Abstract
Prostate cancer is the second leading cause of malignancy-related mortality in males in the United States. As a solid tumor, clinically significant tumor growth and metastasis are dependent on nutrients and oxygen supplied by tumor-associated neovasculature. As such, there is a selective tumorigenic advantage for those neoplasms that can produce angiogenic mediators. We show here that human prostate cancer cell lines can constitutively produce angiogenic CXC chemokines. Tumorigenesis of PC-3 prostate cancer cells was shown to be attributable, in part, to the production of the angiogenic CXC chemokine, interleukin (IL)-8. Neutralizing antisera to IL-8 inhibits PC-3 tumor growth in a human prostate cancer/SCID mouse model. Furthermore, angiogenic activity in PC-3 tumor homogenates was attributable to IL-8. In contrast, the Du145 prostate cancer cell line uses a different angiogenic CXC chemokine, GRO-α, to mediate tumorigenicity. Neutralizing antisera to GRO-α but not IL-8 reduced tumor growth in vivo and reduced the angiogenic activity in tumor homogenates. Thus, prostate cancer cell lines can use distinct CXC chemokines to mediate their tumorigenicity.
Prostate cancer is the second leading cause of malignancy-related mortality in males in the United States. 1 Whereas prostate carcinogenesis is a complex process that involves multiple stages of initiation, promotion, and neoplastic transformation, clinically significant prostate tumor growth is dependent on the delivery of oxygen and nutrients via tumor-associated neovasculature. 2,3 Furthermore, metastasis of prostate cancer has been linked with primary tumor-derived angiogenesis. While autocrine and/or paracrine angiogenic factors must be present to induce tumor-associated angiogenesis, specific angiogenic factors in prostate cancer remain to be elucidated.
CXC chemokines are cytokines whose expression can be induced by inflammatory stimuli in all nucleated cells in the body, 4-9 and these molecules have recently been shown to modulate angiogenesis. Based on the presence or absence of the ‘ELR’ motif (Glu-Leu-Arg) preceding the first conserved cysteine amino acid in the NH2 terminus, this family can be divided into two groups. The first group of CXC chemokines contains the ELR motif and is angiogenic. These include interleukin-8 (IL-8), epithelial-neutrophil activating protein (ENA-78), growth-related genes (GRO-α, GRO-β, and GRO-γ), granulocyte chemotactic protein (GCP-2), and NH2-terminal truncations of platelet basic protein (connective tissue activating protein (CTAP-III), β-thromboglobulin, and neutrophil activating protein (NAP-2)). 10,11 The second group of CXC chemokines, which lack the ELR motif, include interferon-γ-inducible protein (IP-10), monokine induced by γ-interferon (MIG), platelet factor 4 (PF4), and stromal derived factor (SDF-1). 10,11 Interestingly, these ELR-negative CXC chemokines, in general, have been shown to antagonize the angiogenic activities of the ELR-positive CXC chemokines as well as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). 10
IL-8, an angiogenic CXC chemokine, is significantly elevated in non-small cell lung cancer (NSCLC). 12,13 In addition, IL-8 is a significant angiogenic factor contributing to overall tumor-derived angiogenic activity in NSCLC. 13 Furthermore, human gastric carcinomas express high levels of IL-8. 14 The levels of IL-8 in these neoplasms strongly correlate with their vascularity, suggesting that IL-8 produced by gastric tumor cells may regulate neovascularization of gastric carcinoma. Survival of nude mice injected with ovarian carcinomas have been shown to be inversely correlated with the production of IL-8 by the ovarian cancers. 15 Similarly, recent reports indicate that significant levels of IL-8 are present immunohistochemically in prostate cancer specimens but not in benign prostate hyperplasia or normal prostate cells. 16 In a similar manner, GRO-α over-expression has been reported in melanoma lesions. 17 These neoplasms show the expression of the putative receptor for GRO-α, CXCR2, on both the melanoma cells and tumor-infiltrating endothelial cells. Stable transfection of the human GRO-α gene into the murine melan-a-melanocyte line resulted in significantly enhanced tumorigenicity that, in part, was related to their enhanced angiogenic phenotype. 17 Although these studies certainly support the hypothesis that CXC chemokines can mediate tumor-associated angiogenesis, it remains to be elucidated whether their angiogenic behavior is universal to all solid tumors or whether successful anti-tumor therapies can be established by antagonizing the effects of particular angiogenic CXC chemokines. For these reasons, we have analyzed the role CXC chemokines play in mediating tumorigenesis in association with prostate cancer.
We demonstrate here that the human prostate cancer cell lines, PC-3 and Du145, constitutively over-express angiogenic CXC chemokines and that each cell line uses unique CXC chemokines to mediate their tumor growth. Using a human prostate cancer/SCID mouse chimera system, we demonstrate that IL-8 is a major positive regulator of neovascularization and tumorigenesis associated with the growth of PC-3 tumors. In contrast, the Du145 cell line primarily uses a different ELR-CXC chemokine, GRO-α, in a paracrine manner to support an angiogenic environment during tumorigenesis. These data further support the hypothesis that individual CXC chemokines can regulate tumor-associated angiogenesis and demonstrate that anti-CXC chemokine therapy directed at one particular chemokine may not be entirely beneficial even within the same tumor type.
Materials and Methods
Cell Culture
The prostate cancer cell lines were maintained in sterile 150 mm 2 tissue culture flasks in appropriate media as defined by American Type Culture Collection (Rockville, MD). PC-3 cells grow in Ham’s F12K media, Du145 cells in EMEM, and LNCaP cells in RPMI 1640 (Whitaker Biomedical Products, Whitaker, CA) supplemented with 1 mmol/L glutamine, 25 mmol/L HEPES buffer, 100 units/ml penicillin, 100 ng/ml streptomycin (Whitaker Biomedical Products), and 10% fetal calf serum (Harlan, Indianapolis, IN). Cells were cultured and passaged at 37°C in 5% CO2.
In Vitro Enzyme-Linked Immunosorbent Assay (ELISA) Characterization
Approximately 2 × 10 6 prostate cancer cells were plated in 60-mm 2 culture plates in appropriate media. The next day, the media was replaced with fresh growth media, and cell-free supernatants were collected from replicate plates at 24-, 48-, 72-, and 96-hour intervals. Total antigenic levels of CXC chemokines in culture supernatants were assessed by specific ELISAs as previously described. 18,19
Neutralizing Antibodies
The goat anti-human IL-8 antibody was generated against an N-terminal IL-8 peptide by a modification of the previously described protocol to generate rabbit anti-human IL-8 antibodies (Abs). 13,20,21 The rabbit anti-human GRO-α neutralizing antisera was generated as previously described by injecting female New Zealand White rabbits in multiple intradermal sites with recombinant GRO-α in complete Freund’s adjuvant with subsequent boosts given in incomplete adjuvant. The anti-GRO-α and anti-IL-8 antisera each neutralize 30 ng of their respective chemokine at a dilution of 1:1000 without cross-reactivity. These Abs have each been shown to be specific for their respective chemokines and do not cross-react with the murine homologues of these chemokines.
In Vitro Proliferation Assays
5000 PC-3 or Du145 cells were cultured in 96-well flat-bottomed tissue culture plates in their respective media. To the media was added 1, 10, or 100 ng/ml recombinant IL-8 or GROα or vehicle control. In addition, PC-3 and Du145 cells were cultured in the presence of 1:10 or 1:100 dilutions of the neutralizing anti-IL-8 or anti-GROα antisera as well as the same dilutions of a normal preimmune serum control. Final volumes were 200 μl per well. Cells were cultured under these conditions for 24 or 48 hours before 1 μCi of 3-H thymidine was added for 16 hours. Plates were harvested on a Brandel cell harvester and filters were counted in a Beckman LS-1801 β counter. Data in Table 1 ▶ represent at least six replicate wells plated for each condition and each time point.
Table 1.
The Addition or Neutralization of Angiogenic CXC Chemokines Does Not Change the Growth of PC-3 or Du145 Cells in Vitro
| PC-3 | Du 145 | |
|---|---|---|
| Vehicle | 13,596 ± 321 | 14,910 ± 464 |
| +IL-8 (100 ng/ml) | 13,520 ± 443 | 16,836 ± 256 |
| +GRO-α (100 ng/ml) | 14,585 ± 533 | 15,192 ± 1485 |
| +anti-IL-8 Ab (1:10) | 11,438 ± 368 | 11,786 ± 270 |
| +anti-GRO-α (1:10) | 14,912 ± 850 | 13,998 ± 1232 |
| +NRS (1:10) | 15,047 ± 2032 | 15,278 ± 660 |
PC-3 or Du145 cells were cultured for 24 hours in the presence or absence of the indicated concentrations of recombinant angiogenic CXC chemokines or a 1:10 dilution of the neutralizing rabbit anti-murine CXC chemokine antisera before being pulsed with 3-H thymidine for 16 hours. Data were similar when 1 and 10 ng/ml chemokine were added or when the neutralizing antisera was used at a 1:100 dilution. Furthermore, data were similar when cultured for 48 hours prior to pulsing. Data shown represent the mean CPM ± SEM of at least six wells per experimental group.
Human Prostate Cancer/SCID Mouse Model
Male SCID mice between ages 4 to 6 weeks were used. A cohort group of SCID mice were injected with 1 × 10 6 prostate cancer cells in 100 μl of serum free media into each flank region (bilateral). All SCID mice were monitored daily for evidence of illness. Measurement of tumor size by a Thorpe engineer’s caliper (Biomedical Research Instruments, Rockville, MD) was done on a weekly basis. Tumor volume was calculated using the formula, volume = (d1 × d2 × d3) × 0.5236, in which dn represents the three orthogonal diameter measurements. A portion of the tumor was fixed in 4% paraformaldehyde for histological analysis and immunohistochemistry. In the experiments using neutralizing antibodies in vivo, prostate cancer bearing SCID mice received intraperitoneal (i.p.) injections of 0.5 ml of either neutralizing antisera or appropriate control (preimmune) rabbit or goat serum at the time of cell innoculum and every 48 hours thereafter for the duration of the study.
In Vivo Analysis of CXC Chemokines
Two random punch biopsies of each tumor were taken and immediately snap-frozen in liquid nitrogen. Tumor tissue was further processed by homogenization and sonication in an anti-protease “cocktail” of 2 mmol/L phenyl methyl sulfonyl fluoride, and 1 μg/ml each of antipain, aprotinin, leupeptin, and pepstatin A. Samples were then analyzed for antigenic CXC chemokine levels by specific ELISA and normalized to total protein (as measured by the BCA assay, Pierce Chemicals, Rockford, IL) as previously described. 18,19
Corneal Micropocket Model of Angiogenesis
In vivo angiogenic activity of human tumors was assayed in the avascular cornea of Long Evans rat eyes as previously described. 10,12,13,19 Briefly, equal volumes of lyophilized tumor specimens normalized to total protein were combined with sterile Hydron (Interferon Sciences Inc.) casting solution. Five-μl aliquots were pipetted onto the flat surface of an inverted sterile polypropylene specimen container and were polymerized overnight under UV light in a laminar flow hood. Before implantation, pellets were rehydrated in normal saline. Animals were given i.p. ketamine (150 mg/kg) and atropine (250 μg/kg) for anesthesia. Rat corneas were anesthetized with 0.5% proparracaine hydrochloride ophthalmic solution followed by implantation of the Hydron pellet into an intracorneal pocket (1 to 2 mm from the limbus). Six days after implantation, animals received heparin (1000 U) and ketamine (150 mg/kg) i.p. followed by perfusion with 10 ml of colloidal carbon via the left ventricle. Corneas were harvested and photographed. Positive neovascularization responses were defined as sustained directional ingrowth of capillary sprouts and hairpin loops toward the implant. Negative responses were defined as either no growth or only an occasional sprout or hairpin loop displaying no evidence of sustained growth.
Quantification of Vessel Density
Endothelial cells in tumor specimens were enumerated by FACS analysis of PECAM (CD31). Tumor samples were digested to single cell suspensions via mincing followed by enzymatic digestion and agitation (2 hours, 37°C) in Dispase (Collaborative Biomedical Products, Bedford, MA). Cells were then stained and analyzed by flow cytometry for PECAM (CD31) (PharMingen, San Diego, CA) expression using a modification as previously described. 19 The vascularity index was generated by multiplying the percentage of CD31-positive cells (endothelial cells as assessed by flow cytometry) by the tumor volume.
FACS Analysis for CXCR1 and CXCR2 Expression
CXCR1 and CXCR2 receptor expression was analyzed on PC-3 and Du145 cells as well as on normal human peripheral blood neutrophils. The Abs used were from Pharmingen (San Diego, CA). The anti-CXCR1 Ab is clone 5A12, which we used fluorescein isothiocyanate conjugated. The anti-CXCR2 Ab is clone 6C6, which we used PE-conjugated. Samples were analyzed on a Becton-Dickinson FACScan.
Statistical Analysis
The animal studies involved a minimum of 12 human prostate tumors or six SCID mice at each time point or for each manipulation. Data that appeared statistically significant were compared by the Mann-Whitney U test. Data were considered significant if P values were less than 0.05. Results are presented as means ± SEM. Data were analyzed by a Macintosh IIfx computer using the software package InStat for Macintosh (GraphPad Software Co.). R values for correlations were generated using Statview 4.5 (Abacus Concepts Inc., Berkely, CA).
Results
Prostate Cancer Cell Lines Constitutively Express Angiogenic CXC Chemokines
PC-3, 22 Du145, 23 and LNCaP 24 are human prostate cancer cell lines derived from prostate cancer patients. PC-3 was originally derived from a prostatic adenocarcinoma metastatic to bone. Du145 was derived from a prostatic adenocarcinoma metastatic to brain, and LNCaP was derived from a needle biopsy of the supraclavical lymph node of a patient with metastatic prostate cancer. These cell lines were grown to confluence in 60-mm 2 tissue culture dishes, the media was changed, and cell-free supernatants were collected at 24, 48, 72, and 96 hours to evaluate constitutive production of angiogenic CXC chemokines. This conditioned media was analyzed for the presence of IL-8, ENA-78, GRO-α, and GRO-γ by specific ELISA. Figure 1 ▶ represents the ELISA results obtained from the 96-hour conditioned media. Both PC-3 and Du145 prostate cancer cell lines constitutively produced significant levels of angiogenic CXC chemokines. In contrast, LNCaP cells expressed 150- and 25-fold less IL-8 than PC-3 and Du145 cells, respectively. In addition, LNCaP cells expressed 82- and 14-fold less GRO-α than PC-3 and Du145 cells, respectively. Furthermore, the remainder of the angiogenic CXC chemokines were reduced from LNCaP cells. Based on the previous observations that these members of the CXC chemokine family are angiogenic, we hypothesized that the PC-3 and Du145 cell lines would display an angiogenic phenotype.
Figure 1.
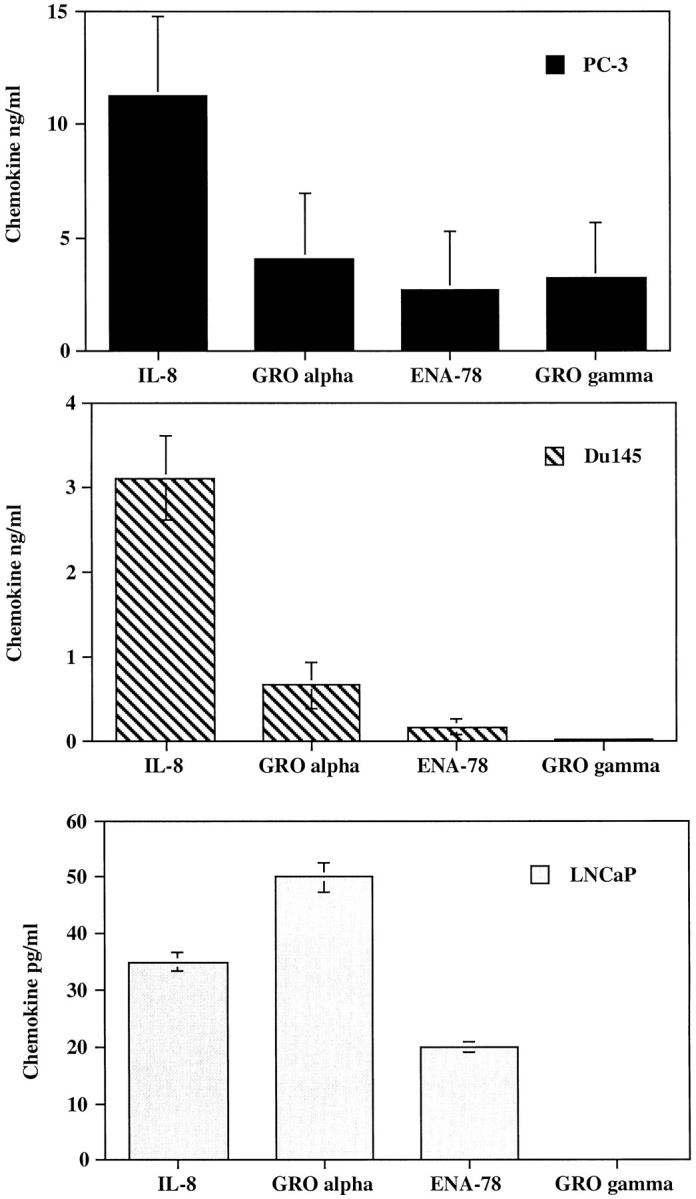
PC-3 and Du145 cells constitutively express significant levels of angiogenic CXC chemokines. ELISA measurements of 96-hour conditioned media from PC-3, Du145, and LNCaP cell lines.
PC-3 and Du145 Cells Are More Tumorigenic than LNCaP Cells
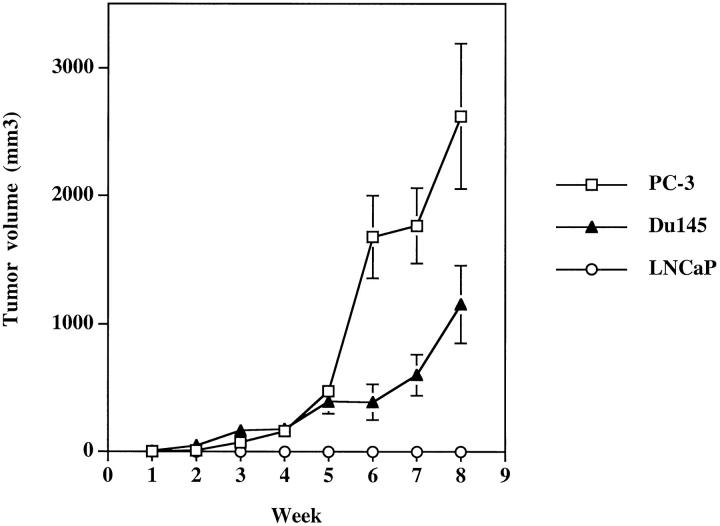
To determine whether the over-expression of angiogenic CXC chemokines by the PC-3 and Du145 cell lines in vitro correlated with their tumorigenicity in vivo, 1 × 10 6 human prostate cancer cells were injected into each flank of male SCID mice. These mice were then carefully monitored for tumor growth for a period of 9 weeks. The experiments were terminated at 9 weeks because of morbidity noted secondary to tumor burden in the animals injected with PC-3 cells. The PC-3 and Du145 cell lines produced easily discernible tumors by week 3, which grew progressively over the 9 weeks of the study (Figure 2) ▶ . In contrast, the LNCaP line showed no discernible tumor growth beyond the original injection site. In addition, metastatic lesions were seen histologically in the lungs of both the PC-3 and Du145 tumor-bearing animals at week 8 (data not shown).
Figure 2.
PC-3 and Du145 cells grow progressively in SCID mice. Male SCID mice were injected bilaterally in the rear flank with 1 × 10 6 PC-3, Du145, or LNCaP cells. Tumors were measured weekly with digital engineer’s calipers.
Production of Specific Angiogenic CXC Chemokines During Tumorigenesis of Human Prostate Cancer in SCID Mice Directly Correlates with Tumor Growth
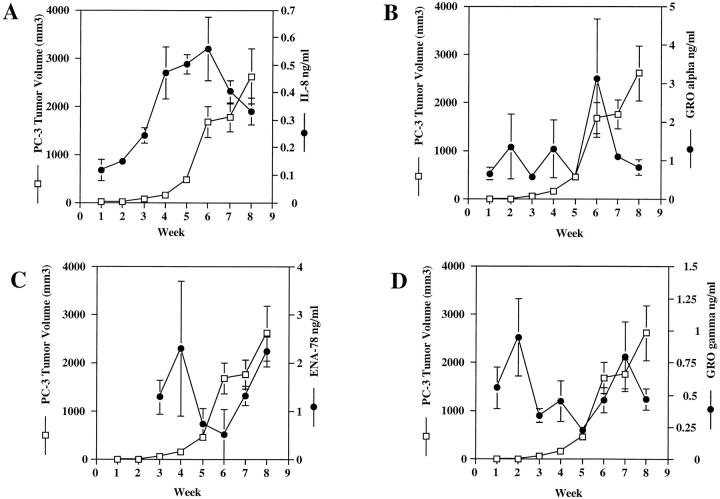
To ascertain whether angiogenic CXC chemokine expression directly correlated with human prostate cancer tumorigenesis in vivo, we xenografted 1 × 10 6 PC-3 or Du145 cells subcutaneously into the flanks of a large cohort of male SCID mice. The above studies suggested that the angiogenic CXC chemokines were constitutively produced at moderate to high levels by PC-3 and Du145 cells in vitro, therefore we postulated that these angiogenic chemokines may be acting in vivo to induce neovascularization and support tumorigenesis. To test this hypothesis, PC-3 or Du145 tumor-bearing mice were sacrificed weekly beginning 1 week after injection, and the level of angiogenic ELR-CXC chemokines in the tumors was measured by ELISA. Figure 3 ▶ demonstrates the correlations between tumor volume and angiogenic CXC chemokine production in vivo in PC-3 tumor-bearing mice. There is a positive correlation between the production of IL-8 and tumor volume (Figure 3A ▶ , r = 0.723 for weeks 1 through 6). This increase in IL-8 also correlated with an increase in tumor mass. In addition, in vivo production of ENA-78 seemed to correlate with the exponential tumor growth seen during weeks 6 to 8 (Figure 3C ▶ , r = 0.973). However, there was apparently no correlation between PC-3 tumor growth and tumor-derived production of GRO-α (Figure 3B ▶ , r = 0.238) or GRO-γ (Figure 3D ▶ , r = −0.787).
Figure 3.
PC-3 tumor growth correlates with the in vivo production of IL-8 and ENA-78. A cohort of male SCID mice were injected with 1 × 10 6 PC-3 cells in the rear flanks bilaterally at day 0. Mice were measured weekly for tumor size. Each week, six mice were sacrificed and tumor biopsies were analyzed by ELISA for specific angiogenic CXC chemokines. Graphs show comparisons between weekly tumor growth and in vivo production of specific angiogenic CXC chemokines.
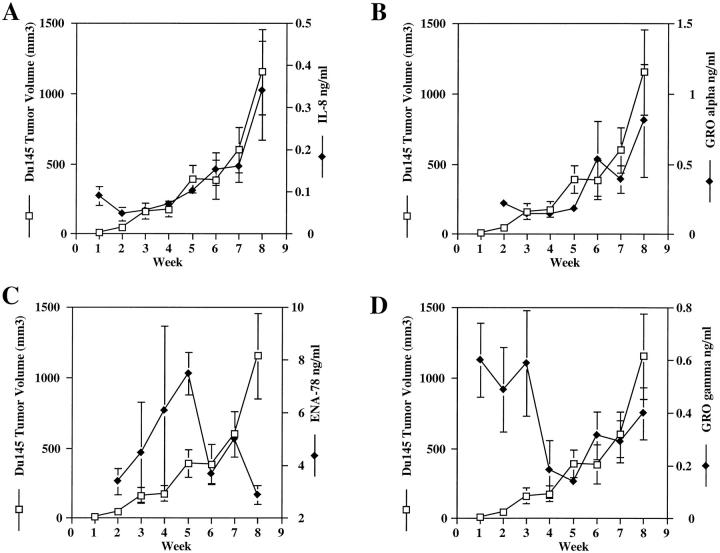
Similar analyses were carried out on Du145 tumor-bearing animals. Figure 4A ▶ demonstrates a positive correlation between tumor-derived IL-8 production and tumor volume (r = 0.955). In addition, Figure 4B ▶ demonstrates a positive correlation between Du145 tumor growth and tumor-derived GRO-α (r = 0.879). As can be seen in Figures 4C and 4D ▶ however, there is no clear evidence that ENA-78 (r = −0.478) and GRO-γ (r = −0.568) are correlated with Du145 tumorigenicity.
Figure 4.
Du145 Tumor growth correlates with the in vivo production of IL-8 and GRO-α. A cohort of male SCID mice were injected with 1 × 10 6 Du145 cells in the rear flanks bilaterally at day 0. Mice were measured weekly for tumor size. Each week, six mice were sacrificed, and tumor biopsies were analyzed by ELISA for specific angiogenic CXC chemokines. Graphs show comparisons between weekly tumor growth and in vivo production of specific angiogenic CXC chemokines.
These data suggested that IL-8 was a common factor expressed by both PC-3 and Du145 cells both in vitro and in vivo, which may contribute to their tumorigenicity. To determine whether IL-8 in vitro was an autocrine growth factor for these cell lines, PC-3 and Du145 cells were cultured in the presence or absence of recombinant IL-8 or neutralizing Abs to IL-8 for 24 and 48 hours. The presence of exogenous IL-8 or depletion of endogenous IL-8 did not significantly alter PC-3 or Du145 cell proliferation (Table 1) ▶ , suggesting that this factor supports tumor growth through a paracrine (angiogenic) mechanism rather than as an autocrine (mitogenic) mechanism.
Inhibition of IL-8 in Vivo Reduces PC-3 Tumorigenicity but Fails to Reduce Du145 Tumorigenicity
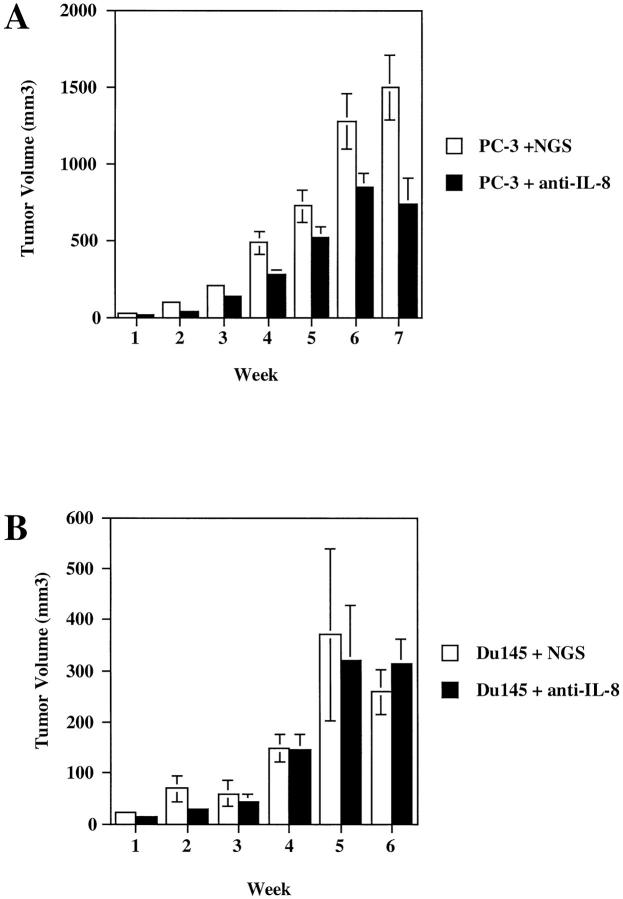
Having established that IL-8 was a common factor expressed by the PC-3 and Du145 cell lines both in vitro and in vivo, we wanted to determine whether IL-8 was directly contributing to the tumorigenicity of these cells in vivo. To do this, two groups of male SCID mice were injected bilaterally as before with either 1 × 10 6 PC-3 or Du145 cells in the rear flanks. Starting at time of tumor cell innoculum, half the animals in each group were given an i.p. injection of a neutralizing polyclonal goat anti-human IL-8 antisera (0.5 ml). The other half of the mice were given an 0.5 ml i.p. injection of normal preimmune goat serum (NGS) as a control. The injections were repeated every 48 hours for the duration of the study, and tumors were measured weekly. Figure 5 ▶ demonstrates the resulting tumor growth (as indicated by tumor volume) in the anti-IL-8- versus NGS-treated animals. Figure 5A ▶ demonstrates that anti-IL-8 treatment clearly blocked tumor progression in PC-3 tumor bearing mice. The mean tumor volume in the NGS-treated PC-3 tumor-bearing mice at week 7 was 1492 ± 211 mm3. The mean volume in the anti-IL-8 treated PC-3 tumor-bearing mice was 736 ± 166 mm3. This change represents a greater than 49% inhibition (P = 0.01). Similarly, the vascularity index was 3.3-fold less in the PC-3 tumor-bearing animals treated with anti-IL-8 (23,749 ± 4640) than in the PC-3 animals treated with NGS (77,218 ± 17,528). Furthermore, when tumor homogenates from the NGS- and anti-IL-8-treated PC-3 tumor-bearing mice were concentrated, normalized to total protein, embedded into a hydron pellet, and implanted into the avascular cornea of a Long Evans rat (the rat corneal micropocket assay; CMP), six of six corneas tested with the NGS-treated homogenates gave positive neovascular responses (Figure 6A) ▶ , whereas in the anti-IL-8-treated tumor homogenates tested, five of six were negative or weakly positive, giving an occasional loop as depicted in Figure 6B ▶ , and one of six corneas tested was positive.
Figure 5.
IL-8 is a tumorigenic factor for PC-3 cells but not for Du145 cells. Male SCID mice were injected with 1 × 10 6 PC-3 (A) or Du145 (B) cells bilaterally in the rear flanks on day 0. Starting on day 0, animals received i.p. injections of 0.5 ml of goat anti-human IL-8 neutralizing antisera or 0.5 ml of NGS as a control. Antibody injections were given i.p. every 48 hours for the duration of the study. Results presented represent weekly tumor measurements.
Figure 6.
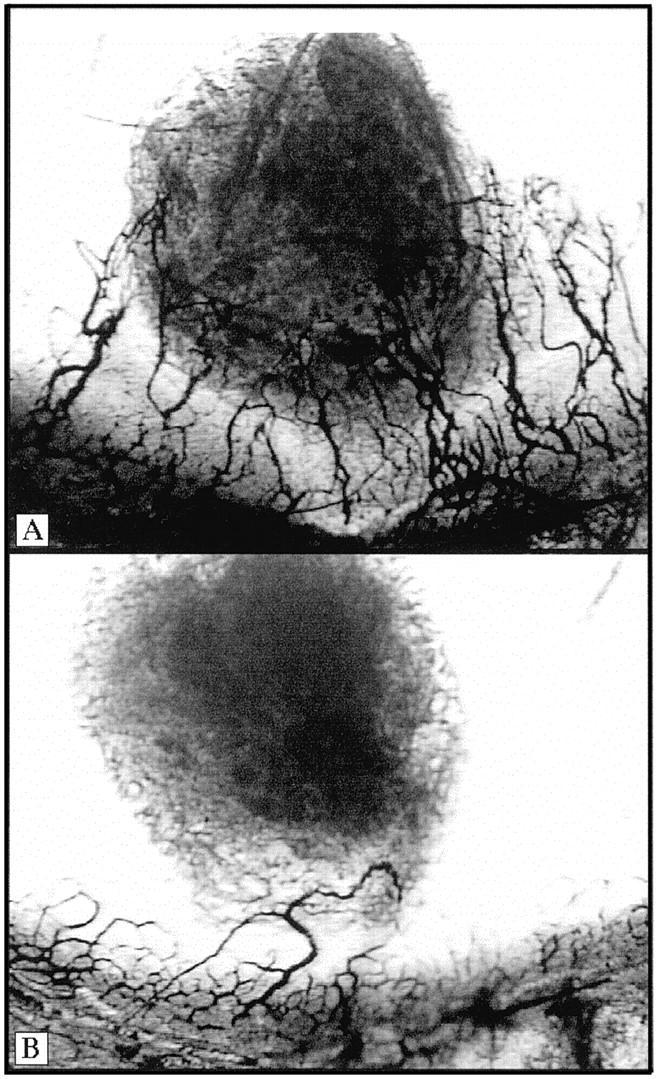
Tumor homogenates from PC-3 tumor-bearing animals treated with anti-IL-8 show less angiogenic activity than NGS-treated tumor homogenates. PC-3 tumor-bearing mice that were treated with either goat anti-human IL-8 or NGS as a control were sacrificed, and tumor homogenates were analyzed in the rat corneal micropocket assay. A demonstrates the neovascular response seen in six of six eyes tested from the NGS-treated mice. B represents the neovascular response seen when the anti-IL-8 treated tumor homogenates were analyzed. In the anti-IL-8 treated mice, two of six eyes tested were negative, three of six showed only an occasional loop as seen in B, and one of six eyes tested was positive.
Contrary to what was seen in the anti-IL-8-treated PC-3 tumor-bearing animals, Du145 tumor-bearing animals treated with either NGS or neutralizing anti-IL-8 showed no statistical difference in tumor progression as measured by tumor volume (Figure 5B) ▶ , nor did they show any histological differences (data not shown). In an effort to confirm that the dose of anti-IL-8 given was sufficient to neutralize the IL-8 being produced by the Du145 tumors in vivo, tumor homogenates from tumor-bearing mice were processed and analyzed by specific ELISA for the expression of the angiogenic CXC chemokines. The production of IL-8 by these Du145 tumor cells was significantly neutralized by the administration of the anti-IL-8 Abs. Du145 tumor homogenates from NGS-treated animals produced 0.751 ± 0.281 ng/ml IL-8, whereas homogenates from anti-IL-8-treated animals were reduced more than 10-fold (0.70 ± 0.01 ng/ml IL-8). Thus, this cannot account for the inability of this anti-IL-8 antisera to reduce Du145 tumorigenicity. There was no difference in the production of other measured CXC chemokines between the anti-IL-8- and NGS-treated Du145 cell-injected animals. Given this unexpected result, we hypothesized that in vivo, Du145 cells use a different CXC chemokine to regulate tumorigenicity. We reasoned, based on the correlations of CXC chemokine expression with tumor growth (Figure 4) ▶ , that the most likely angiogenic CXC chemokine candidate, other than IL-8, to be mediating Du145 tumorigenicity would be GRO-α. As shown in Figure 4 ▶ , GRO-α production positively correlated with Du145 tumor growth in vivo. However, production of ENA-78 and GRO-γ did not. Based on these observations and the knowledge that IL-8 was not a contributing factor to Du145 tumorigenesis, we hypothesized that Du145 cells may use GRO-α to contribute to their tumorigenic phenotype in vivo.
Du145 Prostate Cancer Cells Tumorigenicity Is Dependent on GRO-α not IL-8
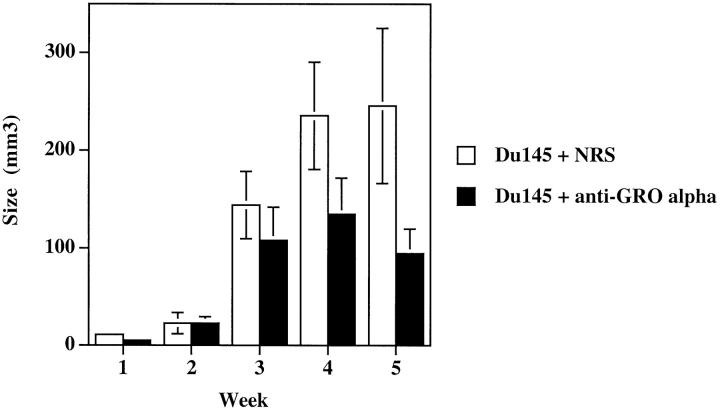
To determine whether GRO-α production in vivo by Du145 cells was contributing to Du145 tumorigenesis, we again used the strategy of a neutralizing antisera to GRO-α to deplete Du145 GRO-α production in vivo. Male SCID mice were injected subcutaneously with 1 × 10 6 Du145 cells on each rear flank. At time of cell innoculum, half of the animals were given a 0.5-ml injection of a rabbit polyclonal anti-human GRO-α antisera and half were given a 0.5-ml injection of normal rabbit serum (NRS) as a control. Animals received injections every 48 hours i.p. for a 5-week period. The Du145 tumor-bearing mice treated with anti-GRO-α antisera displayed a 62% reduction (P = 0.03) in tumor volume as compared with NRS-treated controls (Figure 7) ▶ . The vascularity index for Du145 tumors treated with NRS was 3503 ± 1570 and for Du145 tumors treated with anti-GRO-α, it was 1876 ± 710, a 47% reduction. Because in vitro studies had demonstrated that the addition of recombinant human GRO-α or neutralizing antibodies to GRO-α failed to alter the proliferative capacity of Du145 cells in vitro (Table 1) ▶ , this suggested that the neutralization of tumor-derived GRO-α did not alter the proliferation of the tumor cells directly but rather reduced the angiogenic potential of the tumor microenvironment. These data clearly demonstrate that Du145 cells use GRO-α, a distinctly different CXC chemokine than IL-8, to regulate tumorigenicity in vivo.
Figure 7.
GRO-α is a tumorigenic factor for Du145 cells. Male SCID mice were injected bilaterally in the rear flanks with 1 × 10 6 Du145 tumor cells on day 0. Starting on day 0 animals were given 0.5-ml i.p. injections of either rabbit anti-human GRO-α or NRS as a control. Graph represents weekly tumor measurements.
When tumor homogenates from the Du145-tumor-bearing mice treated with either NRS or anti-GRO-α antisera were tested in the CMP assay, three of five eyes were positive in the Du145 + NRS group. In contrast, tumor homogenates from the Du145 + anti- GRO-α group resulted in a reduced neovascular response or a negative response (Figure 8) ▶ in four of five corneas tested.
Figure 8.
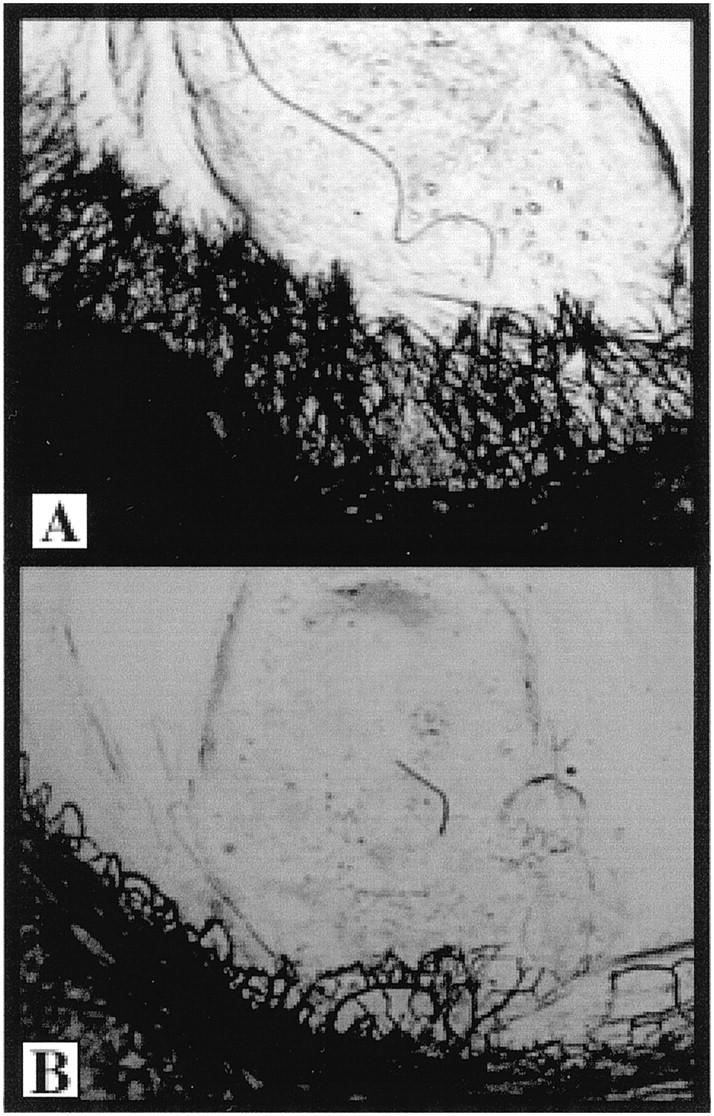
Du145 tumor homogenates from anti-GRO-α treated animals have less angiogenic activity than NRS-treated animals. Tumors were excised from antibody-treated Du145 tumor-bearing mice, homogenized, and analyzed in the corneal micropocket assay. A represents the angiogenic response seen in three of five eyes tested from the NRS-treated animals. B represents the negative response seen in four of five eyes tested from the anti-GRO-α treated animals.
Taken together, these data clearly indicate that the prostate cancer cell lines PC-3 and Du145 express angiogenic activity both in vitro and in vivo. Angiogenic activity of the PC-3 cell line can be inhibited by neutralizing Abs to IL-8, whereas Du145 angiogenic activity can be diminshed by neutralizing antisera to GRO-α.
Discussion
Angiogenic CXC chemokines are present in several human tumors, however, proof that these molecules are directly contributing to the tumorigenicity of the neoplasms has only been recently documented. In NSCLC, IL-8 is a predominant angiogenic factor contributing to the overall tumorigenicity in an animal model using a lung adenocarcinoma cell line, A549. 13 In addition, ENA-78 has been shown to be an angiogenic factor in NSCLC using both the A549 cell line as well as a squamous cell carcinoma cell line, Calu 1. 25 In melanoma, GRO-α has been shown to have both mitogenic and angiogenic effects on tumor growth. 17 Whereas previous reports have shown a correlation between IL-8 expression and metastatic potential using in situ hybridization with PC-3 subclones 26 or shown IL-8 expression in human prostate cancer specimens, 16 we have now directly demonstrated that human prostate cancer tumorigenicity is positively regulated by distinct angiogenic CXC chemokines. It is important to note that these experiments were carried out in SCID mice, thus we have investigated the tumorigenic potential of these molecules in the absence of immune effector cell (T- and B-cell) infiltration. Because these studies used whole Ig to neutralize the CXC chemokines in vivo, one formal possibility to explain the reduced tumor growth could be formation of immune complexes with subsequent ADCC via nonimmune inflammatory cells within the tumor microenviornment. However, a few observations make this hypothesis unlikely. The first is that previous experiments comparing neutralizing or control Ab-treated mice with untreated mice show no histological or tumor volume differences between control Ab-treated and untreated mice, suggesting that there was no obvious immune complex formation. Another observation is that there is no prominent inflammatory cell infiltration seen in these tumors histologically, again suggesting there is no accumulation of ADCC effector cells in this model system. Given that ELR-positive CXC chemokines are known to be neutrophil chemoattractants, it is interesting that there is no marked neutrophil influx in these tumors histologically. This is perhaps explained by the fact that these tumors are secreting high enough circulating levels of these chemokines that the neutrophils are desensitized and unable to sense a local chemotactic gradient. This simulates the phenomenon recently described in the IL-8 transgenic mouse. 27
We have shown that the prostate cancer cell lines, PC-3 and Du145 constitutively express higher levels of angiogenic CXC chemokines than do LNCaP cells and grow progressively in SCID mice. Furthermore, a normal prostate epithelial cell line (PrEC) obtained from Clonetics (San Diego, CA) cultured for 96 hours produced only 0.5 ng/ml IL-8 (25-fold less than PC-3). Futhermore, levels of GRO-α, ENA-78, and GRO-γ were all reduced by at least 20-fold in PrEC as compared with PC-3, suggesting that consitutive over-expression of angiogenic CXC chemokines may be a marker of neoplastic transformation. In addition, we have demonstrated that these molecules work as angiogenic factors for tumor neovascularization and not as mitogenic factors for the tumor cells directly. In contrast, the LNCaP prostate cancer cell line, which does not express high levels of angiogenic CXC chemokines, does not form subcutaneous tumors in SCID mice. These observations suggest that CXC chemokine production contributes to the overall tumorigenicity of prostate cancer in this model. However, different prostate cancer cell lines appear to use distinct angiogenic CXC chemokines to modulate their tumorigenicity. The PC-3 cell line uses IL-8, whereas the Du145 cell line uses GRO-α. Interestingly, the Du145 cell line expresses IL-8 at high levels constitutively in vitro but seems to express GRO-α at higher levels in vivo. Furthermore, the PC-3 cell line produces GRO-α and GRO-γ both in vitro and in vivo, however, these molecules fail to demonstrate a direct correlation with tumor growth in vivo. These data suggest that it may be difficult to determine by in vitro measurements alone which angiogenic CXC chemokines may be important in vivo. Furthermore, CXC chemokine expression alone may not dictate tumorigenic potential per se. It is likely that other metastatic factors such as adhesion molecules and matrix metalloproteinases must also be expressed for successful tumor growth and metastases.
We have now shown that different cancer cell lines from the same general cancer type can use distinct angiogenic CXC chemokines. This difference in utilization may severely limit the usefulness of antineovascular therapies aimed at neutralizing angiogenic CXC chemokine ligands (eg, neutralizing IL-8 Abs). Instead, these data suggest that a better alternative for therapeutic intervention may be to block the action of multiple angiogenic CXC chemokines by using either receptor antagonists or agents that directly inhibit angiogenesis. The receptor for all angiogenic CXC chemokines appears to be CXCR2. Interestingly, this receptor is present not only on tumor cells but also tumor-associated endothelial cells in melanoma. 17 Furthermore, this receptor is associated with head and neck cancers as well as breast cancer . 28,29 Immunotherapy aimed at blocking CXCR2 is attractive because it could potentially limit the angiogenic signal from all angiogenic CXC chemokines. The only caveat to this is that in humans there are two receptors for IL-8. The second is CXCR1, a high affinity receptor for IL-8, which is relatively specific for IL-8, and does not bind most other ELR-positive angiogenic CXC chemokines. 30 The contribution of this receptor to IL-8-mediated tumorigenicity cannot be modeled in mice because there is no CXCR1 homologue in mice. 31 However, the angiogenic signaling of the CXCR1 receptor is questionable because our preliminary data has shown that αCXCR2 Ab blocks human endothelial cell chemotaxis to IL-8 in vitro, whereas αCXCR1 Ab does not (unpublished observation). Of interest, the PC-3 and Du145 cell lines had undetectable levels of CXCR1 and CXCR2 cell surface staining by FACS analysis, whereas human neutrophils were strongly positive for both receptors.
Another potential therapy for tumor-induced angiogenesis may be to block the action with angiostatic molecules. In this regard, it is interesting that there are also angiostatic members of the CXC chemokine family. The angiogenic CXC chemokines all share the Glu-Leu-Arg motif (the ELR motif) immediately preceding the first conserved cysteine amino acid in the primary structure. All CXC chemokine members which do not have the ELR motif are, in fact, angiostatic. 10 The angiostatic members include the molecules PF4, IP-10, and MIG. Interestingly, the PC-3 and Du145 cell lines show very low levels of these molecules when analyzed either in vitro or in vivo (data not shown). Furthermore, because the antichemokine Ab treatment never diminished tumor growth completely, it is reasonable to assume that other, nonchemokine, angiogenic factors may be present. IL-6 has been documented to be a paracrine growth factor for LNCaP cells and an autorcrine growth factor for both PC-3 and Du145 cells, which express the IL-6 receptor. 32,33 Because IL-6 is not an angiogenic factor, it is possible that a combination therapy aimed at eliminating the mitogenic effects of IL-6 as well as the angiogenic effects of the CXC chemokines could be more efficacious. Furthermore, although the LNCaP cell line alone does not form tumors in SCID mice when grown subcutaneously, other investigators have reported significant androgen-dependent LNCaP cell growth in 88% of nude mice when LNCaP cells were injected subcutaneously with 0.25 ml of Matrigel. 34 It would be interesting to see which angiogenic factors are predominating in this instance. Two likely candidates may be VEGF and bFGF. In fact, VEGF and bFGF have been demonstrated in both PC-3 and Du145 cells previously, 16,26,35 and LNCaP growth in Matrigel has been shown to be augmented by bFGF. 36 Interestingly, bFGF, but not VEGF, has been shown to be expressed at higher levels in highly metastatic subclones of prostate cancer cell lines. 26 Using in situ hybridization and immunohistochemistry, we have found that IL-8 is present in a higher percentage of NSCLC tumor cells in a metastatic lesion than in the primary lesion 18 (unpublished observation). It will be interesting to determine whether this observation holds true for prostate cancer as well. Taken together, these data would support the notion that angiogenic CXC chemokines are important angiogenic factors regulating both primary tumorigenesis as well as metastasis and suggest that therapeutic strategies aimed at antagonizing CXC chemokine action should be beneficial in treating many solid tumors.
Footnotes
Address reprint requests to Dr. Robert Strieter, Department of Internal Medicine/Pulmonary, 6301 MSRB III, 1150 W. Medical Center Drive, Ann Arbor, MI 48109-0642. E-mail: rstriete@umich.edu.
Supported by Grants CA79046 (B. B. Moore), CA72543 (D. A. Arenberg), and CA66180, P50HL56402, and P50HL60289 (R. M. Strieter).
References
- 1.Parker S, Tong T, Bolden S, Wingo P: Cancer statistics, 1996. Cancer J Clin 1996, 46:5-27 [DOI] [PubMed] [Google Scholar]
- 2.Maiorana A, Gullino PM: Acquisition of angiogenic capacity and neoplastic transformation in the rat mammary gland. Cancer Res 1978, 38:4409-4414 [PubMed] [Google Scholar]
- 3.Bouck N: Tumor angiogenesis: the role of oncogenes and tumor suppressor genes. Cancer Cells 1990, 2:179-185 [PubMed] [Google Scholar]
- 4.Walz A, Kunkel SL, Strieter RM: CXC chemokines-an overview: chemokines in disease. Edited by AE Koch, RM Strieter. Austin, TX, R.G. Landes, 1996, pp 1–26
- 5.Strieter RM, Kunkel SL: Chemokines and the lung. Lung: Scientific Foundations, 2nd edition. R Crystal, J West, E Weibel, P Weibel. New York, Raven Press, 1997, pp 155–186
- 6.Baggiolini M, Dewald B, Walz A: Interleukin-8 and related chemotactic cytokines. Inflammation: Basic Principles and Clinical Correlates. JI Gallin, IM Goldstein, R Snyderman. New York, Raven Press, Ltd., 1992, pp 247–263
- 7.Miller MD, Krangel MS: Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol 1992, 12:17-46 [PubMed] [Google Scholar]
- 8.Taub DD, Oppenheim JJ: Chemokines, inflammation, and immune system. Therapeutic Immunol 1994, 1:229-246 [PubMed] [Google Scholar]
- 9.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA: The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 1996, 382:829-833 [DOI] [PubMed] [Google Scholar]
- 10.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan S, Roczniak S, Shanafelt A: The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995, 270:27348-27357 [DOI] [PubMed] [Google Scholar]
- 11.Strieter RM, Kunkel SL, Shanafelt AB, Arenberg DA, Koch AE, Polverini PJ: CXC chemokines in regulation of angiogenesis. Chemokines in Disease. AE Koch, RM Strieter. Austin, TX, R.G. Landes, 1996, pp 195–210
- 12.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM: Inhibition of IL-8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994, 179:1409-1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM: Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest 1996, 97:2792-2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler I, Tahara E: Expression of interleukin-8 correlates with vascularity in human gasric carcinomas. Am J Pathol 1998, 152:93-100 [PMC free article] [PubMed] [Google Scholar]
- 15.Yoneda J, Kuniyasu H, Crispens M, Price J, Bucana C, Fidler I: Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst 1998, 90:447-454 [DOI] [PubMed] [Google Scholar]
- 16.Ferrer F, Miller L, Andrawis R, Kurtzman S, Albertsen P, Laudone V, Kreutzer D: Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology 1998, 51:161-167 [DOI] [PubMed] [Google Scholar]
- 17.Luan J, Shattuck-Brandt R, Hagnegahdar H, Owen J, Strieter R, Burdick M, Nirodi C, Beaauchamp D, Johnson K, Richmond A: Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol 1997, 62:588-597 [DOI] [PubMed] [Google Scholar]
- 18.Arenberg DA, Kunkel SL, Burdick MD, Polverini PJ, Strieter RM: Treatment with anti-IL-8 inhibits non-small cell lung cancer tumor growth (meeting abstract). J Invest Med 1995, 43:479A [Google Scholar]
- 19.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass M, Taub DT, Iannetoni MD, Whyte RI, Strieter RM: Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med 1996, 184:981-992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM: Interleukin-8 (IL-8) as a macrophage-derived mediator of angiogenesis. Science 1992, 258:1798-1801 [DOI] [PubMed] [Google Scholar]
- 21.Strieter RM, Kunkel SL, Elner VM, Martonyl CL, Koch AE, Polverini PJ, Elner SG: Interleukin-8: a corneal factor that induces neovascularization. Am J Pathol 1992, 141:1279-1284 [PMC free article] [PubMed] [Google Scholar]
- 22.Kaighn M, Narayan N, Ohnuki Y, Lechner J, Jones L: Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979, 17:16-23 [PubMed] [Google Scholar]
- 23.Mickey D, Stone K, Wunderli H, Mickey G, Bollmer R, Paulson D: Heterotransplantation of a human prostatic adenocarcinoma cell line in nude mice. Cancer Res 1977, 37:4049-4058 [PubMed] [Google Scholar]
- 24.Horoszewicz J, Leong S, Kawinski D, Karr J, Tosenthal H, Chu T, Mirand E, Murphy AG: LNCaP model of human prostatic carcinoma. Cancer Res 1983, 43:1809-1818 [PubMed] [Google Scholar]
- 25.Arenberg D, Keane M, DiGiovine B, Kunkel S, Morris S, Xue Y, Burdick M, Glass M, Iannettoni M, Strieter R: Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 1998, 102:465-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene G, Kitadai Y, Pettaway C, Eschenbach AV, Bucana C, Fidler I: Correlation of metastasis-related gene expression with metastatic potential in human prostate carcinoma cells implanted in nude mice using an in situ messenger RNA hybridization technique. Am J Pathol 1997, 150:1571-1582 [PMC free article] [PubMed] [Google Scholar]
- 27.Simonet WS, Hughes TM, Nguyen HQ, Trebasky LD, Danilenko DM, Medlock ES: Long term impaired neutrophil migration in mice overexpressing human interleukin-8. J Clin Invest 1994, 94:1310-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards B, Eisma R, Spiro J, Lindquist R, Kreutzer D: Coexpression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg 1997, 174:507-512 [DOI] [PubMed] [Google Scholar]
- 29.Youngs S, Ali S, Taub D, Rees R: Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer 1997, 71:257-266 [DOI] [PubMed] [Google Scholar]
- 30.Holmes W, Lee J, Kuang W, Rice G, Wood W: Structure and functional expression of a human interleukin-8 receptor. Science 1991, 253:1278-1280 [PubMed] [Google Scholar]
- 31.Cacalano G, Lee J, Kiikly K, Ryan A, Pitts-Meek S, Hultgren B, Wood W, Moore M: Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science 1994, 265:682-684 [DOI] [PubMed] [Google Scholar]
- 32.Siegsmund MJ, Yamazaki H, Pastan I: Interleukin-6 receptor mRNA in prostate carcinomas and benign prostate hyperplasia. J Urol 1994, 151:1396-1399 [DOI] [PubMed] [Google Scholar]
- 33.Okamoto M, Lee C, Oyasu R: Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res 1997, 57:141-146 [PubMed] [Google Scholar]
- 34.Lim D, Liu X, Sutkowski D, Braun E, Lee C, Kozlowski J: Growth of an androgen-sensitive human prostate cancer cell line, LNCaP, in nude mice. Prostate 1993, 22:109-118 [DOI] [PubMed] [Google Scholar]
- 35.Joseph I, Isaacs J: Potentiation of the antiangiogenic ability of linomide by androgen abation involves down-regulation of vascular endothelial growth factor in human androgen-responsive prostatic cancers. Cancer Res 1997, 57:1054-1057 [PubMed] [Google Scholar]
- 36.Passaniti A, Isaacs J, Haney J, Adler S, Cujdik T, Long P, Kleinman H: Stimulation of human prostatic carcinoma tumor growth in athymic mice and control of migration in culture by extracellular matrix. Int J Cancer 1992, 51:318-324 [DOI] [PubMed] [Google Scholar]