Abstract
Differential gene expression between the androgen sensitive human prostate cancer cell line LNCaP and an insensitive clonal variant, LNCaP-r, was demonstrated by suppression subtractive hybridization. Twenty-one sequences were identified of which 9 are homologous to known genes, 11 are represented by expressed sequence tags (ESTs), and 1 is novel. We present data for 5 of 7 sequences confirmed to be differentially expressed by Northern blot analysis and semiquantitative RT-PCR. Only one gene, fibronectin (FN), was highly overexpressed (>60-fold) in LNCaP-r cells, consistent with previously reported overexpression of FN in prostate cancer. Four sequences were down-regulated in LNCaP-r cells, including an inactive variant of the E2 ubiquitin conjugating enzyme (UEV-1), a novel metalloproteinase-related collagenase (PM5), and a potential tumor suppressor gene (breast basic conserved gene, BBC1). UEV-1 is multifunctional, regulates the cell cycle via cdk1, has homology to MMS2 and likewise functions as a DNA protection protein, and also has homology to TSG101. Aberrant splice variants of TSG101 occur frequently in both breast and prostate cancer, but its mechanism of action is unknown. FN, BBC1, and UEV-1 localize to regions of chromosomal aberration (2q3.4, 16q24.3, and 20q13.2, respectively) associated with advanced prostate cancer and thus may be highly relevant to disease progression.
Androgens stimulate growth of both the normal prostate and prostate cancer (CaP). 1 An initial response to anti-androgenic hormonal therapy is observed in 70–80% of patients with advanced CaP, but progression to an androgen-insensitive (AI) state occurs within 12–18 months 2,3 and after relapse there is no effective curative treatment. 4 Mechanisms underlying the androgen-sensitive (AS)-to-AI transition are unknown 5 but are thought to involve the androgen receptor (AR) gene and its downstream signaling pathways. There are numerous reports of AR mutations resulting in altered AR function (AR mutation database) 6 but controversy exists as to the frequency of their occurrence. 6-9 Overexpression of AR due to gene amplification is observed in 30% of hormone refractory tumors, which would enhance the ability of CaP cells to sustain growth at substantially reduced concentrations of androgens. 10,11 AR-mediated gene transcription is regulated not only by androgens but also by polypeptide growth factors, 12,13 retinoic acid receptors, 14 and cyclic AMP. 15 Each of these factors may also affect AS-to-AI transition. However, this transition may not be attributable exclusively to AR dysfunction but could result from an accumulation of other genetic aberrations that confer a relative growth advantage independent of AR. 5,16 Genetic changes in CaP have been analyzed by allelotyping, loss of heterozygosity, and comparative genomic hybridization to identify target genes underlying the transition of CaP from the AS to the AI state. In early stage CaP, loss of 8p is characteristic whereas loss of 2q, 5q, 10q, 11p, 13q, 16q, 17p, and 20q, gains of 8q and chromosome 7, and mutation or amplification of the AR gene are associated with the progression of metastatic AI CaP. 17,18 In addition to somatic mutations, germline defects account for 9% of all CaP, and one-third of these cases are coincident with the hereditary prostate cancer gene locus at 1q24–25. 19
The androgen-sensitive human CaP cell line LNCaP 20 has been used to study the control of androgen-responsive genes. 21-24 A clonal variant, LNCaP-r, 25 exhibits androgen insensitivity while expressing AR levels similar to those of LNCaP. 26,27
Our objective was to use the LNCaP/LNCaP-r in vitro CaP model reflecting the in vivo situation of CaP progression from the AS to the AI state and to identify candidate genes that were differentially expressed. We have identified three genes, fibronectin (FN), breast basic conserved gene-1 (BBC1), and ubiquitin enzyme variant-1 (UEV-1), which have not previously been reported to be associated with the development of AI CaP. These three genes map to 2q, 16q, and 20q, chromosomal regions known to be altered in advanced CaP.
Materials and Methods
Cell Culture
The prostate carcinoma cell lines LNCaP, PC-3, and DU-145 were purchased from the American Type Culture Collection (Manassas, VA). LNCaP-r 25 was a gift from Dr. van Steenbrugge (Erasmus University, Rotterdam, The Netherlands). The SV40 immortalized primary prostatic epithelial cell lines PNT1A, PNT1B, PNT2, and PSVF1 were a gift from Professor N. Maitland (York University, York, UK). All cell lines were maintained in Dulbecco’s modified Eagle’s medium without phenol red supplemented with 10% fetal bovine serum and cultured to 95% confluence for RNA and DNA extraction.
Isolation of DNA and Arbitrarily Primed-Polymerase Chain Reaction (PCR)
Genomic DNA was isolated from all cell lines using a standard proteinase K procedure 28 and used as a template for arbitrarily primed-PCR. 29 Amplification mixtures consisted of 50 ng DNA, 10 mmol/L Tris-Cl (pH 9.0), 50 mmol/L KCl, 0.01% gelatin (w/v), 1.5 mmol/L MgCl2, 200 μmol/L dNTPs, 1.5 U Taq polymerase (Promega, Madison, WI) and 0.2 μmol/L of either arbitrary primer BS57 or BS58 (Table 1) ▶ in a total volume of 25 μl. Thermal cycling conditions consisted of 1 minute at 94.5°C, 1 minute at 40°C, and 1 minute at 72°C for 45 cycles with a final extension at 72°C for 10 minutes. Amplified products were analyzed on a 2% agarose gel and visualized with ethidium bromide (0.5 μg/ml).
Table 1.
Gene-Specific Primers Used for SQRT-PCR
| Code | Gene | bp | Sequence (5′ → 3′) | Reference |
|---|---|---|---|---|
| G0934 | AR | 19 | AGC TAC TCC GGA CCT TAC G | 34 |
| G0935 | 21 | AGG TGC CAT GGG AGG GTT AG | ||
| B504 | GAPDH | 20 | GCC ACA TCG CTC AGA CAC CA | 34 |
| B505 | 20 | GAT GAC CCT TTT GGC TCC CC | ||
| PSA3 | PSA | 20 | CAC AGA CAC CCC ATC CTA TC | 59 |
| PSA5 | 20 | GAT GAC TCC AGC CAC GAC CT | ||
| FIBS | Fibronectin | 18 | TCA GGA AGC ATC GTT GTG | 55 |
| FIBAS | 21 | ACA CTT TCC TTG TCA TCC TTG | ||
| U2 | UEV-1A/1As | 24 | ATG CCA GGA GAG GTT CAA GCG TCT | 33 |
| U4 | 23 | TTA ATT GCT GTA ACA CTG TCC TT | ||
| BBC1-F | BBC1 | 18 | TTT CCG CTC GGC TGT TTT | 35 |
| BBC1-R | 19 | CGA CTG ATT CCA AGT CCC C | ||
| PM5-F | PM5 | 20 | ACC GAT TCT GCC TGT CCA AG | 46 |
| PM5-R | 20 | TTT TCT CCC TCT CCT GCC TG | ||
| BS57 | 10 | GGAAGCAGCT | 60 | |
| BS58 | 10 | CAGTGAGCGT | ||
| GAP-S | GAPDH | 20 | ACC ACA GTC CAT GCC ATC AC | Clontech |
| GAP-AS | GAPDH | 20 | TCC ACC ACC CTG TTG CTG TA | |
| NP1 | 22 | TCG AGC GGC CGC CCG GGC AGG T | Clontech | |
| NP2R | 20 | AGC GTG GTC GCG GCC GAG GT |
AR, androgen receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PSA, prostate-specific antigen; BBC1, breast basic conserved gene; UEV-1A/1As, ubiquitin enzyme variant.
Isolation of RNA, poly(A)+ RNA, and cDNA Synthesis
Total RNA was isolated from both LNCaP-r and LNCaP cells by the RNAzol B method (Biogenesis, Friendswood, TX) as previously described. 30 Poly(A)+ RNA was purified from total RNA using the Oligotex Direct mRNA purification procedure (Qiagen Ltd., Crawley, UK). Double-stranded cDNA was synthesized from 2 μg of poly(A)+ RNA as previously described, 31 except that no tracer reaction was used and the samples were incubated at 16°C for 2 hours, followed by a further 30 minutes after the addition of T4 polymerase.
Generation of Subtracted Libraries for both LNCaP-r and LNCaP by Subtractive Suppression Hybridization (SSH)
SSH was performed as previously described 31,32 using the PCR-Select Subtraction protocol (Clontech, Palo Alto, CA) according to the manufacturer’s recommendations. Differentially expressed genes were amplified from the subtracted cDNA using a nested PCR protocol (PCR-Select Subtraction). 32 Nested primers (NP1 and NP2R, Table 1 ▶ ) specific to adapters ligated only to the tester cDNA were used to amplify differentially expressed sequences. Thermal cycling conditions were 94°C for 10 seconds, 68°C for 30 seconds, and 72°C for 1.5 minutes for 30 cycles (initial PCR) and 12 cycles (nested PCR). One-third of the subtracted and unsubtracted products were separated on a 1.5% agarose gel.
Evaluation of Subtraction Efficiency
Subtraction efficiency was determined by the PCR analysis of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression in subtracted and unsubtracted cDNA libraries of both LNCaP-r and LNCaP cell lines as described in the PCR-Select Subtraction protocol (Clontech).
Cloning and Colony PCR
Each positively subtracted cDNA library (LNCaP-r and LNCaP) was ligated into the pT-Advantage vector, a TA cloning system, and transformed into TOP10F′ super competent cells (Clontech). Aliquots (100 μl) of Lauria Bertani media supplemented with ampicillin (50 μg/ml) were inoculated with positive colonies and incubated overnight at 37°C. Plasmids containing cloned sequences from each subtracted library were identified using a colony PCR protocol based on the PCR-Select Differential Screening protocol (Clontech). 31 Briefly, inserts were amplified from each clone, 1× AmpliTaq Gold PCR buffer (10 mmol/L Tris (pH 9.0), 50 mmol/L KCl, 0.01% gelatin, 0.1% Triton X-100; Perkin Elmer, Foster City, CA), 1.5 mmol/L MgCl2, 500 mmol/L, 200 μmol/L dNTPs, 0.3 μmol/L each nested primer (NP1 and NP2R) (Table 1) ▶ and 2.5U AmpliTaq Gold polymerase (Perkin Elmer) in a final volume of 20 μl. Thermal cycling was performed at 95°C for 10 minutes (×1), 94°C for 1 minute and 68°C for 3 minutes (×27 cycles). PCR products (5 μl) were separated on a 1.5% agarose gel.
Dot Blots of Positive Colony PCR Products
Each positive colony PCR product was denatured in an equal volume (10 μl) of 0.6 M NaOH and 0.5% bromophenol blue and 1 μl dot-blotted onto a nylon membrane (GeneScreen Plus, DuPont, NEN Research Products, Boston, MA). Two Clontech control cDNAs were used, C1 (340 bp) and C2 (200 bp), corresponding to a human homologue of the mouse testis specific protein (GenBank accession number X52128) and human seminal vesicle-specific semenogelin II mRNA (GenBank accession number M91652). Membranes were prepared in quadruplicate, soaked in 0.5 mol/L Tris (pH 7.4) for >5 minutes, washed with water, and UV cross-linked (120 mJ) in a Stratalinker (Stratagene).
Reverse RNA Dot Blot Analysis and Sequencing Positive Clones
Probes for both subtracted and unsubtracted libraries from both LNCaP-r and LNCaP cell lines were prepared as described in the PCR-Select Differential Screening protocol (Clontech). RNA dot blot membranes were prehybridized in Express Hybridization solution (Clontech) supplemented with 0.2× SSC, 0.01 volume of blocking solution (100 μg/ml denatured sheared salmon sperm DNA and 3 μg/ml primers NP1 and NP2R) (Table 1) ▶ at 72°C for >60 minutes. Probes were labeled by random priming with 32P-dCTP, heat denatured, mixed with SSC and blocking solution (50 μl 20× SSC and 50 μl blocking solution), added to the membranes, and allowed to hybridize at 72°C overnight. Membranes were washed at 68°C four times (20 minutes each) with 100 ml of low-stringency (2× SSC and 0.5% sodium dodecyl sulfate (SDS)) washing solution followed by twice (20 minutes each) with high-stringency (0.2× SSC and 0.5% SDS) washing solution. Membranes were exposed to X-ray film from 1 to 12 hours at −70°C with intensifying screens.
Plasmid DNA was extracted from positive clones, identified by reverse RNA dot blot analysis using the Qiagen MiniPrep purification procedure, and then sequenced using an ABI 377 automatic sequencer (Perkin-Elmer). Sequences were compared with the GenBank, EMBL, dbEST, and THC databases using a BLAST search.
Northern Blot Analysis
Total RNA (30 μg/lane) was separated by denaturing (formaldehyde) gel eletrophoresis, transferred to GeneScreen Plus membranes, and hybridized with 32P-labeled probes of either an EcoRI fragment of DNA from a positive clone or a 0.8-kb EcoRI/HindIII fragment of GAPDH. Membranes were prehybridized for ≥1 hour and hybridized for ≥12 hours in a buffer containing 2× SSC, 50% v/v formamide, 10% dextran sulfate, 0.1% SDS, and 100 μg/ml denatured sheared salmon sperm DNA at 42°C. Posthybridization washes consisted of 2× SSC for 10 minutes at room temperature, followed by 1 or 2 washes with 2× SSC and 1% SDS for 20 minutes at 65°C. In some cases in which higher stringency was required, two extra washes were performed with 0.2× SSC and 1% SDS for 1 hour at 65°C. Membranes were then exposed to film for 1–7 days at −70°C with intensifying screens and the resultant autoradiograms were analyzed on a Molecular Dynamics densitometer with Image Quant software.
Semiquantitative Reverse Transcriptase (SQRT)-PCR
Oligo dT12–18-primed cDNA was synthesized from total RNA as previously described 30 and stored at −20°C until use. Aliquots of the cDNA were used for PCR amplification of either the 1.3-kb of the AR-3′ untranslated region or the 483-bp of GAPDH as previously described for 30 and 25 cycles, respectively. 30 All cDNAs were normalized to give the same relative expression of GAPDH before they were used to determine the relative expression of other genes of interest.
Prostate-specific antigen (PSA) PCR consisted of 1× AmpliTaq Gold PCR buffer (10 mmol/L Tris (pH 9.0), 50 mmol/L KCl, 0.01% gelatin, 0.1% Triton X-100; Perkin Elmer), 1.5 mmol/L MgCl2, 500 mmol/L, 200 μmol/L dNTPs, 0.2 μmol/L each primer (PSA3 and PSA5; Table 1 ▶ ) and 2.5 U AmpliTaq Gold polymerase (Perkin Elmer) in a final volume of 25 μl. A hot start, touch down thermal protocol was used consisting of 1 cycle for 10 minutes at 95°C, 2 cycles for 1 minute at 95°C, 1 minute at 61°C, 2 minutes at 72°C, followed by a decrease in annealing temperature of 2°C every 2 cycles until 55°C, then continued for a further 27 cycles. Fibronectin (FN), basic breast conserved gene (BBC1), and ubiquitin enzyme variant (UEV-1) were amplified using the same reaction conditions as used for the PSA PCR; the primers for each gene are listed in Table 1 ▶ . Thermal cycling for these genes consisted of a hot start protocol, 1 cycle at 95°C for 10 minutes, followed by 25–35 cycles at 95°C for 1 minute, 55–60°C for 1 minute, and 72°C for 2 minutes, with a final extension at 72°C for 5 minutes.
PCR products were separated on a 2% agarose gel and visualized by staining with ethidium bromide (0.5 μg/ml). To verify PCR products, bands migrating at the predicted sizes were gel-purified using the Hybaid II gel purification system (Hybaid, Ashford, UK) and sequenced on an ABI 377.
Results
Characterization of LNCaP and LNCaP-r Cell Lines
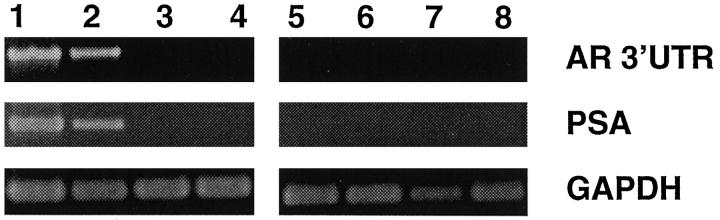
LNCaP and LNCaP-r cell lines express the full length AR message, although the expression is detectably higher in LNCaP than LNCaP-r as determined by SQRT-PCR normalized for GAPDH expression (Figure 1) ▶ . PSA expression, an androgen responsive gene, is approximately threefold lower in LNCaP-r compared to the LNCaP cell line (Figure 1) ▶ . In PC-3, DU-145, and all of the immortalized epithelial prostatic cell lines, AR and PSA expression were undetectable using these PCR conditions (Figure 1) ▶ .
Figure 1.
SQRT-PCR analysis of androgen receptor (AR), prostate specific antigen (PSA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. Lane 1: LNCaP; Lane 2: LNCaP-r; Lane 3: PC-3; Lane 4: DU-145; Lane 5: PNT1A; Lane 6: PNT1B; Lane 7: PSVF1; Lane 8: PNT2.
AP-PCR with primer BS58 resulted in a DNA profile that was similar in LNCaP, LNCaP-r, and PC-3 but different for DU-145 and the immortalized prostatic cell lines (data not shown). With primer BS57 the DNA profile for PC-3 was different than that of LNCaP and LNCaP-r (data not shown). Based on these data it is evident that LNCaP and LNCaP-r have similar predicted DNA profiles.
Identification of Differentially Expressed Genes by SSH
There are clear differences between the subtracted and unsubtracted cDNA libraries (Figure 2A) ▶ . The observable PCR products in the LNCaP and LNCaP-r subtracted libraries ranged between 0.3–0.7 kb and 0.3–1.3 kb, respectively (Figure 2A) ▶ .
Figure 2.
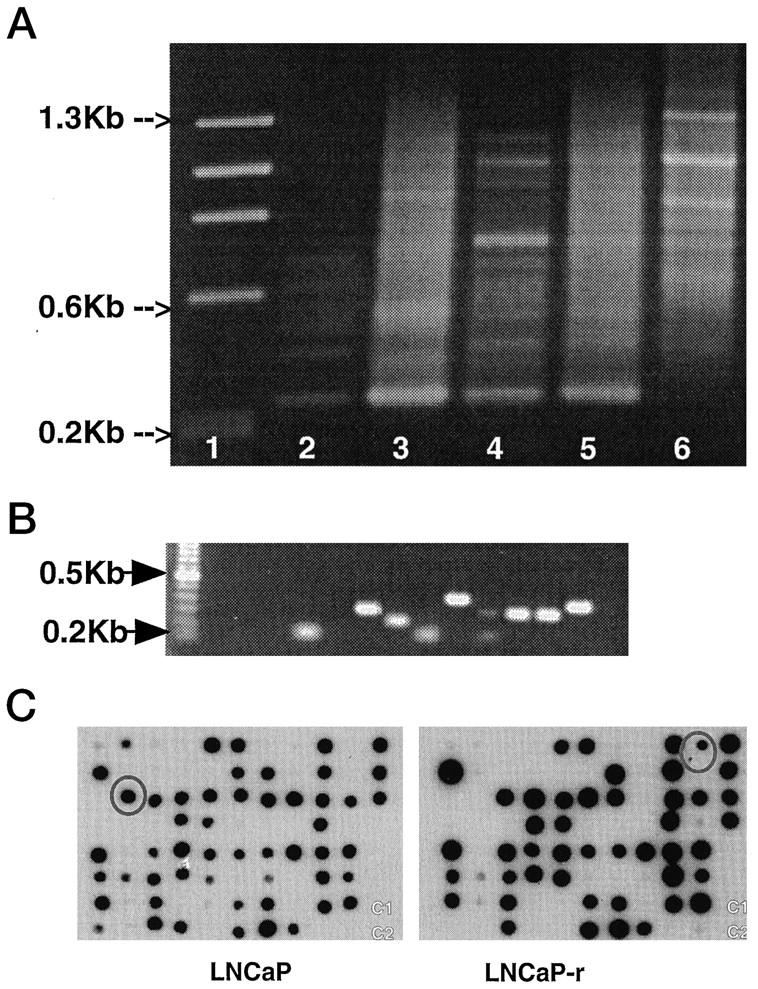
A: Identification of differential gene expression in LNCaP (L) (Lanes 2 and 3) and LNCaP-r (R) (Lanes 4 and 5) cells by SSH before cloning. PCR products of either subtracted (S) (Lanes 2 and 4) or unsubtracted (U) (Lanes 3 and 5) cDNA libraries are separated on a 1.5% TAE agarose gel. Lane 1: φX174 HaeIII markers; Lane 6: positive control for subtraction (φX174 HaeIII markers in placental cDNA). Primers and PCR conditions are described in Materials and Methods. B: An example of colony PCR, the products of which range from 0.2–0.8 kb. C: Reverse Northern screening assay for differentially expressed genes. Replica DNA dot blots were prepared and probed with either DNA from LNCaP (left panel) or LNCaP-r (right panel) subtracted libraries. Clones 49L and 17R are circled on left and right blots, respectively.
Estimation of Subtraction Efficiency
GAPDH is a nondifferentially expressed gene. Using a RT-PCR assay its level of expression in LNCaP/LNCaP-r libraries was used to determine the SSH subtraction efficiency. Its expression in both LNCaP and LNCaP-r subtracted libraries was detectable only after 33 cycles of PCR, whereas in the unsubtracted libraries higher levels were detected after only 18 cycles (data not shown).
Cloning and Colony PCR
A total of 140 colonies were isolated from both LNCaP-r and LNCaP subtracted libraries cloned into the pT-Advantage vector. The average insert size, as determined by colony PCR, was 300 bp (Figure 2B) ▶ . Positive colony PCR products 59 and 28 from LNCaP and LNCaP-r subtracted libraries, respectively, were isolated for reverse Northern analysis.
Reverse RNA Dot Blot Analysis
Quadruplicate filters containing LNCaP selected (49L) and LNCaP-r selected (17R) sequences were probed with either LNCaP or LNCaP-r subtracted libraries (Figure 2C) ▶ and the unsubtracted LNCaP and LNCaP-r cDNA libraries (data not shown). After 4 hours’ exposure at −70°C, 49L and 17R were clearly visible on both blots (Figure 2C) ▶ . A total of 14 LNCaP-positive and 7 LNCaP-r-positive clones (ie, differentially expressed) were isolated on the basis of the reverse RNA dot blot analysis. Positive clones represented approximately 30% of the colony PCR products and the remainder were species common to both the tester and driver cDNA populations. The selective nature of these blots was confirmed by the low background of the filters and by the lack of detection of two negative control cDNAs (C1 and C2) included on each filter (Figure 2C) ▶ even after 12 hours’ exposure (data not shown).
Northern Blot Analysis
To verify differential expression of clones identified by reverse RNA dot blot analysis, EcoRI-excised inserts were labeled and used to probe total RNA from LNCaP and LNCaP-r cells (Table 2 ▶ and Figure 3A ▶ ). The expression levels of the differentially expressed genes between the two cell lines ranged from >6000% (17R) to only 6% (13L) (Table 2 ▶ and Figure 3A ▶ ). The detected transcript sizes are shown in Figure 3A ▶ and the expected transcript sizes based on sequence homology for each clone are listed in Table 2 ▶ . The levels of differential gene expression determined by the inserts from clones 79L and 13L encoding Histone H1.2 and the transcription factor TAFII30, respectively, were below 10%, interpreted as within the range of experimental error. The lowest detectable change in expression, a 25% increase (LNCaP > LNCaP-r) using the insert from clone 85L, corresponded to a human proteasome subunit HsC10-II, which plays a role in the nonlysosomal pathways of protein turnover (Table 2 ▶ and Figure 3A ▶ ).
Table 2.
Characteristics of Differentially Expressed Genes in Androgen Dependent LNCaP and Androgen Independent LNCaP-r Prostate Cancer Cells
| Clone code | Insert (bp) | Homology (bp/bp) | Mapped to | Homology (accession code)* | mRNA size | RNA species† detected (kb) | %‡ | Reference |
|---|---|---|---|---|---|---|---|---|
| 17R | 450 | 352/371 | 3276–3647 | fibronectin (HSFIB1) | :8kb | 9–9.5 (R > L) | 6000 | 55 |
| 12L | 370 | 356/368 | 2066–2436 | HsUEV-1 (HS49278) | :3.5kb | 3.0 (L > R) | 300 | 33 |
| 28R | 300 | 298/300 | 410–708 | BBC1 (HSBBC1) | :1.3kb | 0.5 (R > L) | 200 | 35 |
| 49L | 643 | 634/643 | 1815–2449 | PM5 (HSPM5) | :4kb | 6.0 (L > R) | 168 | 46 |
| 85L | 353 | 304/306 | 388–692 | proteasome subunit HsC10-II (HSPSH1) | :0.7kb | 1.0 (L > R) | 125 | 61 |
| 79L | 284 | 277/279 | 925–1002 | Histone H1.2 (HSH12) | :1.3kb | 1.0 (L > R) | 108 | 62 |
| 13L | 284 | 284/284 | 1517–2028 | TAFII30 (HS13391) | :0.75kb | 1.0 (L > R) | 106 | 63 |
Differentially expressed genes were cloned and their expression verified by reverse Northern and Northern blot analysis. The inserts were sequenced and homology to other genes determined with a BLAST search of GenBank, EMBL, dbEST, and THC databases.
*All codes relate to the EMBL database.
†In which cell type the expression is greater, LNCaP (L) or LNCaP-r (R).
‡% increase normalized for GAPDH expression and the average of two samples for both LNCaP and LNCaP-r.
Figure 3.
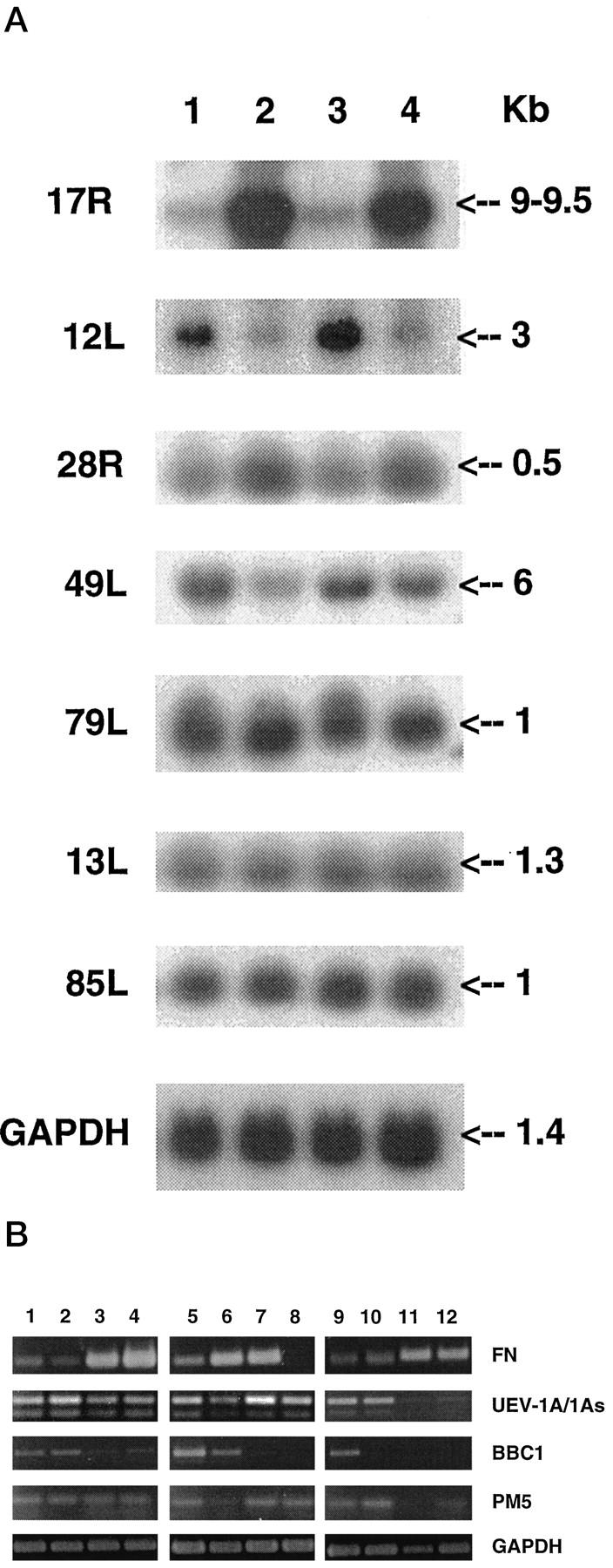
A: Northern blot analysis of differentially expressed sequences (Table1) in paired isolates of LNCaP and LNCaP-r. Total RNA (30 μg) for LNCaP and LNCaP-r was electrophoresed on a 1% denaturing gel, blotted onto nylon filters, and probed with EcoRI inserts isolated from the clones listed in Table 1 ▶ . All blots were reprobed with a control GAPDH cDNA probe (EcoRI-HindIII). Arrows, positions, and estimated sizes of detected mRNAs. Lanes 1 and 3: LNCaP; Lanes 2 and 4: LNCaP-r. B: SQRT-PCR analysis of fibronectin (FN), ubiquitin enzyme variants (UEV-1A/1As), breast basic conserved gene (BBC-1), PM5, and GAPDH. Lanes 1, 2, and 5: LNCaP; Lanes 3, 4, and 6: LNCaP-r; Lane 7: PC-3; Lane 8: DU-145; Lane 9: PNT1A; Lane 10 : PNT1B; Lane 11: PSVF1; Lane 12: PNT2.
SQRT-PCR analysis was used to verify specific differential expression of FN, UEV-1, BBC1, and PM5 genes encoded by clones 17R, 12L, 28R, and 49L, respectively. Results are detailed below in order of decreasing differential expression as demonstrated between LNCaP and LNCaP-r cell lines.
Fibronectin (Clone 17R)
There was a >60-fold increase in FN expression in LNCaP-r compared to LNCaP by Northern blotting (Figure 3A) ▶ , verified by SQRT-PCR using two sets of primers spanning nucleotides 3323–3690 (Figure 3B) ▶ and the FN IIICS region (data not shown). The hormone-insensitive cell lines PC-3 and DU-145 were also analyzed and abundant expression of FN was detected in the PC-3 cell line but there was no detectable FN in DU-145 (Figure 3B) ▶ . The SV40 immortalized cell lines PSVF1 and PNT2 expressed FN at levels comparable to those found in PC-3 and LNCaP-r, although lower FN levels were observed in PNT1A and PNT1B cells (Figure 3B) ▶ .
UEV-1 (Clone 12L)
A threefold difference in expression, LNCaP > LNCaP-r, was demonstrated by Northern analysis using the insert from clone 12L (Figure 3A) ▶ , which maps to nucleotides 2066–2436 in the 3′UTR of UEV-1. 33 Published primer sequences were used to determine which of the four known splice variants is responsible for this differential gene expression. 33 Differential expression (LNCaP > LNCaP-r) was verified with primers specific for splice variants 1A/1As (Figure 3B) ▶ and not with primers for 1B/1Bs variants (data not shown). Expression was highest in PC-3, moderate in LNCaP, DU-145, PNT1A, and PNT1B, lower in LNCaP-r, and undetectable in PSVF1 and PNT2 (Figure 3B) ▶ . Expression of UEV-1A does not appear to correlate with either AR or PSA gene expression in these cell lines.
BBC1 (Clone 28R)
The insert of clone 28R mapped to the 3′ coding region (nucleotides 410–709) of the 1.3-kb BBC1, 34 which shares homology with multiple ESTs of unknown function. Northern analysis using the EcoRI fragment from clone 28R revealed a twofold increase in expression (LNCaP-r > LNCaP) of a 0.5-kb gene (Figure 3A) ▶ . SQRT-PCR analysis with BBC1-specific primers 35 indicated that BBC1 (full coding sequence) was down-regulated in LNCaP compared with LNCaP-r (Figure 3B) ▶ , in contrast to the Northern blotting data (Figure 3B) ▶ , suggesting that the differential expression identified by SSH is due to the expression of one or more BBC1-like sequences. However, BBC1 expression in PC-3, DU-145, and the SV40 immortalized cell lines was detectable only in PNT1A cells (Figure 3B) ▶ .
PM5 (Clone 49L)
PM5 expression was 68% higher in LNCaP than LNCaP-r cells, as determined by Northern blot analysis, a difference that is difficult to verify by PCR (Figure 3B ▶ , lanes 1–6). Its levels varied in the other cell lines examined (Figure 3B) ▶ . The PM5 profile in the SV40 immortalized cells correlates directly with UEV-1A and inversely with FN expression.
Discussion
We have used SSH and identified five genes that are differentially expressed between LNCaP and LNCaP-r cell lines. SSH normalizes and positively selects differentially expressed genes in contrast to differential display, in which the relative abundance of genes affects the results. 36 In conjunction with reverse RNA dot blot analysis, SSH offers a high-throughput screening procedure for both high- and low-abundance transcripts. 31,32 The high background of clones common to both tester and driver is due to limited differences between the tester and driver mRNAs, as LNCaP-r is a clone derived from LNCaP. From an initial 140 clones isolated, approximately 15% were positive at the reverse Northern stage and a total of 10% after Northern blot analysis.
We have demonstrated that LNCaP-r cells express AR mRNA at levels similar to those found in LNCaP, but the expression of PSA is dramatically reduced in the former. LNCaP-r cell growth is independent of steroids and not stimulated by the synthetic androgen R1881. 26 Therefore, genes differentially expressed between these two CaP cell lines are potentially representative of genes expressed in vivo in hormone refractory tumors which express functional AR but are not androgen-sensitive. 7,37-39
FN-integrin interactions are important in tumor migration, invasion, and metastasis. Peptide and antibody inhibitors of FN and integrin interactions are effective inhibitors of metastasis 40 and reduced cellular expression of FN is associated with carcinogenesis in some cancers, such as colorectal and breast tumors. 41 In contrast, levels of FN are significantly greater in CaP tissue than in normal or hyperplastic prostate tissue 42,43 and MDA PCa2a prostate cancer cells have been shown to express high levels of FN. 44 We have demonstrated that LNCaP-r cells overexpress FN (>60-fold as compared with LNCaP). FN-integrin mediated cell adhesion triggers intracellular signaling events, such as activation of the ERK2/MAP kinase cascade via focal adhesion kinase (FAK), which modulate FN-mediated gene transcription. 45
PM5 (clone 49L) is a 4-kb metalloproteinase-like collagenase that was isolated from a melanoma cDNA library by screening with oligonucleotide sequences to the metal-binding domain of human fibroblast collagenase. Expression in CaP has not been reported before but there is no difference in PM5 mRNA expression in normal and malignant colorectal tissue. 46 In this study PM5 expression was differentially expressed between LNCaP and LNCaP-r cells. The substrate for this enzyme is unknown and it is conceivable that it may degrade a matrix which promotes motility; its down-regulation in CaP would be consistent with tumor progression.
BBC-1, which is located at 16q24.3, was initially suggested to be a potential tumor suppressor gene in breast cancer, 34 although recent data have given rise to dispute about this role. 35 In CaP loss of heterozygosity at 16q has been observed in several studies 47-50 and 16q allelic loss is significantly higher in cancer death cases than in early stage tumors. 49 Loss of heterozygosity at 16q24.3 is significantly higher in metastatic than localized prostate tumors. 51 Expression of BBC1 was down-regulated in LNCaP compared with LNCaP-r cells, supporting further study of BBC1 as a tumor suppressor gene in CaP.
UEV-1 (or CROC-1), 33,52 an inactive variant of the E2-conjugating enzymes, is located on chromosome 20q13.2 and is expressed as four isoforms generated by alternative splicing. 33 UEV-1A is down-regulated in differentiating colon carcinoma cells and expressed in a cell cycle-dependent manner. 33 We have demonstrated that the threefold higher expression of UEV-1 in LNCaP compared with LNCaP-r cells is due to variations in the 1A/1As and not the 1B/1Bs isoforms. Constitutive overexpression of the UEV-1A gene inhibits the activity of the mitotic kinase cdk-1, resulting in G2-M growth arrest. 33 LNCaP-r cells possess a higher growth rate than LNCaP cells (data not shown), an effect which may be due to diminished inhibition of cdk-1 by reduced levels of UEV-1A. A similar effect in vivo would provide a growth advantage for hormone-insensitive CaP cells.
The role of UEV-1 is not limited to regulation of the cell cycle. This gene was originally identified by its ability to transactivate a c-fos promoter. 52,53 Thompson et al 54 have demonstrated that UEV-1A protects cells from DNA-damaging agents and acts through a pathway common to the human MMS2 gene. 53 Down-regulation of UEV-1A, as we have observed, could render LNCaP-r cells susceptible to mutation. UEV-1 is structurally related to the tumor suppressor gene TSG101 55 and deletions and aberrant splicing patterns of the TSG101 gene have been reported in both breast cancer 56,57 and CaP. 58 Cher et al 17 demonstrated that loss at chromosome 20q is frequently associated with advanced CaP, further implicating UEV-1 as a candidate gene in the progression to AI.
In conclusion, we have identified genes (FN, UEV 1, and BBC1) that correspond to regions of genetic alteration found in both LNCaP-r cells and advanced CaP. The functional role of these genes in the development of AI CaP needs further evaluation.
Footnotes
Address reprint requests to Prof. G.W.H. Stamp, Department of Histopathology, Imperial College School of Medicine, Hammersmith Campus, Du Cane Road, London W12 ONN, UK. E-mail: gstamp@rpms.ac.uk.
References
- 1.Bruchovsky N, Lesser B, Van Doorn E, Craven S: Hormonal effects on cell proliferation in rat prostate. Vitam Horm 1975, 33:61-102 [DOI] [PubMed] [Google Scholar]
- 2.Stearn ME, McGarvey T: Biology of disease: prostate cancer: therapeutics, dignostic and basic studies. Lab Invest 1992, 67:540-552 [PubMed] [Google Scholar]
- 3.Newling DW: The management of hormone refractory prostate cancer. Eur Urol 1996, 29(Suppl 2):69-74 [DOI] [PubMed] [Google Scholar]
- 4.Fossa SD: Management of hormone resistant prostate cancer. Acta Urol Belg 1994, 62:73-76 [PubMed] [Google Scholar]
- 5.Rinker-Schaeffer CW, Partin AW, Isaacs WB, Coffey DS, Isaacs JT: Molecular and cellular changes associated with the acquisition of metastatic ability by prostatic cancer cells. Prostate 1994, 25:249-265 [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb B, Trifiro M, Lumbroso R, Pinsky L: The androgen receptor gene mutations database. Nucleic Acids Res 1997, 25:158-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, Verleun-Mooijman MC, Trapman J, Brinkmann AO, Santerse AB, Schroder FH, van der Kwast TH: Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol 1994, 144:735-746 [PMC free article] [PubMed] [Google Scholar]
- 8.Gaddipati JP, McLeod DG, Heidenberg HB, Sesterhenn IA, Finger MJ, Moul JW, Srivastava S: Frequent detection of codon 877 mutation in the androgen receptor gene in advanced prostate cancers. Cancer Res 1994, 54:2861-2864 [PubMed] [Google Scholar]
- 9.Tilley WD, Buchanan G, Hickey TE, Bentel JM: Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res 1996, 2:277-285 [PubMed] [Google Scholar]
- 10.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP: In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet 1995, 9:401-406 [DOI] [PubMed] [Google Scholar]
- 11.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP: Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res 1997, 57:314-319 [PubMed] [Google Scholar]
- 12.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H: Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 1994, 54:5474-5478 [PubMed] [Google Scholar]
- 13.Reinikainen P, Palvimo JJ, Janne OA: Effects of mitogens on androgen receptor-mediated transactivation. Endocrinology 1996, 137:4351-4357 [DOI] [PubMed] [Google Scholar]
- 14.Young CY, Murtha PE, Andrews PE, Lindzey JK, Tindall DJ: Antagonism of androgen action in prostate tumor cells by retinoic acid. Prostate 1994, 25:39-45 [DOI] [PubMed] [Google Scholar]
- 15.Ikonen T, Palvimo JJ, Kallio PJ, Reinikainen P, Janne OA: Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology 1994, 135:1359-1366 [DOI] [PubMed] [Google Scholar]
- 16.Kundson AG: All in the cancer family. Nat Genet 1993, 5:103. [DOI] [PubMed] [Google Scholar]
- 17.Cher ML, MacGrogan D, Bookstein R, Brown JA, Jenkins RB, Jensen RH: Comparative genomic hybridization, allelic imbalance, and fluorescence in situ hybridization on chromosome 8 in prostate cancer. Genes Chromosomes Cancer 1994, 11:153-162 [DOI] [PubMed] [Google Scholar]
- 18.Lalani E-N, Stubbs AP, Stamp GWH: Molecular changes in prostate cancer. Kirkham N Lemoine NR eds. Progress in Pathology 4. 1998, :pp 113-136 Churchill Livingstone, New York [Google Scholar]
- 19.Smith JR, Freije D, Carpten JD, Gronberg H, Xu J, Isaacs SD, Brownstein MJ, Bova GS, Guo H, Bujnovszky P, Nusskern DR, Damber JE, Bergh A, Emanuelsson M, Kallioniemi OP, Walker-Daniels J, Bailey-Wilson JE, Beaty TH, Meyers DA, Walsh PC, Collins FS, Trent JM, Isaacs WB: Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 1996, 274:1371-1374 [DOI] [PubMed] [Google Scholar]
- 20.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA: The LNCaP cell line: a new model for studies on human prostatic carcinoma. Prog Clin Biol Res 1980, 37:115-132 [PubMed] [Google Scholar]
- 21.Wolf DA, Schulz P, Fittler F: Transcriptional regulation of prostate kallikrein-like genes by androgen. Mol Endocrinol 1992, 6:753-762 [DOI] [PubMed] [Google Scholar]
- 22.Wolf DA, Kohlhuber F, Schulz P, Fittler F, Eick D: Transcriptional down-regulation of c-myc in human prostate carcinoma cells by the synthetic androgen mibolerone. Br J Cancer 1992, 65:376-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf DA, Herzinger T, Hermeking H, Blaschke D, Horz W: Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol Endocrinol 1993, 7:924-936 [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Rudra-Ganguly N, Miller GJ, Moffatt KA, Cote RJ, Roy-Burman P: Steroid hormone induction and expression patterns of L-plastin in normal and carcinomatous prostate tissues. Am J Pathol 1997, 150:2009-2018 [PMC free article] [PubMed] [Google Scholar]
- 25.Hasenson M, Hartley AB, Kihlfors C, Lundin A, Gustafsson JA, Pousette A: Effect of hormones on growth and ATP content of a human prostatic carcinoma cell line, LNCaP-r. Prostate 1985, 7:183-194 [DOI] [PubMed] [Google Scholar]
- 26.van Steenbrugge GJ, Groen M, van Dongen JW, Bolt J, van der Korput H, Trapman J, Hasenson M, Horoszewicz J: The human prostatic carcinoma cell line LNCaP and its derivatives: an overview. Urol Res 1989, 17:71-77 [DOI] [PubMed] [Google Scholar]
- 27.Pousette A, Carlstrom K, Henriksson P, Grande M, Stege R: Use of a hormone-sensitive (LNCaP) and a hormone-resistant (LNCaP-r) cell line in prostate cancer research. Prostate 1997, 31:198-203 [DOI] [PubMed] [Google Scholar]
- 28.Sambrook JJ, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press, 1989
- 29.Welsh J, Chada K, Dala SS, Cheng R, Ralph R, McClelland M: Arbitrarily primed PCR fingerprinting of RNA. Nucleic Acids Res 1992, 21:2627-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubbs AP, Lalani EL, Stamp GWH, Hurst H, Abel P, Waxman J: Second messenger up-regulation of androgen receptor gene transcription is absent in androgen insensitive human prostatic carcinoma cell lines, PC-3 and DU-145. FEBS Lett 1996, 383:237-240 [DOI] [PubMed] [Google Scholar]
- 31.von Stein OD, Thies W, Hofmann M: A high throughput screening for rarely transcribed differentially expressed genes. Nucleic Acids Res 1997, 25:2598-2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert PD: Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA 1996, 93:6025-6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sancho E, Vila MR, Sanchez-Pulido L, Lozano JJ, Paciucci R, Nadal M, Fox M, Harvey C, Bercovich B, Loukili N, Ciechanover A, Lin SL, Sanz F, Estivill X, Valencia A, Thomson TM: Role of UEV-1, an inactive variant of the E2 ubiquitin-conjugating enzymes, in in vitro differentiation and cell cycle behavior of HT-29-M6 intestinal mucosecretory cells. Mol Cell Biol 1998, 18:576-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams SM, Helps NR, Sharp MG, Brammar WJ, Walker RA, Varley JM: Isolation and characterization of a novel gene with differential expression in benign and malignant human breast tumours. Hum Mol Genet 1992, 1:91-96 [DOI] [PubMed] [Google Scholar]
- 35.Moerland E, Breuning MH, Cornelisse CJ, Cleton-Jansen AM: Exclusion of BBC1 and CMAR as candidate breast tumour-suppressor genes. Br J Cancer 1997, 76:1550-1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang P, Pardee AB: Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 1992, 257:967-971 [DOI] [PubMed] [Google Scholar]
- 37.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, Trapman J: Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer 1991, 48:189-193 [DOI] [PubMed] [Google Scholar]
- 38.Chang GT, Blok LJ, Steenbeek M, Veldscholte J, van Weerden WM, van Steenbrugge GJ, Brinkmann AO: Differentially expressed genes in androgen-dependent and -independent prostate carcinomas. Cancer Res 1997, 57:4075-4081 [PubMed] [Google Scholar]
- 39.Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A: Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res 1995, 55:3068-3072 [PubMed] [Google Scholar]
- 40.Akiyama SK, Olden K, Yamada KM: Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev 1995, 14:173-189 [DOI] [PubMed] [Google Scholar]
- 41.Steel DM, Harris H: The effect of antisense RNA to fibronectin on the malignancy of hybrids between melanoma cells and normal fibroblasts. J Cell Sci 1989, 93:515-524 [DOI] [PubMed] [Google Scholar]
- 42.Sonmez H, Suer S, Karaarslan I, Baloglu H, Kokoglu E: Tissue fibronectin levels of human prostatic cancer, as a tumor marker. Cancer Biochem Biophys 1995, 15:107-110 [PubMed] [Google Scholar]
- 43.Suer S, Sonmez H, Karaaslan I, Baloglu H, Kokoglu E: Tissue sialic acid and fibronectin levels in human prostatic cancer. Cancer Lett 1996, 99:135-137 [DOI] [PubMed] [Google Scholar]
- 44.Rajagopal S, Navone NM, Troncoso P, Fritsche HA, Chakrabarty S: Modulation of cellular proliferation and production of prostate-specific antigen and matrix adhesion molecules in human prostate carcinoma cells by polypeptide growth factors: comparative analyses of MDA PCa2a with established cell lines. Int J Oncol 1998, 12:589-595 [DOI] [PubMed] [Google Scholar]
- 45.Schlaepfer DD, Jones KC, Hunter T: Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol 1998, 18:2571-2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Templeton NS, Rodgers LA, Levy AT, Ting KL, Krutzsch HC, Liotta LA, Stetler-Stevenson WG: Cloning and characterization of a novel human cDNA that has DNA similarity to the conserved region of the collagenase gene family. Genomics 1992, 12:175-176 [DOI] [PubMed] [Google Scholar]
- 47.Cher ML, Bova GS, Moore DH, Small EJ, Carroll PR, Pin SS, Epstein JI, Isaacs WB, Jensen RH: Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Res 1996, 56:3091-3102 [PubMed] [Google Scholar]
- 48.Brothman AR: Cytogenetic studies in prostate cancer: are we making progress? Cancer Genet Cytogenet 1997, 95:116-121 [DOI] [PubMed] [Google Scholar]
- 49.Suzuki H, Komiya A, Emi M, Kuramochi H, Shiraishi T, Yatani R, Shimazaki J: Three distinct commonly deleted regions of chromosome arm 16q in human primary and metastatic prostate cancers. Genes Chromosomes Cancer 1996, 17:225-233 [DOI] [PubMed] [Google Scholar]
- 50.Visakorpi T, Kallioniemi AH, Syvänen AC, Hyytinen ER, Karhul R, Tammela T, Isola JJ, Kallioniemi O-P: Genetic changes in primary and recurrent prostate cancer by comparative genomic hybridisation. Cancer Res 1995a, 55:342-347 [PubMed] [Google Scholar]
- 51.Latil A, Cussenot O, Fournier G, Driouch K, Lidereau R: Loss of heterozygosity at chromosome 16q in prostate adenocarcinoma: identification of three independent regions. Cancer Res 1997, 57:1058-1062 [PubMed] [Google Scholar]
- 52.Rothofsky ML, Lin SL: CROC-1 encodes a protein which mediates transcriptional activation of the human FOS promoter. Gene 1997, 195:141-149 [DOI] [PubMed] [Google Scholar]
- 53.Xiao W, Lin SL, Broomfield S, Chow BL, Wei YF: The products of the yeast MMS2 and two human homologs (hMMS2 and CROC-1) define a structurally and functionally conserved Ubc-like protein family. Nucleic Acids Res 1998, 26:3908-3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson TM, Khalid H, Lozane JJ, Sanch E, Ariño J: Role of UEV-1A, a homologue of the tumour suppressor protein TSG101, in protection from DNA damage. FEBS Lett 1998, 423:49-52 [DOI] [PubMed] [Google Scholar]
- 55.Kornblihtt AR, Umezawa K, Vibe-Pedersen K, Baralle FE: Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J 1985, 4:1755-1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Cohen SN: TSG101: a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell 1996, 85:319-329 [DOI] [PubMed] [Google Scholar]
- 57.Lee MP, Feinberg AP: Aberrant splicing but not mutations of TSG101 in human breast cancer. Cancer Res 1997, 57:3131-3134 [PubMed] [Google Scholar]
- 58.Sun Z, Pan J, Bubley G, Balk SP: Frequent abnormalities of TSG101 transcripts in human prostate cancer. Oncogene 1997, 15:3121-3125 [DOI] [PubMed] [Google Scholar]
- 59.Katz AE, Seaman E, Olsson CA, O’Toole KM, Rafto AJ, McMahon D, Cama C, Benson MC, Pearlman H, Buttyan R: Molecular staging of prostate cancer with use of an enhanced reverse transcriptase-PCR assay. Urology 1994, 43:765-775 [DOI] [PubMed] [Google Scholar]
- 60.Sokolov BP, Prockop DJ: A rapid and simple PCR-based method for isolation of cDNA’s from differentially expressed genes. Nucleic Acids Res 1994, 25:4009-4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nothwang HG, Tamura T, Tanaka K, Ichihara A: Sequence analyses and inter-species comparisons of three novel human proteasomal subunits, HsN3, HsC7-I and HsC10-II, confine potential proteolytic active-site residues. Biochim Biophys Acta 1994, 1219:361-368 [DOI] [PubMed] [Google Scholar]
- 62.Eick S, Nicolai M, Mumberg D, Doenecke D: Human H1 histones: conserved and varied sequence elements in two H1 subtype genes. Eur J Cell Biol 1989, 49:110-115 [PubMed] [Google Scholar]
- 63.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L: Human TAFII30 is present in a distinct TFIID complex, and is required for transcriptional activation by the estrogen receptor. Cell 1994, 79:107-117 [DOI] [PubMed] [Google Scholar]