Abstract
Because the mechanisms of lymphocyte accumulation in the lungs of children with AIDS-associated lymphocytic interstitial pneumonia (LIP) are unknown, we studied the relative contributions of known adhesion pathways in mediating lymphocyte adherence to endothelium and the potential role of human herpesviruses in the expansion of these lesions. LIP was characterized by lymphoid hyperplasia of the bronchus-associated lymphoid tissue (BALT) and infiltration of the pulmonary interstitium with CD8+ T lymphocytes. In some individuals there was expansion of the alveolar septae with dense aggregates of B lymphocytes, many containing the Epstein-Barr viral (EBV) genome. Patients with concurrent EBV infection also demonstrated large-vessel arteriopathy characterized by thickening of the intimae with collagen and smooth muscle. Venular endothelium from the lung of children with LIP, but not uninflamed lung from other children with AIDS or lung from children with nonspecific pneumonitis, expressed high levels of vascular cell adhesion molecule-1 (VCAM-1) protein. In turn, inflammatory cells expressing very late activation antigen-4 (VLA-4), the leukocyte ligand for VCAM-1, were the predominant perivascular infiltrate associated with vessels expressing VCAM-1. Expression of other endothelial adhesion molecules, including intracellular adhesion molecule-1 and E-selectin, was not uniformly associated with LIP. Using a tissue adhesion assay combined with immunohistochemistry for VCAM-1, we show that CD8+ T cell clones that express VLA-4 bind preferentially to pulmonary vessels in sites of LIP: vessels that expressed high levels of VCAM-1. When tissues and cells were pretreated with antibodies to VCAM-1 or VLA-4, respectively, adhesion was inhibited by ≥80%. Thus, infiltration of alveolar septae with CD8+ T cells was highly correlative with VCAM-1/VLA-4 adhesive interactions, and focal expansion of B cells was coincidental to co-infection with EBV.
The high death rate seen in children infected with human immunodeficiency virus type 1 (HIV) at birth and during the first year of life is most often attributed to pneumonia caused by opportunistic infections. 1,2 After the first year, the most frequently seen pulmonary complications are chronic lymphoid processes. 3-6 Lymphocytic interstitial pneumonia (LIP) is recognized as an AIDS-defining diagnosis in children under 13, as specified by the Centers for Disease Control and Prevention (CDC) pediatric AIDS case definition. 7 Because children with LIP generally have a longer average survival time than those who acquire opportunistic pulmonary infections, 6 this distinction is important for prognosis and treatment. Still, little is known regarding the pathogenesis of LIP.
Aggressive B-cell non-Hodgkin lymphomas and fatal polyclonal lymphoproliferative disorders occur with increased frequency among children with HIV AIDS. 5,8-11 Many of these neoplastic and pre-neoplastic conditions have been attributed to co-infection with human herpesviruses. 12-15 Interactions between herpesviruses and HIV have been demonstrated in vitro through experiments showing transactivation, CD4 up-regulation, Fc receptor induction, pseudotype formation, cytokine production, and antigen presentation. 16-18 Still, the role of human herpesviruses in LIP, strictly as opportunists or as co-factors in HIV disease, is yet unknown.
Cell-to-cell and cell-to-extracellular matrix interactions are important in cell migration. The binding of lymphocytes to vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and/or intracellular adhesion molecule-1 (ICAM-1) expressed on vascular endothelial cells accounts for a significant portion of the binding of lymphocytes to endothelium. 19-22 Collectively, these molecules differ in their leukocyte binding repertoire. The integrin α4β1 that defines very late activation antigen-4 (VLA-4), is the leukocyte ligand for VCAM-1 and is expressed by a restricted set of cell types, including monocytes 21,23 and lymphocytes, 22,24,25 and is present at low levels on some polymorphonuclear cells. 26,27 E-selectin also mediates the adhesion of polymorphonuclear cells, 28 monocytes, 29 and certain lymphocyte subsets. 19,30 In contrast, ICAM-1 promotes the adhesion of all leukocytes that bear the surface receptor leukocyte function-associated antigen-1 (LFA-1). 31,32
The release of chemotaxins in concert with the expression and avidity of specific adhesion molecules are mechanisms that influence inflammatory cell migration and localization of cells to specific tissue sites, as blocking of adhesion molecule receptors in vivo can influence subsequent cellular infiltration. 21,22,33 Inasmuch as cell trafficking and extravasation of lymphocytes to the lung may be controlled by the differential expression of these endothelial and/or leukocyte adhesion molecules, dysregulation of these processes can lead to immunopathological disease. Herein, we show that pulmonary infiltration by CD8+ T lymphocytes was highly associative with VCAM-1/VLA-4 adhesive interactions and, although HIV infection and AIDS were requisite for the condition of LIP, co-infection with Epstein-Barr virus (EBV) significantly compounded the severity and character of disease.
Materials and Methods
Patients and Tissue Samples
Lung was obtained at autopsy or by open-chest biopsy from 11 prepubescent children (median age, 3.6 years) with perinatally acquired HIV-1 infection and classified as P2-C (symptomatic) according to the CDC Control and Prevention classification system. All subjects had radiological evidence of pulmonary interstitial reticulonodular infiltration (widespread subsegmental consolidations) in the absence of pulmonary opportunistic infections, including Pneumocystis carinii, or neoplasia by standard histopathological, cytological, and/or microbiological culture and staining techniques. Exceptions were patients with human herpesvirus infections, where virus was detected prospectively in lung by in situ hybridization. Of these 11 cases, 6 had histological evidence of moderate to severe LIP, and of these 6, 3 showed vascular lesions manifest by thickening of the arterial intimae (Table 1) ▶ . The microscopic features of the vascular lesions and their association with HIV and other infectious agents were not known before the initiation of the study. Samples from the remaining five children were diagnosed with mild nonspecific interstitial pneumonitis, and none had pulmonary vascular lesions. In addition, postmortem lung was obtained from children without HIV infection or pneumonia (n = 4) and from children with AIDS but without LIP or pulmonary arteriopathy (n = 2). For in situ hybridization and most immunohistochemical analyses, representative sections of lung were fixed in 10% neutral buffered formalin, embedded in paraffin wax, sectioned to a thickness of 5 μm, and mounted on silane-treated (triethoxyaminopropyl-silane, American HistoLabs, Gaithersburg, MD) glass microscope slides. Lung tissue was also snap-frozen in 2-methylbutane and embedded in OCT compound (Miles, Elkhart, IN) for use with antibodies that precluded aldehyde fixation. Serial sections were cut on a cryostat microtome at a thickness of 7 μm for immunohistochemical analyses and 10 μm for tissue adhesion assays and allowed to air dry at room temperature for 2 hours.
Table 1.
Histomorphological and Immunohistochemical Characteristics of Inflammatory and Vascular Lesions in Children with AIDS-Associated Lymphocytic Interstitial Pneumonia
| Case | LIP | Mϕ ISH/IHC | VCAM-1 | Arteriopathy | |||
|---|---|---|---|---|---|---|---|
| Vasculitis | Initimal fibrosis | Fragmentation of elastic tissue | Fibrosis and/or calcification of media | ||||
| 1 | +++*† | ++++/++++ | 8 | + | + | + | − |
| 2 | +++* | ++++/++++ | 6 | + | + | + | − |
| 3 | ++ | ++/+ | 6 | − | − | − | − |
| 4 | ++‡ | +/+ | 2 | − | − | − | − |
| 5 | ++ | +/− | 3 | − | − | − | − |
| 6 | +++* | +++/+++ | 8 | + | + | + | + |
Specimens were obtained from W. Travis, National Institutes of Health, Bethesda, MD, and S. Brodie, Department of Laboratory Medicine, University of Washington, Seattle, WA.
LIP was scored as follows: +, multifocal interstitial leukocytes; ++, multifocal and intermittent confluent areas of leukocyte infiltration; +++, confluent areas of alveolar septal thickening with leukocytes and lymphocyte aggregates with or without germinal centers. ISH/IHC, in situ hybridization for HIV mRNA/immunohistochemistry for HIV gag p24 protein. Results represent an average of 10 microscopic fields (10×) as follows: −, no positive cells; +, 1 to 5 positive cells; ++, 5 to 10 positive cells; +++, 10 to 15 positive cells; ++++, greater than 15 positive cells. For VCAM-1, immunoreactive vessels were assigned scores as follows: 0, no vessels; 1, 1 to 4 vessels; 2, 5 to 10 vessels; 3, 11 to 15 vessels; 4, 16 to 20 vessels; 5, >20 vessels. Staining intensity was scored as follows: 0, no reactivity; 1, faint; 2, moderate, with or without 4 vessels being intense; 3, intense staining of >4 pulmonary vessels. The sum of these two values was then used as the final score, which had a theoretical range from 0 to 8. Arteriopathy was characterized by special stains for inflammation within the vascular wall (vasculitis), intimal fibrosis, fragmentation of elastic tissue and/or fibrosis and calcification of media. These categories were scored as positive (+) or negative (−).
* Concurrent EBV.
† Concurrent KSHV.
‡ Concurrent human CMV.
Histochemistry
Tissues were evaluated by hematoxylin and eosin (H&E) stains for patterns of leukocyte infiltration consistent with LIP. In addition, special stains, including Masson’s trichrome for collagen, Weigert’s resorcin fuchsin for elastic fibers, Von Kossa’s for mineral, Congo red for amyloid, and phosphotungstic acid hematoxylin for fibrin, were used to characterize the composition of vascular lesions in paraffin-embedded tissues. When available, frozen sections were also evaluated for lipid by staining with oil red o.
Immunohistochemical procedures were used to 1) identify cell types within inflammatory and/or arteriosclerotic lesions, 2) identify cells expressing viral antigens, and 3) determine the level of expression of endothelial and leukocyte adhesion molecules in histological sections of lung from individuals identified in Table 1 ▶ . Immunophenotyping was performed following standard procedure, 34,35 which included an antigen retrieval step when necessary (steaming in 1 mmol/L citrate buffer, pH 6.0, for 10 to 20 minutes) and used monoclonal antibodies (MAbs; 10 to 20 μg/ml; MAb clones and sources are identified in figure legends) to viral and cellular antigens. Briefly, primary MAbs were applied overnight at 4°C followed by detection with isotype-specific secondary antibody and an ABC peroxidase technique using 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA) as the chromogen. To control for nonspecific binding, isotype-matched antibodies for irrelevant antigens were used in substitution of the primary antibody. Stained tissues were evaluated by incident light microscopy.
Tissue Adhesion Assay
Leukocyte adhesion to vascular endothelium was assessed by a modified tissue adhesion assay first described by Stamper and Woodruff 36 and used more recently by Sasseville et al. 21 Briefly, frozen sections were pretreated with RPMI 1640 containing 10% fetal bovine serum for 10 minutes at 4°C. Next, 10 6 CD20+ B cells (human B cell line Ramos) or 10 6 CD8+ T cells (primary clones LN2A3, LN33D7–797, and LN9E12, as described previously 37 ) were diluted in 100 μl of medium and applied to each tissue section, and the section and cells were gently shaken for 30 minutes at 4°C. After mitogen stimulation for 48 hours (5 μg/ml pokeweed mitogen for B cells and 5 μg/ml phytohemagglutinin for T cells; Sigma Chemical Co., St. Louis, MO), the clones were shown to express both VLA-4 α4 and β1 subunits when assessed by immunocytochemistry (Figure 1P ▶ , inset) and by flow cytometry. In addition, unstimulated BCBL-2 cells, a B cell lymphoma-derived cell line, 38,39 did not express VLA-4α4β1 and were therefore used to control for nonspecific adhesion. The sections were then gently rinsed in cold 0.15 mol/l PBS, fixed in 1% glutaraldehyde in PBS for 15 minutes at 4°C, rinsed in PBS/0.2% gelatin (pH 7.2), and subsequently stained with 0.5% toluidine blue/30% ethanol for 15 to 20 seconds. After rinsing in 100% ethanol, the stained sections were mounted and examined microscopically. Experiments were performed a minimum of five times.
Figure 1.
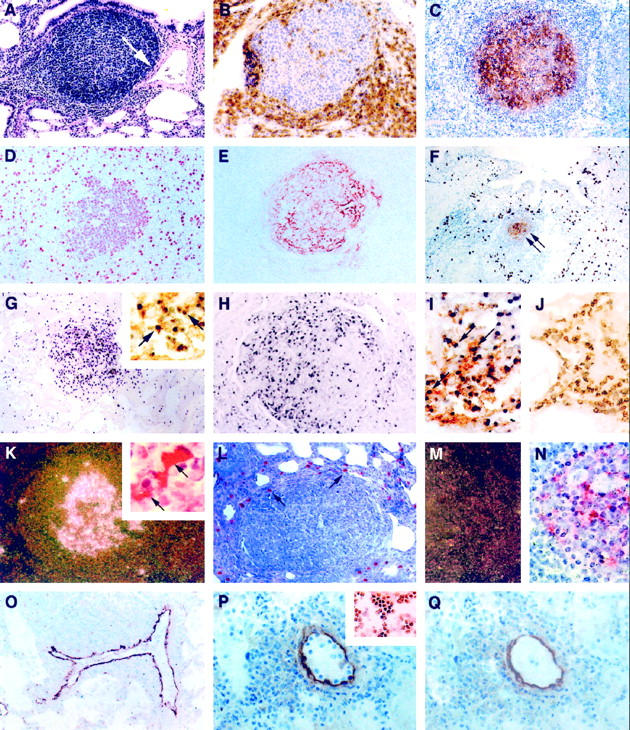
Infiltrative and proliferative lesions associated with pediatric lymphocytic interstitial pneumonia. Immunoperoxidase (IP) procedures used 3,3′-diaminobenzidine as the chromogen, and in situ hybridization (ISH) procedures used anti-digoxigenin-alkaline phosphatase (αDIG-AP) or 125I-labeled CTP as an indicator. A: Widespread thickening of the pulmonary interstitium with mononuclear leukocytes and a single peribronchiole and perivascular aggregate of lymphocytes. Note that the expansion of lymphocytes is associated with constriction of a medium-sized artery (arrow) H&E, magnification, ×100. B: Perifollicular aggregation of CD8+ T lymphocytes. IP, CD8 (clone C8/144B, DAKO); magnification, ×250. C: Lymphoid aggregate showing a predominance of immature B cells within the germinal center. IP, CD19 (clone 4G7/2E, Novocastra, Newcastle, UK); magnification, ×100. D to F: Most germinal center lymphoblasts were mitotically active (D; IP, PCNA (clone PC10, DAKO); magnification, ×250) and surrounded well developed networks of FDC (E and F, arrows; IP, CD21 (clone 1F8, DAKO); magnification, ×100). F: Alveolar and interstitial macrophages were found in high numbers on the periphery of lymphoid follicles. IP, CD68 (KP1, DAKO); magnification, ×100. G: A high percentage of cells (>20%) within the follicular germinal center of lymphoid aggregates contained high copy numbers of EBV EBER-1 RNA. ISH αDIG-AP for EBER-1; magnification, ×100. Most of these EBV RNA-positive cells were of B cell lineage (G, inset; combined ISH αDIG-AP for EBER-1 and IP for CD20; magnification, ×400), and many expressed EBV latent membrane proteins (H, IP for EBV LMP (clone CS1–4, DAKO); magnification, ×200). I: EBV also co-localized to follicular dendritic cells (arrows; combined ISH αDIG-AP for EBV RNA and IP for CD21 FDC; magnification, ×400), but not to interstitial T lymphocytes J; combined ISH αDIG-AP for EBV RNA and IP for CD3; magnification, ×400). K: HIV mRNA was observed within follicular germinal centers (125I ISH for HIV-1 mRNA, dark-field; magnification, ×40) in association with FDC (combined 125I ISH for HIV-1 mRNA and IP for CD21 FDC; inset, ×1000); however, cells that expressed viral antigens were found mostly on the periphery of lymphoid aggregates in areas normally occupied by macrophages (L, arrows; IP for HIV gag p24 (clone Kal-1, DAKO; magnification, ×200). M and N: Serial tissue sections hybridized with noncomplementary HIV RNA probes transcribed in sense-strand orientation did not form hybrids (M, 125I ISH for HIV-1 RNA, dark-field; magnification, ×40; N, 125I ISH for HIV-1 RNA; magnification, ×500). O: VCAM-1 expression was heightened in vessels with perivascular inflammatory foci. IP, CD106 (clone 51–10C9, PharMingen); magnification, ×100. P: Tissue adhesion assay demonstrating that CD8+ T cell clones that co-express the VLA-4 ligand (inset, IP for VLA-4α4/β1; magnification, ×200) selectively adhere to venular endothelium in the lung from a child with severe LIP (IP for VCAM-1; magnification, ×400). Q: Pretreatment of sequential tissue sections (shown in P) with blocking antibodies to VCAM-1 and incubation of CD8+ T cell clones with blocking antibodies to VLA-4α4/β1 inhibited >80% of this adhesion. After the adhesion assay, the tissues were stained with antibody to VCAM-1 to demonstrate presence of VCAM-1 expression. IP for VCAM-1; magnification, ×400.
VCAM-1 was the only endothelial adhesion molecule routinely expressed in LIP and was not up-regulated in tissues without lymphocytic lesions. Also, CD8+ T cells were the primary infiltrate in LIP. Thus, our investigation focused primarily on the neutralizing or blocking effects of antibodies to VCAM-1 on tissues and antibodies to VLA-4α4β1 on CD8+ T cell lines. Still, a similar approach was used to assess the potential role of other adhesion pathways in LIP, including ICAM-1/LFA-1α1β2 and E-selectin. Briefly, before the tissue-cell incubations, tissue sections, cells, or both were incubated for 30 minutes at 4°C with saturating concentrations (20 to 40 μg/ml) of MAbs to VCAM-1 (clone 51–10C9, PharMingen, San Diego, CA) or the α4β1 subunits of VLA-4 (clones HP2/1 and Lia1/2, respectively, Coulter-Immunotech, Westbrook, ME). Monoclonal antibodies to CD31 (pan-endothelial (clone JC/70A), DAKO, Carpinteria, CA), and CD45 (leukocyte common antigen, (clone HI30), PharMingen, San Diego, CA) were used as controls on tissues and cell lines, respectively.
In Situ Hybridization
HIV-1 RNA probes were synthesized with 125I-labeled CTP (Amersham Corp., Arlington heights, IL). Five sense and five antisense probes, in all representing 90% of the HIV-1 genome, were synthesized using pGem 3 subcloned restriction fragments of the clone HXB2, as described previously. 40 The probes were then applied to tissues as a cocktail. For EBV, 30-bp antisense oligonucleotide probes, one complementary to the EBER-1 gene transcript and another to the BHLF-1 gene transcript were prepared by labeling the 3′ end with digoxigenin-11-dUTP (DIG) using a DIG-tailing reaction (Genius 6 kit, Boehringer Mannheim, Indianapolis, IN). The probes were applied to tissues as described previously. 41 The EBER-1 gene is actively transcribed in latently infected cells, and the BHLF-1 gene is expressed early in the EBV life cycle and therefore identifies EBV-infected cells in the replicative phase of the virus life cycle. In addition, DIG-labeled oligonucleotide probes specific for the cytomegalovirus (CMV) early gene transcript were used following manufacturer recommendations (Novocastra, Newcastle, UK) and as reported previously. 42 Expression of the CMV early gene transcript precedes DNA replication, and therefore, the probe allows for the earliest detection of permissive infection. Last, DIG-labeled RNA probes complementary to the Kaposis sarcoma herpesvirus (KSHV) T0.7 transcript (155 bp) were constructed as described previously. 38,39 The T0.7 gene product is transcribed at a high molar ratio during viral latency. Positive and negative controls consisted of cytocentrifuge preparations of primary peripheral blood mononuclear cells (PBMCs) and/or lung tissues known to be positive or negative for the respective virus by DNA extraction followed by solution-based polymerase chain reaction (PCR) and liquid hybridization. Hybridization controls consisted of noncomplementary (sense-strand) 125I-labeled RNA probes for HIV and noncomplementary DIG-labeled probes for KSHV, EBV, and CMV of similar length and G/C content as the antisense probe. After development, the slides were examined by incident light and/or dark-field microscopy.
Semiquantitative Analysis
LIP was defined as infiltration of the pulmonary interstitium with mostly CD8+ T lymphocytes and in some individuals by expansion of the alveolar septae with dense aggregates of polymorphic and polyclonal (CD19+ and CD20+) B lymphocytes. The severity of LIP was scored using a system we described previously 35,43 : +, multifocal interstitial leukocytes; ++, multifocal and intermittent confluent areas of leukocyte infiltration; and +++, confluent areas of alveolar septal thickening with leukocytes and multifocal areas of lymphocyte aggregation (Table 1) ▶ .
The degree of expression of endothelial VCAM-1, ICAM-1, and E-selectin was assessed semiquantitatively using a method modified from that previously described. 44 Briefly, both numbers of immunoreactive pulmonary vessels in a 0.5-cm 2 sample and intensity of staining were used as criteria. For relative numbers of immunoreactive vessels, assigned scores included the following: 0, no vessels; 1, 1 to 4 vessels; 2, 5 to 10 vessels; 3, 11 to 15 vessels; 4, 16 to 20 vessels; and 5, >20 vessels. The scores for staining intensity were as follows: 0, no reactivity; 1, faint; 2, moderate (±4 vessels being intense); 3, intense staining of >4 pulmonary vessels. The sum of these two values for numbers and intensity of immunoreactive vessels were then used as the final score, which had a theoretical range from 0 to 8. Each sample was evaluated independently and in a blind fashion by two reviewers (S.J. Brodie and K. Diem). The severity of LIP, extent of macrophage viral burden, and levels of vessel immunoreactivity were compared using a Spearman nonparametric correlation analysis where r values >0.7 and P > 0.05 were considered significant.
Cell adhesion to tissue sections was assessed by computerized image analysis. Images from 10 representative 10× microscopic fields were transmitted to a computer equipped with a digital imaging board and software for determination of point count size from which a percentage of specifically stained cells per linear unit (0.2 mm) of endothelium was then determined. We chose 0.2 mm as the unit of measurement. This allowed for the inclusion of most intralesional pulmonary venules. Next, a binding coefficient was determined by the quotient of bound cells/vascular dimension unit. 21 Cells that bound to venular endothelium were defined as those adhering exclusively to the luminal (apical) endothelial surface and were differentiated from resident or inflammatory leukocytes by concurrent assessment of untreated consecutively sectioned tissues. At least 10 vessels ≥0.2 mm in circumference from each tissue section were used to obtain a mean binding coefficient (MBC). Data were analyzed by one-way analysis of variance (ANOVA) to test whether the mean differed among groups of individuals categorized by HIV status and histopathology (Table 2) ▶ . A Bonferroni multiple comparison test was then used as a post-test to compare pairs of group means where P > 0.05 was considered significant. Inhibition of cell adhesion was measured as the percentage of MBC obtained using blocking or control antibodies relative to the MBC obtained from a serial tissue section in the same assay without antibody treatment. To determine whether cell binding occurred on endothelium expressing VCAM-1, ICAM-1, or E-selectin, after performing the adhesion assay, tissue sections were post-fixed in 0.5% glutaraldehyde. An immunoperoxidase technique was then performed on the same tissue section using antibodies to VCAM-1 (clone 51–10C9, PharMingen, San Diego, CA), ICAM-1 (clone HA58 (CD54), PharMingen) or E-selectin (clone 68-5H11 (CD62E), PharMingen), respectively. Immediately after chromogen development, the tissue sections were post-fixed in 2% paraformaldehyde/0.5% PBS for 10 minutes at 25°C, washed in PBS/0.2% gelatin, stained for 15 to 20 seconds in toluidine blue/30% ethanol, rinsed two times in 100% ethanol, and mounted.
Table 2.
Mean Binding Coefficient (MBC) for CD8+ T Cell Clones and Ramos Cells (B Cells) to Pulmonary Vessels in Children with AIDS-Associated LIP, Nonspecific Pneumonitis, and Histologically Normal Lung
| Group | Cell lines | |
|---|---|---|
| CD8+ T cells | CD20+ B cells (Ramos) | |
| HIV+, LIP (n = 6) | 22.2 ± 1.9 | 12.8 ± 1.2 |
| HIV+, nonspecific pneumonitis (n = 5) | 2.3 ± 0.7 | 1.0 ± 0.3 |
| HIV+, normal lung (n = 2) | 0.7 ± 0.2 | 0.0 |
| HIV−, normal lung (n = 4) | 0.8 ± 0.1 | 0.0 |
Tissue adhesion assays used to determine MBCs were performed a minimum of five times. Values represent average MBC ± SEM per linear unit (0.2 mm) of venular endothelium. Data were analyzed by one-way ANOVA to test whether the mean differed among groups. A Bonferroni multiple comparison test was then used as a post-test to compare pairs of group means. Differences were present in HIV+ children with LIP when comparing the MBC of CD8+ T cell clones and Ramos B cells with other groups (P < 0.001). All other intra-group comparisons were insignificant (P > 0.05).
Results
LIP Is an Infiltrative and Proliferative Disease
The six subjects with LIP (Table 1) ▶ all showed widespread hyperplasia of bronchus-associated lymphoid tissue (BALT) and lymphocyte infiltration of alveolar septae (Figures 1A and 3A) ▶ ▶ . BALT, defined as resident organized lymphoid tissue of the lung, was observed only in a small percentage (one of six) of children without evident pulmonary infection. This finding is consistent with other studies where BALT was present in human fetal and infant lung only when there was evidence of antigen stimulation. 45 Lymphocytes of CD8+ T cell lineage represented the predominant intraseptal infiltrate (Figure 1B) ▶ . Three of these six individuals also showed dense interstitial perivascular aggregates of polymorphic B lymphocytes (Figure 1C) ▶ , most in replicative stages of the cell cycle (Figure 1D) ▶ and all surrounding well developed networks of follicular dendritic cells (FDCs; Figure 1, E and F ▶ (double arrow)). These aggregates of B cells were predominantly immunoblasts and showed normal to slightly elevated levels of mitotic activity (two to four mitotic figures per 100 cells). In addition, 20% to 30% of these B cells contained EBV EBER-1 gene transcripts (Figure 1G) ▶ and/or EBV latent membrane proteins (Figure 1H) ▶ . Herpesviral gene products localized to B cells (Figure 1G ▶ , inset) and FDCs (Figure 1I) ▶ and not to other cell types, including T cells (Figure 1J) ▶ . HIV mRNA (Figure 1K) ▶ and protein antigens also localized within follicular germinal centers (Figure 1K) ▶ in association with FDCs. Loosely scattered cells within the thickened pulmonary interstitium and pockets of cells on the periphery of lymphocyte aggregates contained high copy numbers of HIV gag proteins (Figure 1L) ▶ , areas shown previously to harbor alveolar and interstitial macrophages (Figure 1F) ▶ . By combining immunohistochemistry for cell-surface antigens with in situ hybridization for HIV mRNA, cells that contained high viral copy numbers were mostly FDCs in germinal centers (Figure 1K ▶ , inset) and macrophages within interseptal spaces.
Figure 3.
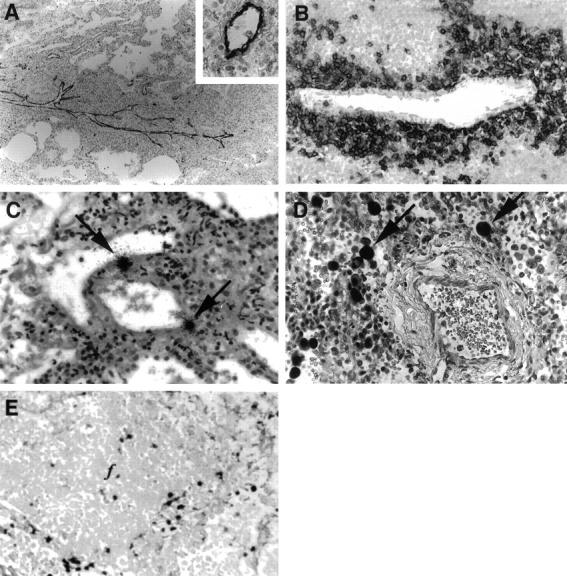
Mechanisms of lymphocyte infiltration and localization of virus in pediatric lymphocytic interstitial pneumonia. A: Longitudinal section of a vein showing VCAM-1 expression and associated mononuclear cell infiltrates. IP for VCAM-1 (clone 51–10C9, PharMingen); magnification, ×100. The inset shows a transverse section of a venule and demonstrates the uniform expression of endothelial VCAM-1. IP for VCAM-1; magnification, ×400. B: Perivenular aggregation of CD8+ T lymphocytes in areas of interstitial collapse. IP for CD8 (clone C8/144B, DAKO); magnification, ×400. C: Localization of cells harboring HIV mRNA (arrows) within a thin-walled vein. 125I ISH for HIV-1 mRNA; magnification, ×100. D: Expression of CMV early gene RNA in cytomegaloid cells (arrows) adjacent to a vein and in a region of interstitial collapse and lymphocyte infiltration. ISH αDIG-AP for EBV EBER-1 RNA; magnification, ×400. E: Expression of KSHV latent gene transcripts in cells associated with follicular aggregation. ISH αDIG-AP for KSHV T0.7 RNA; magnification, ×400.
Arterial Intimal Proliferation
Pulmonary arteritis and/or thickening of the arterial intimae was strongly correlative with severe LIP (Table 1) ▶ . Lesions were present in mostly medium-sized arteries and included components of inflammation, intimal fibrosis, fragmentation of elastic tissue, and/or fibrosis or calcification of the media. In the most severely affected vessels, the endothelium was focally detached from the underlying intimae and the cells were slightly rounded. Vessels demonstrating intimal thickening showed variable degrees of luminal narrowing (Figure 2A) ▶ with fragmentation and/or duplication of the internal elastic lamina (Figure 2B) ▶ . The thickened intimae consisted of mostly collagen (Figure 2C) ▶ , smooth muscle (Figure 2D) ▶ , and occasional macrophages, some harboring the HIV genome (Figure 2E) ▶ . In addition, one patient (case 6) showed extensive mineral deposits that localized mostly within the adventitia of medium-sized arteries (Figure 2F) ▶ . Other special stains revealed the absence of amyloid, lipid, and fibrin within the vascular wall. Platelet and fibrin thrombi could be visualized within intimal plaques and within the lumen of smaller arteries. Fibrinoid necrosis, a feature of pulmonary infarction, was not present in any of the samples examined, nor did any of the subjects show lesions suggestive of pulmonary hypertension and/or congestive heart failure, such as widespread pulmonary edema. Tissues from children diagnosed with nonspecific interstitial pneumonitis, a common sequelae to HIV infection in adults, 46 and children with AIDS but without lung involvement had no evidence of vascular disease. Collectively, these findings suggest an association between pediatric LIP and arteriopathy in children with HIV-induced immune suppression.
Figure 2.
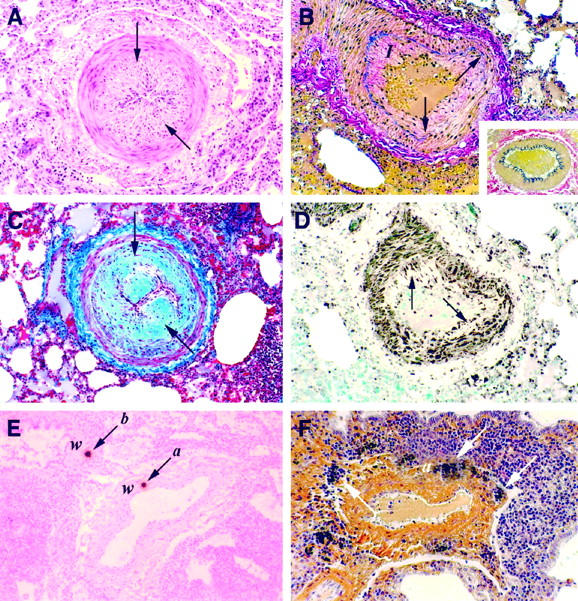
Histochemical and immunochemical characterization of pulmonary arterial lesions. A: Marked intimal thickening (arrows) with luminal narrowing. H&E; magnification, ×100. B: Disruption of the internal elastic laminae (arrows) due to expansion of the tunica intimae (I). Weigert’s resorcin fuchsin; magnification, ×100. Vessel showing normal intimae and normal elastic laminae. Weigert’s resorcin fuchsin; magnification, ×200 (inset). C and D: Advanced narrowing of the vascular lumen (arrows) attributable to intimal fibrous (C; Masson’s trichrome; magnification, ×100) and smooth muscle cell proliferation (D; immunoperoxidase for α-actin (clone 1A4, DAKO); magnification, ×100. E: Localization of cells harboring high copy number HIV gag mRNA (arrows) within the wall (w) of a medium-sized artery (a) and bronchiole (b). 125I ISH; magnification, ×100. F: Mineral deposition surrounding the tunica adventitia (a) of a medium-sized artery. Von Kossa’s; magnification, ×100.
VCAM-1/VLA-4 Adhesive Interactions
Three common and distinct pathways mediating mononuclear cell adhesion to pulmonary endothelium were investigated. VCAM-1 expression was significantly heightened in vascular endothelium in children with LIP and was most pronounced in veins with perivascular inflammatory foci (Figures 1O and 3A) ▶ ▶ and included all tissues with arteriosclerotic lesions (Table 1) ▶ . Interestingly, arterial endothelium from vessels with thickened intimae expressed low or undetectable levels of VCAM-1, and there was no evidence of cells with HIV gag mRNA or p24 antigen expression within the vascular wall. When ranking the intensity of VCAM-1 expression with a range in score from 0 to 8, the mean score for children with LIP was 5.5 ± 1.0. Tissues with severe LIP, characterized by EBV-associated lymphoproliferation and arteriopathy, showed the most extensive and intense VCAM-1 staining (7.3 ± 0.7). In contrast, HIV-infected children without LIP, including those with nonspecific interstitial pneumonitis (1.6 ± 0.5) and those without pulmonary lesions (0.9 ± 0.4) showed low levels of VCAM-1 immunoreactivity and were no different from that of HIV-seronegative children without pulmonary lesions (0.7 ± 0.2; P > 0.05, ANOVA). Thus, only in tissues with LIP was there a significant difference in VCAM-1 expression over background (P < 0.05, ANOVA). VLA-4, the leukocyte ligand for VCAM-1, was expressed at high density on perivascular lymphocytes (Figure 3B) ▶ , most of which also bore the CD8 surface receptor.
In contrast with VCAM-1, widespread and uniform expression of endothelial ICAM-1 was observed in sections of lung from all 11 test subjects, as well as samples from the 2 HIV-positive and 4 HIV-negative patients without pulmonary lesions (not shown). Variations in staining intensity were not appreciable. Interestingly, ICAM-1 was also expressed on smooth muscle cells within the intimae of affected vessels. In turn, E-selectin was only weakly and sporadically expressed on endothelial surfaces (not shown) and mostly from patients with nonspecific pneumonitis, many of which also showed chronic-active inflammation.
Using a tissue adhesion assay, we show that CD8+ T cell clones selectively adhere to endothelium in lung from children with LIP (Figure 1P) ▶ . The MBC for CD8+ T cells (VLA-4α4β1 positive) was significantly greater when comparing individuals with LIP (22.2 ± 1.9) with those with nonspecific pneumonitis (2.3 ± 0.7; P = 0.02, ANOVA) or to individuals without pulmonary lesions (0.7 ± 0.2; P < 0.001, ANOVA; Table 2 ▶ ). There was no difference when comparing individuals with moderate (20.5 ± 2.6; n = 3) and severe (23.8 ± 3.1; n = 3) LIP (P = 0.4, ANOVA). Similar observations were made between the same groups when comparing tissues for Ramos cell (B cell) adhesion, although the average MBCs were much lower than those of CD8+ T cell clones (Table 2) ▶ . This decrease in binding efficiency of B cells coincided with a marked reduction in the expression of VLA-4α4β1. In all, tissues with a high ratio of immunoreactive vessels and elevated staining intensity for VCAM-1 demonstrated the most severe lesions of LIP and conferred the greatest adhesive interactions to cells expressing the VLA-4 ligand.
Pretreatment of CD8+ T cell clones with blocking antibodies to VLA-4α4β1 (clones HP2/1 and Lia1/2 (CDw49d/CD29), Coulter-Immunotechnology, Westbrook, ME) and/or pretreatment of tissues with LIP using blocking antibodies to VCAM-1 (clone 51–10C9 (CD106), PharMingen) resulted in >80% inhibition of this adhesion (Figure 1Q) ▶ . For example, children with LIP had an average MBC of 22.2 ± 1.9 for CD8+ T cell clones per linear unit (0.2 mm) of pulmonary venular endothelium (Table 2) ▶ . When these tissues and cells were pretreated with MAbs to VCAM-1 or VLA-4α4/β1, respectively, the MBC dropped to <5. In turn, Ramos cells, which also expressed VLA-4α4/β1 but at a much lower intensity than CD8+ T cell clones, also bound less efficiently to endothelium in serial sections of the same tissues. Unstimulated cells bound less efficiently than mitogen-stimulated cells, and unstimulated BCBL-2 cells did not adhere to vascular endothelium under any application. LFA-1 was also expressed on CD8+ T cell clones and Ramos cells. However, in the absence of VCAM-1 expression, neither cell type bound appreciably to endothelium that expressed ICAM-1 alone (T cells, MBC = 2.4 ± 0.8; Ramos cells, MBC = 0.8 ± 0.2; clone HA58 (CD54), PharMingen) or vessels that co-expressed ICAM-1 and E-selectin (T cells, MBC = 1.5 ± 0.6; Ramos cells, MBC = 0.7 ± 0.2). Similarly, pretreatment of lymphocyte cell lines with saturating concentrations of antibodies to LFA-1α1β2 (clone HI111 (CD11a), PharMingen, and clone MHM23 (CD18), DAKO) did not significantly affect the binding of these cells when applied to tissues with vessels that expressed ICAM-1 or E-selectin alone (T cells, MBC = 2.1 ± 0.7; Ramos cells, 0.8 ± 0.1; P > 0.05). Cells and tissues when incubated with antibodies to CD45 and CD31, respectively, as a control for nonspecific blocking of cell-to-tissue adhesion, did not alter the MBC in the tissue adhesion model (P > 0.05).
Virus Localization by In Situ Hybridization
Mononuclear cells bearing HIV transcripts were observed, although infrequently, within the wall of arteries (Figure 2E) ▶ and veins (Figure 3C) ▶ and mostly in sites of perivascular infiltration. The majority of cells with detectable HIV mRNA had morphological and phenotypic (CD68+) features of macrophages and localized within the interstitial and alveolar spaces (Figure 1, K and L) ▶ . The number of HIV-infected intralesional monocyte/macrophages was strongly correlative with the severity of LIP (r = −0.86; P = 0.01, Spearman rank correlation coefficient, two-tailed analysis); severe lesions with both infiltrative and proliferative components (cases 1, 2, and 6) showed the greatest number of cells with detectable HIV gag gene expression (Table 1) ▶ .
Because γ-herpesviruses have also been linked to LIP and more recently with arteriopathy, 47,48 we examined lung for the presence of viral transcripts and protein antigens indicative of CMV, KSHV, and/or EBV infections. We also examined the effects of concurrent herpesvirus infection on adhesion molecule expression. The child with CMV pneumonitis (case 4) had no evidence of arteriopathy and showed only low levels of endothelial VCAM-1 (score 2.0). Although, VCAM-1 expression in this patient was most pronounced in vessels within areas of heavy lymphocyte infiltration, the distribution of cells expressing CMV RNA was widespread (Figure 3E) ▶ and included histologically normal areas of the lung. The role of KSHV in lesion development was also unclear as cells containing the T0.7 transcript were detected in only 1 of 11 subjects (case 1), and there were no patterns of virus localization relative to sites of lymphoproliferation, arteriosclerotic vessels, and/or vessels expressing VCAM-1. In contrast, the three patients with severe LIP (Table 1) ▶ characterized by dense intraseptal aggregates of lymphoblasts (Figure 1C) ▶ , showed that as many as 20% of these cells harbored detectable levels of EBV EBER-1 RNA (Figure 1G) ▶ and/or expressed EBV latent membrane protein 1 (Figure 1H) ▶ . EBV was not detected in bronchiolar or alveolar epithelium, as reported by others, 49 nor was it observed in T cells (Figure 1J) ▶ .
Discussion
Determining which adhesion molecule pathway(s) are involved in a particular inflammatory disease process has important implications for understanding events responsible for leukocyte recruitment and for therapeutic intervention. We show uniform expression of VCAM-1 in tissues with LIP and not in control tissues, including those with nonspecific pneumonitis. Moreover, expression of VCAM-1 on postcapillary venules showed the highest degree of spatial localization with infiltrates. Using a tissue adhesion assay, CD8+ T cell attachment was blocked by incubating tissues with antibody to VCAM-1 and was maximal when blocking antibodies to both α4 and β1 subunits of VLA-4 were applied concurrently. Collectively, these results suggest strongly that VCAM-1/VLA-4 adhesive interactions are important in lymphocyte trafficking in LIP. Moreover, differences in host factors in children that allow for higher viral loads 50,51 and heightened cytokine responsiveness 52 may ultimately influence the progression of LIP.
ICAM-1 mediates an LFA-1-dependent pathway 31 and, like VCAM-1, may be important in lymphocyte emigration to sites of inflammation or immune reaction. 23,53 However, unlike VCAM-1, ICAM-1 is constitutively expressed and is present on a variety of other cell types. Hence, the role of ICAM-1 in inflammation may be difficult to assertain. Moreover, because ICAM-1 was also expressed in normal areas of diseased tissue, it was unlikely to have played a major role in leukocyte infiltration in children with LIP. E-selectin may also play a role in the initial interaction of certain subpopulations of T lymphocytes with activated endothelium. 19,30 We show that E-selectin was expressed on venular endothelium from tissues with nonspecific pneumonitis, most often when there was a prominent neutrophil component, but was absent or only weakly expressed in tissues with LIP. Blockade of E-selectin and ICAM-1/LFA-1α1β2 did not significantly alter lymphocyte attachment to pulmonary endothelium. Thus, ICAM-1 and E-selectin did not appear to contribute substantially to lymphocyte trafficking in LIP. Still, we cannot discount that these and other adhesion pathways, although having a minor contribution in the adhesion assay when examined individually, may work in concert in vivo to contribute to lymphocyte-endothelial adhesion. It is also conceivable that our assays were insensitive to reveal relatively low-affinity interactions that may occur with selectins and their ligands.
A variety of herpesviruses have also been implicated in pediatric LIP 5,8,12,13 and expansion of BALT. 54 We show that EBV localized to B lymphoblasts in all tissues with lymphoid aggregates and also within FDCs in lesions with germinal center formation. This high incidence of EBV infection and virus replication may result from defective regulation of EBV in patients with AIDS or AIDS-related disorders. 55 Patients with LIP typically show high numbers of EBV-infected B cells in circulation as a consequence of profound defects in T cell immunity. 55,56 Children with AIDS or AIDS-related disorders, including those with LIP, are also predisposed to EBV-associated non-Hodgkin’s lymphoma. 14,15,56 Consequently, it has been proposed that LIP may represent an intermediate process between benign and malignant transformation. 57 Co-infection of B cells with HIV and EBV has also been described as a possible co-factor in the progression from polyclonal B cell proliferation to lymphoma as both the up-regulation of c-myc and activation of EBV can occur as a result of HIV infection. 11 Interestingly, when comparing the intensity and avidity of VCAM-1 expression and cell adhesion (MBCs) in children with LIP there was no difference when examining tissues with EBV-associated lymphoblastic lesions (+++LIP) and those without (++LIP). This further suggests that this B cell component of LIP does not occur as the result of infiltration, but results from local expansion of B cells.
The pulmonary arterio-occlusive lesions that we describe appear to be unique to children with AIDS and, interestingly, were observed only in children with concurrent EBV infection. Similar lesions have been described in macaques experimentally infected with SIVmac 58 and more recently in transgenic mice carrying a replication-defective HIV-1 provirus. 59 Newly described γ-herpesviruses have also been linked to arteriopathy in SIV-infected macaques 48 and large-vessel arteritis in mice. 47 Various types of synergy have been described between retroviruses and herpesviruses. 16-18 Of potential relevance to vascular disease, HIV-1 tat protein was shown to promote the growth of normal vascular cells and spindle cells derived from AIDS-associated Kaposi’s sarcoma, 60 a tumor of vascular origin and common neoplasm in AIDS patients and a disease recently attributed to γ-herpesvirus infection. 61
Studies of Kaposi’s sarcoma cell lines indicate that KS cells release a variety of cellular growth factors. 62 EBV-infected and/or EBV-activated lymphocytes also liberate angiogenic cytokines, including endothelial growth factor and fibroblast growth factor. 63,64 Thus, we examined sections of lung with histological evidence of arteriopathy for the γ-herpesviruses KSHV and EBV by in situ hybridization. Vascular lesions were characterized by intimal thickening resulting from collagen and smooth muscle deposition and affected mostly medium-sized arteries. Only 1 of 11 subjects demonstrated cells carrying the KSHV latent genome, and unlike EBV where high levels of virus localized to B cells within lymphoid aggregates, there was no pattern to the distribution of KHSV in this patient, and viral transcripts were not detected in individuals with vasculopathy. Previous studies of ours have shown KSHV latent gene transcripts in prostate glandular epithelium of HIV-infected men but only at very low viral copy number. 38,39 In contrast, both latent and transcriptionally active EBV infection of B cells occurred in all individuals with arterio-occlusive disease. Yet, unlike HIV, EBV was not detected within the vascular wall of affected vessels. Others have shown the absence of EBV in large-vessel disease. 65 We also examined lung for CMV, a β-herpesvirus capable of up-regulating HIV replication in macrophages 16,66 and thought to induce smooth muscle proliferation in inflammatory aortic aneurysms. 67 Cytomegaloid cells carrying the CMV early gene were identified in the pulmonary interstitium but only in a single patient and not in association with vascular lesions or within sites of lymphoid aggregation. Collectively, these findings suggest that EBV, and not KSHV or CMV, may play a role in pulmonary arterial disease in immunocompromised children; however, the mechanism for this potential interaction is unclear.
Herein, we show that pediatric LIP is a multifaceted disease with infiltrative and proliferative components. Infiltration of CD8+ T lymphocytes into the pulmonary interstitium was highly correlative with VCAM-1/VLA-4 adhesive interactions between pulmonary endothelium and blood leukocytes. In addition, individuals with arteriosclerotic lesions demonstrated multifocal aggregates of B cells that occurred in association with high levels of B cell infection with EBV. Although HIV infection and AIDS were requisite for the condition of LIP, the mechanism(s) of induction of VCAM-1 and role of EBV, as a viral co-factor or simply as an opportunistic agent, requires further investigation.
Acknowledgments
We thank Tamarind Keating for technical assistance and Dr. Deborah Lewinsohn for providing T cell clones.
Footnotes
Address reprint requests to Dr. Scott J. Brodie, University of Washington School of Medicine, Department of Laboratory Medicine, Vaccine/Virology Division, Retrovirology Laboratory, Room T293X, Seattle, WA 98195. E-mail: sjbrodie@u.washington.edu.
Supported by funds from the National Institutes of Health, NIAID (AI36613, AI41535, and AI30731).
References
- 1.Bye MR, Cairns-Bazarian AM, Ewig JM: Markedly reduced mortality associated with corticosteroid therapy of Pneumocystis carinii pneumonia in children with acquired immune deficiency syndrome. Arch Pediatr Adolesc Med 1995, 148:638-641 [DOI] [PubMed] [Google Scholar]
- 2.Blanche S, Newell ML, Mayaux MJ, Dunn DT, Teglas JP, Rouzioux C, Peckham CS: Morbidity and mortality in European children vertically infected with HIV-1: the French Pediatric HIV Infection Study Group and European Collaborative Study. J Acquir Immune Defic Syndr Hum Retrovirol 1997, 14:442-450 [DOI] [PubMed] [Google Scholar]
- 3.Scott GB, Hutto C, Makuch RW, Mastrucci MT, O’Connor T, Mitchell CD, Trapido EJ, Parks WP: Survival in children with perinatally acquired immunodeficiency virus type 1 infection. N Engl J Med 1989, 321:1791-1796 [DOI] [PubMed] [Google Scholar]
- 4.Marolda J, Pace B, Bonforte RJ, Kotin NM, Rabinowitz J, Kattan M: Pulmonary manifestations of HIV infection in children. Pediatr Pulmonol 1991, 10:231-235 [DOI] [PubMed] [Google Scholar]
- 5.Moran CA, Suster S, Pavlova Z, Mullick FG, Koss MN: The spectrum of pathological changes in the lung in children with the acquired immunodeficiency syndrome: an autopsy study of 36 cases. Hum Pathol 1994, 25:877-882 [DOI] [PubMed] [Google Scholar]
- 6.McSherry GD: Human immunodeficiency-virus-related pulmonary infections in children. Semin Respir Infect 1996, 11:173-183 [PubMed] [Google Scholar]
- 7.Pitt J: Lymphocytic interstitial pneumonia. Pediatr Clin North Am 1991, 38:89-95 [DOI] [PubMed] [Google Scholar]
- 8.Rubinstein A, Morecki R, Silverman B, Charytan M, Krieger BZ, Andiman W, Ziprkowski MN, Goldman H: Pulmonary disease in children with acquired immune deficiency syndrome and AIDS-related complex. J Pediatr 1986, 108:498-503 [DOI] [PubMed] [Google Scholar]
- 9.Joshi VV, Kauffman S, Oleske JM, Fikrig S, Denny T, Gadol C, Lee E: Polyclonal polymorphic B-cell lymphoproliferative disorder with prominent pulmonary involvement in children with acquired immune deficiency syndrome. Cancer 1987, 59:1455-1462 [DOI] [PubMed] [Google Scholar]
- 10.Joshi VV, Oleske JM, Connor EM: Morphologic findings in children with acquired immune deficiency syndrome: pathogenesis and clinical implications. Pediatr Pathol 1990, 10:155-165 [DOI] [PubMed] [Google Scholar]
- 11.Laurence J, Astrin SM: Human immunodeficiency virus induction of malignant transformation in human B lymphocytes. Proc Natl Acad Sci USA 1991, 88:7635-7639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andiman WA, Eastman R, Martin K, Katz BZ, Rubinstein A, Pitt J, Miller G: Opportunistic lymphoproliferation associated with Epstein-Barr viral DNA in infants and children with AIDS. Lancet 1985, 2:1390-1393 [DOI] [PubMed] [Google Scholar]
- 13.Barbera JA, Hayashi S, Hegele RG, Hogg JC: Detection of Epstein-Barr virus in lymphocytic interstitial pneumonia by in situ hybridization. Am Rev Respir Dis 1992, 145:940-946 [DOI] [PubMed] [Google Scholar]
- 14.Hamilton-Dutoit SJ, Raphael M, Audouin J, Diebold J, Lisse I, Pedersen C, Oksenhendler E, Marelle L, Pallesen G: In situ demonstration of Epstein-Barr virus small RNAs (EBER-1) in acquired immunodeficiency syndrome-related lymphomas: correlation with tumor morphology and primary site. Blood 1993, 82:619-624 [PubMed] [Google Scholar]
- 15.Nadal D, Caduff R, Frey E, Hassam S, Zimmermann DR, Seigneurin JM, Pluss HJ, Seger RA: Non-Hodgkin’s lymphoma in four children infected with the human immunodeficiency virus: association with Epstein-Barr virus and treatment. Cancer 1994, 73:224-230 [DOI] [PubMed] [Google Scholar]
- 16.Toth FD, Mosborg-Peterson P, Kiss J, Aboagye-Mathiesen G, Hager H, Juhl CB, Gergely L, Zdravkovic M, Aranyosi J, Lampe L, Ebbesen P: Interactions between human immunodeficiency virus type 1 and human cytomegalovirus in human term syncytiotrophoblast cells coinfected with both viruses. J Virol 1995, 69:2223-2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths PD: Herpesviruses and AIDS. J Antimicrob Chemother 1996, 37(Suppl B):87-95 [DOI] [PubMed] [Google Scholar]
- 18.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M: Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science 1997, 276:1874-1878 [DOI] [PubMed] [Google Scholar]
- 19.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC: ELAM-1 is an adhesion molecule for skin homing T cells. Nature 1991, 349:796-799 [DOI] [PubMed] [Google Scholar]
- 20.Fries JWU, Williams AJ, Atkins RC, Newman W, Lipscomb MF, Collins T: Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol 1993, 143:725-737 [PMC free article] [PubMed] [Google Scholar]
- 21.Sasseville VG, Newman WA, Brodie SJ, Hesterberg P, Pauley D, Ringler DJ: Monocyte adhesion to endothelium in SIV-induced AIDS encephalitis is mediated by VCAM-1/α4β1 integrin interactions. Am J Pathol 1994, 144:27-40 [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I: Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med 1995, 179:1145-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlos T, Kovach N, Schwartz B, Rosa M, Newman B, Wayner E, Benjamin C, Osborn L, Lobb R, Harlan J: Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule 1. Blood 1991, 77:2266-2271 [PubMed] [Google Scholar]
- 24.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR: VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 1990, 60:577-584 [DOI] [PubMed] [Google Scholar]
- 25.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA, Jr: Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med 1993, 177:57-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Issekutz TB, Miyasaka M, Issekutz AC: Rat blood neutrophils express very late antigen 4 and it mediates migration to arthritic joint and dermal inflammation. J Exp Med 1996, 183:2175-2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinhardt PH, Elliott JF, Kubes P: Neutrophils can adhere via α4β1-integrin under flow conditions. Blood 1997, 89:3837-3846 [PubMed] [Google Scholar]
- 28.Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B: Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science 1989, 243:1160-1165 [DOI] [PubMed] [Google Scholar]
- 29.Ley K, Tedder TF: Leukocyte interactions with vascular endothelium: new insights into selectin-mediated attachment and rolling. J Immunol 1995, 155:525-528 [PubMed] [Google Scholar]
- 30.Shimizu Y, Shaw S, Graber N, Gopal TV, Horgan KJ, Van Severnter GA, Newman W: Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature 1991, 349:799-802 [DOI] [PubMed] [Google Scholar]
- 31.Dustin ML, Springer TA: Lymphocyte function associated antigen-1 (LFA-1) interaction with intracellular adhesion adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol 1988, 107:321-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briscoe DM, Cotran RS, Pober JS: Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo: correlation with CD3+ T cell infiltration. J Immunol 1992, 149:2954-2960 [PubMed] [Google Scholar]
- 33.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N: Prevention of experimental autoimmune encephalitis by antibodies against α4β1 integrin. Nature 1992, 356:63-66 [DOI] [PubMed] [Google Scholar]
- 34.Brodie SJ, Sasseville VG, Reimann K, Simon MA, Sehgal PK, Ringler DJ: Macrophage function in simian AIDS: killing defects in vivo are independent of macrophage infection, associated with alterations in Th phenotype and are reversible with IFN-γ. J Immunol 1994, 153:5790-5801 [PubMed] [Google Scholar]
- 35.Brodie SJ, Pearson LD, Zink MC, Bickle HM, Anderson BC, Marcom KA, DeMartini JC: Ovine lentivirus expression and disease: virus replication, but not entry, is restricted to macrophages of specific tissues. Am J Pathol 1995, 146:1-13 [PMC free article] [PubMed] [Google Scholar]
- 36.Stamper HB, Jr, Woodruff JJ: Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med 1976, 144:828-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brodie SJ, Lewinsohn DA, Patterson BK, Jiyamapa D, Krieger J, Corey L, Greenberg PD, Riddell SR: In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nature Med 1999, 5:34-41 [DOI] [PubMed] [Google Scholar]
- 38.Diamond C, Brodie SJ, Koelle DM, Meei-Li Huang, Diem K, Muthui D, Krieger JN, Corey L: Human herpes virus eight in prostate tissue on men with Kaposi’s sarcoma. J Virol 1998, 72:6223-6227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodie SJ, Krieger JN, Diamond C, Diem K, Corey L: Kaposi’s sarcoma associated human herpes virus eight: localization of viral gene expression and viral latency. Techniques in Localization of Gene Expression. Edited by Patterson BK. Boston, Birkhauser, 1999, in press
- 40.Fox CH, Fox M: In situ hybridization for detection of HIV RNA. Current Protocols in Immunology, vol 3. Edited by Coligan JE. New York, Wiley & Sons, 1993, pp 12.8.1–12.8.17 [DOI] [PubMed]
- 41.Chang KL, Chen YY, Shibata D, Weiss LM: Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol 1992, 1:246-255 [PubMed] [Google Scholar]
- 42.Wu TC, Lee WA, Pizzorno MC, Au WC, Chan YJ, Hruban RH, Hutchins GM, Hayward GS: Localization of the human cytomegalovirus 2.7-kb major early β-gene transcripts by RNA in situ hybridization in permissive and nonpermissive infections. Am J Pathol 1992, 141:1247–1254 [PMC free article] [PubMed]
- 43.Brodie SJ, Marcom KA, Pearson LD, Anderson BC, de la Concha-Bermejillo A, Ellis JA, DeMartini JC: The effects of virus load in the pathogenesis of lentivirus-induced lymphoid interstitial pneumonia. J Infect Dis 1992, 166:531-541 [DOI] [PubMed] [Google Scholar]
- 44.Silber A, Newman W, Reimann KA, Hendricks E, Walsh D, Ringler DJ: Kinetic expression of endothelial adhesion molecules and relationship to leukocyte recruitment in two cutaneous models of inflammation. Lab Invest 1994, 70:163-175 [PubMed] [Google Scholar]
- 45.Gould SJ, Isaacson PG: Bronchus-associated lymphoid tissue (BALT) in human fetal and infant lung. J Pathol 1993, 169:229-234 [DOI] [PubMed] [Google Scholar]
- 46.Travis WD, Fox CH, Devaney KO, Weiss LM, O’Leary TJ, Ognibene FP, Suffredini AF, Rosen MJ, Cohen MB, Shelhamer J: Lymphoid pneumonitis in 50 adult patients with the human immunodeficiency virus: lymphocytic interstitial pneumonitis versus nonspecific interstitial pneumonitis. Hum Pathol 1992, 23:529-541 [DOI] [PubMed] [Google Scholar]
- 47.Weck KE, Dal-Canto AJ, Gould JD, O’Guin AK, Roth KA, Saffitz JE, Speck SH, Virgin HW: Murine γ-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-γ responsiveness: a new model for virus-induced vascular disease. Nature Med 1997, 3:1346-1353 [DOI] [PubMed] [Google Scholar]
- 48.Desrosiers RC, Sasseville VG, Czajak SC, Zhang X, Mansfield KG, Kaur A, Johnson RP, Lackner AA, Jung JU: A herpesvirus of rhesus monkeys related to human Kaposi’s sarcoma-associated herpesvirus. J Virol 1997, 71:9764-9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drut R, Drut RM: EBV-associated sarcoma in a pediatric renal transplant recipient. Pediatr Pathol 1994, 14:863-872 [DOI] [PubMed] [Google Scholar]
- 50.Sperduto AR, Bryson YJ, Chen ISY: Increased susceptibility of neonatal monocyte/macrophages to HIV-1 infection. AIDS Res Hum Retrovir 1993, 9:1277-1285 [DOI] [PubMed] [Google Scholar]
- 51.Pearce TE, Nowakowski M, Eden E, Huang ZB, Steiner P, Shahabuddin M, Potash MJ, Volsky DJ: Uniform detection of HIV-1 in alveolar macrophages of pediatric but not adult AIDS patients. J Leukoc Biol 1993, 53:722-726 [DOI] [PubMed] [Google Scholar]
- 52.Sei S, Akiyoshi H, Bernard J, Venzon DJ, Fox CH, Schwartzentruber DJ, Anderson BD, Kopp JB, Mueller BU, Pizzo PA: Dynamics of virus versus host interaction in children with human immunodeficiency infection type 1 infection. J Infect Dis 1996, 173:1485-1490 [DOI] [PubMed] [Google Scholar]
- 53.Graber N, Gopal TV, Wilson D, Beall LD, Polte T, Newman W: T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol 1990, 145:819-830 [PubMed] [Google Scholar]
- 54.Koss MN: Pulmonary lymphoid disorders. Semin Diagn Pathol 1995, 12:158-171 [PubMed] [Google Scholar]
- 55.Birx DL, Redfield RR, Tosato G: Defective regulation of Epstein-Barr virus infection in patients with AIDS or AIDS-related disorders. N Engl J Med 1986, 314:874-879 [DOI] [PubMed] [Google Scholar]
- 56.Carbone A, Gloghini A, Volpe R, Boiocchi M, Tirelli U: High frequency of Epstein-Barr virus latent membrane protein-1 expression in acquired immunodeficiency syndrome-related Ki-1 (CD30)-positive anaplastic large-cell lymphomas. Am J Clin Pathol 1994, 101:768-772 [DOI] [PubMed] [Google Scholar]
- 57.Banerjee D, Ahmad D: Malignant lymphoma complicating lymphocytic interstitial pneumonia: a monoclonal B-cell neoplasm arising in a polyclonal lymphoproliferative disorder. Hum Pathol 1982, 13:780-782 [DOI] [PubMed] [Google Scholar]
- 58.Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS, Ringler DJ: Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest 1992, 67:338-349 [PubMed] [Google Scholar]
- 59.Tinkle BT, Ngo L, Luciw PA, Maciag T, Jay G: Human immunodeficiency virus-associated vasculopathy in transgenic mice. J Virol 1997, 71:4809-4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albini A, Barillari G, Benelli R, Gallo RC, Ensoli B: Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci USA 1995, 92:4838-4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore PS, Chang Y: Detection of human herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and those without HIV infection. N Engl J Med 1995, 332:1181-1185 [DOI] [PubMed] [Google Scholar]
- 62.Salahuddin SZ, Nakamura S, Biberfield P, Kaplan MH, Markham PD, Larsson L, Gallo RC: Angiogenic properties of Kaposi’s sarcoma-derived cells after long-term culture in vitro. Science 1988, 242:430-433 [DOI] [PubMed] [Google Scholar]
- 63.Barillari G, Buonaguro L, Fiorelli V, Hoffman J, Michaels F, Gallo RC, Ensoli B: Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression: implications for AIDS-Kaposi’s sarcoma pathogenesis. J Immunol 1992, 149:3727-3734 [PubMed] [Google Scholar]
- 64.Cen H, Williams PA, McWilliams HP, Breinig MC, Ho M, McKnight JL: Evidence for restricted Epstein-Barr virus latent gene expression and anti-EBNA antibody response in solid organ transplant recipients with posttransplant lymphoproliferative disorders. Blood 1993, 81:1393-1403 [PubMed] [Google Scholar]
- 65.Tanaka S, Komori K, Okadome K, Sugimachi K, Mori R: Detection of active cytomegalovirus infection in inflammatory aortic aneurysms with RNA polymerase chain reaction. J Vasc Surg 1994, 20:235-243 [DOI] [PubMed] [Google Scholar]
- 66.Lathey JL, Spector DH, Spector SA: Human cytomegalovirus-mediated enhancement of human immunodeficiency virus type-1 production in monocyte-derived macrophages. Virology 1994, 199:98-104 [DOI] [PubMed] [Google Scholar]
- 67.Yonemitsu Y, Nakagawa K, Tanaka S, Mori R, Sigimachi K, Sueishi K: In situ detection of frequent and active infection of human cytomegalovirus in inflammatory abdominal aortic aneurysms: possible pathogenic role in sustained chronic inflammatory reaction. Lab Invest 1996, 74:723-736 [PubMed] [Google Scholar]


