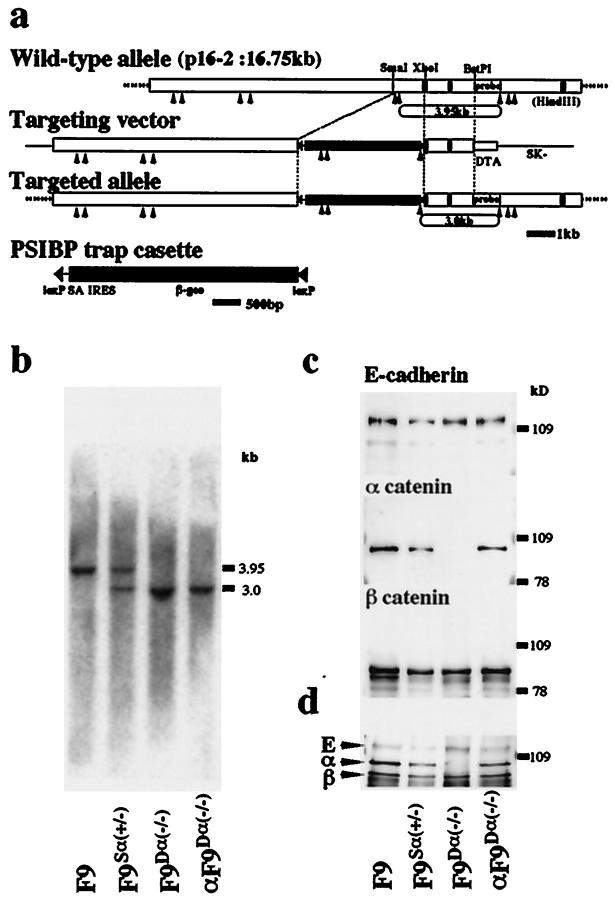
Figure 1.
Targeted inactivation of the α-catenin gene in mouse F9 cells. a: The structures of the wild-type allele, targeting vector, targeted allele, and the PSIBP trap-selection cassette are shown together with the pertinent restriction sites. Closed boxes represent the three α-catenin exons in clone p16-2. Insertion of the PSIBP trap cassette resulted in generation of a new HindIII fragment of 3.0 kb in contrast to the 3.9-kb wild-type allele. b: Southern blotting analysis of DNA derived from each cell clone. Genomic DNA was digested with HindIII and analyzed using the BstPI-HindIII fragment indicated in a as a probe. c: Western blotting analysis of each cell clone with anti-E-cadherin, anti-α-catenin and anti-β-catenin mAbs. d: Immunoprecipitation analysis of each cell clone with anti-E-cadherin mAb. E, E-cadherin; α, α-catenin; β, β-catenin.
Antibodies
The following primary antibodies were used: function-blocking rat anti-mouse E-cadherin mAb (ECCD-1), 28 rat anti-mouse E-cadherin mAb (ECCD-2), 29 rat anti-mouse α-catenin mAb (α18)30; rat anti-mouse occludin mAb (MOC37), 27 mouse anti-β-catenin mAb (Transduction Lab., Lexington, KY), and mouse anti-cytokeratin 18 mAb (Ks 18.04, Progen Biotechnik, Heidelberg, Germany). As the secondary antibody, FITC-conjugated goat anti-rat IgG (Tago, Inc., Burlingame, CA) and rhodamine-conjugated goat anti-mouse IgG (Amersham Pharmacia Biotech, Buckinghamshire, UK) were used. Immunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting analysis, and immunostaining were performed as described. 24
Cell Dissociation and Aggregation Assay
Cell dissociation and aggregation assays were performed as described previously. 30, 31 In some cell aggregation experiments, ECCD-1 was added to the culture medium.
Partial Purification of Signet Ring Cells and Histochemical Analysis
Day 10 embryoid body culture medium of F9Dα(−/−) cells was settled in a centrifuge tube to remove large cell aggregates and the supernatant was then moved to another tube and centrifuged at 1000 × g for 5 minutes. The cell pellet with signet ring cells was washed and resuspended in HEPES-buffered magnesium-free saline. This signet ring cell-rich fraction was smeared on glass slides, air-dried, and fixed with 10% formaldehyde. Slides were stained with hematoxylin and periodic acid-Schiff.
Ultrathin Section Electron Microscopy
Embryoid bodies and pellets of signet ring cells were prepared for ultrathin sectioning as described previously. 27 Ultrathin sections were cut with a diamond knife, double-stained with uranyl acetate and lead citrate, and then examined using a 1200EX electron microscope (JOEL, Tokyo, Japan) at an acceleration voltage of 100 kV.
Results
F9 cells targeted at one α-catenin allele (F9Sα(+/−)) were generated by replacing part of the α-catenin genomic DNA with the PSIBP trap-selection cassette in sense orientation (Figure 1a ▶ , see Methods). To isolate F9 cells homozygous for this replacement (F9Dα(−/−)), F9Sα(+/−) cells were retransfected with the α-catenin-targeting vector by electroporation, then cultured in the presence of increasing concentrations of G418. Heterozygous and homozygous genotypes were demonstrated by Southern blotting (Figure 1b) ▶ , and the loss of expression of α-catenin protein in F9Dα(−/−) cells was confirmed by immunoblotting with the anti-α-catenin monoclonal antibody (mAb) α18 (Figure 1c) ▶ . Immunoblotting with anti-E-cadherin (ECCD-2) and anti-β-catenin mAbs showed that the levels of expression of these molecules in F9Dα(−/−) cells were comparable to those in parental F9 or F9Sα(+/−) cells (Figure 1c) ▶ . Immunoprecipitation with anti-E-cadherin mAb showed that E-cadherin formed a complex with α- and β-catenin in wild-type F9 or F9Sα(+/−) cells (Figure 1d) ▶ . Even in F9Dα(−/−) cells, E-cadherin formed a complex with β-catenin despite the absence of α-catenin (Figure 1d) ▶ . Southern blotting analysis of DNA derived from F9Dα(−/−) cells using the neo fragment as probe showed that a single copy of the targeting vector was inserted in the α-catenin locus and random integration of the targeting vector did not occur (data not shown). To confirm that F9Dα(−/−) cells have no severe mutations other than the lack of α-catenin expression, we introduced an expression vector encoding full-length α-catenin into F9Dα(−/−) cells, then isolated cells re-expressing similar amounts of α-catenin to wild-type F9 cells, αF9Dα(−/−). The expressed α-catenin was derived from the introduced expression vector because the targeted locus in αF9Dα(−/−) cells retained the homozygous genotype (Figure 1b) ▶ . In these αF9Dα(−/−) cells, expressed α-catenin formed a complex with E-cadherin and β-catenin (Figure 1c) ▶ . Because these αF9Dα(−/−) cells were indistinguishable from the wild-type F9 cells in all assays performed here (see below), one of the clones, αF9Dα(−/−)-4-4, was used as a representative line of normal F9 cells throughout subsequent experiments.
In low density monolayer culture, αF9Dα(−/−) and F9Dα(−/−) cells showed different morphologies. αF9Dα(−/−) cells formed small and compact colonies (Figure 2a) ▶ similar to those of parental F9 cells. In contrast, F9Dα(−/−) cells showed a scattered morphology, suggesting dysfunction of their cadherin-catenin adhesion system (Figure 2f) ▶ . In fact, cell dissociation assay showed that αF9Dα(−/−) cells were hardly dissociated into single cells, while F9Dα(−/−) cells were readily dissociated (Figure 2, b and g) ▶ . Cell aggregation assay, however, showed that both αF9Dα(−/−) and F9Dα(−/−) cells formed cell aggregates of a similar size and in similar numbers (Figure 2, c and h) ▶ . After 1 day of aggregation, both types of cells formed spherical aggregates (data not shown). A function-blocking monoclonal anti-E-cadherin antibody (ECCD-1) inhibited this aggregate formation (Figure 2, d and i) ▶ . When cells were immunocytochemically stained for E-cadherin (Figure 2, e and j) ▶ or β-catenin (data not shown), the antigens were found to be concentrated in the boundaries between cells. These results demonstrated that the strong state of cell adhesion activity mediated by E-cadherin-catenin complex was dependent on α-catenin, whereas the weak state was not.
Figure 2.
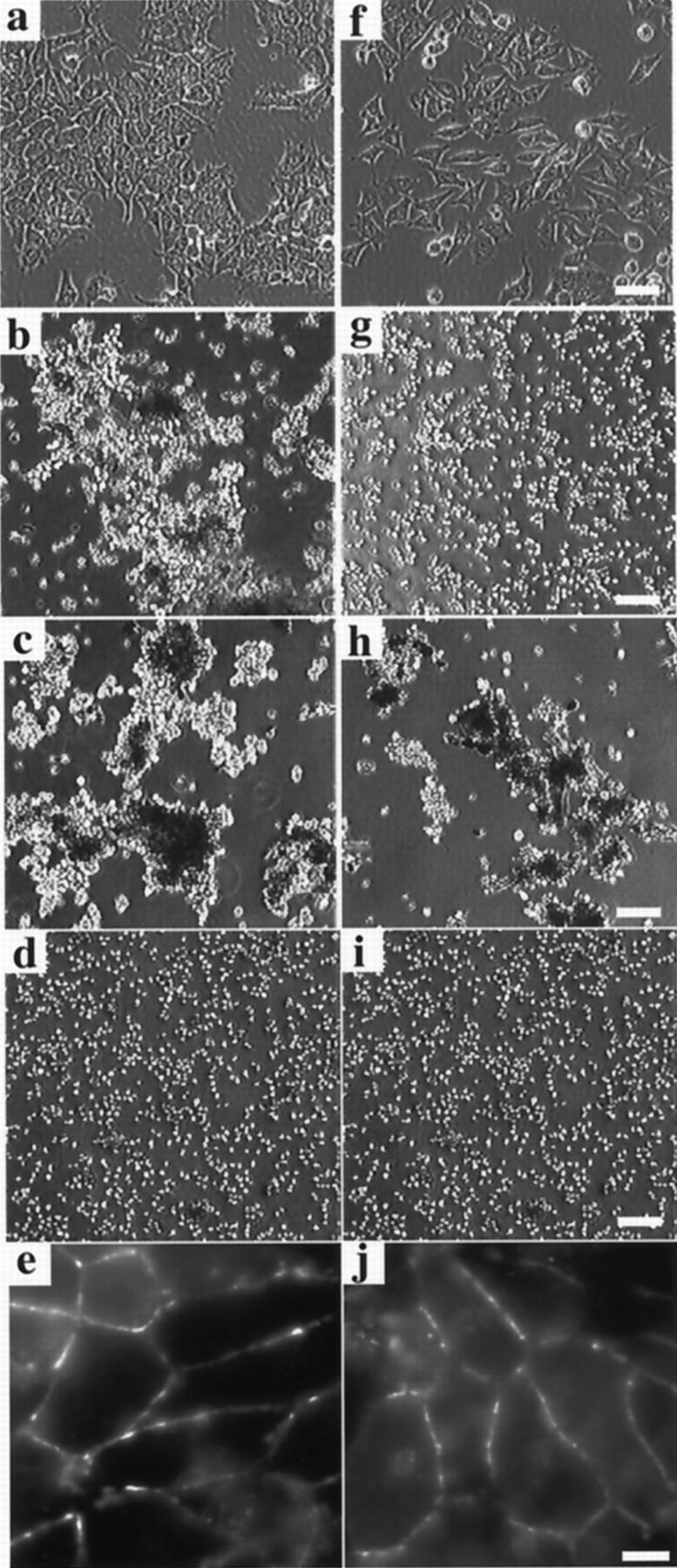
Cell adhesion properties of α-catenin-deficient cells. a–e: αF9Dα(−/−), α-catenin-positive control cells. f–j: F9Dα(−/−), α-catenin-deficient cells. a and f: Phase contrast images. Bar, 100 μm. b and g: Cell dissociation assay. Bar, 100 μm c, d, h, and i: Cell aggregation assay. Bar, 100 μm. In d and i, function-blocking anti-E-cadherin mAb, ECCD-1, was added to culture medium. e and j: Immunostaining with the anti-E-cadherin mAb ECCD-2. Bar, 25 μm.
Because cell-cell adhesion has been implicated in the differentiation of F9 cells into visceral endoderm with an epithelial structure, 32 we examined the differentiation of αF9Dα(−/−) and F9Dα(−/−) cell aggregates grown in the presence of retinoic acid. After culture in the presence of 5 × 10−8 mol/L retinoic acid for 5 days αF9Dα(−/−) cell aggregates were surrounded by an outer layer with a smooth surface (Figure 3 ▶ (top) a). In contrast, F9Dα(−/−) cell aggregates were irregularly surrounded by round-shaped cells and did not develop a clear outer layer (Figure 3 ▶ (top) e). Electron microscopic analysis showed that the outer surface cells of αF9Dα(−/−) cell aggregates consisted of polarized epithelial cells, characterized by numerous microvilli on the outer (apical) surface and well-developed junctional complex at cell-cell boundaries 18 (Figure 3 ▶ (top), b and c). The outer surface cells of F9Dα(−/−) cell aggregates, however, did not show such epithelial morphology (Figure 3 ▶ (top) f). These cells were spherical, had microvilli sparsely distributed over the entire cell surface, and were morphologically indistinguishable from the inner cell mass. No junctional complex was found at cell-cell boundaries of these cells (Figure 3 ▶ (top) g).
Figure 3.
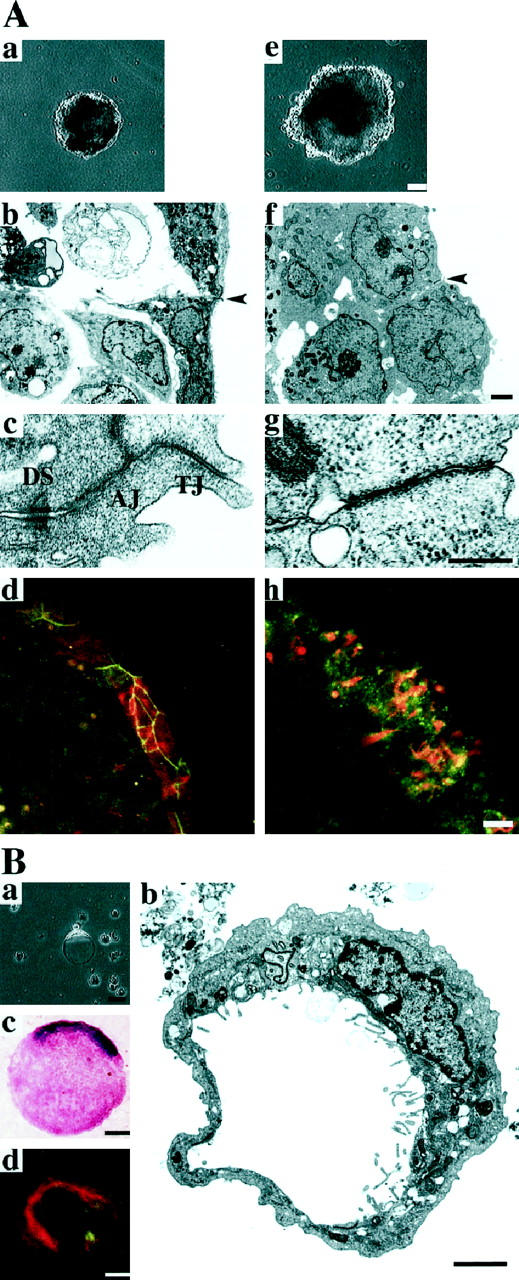
Top (A): Visceral endoderm differentiation in αF9Dα(−/−) and F9Dα(−/−) cell aggregates. a–d: αF9Dα(−/−) cell aggregates. e–h: F9Dα(−/−) cell aggregates after 5 days in culture in the presence of 5 × 10−8 M retinoic acid. a and e: Phase contrast images. Bar, 100 μm. b and f: Electron micrographs. Bar, 5 μm. c and g: Higher magnification of cell-cell contact sites indicated by arrowheads in b and f, respectively. Bar, 500 nm. d and h: Immunostaining with anti-occludin and anti-cytokeratin 18 mAbs. Expression of both occludin (green) and cytokeratin 18 (red) was detected in outer surface cells of both aggregates. Bar, 20 μm. Bottom (B): Signet ring cells derived from differentiated F9Dα(−/−) cell aggregates. a Phase contrast image. b Electron micrograph. c Periodic acid-Schiff and hematoxylin staining. d Immunostaining with anti-occludin and anti-cytokeratin 18 mAbs. Expression of both occludin (green) and cytokeratin 18 (red) was detected in signet ring cells. Bar, 20 μm.
We next used immunohistochemical analysis to examine expression of the epithelial marker proteins cytokeratin 18 and occludin. Cytokeratin 18 is one of the constituents of intermediate filaments and is expressed mainly in epithelial cells. 32 Occludin is a major membrane protein of tight junctions and is predominantly expressed in epithelial and endothelial cells. 33 The outer layer cells of αF9Dα(−/−) cell aggregates expressed both cytokeratin 18 and occludin (Figure 3 ▶ (top) d). The expressed occludin was clearly localized at cell-cell adhesion sites, probably at tight junctions, in a linear pattern. Cytokeratin 18 and occludin expression were also observed in the outer surface cells of F9Dα(−/−) cell aggregates (Figure 3 ▶ (top) h). Occludin, however, was localized in an irregular dot-like pattern. α-Fetoprotein, a visceral endoderm-specific product, 18 was also detected in both F9Dα(−/−) and αF9Dα(−/−) cell aggregates after 8 days of culture in the presence of retinoic acid (data not shown). These results indicated that, in the presence of retinoic acid, α-catenin-deficient cells did not show epithelial morphology but differentiated into visceral endoderm as determined by the expression of epithelial marker proteins.
On culture in the presence of retinoic acid, many cells were scattered from F9Dα(−/−) cell aggregates. Most of these scattered cells expressed epithelial marker proteins (data not shown, see Figure 3 ▶ (bottom) d). Some of the scattered cells showed a unique signet ring cellular configuration (Figure 3 ▶ (bottom) a). Electron microscopic examination demonstrated that these cells had an intracytoplasmic lumen pushing the nucleus to one side (Figure 3 ▶ (bottom) b). Numerous microvilli were often observed on the surface of this intracytoplasmic lumen. Signet ring cells have been reported to be present in mucin-producing carcinomas. Signet ring cells derived from F9Dα(−/−) cell aggregates showed positive staining with periodic acid-Schiff, a routine mucin staining method (Figure 3 ▶ (bottom) c), and, for cytokeratin 18 and occludin, epithelial marker proteins (Figure 3 ▶ (bottom) d). Signet ring cells were hardly produced from F9Dα(−/−) cell aggregates cultured in the absence of retinoic acid (data not shown). These observations indicated that the signet ring cells originated from F9Dα(−/−) cells differentiated into visceral endoderm.
Discussion
Loss of α-catenin expression is thought to be one of direct causes of the scattered phenotype of diffuse type adenocarcinoma cells. 15 Consistent with this hypothesis, α-catenin-deficient F9 cells lost their adhesiveness and scattered from cell aggregates when cultured under conditions suitable for epithelial differentiation. Because these scattered cells expressed epithelial marker proteins, they were differentiated into epithelial cells but failed to form epithelium. Undifferentiated F9 cells, however, showed the weak state of cadherin-dependent cell adhesion activity and formed spherical aggregates in suspension culture. These observations suggested that E-cadherin/β-catenin complex without α-catenin is not sufficient for the maintenance of cell-cell adhesion between F9 cells differentiated into epithelial cells, although it mediates the weak state of cell adhesion between undifferentiated F9 cells. α-Catenin plays critical roles in junctional complex formation between epithelial cells. 14 This junctional complex formation may be important for the maintenance of cell-cell adhesion between epithelial cells.
Morphological analysis showed that α-catenin-deficient F9 cells formed a signet ring cell configuration when they were differentiated into epithelial cells. Because these cells were positively stained with periodic acid-Schiff, they were thought to resemble a type of traditional signet ring cell carcinoma. Numerous microvilli were often observed on the intracytoplasmic luminal surface of these signet ring cells. This observation suggested the maintenance of epithelial cell polarity in these cells, even though they grew as single round cells. This is consistent with a previous observation obtained by electron microscopic analysis of signet ring adenocarcinoma cells in serous effusions. 16
Several human gastric carcinoma cell lines with signet ring cell properties, such as HSC-43 17 and HSC-39, 34 have been established. In these cell lines, abnormalities of the cadherin-catenin system were frequently observed. For example, the expression of α-catenin was not detected in HSC-43. 7 In HSC-39, β-catenin molecules lacking the α-catenin-binding domain were expressed and E-cadherin/β-catenin complex did not interact with α-catenin. 35 The present results also demonstrated that the exogenous expression of α-catenin in α-catenin-deficient F9 cells prevented signet ring cell formation. These observations strongly suggested that dysfunction of α-catenin in epithelial cells is a direct cause of signet ring cell formation. It remains unclear how α-catenin-deficient cells form signet ring cellular configurations. Further studies addressing this question may provide important information regarding the oncogenic mechanisms of signet ring cell formation and the molecular mechanisms of epithelial cell morphogenesis.
We have provided clear evidence that a single mutation in the α-catenin gene may be a direct cause not only of the scattered properties of cells but also of signet ring cell formation in diffuse type adenocarcinoma. Because signet ring cell formation was regulated by addition of retinoic acid in the α-catenin-deficient F9 cell system, these cells are a promising new in vitro model system for studying oncogenic mechanisms and for diagnosis of signet ring cell carcinoma.
Acknowledgments
We thank all the members of our laboratory (Department of Cell Biology, Faculty of Medicine, Kyoto University) for helpful discussions. We also thank Dr. M. Takeichi for his gifts of anti-E-cadherin mAbs ECCD-1 and ECCD-2 and Drs. S. Nagata and T. Nakano for their gift of the pEF-MC1neo vector.
Footnotes
Address reprint requests to Akira Nagafuchi, Ph.D., Department of Cell Biology, Faculty of Medicine, Kyoto University, Sakyo-ku, Kyoto 606, Japan. E-mail: naga-san@mfour.med.kyoto-u.ac.jp.
Supported by a Grant-in-Aid for Cancer Research and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, and by Special Coordination Funds for Promoting Science and Technology from the Science and Technology Agency of Japan.
References
- 1.Nagafuchi A, Tsukita S, Takeichi M: Transmembrane control of cadherin-mediated cell-cell adhesion. Semin Cell Biol 1993, 4:175-181 [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T, Shiozaki H, Inoue M, Tamura S, Oka H, Doki Y, Iihara K, Matsui S, Iwazawa T, Nagafuchi A, Tsukita S, Mori T: E-cadherin and α-catenin expression in human esophageal cancer. Cancer Res 1994, 54:291-296 [PubMed] [Google Scholar]
- 3.Nakanishi Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S: Expression of E-cadherin, α-catenin, β-catenin and plakoglobin in esophageal carcinomas and its prognostic significance: immunohistochemical analysis of 96 lesions. Oncology 1997, 54:158-165 [DOI] [PubMed] [Google Scholar]
- 4.Shiozaki H, Iihara K, Oka H, Kadowaki T, Matsui S, Gofuku J, Inoue M, Nagafuchi A, Tsukita S, Mori T: Immunohistochemical detection of α-catenin expression in human cancers. Am J Pathol 1994, 144:667-674 [PMC free article] [PubMed] [Google Scholar]
- 5.Ochiai A, Akimoto S, Shimoyama Y, Nagafuchi A, Tsukita S, Hirohashi S: Frequent loss of α catenin expression in scirrhous carcinomas with scattered cell growth. Jpn J Cancer Res 1994, 85:266-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimoyama Y, Nagafuchi A, Fujita S, Gotoh M, Takeichi M, Tsukita S, Hirohashi S: Cadherin dysfunction in a human cancer cell line: possible involvement of loss of α-catenin expression in reduced cell-cell adhesiveness. Cancer Res 1992, 52:5770-5774 [PubMed] [Google Scholar]
- 7.Yasui W, Kuniyasu H, Akama Y, Kitahara K, Nagafuchi A, Ishihara S, Tsukita S, Tahara E: Expression of E-cadherin, α- and β-catenins in human gastric carcinomas: correlation with histology and tumor progression. Oncology Reports 1995, 2:111-117 [PubMed] [Google Scholar]
- 8.Oda T, Kanai Y, Shimoyama Y, Nagafuchi A, Tsukita S, Hirohashi S: Cloning of the human α-catenin cDNA and its aberrant mRNA in a human cancer cell line. Biochem Biophys Res Commun 1993, 193:897-904 [DOI] [PubMed] [Google Scholar]
- 9.Morton RA, Ewing CM, Nagafuchi A, Tsukita S, Isaacs WB: Reduction of E-cadherin levels and deletion of the α-catenin gene in human prostate cancer cells. Cancer Res 1993, 53:3585-3590 [PubMed] [Google Scholar]
- 10.Vermeulen SJ, Bruyneel EA, Bracke ME, De Bruyne GK, Vennekens KM, Vleminckx KL, Berx GJ, van Roy FM, Mareel MM: Transition from the noninvasive to the invasive phenotype and loss of α-catenin in human colon cancer cells. Cancer Res 1995, 55:4722-4728 [PubMed] [Google Scholar]
- 11.Bullions LC, Notterman DA, Chung LS, Levine AJ: Expression of wild-type α-catenin protein in cells with a mutant α-catenin gene restores both growth regulation and tumor suppressor activities. Mol Cell Biol 1997, 17:4501-4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Hengel J, Gohon L, Bruyneel E, Vermeulen S, Cornelissen M, Mareel M, van Roy F: Protein kinase C activation upregulates intercellular adhesion of α-catenin-negative human colon cancer cell variants via induction of desmosomes. J Cell Biol 1997, 137:1103-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M: Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell 1992, 70:293-301 [DOI] [PubMed] [Google Scholar]
- 14.Watabe M, Nagafuchi A, Tsukita S, Takeichi M: Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol 1994, 127:247-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirohashi S: Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 1998, 153:333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duane GB, Kanter MH: Light and electron microscopic characteristics of signet-ring adenocarcinoma cells in serous effusions and their distinction from mesothelial cells. Acta Cytol 1985, 29:211-218 [PubMed] [Google Scholar]
- 17.Yanagihara K, Kamada N, Tsumuraya M, Amano F: Establishment and characterization of a human gastric scirrhous carcinoma cell line in serum-free chemically defined medium. Int J Cancer 1993, 54:200-207 [DOI] [PubMed] [Google Scholar]
- 18.Hogan BL, Taylor A, Adamson E: Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature 1981, 291:235-237 [DOI] [PubMed] [Google Scholar]
- 19.Robberson BL, Cote GJ, Berget SM: Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol 1990, 10:84-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghattas IR, Sanes JR, Majors JE: The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol 1991, 11:5848-5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrich G, Soriano P: Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 1991, 5:1513-1523 [DOI] [PubMed] [Google Scholar]
- 22.Sternberg N, Hamilton D, Hoess R: Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J Mol Biol 1981, 150:487-507 [DOI] [PubMed] [Google Scholar]
- 23.Yagi T, Ikawa Y, Yoshida K, Shigetani Y, Takeda N, Mabuchi I, Yamamoto T, Aizawa S: Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin A-fragment gene in negative selection. Proc Natl Acad Sci USA 1990, 87:9918-9922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagafuchi A, Takeichi M, Tsukita S: The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell 1991, 65:849-857 [DOI] [PubMed] [Google Scholar]
- 25.Visvader JE, Elefanty AG, Strasser A, Adams JM: GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J 1992, 11:4557-4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyamada Y, Komatsu K, Kimura H, Mori M, Oyamada M: Differential regulation of gap junction protein (connexin) genes during cardiomyocytic differentiation of mouse embryonic stem cells in vitro. Exp Cell Res 1996, 229:318-326 [DOI] [PubMed] [Google Scholar]
- 27.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S: Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 1998, 141:397-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida-Noro C, Suzuki N, Takeichi M: Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol 1984, 101:19-27 [DOI] [PubMed] [Google Scholar]
- 29.Shirayoshi Y, Nose A, Iwasaki K, Takeichi M: N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct Funct 1986, 11:245-252 [DOI] [PubMed] [Google Scholar]
- 30.Nagafuchi A, Ishihara S, Tsukita S: The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-α catenin fusion molecules. J Cell Biol 1994, 127:235-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M: Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 1987, 329:341-343 [DOI] [PubMed] [Google Scholar]
- 32.Grover A, Rosentraus MJ, Sterman B, Snook ME, Adamson ED: An adhesion-defective variant of F9 embryonal carcinoma cells fails to differentiate into visceral endoderm. Dev Biol 1987, 120:1-11 [DOI] [PubMed] [Google Scholar]
- 33.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S: Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993, 123:1777-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanagihara K, Seyama T, Tsumuraya M, Kamada N, Yokoro K: Establishment and characterization of human signet ring cell gastric carcinoma cell lines with amplification of the c-myc oncogene. Cancer Res 1991, 51:381-386 [PubMed] [Google Scholar]
- 35.Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, Nagafuchi A, Tsukita S, Shibamoto S, Ito F, Takeichi M, Matsuda H, Hirohashi S: A truncated β-catenin disrupts the interaction between E-cadherin and α-catenin: a cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res 1994, 54:6282-6287 [PubMed] [Google Scholar]