Abstract
Monocyte chemotactic protein (MCP)-1 is postulated to play a role in cellular recruitment during inflammatory reactions. C-C chemokine receptor 2 (CCR2) is considered the major G-protein coupled receptor for MCP-1/JE. We reported that mice with knockout of the CCR2 gene display partially impaired type-1 granuloma formation. The present study similarly examined the effect of CCR2 deficiency on synchronously developing type-2 (Th2) cytokine-mediated lung granulomas elicited by embolization of beads coated with Ags of Schistosoma mansoni eggs. Systemically, blood monocytes were reduced by about half throughout the 8-day study period. At the local level, granuloma size and macrophage content were impaired during the early growth phase (days 1 to 2). By day 4, granuloma sizes were similar to controls. In granulomatous lungs, CCR2 knockout increased mRNA for CCR2 agonists, MCP-1, MCP-3, and MCP-5, but reduced IL-4 and IFNγ mRNA. The latter was possibly related to decreased CD4+ T cell recruitment. Regionally, draining lymph nodes showed panlymphoid hyperplasia with impaired production of IFNγ, IL-2, and IL-4, but not IL-5, IL-10, or IL-13. Analysis of procollagen gene expression indicated transient impairment of procollagen III transcripts on day 4 of granuloma formation. These findings indicate that agonists of CCR2 contribute to multiple facets of type-2 hypersensitivity granulomatous inflammation.
Due to their potent leukocyte chemotactic activity, the class of 8- to 10-kd protein molecules known as chemokines are postulated to be important mediators of inflammation. 1,2 In recent years they have been extensively investigated and a host of these molecules are known. Based upon positional conservation of cysteine residues, two major classes are recognized, the C-X-C and C-C chemokines. Recently, a group of G-protein-coupled transmembrane receptors for these molecules has been described that shows both selective and promiscuous binding of particular chemokines. Among the C-C group of chemokines, monocyte chemoattractant protein (MCP)-1 appears to bind selectively to C-C chemokine receptor 2 (CCR2). 3 However, other C-C chemokines are known to bind this receptor.
As a potent chemoattractant for mononuclear phagocytes, MCP-1 may potentially recruit macrophages during chronic inflammation and indeed a number of reports seem to support this notion. 4-10 We have previously shown that T-cell-mediated, hypersensitivity-type granuloma formation can be classified on the basis of cytokine participation into type-1/Th1 dominant, and type-2/Th2 dominant. 11 More recently, we examined type-1 mycobacterial Ag-elicited granuloma formation in mice with targeted knockout of the CCR2 receptor and showed a transient defect in local macrophage recruitment as well as a defect in regional IFNγ production. 10 That study indicated that CCR2 was involved at least temporally in local macrophage recruitment and regional T cell maturational events during a Th1 response. In a previous study using anti-MCP-1 antibodies, we showed that MCP-1 also appears to participate in Th2 (type-2) cytokine-mediated granulomas at both local and regional levels. 7 Subsequent studies using MCP-1 knockout mice showed similar results using Schistosoma mansoni egg challenge. 9 In the present study, we performed a detailed analysis of local, regional, and systemic parameters during synchronized type-2 granuloma formation in mice targeted for knockout of the CCR2 receptor. The results indicate a broad role for CCR2 agonists in hypersensitivity-type granuloma formation, ranging from chemotaxis to regulation of matrix synthesis.
Methods
Animals
CCR2 knockout mice were generated from 129 strain embryonic stem cells using targeting vectors as previously described. 10 Control animals consisted of age-matched nonmutant 129 X B6 F1 mice. Mice were maintained in isolator cages under specific pathogen-free conditions and provided with food and water ad libitum.
Sensitization and Granuloma Induction
Pulmonary granulomas with predominantly type-2 cytokine involvement were generated as described. 11 Briefly, mice were sensitized by i.p. injection of 3000 S. mansoni eggs suspended in 0.5 ml phosphate-buffered saline (PBS). Fourteen to 16 days later, sensitized mice were challenged by i.v. with 6000 Sepharose 4B beads (in 0.5 ml PBS) covalently coupled with soluble schistosome egg antigens (SEA) obtained from the World Health Organization (Geneva, Switzerland).
Granuloma Dispersal and Draining Lymph Node Culture
Groups of mice were killed at days 1, 2, 4, and 8 of granuloma formation. Following perfusion with cold RPMI, lungs (excluding trachea and major bronchi) were excised. The right upper lung of each mouse was snap-frozen in liquid N2 for mRNA isolation. The left lower lobe was post-inflated and formalin-fixed. The remaining lung lobes were placed in cold RPMI medium; granulomas were then isolated and dispersed as previously described. 7,11 For differential counting, duplicate cytospin preparations were prepared from the remaining dispersed granuloma cells and stained with Wright’s stain. Blood was also collected from each animal and total white cell count and differential analysis performed.
Mediastinal lymph nodes were collected at the time of lung harvest and teased into single cell suspension. After washing, the cells were cultured in RPMI-1640 medium (JRH Biosciences, Lenexa, KS) containing 10% FBS (Intergen, Purchase, NY), 10 mmol/L glutamine, and 100 mg/ml streptomycin and 100 U/ml penicillin (RPMI-FBS) at 5 × 106/ml in the presence or absence of 5 μg/ml SEA, then cultured as above for 24 hours. Supernates were collected by centrifugation and stored at −45°C.
Granuloma Measurement
Granulomas were measured blindly from formalin-inflated lungs that were paraffin-embedded, sectioned, and stained with hematoxylin and eosin. Granuloma area was measured by computerized morphometry. A minimum of 20 lesions was measured per lung.
Cell Identification by Flow Cytometry
Dispersed granulomas and mediastinal lymph node suspensions were subjected to flow cytometry using two- and three-color fluorescent analysis. Anti-CD3, -CD4, -CD8, -CD14, -CD19, and Mac-3 fluorescent-labeled antibodies were obtained commercially (Pharmingen, San Diego, CA). Samples were stained and analyzed immediately using a FACSCAN (Becton-Dickinson, Franklin Lakes, NJ) and WinList analysis program as described previously. 12
RNA Extraction
Using a modified method of Chirgwin et al 13 and Jonas et al, 14 total cellular RNA was extracted from perfused lung lobes excluding major bronchi that had been snap-frozen with liquid N2. The frozen tissues were suspended in extraction buffer (25 mmol/L Tris, pH 8.0, 4.2 mol/L guanidine isothiocyanate, 0.5% Sarkosyl, and 0.1 mol/L 2-mercaptoethanol), homogenized, then added to an equal volume of extraction buffer (100 mmol/L Tris, pH 8.0, 10 mmol/L EDTA and 1% sodium dodecyl sulfate (SDS). The mixture was then serially extracted with chloroform-phenol and chloroform-isoamyl alcohol. The RNA is next precipitated at −70°C in ethyl alcohol, washed, and reprecipitated. The pellet was finally dissolved in DEPC water and RNA concentrations determined spectrophotometrically prior to storage at −70°C.
Primers and Probes
Primers and probes (18-22 mer) were designed based upon nucleotide sequences downloaded from the NCBI data bank and using primer design software (Premier Biosoft International, Palo Alto, CA). Designed primer and probe sequences for each of the target RNA species examined are shown in Table 1 ▶ . All primers and probes were prepared by Genosys Biotechnologies Inc. (The Woodlands, TX). Probes were biotinylated with biotin-UTP using a standard 3′-end labeling kit (Boehringer Mannheim, Indianapolis, IN), unincorporated biotin was removed with QuickSpin columns (Boehringer Mannheim). Incorporation was confirmed by nitrocellulose blotting followed by streptavidin-alkaline phosphatase detection.
Table 1.
Primer and Probe Sequences Used for PCR Amplification and Detection of Cytokine Transcripts
| Mouse gene | Sequence (5′ to 3′) | Product size | Product span | Probe span | |
|---|---|---|---|---|---|
| IFNγ | sense | AGTGGCATAGATGTGGAAGAAA | 247 | 228–474 | 267–288 |
| antisense | GACCTCAAACTTGGCAATACTC | ||||
| probe | ATCTGGAGGAACTGGCAAAAGG | ||||
| IL-4 | sense | CTGACGGCACAGAGCTATTGA | 252 | 68–319 | 181–199 |
| antisense | TATGCGAAGCACCTTGGAAGC | ||||
| probe | GAGATCATCGGCATTTTGA | ||||
| MCP-1/JE* | sense | TTAACGCCCCACTCACCTGCTG | 107 | 1377–1483 | 1417–1438 |
| antisense | GCTTCTTTGGGACACCTGCTGC | ||||
| probe | GATGATCCCAATGAGTAGGCTG | ||||
| MCP-3 | sense | GTGCCTGAACAGAAACCAACCT | 148 | 353–500 | 393–415 |
| antisense | CATTCCTTAGGCGTGACCATT | ||||
| probe | TTCCTCACCGCTGTTCTTTCTG | ||||
| MCP-5 | sense | TTGGCTGGACCAGATGCG | 118 | 116–233 | 204–226 |
| antisense | GGGACACTGGCTGCTTGTGA | ||||
| probe | ACAGGAGAATCACAAGCAGCCA | ||||
| CCR2 | sense | TCATCCACGGCATACTATCAA | 343 | 120–462 | 262–282 |
| antisense | TATTCCCAAAGACCCACTCAT | ||||
| probe | CCTGCCTCCACTCTACTCCCT | ||||
| Procollagen I | sense | TCGTGACCGTGACCTTGCG | 255 | 255–509 | 327–348 |
| antisense | GAGGCACAGACGGCTGAGTAGG | ||||
| probe | CGANAGCAGCCGCAAGAACCCT | ||||
| Procollagen III | sense | GGCTGATGTACACATGCTCC | 259 | 192–450 | 275–296 |
| antisense | GCTCAGAGTAGCACCATCAG | ||||
| probe | TTGCTTTTACTGCTGAGGGGAT | ||||
| Cyclophilin | sense | ACCTAAAGTCACAGTCAACG | 327 | 133–459 | 296–316 |
| antisense | TGGTGTCTTTGCCTGCATTG | ||||
| probe | CATCGTGTCATCAAGGACTTC |
*Based on MCP-1/JE exon 1371–1488.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) ELISA Detection of Cytokine mRNA
The isolated RNA was first reverse transcribed (RT) to DNA as follows. To 25 μg of RNA (in 25 μl of DEPC water) were added 3.6 μl of RNAsin (Boehringer Mannheim) and 10 μl of random hexamer solution (500 mg/ml, Promega, Madison, WI) followed by heating to 70°C for 5 minutes in a thermocycler (9600, Perkin-Elmer Corp., Norwalk, CT). The temperature was then reduced to 43°C and 69 μl of a first-strand buffer (Gibco BRL, Grand Island, NY) containing dTT, dNTPs, and 1000 U murine Moloney leukemia virus reverse transcriptase was added. 15 The mixture was incubated for 2 hours, then heated to 95°C to stop the reaction. The DNA was then subjected to PCR. 16 Briefly, 5 μl of DNA were added to 95 μl PCR buffer containing unlabeled dNTPs (0.2 mmol/L of each) plus digoxigenin-labeled dUTP, 1 μg sense primer, 1 μg antisense primer, and 5 U Taq polymerase (all from Boehringer Mannheim) in a thin-walled PCR tube. Amplification was performed in a thermocycler as follows: 4 minutes at 95°C, followed by up to 23 cycles of 1 minute at 95°C, 2 minutes at 57°C, and 1 minute at 72°C. After cycling there was a DNA extension period of 6 minutes at 72°C. Samples were stored at −20°C prior to analysis.
Detection of PCR products was performed as follows. Initially a series of amplification reactions using unlabeled dNTPs was performed and the products analyzed by standard agarose gel electrophoresis to confirm that primers yielded predicted products. Once confirmed, la- beled products were generated and detected by PCR-ELISA. 17 Briefly, 10–60 μl of amplified product were added to a sterile microfuge tube containing 40 μl of denaturing buffer (Boehringer Mannheim) and incubated for 10 minutes. Next, 500 μl of hybridization buffer containing 4 ng/ml of appropriate (target gene) biotinylated probe was added. Negative controls included tubes with no DNA or DNA with inappropriate probe. The solution was mixed and 200-μl portions were distributed into duplicate wells of a multiwell, streptavidin-coated plate and incubated for 3 hours at 42°C. The plate was then washed and any bound product detected with peroxidase labeled anti-digoxigenin Ab by standard colorimetric reaction using 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate. A 96-well plate ELISA reader was used to measure O.D. at 405 nm. The O.D. was directly proportional to levels of target PCR product, which was normalized to levels of a housekeeping gene, cyclophilin.
Cytokine Measurement
Interleukins 2, 4, 5, 10, and 13 and interferon-γ were measured by ELISA using commercially available reagents (Pharmingen; R&D Systems, Minneapolis, MN); sensitivities ranged from 10 to 50 pg/ml. Commercially available recombinant murine cytokines served as standards in all assays (Genzyme, Cambridge, MA; Preprotech Inc., Rocky Hill, NJ; R&D Systems).
Statistics
Student’s t-test (two-tailed) was used to compare control with experimental groups. Values of P > 0.05 were considered to indicate lack of significance.
Results
CCR2−/− Mice Displayed Impaired Monocytosis and Defective Early Phase Monocyte Recruitment to Type-2 Granulomas
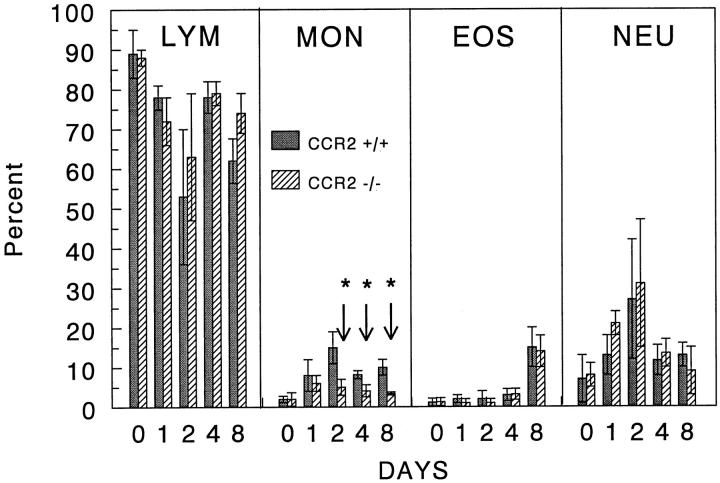
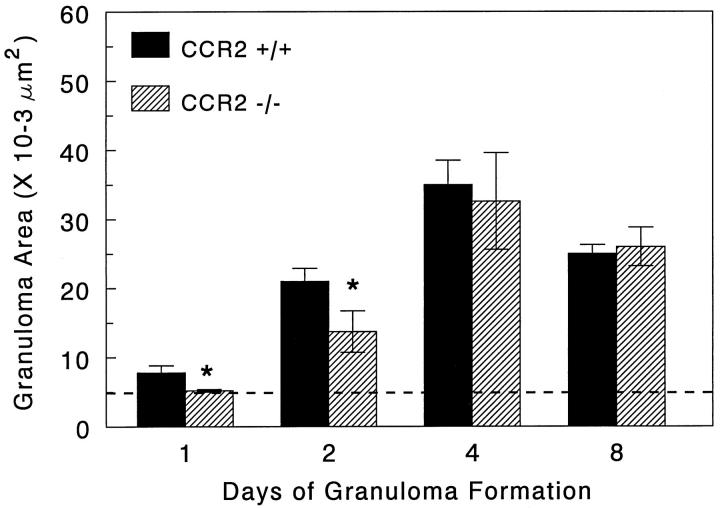
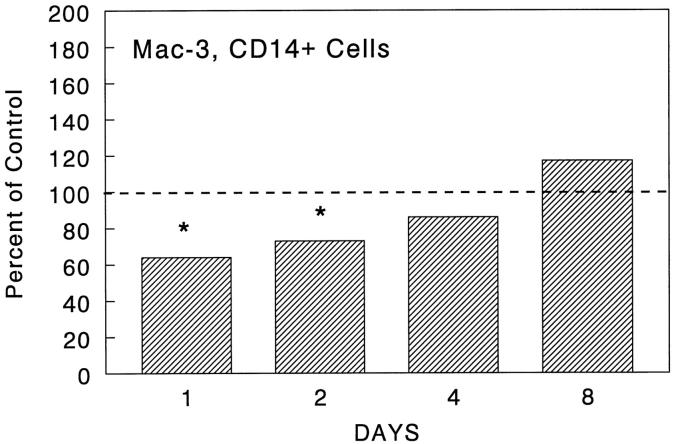
In order to determine the effect of CCR2 knockout on leukocyte mobilization, we assessed blood leukocyte differentials and granuloma size and composition during type-2 granuloma formation. As shown in Figure 1 ▶ , the day 0 baseline levels of blood leukocyte populations were comparable among groups. Thereafter, granuloma induction caused peripheral increases in monocytes, eosinophils, and neutrophils in control mice. Of these populations, the percentage of circulating monocytes in knockout mice was reduced by 50–70% during the 8-day study period. This decrease was absolute and relatively specific to monocytes; total leukocyte counts were comparable or lower in knockout mice (data not shown). Despite the persistent decrease in circulating monocytes, granuloma sizes were only transiently impaired on days 1 and 2 with the mean area being reduced to near that of the bead diameter on day 1 and reaching only 30% of controls on day 2 (Figures 2 and 3) ▶ ▶ . Analysis of lesion composition revealed that this impairment was due to a 50% reduction in granuloma macrophages (Figure 4) ▶ . This effect was selective in that there was no reduction in lymphocytes, eosinophils, or neutrophils. By day 4, both granuloma size and composition had returned to control levels. Although not totally specific for macrophages, CD14 and Mac-3 markers were evaluated by flow cytometry. Cells coexpressing these markers were likewise reduced during the early recruitment stage (Figure 5) ▶ . Thus, CCR2 deficiency was associated with re-duced circulating monocytes and transient monocyte/macrophage recruitment defect in the early phase of granuloma formation.
Figure 1.
Peripheral blood leukocyte differentials in wild type and CCR2 knockout mice during type-2 lung granuloma formation. Bars are mean percentages ± SE derived from 4 to 5 individual mice per group. Asterisks indicate statistically significant changes, P < 0.05.
Figure 2.
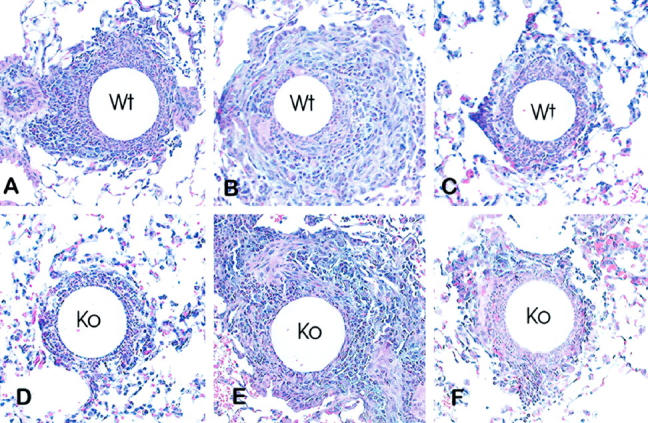
Histologic appearance of type-2 lung granulomas in wild type and CCR2 knockout mice. A, B, and C: Lesions of wild type mice on days 2, 4 and 8 respectively. D, E, and F: Lesions of knockout mice on days 2, 4, and 8, respectively. Day 2 knockout lesions contained fewer mononuclear cells. Hematoxylin and eosin stain. Magnification, ×200.
Figure 3.
Effect of CCR2 knockout on type-2 lung granuloma sizes. Bars are means ± SE derived from 4 to 6 mice per group. A minimum of 20 lesions was measured from each lung. Dashed line indicates size of bead nidus. Asterisks indicate statistically significant changes, P < 0.05.
Figure 4.
Leukocyte composition of granulomas in wild-type and CCR2 knockout mice during type-2 lung granuloma formation. Bars are mean percentages ± SE derived from 4 to 5 individual mice per group. Asterisks indicate statistically significant changes, P < 0.05.
Figure 5.
Effect of CCR2 knockout on recruitment of Mac-3/CD14+ cells during type-2 lung granuloma formation. Dispersed granuloma cells were stained with fluorescent-labeled antibodies to known mouse macrophage markers, Mac-3 and CD14, then subjected to flow cytometry. Bars show levels of expression in knockout preparations as compared to the wild-type control, normalized as 100 percent. Values were derived from two experiments with a total of 4 mice per group. Asterisks indicate statistically significant changes, P < 0.05.
CCR2−/− Mice Showed Enhanced Chemokine mRNA Expression in Granulomatous Lungs
In order to explore potential compensatory changes in knockout mice, we assessed granulomatous lungs for mRNA specific for MCP-1/JE and other CCR2 chemokine agonists, MCP-3 and MCP-5. Message levels were determined on days 2, 4, and 8 of granuloma formation using semiquantitative RT-PCR ELISA assay. As shown in Figure 6 ▶ , wild-type transcript expression for MCP-1/JE and MCP-3 was generally 1.5- to 2-fold above granuloma-free lungs. Message for MCP-5 was less pronounced, ranging from 30–40% above noninflamed lungs. In all cases, CCR2 knockout caused profound augmentations. This was especially notable for MCP-1/JE and MCP-3, which ranged from 3- to 7-fold above levels in unchallenged lungs on days 4 and 8. Increases were seen on all days for MCP-5 transcripts. These findings suggested that CCR2 participates in a feedback inhibition of chemokine synthesis.
Figure 6.

Expression of MCP-1/JE, MCP-3, and MCP-5 mRNA in lungs with type-2 granuloma formation. Bars show the mean ratios of housekeeping gene normalized signal of granulomatous to unchallenged lungs, which were valued at 1. Bars are means ± SE derived from two separate experiments assayed in duplicate. In each experiment, equal amounts of mRNA were pooled from 3 to 4 mice, then subjected to PCR-ELISA for the indicated cytokines. Asterisks indicate P < 0.05.
CCR2−/− Mice Showed Altered Cytokine mRNA Expression in Granulomatous Lungs
In order to determine if CCR2 knockout influenced the local expression of cytokines, we compared levels of IFNγ and IL-4 transcripts in lungs with type-2 granulomas. Figure 7 ▶ shows the expression of these transcripts and those for CCR2. As expected, knockout mice showed no expression of CCR2 mRNA. Interestingly, among the control groups there was increasing CCR2 transcript expression during the response, especially on days 4 and 8. With regard to IFNγ mRNA, wild-type mice showed about two-fold increases on days 4 and 8. Consistent with the type-2 response, wild-type mice also showed strong IL-4 expression over the study period, ranging from two- to three-fold above granuloma-free control mice. Knockout mice displayed definite alterations in these patterns. On day 2, IFNγ message was enhanced in knockout mice, possibly reflecting the reduced component of macrophages. The latter may contribute RNA species that dilute IFNγ mRNA or produce factors that inhibit IFNγ synthesis by lymphocytes. By day 4, IFNγ transcripts declined by 40%, but recovered to control levels by day 8. In contrast, CCR2 deficiency showed greater effect on IL-4 message. Initially on day 2, IL-4 mRNA was comparable, but then declined by about 40–50% among knockout mice on days 4 and 8. Thus, CCR2 and presumably its ligands regulated levels of local cytokine production or numbers of cytokine-producing cells.
Figure 7.
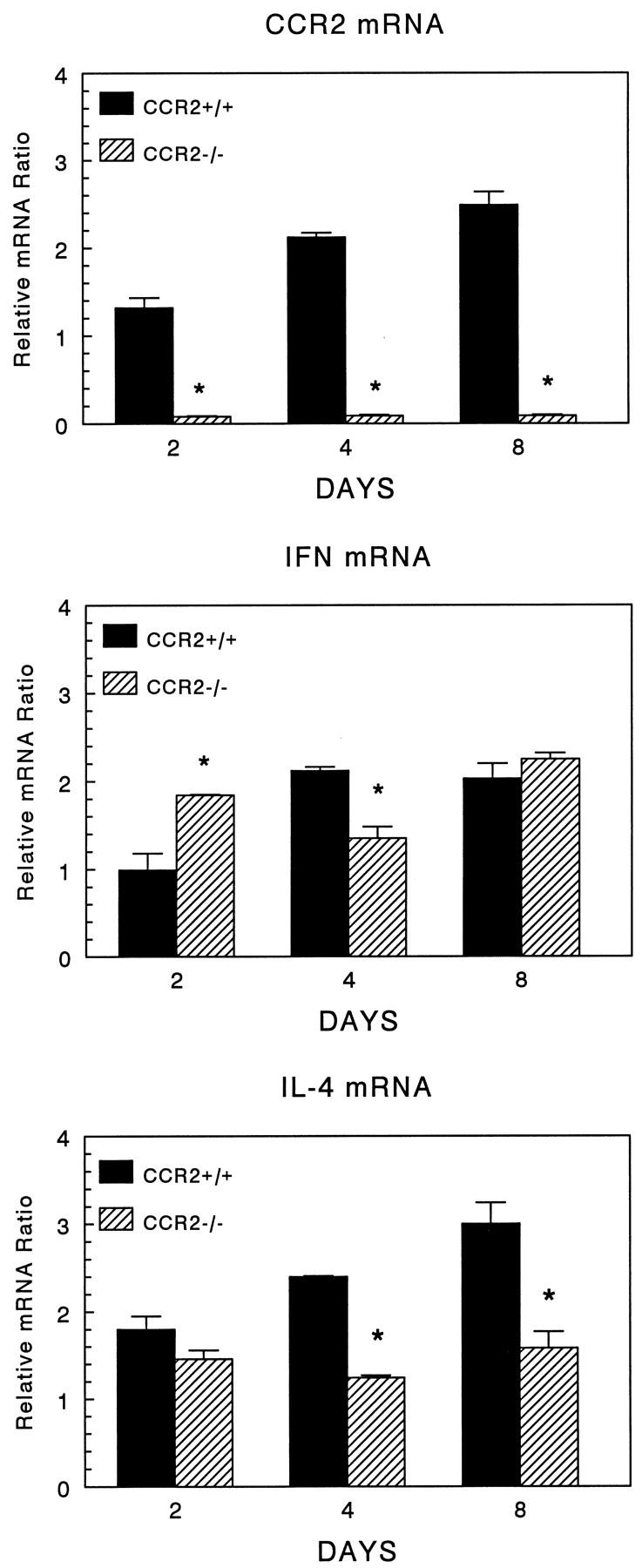
Expression of CCR2, IFNγ, and IL-4 mRNA in lungs with type-2 granuloma formation. Bars show the mean ratios of housekeeping gene normalized signal of granulomatous to unchallenged lungs, which were valued at 1. Bars are means ± SE derived from two separate experiments assayed in duplicate. In each experiment, equal amounts of mRNA were pooled from 3 to 4 mice, then subjected to PCR-ELISA for the indicated cytokines. Asterisks indicate P < 0.05.
Type-2 Granulomas Showed Altered T Cell Subpopulations in CCR2−/− Mice
In order to detect changes in lymphocyte recruitment, flow cytometric analysis of granuloma lymphocyte populations was performed. Figure 8 ▶ summarizes the gated analysis of granuloma lymphocytes. A significant component of CD19+ B cells appeared in granulomas, ranging from 20–30% of lymphocytes (upper panel). This population was comparable between control and knockout mice over the study period. The bulk of the remaining lymphocytes were CD3+ T cells. A subpopulation analysis of CD3+CD4+ and CD3+CD8+ cells showed a small but significant reduction in the percentage of CD4+ on day 4 with a corresponding increase in CD8+ cells (middle and lower panels). It is unclear if this was due to impaired CD4+ T cell expansion or increased CD8+ T cell recruitment.
Figure 8.
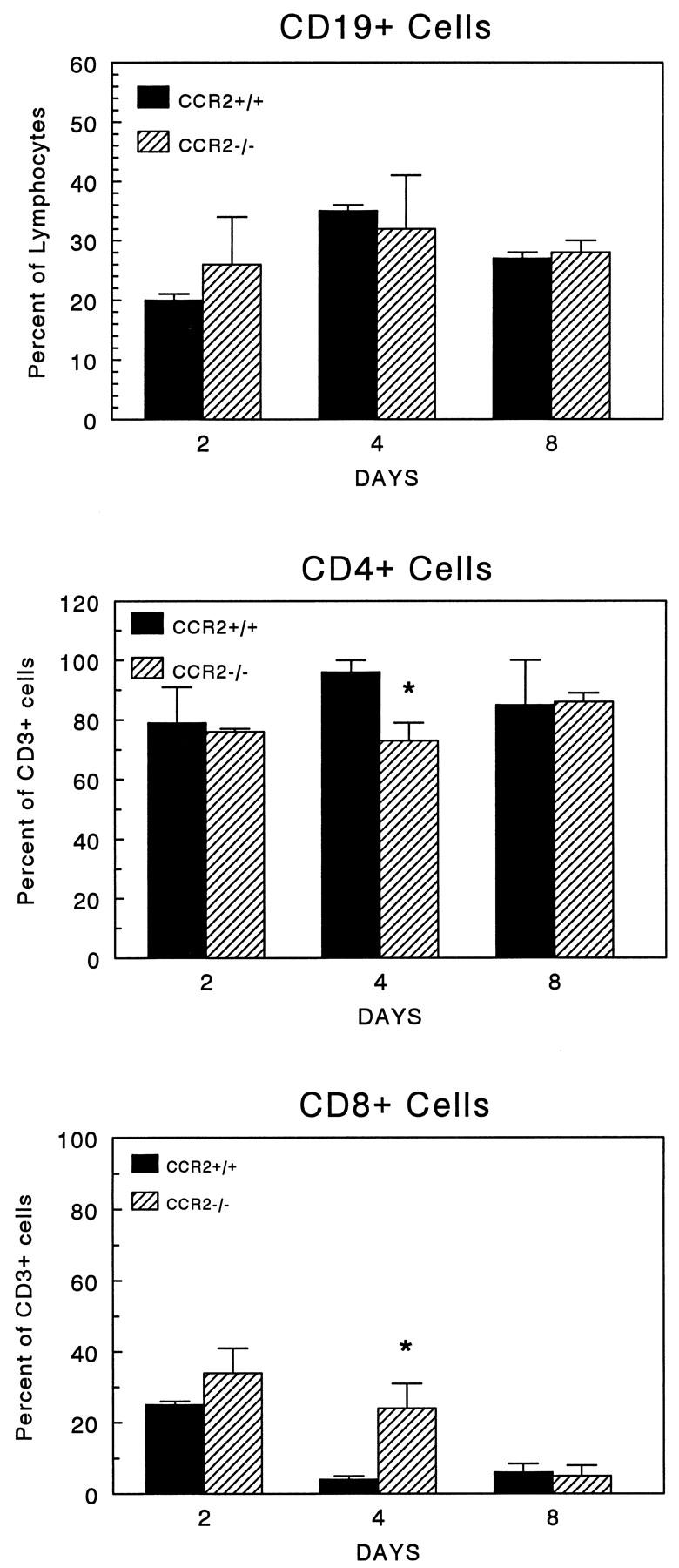
Effect of CCR2 knockout on recruitment of CD19+, CD4+, and CD8+ lymphocytes during type-2 lung granuloma formation. Dispersed granuloma cells were stained with fluorescent-labeled antibodies to CD19, CD3, CD4, and CD8, then subjected to flow cytometry. Data were derived from analysis of gated lymphocytes. Upper panel: Proportion of CD19+ B cells: Middle panel: Proportion of CD3+ T cells bearing CD4 only. Lower panel : proportion of CD3+ T cells bearing CD8 only. Bars are means ± SE derived from two experiments with a total of 4 mice per group. Asterisks indicate P < 0.05.
CCR2−/− Mice Showed Altered Cytokine Expression in Draining Lymph Node Cultures
We and others have reported that chemokines can modulate cytokine production by lymphocytes. 18-20 In addition, we recently demonstrated that draining lymph node cells of CCR2−/− mice display defective IFNγ production during the course of type-1 (Th1) granuloma formation. 10 In a similar fashion, we assessed cytokine profiles in CCR2 knockout during type-2 (Th2) granuloma formation. Table 2 ▶ shows spontaneous and Ag-elicited levels of IFNγ and interleukins 2, 4, 5, and 13 in lymph node cultures. In accord with a Th2 response, the wild-type profile was dominated by type-2 related cytokines IL-4, IL-5, IL-10, and IL-13, which were produced in greatest amounts on days 4 and 8. With regard to IFN-γ and IL2, the CCR2 knockout mice displayed virtually complete impairment, especially after day 2, similar to our finding in the type-1 response. 10 Interestingly, partial impairment also extended to spontaneous and elicited IL-4 production throughout the study period. Some impairment of spontaneous IL-5 and IL-13 was also seen on day 4. In contrast, Ag-elicited IL-5 and IL-13 were initially enhanced on day 2 and thereafter were statistically no different than controls.
Table 2.
Effect of CCR2 Knockout on Draining Lymph Node Cytokine Profiles during Type-2 Granuloma Formation
| Day of granuloma formation and genotype | Ag | Cytokine (ng/ml) | |||||
|---|---|---|---|---|---|---|---|
| IFNγ | IL-2 | IL-4 | IL-5 | IL-10 | IL-13 | ||
| Day 2 | |||||||
| CCR2+/+ | − | 0.06 ± 0.09 | 0.10 ± 0.08 | 0.02 ± 0.01 | 0.07 ± 0.01 | <0.01† | 0.07 ± 0.07 |
| CCR2−/− | − | <0.05† | 0.05 ± 0.01 | <0.01† | 0.33 ± 0.01 | <0.01† | 0.13 ± 0.04 |
| CCR2+/+ | + | 0.03 ± 0.02 | 0.36 ± 0.01 | 0.08 ± 0.04 | 0.11 ± 0.03 | 0.05 ± 0.01 | 0.17 ± 0.08 |
| CCR2−/− | + | 0.07 ± 0.08 | 0.43 ± 0.02 | <0.01† | 0.72 ± 0.01* | 0.22 ± 0.04* | 0.44 ± 0.02* |
| Day 4 | |||||||
| CCR2+/+ | − | 0.23 ± 0.26 | 0.09 ± 0.14 | 0.02 ± 0.01 | 0.55 ± 0.02 | <0.01† | 0.66 ± 0.20 |
| CCR2−/− | − | <0.05† | <0.01† | <0.01† | 0.17 ± 0.01* | <0.01† | <0.05† |
| CCR2+/+ | + | 0.17 ± 0.05 | 0.41 ± 0.04 | 0.21 ± 0.04 | 6.25 ± 0.47 | 1.20 ± 0.13 | 5.17 ± 0.49 |
| CCR2−/− | + | <0.05† | 0.09 ± 0.01* | 0.14 ± 0.03* | 5.93 ± 1.80 | 1.40 ± 0.13 | 8.40 ± 2.80 |
| Day 8 | |||||||
| CCR2+/+ | − | 0.08 ± 0.01 | 0.07 ± 0.10 | 0.03 ± 0.01 | 0.31 ± 0.03 | <0.01† | 0.16 ± 0.09 |
| CCR2−/− | − | 0.01 ± 0.02* | 0.02 ± 0.02* | <0.01† | 0.21 ± 0.01 | <0.01† | 0.02 ± 0.02* |
| CCR2+/+ | + | 0.19 ± 0.11 | 1.36 ± 0.03 | 0.06 ± 0.03 | 7.46 ± 0.79 | 0.9 ± 0.1 | 4.92 ± 0.49 |
| CCR2−/− | + | <0.05† | 0.75 ± 0.05* | <0.01† | 8.20 ± 0.41 | 0.5 ± 0.3 | 4.45 ± 0.78 |
Values are mean ± SD of triplicate determinations. Lymph node cells of 5 to 6 mice were pooled and cultured for 36 hours in the absence or presence of 5 μg/ml SEA; supernates were then collected and frozen before assay of the indicated cytokines.
*P < 0.05.
†Below assay detection level.
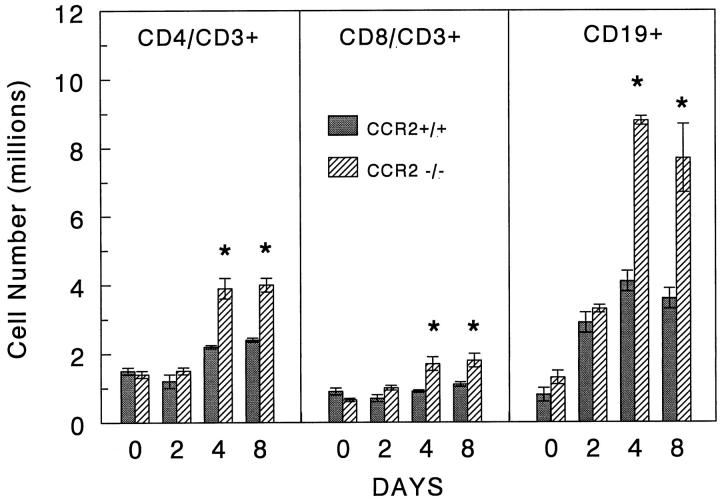
Flow cytometric analysis of the lymph node cells showed no major differences in the relative proportions of B cell and T cell subpopulations. However, knockout mice displayed a significantly greater hyperplastic response with augmentation of all cell populations on days 4 and 8 (Figure 9) ▶ .
Figure 9.
Effect of CCR2 knockout on the expansion of CD19+, CD4+, and CD8+ lymphocytes in mediastinal lymph nodes during type-2 lung granuloma formation. Cell suspensions of mediastinal lymph nodes were quantified, stained with fluorescent-labeled antibodies to CD19, CD3, CD4, and CD8, then subjected to flow cytometry. Bars show yield per mouse in millions. Data were derived from analysis of gated lymphocytes. Shown are means ± SE derived from two experiments with a total of 4 to 5 mice per group. Asterisks indicate P < 0.05.
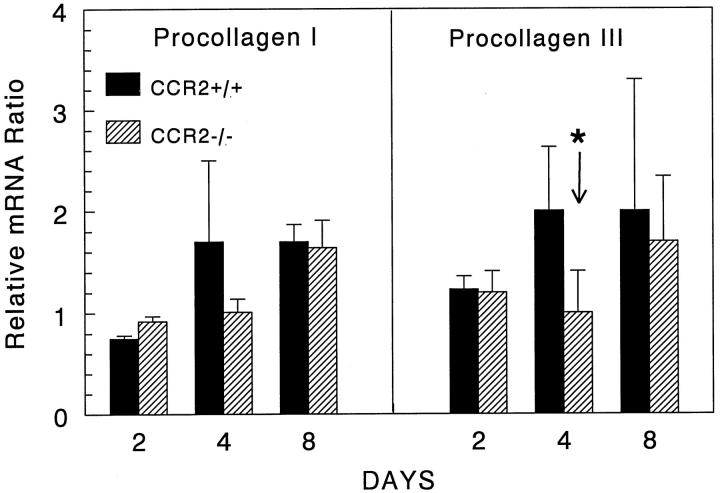
CCR2−/− Mice Showed Delayed Collagen Gene Expression during Granuloma Formation
We next examined potential effects of CCR2 deficiency on the resolution or healing phase of granuloma formation. In view of studies showing that MCP-1/JE stimulates collagen gene expression, 21 we analyzed procollagens I and III transcripts by RT-PCR-ELISA. As shown in Figure 10 ▶ , wild-type mice displayed increased mRNA for both procollagen I and III on days 4 and 8 of granuloma formation. In contrast, CCR2-deficient mice did not display increased expression until day 8, by which time levels were comparable to those of control animals. The impairment on day 4 was statistically significant for procollagen III and was observed as a trend for procollagen I. Thus, the effect of CCR2 deficiency extended to the resolution phase of granuloma formation by delaying the onset of maximal collagen synthesis.
Figure 10.
Expression of procollagen I and III mRNA in lungs with type-2 granuloma formation. Bars show the mean ratios of housekeeping gene normalized signal of granulomatous to unchallenged lungs, which were valued at 1. Bars are means ± SE derived from two separate experiments assayed in duplicate. In each experiment, equal amounts of mRNA were pooled from 3 to 4 mice, then subjected to PCR-ELISA for the indicated cytokines. Asterisks indicate P < 0.05.
Discussion
The C-C chemokine receptor CCR2 is considered the primary receptor for murine MCP-1/JE. 3 Consequently, targeted deletion of this receptor was performed as one approach to study the function of MCP-1-mediated physiological events. Developmentally, such mice are normal; however, we recently reported that they display defective macrophage recruitment and impaired type-1 cytokine production. 10 Previous studies using polyclonal anti-MCP-1 Abs 7,22 and MCP-1 knockout mice 9 suggested that MCP-1 also supports Th2 responses; therefore, the present study was undertaken to further analyze the evolution of type-2 cytokine-mediated inflammation using mice with CCR2 gene knockout. Our findings provide evidence that CCR2 and presumably its agonists participate in multiple aspects of hypersensitivity-type granuloma formation including local cellular recruitment, cytokine production, regional lymphoid proliferation, and collagen-gene expression.
With regard to local cellular mobilization, our synchronized model of granulomatous inflammation revealed that CCR2 knockout mice display impaired mononuclear phagocyte recruitment only in the early phase of granuloma formation suggesting that MCP-1 has a restricted window of chemotactic function. This was likewise observed for the type-1 mycobacterial lesion. 10 The eventual recovery of macrophage recruitment by day 4 suggests that other chemotactic factors and receptors must supplant the function of CCR2. Indeed, we demonstrated increased transcripts for MCP-3 that can utilize receptors other than CR2 and potentially compensate for MCP-1. 23 We previously reported that treatment with a polyclonal anti-MCP-1 antibody preparation abrogated type-2 granuloma size with the effect persisting until day 4. 7 The seemingly greater potency of the Ab was possibly due to nonspecificity, since this preparation has subsequently been shown to cross-react with MCP-3 and its effect would not necessarily be undermined by CCR2 deficiency. However, increases in other promiscuous chemokines could compensate for MCP-1. Because MCP-3 transcripts were readily demonstrable, it could be among a spectrum of chemokines that supplant or compensate for MCP-1 and MCP-5. 23,24 Compensatory factors, however, could not overcome the early macrophage recruitment defect. In an analogous fashion, mice with disruption of the eotaxin gene displayed only early phase impairment of eosinophil recruitment, whereas later secondary recruitment was unaffected. 25
Interestingly, the apparently compensated lesion on day 4 did undergo changes in T cell subpopulations resulting in a decreased CD4/CD8 T cell ratio. Because MCP-1/JE is reportedly chemotactic for Th cells, this effect may have been caused by impaired CD4+ T cell recruitment. 26 Alternatively, the early macrophage recruitment defect may have resulted in downstream changes in T cell populations. Local macrophages may be required to support proliferation or produce secondary waves of T cell chemotactic signals. Based upon our observations to date, we hypothesize that local recruitment during the initiation, mobilization, sustenance, and resolution stages of chronic inflammation may be mediated by successive waves of chemotactic mediators. Efforts are presently underway in our laboratory to test this hypothesis.
Although CCR2 was transiently required for local macrophage recruitment, its absence resulted in a chronic partial monocytopenia in the peripheral blood. As previously reported, CCR2 deficiency does not cause myelosuppression, 10 so MCP-1 presumably promotes monocyte production or the release of monocytes from the marrow during an inflammatory stress. It should be emphasized that the observed impairment was consistently about 50% and ultimately did not prevent granulomas from attaining maximal cellularity. The potential role of MCP-1 in regulating peripheral blood monocytosis merits further investigation.
Our finding that CCR2 knockout mice display altered cytokine production in draining lymph nodes and granulomas provides further support for the notion that chemokines influence lymphoid maturational or activation events. As reported for the mycobacterial response, 10 IFNγ and IL-2 were notably abrogated in lymph node cultures of knockout mice and this effect extended to IL-4 in the type-2 response. The latter is consistent with the effect of anti-MCP-1 Ab treatment 7 and MCP-1 knockout. 9 The partial IL-4 abrogation caused by CCR2 deficiency did not impair type-2 granuloma formation after day 2 nor the production of other Ag-elicited Th2-related cytokines such as IL-5 and IL-13. However, it was noted that spontaneous production of IL-5 and IL-13 was reduced on day 4, suggesting a subtle but ineffectual defect. This is not surprising, because alternative sources of IL-4 or compensatory mechanisms can support the type-2 responses. 24,27 In addition, it is known that endogenous IFNγ limits S. mansoni egg Ag-induced inflammation and cytokines production by means of cross-regulatory inhibition. 28,29 Therefore, any concurrent abrogation of IFNγ might be predicted to offset impairment conferred by decreased levels of IL-4. The overall increased cell proliferation in knockout lymph nodes likely reflects the absence of IFNγ-mediated cross-regulation of the Th2 response. 28 It was of interest that overall reductions of IFNγ mRNA in granulomatous lungs were less pronounced than protein levels in lymph node cultures. At present, we have no definitive explanation. The relationship between mRNA levels and protein secretion remains unclear and it is possible that cytokine producing cells are enriched at sites of granuloma formation.
In our previous study, 7 anti-MCP-1 Ab treatment caused greater abrogation of Th2 cytokines than we observed in CCR2 knockout mice. This observation has subsequently been repeated with new, highly specific anti-murine MCP-1/JE Abs (data not shown). Therefore, the disparity likely reflects different experimental conditions. This is an especially important consideration because congenital CCR2 deficiency likely influences both induction and elicitation phases of immunity. Notably, our findings in CCR2 knockout mice agree well with in vitro studies showing that MCP-1 promotes both IL-4 and IFNγ during primary and secondary lymphocyte stimulation in vitro. 19 Thus, CCR2 potentially contributes to both sensitization and elicitation phases of immunity and its absence could impair both IFNγ and IL-4. However, as we observed, compensatory mechanisms ultimately established a CCR2/MCP-1-independent response. Our previous Ab-mediated depletion study examined the elicitation phase of the type-2 response which, not having been deprived of CCR2-mediated stimulation during induction, likely will have a greater MCP-1/JE dependence and sensitivity to anti-MCP-1/JE treatment. In any case, both MCP-1 depletion and CCR2 knockout studies point to a role for MCP-1/JE in the generation or activity of IFNγ- and IL-4-producing lymphoid cells.
As mentioned above, MCP-1/JE is a known lymphocyte chemoattractant 26,30 and it is possible that local cytokine defects of CCR2 knockout mice are related to this chemotactic function. Studies by Qin et al suggest that CCR2 is more densely expressed on CD26+ (activated/memory) than naive T cells. 31,32 If this is the case, then our findings may reflect memory T cells with suboptimal homing to granulomas. In addition, because chemokines positively regulate T cell adhesion molecules 33,34 then CCR2 deficiency could result in impaired T cell migration events. Adhesion defects might also compromise T cell stimulation. Specifically, MCP-1 is reported to enhance β1 integrin binding to fibronectin, 35 which not only promotes T cell migration but is also known to be a potent costimulatory factor. 36,37 Reduced integrin-mediated stimulation could result in partial impairments of T cell activation, maturation, and cytokine production.
Finally, our finding that granulomatous lungs of CCR2 knockout mice have delayed onset of procollagen transcripts suggested that agonists of this receptor participate in the healing or resolution phase of granuloma formation. The eventual recovery of procollagen transcripts on day 8 suggests that the CCR2 defect is overcome or supplanted by other mediators. This may be a simple indirect effect of the early impairment of macrophage and lymphocyte recruitment slowing the efficiency of a variety of downstream events that support collagen synthesis. However, our finding is also consistent with the report of Gharee-Kermani et al demonstrating that MCP-1 is a direct mediator of collagen gene expression. 21 We have consistently noted more severe fibrosis in resolving type-2 lesions, which express greater levels of MCP-1/JE than type-1 lesions. 7 Studies by Hogaboam et al indicate that fibroblasts of type-2 lesions express CCR2, MCP-1/JE, and procollagen mRNA in response to IL-4 (manuscript submitted). Such a clustered expression implies that IL-4 may induce an autocrine feedback circuit among type-2 fibroblasts that promotes collagen synthesis.
In conclusion, analysis of type-2, T-cell-mediated hypersensitivity granuloma formation in CCR2 knockout mice suggests that MCP-1/JE participates at multiple stages and facets of this chronic immunoinflammatory response. Specifically, we have demonstrated contribution to monocyte mobilization to peripheral blood, early phase macrophage recruitment, cytokine production, and procollagen gene expression. Thus, chemokines appear to have a broad role in T-cell-mediated inflammation.
Footnotes
Supported by the Department of Veterans Affairs and in part by the National Institutes of Health (HL-52773)
Dr. Curtis is a career investigator of the American Lung Association of Michigan.
Address to Stephen W. Chensue, M.D., Ph.D., Pathology and Laboratory Medicine 113, Veterans Affairs Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105. E-mail: schensue@umich.edu.
References
- 1.Taub DD, Oppenheim JJ: Chemokines, inflammation and the immune system. Therapeutic Immunol 1994, 1:229-246 [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 [DOI] [PubMed] [Google Scholar]
- 3.Boring L, Gosling J, Monteclaro FS, Lusis AJ, Tsou C-L, Charo IF: Molecular cloning and functional expression of murine JE (monocyte chemotactic protein 1) and murine macrophage inflammatory protein 1a receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J Biol Chem 1996, 271:7551-7558 [DOI] [PubMed] [Google Scholar]
- 4.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D: Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA 1991, 88:5252-5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Burdick MD, Pope RM, Strieter RM: Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest 1992, 90:772-779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flory CM, Jones ML, Warren JS: Pulmonary granuloma formation in the rat is partially dependent on monocyte chemoattractant protein 1. Lab Invest 1993, 69:396-404 [PubMed] [Google Scholar]
- 7.Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL: Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol 1996, 157:4602-4608 [PubMed] [Google Scholar]
- 8.Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, Flavell R, Gladue R, Rollins B: In vivo properties of monocyte chemoattractant protein-1. J Leuk Biol 1997, 62:577-580 [DOI] [PubMed] [Google Scholar]
- 9.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ: Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 1998, 187:601-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF: Impaired monocyte migration and reduced type-1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997, 100:2552-2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chensue SW, Warmington K, Ruth J, Lincoln P, Kuo MC, Kunkel SL: Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol 1994, 145:1105-1113 [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis JL, Kim S, Scott PJ, Buechner-Maxwell VA: Adhesion receptor phenotypes of murine CD4+ T cells during a pulmonary immune response to sheep erythrocytes. Am J Respir Cell Mol Biol 1995, 12:520-530 [DOI] [PubMed] [Google Scholar]
- 13.Chirgwin JM, Przbyca AE, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 18:5294-5299 [DOI] [PubMed] [Google Scholar]
- 14.Jonas ET, Sargent D, Dawid IB: Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci USA 1985, 82:5413-5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krug MS, Berger SL: First strand cDNA synthesis primed with oligo (dT). Methods Enzymol 1987, 152:316-325 [DOI] [PubMed] [Google Scholar]
- 16.Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA: Primer-directed enzymatic amplification of DNA with thermostable DNA polymerase. Science 1988, 239:487-491 [DOI] [PubMed] [Google Scholar]
- 17.Venturoli S, Zerbini M, La Placa M, Jr, D’Antuono A, Negosanti M, Gentilomi G, Gallinella G, Manaresi E, Musiani M: Evaluation of immunoassays for the detection and typing of PCR ampified human papillomavirus DNA. J Clin Pathol 1998, 51:143-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ: Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol 1996, 156:2095-2103 [PubMed] [Google Scholar]
- 19.Lukacs NW, Chensue SW, Karpus WJ, Lincoln P, Keefer C, Strieter RM, Kunkel SL: C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am J Pathol 1997, 150:1861-1868 [PMC free article] [PubMed] [Google Scholar]
- 20.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA: Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol 1997, 158:4129-4136 [PubMed] [Google Scholar]
- 21.Gharaee-Kermani M, Denholm EM, Phan SH: Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 1996, 271:17779-17784 [DOI] [PubMed] [Google Scholar]
- 22.Chensue SW, Warmington KS, Lukacs NW, Lincoln PM, Burdick MD, Strieter RM, Kunkel SL: Monocyte chemoattractant protein expression during schistosome egg granuloma formation. Sequence of production, localization, contribution and regulation. Am J Pathol 1995, 146:130-138 [PMC free article] [PubMed] [Google Scholar]
- 23.Combadiere C, Ahuja SK, Van Damme J, Tiffany HL, Gao JL, Murphy PM: Monocyte chemoattractant protein-3 is a functional ligand for CC chemokine receptors 1 and 2B. J Biol Chem 1995, 270:29671-29675 [DOI] [PubMed] [Google Scholar]
- 24.Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL: 1997. Mycobacterial and Schistosomal antigen-elicited granuloma formation in interferon-γ and interleukin-4 knockout mice: Analysis of local and regional cytokine and chemokine networks. J Immunol 1997, 159:3565–3573 [PubMed]
- 25.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P: Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med 1997, 185:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siveke JT, A. Hamann A: T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol 1998, 160:550-554 [PubMed] [Google Scholar]
- 27.Arase H, Arase N, Nakagawa K, Good RA, Onoe K: NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur J Immunol 1993, 23:307-310 [DOI] [PubMed] [Google Scholar]
- 28.Lukacs NW, Boros DL: Lymphokine regulation of granuloma formation in murine Schistosomiasis mansoni. Clin Immunol Immunopathol 1993, 68:57-63 [DOI] [PubMed] [Google Scholar]
- 29.Chensue SW, Warmington KS, Ruth J, Lincoln PM, Kunkel SL: Cross-regulatory role of γ-IFNγ, IL-4, and IL-10 in schistosome egg granuloma formation: in vivo regulation of Th activity and inflammation. Clin Exp Immunol 1994, 98:395-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA: Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 1994, 91:3652-3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oin S, LaRosa G, Campbell JJ, Smith-Heath H, Kassam N, Shi X, Zeng L, Buthcher EC, Mackay CR: Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol 1996, 26:640-647 [DOI] [PubMed] [Google Scholar]
- 32.Mackay CR: Chemokines receptors and T cell chemotaxis. J Exp Med 1996, 184:799-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd AR, Oppenheim JJ, Kelvin DJ, Taub DD: Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol 1996, 156:932-938 [PubMed] [Google Scholar]
- 34.Carr MW, Alon R, Springer TA: The C-C chemokine MCP-1 differentially modulates the avidity of beta 1 and beta 2 integrins on T lymphocytes. Immunity 1996, 4:179-187 [DOI] [PubMed] [Google Scholar]
- 35.Shimizu Y, van Seventer GA, Horgan KJ, Shaw S: Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol 1990, 145:59-67 [PubMed] [Google Scholar]
- 36.Brunmark A, O’Rourke AM: Augmentation of mature CD4+ T cell responses to isolated antigenic class II proteins by fibronectin and intercellular adhesion molecule-1. J Immunol 1997, 159:1676-1685 [PubMed] [Google Scholar]
- 37.Ostergaard HL, Ma EA: Fibronectin induces phosphorylation of a 120-kDa protein and synergizes with the T cell receptor to activate cytotoxic T cell clones. Eur J Immunol 1995, 25:252-256 [DOI] [PubMed] [Google Scholar]