Abstract
Recently, monoclonal antibodies against the human vascular endothelial growth factor receptor VEGFR-3 were shown to provide a specific antigenic marker for lymphatic endothelium in various normal tissues. In this study we have investigated the expression of VEGFR-3 and its ligand VEGF-C in normal breast tissue and in breast tumors by immunohistochemistry. VEGFR-3 was weakly expressed in capillaries of normal breast tissue and in fibroadenomas. In intraductal breast carcinomas, VEGFR-3 was prominent in the “necklace” vessels adjacent to the basal lamina of the tumor-filled ducts. VEGF receptor 1 and 2 as well as blood vessel endothelial and basal lamina markers were colocalized with VEGFR-3 in many of these vessels. Antibodies against smooth muscle α-actin gave a weak staining of the necklace vessels, suggesting that they were incompletely covered by pericytes/smooth muscle cells. A highly elevated number of VEGFR-3 positive vessels was found in invasive breast cancer in comparison with histologically normal breast tissue (P < 0.0001, the Mann-Whitney test). VEGF-C was located in the cytoplasm of intraductal and invasive cancer cells. The results demonstrate that the expression of VEGFR-3 becomes up-regulated in the endothelium of angiogenic blood vessels in breast cancer. The results also suggest that VEGF-C secreted by the intraductal carcinoma cells acts predominantly as an angiogenic growth factor for blood vessels, although this paracrine signaling network between the cancer cells and the endothelium may also be involved in modifying the permeabilities of both blood and lymphatic vessels and metastasis formation.
Vascular endothelial growth factor (VEGF) is a well-known hypoxia-induced stimulator of endothelial cell growth and angiogenesis, 1,2 which is also up-regulated by various hormones and cytokines, such as transforming growth factor-β. 3 VEGF is a ligand for two tyrosine kinase receptors named VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1). VEGFR-2 is mainly expressed in endothelial cells, whereas VEGFR-1 can also be found in monocytes. 4 The inhibition of VEGF activity by specific monoclonal antibodies has been reported to reduce the growth of experimental tumors and their blood vessel density. 5 Primary breast cancers are known to express several angiogenic polypeptides of which VEGF was the most abundant. 6,7 Tumor cells contained high levels of VEGF mRNA in both invasive and noninvasive, ductal (in situ) breast carcinomas. 8 The endothelial cells adjacent to the in situ carcinomas expressed VEGFR-1 and VEGFR-2 mRNA in a continuous pattern. Thus, VEGF and its receptors may contribute to the angiogenic progression of malignant breast tumors because correlations have been found between tumor vascular density and the prognosis of the disease. 9
VEGFR-3 is a receptor tyrosine kinase that is similar to the two VEGF receptors in structure but does not bind VEGF, 10 placenta growth factor (PlGF), 11 or VEGF-B. 12 VEGFR-3 is initially expressed in all embryonic endothelia, but its expression in the blood vessel endothelium decreases during development, and it becomes largely restricted to the lymphatic endothelium in adult tissues. 13 In the early embryos, VEGFR-3 plays an important role in blood vessel development. 14 We have shown that monoclonal antibodies against VEGFR-3 provide the first specific antigenic marker for lymphatic endothelial cells in several normal tissues. 15 In adults, very little or no staining was observed in most blood vessel endothelia, whereas increased expression was found in Kaposi’s sarcoma spindle cells and in vascular skin tumors. 15,16 In addition, increased VEGFR-3 mRNA has been found in metastatic lymph nodes and in lymphangiomas. 13
The two known ligands of VEGFR-3 have a high degree of homology to VEGF. Thus, they have been named as VEGF-C 17 and VEGF-D 18 and consist of proteolytically processed polypeptides, which form disulfide-linked dimers. 18,19 Experiments in transgenic mice have shown that VEGF-C is a growth factor for the developing lymphatic vessels, 20,21 although it can also bind to VEGFR-2 expressed in blood vessel endothelia and induce capillary endothelial cell migration in culture 17,19 and angiogenesis ischemic rabbit hindlimb 22 and in mouse cornea. 23 Expression of VEGF-C mRNA has been detected also in malignant human tumors, including nearly half of the breast cancers analyzed. 24
While active angiogenesis is known to be a prerequisite for tumor growth beyond a few mm 3 in size, 25 lymphangiogenesis in normal or pathological adult tissues, including malignant tumors has not been reported. 25-27 It has been suggested that solid tumors may compress the nearby lymphatic vessels, which cannot penetrate the tumor mass because of an elevated interstitial fluid pressure within the tumor. 28 If lymphangiogenesis takes place during cancer progression, cancers with active lymphangiogenesis could be predisposed to metastatic spread via the lymphatic system and thus to poor survival. We wanted to use VEGFR-3 as a marker to study the lymphatic vasculature of breast carcinomas. To our surprise, we found that in normal breast tissue, VEGFR-3 was very weakly expressed in blood capillary endothelium in addition to lymphatic endothelia. In breast carcinomas, VEGFR-3 was also expressed in the lymphatic endothelium, but more detailed analysis showed that the expression of this receptor becomes up-regulated in angiogenic capillaries and that some of the nearby tumor cells express the VEGF-C protein.
Materials and Methods
Freshly Frozen Tissue Samples
Freshly frozen breast tissue samples were retrieved from the files of the Department of Pathology, University of Helsinki. The samples consisted of ductal carcinoma (n = 6), lobular carcinoma (n = 6), intraductal carcinoma (n = 8), fibroadenoma (n = 4), and histologically normal breast tissue (n = 12). Two of the ductal carcinomas were grade I, three were grade II, and one was grade III. All samples had been frozen immediately after surgical excision in liquid nitrogen and stored at −70°C.
Immunohistochemistry
Mouse monoclonal antibodies (mAbs) against human VEGFR-3 were produced as described earlier. 15 The VEGFR-3 extracellular protein domain (VEGFR-3EC) was expressed via a recombinant baculovirus in insect cells and purified from the culture medium. Mouse monoclonal antibodies against VEGFR-3EC were then produced using standard methods, and the immunoglobulin fraction was purified by protein A affinity chromatography from hybridoma ascites fluid or Tecnomouse® culture supernatants.
Five-μm cryosections of the tissues were air-dried and fixed in cold acetone for 10 minutes. The sections were rehydrated in phosphate-buffered saline (PBS) and incubated for 30 minutes in 5% normal horse serum at room temperature. The sections were then incubated for 2 hours in a humid atmosphere at room temperature with the 9D9 mAbs at the concentration of 1.0 μg/ml. Other anti-VEGFR-3 mAb against distinct epitopes of the VEGFR-3EC were also studied; clones 2E11 and 7B8 were used at the concentrations of 9.5 and 8.5 μg/ml, respectively. A subsequent incubation for 30 minutes in biotinylated anti-mouse serum was followed by a 60-minute incubation using reagents of the Vectastain Elite Mouse IgG ABC kit (Vector Laboratories, Burlingame, CA). Peroxidase activity was developed with 3-amino-9-ethyl carbazole (AEC, Sigma, St. Louis, MO) for 10 minutes. Finally, the sections were stained with hematoxylin for 20 seconds. Negative controls were done by omitting the primary antibody or by using irrelevant primary antibodies of the same isotype. The purified baculoviral immunogen 15 was used to block the binding of the 9D9 antibodies as another negative control. In these experiments the antibodies were incubated overnight with a 10-fold molar exess of the VEGFR-3EC protein in PBS. After centrifugation for 4 minutes at 4000 rpm, 4°C, the supernatant was carefully collected and then used as primary antibody. The 5-μm cryosections adjacent to the ones stained with the anti-VEGFR-3 antibodies were immunostained for PAL-E (0.15 μg/ml, Monosan, Uden, The Netherlands), laminin (1:4000 dilution of the supernatant of clone LAM-89, Sigma), collagen XVIII (1.9 μg/ml), α-smooth muscle actin (SMA, 0.5 μg/ml, clone 1A4, Sigma), VEGFR-1 (1:200 dilution of the supernatant of clone 19), or VEGFR-2 (dilution 1:100). 29
Following the staining procedures, all samples were examined by a trained pathologist (P.H.). The blood vascular densities were obtained from the slides stained for PAL-E, 27 following the guidelines recommended by Gasparini and Harris. 30 The VEGFR-3 positive vessel densities were studied in the same way. A slide was first scanned at low magnification, and intratumoral vessel density was then assessed by counting the number of stained vessels per a ×400-magnification high power field (hpf) in the areas with the highest vascular density (“vascular hotspots”) or in the areas with highest VEGFR-3 positive vessel density. A minimum of five fields was counted per slide, after which the three highest counts were averaged.
Double-Staining Procedures
VEGFR-3 and PAL-E Double Staining
Double staining with PAL-E and VEGFR-3 mAbs was used to differentiate immunohistochemical staining of lymphatic and blood vessels in two intraductal carcinomas. Briefly, acetone-fixed 5-μm cryosections were incubated for 1 hour with anti-PAL-E antibodies with biotinylated horse anti-mouse antibody (Vectastain Elite Mouse IgG ABC kit, Vector Laboratories) for 30 minutes with ABC-peroxidase (Vectastain, 1:100) for 45 minutes and developed finally with 3-amino-9-ethyl carbazole (AEC) for 10 minutes. For the second step, the sections were incubated with anti-VEGFR-3 antibodies for 1 hour (0.14 μg/ml), followed by biotinylated anti-mouse antibody for 30 minutes (1:200 dilution of the supernatant of clone), ABC-peroxidase for 30 minutes (1:100), biotinylated tyramin solution (1:2.000) containing 0.01% peroxide for 5 minutes, ABC-alkaline phosphatase (1:100) for 20 minutes, and developed with Fast Blue (Sigma) for 20 minutes, according to a procedure previously described for in situ hybridization signal enhancement. 31 Five-μm cryosections adjacent to the double stained ones were also immunostained with VEGFR-3 antibodies only, as described above.
VEGFR-3 and Ki-67 Double Staining
To study if the endothelial cells in VEGFR-3 positive necklace vessels in intraductal carcinoma were undergoing angiogenesis, two intraductal samples were chosen for double staining for the nuclear proliferation marker Ki-67 (0.5 mg/ml, Dako Immunoglobulins, Glostrup, Denmark) and VEGFR-3. Five-μm frozen sections were rehydrated and incubated for 20 minutes in 5% normal goat serum at room temperature. The sections were incubated for 1 hour at room temperature with the rabbit polyclonal Ki-67 antibody at a concentration of 5 μg/ml. A subsequent incubation for 30 minutes in biotinylated anti-rabbit serum was followed by a 30-minute incubation using reagents of the Vectastain Elite ABC anti-rabbit kit (Vector Laboratories). Peroxidase activity was developed with 3-amino-9-ethyl carbazole (Sigma) for 10 minutes. Subsequently, the sections were rehydrated and incubated for 20 minutes in 5% in normal horse serum at room temperature. The sections were incubated for 1 hour with the anti-VEGFR-3 monoclonal antibodies at the concentration of 2.6 μg/ml. A subsequent incubation for 30 minutes in biotinylated anti-mouse serum was followed by a 30-minute incubation using reagents of the Vectastain Elite ABC anti-mouse IgG kit (Vector Laboratories). Peroxidase activity was developed with 3,3′-diaminobenzidine (Sigma) for 10 minutes. Finally, the sections were stained with hematoxylin. Negative controls were performed by omitting the primary antibodies or by using irrelevant primary antibodies.
Production, Analysis, and Use of Polyclonal Anti-VEGF-C Antibodies
Polyclonal antibodies were produced in rabbits against a synthetic peptide corresponding to the amino acid residues 2–18 of the N-terminus of mature secreted human VEGF-C (residues 104–120 of the VEGF-C prepropeptide) as described earlier. 17,19 The antisera were affinity-purifed using the immunogenic polypeptide coupled to an epoxy-activated sepharose-6B column and tested for specific staining of VEGF-C using cells infected with an adenoviral vector expressing VEGF-C or control β-galactosidase (unpublished data of Michael Jeltsch, Seppo Ylä-Herttuala and the authors). The possibility of a cross-reaction between VEGF-C and VEGF-D was studied by immunoprecipitation analysis. 293T cells grown in Dulbecco’s modified Eagle’s medium − 10% fetal calf serum were transfected using the calcium phosphate precipitation method with equivalent amounts of plasmids pcDNA3.1-VEGF-C, pcDNA3.1-VEGF-D, CMV-βgal, pIg-βgal, pIg-VEGFR-3(1–3 loop), and pIg-VEGFR-1(1–5 loop). When producing the receptor bodies, serum-free culture medium containing 0.2% bovine serum albumin was changed to the cells 48 hours after transfection, and after an additional 30 hours the medium was collected, clarified by centrifugation, and used in the experiments. Metabolic labeling of VEGF-C and VEGF-D produced by the transfected cells was carried out by addition of 100 μCi/ml of Pro-Mix L-[35S] in vitro cell labeling mix (Amersham) to culture medium devoid of cysteine and methionine. After 6 hours, the medium was collected and immunoprecipitated using affinity purifed rabbit antibodies against VEGF-C or rabbit anti-VEGF-D antisera (a generous gift from Drs. Marc Achen and Steven Stacker).
The eight intraductal carcinomas and all of the invasive carcinomas analyzed for VEGFR-3 were chosen for further analyses of the expression of VEGF-C. Five-μm cryosections adjacent to the sections stained with the anti-VEGFR-3 antibodies were air-dried and fixed in cold acetone for 10 minutes. The sections were rehydrated in PBS and incubated for 30 minutes in 5% normal goat serum and then for 2 hours in a humid atmosphere at room temperature with the rabbit polyclonal antibodies against human VEGF-C diluted 1:200 in PBS. A subsequent incubation for 30 minutes in biotinylated anti-rabbit serum was followed by a 60-minute incubation using reagents of the Vectastain Elite Rabbit IgG ABC kit (Vector Laboratories). The sections were further processed as described above. As a negative control, the purified immunogen was used to block the binding of the VEGF-C antibodies. In these experiments, VEGF-C antibodies were incubated overnight with a 10-fold molar excess of the VEGF-C protein in PBS. After centrifugation for 4 minutes at 4000 rpm, 4°C, the supernatant was carefully collected and used in the immunostainings.
For comparison, two intraductal carcinomas were immunostained with commercially available anti-VEGF-C polyclonal antibodies (c = 200 μg/ml, Santa Cruz Biotechnology, Santa Cruz, California) raised against the amino terminus of the human VEGF-C precursor. After acetone fixation sections were incubated with 5% normal rabbit serum and with 4.0 μg/ml anti-VEGF-C antibodies for 2 hours at room temperature, a subsequent incubation for 30 minutes in biotinylated anti-goat serum was done and was followed by a 60-minute incubation using reagents of the Vectastain Elite Goat IgG ABC kit (Vector Laboratories).
Production of Collagen Type XVIII Monoclonal Antibodies
Monoclonal antibodies to human type XVIII collagen were generated by DiaBor Ltd. (Oulu, Finland) by immunization of mice with the recombinant polypeptide QH48.18, 32 corresponding to the common region of the N-terminal NC1 domain of human type XVIII collagen. The clones were screened by enzyme-linked immunosorbent assay and Western analysis using the polypeptide QH48.18 and also by immunofluorescence staining of frozen human tissue sections (data not shown). The screening of the hybridoma clones resulted in three monoclonal antibodies, which were positive in all three assays mentioned (enzyme-linked immunosorbent assay, Western, immunofluorescence staining). One of the antibodies that gave the strongest signals, DB144-N2, was used in subsequent experiments. It gave an identical staining pattern to that of the polyclonal anti-all hu(XVIII), eg, in adult human skin and kidney samples (data not shown). 33
Results
VEGFR-3 in Histologically Normal Breast Tissue and in Benign Fibroadenomas
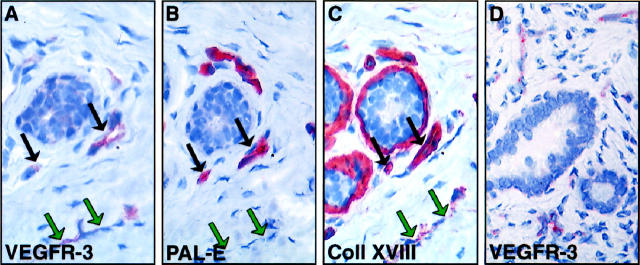
Immunohistochemical staining of VEGFR-3 in normal breast tissue showed a very weak staining in capillaries of the interductal stroma. These vessels did not form any specific pattern but were scattered throughout the stroma (Figure 1A) ▶ . The density of the VEGFR-3 positive vessels in the normal breast tissue samples ranged from 6 to 17 per hpf, median 9 (n = 12). Most of such vessels were strongly stained for the blood vascular endothelial marker PAL-E and for the basal lamina component, collagen XVIII (black arrows in Figure 1 ▶ ), suggesting that VEGFR-3 was expressed weakly in the blood vessels of normal breast tissue. However, some thin vessels in the stroma, which were clearly stained for VEGFR-3 were negative for PAL-E and only weakly positive for the collagen type XVIII, suggesting that they were lymphatic vessels (green arrows). VEGFR-3 positive vessels were also uniformly found in benign fibroadenomas (Figure 1D) ▶ in which their density (median 8 vessels per hpf; range 3 to 19; n = 4) did not differ from that of the histologically normal breast tissue (median 8 versus 9; P > 0.1, the Mann-Whitney test).
Figure 1.
Immunostaining for VEGFR-3 (A), PAL-E (B), and collagen XVIII (C) in adjacent sections of histologically normal breast tissue. Careful examination of the staining patters shows that most of the weakly VEGFR-3 positive vessels are also stained for PAL-E and collagen XVIII (black arrows). One of the vessels, indicated by green arrows, is stained for VEGFR-3 but not for the PAL-E and only very weakly for collagen XVIII and therefore presumably lymphatic. In a benign fibroadenoma, numerous VEGFR-3 positive vessels can be observed (arrows in D). Magnifications, ×400.
VEGFR-3 Positive Vessels in Intraductal Carcinomas
In intraductal carcinomas, a special pattern of strongly stained VEGFR-3 positive vessels was observed. The vessels formed arch-like structures around the affected ducts (Figure 2A) ▶ . This necklace pattern was also observed in staining of adjacent sections for the blood vessel endothelial marker, PAL-E (Figure 2B) ▶ , suggesting that VEGFR-3 expression was enhanced in capillary endothelium. To more definitively differentiate between blood and lymphatic vessels and to search for the presence of smooth muscle cells and pericytes in the vessel walls, additional stainings were done using antibodies against smooth muscle α-actin and basal lamina components laminin and type XVIII collagen. According to this staining, the small vessels close to the intraductal carcinomas expressed simultaneously VEGFR-3 and the basal lamina proteins but stained more weakly for SMA, suggesting that they are incompletely covered by pericytes/smooth muscle cells in the vessel wall (black arrows in Figure 2, C-F ▶ ). In contrast, larger blood vessels at some distance from the intraductal lesions were in general negative for VEGFR-3 but were positive for laminin, collagen XVIII, and SMA (red arrows). In addition, vessels were found that were positive for VEGFR-3 but only very weakly stained for the basal lamina proteins laminin and type XVIII collagen and not at all for SMA (green arrows). These were considered to represent lymphatic vessels.
Figure 2.
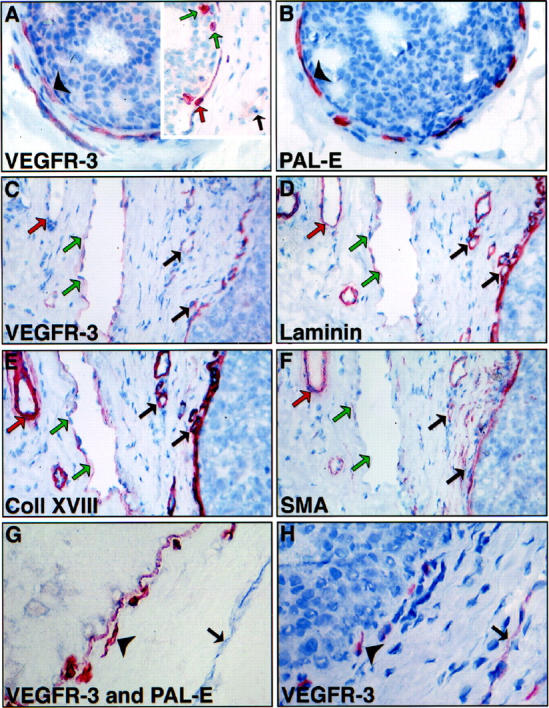
Immunohistochemical characterization of VEGFR-3 expressing vessels in intraductal carcinoma. In adjacent sections (A, B), VEGFR-3 and PAL-E decorate a similar pattern of necklace vessels (arrowheads) around the duct filled with carcinoma cells. In double staining for VEGFR-3 and Ki-67, several nucleae of the tumor cells inside the ducts are strongly positive for Ki-67 (A, inset, green arrows) as are several endothelial cell nucleae of the necklace vessels (red arrow). Some distance away, an apparently quiescent, VEGFR-3 positive vessel with a Ki-67 negative nucleus is marked with a black arrow. Another set of adjacent sections was compared with staining for VEGFR-3 (C), laminin (D), collagen XVIII (E), and SMA (F). Double staining for PAL-E and VEGFR-3 (G) and comparison with adjacent section stained for VEGFR-3 only (H). The vessels adjacent to the affected ducts are double-positive (arrowheads), whereas a VEGFR-3 positive vessel is present a short distance away from the affected duct in the interductal stroma (arrows). Note that basal lamina is positive for PAL-E in the double staining procedure. Magnification, ×400 (A, B); ×320 (C, D, E, F); ×480 (G, H).
Differential Double Staining of Blood and Lymphatic Vessels
Two intraductal carcinomas were chosen for the immunohistochemical double-staining procedure to more clearly differentiate lymphatic vessels from blood vessels. 27 Using this method, the VEGFR-3 positive vessels are stained blue, whereas the PAL-E positive vessels and basal laminae are stained brown. Both tested samples showed a similar pattern of staining: the vessels lining the tumor filled ducts were predominantly PAL-E positive (Figure 2 ▶ , arrowhead in G and H), whereas the presumably lymphatic, VEGFR-3 positive vessels a short distance away in the interductal stroma were PAL-E negative (Figure 2 ▶ , black arrows in G and H). To exclude misinterpretation due to possible double-staining artifacts, adjacent 5-μm sections were stained using anti-VEGFR-3 alone. This staining confirmed that several of the PAL-E positive blood vessles are also positive for VEGFR-3.
Double Staining Using Antibodies Against Ki-67 and VEGFR-3
To show if the endothelial cells of the necklace vessels in intraductal carcinomas were undergoing angiogenic growth, double staining of the nuclear proliferation marker Ki-67 and VEGFR-3 was carried out for two intraductal carcinomas. Using this method, the nucleae in S phase are stained red, and VEGFR-3 positive endothelial cells are stained dark brown. Both of the samples showed a similar pattern of staining; several nucleae of the tumor cells inside the ducts were strongly positive for Ki-67 (Figure 2A ▶ , inset, green arrows) as were several endothelial cell nucleae of the necklace vessels (red arrow). Some distance away, the VEGFR-3 positive vessels had only Ki-67 negative nucleae (black arrow), suggesting that they were in a quiescent state.
Specificity of Anti-VEGF-C Antibodies
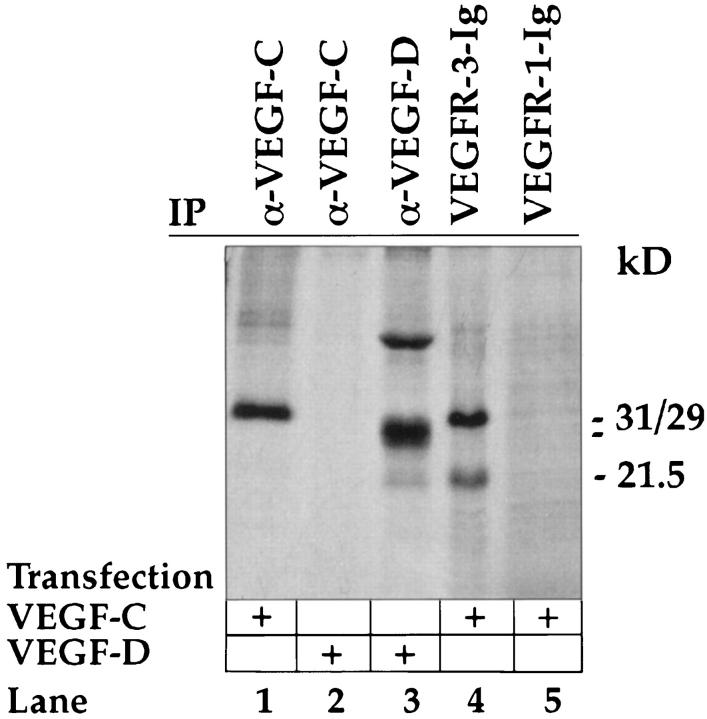
The rabbit antisera against human VEGF-C have been previously characterized and found to lack cross-reactivity against VEGF or VEGF-B. 17,19 To test for a possible cross-reaction with the related VEGF-D polypeptides, immunoprecipitations were carried out. Anti-VEGF-C antibodies precipitated the proteolytically processed 29- and 31-kd VEGF-C polypeptides from metabolically labeled conditioned media of transfected 293T cells (Figure 3 ▶ , lane 1) but did not react with the fully processed 21.5-kd form, which was however bound by the VEGFR-3-Ig receptor bodies (Figure 3 ▶ , lane 4). On the other hand, anti-VEGF-C did not recognize VEGF-D (Figure 3 ▶ , lane 2) unlike the anti-VEGF-D antibodies (Figure 3 ▶ , lane 3), indicating a lack of cross-reaction with VEGF-D.
Figure 3.
Analysis of anti-VEGF-C antibodies by immunoprecipitation. Metabolically labeled conditioned medium of 293 T cells transfected with expression vectors for VEGF-C or VEGF-D was precipitated with the indicated antibodies (α-) or receptor bodies (-Ig) detailed in the materials and methods and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Note the lack of precipitation of VEGF-D by the anti-VEGF-C antibodies. Note also that the VEGFR-3-Ig precipitates both 29/31 kd and 21.5 kd forms of VEGF-C, which are not recognized by VEGFR-1-Ig.
VEGF-C in the Intraductal Carcinoma Cells and Its Receptors in Adjacent Vessels
Polyclonal affinity-purified antibodies against human VEGF-C were used to stain the eight intraductal carcinoma samples. According to the results obtained, all samples contained at least some VEGF-C, but considerable heterogeneity was observed in the intensity of staining and in the expression patterns. In some cases, most of the carcinoma cells were strongly positive for VEGF-C (Figure 4A) ▶ , whereas in others, only some carcinoma cells gave a staining signal (Figure 4B) ▶ . In contrast, very little or no staining was observed in the normal tissues surrounding the affected ducts, although weak signal was also obtained in unaffected normal ductal epithelium (data not shown). Antigen-blocking experiments indicated that the staining for VEGF-C was specific (inset in Figure 4B ▶ ). Antibodies against the amino terminus of the VEGF-C precursor form of human origin showed a similar pattern of staining (data not shown). The other VEGF-C receptor, VEGFR-2, as well as the other VEGF receptor VEGFR-1 were expressed in the same necklace vessels adjacent to the intraductal carcinoma cells (Figure 4, C-F ▶ ).
Figure 4.
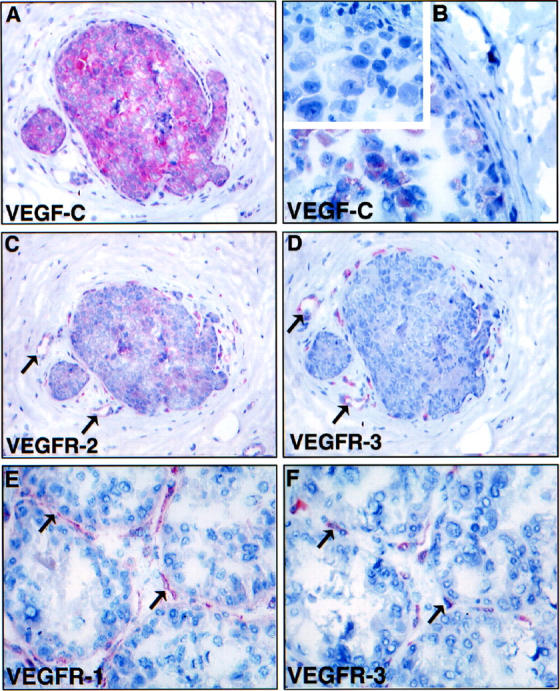
Comparison of VEGF-C (A, B) and VEGFR (C-F) stainings in intraductal carcinoma. Note the expression of VEGF-C in all (A) or part (B) of the carcinoma in situ cells. Negative control using anti-VEGF-C antibodies blocked with a 10-fold molar excess of the VEGF-C protein gave no staining of the carcinoma cells (inset in B). C and D show sections adjacent to A stained for VEGFR-2 and VEGFR-3, respectively. Note that the necklace vessels are positive for both VEGFR-2 and VEGFR-3 in the vicinity of VEGF-C expressing carcinoma cells. E and F show a similar comparison for VEGFR-1 and VEGFR-3, respectively. Note that the staining for VEGFR-3 appears more discontinuous. Magnification, ×300 (<label;A, C, D>); ×400 (B); ×350 (E, F).
VEGFR-3 Positive Vessels and VEGF-C in Invasive Breast Carcinoma
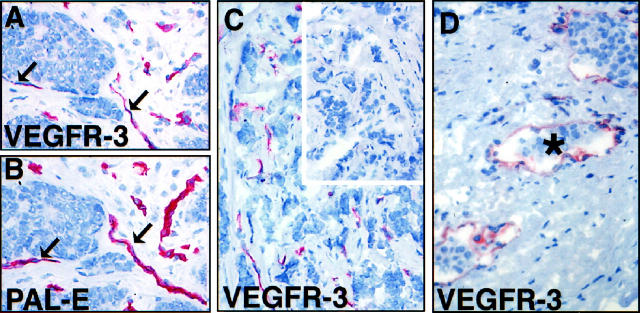
Strongly stained VEGFR-3 positive vessels were also present in all invasive ductal carcinomas and lobular carcinomas studied (Figure 5, A-C and D ▶ , respectively). The VEGFR-3 positive vessels did not appear to form any specific distribution pattern; most of these vessels were also immunoreactive for the PAL-E antigen (Figure 5B) ▶ . The intratumoral VEGFR-3 positive vessel density (median 21, range 9 to 56 vessels per hpf; n = 12) was significantly elevated in the invasive breast carcinomas when compared with normal breast tissue (median 21 versus 9; P < 0.0001, the Mann-Whitney test). Occasionally, invasion of the carcinoma cells into the VEGFR-3 positive vessels could be observed (asterisk in Figure 5D ▶ ).
Figure 5.
VEGFR-3 in invasive ductal (A-C) and lobular (D) breast cancer. The intratumoral VEGFR-3 positive vessels (A) surround tumor cell clusters in ductal breast carcinoma and are predominantly positive for PAL-E (black arrows in B). Also an invasive growth pattern is associated with VEGFR-3 positive vessels (C), whereas no staining is observed using anti-VEGFR-3 antibodies blocked with a 10-fold molar excess of the VEGFR-3 protein (inset in C). Occasionally, invasion of the carcinoma cells into the VEGFR-3 positive vessels could be observed (D). Magnification, ×200.
Immunostaining for VEGF-C varied strongly among the invasive carcinomas studied (n = 12). Some carcinoma cells were strongly positive for VEGF-C (data not shown), whereas others stained very weakly or, in some cases, no staining was observed. As in the intraductal carcinomas, very little or no staining was observed in the connective tissue in these sections.
Discussion
The present study demonstrates that VEGFR-3, which has been described to be a predominantly lymphatic endothelial marker in most adult tissues, is very weakly expressed also in capillary endothelium of normal breast tissue. However, in intraductal carcinomas, a necklace pattern of strongly stained VEGFR-3 positive vessels was detected lining the tumor-filled ducts. Most of these vessels expressed the blood vessel endothelial marker PAL-E and the basal lamina components laminin and collagen XVIII but apparently had less pericytes/smooth muscle cells than blood vessels located further away from the tumor cells, as shown by staining using antibodies against SMA. Several nucleae in these vessels were also positive for the nuclear proliferation marker Ki-67. These features suggest that the necklace vessels were undergoing angiogenesis.
A second group of vessels lying a distance away from the affected ducts were positive for VEGFR-3 but very weakly positive for the basal lamina components and negative for PAL-E, suggesting that they are lymphatic vessels. These vessels also lacked SMA-positive pericytic components. Also in invasive breast carcinomas, VEGFR-3 was up-regulated in PAL-E positive blood vessels, although the vessel patterns seen were more randomly organized in the connective tissue stroma around the tumor cells. The results suggest that VEGFR-3 expression is up-regulated in breast carcinomas during angiogenesis associated with tumor growth. The highly elevated number of VEGFR-3 positive vessels found in carcinoma in situ is compatible with the hypothesis that the carcinoma cells produce factors, which activate the growth of blood vessels in the immediate vicinity of the carcinoma cells.
VEGF-C is obviously a candidate growth factor for the VEGFR-3 and VEGFR-2 positive vessels in the carcinomas. Indeed, we found that both intraductal and invasive carcinoma cells contain the VEGF-C protein. This is in agreement with our recent study in which nearly half of unselected malignant invasive tumors contained VEGF-C mRNA in Northern blotting analyses. 24 So far, VEGF-C has been considered to be a relatively specific lymphatic endothelial growth factor in normal tissues. Expression of a VEGF-C transgene in the basal layer of mouse skin keratinocytes caused a selective development of a hyperplastic subpapillary lymphatic network, 20 and recombinant VEGF-C protein also promoted predominantly lymphangiogenesis in the chick chorioallantoic membrane. 21
VEGF-C is synthesized as a precursor protein, which requires proteolytic cleavages for full activity. In a recent study we showed that proteolytic processing of the VEGF-C precursor is required for activation of VEGFR-2, whereas a partially processed precursor can also activate VEGFR-3. 19 The fully processed VEGF-C was less potent in stimulating capillary endothelial cell growth but almost as active as VEGF in promoting capillary endothelial cell migration. In malignant tumors, the fully processed VEGF-C may be available and may participate in the stimulation of angiogenesis via both of its two receptors, VEGFR-3 and VEGFR-2, which according to the present data are up-regulated in angiogenic blood capillary endothelium. Such a paracrine relationship appears to exist also for VEGF and its two receptors VEGFR-1 and VEGFR-2, which have been shown to be expressed at mRNA level by breast carcinoma cells and the endothelia of adjacent vessels. 8
In some cases we observed invasion of tumor cells into the VEGFR-3 positive vessels, which had a morphology characteristic of the lymphatic vessels and lacked red blood cells. It is well established that breast cancer may spread via the lymphatic vessels into the axillary lymph nodes. Lymph node-positive cancer is associated with an inferior survival rate as compared with node-negative disease, and the number of involved axillary lymph nodes is still probably the most important and most widely used independent prognostic factor in breast cancer. 34 Even small breast cancers may be associated with axillary nodal metastases. About 23% of cancers that are 2 cm or smaller in size are associated with positive axillary nodes in conventional histopathological examination, 35 and even a higher frequency of metastatic nodes may be found if the nodes are investigated by serial sectioning and immunohistochemistry. Interestingly, enhanced expression of the VEGFR-3 mRNA has been found in the lymphatic endothelium of metastatic lymph nodes. 13 This could be due to activation of the lymphatic endothelium by cytokines secreted by the cancer cells, but it could also be a consequence of a relative obstruction of the lymphatic flow.
Relatively little is known about the mechanisms of metastatic spread via the lymphatics. It appears that the tumor cells need to penetrate the lymphatic endothelium twice to translocate into the interstitium of a lymph node. Because lymphatic vessels do not contain tight junctions or continuous basal laminae, 36 their penetration may only require tumor cell adhesion to the endothelium and transmigration through intracellular gaps that have been described to occur between lymphatic endothelial cells (reviewed in Ref. 37 ). It is not yet known how such interendothelial gaps are regulated, but VEGF has been shown to increase microvascular permeability by enhancing the transmigration activity of the vesicular-vacuolar organelles. 38 Both VEGF and VEGF-C are strong vascular permeability factors, 19 and VEGF-C might also have a similar function in the lymphatic system. In general, VEGF-C and VEGFR-3 could be components of a paracrine signaling network between cancer cells and the endothelium, and they may be involved in modifying the permeabilities of both blood and lymphatic vessels and metastasis formation.
We conclude that the number of VEGFR-3 positive vessels is elevated in breast cancer. Part of the VEGFR-3 positive vessels are angiogenic blood vessels with up-regulated expression of VEGFR-3 in the endothelial cells. However, a part of the VEGFR-3 positive structures appear to be lymphatic vessels, although we found no evidence that lymphangiogenesis occurs in breast cancers. The presence of VEGF-C in intraductal carcinoma cells as well as the VEGFR-3 positive capillaries surrounding the affected ducts suggest that VEGF-C secreted by the cancer cells acts predominantly as an angiogenic growth factor, but it could also affect the lymphatic vessels during tumor metastasis into the axillary lymph nodes.
Acknowledgments
We thank Drs. Marc Achen and Steven Stacker for providing us VEGF-D reagents and Kati Konola, Paula London, Pipsa Ylikantola, and Eija Koivunen for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Kari Alitalo, Molecular/Cancer Biology Laboratory, Haartman Institute, P.O. Box 21 (Haartmaninkatu3), University of Helsinki, FIN-00014 Helsinki, Finland. E-mail: kari.alitalo@helsinki.fi.
Supported by the Finnish Academy of Sciences, the Finnish Cancer Research Foundation, the Helsinki University Central Hospital Research Funds (TYH8105), and the European Union (Biomedicine Grant PL 963380). M. Rehn and T. Pihlajaniemi were supported by the Sigrid Juselius Foundation and FibroGen Inc. (South San Francisco, CA).
References
- 1.Ferrara N, Davis-Smyth T: The biology of vascular endothelial growth factor. Endocr Rev 1997, 18:4-25 [DOI] [PubMed] [Google Scholar]
- 2.Shibuya M: Role of VEGF-FLT receptor system in normal and tumor angiogenesis. Adv Cancer Res 1995, 67:281-316 [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N: The role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 1999, In press [DOI] [PubMed]
- 4.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W: The vascular endothelial growth factor receptor Flt-1 mediates biological activities: Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996, 271:17629-17634 [DOI] [PubMed] [Google Scholar]
- 5.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N: Inhibition of vascular endothelial growth factor induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362:841-844 [DOI] [PubMed] [Google Scholar]
- 6.Relf M, LeJeune S, Scott P, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris A: Expression of the angiogenic factors vascular endothelial growth factor, acidic and basic fibroblast growth factor, tumor growth factor β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 1997, 57:963-969 [PubMed] [Google Scholar]
- 7.Bicknell R, Lewis C, Ferrara N: Tumour Angiogenesis. 1997. Oxford University Press, Oxford
- 8.Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, Senger DR, Connolly JL, Schnitt SJ: Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol 1995, 26:86-91 [DOI] [PubMed] [Google Scholar]
- 9.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G: Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992, 84:1875-1887 [DOI] [PubMed] [Google Scholar]
- 10.Pajusola K, Aprelikova O, Pelicci G, Weich H, Claesson-Welsh L, Alitalo K: Signalling properties of FLT4, a proteolytically processed receptor tyrosine kinase related to two VEGF receptors. Oncogene 1994, 9:3545-3555 [PubMed] [Google Scholar]
- 11.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M: Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ 1996, 7:213-221 [PubMed] [Google Scholar]
- 12.Olofsson B, Korpelainen E, Mandriota S, Pepper MS, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U: VEGF-B binds to VEGFR-1, and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA 1998, 95:11709-11714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VM, Fang G-H, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to endothelium of lymphatic vessels during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K: Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998, 282:946-949 [DOI] [PubMed] [Google Scholar]
- 15.Jussila L, Valtola R, Partanen T, Salven P, Heikkilä P, Matikainen M-T, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K: Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res 1998, 58:1599-1604 [PubMed] [Google Scholar]
- 16.Lymboussaki A, Partanen TA, Olofsson B, Thomas-Crusells J, Fletcher C, deWaal R, Kaipainen A, Alitalo K: Expression of the vascular endothelial growth factor receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol 1998, 153:395-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 18.Achen M, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks A, Alitalo K, Stacker S: Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 1998, 95:548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K: Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 1997, 16:3898-3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 21.Oh S-J, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J: VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol 1997, 188:96-109 [DOI] [PubMed] [Google Scholar]
- 22.Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, Principe N, Kearney M, Hu J-H, Isner JM: Vascular endothelial growth factor-C (VEGF-C/VEGFR-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol 1998, 153:381-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi J-H, Claesson-Welsh L, Alitalo K: Vascular endothelial growth factor induces angiogenesis in vivo. Proc Natl Acad Sci USA 1998, 95:14389-14394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salven P, Lymboussaki A, Heikkilä P, Joensuu H, Alitalo K: Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol 1998, 153:103-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 26.Jain R: Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst 1989, 81:570-576 [DOI] [PubMed] [Google Scholar]
- 27.de Waal R, van Altena M, Erhard H: Lack of lymphangiogenesis in human primary cutaneous melanoma: consequences for the mechanism of lymphatic dissemination. Am J Pathol 1997, 150:1951-1957 [PMC free article] [PubMed] [Google Scholar]
- 28.Baish J, Netti P, Jain R: Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc Res 1997, 53:128-41 [DOI] [PubMed] [Google Scholar]
- 29.Simon M, Röckl W, Hornig C, Gröne EF, Theis H, Weich HA, Fuchs E, Yayon A, Gröne H-J: Receptors of vescular endothelial growth factor/vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and (125 I) VEGF binding sites. J Am Soc Nephrol 1998, 9:1032-1044 [DOI] [PubMed] [Google Scholar]
- 30.Gasparini G, Harris A: Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol 1995, 13:765-782 [DOI] [PubMed] [Google Scholar]
- 31.Kerstens H, Poddighe P, Hanselaar A: A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem 1995, 43:347-352 [DOI] [PubMed] [Google Scholar]
- 32.Saarela J, Ylikärppä R, Rehn M, Purmonen S, Pihlajaniemi T: Complete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol 1998, 16:319-328 [DOI] [PubMed] [Google Scholar]
- 33.Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T: The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol 1998, 153:611-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeVita V, Veronesi U, Pinedo H: Cancer of breast. Cancer Principles & Practice of Oncology, ed 5. 1997, p 1590
- 35.Barth A, Craig P, Silverstain M: Predictors of axillary node metastases in patients with T1 breast carcinoma. Cancer 1997, 15:1918-1922 [PubMed] [Google Scholar]
- 36.Leak LV: Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvisc Res 1970, 2:361-391 [DOI] [PubMed] [Google Scholar]
- 37.O’Morchoe C, O’Morchoe P: Differences in lymphatic and blood capillary permeability: ultrastructural-functional correlations. Lymphology 1987, 20:205-209 [PubMed] [Google Scholar]
- 38.Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF: The vesiculo-vacuolar organelle (VVO)-a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. Leuk Biol 1996, 59:100-115 [PubMed] [Google Scholar]