Abstract
Complement plays an important role in many acute inflammatory responses. In the current studies it was demonstrated that, in the presence of either C5a or sublytic forms of the complement-derived membrane attack complex (MAC), rat alveolar macrophages costimulated with IgG immune complexes demonstrated synergistic production of C-X-C (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) and C-C (macrophage inflammatory protein-1α and monocyte chemoattractant-1) chemokines. In the absence of the costimulus, C5a or MAC did not induce chemokine generation. In in vivo studies, C5a and MAC alone caused limited or no intrapulmonary generation of chemokines, but in the presence of a costimulus (IgG immune complexes) C5a and MAC caused synergistic intrapulmonary generation of C-X-C and C-C chemokines but not of tumor necrosis factor α. Under these conditions increased neutrophil accumulation occurred, as did lung injury. These observations suggest that C5a and MAC function synergistically with a costimulus to enhance chemokine generation and the intensity of the lung inflammatory response.
Complement is an integral buttress of the inflammatory reaction. Some functions of complement include opsonization, accelerated clearance of circulating immune complexes, lysis of nucleated and non-nucleated cells, and lysis of gram-negative bacteria. In addition to these activities, the complement-generated anaphylatoxins, C3a and C5a, are capable of increasing vascular permeability, inducing smooth muscle contraction, and, especially in the case of C5a, causing accumulation of neutrophils. 1 The importance of anaphylatoxins as major participants in inflammatory processes is suggested by elevated plasma levels of C3a and C5a in local and systemic inflammatory conditions. The presence of C5a in plasma has been found after polytrauma, abdominal surgery, and acute pancreatitis, and in patients with “shock lung” following trauma, thermal burns, or sepsis. 2,3 Elevated plasma levels of C3a and C5a as well as MAC have been described in patients with adult respiratory distress syndrome. 4-6
The role of complement activation products in inflammatory responses has been confirmed by a number of experimental studies. C5a has been demonstrated to be required for the full development of injury in a variety of inflammatory models. 7-9 Although C5a is traditionally known for its powerful chemotactic activity for neutrophils, C5a and MAC have also been shown to activate endothelial cells, resulting in surface expression of P-selectin. 10,11 Additionally, both C3a and C5a have been shown to induce the release of cytokines interleukin-1 (IL-1) or tumor necrosis factor α (TNFα), prostaglandins, and leukotrienes from phagocytes. 7 MAC in sublytic concentrations enhances production of endothelial intercellular adhesion molecule-1 (ICAM-1) and E-selectin in the presence of low concentrations of TNFα and directly induces endothelial cell production of interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1). 11-13 MAC can directly activate monocytes, leading to cytokine and oxidant production. 14 These findings underscore the diverse roles of complement activation products in the inflammatory cascade that leads to tissue injury.
Although studies of the role of complement in acute inflammatory responses have suggested that complement depletion or blockade is effective in suppressing the inflammatory response, less is known about the relationship of complement activation products to chemokine generation and attendant effects on lung injury. In IgG immune complex-induced alveolitis in rats, injury has been shown to require whole complement (based on complement depletion procedures), C5a, neutrophils, and chemokines. 15-18 In the current study we sought to determine whether C5a or MAC, alone or together with a costimulus (IgG immune complexes), would affect chemokine generation in vitro and in vivo. In the findings presented here, neither C5a nor MAC alone caused significant C-X-C or C-C chemokine generation either in vitro or in vivo. However, in the presence of a costimulus chemokine generation was greatly enhanced. In the lung, this led to intensified neutrophil recruitment and enhanced lung injury.
Materials and Methods
Reagents
Chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified. Human recombinant C5a was purchased from Fluka Chemical Corp. (Ronkonkoma, NY). Purified human complement components (C5–9) were obtained from Quidel, Inc. (San Diego, CA).
Isolation and Culture Conditions for Alveolar Macrophages
Rat alveolar macrophages were isolated by repeatedly lavaging lungs of anesthetized, healthy animals. After centrifugation of bronchoalveolar lavage (BAL) fluids, cells were resuspended in Dulbecco’s modified Eagle’s medium (Whittaker Bioproducts, Wakersville, MA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Inc., Logan, UT), plated in 48-well tissue culture plates (Corning, Inc., New York, NY) at a concentration of 1 × 10 6 cells/well, and allowed to settle for at least 1 hour. Wells were then washed with medium to remove nonadherent cells and treatment conditions were applied as indicated, with a total volume of 1 ml per well. Immune complexes were formed by incubation of bovine serum albumin (BSA) with rabbit anti-BSA in a molar ratio of 1:4. The precipitate was centrifuged and then resuspended in medium at a final concentration of 10 μg/ml. C5a was added to some wells in a concentration of 50 ng/ml (5 nmol/L). Assembly of the MAC complex was accomplished as previously described. 14 Formation of the C5b-like activation product was accomplished by oxidation of C5 with chloramine-T. 19 Briefly, 100 μg of C5 were incubated with 100 μl of 0.32 mmol/L chloramine-T in 100 μl veronal-buffered saline (pH 7.4). Oxidation was stopped by addition of 100 μl methionine (1 mmol/L). Formation of the C5–6 complex was achieved by addition of 200 μg C6 and incubation for 24 hours at 37°C. For assembly of the MAC, some wells containing alveolar macrophages were incubated with 10 μg C5–6 activation product together with 20 μg C7. For incomplete assembly of MAC, the addition of C7 was omitted. After a medium change to remove excess C5–7 complement components, C8 and C9 were added (20 μg each) to wells and allowed to incubate for 30 minutes. A final medium change to remove excess complement components was followed by addition of immune complexes. At this concentration of MAC no cytolysis occurred, as demonstrated microscopically with trypan blue staining (data not shown). As negative controls, macrophages were treated with only BSA, anti-BSA, C5a, or MAC. After an incubation period of 4 hours cell supernatant fluids were collected and used for chemokine quantitation.
TNFα Quantitation
Cell culture supernatants and BAL fluids were evaluated for TNFα activity using a standard WEHI cell cytotoxicity assay as previously reported. 20
Animal Model of Lung Injury and Intrapulmonary Assembly of MAC
Male Long-Evans specific pathogen-free rats (275–300 g, Charles River Breeding Laboratories, Inc., Portage, MI) were anesthetized by intraperitoneal ketamine (15 mg/100 mg body weight). Lung injury was produced by intrapulmonary deposition of IgG immune complexes as described elsewhere. 15 Briefly, 1.25 mg of polyclonal goat anti-BSA in a total volume of 300 μl were instilled intratracheally followed by intravenous administration of 10 mg BSA together with trace amounts of 125I-BSA. Negative control animals received anti-BSA intratracheally in the absence of the intravenous infusion of 10 mg BSA. Some animals received 2.5 μg C5a intratracheally together with the anti-BSA. Special procedures for intrapulmonary assembly of MAC were required in order not to preassemble the entire complex, which could not be inserted into cell membranes. 21 For in vivo assembly of the MAC, 15 μg C5–6 complex (formed as described above) were instilled intratracheally during inspiration together with 30 μg C7 in a volume of 200 μl. For incomplete assembly of MAC a control group received C5–6 intratracheally in a volume of 200 μl without addition of C7. After 10 minutes, C8 and C9 were then intratracheally administered (30 μg each) together with anti-BSA in a total volume of 200 μl (as described above), followed by the intravenous administration of BSA. Negative control animals received complement preparations in the same volumes together with anti-BSA, which were instilled intratracheally (with omission of the intravenous administration of BSA). Animals tolerated the two intratracheal challenges with no apparent difficulty; postsurgical recovery was comparable to animal groups receiving a single administration of 300 μl anti-BSA intratracheally. Animals were sacrificed after 4 hours and BAL fluids collected by instillation of 5 ml phosphate-buffered saline (PBS) (pH 7.4), flushing, and withdrawing 3 times via an intratracheal cannula. After adding a protease inhibitor cocktail (1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, 1 μg/ml pepstatin) samples were centrifuged at 3000 × g for 10 minutes, pellets were resuspended for neutrophil counts, and supernatants used for chemotaxis evaluation and chemokine quantitation. For the measurement of vascular permeability, the lung vasculature was flushed with 10 ml PBS via pulmonary artery injection. Vascular permeability indices, as a measure of lung injury, were determined by comparing the amount of 125I-BSA present in lung parenchyma to the amount present in 1 ml blood obtained from the inferior vena cava at the time of death.
Quantitation of Chemokines
Chemokine detection was performed using antibody-sandwich enzyme-linked immunosorbent assays (ELISA). Purification of antibodies used for the macrophage inflammatory protein-2 (MIP-2) and cytokine-induced neutrophil chemoattractant (CINC) ELISA and the biological activity of these antibodies have been described elsewhere. 17 MIP-2 anti-serum was obtained from rabbit serum. Rabbit serum rich in anti-CINC was kindly provided by Dr. Arthur Whittwer (Monsanto Corp., St. Louis, MO). The antibody and standard protein used for macrophage inflammatory protein-1α (MIP-1α) ELISA quantitation was purchased from Peprotech, Inc. (Rocky Hill, NJ). Anti-MCP-1 antibodies were acquired from PharMingen (San Diego, CA). rrMCP-1 protein was purchased from BioSource International (Camarillo, CA). Biotinylation steps were performed with EZ-Link NHS-LC-Biotinylation Kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. Immulon 4 Removawell Strips (Dynatech Laboratories, Inc., Chantilly, VA) were coated with 10 μg/ml 50 μl/well anti-rat MIP-2 antibody using a 0.1 mol/L carbonate coating buffer (pH 9.2). After overnight coating (4°C) plates were washed 3 times (microplate autowasher EL 403, Bio-Tek Instruments, Inc., Winooski, VT) and nonspecific binding blocked with 2% BSA in PBS. Samples and standards were loaded into microtiter wells in a volume of 100 μl per well and incubated for 1 hour at 37°C. After four washing steps, 100 μl 5 μg/ml biotinylated antibody were added per well and incubated for 1 hour. After another incubation with a streptavidin horseradish-peroxidase conjugate (Zymed Laboratories, South San Francisco, CA), the assay was developed by using OPD-substrate (o-phenylenediamine dihydrochloride). The reaction was stopped with 3 mol/L sulfuric acid (50 μl/well) and the color reaction measured at 490 nm (microplate EL 340, Bio-Tek Instruments). The same ELISA technique and reagents were used for developing immunoassays to measure the expressions of CINC, MIP-1α, and MCP-1. All concentrations and amounts of antibody solutions for CINC quantitation were used following the ELISA protocol described above. For MIP-1α, 60 μl/well of 5 μg/ml antibody was used for coating, and samples were diluted 1:2. The biotinylated secondary antibody was used at a concentration of 25 μg/ml. For MCP-1 detection the concentrations used were 10 μg/ml for coating antibody (100 μl/well) and 0.5 μg/ml for secondary antibody. For this ELISA, all incubations were carried out at room temperature. Incubation times were 2 to 4 hours. All other procedures were performed as described above.
Chemotaxis Assay
Human neutrophils were isolated from citrate-treated human whole blood by a Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden) gradient. Following dextran sedimentation and hypotonic red blood cell lysis, cells were fluorescein-labeled with BCECF (2′,7′-bis [2-carboxyethyl]-5-[and 6]-carboxy-fluorescein acetoxymethyl ester) (Molecular Probes, Inc., Eugene, OR). Labeled neutrophils were then loaded in 96-well minichambers (NeuroProbe, Inc., Cabin John, MD) on top of a polycarbonate membrane with a pore size of 3 μm to facilitate migration to a well of BAL fluid samples below the membrane and the chambers were incubated at 37°C for 30 minutes. The number of cells that migrated through the polycarbonate filter was measured with a cytofluorometer (Cytofluor II, PerSeptive Biosystems, Inc., Framingham, MA). For each measurement, samples were done in quadruplicate.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts of whole lung tissues were prepared by the method of Deryckere and Gannon. 22 Nuclear extracts of alveolar macrophages were prepared as previously described. 13 Protein concentrations were determined by bicinchoninic acid assay with trichloroacetic acid precipitation using BSA as a reference standard (Pierce). Double-stranded NF-κB consensus oligonucleotide (5′-AGT GAG GGG ACT TTC CCA GGC-3′, Promega, Madison, WI) was end-labeled with γ[32P] ATP (3000 Ci/mmol at 10 mCi/ml, Amersham, Arlington Heights, IL). Binding reactions containing equal amounts of protein (10 μg) and 35 fmol (∼50,000 cpm, Cherenkov counting) of oligonucleotide were performed for 30 minutes in binding buffer (4% glycerol, 1 mmol/L MgCl2, 0.5 mmol/L EDTA (pH 8.0), 0.5 mmol/L dithiothreitol, 50 mmol/L NaCl, 10 mmol/L Tris (pH 7.6), 50 mg/ml poly [dI-dC], Pharmacia, Piscataway, NJ). Reaction volumes were held constant to 15 μl. Reaction products were separated in a 4% polyacrylamide gel and analyzed by autoradiography.
Statistical Analysis
In groups with equal variances, data sets were analyzed using one-way analysis of variance. Individual group means were then compared with the Tukey multiple comparison test. In groups containing unequal variances, Kruskal-Wallis analysis of variance was performed followed by Dunnett’s method for multiple comparison. All values were expressed as mean ± SE. Significance was assigned where P < 0.05.
Results
In Vitro Chemokine Responses as a Function of Doses of Immune Complexes
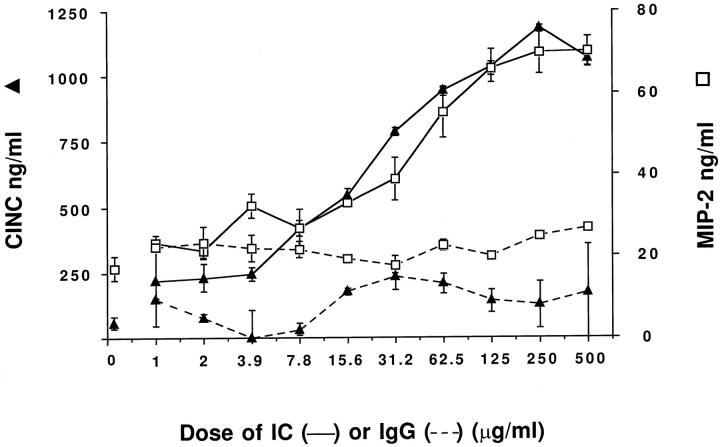
To obtain dose-response relationships for chemokine generation, 1 × 10 6 rat alveolar macrophages were incubated for 4 hours with IgG immune complexes (over a range of 1–500 μg/ml) or with similar amounts of IgG anti-BSA (in the absence of BSA). The CINC and MIP-2 responses are shown in Figure 1 ▶ . Macrophages with no additions generated 44.8 ± 7.9 and 16.9 ± 1.3 ng/ml of CINC and MIP-2, respectively. In the presence of 1–500 μg anti-BSA (but absence of BSA), CINC and MIP-2 responses never exceeded 350 and 25 ng/ml, respectively. In contrast, in the presence of IgG immune complexes, the CINC and MIP-2 responses were dependent on the amounts of immune complexes added. A dose response was found with immune complexes at concentrations >10 μg/ml. A plateau in the response occurred with immune complexes in the range of 125–500 μg/ml. In all subsequent experiments the in vitro concentrations of IgG immune complexes used were 10 μg/ml.
Figure 1.
Dose-responses for chemokine generation in vitro by rat alveolar macrophages (1 × 106/well in triplicate in this and all succeeding studies), using increasing doses of IgG immune complexes (solid lines). Levels of MIP-2 (□) and CINC (▴) are shown. Dashed lines show chemokine expression with increasing doses of IgG alone (in the absence of antigen, BSA).
In Vitro Dose Response Effects of C5a or MAC on IgG Immune Complex-Induced Production of CINC and MIP-2
Alveolar macrophages (1 × 106) were incubated with a fixed amount (10 μg/ml) of IgG immune complexes and with increasing amounts of C5a or MAC to establish the doses of the complement activation products to be used in subsequent experiments. C5a was used over a dose range of 25–150 ng/ml, whereas MAC was used under conditions employing 2.5–10 μg/ml C5 for formation of MAC. In the absence of C5a or MAC, baseline levels of CINC and MIP-2 in cells stimulated with 10 μg IgG anti-BSA alone were 79 ± 2.7 and 16.3 ± 6.7 ng/ml, respectively (Figure 2) ▶ . Stimulation of macrophages with 10 μg immune complexes/ml alone caused CINC and MIP-2 levels to rise to 1013 ± 32 and 62.4 ± 0.5 ng/ml, respectively. The presence of 50 ng/ml C5a with IgG immune complexes resulted in a clear increase in chemokine production (Figure 2A) ▶ . There was a declining response at higher concentrations of C5a, a finding similar to the bell-shaped dose-response curve for C5a in neutrophil chemotaxis assays which show a peak at approximately 10 nmol/L C5a. 16 No concentration of C5a, in the absence of immune complexes, induced significant CINC or MIP-2 generation above that found in nonstimulated cells. For all subsequent in vitro experiments, C5a was used at a dose of 50 ng/ml.
Figure 2.
Dose-responses for chemokine production in vitro by rat alveolar macrophages after stimulation with a constant dose (10 μg/ml) of IgG immune complexes (IC) together with increasing doses of C5a (25–150 ng/ml), frame A, or MAC (based on 2.5–10 μg C5/ml), frame B. MAC was assembled with a twofold excess of complement components C6-C9 to avoid cytolytic effects. Background chemokine production after stimulation with IgG alone (10 μg/ml) in the absence of complement components is shown with an asterisk.
The assembly of the MAC on cell membranes of alveolar macrophages (1 × 106) followed by addition of IgG immune complexes led to a generation of C-X-C chemokines in a dose-dependent manner (Figure 2B) ▶ . Distinct from the pattern found with C5a, increasing doses of MAC resulted in progressively increasing production of CINC and MIP-2 by macrophages. MAC alone at any of the concentrations used failed to show an increase CINC or MIP-2 levels above those of nonstimulated macrophages. At the highest dose of MAC used, no detectable cell lysis occurred as assessed by trypan blue exclusion (data not shown). Based on these findings, the assembly of MAC for all in vitro studies used 10 μg/ml C5 with a twofold molar excess of complement components C6, C7, C8, and C9.
Immune Complex-Induced Chemokine Production In Vitro in the Presence of C5a or MAC
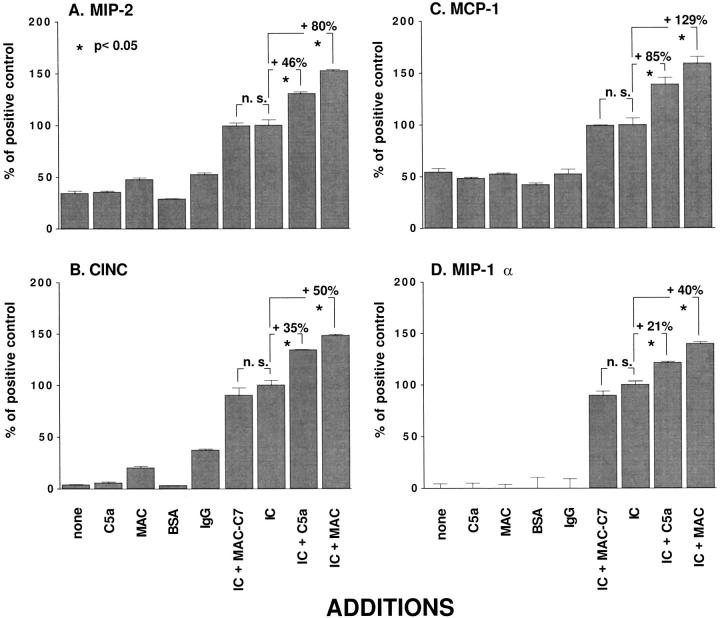
Using the conditions established by the data in Figure 2 ▶ , rat alveolar macrophages were incubated with medium alone (“untreated or none”) or with C5a (50 ng/ml) alone, with MAC (containing 10 μg/ml C5) alone, with BSA (2 μg/ml) alone, with anti-BSA alone (IgG, 10 μg/ml), or with immune complexes (10 μg/ml) alone, or with immune complexes in combination with C5a or MAC, or with incompletely assembled MAC (MAC in absence of C7, MAC − C7) at the doses described above. Chemokine responses in the culture supernatant fluids were determined by ELISA. The results are shown in Figure 3 ▶ . For the C-X-C chemokines (MIP-2 and CINC, frames A and B) and for the C-C chemokines (MCP-1 and MIP-1α, frames C and D), very low levels of chemokines were found in supernatant fluids from untreated macrophages or in supernatant fluids from cells treated only with C5a, MAC, BSA, or anti-BSA. In all cases, chemokine production by alveolar macrophages was significantly increased after treatment with IgG immune complexes. Treatment with MAC − C7 along with immune complexes caused showed no significant differences when compared to addition of IgG immune complexes alone. For the sake of simplicity, the results reported in Figure 3 ▶ represent percent changes relative to macrophages stimulated with IgG immune complexes alone (IC). In the presence of C5a or MAC, immune complex-induced MIP-2 production increased by 46% and 80%, respectively. In the case of CINC, the presence of C5a- or MAC-augmented immune complex-induced production by 35% and 50%, respectively. When MCP-1 was assessed, the presence of C5a or MAC enhanced immune complex-induced production by 85% and 129%, respectively. A similar pattern was observed for MIP-1α. The addition of C5a or MAC increased immune complex-induced MIP-1α production by 21% and 40%, respectively. Thus, in all cases C5a or MAC present together with IgG immune complexes caused synergistic production of C-X-C (MIP-2 and CINC) and C-C (MCP-1 and MIP-1α) chemokines by alveolar macrophages.
Figure 3.
Chemokine generation in vitro after stimulation of rat alveolar macrophages (1 × 106/well) with 10 μg/ml IgG immune complexes (IC) for 4 hours. C5a was used at a concentration of 50 ng/ml. MAC was assembled on cell surfaces of alveolar macrophages in sublytic concentrations based on 10 μg C5/ml, with a twofold molar excess of components C6 to C9, in specified groups without C7 (MAC – C7). MAC was formed in the absence of C7. In this and in all succeeding relevant figures, where the y-axes are labeled “% of positive control,” values obtained with each treatment are shown as a percentage of values obtained when cells were treated with IgG immune complexes (IC, positive control). In addition, values obtained with no treatment (none) were subtracted from each data point before making comparisons between the relevant groups. The absolute values (ng/ml) for MIP-2, CINC, MCP-1, and MIP-1α obtained when macrophages were treated with IgG immune complexes (IC) were: 49.5 ± 2.72, 1158.0 ± 53.3, 22.6 ± 1.52, and 78.2 ± 2.86, respectively.
Enhanced in Vivo Production of Lung Chemokines in the Presence of C5a or MAC and Immune Complexes
In these experiments C5a or MAC was instilled into either untreated rat lungs or rat lungs undergoing IgG immune complex deposition as described above. The effects on intrapulmonary chemokine levels in BAL fluids were then determined. The results, shown in Figure 4 ▶ , are presented as percentage changes compared to results with immune complexes alone. Chemokine content in BAL fluids were quantitated by ELISA 4 hours after initiation of lung reactions. In all cases, chemokine levels in BAL fluids of lungs receiving C5a or MAC alone were significantly lower than the levels induced by the in vivo intrapulmonary deposition of IgG immune complexes. Furthermore, the presence of MAC − C7 together with IgG immune complexes did not yield chemokine levels that were different from those induced by IgG immune complexes alone. However, coadministration of C5a increased IgG immune complex-induced production of MIP-2, CINC, MCP-1, and MIP-1α by 56%, 26%, 80%, and 49%, respectively. Similarly, cotreatment with MAC enhanced IgG immune complex-induced generation of MIP-2, CINC, MCP-1, and MIP-1α by 65%, 35%, 75%, and 49%, respectively. Thus, coadministration of C5a or MAC with IgG immune complexes caused synergistic intrapulmonary generation of chemokines.
Figure 4.
Chemokine levels in bronchoalveolar lavage (BAL) fluids 4 hours after deposition of IgG immune complexes (IC) in lung. The administered amount of C5a was 2.0 μg (as determined by the data in Figure 6 ▶ ). The assembly of MAC was achieved by intratracheal instillation of 15 μg C5b-like product (oxidized C5) together with C6 and C7 (omitted in some animals), each at 30 μg, followed by a second instillation after 10 minutes of C8 and C9, each at 30 μg. In the IgG immune complex-treated groups (IC) the BAL values (ng/ml) for these four chemokines were 51.3 ± 3.91, 2210.0 ± 67.1, 13.0 ± 1.10, and 63.0 ± 2.97, respectively. For each column, n = 4.
Comparison of Neutrophil Chemotactic Activities of C5a and MAC
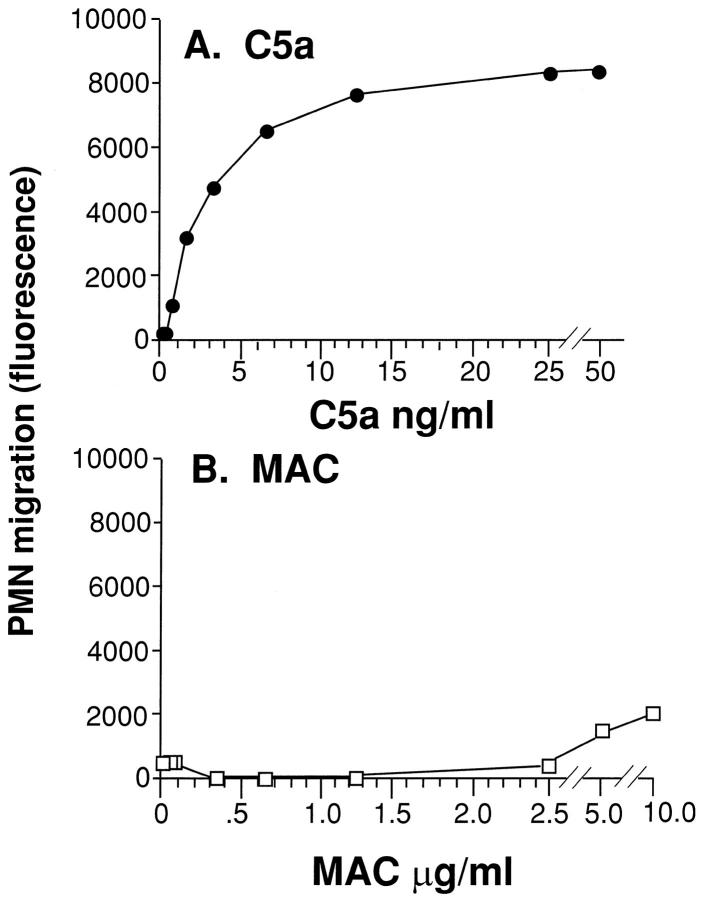
To determine whether the MAC preparation contained intrinsic chemotactic activity for neutrophils (as compared to C5a) which might account for in vivo effects, neutrophil responses to increasing doses of C5a (0.2–50 ng/ml) or MAC (0.04–10 μg/ml C5) were compared. C5a demonstrated the expected chemotactic activity above 1 ng/ml, with a plateau at 50 ng/ml (Figure 5A) ▶ , a finding that is consistent with earlier reports. 16 In the case of MAC, no significant chemotactic activity could be detected over the entire dose range used (Figure 5B) ▶ . Therefore, the increased accumulation of neutrophils in lungs containing IgG immune complexes together with MAC (as described below) cannot be explained by neutrophil chemotactic activity of exogenous MAC.
Figure 5.
Intrinsic neutrophil chemotactic activity of human C5a and MAC. Fluorescein-labeled human neutrophils were exposed to the chemotactic activity of increasing concentrations of C5a (0.2–50 ng/ml) or preformed MAC (0.04–10 μg/ml). The amount of neutrophils that migrated after 30 minutes through a filter containing pores of 3 μm was assessed by measuring fluorescence in a cytofluorometer. The results are typical of data collected in two separate and independent experiments. Each data point was done in quadruplicate. SE values are not displayed because of the very small variances (<10% of x).
Effects of Exogenous C5a and MAC on Lung Injury after Immune Complex Deposition
To establish whether C5a could affect development of lung injury (as defined by neutrophil accumulation and albumin leakage) caused by intrapulmonary deposition of IgG immune complexes, several different doses of C5a were administered intratracheally with the anti-BSA. By itself, airway instillation of 2–8 μg C5a failed to cause an increase in the permeability index when compared to the negative control group (Figure 6) ▶ . When C5a was administered intratracheally with IgG immune complex (IC) in a dose range from 1.0–8.0 μg, the 2.0 μg dose of C5a significantly increased the permeability index by 125%. At higher in vivo doses of C5a these enhancing effects were lost, similar to the in vitro effects of C5a at high concentrations as shown in Figure 2 ▶ . In subsequent studies, we assessed the effects of C5a (2.0 μg) and MAC (the latter in the presence or absence of C7, MAC versus MAC − C7, respectively) on lung inflammatory responses triggered by intrapulmonary deposition of IgG immune complexes, using the endpoints of lung vascular permeability and numbers of neutrophils in BAL fluids. The coadministration of C5a or MAC with the immune complexes caused 150% and 200% increases in the permeability index, respectively (Figure 7A) ▶ . The coadministration of the incomplete MAC (MAC − C7) had no effect on IgG immune complex-induced lung injury.
Figure 6.
Lung vascular permeability in presence of a dose range (1.0–8.0 μg) of exogenously administered C5a during IgG immune complex (IC) deposition in lungs. Trace amounts of 125I-BSA were injected intravenously and albumin leak into lung (permeability index) was determined after 4 hours.
Figure 7.

Enhanced permeability changes and accumulation of neutrophils (PMN) in BAL fluids after intrapulmonary deposition of IgG immune complexes (IC) using 1.25 mg anti-BSA in the absence or presence of C5a (2.0 μg) or MAC (based on 15 μg oxidized C5 with a twofold excess of complement components C6-C9). Cell content is shown as PMN/ml with a total volume of 3.0 ml. For each condition, n = 4.
In this model, the development of lung injury, including increased vascular permeability, is a direct result of pulmonary accumulation of neutrophils. 15 To investigate whether enhanced lung injury induced by C5a or MAC was related to increased neutrophil recruitment, we assessed the effects of these treatments on the number of neutrophils retrieved by BAL. (Figure 7B) ▶ . Combined treatment with IgG immune complexes together with 2.0 μg C5a resulted in a substantial increase (114%) in BAL neutrophils. Similarly, coadministration of MAC resulted in a 158% increase in BAL neutrophils, whereas co-treatment with MAC − C7 had no effect. Thus, intrapulmonary administration of C5a or MAC increases the number of neutrophils recruited into lung and exaggerates the extent of lung injury.
In companion studies, MAC was fully assembled in vitro, forming soluble MAC (sMAC), which was then given intratracheally before airway instillation of anti-BSA. Under these conditions, the mean permeability index of the sMAC-treated group was 0.25 ± 0.02 was not different from rats that received only IgG immune complexes (permeability index 0.28 ± 0.03). Thus, as would be expected, sMAC, which has little ability to bind to cell surfaces, is not capable of enhancing lung injury after intrapulmonary deposition of IgG immune complexes.
In Vivo Effects of C5a and MAC on BAL Fluid Chemotactic Activity and TNFα
To obtain additional information on the mechanisms involved in enhanced neutrophil recruitment, total neutrophil chemotactic activity in BAL fluids was assessed. Enhanced levels of chemotactic activity were anticipated on the basis of the chemokine data described in Figure 4 ▶ . BAL fluids from untreated negative controls were devoid of detectable neutrophil chemotactic activity. A barely measurable increase in chemotactic activity was found in BAL fluids of animals 4 hours after receiving C5a or MAC alone. As expected, substantial chemotactic activity for neutrophils was found in BAL fluids from rats receiving intrapulmonary deposition of immune complexes. Coadministration of C5a or MAC resulted in 62% and 84% increases in chemotactic activity, respectively, when compared to BAL fluids from rats receiving only IgG immune complexes. Chemotactic activity of BAL fluids from rats treated with IgG immune complexes and inactive MAC (MAC − C7) was not different from chemotactic activity in BAL fluids from rats treated with IgG immune complexes alone. Increases in chemotactic activity were not due to increased endogenous production of C5a under the used conditions, because an ELISA specific for rat C5a 16 demonstrated no significant differences between the various treatment groups. Rat C5a levels in BAL fluids from untreated rats were 0.93 ± 0.92 ng/ml, rising to 4.75 ± 0.79 ng/ml after IgG immune complex deposition. In the presence of either C5a or MAC, IgG immune complex-induced lung injury resulted in levels of rat C5a of 6.47 ± 1.03 ng/ml, and 6.34 ± 1.59 ng/ml, respectively (for both, P > 0.05 when compared to the levels of C5a in the absence of C5a or MAC). Thus, consistent with the data in Figure 4 ▶ , increases in chemotactic activity appeared to be associated with increased chemokine content in BAL fluids.
To further investigate the mechanisms of enhanced lung injury caused by the presence of C5a or MAC, levels of TNFα were assessed in BAL fluids. In contrast to chemokines, the presence of C5a or MAC caused no differences in IgG immune complex-induced BAL TNFα levels. Whereas TNFα in BAL fluids of negative control animals were below 0.25 ng/ml, deposition of IgG immune complexes caused TNFα levels to rise nearly 12-fold to 3.1 ± 0.29 ng/ml. The presence of either C5a or MAC with IgG immune complexes was associated with TNFα levels of 2.86 ± 0.24, and 3.16 ± 0.25 ng/ml, respectively. Thus, the in vivo injury-enhancing effects of C5a or MAC appear to be due to changes in BAL levels of chemokines rather than changes in levels of TNFα or rat C5a.
Effects of C5a and MAC on NF-κB Activation
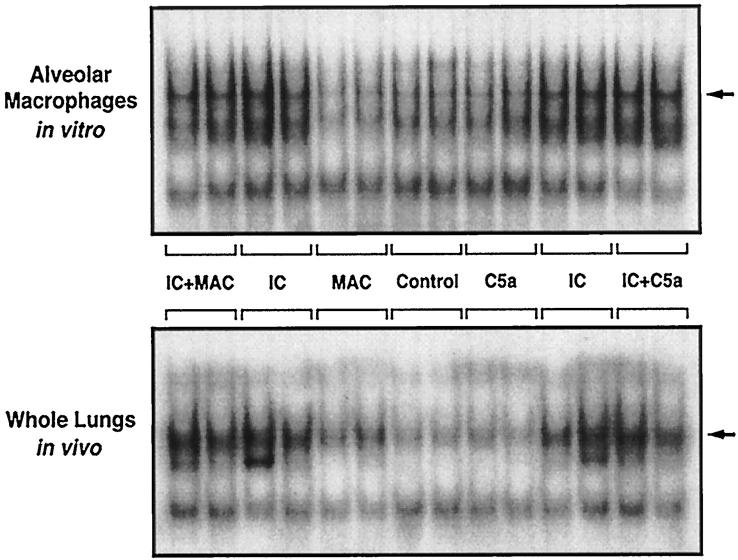
Because activation of the transcription factor NF-κB is known to occur after intrapulmonary deposition of IgG immune complexes, 23 we evaluated the effects of C5a and MAC on NF-κB activation in alveolar macrophages and lung nuclear extracts. As shown in Figure 9 ▶ (upper frame), alveolar macrophages treated in vitro with either C5a or MAC alone exhibited no consistent activation of NF-κB when compared to negative control groups of untreated macrophages. Treatment with IgG immune complexes caused nuclear translocation of NF-κB. Coadministration of C5a or MAC had no effect on the activation of NF-κB. Similar results were found in vivo in lung nuclear extracts (Figure 9 ▶ , lower frame). C5a or MAC alone had little effect on NF-κB activation in whole lungs. The deposition of IgG immune complexes resulted in marked activation of NF-κB. However, coadministration of C5a or MAC had no effect on this level of activation. These data are consistent with earlier studies in which IgG immune complex-induced NF-κB activation in rat lung occurs in the face of complement depletion. 23
Figure 9.
NF-κB activation 1 hour after in vitro or in vivo stimulation with IgG immune complexes in the absence or presence of C5a or MAC, as detected by the electrophoretic mobility shift assay (EMSA). Nuclear extracts of alveolar macrophages or whole lung nuclei were used. The position of NF-κB (p50, p65) is indicated by the arrows.
Discussion
The inflammatory response and accompanying injury in the IgG immune complex-induced model of lung injury has been shown to require availability of the complement system as determined by complement depletion procedures. 15 In vivo blockade of C5a resulted in greatly but not totally suppressed injury in this model, suggesting a dominant proinflammatory role for this anaphylatoxin. 16 The incomplete ability of anti-C5a to suppress lung injury in the IgG immune complex model in comparison to total suppression of injury by anti-C5a in the model of lung injury following systemic activation of complement 16 suggests the former model may be both C5a- and MAC-dependent, whereas in the latter model C5a seems to be the chief and perhaps only complement activation product involved. It has been shown in vitro that C5a and/or sublytic concentrations of MAC can stimulate endothelial cells to express adhesion molecules (P-selectin, E-selectin, ICAM-1) and chemokines (IL-8 and MCP-1). 10-12 In the face of recent observations in the IgG immune complex model of lung injury indicating that blockade of C5a reduces intrapulmonary generation of TNFα 16 and because injury in the same model has been shown to require chemokines, 17,18 the extent to which complement components may be involved in chemokine generation is uncertain. In vitro studies showed that C5a and MAC synergistically enhanced C-X-C and C-C chemokine production in alveolar macrophages stimulated with IgG immune complexes. Importantly, neither C5a nor MAC alone induced macrophage production of chemokines. Similar findings were obtained in vivo in the IgG immune complex model of lung inflammatory injury.
Our experiments appear to represent the first successful in vivo assembly of exogenously administered MAC components leading to enhanced chemokine expression and lung injury. This has been made possible by the ability to administer late-acting complement components repeatedly into the airways of rats. We chose to assemble MAC using oxidized C5, which results in an altered form of C5 with the properties of C5b. 19 This altered molecule is not cleaved, has the functional activity of C5b, and interacts with C6 to form the C5, C6 complex, which is then reactive with C7, C8, and C9. At sufficient concentrations of C7–9, this MAC preparation demonstrates properties of a cytolytic complex. This strategy avoids complications related to the generation of C3a or C5a when C3 and C5 convertases are assembled using purified complement components. For the in vivo assembly of MAC, it was crucial to administer components of MAC in a fashion that would allow the assembly of the complex (starting with C5 and C6, followed by addition of C7, C8, and C9) on cell surfaces to avoid formation of the soluble but biologically inactive MAC. The cell-lysing ability of MAC is based on the ring-like structure formed on cell membranes. 21 The extent of cytolysis induced by MAC has been linked to the number of C9 molecules present. As many as 16 C9 molecules have been described as being arranged in individual ring lesions on cell membranes, 24 with at least four C9 molecules being necessary per C5b-8 complex to form a lytic lesion through which small and large intracellular molecules can pass to the exterior of the cell. 25 In functional immunochemical assays, three to four C9 molecules on average have been shown to be present in the C5b-9 complex on complement-lysed erythrocytes. 26 Nucleated cells often show a resistance to lysis by complement, largely dependent on their metabolic state at any given moment as well as on the surface expression of complement inhibitors such as decay-accelerating factor. In our studies, two C9 molecules were offered per C5b-8 complex, thereby minimizing any cytolytic activity of MAC. Pre-assembled soluble C5b-9 complexes (sMAC) are not inserted into cell membranes and are known to be functionally inert. 21 Assembled MAC lacking any one of the individual five complement components is inactive in cell lysis and in proinflammatory effects. 27 The C5b-8 complex has some lytic activity, but this is minimal when compared to the C5b-9 complex. The requirement for all subunits of the MAC is also reflected in our results. Administration of MAC lacking C7 (MAC − C7) along with IgG immune complexes failed to show the phlogistic effects associated with the fully assembled MAC. Furthermore, intrapulmonary instillation of in vitro preassembled sMAC failed to induce the intensification of immune complex-induced lung injury. Thus, functional enhancement of the inflammatory response in the in vivo lung model suggests that complete assembly of the terminal complement complex on membranes of intrapulmonary cells has occurred.
In addition to lung macrophages, alveolar epithelial Type II cells have also been described as expressing cytokines and inducible nitric oxide synthase. 28-30 Intrapulmonary assembly of MAC may occur on Type II cells as well as on lung macrophages. It appears that C5a receptors are more widespread in lung than previously assumed, in light of recent studies suggesting the presence of C5a receptors on bronchial and alveolar epithelial cells 31,32 in addition to the well-known C5a receptor expression on myeloid cells. Our in vitro studies clearly show that C5a and MAC enhance chemokine production in alveolar macrophages stimulated with IgG immune complexes. Whether the in vivo chemokine responses are limited to effects on macrophages or also involve other lung cells remains to be determined. It is known that bronchial epithelial cells express C5a receptors and react to this ligand with generation of IL-8. 32 This might account for small but limited increases in MIP-2 and CINC levels in animals stimulated with C5a in the absence of immune complexes, because alveolar macrophages did not respond to C5a stimulation in the absence of other agonists with increased chemokine production. Because of the known distribution of Fc receptors on myeloid cells and because of the known requirement for FcR in IgG immune complex-induced tissue injury, 33 it seems likely that the lung inflammatory responses noted require both C5a receptors and FcR.
The rather narrow dose range within which C5a produced enhanced chemokine generation by immune complex-stimulated alveolar macrophages (Figure 2A) ▶ is similar to the C5a dose response for neutrophil chemotaxis, 34,16 endothelial cell production of superoxide, 35 and endothelial cell expression of P-selectin. 10 The loss of function observed at high doses of C5a may reflect cell activation, which results in a refractory cell response. In contrast, with increasing doses of MAC no such decline in macrophage response was found (Figure 2B) ▶ . This is probably explained by the fact that C5a interacts with the G-protein-associated, seven-membrane-spanning C5aR, whereas MAC is inserted directly into the cell membrane in the absence of any known receptor. Thus, it would appear that there is no measurable limit of the extent to which MAC may induce cell activation, at least under the experimental conditions used.
Intratracheal instillation of human C5a or C5adesArg has been shown to be ineffective in producing lung injury in rabbits; the induction of injury requires an additional stimulus. 36 In the current studies, C5a or MAC by themselves were unable to cause neutrophil recruitment and lung injury. We found that when C5a or MAC was administered intratracheally with IgG immune complexes, BAL fluids contained significantly greater neutrophil chemotactic activity compared to treatment with IgG immune complexes alone (Figure 8) ▶ . We subsequently showed that this enhanced chemotactic activity was associated with augmented lung production of the neutrophil-attracting C-X-C chemokines MIP-2 and CINC (Figure 4) ▶ . The fact that enhanced BAL levels of MIP-2 and CINC occurred without changes in BAL levels of TNFα or C5a suggest that increased neutrophil influx is directly related to synergistic production of MIP-2 and CINC.
Figure 8.
Neutrophil chemotactic activity in BAL fluids obtained 4 hours after onset of lung injury, determined by the use of fluorescein-labeled neutrophils. When no additions to the lung were made (none), <10 units fluorescence were obtained in BAL fluids, whereas in lungs where IgG immune complex was deposited, BAL fluid values obtained were 2980 ± 262.
In the context of the IgG immune complex-induced lung injury model, NF-κB has been shown to be upregulated within 30 minutes after the onset of injury, with peak of expression at 4 hours. 37 MIP-2 and CINC expression in this same lung model has a very similar time course, peaking between 2 and 4 hours. 17 For CINC induction, direct regulation through NF-κB has been demonstrated. 38 NF-κB participation as an inducible transcriptional activator has been linked not only to the early response cytokines TNFα and IL-1 39,40 but also to IL-8 (or the rat homologs, MIP-2 and CINC), perhaps also extending to MCP-1 and MIP-1α. 13,41-43 For these reasons we sought to determine if synergistic increases of chemokine production in vitro and in vivo would be reflected by measurable increases in NF-κB activation. The presence of C5a or MAC did not significantly enhance IgG immune complex-induced NF-κB activation in macrophages in lung nuclear extracts. The slight increase of NF-κB activation in whole lungs from animals treated with intratracheally instilled MAC (Figure 9) ▶ might be related to the observation that MAC induces NF-κB up-regulation in endothelial cells. 13 It is possible that the experimental conditions used did not permit detection of subtle changes in NF-κB activation. We have previously shown that C5a alone does not activate NFκB in lung, 44 which the current study confirmed. In our current study we were also unable to obtain convincing evidence that MAC, alone or in conjunction with IgG immune complexes, causes augmented NF-κB activation in lung.
In summary, alveolar macrophages stimulated in vitro with IgG immune complexes in the presence of C5a or MAC demonstrate synergistic production of C-X-C and C-C chemokines. Synergistic enhancement of lung injury also occurs in vivo when C5a or MAC is present together with IgG immune complexes, accompanied by enhanced increases in CINC and MIP-2 levels in the lung. These changes are associated with increased neutrophil recruitment into lungs and intensified lung injury. These findings imply an important function of complement activation products in amplification of an inflammatory process via enhanced generation of C-X-C and C-C chemokines.
Footnotes
Address reprint requests to Peter A. Ward, M.D., Department of Pathology, University of Michigan Medical School, 1301 Catherine Road, Ann Arbor, MI 48109-0602. E-mail: pward@umich.edu.
Supported by National Institutes of Health grants GM-29507 and HL-31963.
References
- 1.Koehl J, Bitter-Suermann D: Anaphylatoxins. Whaley K Loos M Weiler JM eds. Complement in Health and Disease. 1993, :pp 299-324 Kluwer Academic Publishers Dordrecht, The Netherlands [Google Scholar]
- 2.Craddock PR: Complement, granulocytes, and shock lung. Am J Emerg Med 1984, 2:78-81 [DOI] [PubMed] [Google Scholar]
- 3.Duchateau J, Haas M, Schreyen H, Radoux L, Sprangers I, Noel FX, Braun M, Lamy M: Complement activation in patients at risk of developing the adult respiratory distress syndrome. Am Rev Respir Dis 1984, 130:1058-1064 [DOI] [PubMed] [Google Scholar]
- 4.Weigelt JA, Chenoweth DE, Borman KR, Norcross JF: Complement and the severity of pulmonary failure. J Trauma 1988, 28:1013-1019 [DOI] [PubMed] [Google Scholar]
- 5.Langlois PF, Gawryl MS: Accentuated formation of the terminal C5b-9 complement complex in patient plasma precedes development of the adult respiratory distress syndrome. Am Rev Respir Dis 1988, 138:368-375 [DOI] [PubMed] [Google Scholar]
- 6.Langlois PF, Gawryl MS, Zeller J, Lint T: Accentuated complement activation in patient plasma during the adult respiratory distress syndrome: a potential mechanism for pulmonary inflammation. Heart Lung 1989, 18:71-84 [PubMed] [Google Scholar]
- 7.Frank MM, Fries LF: The role of complement in inflammation and phagocytosis. Immunol Today 1991, 12:322-326 [DOI] [PubMed] [Google Scholar]
- 8.Ward PA: Role of complement in lung inflammatory injury. Am J Pathol 1996, 149:1081-1086 [PMC free article] [PubMed] [Google Scholar]
- 9.Mulligan MS, Schmid E, Till GO, Hugli TE, Friedl HP, Roth RA, Ward PA: C5a-dependent upregulation in vivo of lung vascular P-selectin. J Immunol 1997, 158:1857-1861 [PubMed] [Google Scholar]
- 10.Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, Eddy SM, Ward PA: C5a-induced expression of P-selectin in endothelial cells. J Clin Invest 1994, 94:1147-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori R, Hamilton KK, McEver RP, Sims PJ: Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem 1989, 264:9053-9060 [PubMed] [Google Scholar]
- 12.Kilgore KS, Shen JP, Miller BF, Ward PA, Warren JS: Enhancement by the complement membrane attack complex of TNFα-induced endothelial cell expression of E-selectin and ICAM-1. J Immunol 1995, 155:1434-1441 [PubMed] [Google Scholar]
- 13.Kilgore KS, Schmid E, Shanley TP, Flory CM, Maheswari V, Tramontini NL, Cohen H, Ward PA, Friedl HP, Warren JS: Sublytic concentrations of the membrane attack complex (MAC) of complement induce endothelial interleukin 8 (IL-8) and monocyte protein 1 (MCP-1) through nuclear factor-κB activation. Am J Pathol 1997, 150:2019-2031 [PMC free article] [PubMed] [Google Scholar]
- 14.Hänsch GM, Seitz M, Betz M: Effect of the late complement component C5b-9 on human monocytes: release of prostanoids, oxygen radicals and of a factor inducing cell proliferation. Int Arch Allergy Appl Immunol 1987, 82:317-320 [DOI] [PubMed] [Google Scholar]
- 15.Johnson KJ, Ward PA: Acute immunologic pulmonary alveolitis. J Clin Invest 1974, 54:349-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, Ward PA: Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest 1996, 98:503-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA: Requirement for the C-X-C chemokines: MIP-2 and CINC in IgG immune complex-induced lung injury. J Immunol 1997, 158:3439-3448 [PubMed] [Google Scholar]
- 18.Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA: Role of macrophage inflammatory protein-1α (MIP-1α) in acute lung injury in rats. J Immunol 1995, 154:4793-4802 [PubMed] [Google Scholar]
- 19.Vogt W, Zimmermann B, Hesse D, Nolte R: Activation of the fifth component of human complement, C5, without cleavage, by methionine oxidizing agents. Mol Immunol 1992, 29:251-256 [DOI] [PubMed] [Google Scholar]
- 20.Espevik T, Nissen-Meyer J: A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor, tumor necrosis factor from human monocytes. J Immunol Methods 1986, 95:99-105 [DOI] [PubMed] [Google Scholar]
- 21.Morgan BP: Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J 1989, 264:1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deryckere F, Gannon F: A one hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. BioTechniques 1994, 16:405-410 [PubMed] [Google Scholar]
- 23.Lentsch AB, Czermak BJ, Bless NM, Ward PA: NF-κB activation during IgG immune complex-induced lung injury: requirements for TNFα and IL-1β but not complement. Am J Pathol 1998, 152:1327-1336 [PMC free article] [PubMed] [Google Scholar]
- 24.Podack ER: Cytolytic Lymphocytes and Complement. Edited by Podack ER. Boca Raton, FL, CRC Press, 1988, pp 173–184
- 25.Ramm LE, Whitlow MB, Mayer MM: The relationship between channel size and number of C9 molecules in the C5b-9 complex. J Immunol 1985, 134:2594-2599 [PubMed] [Google Scholar]
- 26.Stewart JL, Monahan JB, Brickner A, Sodetz JM: Measurement of the ratio of the eight and ninth components of human complement on complement-lysed membranes. Biochemistry 1984, 23:4016-4022 [DOI] [PubMed] [Google Scholar]
- 27.Morgan BP: Cellular responses to the membrane attack complex. Whaley K Loos M Weiler JM eds. Complement in Health and Disease. 1993, :pp 325-351 Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 28.Driscoll KE, Hassenbein DG, Carter J, Poynter J, Asquith TN: Macrophage inflammatory proteins 1 and 2: expression by rat alveolar macrophages, fibroblasts, and epithelial cells and in rat lung after mineral dust exposure. Am J Respir Cell Mol Biol 1993, 8:311-318 [DOI] [PubMed] [Google Scholar]
- 29.Paine R, III, Rolfe MW, Standiford TJ, Burdick MD, Rollins BJ, Strieter RM: MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol 1993, 150:4561-4570 [PubMed] [Google Scholar]
- 30.Warner RL, Paine R, III, Christensen PJ, Marletta MA, Richards MK, Wilcoxen SE, Ward PA: Lung sources and cytokine requirements for in vivo expression of inducible nitric oxide synthase. Am J Respir Cell Mol Biol 1995, 12:649-661 [DOI] [PubMed] [Google Scholar]
- 31.Wetsel RA: Expression of the complement C5a anaphylatoxin receptor (C5aR) on nonmyeloid cells. Immunol Lett 1995, 44:183-187 [DOI] [PubMed] [Google Scholar]
- 32.Floreani AA, Heires AJ, Welniak LA, Miller-Lindholm A, Clark-Pierce L, Rennard SI, Morgan EL, Sanderson SD: Expression of receptors for C5a anaphylatoxin (CD88) on human bronchial epithelial cells: enhancement of C5a-mediated release of IL-8 upon exposure to cigarette smoke. J Immunol 1998, 160:5073-5081 [PubMed] [Google Scholar]
- 33.Sylvestre DL, Ravetch JV: Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science 1994, 265:1095-1098 [DOI] [PubMed] [Google Scholar]
- 34.Cui L, Carney DF, Hugli TE: Primary structure and functional characterization of rat C5a: an anaphylatoxin with unusually high potency. Protein Sci 1994, 3:1169-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy HS, Shayman JA, Till GO, Maroughui M, Owens CB, Ryan US, Ward PA: Superoxide responses of endothelial cells to C5a and TNFα: divergent signal transduction pathways. Am J Physiol 1992, 263:L51-L59 [DOI] [PubMed] [Google Scholar]
- 36.Henson PM, McCarthey K, Larsen GL, Webster RO, Giclas PC, Dreisin RB, King TE, Shaw JO: Complement fragments, alveolar macrophages, and alveolitis. Am J Pathol 1979, 97:93-110 [PMC free article] [PubMed] [Google Scholar]
- 37.Lentsch AB, Shanley TP, Sarma V, Ward PA: In vivo suppression of NF-κB, and preservation of IκBα by interleukin-10, and interleukin 13. J Clin Invest 1998, 100:2243-2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW: In vivo antioxidant treatment suppresses NF-κB activation, and neutrophilic lung inflammation. J Immunol 1996, 157:1630-1637 [PubMed] [Google Scholar]
- 39.Hohmann HP, Remy R, Poschl B, van Loon APG: Tumor necrosis factors-α and -β bind to the same two types of tumor necrosis factor receptors and maximally activate the transcription factor NF-κB at low receptor occupancy and within minutes after receptor binding. J Biol Chem 1990, 265:15183-15188 [PubMed] [Google Scholar]
- 40.Hiscott J, Marois J, Garoufalis J, D’Addario M: Characterization of a functional NF-κB site in the human interleukin 1 β promoter: evidence for a positive autoregulatory loop. Mol Cell Biol 1993, 13:6231-6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukaida N, Mahe Y, Matsushima K: Cooperative interaction of NF-κB and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem 1990, 265:21128-21133 [PubMed] [Google Scholar]
- 42.Mahe Y, Mukaida N, Kuno K, Akiyama M, Ikeda N, Matsushima K, Murakami S: Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on NF-κB, and CCAAT/enhancer-binding protein-like cis-elements. J Biol Chem 1991, 266:13759-13763 [PubMed] [Google Scholar]
- 43.Grove M, Plumb M: C/EBP, NF-κB, and c-Ets family members, and transcriptional regulation of the cell-specific, and inducible macrophage inflammatory protein 1 α immediate-early gene. Mol Cell Biol 1993, 13:5276-5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lentsch AB, Czermak BJ, Bless, Nicolas M, Ward PA: NF-κB activation during IgG immune complex-induced lung injury: requirements for TNFα and IL-1β but not complement. Am J Pathol 1998, 152:1327-1336 [PMC free article] [PubMed] [Google Scholar]