Abstract
Apoptosis (programmed cell death) serves an important role in the normal morphogenesis, immunoregulation, and homeostatic mechanisms in both normal and neoplastic cells. Caspase-3/CPP32, a member of the ICE/Ced-3-family of cysteine proteases, is an important downstream mediator of several complex proteolytic cascades that result in apoptosis in both hematopoietic and nonhematopoietic cells. Previous studies have demonstrated that caspase-3 is commonly expressed in classical Hodgkin’s disease (CHD); however, the biological significance of its expression in Hodgkin’s disease is unknown. In this report, the expression of caspase-3 in nodular lymphocyte predominance Hodgkin’s disease (NLPHD) was evaluated by immunohistochemistry; in addition, we investigated the role of caspase-3 in CD95 (Fas)-mediated apoptosis in three CHD cell lines. Formalin-fixed, paraffin-embedded tissue sections from 11 cases of NLPHD were immunostained for caspase-3 using a polyclonal rabbit antibody that detects both the 32-kd zymogen and the 20-kd active subunit of the caspase-3 protease. Only 1/11 cases of NLPHD demonstrated caspase-3 immunopositivity in lymphocytic/histiocytic cells. Caspase-3 expression was also evaluated in three CHD cell lines, HS445, L428, and KMH2. Whereas caspase-3 expression was detected in HS445 and L428 cell lines, no expression was found in KMH2 cells by immunohistochemical staining. Treatment of HS445 and L428 cell lines for 72 hours with agonistic CD95 monoclonal antibody induced marked apoptosis that was significantly inhibited by pretreatment with the caspase-3 inhibitor, DEVD-FMK, as determined by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay and flow cytometric analysis of 7-amino-actinomycin D staining. In addition, a significant increase in caspase-3 activity as determined by an enzyme colorimetric assay was detected in HS445 and L428 cells after 48 hours of CD95 stimulation. In marked contrast, treatment of caspase-3-deficient KMH2 cells with anti-CD95 mAb did not demonstrate an increase in caspase-3 activity or induce apoptosis. These data demonstrate caspase-3 is important for CD95-mediated apoptosis in CHD cell lines. In addition, the majority of NLPHD cases examined in this study failed to express detectable levels of caspase-3, suggesting these tumor cells may be resistant to apoptotic stimuli dependent on caspase-3 activity. Furthermore, these data suggest the differential expression of caspase-3 noted between NLPHD and CHD may provide additional evidence that each is a unique disease entity.
Increased understanding of the physiological and pathological processes of programmed cell death, or apoptosis, at the molecular level will provide insights into carcinogenesis and potentially create new opportunities for development of novel prognostic markers and therapeutic tools for the treatment of various neoplasms. One of the earliest cell death-regulating genes to be identified was the proto-oncogene Bcl-2, an apoptosis inhibitor that appears to block a step in an evolutionarily conserved pathway involved in apoptosis. 1-2 Subsequent investigations led to the isolation of a homologue of Bcl-2 in the nematode Caenorhabitis elegans. This homologue, called Ced-9, is necessary for the survival of all cells in this organism. 3 Ced-9 opposes the actions of two cell death-promoting genes, Ced-3 and Ced-4, which are critical for apoptosis in C. elegans. 4 The gene product of Ced-3 demonstrates homology to the mammalian interleukin-1β-converting enzymes (ICE), a group of cysteine proteases. 5 Ced-4 is thought to be homologous to Apaf-1, a mammalian protein that can associate with several death proteases to promote apoptosis. 6
To date at least 13 members of the ICE/Ced-3 family (caspases) have been identified, the majority of which, on activation, are involved in the induction and execution phases of apoptosis. 7,8 Of these cysteine proteases, caspase-3 (CPP32, Yama, apopain) is believed to be one of the most commonly involved in the execution of apoptosis in various cell types. 7 On cleavage by other caspases, caspase-3 gives rise to two active subunits with molecular masses of 17–20 kd and 10–12 kd. 7,9 These subunits assemble to form an enzymatically active tetrameric complex. 9 Activation of caspase-3 has been described in a number of cell types undergoing apoptosis induced by a variety of stimuli, including CD95 (Fas/Apo-1) signaling. 10,11
CD95, a cell surface protein receptor belonging to the tumor necrosis factor (TNF)/nerve growth factor receptor family, is an important molecule in the induction of apoptosis in both hematopoietic and nonhematopoietic cells. 9-15 Mutations in the gene that codes for CD95 have been linked to the development of autoimmune disease and lymphoproliferative disorders in both humans and animal models. 16-18 Previous studies demonstrated that crosslinking of the CD95 receptor on the cell surface by agonistic antibody or by its ligand, CD95L, induced apoptosis that was dependent on caspase activation. 19-23 Furthermore, the inhibition of CD95-mediated apoptosis by blocking proteolysis of caspase-3 by viral proteins is suggested to play a role in the pathogenesis of various neoplasms. 10,11,14,15
The role of caspases, including caspase-3, applied to apoptotic processes in Hodgkin’s disease is currently undefined. In this report, we demonstrate caspase-3 plays an important role in CD95-mediated apoptosis in classical Hodgkin’s disease (CHD) cell lines. Furthermore, we demonstrate that nodular lymphocyte predominance Hodgkin’s disease (NLPHD) lacks caspase-3 expression by immunophenotypic analysis. The lack of caspase-3 expression in NLPHD may contribute to the development and pathogenesis of this disease by imbuing tumor cells with resistance to caspase-3-dependent apoptotic pathways.
Materials and Methods
Case Selection, Histological Examination, and Immunohistochemistry of NLPHD
Formalin-fixed, paraffin-embedded tissue sections from 11 cases of NLPHD were selected from the surgical pathology files of Loyola University Medical Center and the University of Michigan Medical School for immunohistochemical determination of caspase-3. Diagnosis of NLPHD was performed using established criteria on lymph node biopsy histology and immunohistochemistry. 24,25 NLPHD was diagnosed by the finding of typical nodular architecture and lymphocytic/histiocytic (L&H) cells with the appropriate CD20- and CD45RB-positive immunophenotype.
Morphology assessment of NLPHD cases was performed on 4-μm tissue sections with hematoxylin-eosin. Immunoperoxidase staining of lymph node sections with the antibodies listed in Table 1 ▶ was performed using a Ventana 320 automated stainer (Ventana Medical Systems, Tucson, AZ) and a streptavidin/horseradish peroxidase detection kit (Ventana), with microwave antigen retrieval and trypsin pretreatment used as necessary. The chromogen was 3,3′-diaminobenzidine tetrahydrochloride (DAB).
Table 1.
Antibodies Used in Immunohistochemical Staining of NLPHD
| Antibody | Source | Dilution |
|---|---|---|
| LCA (PD7/26/16 and 2B11) | DAKO (Carpinteria, CA) | 1:50 |
| CD30 (Ber-H2) | DAKO | 1:40 |
| CD20 (L26) | DAKO | 1:100 |
| EMA (E29) | DAKO | 1:100 |
| CD15 (Leu-M1) | Becton Dickinson, (San Jose, CA) | 1:50 |
| CD45RO (A6) | Zymed Laboratories (San Francisco, CA) | 1:50 |
Cell Lines
The CHD cell lines KMH2, L428, and HS445 were used in this study. KMH2 and L428 cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). HS445 and Jurkat cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cell lines were cultured in RPMI 1640 (Gibco-BRL, Grand Island, NY) supplemented with 20% (v/v) heat-inactivated fetal bovine serum (Sigma Chemical Co., St. Louis, MO), 2 mmol/L L-glutamine (Gibco-BRL), 25 mmol/L Hepes (Sigma), and antibiotic-antimycotic solution (Sigma). All cell lines were maintained at 37°C in a humidified incubator at 5% CO2.
Immunohistochemical Analysis for Caspase-3 Expression
Four-micron-thick formalin-fixed, paraffin-embedded tissue sections from each case of NLPHD were deparaffinized in xylene, hydrated in graded alcohol, and pretreated for antigen retrieval in 10 mmol/L citrate buffer, pH 6.0, for 10 minutes. Cytospins from CHD cell lines were fixed in a 1:1 mixture of acetone and methanol for 10 minutes. Staining was performed using polyclonal rabbit anti-human CPP32 (1:200 titer, DAKO Corp., Carpinteria, CA) and a Vectastain ABC peroxidase, rabbit IgG detection kit (Vector Laboratories, Burlingame, CA) with 3-amino 9-ethyl carbazole (AEC) as the chromogen. The chromogen DAB was used for paraffin-embedded specimens. Formalin-fixed, paraffin-embedded tissue sections from three cases of caspase-3-positive nodular sclerosis Hodgkin’s disease and a reactive tonsil were used as positive controls for caspase-3 staining.
Apoptosis Induction and Detection
For apoptosis assays, 1 × 10 6 cells from each cell line were cultured in 24-well tissue culture plates (Falcon, Lincoln Park, NJ) and incubated with 500 ng/ml of agonistic anti-CD95 monoclonal antibody (mAb) (clone CH11, mouse IgM, Upstate Biotechnology, Lake Placid, NY) for indicated time periods, with or without 1 hour preincubation with 10 μmol/L caspase-3 peptide inhibitor Ac-Asp-Glu-Val-Asp-fluoromethyl ketone (DEVD-FMK, Clontech, Palo Alto, CA).
Detection of apoptosis in CHD cell lines by terminal deoxynucleotidyl tranferase-mediated dUTP nick end-labeling (TUNEL) was quantitated using the ApopTag in situ apoptosis peroxidase detection kit (Oncor, Gaithersburg, MD). Cytospin preparations of cells were fixed in 1% formaldehyde for 15 minutes followed by 1 hour fixation in 70% ethanol at −20°C. After a brief wash in FA buffer (Difco Laboratories, Detroit, MI), each slide was incubated at room temperature (RT) for 10 minutes with equilibration buffer followed by 1 hour incubation at 37°C with TdT enzyme (or deionized water (dH2O) for negative controls) diluted with the reaction buffer. The TdT reaction was stopped with stop/wash buffer and each specimen was briefly washed with FA buffer before 30 minute incubation with anti-digoxigenin-peroxidase at RT. After a series of washes with FA buffer, each slide was developed with DAB/hydrogen peroxide (Sigma) color substrate for 6 minutes at RT. All slides were counterstained with hematoxylin. A CD95-sensitive Jurkat T cell line was used as a positive control for apoptosis. A positive reaction for apoptosis was characterized by brown/black coloration of the nuclear or perinuclear region of the cell. Apoptotic cells were quantitated by 1000-cell count at 400× magnification.
The 7-Amino Actinomycin D (7-AAD) staining method to measure cell viability was performed per manufacturer’s protocol using Via-Probe 7-AAD (PharMingen, San Diego, CA). Briefly, anti-CD95 mAb-treated and untreated cells (1 × 10 6 cell/ml) were washed twice in cold PBS and resuspended in 1× binding buffer (10 mmol/L Hepes/NaOH (pH 7.4), 140 mmol/L NaCL, and 2.5 mmol/L CaCl2). Resuspended cells were then incubated for 20 minutes at 20–25°C in the dark with 5 μl of 7-AAD. Samples (30,000 events per sample) were then quantitated on an Epics XL-MCL flow cytometer (Coulter, Miami Lakes, FL), recorded in LIST mode, and registered on logarithmic scales. 7-AAD emission was detected in the FL-3 channel (>650 nm). Analysis was performed using Coulter System II software.
Determination of Caspase-3 Activity in Cell Lines
Caspase-3 activity was determined using the ApoAlert CPP32/Caspase-3 colorimetric assay kit (Clontech). After a 48-hour incubation with anti-CD95 mAb, duplicate samples of untreated and treated cells (2 × 10 6 cells) were washed in cold PBS, resuspended in 50 μl cell lysis buffer, and incubated on ice for 10 minutes. Cell lysates were pelleted, followed by transfer of the supernatants to microcentrifuge tubes. Fifty microliters of 2× reaction buffer with 5 mmol/L DTT and 5 μl of 1 mmol/L DEVD-p-nitroanilide (pNA)-conjugated CPP32 substrate were added to each tube, followed by 1 hour incubation in a water bath at 37°C. A control reaction of treated cells without DEVD-pNA was included. Optical density (OD) for each specimen was determined at 405 nm using the EL 312e microplate reader (Bio-Tek Instruments, Winooski, VT). For quantification of protease activity, sample values were plotted on a calibration curve derived from the OD values obtained from each of five standards (range: 0–20 nmole pNA). For each sample, units of CPP32 activity were determined by the following formula:
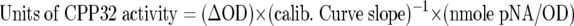 |
where ΔOD is the change in optical density from the control reaction without conjugated substrate.
Results
Histology and Immunohistochemical Characterization of NLPHD Cases
All 11 specimens demonstrated architectural changes consistent with NLPHD and were composed predominantly of large nodules with focal areas of diffuse effacement. Typical nodules contained characteristic L&H cells intermixed in a background of small lymphocytes and occasional epithelioid histiocytes separated by compressed intervening paracortical areas composed of small lymphocytes and scattered plasma cells.
In all specimens, L&H cells demonstrated positive staining for CD20 and LCA. In seven specimens, L&H cells expressed EMA, with one case also expressing CD30. In all cases, L&H cells were negative for CD15 and CD45RO.
Most NLPHD Cases Failed to Express Caspase-3 by Immunohistochemistry
In 10 of 11 cases of NLPHD, including the case which expressed CD30, L&H cells were negative for caspase-3 expression by immunohistochemical staining as represented in Figure 1A ▶ . In one case, caspase-3 immunopositivity was detected in the cytoplasm in a few scattered L&H cells (Figure 1B) ▶ . In contrast, caspase-3 expression was demonstrated both in Hodgkin Reed-Sternberg (HRS) cells and in background lymphocytes in three cases of nodular sclerosis Hodgkin’s disease (Figure 1C) ▶ . In addition, tonsil tissue positive controls demonstrated caspase-3 immunopositivity concentrated predominantly in germinal center cells of secondary follicles (Figure 1D) ▶ .
Figure 1.
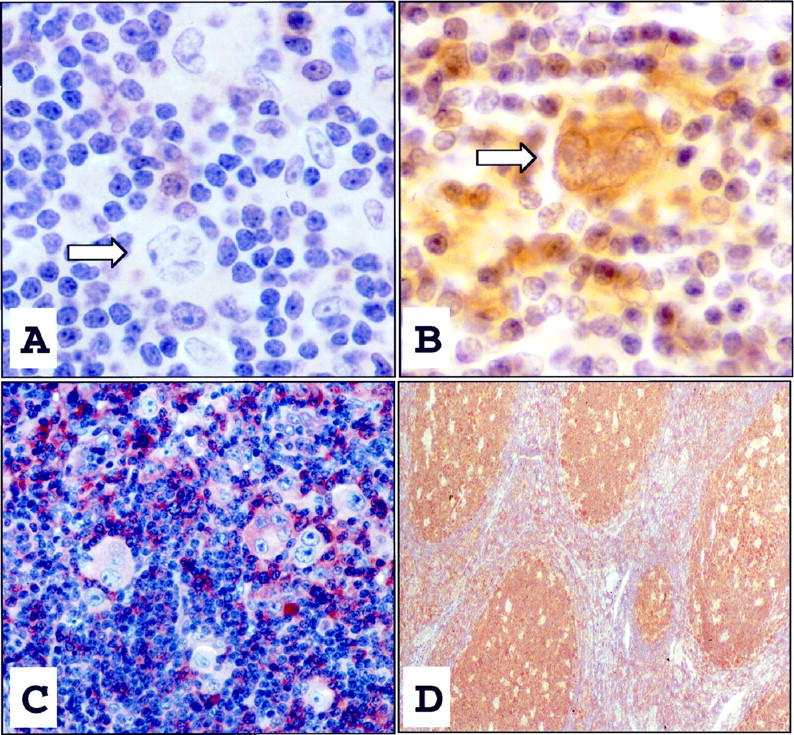
This L&H cell (arrow), as seen in the majority of NLPHD cases, was immunohistochemically negative for caspase-3, whereas scattered plasma cells and lymphocytes expressed caspase-3 (A, DAB ×1000). A single L&H cell (arrow) from one case of NLPHD displayed cytoplasmic expression of caspase-3 (B, DAB ×1000). Control cases of nodular sclerosis Hodgkin’s disease demonstrated diffuse caspase-3-immunopositivity of HRS cells and intense positive immunostaining of lymphocytes and plasma cells within the surrounding infiltrate (C, DAB ×400). Reactive follicular centers in tonsil controls also displayed intense positive staining for caspase-3 (D, DAB, ×200).
Caspase-3 Was Detectable in CHD Cell Lines
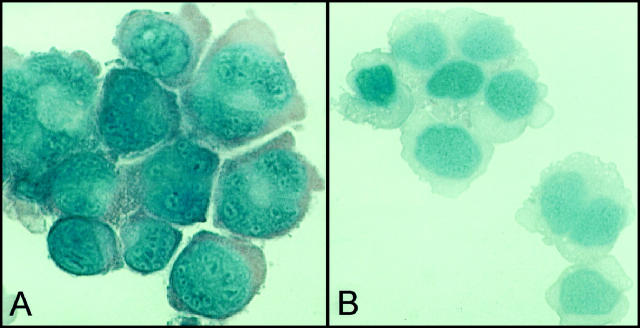
Three CHD cell lines (HS445, L428, and KMH2) were analyzed for caspase-3 expression by immunohistochemistry. HS445 and L428 consistently demonstrated substantial cytoplasmic immunostaining for caspase-3 (Figure 2 ▶ and data not shown). However, in contrast, repeated immunohistochemistry assays failed to detect expression of caspase-3 in the KMH2 cell line (Figure 2) ▶ .
Figure 2.
Immunohistochemical detection of caspase-3 on cytospin preparations of the L428 cell line (A) displayed strong cytoplasmic positive staining for caspase-3 (AEC, ×400); however, the KMH2 cell line (B) failed to express caspase-3 (AEC, ×400). Isotype control antibody staining was negative (data not shown).
Caspase-3 Was Proteolytically Cleaved and Activated during CD95-Mediated Apoptosis in Caspase-3-Positive CHD Cell Lines
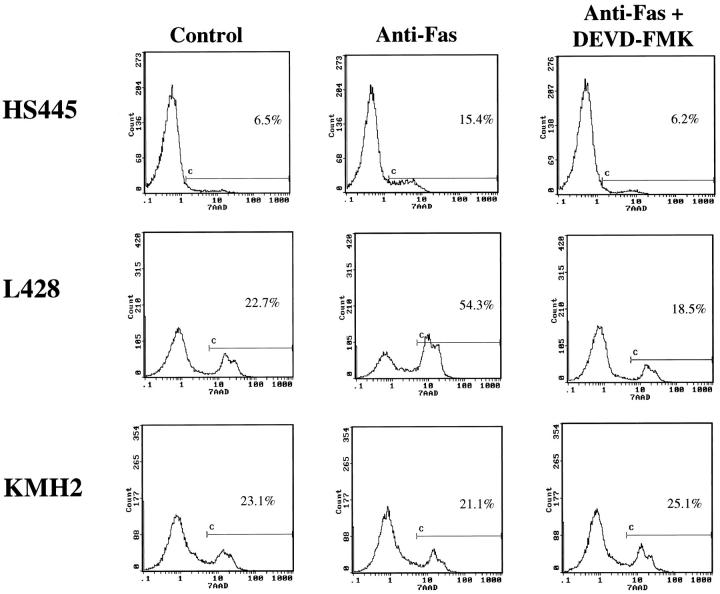
Activation of the CD95 receptor by ligand or agonistic mAb is known to induce apoptosis with concomitant proteolytic cleavage and activation of caspases, including caspase-3, in CD95-positive neoplasms. 19-23 To investigate the effect of CD95 stimulation with potential activation of caspase-3 in Hodgkin’s disease, we examined the effect of agonistic CD95 mAb on CHD cell lines. The HS445 and L428 cell lines displayed a significant increase in apoptosis after 72 hours’ treatment with anti-CD95 mAb as quantitated by both flow cytometric analysis with 7-AAD (Figure 3) ▶ staining and the TUNEL assay (Figure 4 ▶ , Table 2 ▶ ). CD95-induced apoptosis was significantly inhibited in these cells by the caspase-3 peptide inhibitor, DEVD-FMK, as demonstrated by the decrease in number of apoptotic cells to nearly background levels (Figures 3 and 4 ▶ ▶ , Table 2 ▶ ). In contrast, the KMH2 cell line demonstrated consistent resistance to anti-CD95 mAb treatment with no effect by the addition of DEVD-FMK (Figure 3 ▶ and Table 2 ▶ ). Resistance of KMH2 cells to CD95-mediated apoptosis was not due to lack of CD95 expression, as all three cell lines expressed similarly high levels of CD95 as determined by flow cytometric staining. 26
Figure 3.
Flow cytometric analysis of 7-AAD staining in anti-CD95 (Fas)-treated CHD cell lines. Increased cell death was observed after 72 hours of treatment with 500 ng/ml agonistic CD95 mAb (CH11) in HS445 and L428 cell lines compared to untreated control cells. Pretreatment of cells with caspase-3 peptide inhibitor, DEVD-FMK, significantly decreased cell death in anti-CD95-treated cells to near background levels. In contrast, no significant increase in cell death was observed after anti-CD95 treatment of KMH2 cells as compared to untreated cells. The x axis represents fluorescence intensity (log scale) and the y axis represents relative cell number. These data are representative of at least three separate experiments performed.
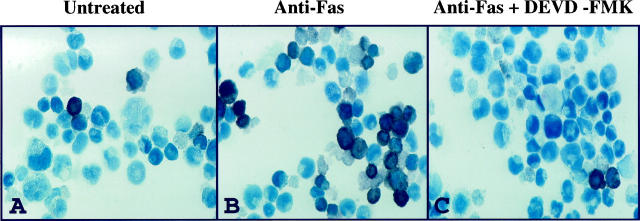
Figure 4.
TUNEL assay for apoptosis. Compared to untreated cells (A), the L428 cell line displayed a considerable increase in apoptosis (dark brown/black cells) after 72 hour incubation with 500 ng/ml agonistic CD95 (Fas) mAb (B). Pretreatment of L428 cells with 10 μM DEVD-FMK decreased the number of apoptotic cells to near baseline levels (C). Stimulation of KMH2 cells with anti-CD95 mAb with or without DEVD-FMK pretreatment showed no increase in apoptosis compared to untreated cells (data not shown).
Table 2.
Apoptosis Rates Induced in Anti-CD95 mAb Treated or Untreated CHD Cell Lines as Determined by TUNEL Assay
| CHD cell line | Apoptosis* | ||
|---|---|---|---|
| Untreated | Anti-CD95 | Anti-CD95+ DEVD-FMK | |
| HS445 | 7.5% | 20.5% | 9.9% |
| L428 | 7.8% | 30.2% | 10.8% |
| KMH2 | 1.0% | 1.3% | 1.0% |
*1 × 106 cells were untreated or treated with 500 ng/ml anti-CD95 mAb (CH11) for 72 hours with or without pretreatment with 10 μM caspase-3 peptide inhibitor, DEVD-FMK. Apoptosis was quantitated by TUNEL staining as described in Materials and Methods.
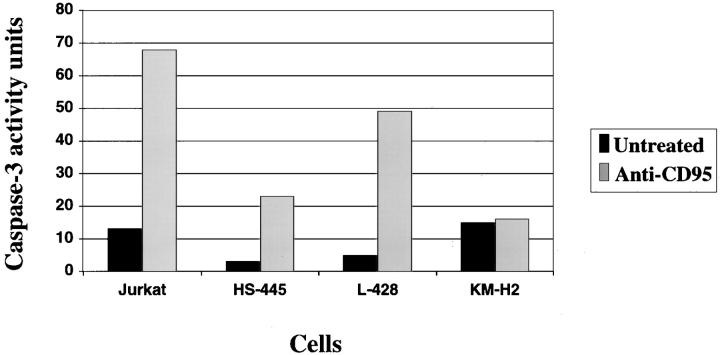
The significant inhibition of apoptosis by a caspase-3 inhibitor in HS445 and L428 cells, and the lack of apoptosis induced by caspase-3-deficient KMH2 cells suggests caspase-3 is important for CD95-mediated apoptosis in CHD cell lines. To further substantiate caspase-3 cleavage and activation in CD95-mediated apoptosis in CHD, each cell line was evaluated for changes in caspase-3 activity before and after treatment with anti-CD95 mAb using an enzyme colorimetric assay (Figure 5) ▶ . Forty-eight-hour treatment revealed approximately tenfold increases in caspase-3 activity in HS445 and L428 cells in contrast to no difference detected in treated KMH2 cells. Positive control CD95-sensitive Jurkat T cells displayed a fivefold increase in caspase-3 activity after 24 hours of treatment with anti-CD95 mAb.
Figure 5.
Caspase-3 activity in anti-CD95-treated CHD cell lines. Positive control for caspase-3 activity is demonstrated in Jurkat T cells by fivefold induction of caspase-3 activity after 24 hours of agonistic CD95 mAb treatment. HS445 and L428 cell lines had approximately ten-fold increases in caspase-3 activity after 48 hours’ treatment with anti-CD95 mAb compared to untreated controls. In contrast, no significant increase in caspase-3 activity was detected in anti-CD95-treated KMH2 cells when compared to untreated cells. These data are representative of three separate experiments.
Discussion
Among the caspases identified in humans thus far, caspase-3 is probably one of the most relevant and best studied as regards to apoptosis in hematopoietic cells. Caspase-3 (CPP32, Yama, apopain) has been shown to be a key effector molecule in the downstream execution of various apoptotic stimuli. 9-11,27-30 Activated caspase-3 cleaves and inactivates many vital cellular proteins during apoptosis including kinases and proteins associated with cellular structure, cell cycle, and DNA repair. One such well characterized caspase-3 death substrate is poly(ADP-ribose) polymerase (PARP), an enzyme involved in DNA repair, genome surveillance, and integrity. 31,32 In addition, caspase-3 appears to indirectly activate endonucleases implicated in internucleosomal DNA cleavage by removing the negative regulatory effect of PARP. 31
The cleavage and activation of caspase-3 during apoptosis has been well documented in neoplastic cells. Caspase-3 activation and subsequent cleavage of its substrates, protein kinase C-δ (PKC-δ) and PARP, was demonstrated by chemotherapeutic drug treatment in human leukemic cell lines. 33 Cross-resistance to CD95- and chemotherapeutic drug-induced apoptosis due to lack of caspase activation including caspase-3 was demonstrated in a human acute T-cell leukemia line, CEM. 34 Also, MCF breast carcinoma cells lacking expression of caspase-3 were resistant to apoptotic stimuli. 35 Thus, the expression and activation of caspase-3 appears to be critical for the execution of various apoptotic stimuli in neoplasms.
Hodgkin’s disease accounts for 14% of malignant lymphomas. Currently, one-third of advanced Hodgkin’s disease patients are resistant to conventional therapies. 36 Our knowledge of the expression and function of apoptosis-related proteins such as caspases and how they may contribute to the pathogenesis and treatment of this malignancy is limited. Previous immunohistochemical studies in situ demonstrated that caspase-3 is commonly expressed in CHD. 37 However, the examination of caspase-3 expression in NLPHD has been limited. Furthermore, the overall biological significance of caspase-3 in Hodgkin’s disease is unknown. Therefore, in this study we examined the expression of caspase-3 in NLPHD and determined its functional significance in CHD cell lines.
We first examined the in situ expression of caspase-3 in NLPHD. By immunohistochemistry, we identified caspase-3 immunopositivity in scattered L&H cells from only 1 of 11 cases of NLPHD. These findings are in agreement with the study of Chhanabhai and colleagues, who found no expression of caspase-3 in L&H cells from 6 cases of NLPHD. 37 In addition, these authors observed the HRS in the majority of cases of CHD were positive for caspase-3 expression. 37 These latter observations differ from the immunohistochemical findings of Xerri et al in which only 3 of 16 cases of HRS of CHD (nodular sclerosis and mixed cellularity type) were caspase-3-immunopositive. 38 The reason for the difference in caspase-3 expression in CHD noted between these groups is presently unclear.
Our immunohistochemical analysis of HRS cells of three CHD cell lines revealed substantial expression of caspase-3 in HS445 and L428, but only weak expression in KMH2 cells. These findings concur with Western blot analysis of these cell lines, which revealed expression of the 32-kd zymogen form of caspase-3 in HS445 and L428, but virtually undetectable expression in KMH2 cells. 26
To address the biological significance of caspase-3 in Hodgkin’s disease, we investigated the role of caspase-3 in CD95-mediated apoptosis in CHD lines. After stimulation of the CD95 receptor by agonistic CD95 mAb, significant apoptosis was induced in caspase-3 positive cell lines HS445 and L428 by TUNEL and 7-AAD assays. However, KMH2 cells, which virtually failed to express caspase-3 by immunohistochemistry, were consistently resistant to CD95 stimulation, suggesting that resistance to CD95-mediated apoptosis in this cell line may be due to a deficiency of caspase-3 26 (manuscript in preparation).
To establish caspase-3 as a key mediator in CD95-induced apoptosis in CHD cell lines, enzyme assays specific for caspase-3 activity were performed. Approximately tenfold increases in caspase-3 activity were observed in HS445 and L428 after 48 hours’ incubation with anti-CD95 mAb, compared to no increase in KMH2 cells. In addition, we pretreated each cell line with the caspase-3 peptide inhibitor DEVD-FMK before CD95 activation. Previous studies in other experimental systems have demonstrated that DEVD inhibitors have specificity for caspase-3 by bearing similarities to the cleavage site of the caspase-3 substrate, PARP. 9,11,27-31,39-41 The addition of DEVD-FMK to cultures of HS445 and L428 significantly decreased CD95-mediated apoptosis; however, there was no effect on KMH2 cells. These findings in CHD lines correlate with previous studies which demonstrated caspase-3 is proteolytically cleaved and activated and plays a key role in CD95-mediated apoptosis in other experimental systems. 9-11,27-30,42 However, it should be noted that CD95-induced apoptosis may occur without activation of caspase-3, suggesting the existence of alternate apoptosis execution pathways in response to CD95 signaling. 43
Most investigations related to apoptosis in Hodgkin’s disease have focused on the expression of mitochondrial apoptosis regulatory proteins Bcl-2, Bcl-x, and Bax. 44-49 These studies revealed variable expression of Bcl-2 44-48 but frequent expression of the pro-apoptotic protein Bax 47 and the apoptosis antagonist Bcl-xL. 48-49 Previous investigations of CD95 expression by HRS cells have been limited; however, these studies revealed that CD95 is expressed on HRS cells in the majority of cases of CHD. 50-53 In this report, we demonstrate that CHD cell lines expressing CD95 can undergo apoptosis by CD95 stimulation. A recent study assayed CD95-induced apoptosis in fresh tissue samples with Hodgkin’s disease; however, the HRS cells were not specifically analyzed. 51 Because HRS cells usually constitute less than 1% of involved tissue, it is difficult to assay CD95 stimulation of HRS cells directly without selective separation.
The absence of caspase-3 expression in L&H cells is similar to that seen in several indolent B-cell NHLs, most notably follicular center lymphoma (FCL), grade I. 38,54 Recent studies have noted clinical similarities between NLPHD and indolent B-cell NHL. 55-57 However, NLPHD differs from the majority of low-grade B-cell NHLs with respect to treatment response. Most NLPHD patients are cured and rarely show progressive disease, in contrast in the majority of FCL patients. Furthermore, although bcl-2 is commonly overexpressed in FCLs, NLPHD typically lacks expression of this protein. 24,46,58 The combined high and low expression of bcl-2 and caspase-3 protein, respectively, in low-grade FCL suggests the incurability of many of these lymphomas may be directly related to the overexpression of anti-apoptotic proteins (eg, Bcl-2) combined with the lack of downstream apoptotic mediators such as caspase-3. Furthermore, overexpression of Bcl-2 and Bcl-xL in cell lines can also block cleavage and activation of caspase-3. 27,29,59-61
The lack of caspase-3 expression in NLPHD may also be an important mechanism of resistance to apoptosis. Furthermore, the differential expression of caspase-3 between CHD and NLPHD suggests that each may be a distinct disease entity, and may account for some of the clinical differences between these two disorders. Additional studies to define the expression and function of caspases and their relationship to other apoptosis-related proteins may provide novel insights into the pathogenesis and treatment resistance of this malignancy.
Acknowledgments
We thank Barbara Rozhon for assistance in preparing the manuscript, Luann Desautel of the Loyola University Medical Center Flow Cytometry Laboratory for technical assistance with 7-AAD staining, and Heide Guzlas for expert photographic assistance. The authors gratefully acknowledge Dr. Tom Ellis for critical review of this manuscript.
Footnotes
Address reprint requests to Serhan Alkan, M.D., Department of Pathology, Loyola University Medical Center, EMS Building, Suite 2230, 2160 S. First Avenue, Maywood, IL 60153-5385. E-mail: SALKAN@luc.edu.
References
- 1.Tsujimoto Y, Croce CM: Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA 1986, 83:5214-5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaul DL, Cory S, Adams JM: Bcl-2 gene promotes haematopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988, 335:440-442 [DOI] [PubMed] [Google Scholar]
- 3.Hengartner MO, Horvitz HR: C. elegans survival gene ced-9 encodes a functional homologue of the mammalian proto-oncogene bcl-2. Cell 1994, 76:665-676 [DOI] [PubMed] [Google Scholar]
- 4.Shaham S, Horvitz HR: Developing Caenorhabditis elegans neurons may contain both cell-death protective and killer activities. Genes Dev 1996, 10:578-591 [DOI] [PubMed] [Google Scholar]
- 5.Yuan J, Shaham S, Ledoux S, Ellis JM, Horvitz JR: C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell 1993, 75:641-652 [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Benedict MA, Wu D, Inohara N, Nunez G: Bcl-XL interacts with Apaf-1, and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci USA 1998, 95:4386-4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J: Human ICE/CED-3 protease nomenclature. Cell 1996, 87:171. [DOI] [PubMed] [Google Scholar]
- 8.Humke EW, Ni J, Dixit VM: ERICE, a novel FLICE-activatable caspase. J Biol Chem 1998, 273:15702-15707 [DOI] [PubMed] [Google Scholar]
- 9.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW: CPP32/apopain is a key interleukin 1β converting enzyme-like protease involved in Fas- mediated apoptosis. J Biol Chem 1996, 1841–1844 [DOI] [PubMed]
- 10.Enari M, Hug H, Nagata S: Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature 1995, 375:78-81 [DOI] [PubMed] [Google Scholar]
- 11.Enari M, Talanian RV, Wong WW, Nagata S: Sequential activation of ICE-like and CPP32- like proteases during Fas-mediated apoptosis. Nature 1996, 380:723-726 [DOI] [PubMed] [Google Scholar]
- 12.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S: The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66:233-243 [DOI] [PubMed] [Google Scholar]
- 13.Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, Li-Weber M, Richards S, Dhein J, Trauth BC: Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem 1992, 267:10709-10715 [PubMed] [Google Scholar]
- 14.Smith CA, Farrah T, Goodwin RG: The TNF receptor superfamily of cellular, and viral proteins: activation, costimulation and death. Cell 1994, 76:959-962 [DOI] [PubMed] [Google Scholar]
- 15.Bump NJ, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P: Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science 1995, 269:1885-1888 [DOI] [PubMed] [Google Scholar]
- 16.Nagata S, Suda T: Fas and Fas ligand: lpr and gld mutations. Immunol Today 1995, 16:39-43 [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S: Generalized lymphoproliferative disease in mice caused by a point mutation in Fas ligand. Cell 1994, 76:969-976 [DOI] [PubMed] [Google Scholar]
- 18.Gronbaek K, thor Straten P, Ahrenkeil V, Klarskov Anderson M, Ebbe Hansen N, Zeuthen J, Hou-Jensen K, Guldenberg P: Somatic fas mutations in non-Hodgkin’s lymphomas: association with extranodal disease and autoimmunity. Blood 1998, 92:3018-3024 [PubMed] [Google Scholar]
- 19.Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH: Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989, 245:301-305 [DOI] [PubMed] [Google Scholar]
- 20.Yonehara S, Ishii A, Yonehara M: A cell killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med 1989, 169:1747-1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suda T, Nagata S: Purification and characterization of the Fas ligand that induces apoptosis. J Exp Med 1994, 179:873-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suda T, Takahashi T, Golstein P, Nagata S: Molecular cloning and expression of the Fas ligand, a novel member of the TNF family. Cell 1993, 75:1169-1178 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S: Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol 1994, 6:1567-1574 [DOI] [PubMed] [Google Scholar]
- 24.von Wasielewski R, Werner M, Fischer R, Hansmann ML, Hubner K, Hasenclever D, Franklin J, Sextro M, Diehl V, Georgii A: Lymphocyte-predominant Hodgkin’s disease: An immunohistochemical analysis of 208 reviewed Hodgkin’s disease cases from the German Hodgkin’s Study Group. Am J Pathol 1997, 150:793-803 [PMC free article] [PubMed] [Google Scholar]
- 25.Harris NL, Jaffe ES, Stein H, Banks P, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 26.Alkan S, Hsi ED, Wrone-Smith T: CD95 (Fas antigen: APO-1) mediated apoptosis on Hodgkin’s disease cell lines. Mod Pathol 1998, 11:162A (abstr. 950)
- 27.Armstrong RC, Aja T, Xiang J, Gaur S, Krebs JF, Hoang K, Bai X, Korsmeyer SJ, Karanewski DS, Fritz LC, Tomaselli KJ: Fas-induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem 1996, 271:16850-16855 [DOI] [PubMed] [Google Scholar]
- 28.Los M, Van de Craen M, Penning LC, Schenk H, Westendrop M, Baeuerle PA, Droge W, Krammer PH, Fiers W, Schulze-Osthoff K: Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature 1995, 375:81-83 [DOI] [PubMed] [Google Scholar]
- 29.Chinnaiyan AM, Orth K, O’Rourke K, Duan H, Poirier GG, Dixit VM: Molecular ordering of the cell death pathway: Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem 1996, 271:4573-4576 [DOI] [PubMed] [Google Scholar]
- 30.Darmon AJ, Bleackley RC: An interleukin-1β converting enzyme-like protease is a key component of Fas-mediated apoptosis. J Biol Chem 1996, 271:21699-21702 [DOI] [PubMed] [Google Scholar]
- 31.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA: Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376:37-43 [DOI] [PubMed] [Google Scholar]
- 32.Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM: Yama/CPP32b, a mammalian homologue of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly (ADP-ribose) polymerase. Cell 1995, 81:801-809 [DOI] [PubMed] [Google Scholar]
- 33.Datta R, Banach D, Kojima H, Talanian RV, Alnemri ES, Wong WW, Kufe DW: Activation of the CPP32 protease in apoptosis induced by 1-β-D-Arabinofuranosylcytosine and other DNA-damaging agents. Blood 1996, 6:1936-1943 [PubMed] [Google Scholar]
- 34.Los M, Herr I, Friesen C, Fulda S, Schulze-Osthoff K, Debatin K-M: Cross-resistance of CD95- and drug-induced apoptosis as a consequence of deficient activation of caspases (ICE/Ced-3 proteases). Blood 1997, 8:3118-3129 [PubMed] [Google Scholar]
- 35.Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC: Cell-specific induction of apoptosis by microinjection of cytochrome c. J Biol Chem 1997, 48:30299-30305 [DOI] [PubMed] [Google Scholar]
- 36.Hasenclaver D, Diehl V: A prognostic score for advanced Hodgkin’s disease. New Engl J Med 1998, 339:1506-1514 [DOI] [PubMed] [Google Scholar]
- 37.Chhanabhai M, Krajewski S, Krajewska M, Wang HG, Reed JC, Gascoyne RD: Immunohistochemical analysis of interleukin-1β-converting enzyme/Ced-3 family protease CPP32/Yama/Caspase 3 in Hodgkin’s disease. Blood 1997, 90:2451-2455 [PubMed] [Google Scholar]
- 38.Xerri L, Devilard E, Ayello C, Brousset P, Reed JC, Emile J-F, Hassoun J, Parmentier S, Birg F: Cysteine protease CPP32, but not Ich1-L, is expressed in germinal center B cells and their neoplastic counterparts. Hum Pathol , 28:912-921 [DOI] [PubMed] [Google Scholar]
- 39.Livingston DJ: In vitro and in vivo studies of ICE inhibitors. J Cell Biochem 1997, 64:19-26 [PubMed] [Google Scholar]
- 40.Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y: Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene 1996, 12:2251-2257 [PubMed] [Google Scholar]
- 41.Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW: Substrate specificities of caspase family proteases. J Biol Chem 1997, 272:9677-9682 [DOI] [PubMed] [Google Scholar]
- 42.Zhivotovsky B, Burgess DH, Schlegel J, Porn MI, Vanags D, Orrenius S: Proteases in Fas-mediated apoptosis. J Cell Biochem 1997, 64:43-49 [PubMed] [Google Scholar]
- 43.Monney L, Otter I, Olivier R, Ozer HL, Hass AL, Omura S, Borner C: Defects in the ubiquitin pathway induce caspase-independent apoptosis blocked by Bcl-2. J Biol Chem 1998, 273:6121-6131 [DOI] [PubMed] [Google Scholar]
- 44.Gupta RK, Lister TA, Bodmer JG: The t(14;18) chromosomal translocation and Bcl-2 protein expression in Hodgkin’s disease. Leukemia 1994, 8:1337-1341 [PubMed] [Google Scholar]
- 45.Bhagat SK, Medeiros LJ, Weiss LM, Wang J, Raffeld M, Stetler-Stevenson M: Bcl-2 expression in Hodgkin’s disease. Correlation with the t(14;18) translocation and Epstein-Barr virus. Am J Clin Pathol 1993, 99:604-608 [DOI] [PubMed] [Google Scholar]
- 46.Alkan S, Ross CW, Hanson CA, Schnitzer B: Epstein-Barr virus bcl-2 protein overexpression are not detected in the neoplastic cells of nodular lymphocyte predominance Hodgkin’s disease. Mod Pathol 1995, 8:544-547 [PubMed] [Google Scholar]
- 47.Rigal-Huguet F, Gopas J, Prinsloo I, Pris J, Delsol G, Reed JC, Schlaifer D, Brousset P, Benharroch D, Krajewski S, Laurent G, Meggetto F: Frequent expression of the cell death-inducing gene Bax in Reed-Sternberg cells of Hodgkin’s disease. Blood 1996, 87:2470-2475 [PubMed] [Google Scholar]
- 48.Schlaifer D, March M, Krajewski S, Laurent G, Pris J, Delsol G, Reed JC, Brousset P: High expression of the bcl-x gene in Reed-Sternberg cells of Hodgkin’s disease. Blood 1995, 85:2671-2674 [PubMed] [Google Scholar]
- 49.Schlaifer D, Krajewski S, Rigal-Huguet F, Laurent G, Pris J, Delsol G, Reed JC, Brousset P: Bcl-x gene expression in Hodgkin’s disease. Leuk Lymphoma 1996, 23:143-146 [DOI] [PubMed] [Google Scholar]
- 50.Kubonishi I, Daibata M, Sakuma I, Yoshino T, Sonobe H, Ohtsuki Y, Miyoshi I: Expression of Fas and apoptosis of a Hodgkin’s disease cell line (HD-70). Int J Hematol 1997, 65:305-307 [PubMed] [Google Scholar]
- 51.Xerri L, Devilard E, Hassoun J, Haddad P, Birg F: Malignant and reactive cells from human lymphomas frequently express Fas ligand but display a different sensitivity to Fas-mediated apoptosis. Leukemia 1997, 11:1868-1877 [DOI] [PubMed] [Google Scholar]
- 52.Nguyen PL, Harris NL, Ritz J, Robertson MJ: Expression of CD95 antigen and Bcl-2 protein in non-Hodgkin’s lymphomas and Hodgkin’s disease. Am J Pathol 1996, 148:847-853 [PMC free article] [PubMed] [Google Scholar]
- 53.Dirks W, Schone S, Uphoff C, Quentmeier H, Pradella S, Drexler HG: Expression and function of CD95 (FAS/APO-1) in leukaemia-lymphoma tumour lines. Br J Haematol 1997, 96:584-593 [DOI] [PubMed] [Google Scholar]
- 54.Krajewski S, Gascoyne RD, Zapata JM, Krajewska M, Kitada S, Chhanabhai M, Horsmann D, Berean K, Piro LD, Fugier-Vivier I, Liu YJ, Wang HG, Reed JC: Immunolocalization of the ICE/Ced-3-family protease, CPP32 (Caspase 3), in non-Hodgkin’s lymphomas, chronic lymphocytic leukemias, and reactive lymph nodes. Blood 1997, 89:3817-3825 [PubMed] [Google Scholar]
- 55.Pinkus GS, Said JW: Hodgkin’s disease, lymphocyte predominance type, nodular- further evidence for a B-cell derivation. Am J Pathol 1988, 133:211-217 [PMC free article] [PubMed] [Google Scholar]
- 56.Grossman DM, Hanson CA, Schnitzer B: Simultaneous lymphocyte predominant Hodgkin’s disease and large-cell lymphoma. Am J Surg Pathol 1991, 15:668-676 [DOI] [PubMed] [Google Scholar]
- 57.Sextro M, Diehl V, Franklin J, Hansmann ML, Anagnostopoulous I, Marafioti T, Stein H: Lymphocyte predominant Hodgkin’s disease: a workshop report. Ann Oncol 1996, 7:61-65 [DOI] [PubMed] [Google Scholar]
- 58.Algara P, Martinez P, Sanchez L, Villuendas R, Orradre JL, Oliva H, Piris MA: Lymphocyte predominance Hodgkin’s disease (nodular paragranuloma): a bcl-2 negative germinal centre lymphoma. Histopathology 1991, 19:69-75 [DOI] [PubMed] [Google Scholar]
- 59.Ibrado AM, Huang Y, Fang G, Liu L, Bhalla K: Overexpression of Bcl-2 or Bcl-xL inhibits Ara-C-induced CPP32/Yama protease activity and apoptosis of human acute myelogenous leukemia HL-60 cells. Cancer Res 1996, 56:4743-4748 [PubMed] [Google Scholar]
- 60.Memon SA, Moreno MB, Petrak D, Zacharchuk CM: Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol 1995, 155:4644-4652 [PubMed] [Google Scholar]
- 61.Moreno MB, Memon SA, Zacharchuk CM: Apoptosis signaling pathways in normal T cells: differential activity of Bcl-2 and IL-1β-converting enzyme family protease inhibitors on glucocorticoid- and Fas-mediated cytotoxicity. J Immunol 1996, 157:3845-3849 [PubMed] [Google Scholar]