Abstract
Microsatellite instability (MSI) characterizes colorectal carcinomas (CRCs) in hereditary nonpolyposis colorectal cancer (HNPCC) syndrome and a proportion of sporadic CRCs. These MSI+ CRCs share several clinicopathological features, including a reputation for better survival rates than MSI− cases and a pronounced stromal inflammatory reaction of still undefined nature. In the present study, the presence, spatial distribution, and activation status of infiltrating cytotoxic effectors were investigated comparatively in 18 MSI+ and 37 MSI− CRCs by immunohistochemistry. The frequency of apoptosis was also evaluated by morphology and in situ end-labeling. MSI+ cases carried significantly higher numbers of cytotoxic lymphocytes infiltrating within neoplastic epithelial structures, as shown by immunostaining for CD3 (15.1 ± 6.2 versus 4.6 ± 4.1, P < 0.001), CD8 (13 ± 6.4 versus 3.7 ± 3.8, P < 0.001), and TIA-1 (11.2 ± 6.5 versus 1.9 ± 1.7, P < 0.001). These cytotoxic effectors were globally more activated in MSI+ than in MSI− tumors, as revealed by the expression of granzyme B (5.3 ± 4.5 versus 0.6 ± 1.3, P < 0.001). In MSI+ CRCs, the number of intraepithelial activated cytotoxic lymphocytes was significantly correlated with the proximal location of the tumor, a poorly differentiated phenotype, and the presence of peritumor lymphoid nodules. Multivariate analysis revealed that MSI was the major determinant of the presence of activated cytotoxic intraepithelial lymphocytes. Moreover, MSI+ CRCs also showed a significantly higher percentage of tumor cells undergoing apoptotic cell death (4.1 ± 2.1 versus 2.6 ± 1.1, P < 0.0001, by the TUNEL method), often located in close proximity of activated cytotoxic lymphocytes. These results are consistent with the presence of anti-tumor cytotoxic immune responses in most of MSI+ CRCs, a phenomenon that may at least in part contribute to the survival advantage ascribed to these patients.
Most colorectal carcinomas (CRCs) in hereditary nonpolyposis colorectal cancer (HNPCC) syndrome 1,2 and a proportion (15% to 28%) of sporadic CRCs 3-5 show a particular form of genetic instability, also termed microsatellite instability (MSI), which leads to the accumulation of deletion and insertion mutations at simple repeated sequences. This is the result of the failure to correct mistakes that may occur during DNA replication as a consequence of defects in the mismatch repair system. 6 Five causative genes for HNPCC, namely, hMSH2, hMLH1, hPMS1, hPMS2, and hMSH6, have recently been identified as homologues of bacterial DNA mismatch repair genes (reviewed in Ref. 7 ). Sporadic CRCs with microsatellite instability (MSI+ cases) share some clinicopathological features with those occurring in HNPCC patients, such as the location in the proximal colon, the mucinous histotype, and poor differentiation. 5,8,9 In particular, it has been reported that patients with HNPCC 10-14 or MSI+ CRCs 3,5 show apparently better survival rates than cases carrying MSI− tumors. One attractive hypothesis to explain this phenomenon is constituted by the possibility that, besides favoring the occurrence of mutations at genes critical for oncogenesis, the mutator phenotype may also increase the production of abnormal peptides able to elicit cytotoxic immune responses against tumor cells. Of interest, recent histopathological findings have indicated that MSI+ CRCs, either from HNPCC kindreds or sporadic cases, are characterized by the presence of a pronounced stromal inflammatory reaction. 8,9 A similar lymphoid infiltrate is only infrequently observed in MSI− tumors. 8,9 Nevertheless, only limited information is currently available on the presence and activation status of cytotoxic effectors in these tumors.
Recent experimental data have brought considerable progress in our understanding of the molecular pathways involved in the process of cell killing mediated by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. 15 In particular, it has been demonstrated that the granule exocytosis pathway is one of the main mechanisms of cellular cytotoxicity. 15 In this pathway, the recognition and tight binding of a susceptible target cell by a CTL or NK cell induces the release of electron-dense cytoplasmic granules by the effector cells. These secretory granules contain perforin, a protein that undergoes Ca2+-dependent polymerization on the target cell membrane forming a channel that allows the exocytosis of other granule constituents. 16 These latter include granzymes, 17 a family of neutral serine proteases, and the protein TIA-1, 18,19 which are critical for the induction of DNA fragmentation and apoptosis of the target cell. Expression of the TIA-1 protein is characteristic of cytotoxic cells, independently of their activation status, whereas perforin and granzymes are produced only by activated effectors. 20 Recently, monoclonal antibodies specifically reacting with these granule proteins became available, thus allowing the identification of activated CTLs or NK cells in situ.
The aim of the present investigation was to better define the nature of the lymphoid infiltrate that characterizes MSI+ CRCs. In particular, we sought to determine whether the presence, spatial distribution, and activation status of cytotoxic effectors infiltrating MSI+ CRCs could be compatible with a tumor-specific immune response. The frequency of apoptotic tumor cells was also comparatively analyzed in MSI+ and MSI− CRCs by in situ end-labeling.
Materials and Methods
Tissue Specimens
This study includes 55 primary CRCs from 55 patients (35 males and 20 females; mean age, 60.3 ± 13.9 and 61.2 ± 18.9 years, respectively). Twenty-eight carcinomas were located in the proximal colon (cecum, ascending colon, hepatic flexure, and transverse colon), whereas 27 had distal location (splenic flexure, descending colon, sigmoid, and rectum). Eleven tumors (seven proximal and four distal) were obtained from a series of CRC patients with a suspicion of genetic predisposition based on tumor family history and/or very early age of tumor onset (mean age, 38.5 ± 11.4 years). Five of them were identified as heterozygous carriers of constitutional pathogenetic MLH1 or MSH2 gene mutations. 21,22 The other 44 cases were selected from a series of apparently sporadic CRC patients (mean age, 66.2 ± 11.1 years), with unavailable family history, and were enriched in right-sided tumors (21/44) to increase the probability of including MSI+ cases. 8 Conventional histopathological parameters, including Duke’s stage, grading, and histotype, were also considered. The presence of peritumor lymphoid nodules, also called Crohn’s-like reaction, 23 was identified and scored as described by Graham and Appleman. 23 A CRC was defined as mucinous when more than 30% of the tumor area was composed of mucus lakes with interspersed neoplastic cells. Medullary carcinoma was classified as described by Rüschoff. 24 Thirteen tumors were classified as mucinous whereas three CRCs were of the medullary type.
MSI Analysis
DNA was extracted from peripheral blood lymphocytes and paraffin-embedded normal and tumor tissues and analyzed for evidence of genetic instability at a minimum of six of seven microsatellite loci, including tetra- (L-myc on 1p), 25 tri- (DM on 19p), 26 di- (D1S170 on 1p, CA21 on 2p, and D3S1611 on 3p), 27,28 and mono-nucleotide repeats (BAT-13 and BAT-26 on 2p). 29 Polymerase chain reaction was carried out in the presence of [α-33P]dATP (Amersham, Little Chalfont, UK) as described previously. 30 Genetic instability was determined as mobility shift of 33P-labeled polymerase chain reaction products, by comparison between tumor and corresponding normal DNAs. If two or more markers were positive, tumors were considered as having high MSI and were defined as MSI+. Tumors with only one positive marker (low MSI) and CRCs with stable microsatellite sequences were defined as MSI−.
Immunohistochemistry
Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded material as previously described in detail. 31 Briefly, sections were deparaffinized in xylene, rehydrated, washed in phosphate-buffered saline (PBS), and, for selected antigens, immersed in 0.01 mol/l citrate buffer, pH 6, or 0.1 mmol/l EGTA and microwaved for 5 minutes at 750 W three times. The sections were then kept for 15 minutes at room temperature (RT) before further PBS washing and immunostaining with a standard streptavidin-biotin-peroxidase procedure and diaminobenzidine (DAB) color reaction. Double-immunostaining procedures were performed on selected samples; the usual streptavidin-peroxidase procedure with brown DAB color reaction was followed by a second cycle of microwaving and a streptavidin-alkaline phosphatase immunostaining with Fast Red as chromogen. The following antibodies were used: anti-CD3 (polyclonal, 1:1000), anti-CD8 (clone cd8/144b, 1:25), and anti-β2-microglobulin (β2M) (polyclonal A072, 1:5000) obtained from DAKO (Glostrup, Denmark); anti-CD4 (clone IF6, 1:100) from Novocastra (Newcastle, UK); anti-granzyme B (GrB; clone GrB7, 1:20) from Monosan (Leiden, The Netherlands); anti-perforin (clone KM585 P1–8, 1:1000) from Kamiya (Nippon); anti-TIA-1 (1:1000) from Coulter Corp. (Hialeah, FL); anti-CD56 (clone 123C3.D5, 1:100) from Neomarker (Freemont, CA); and anti-HLA-ABC monomorphic with preference with HLA-B heavy chain (clone HC10, 1:800), a kind gift of Dr. S. Ferrone (Department of Microbiology and Immunology, New York Medical College, Valhalla, NY). A cocktail of the CAM5.2 (1:50; Becton and Dickinson, Milan, Italy) and the AE1 (1:200; Neomarker) antibodies was used for cytokeratin detection in double-labeling experiments. Nonspecific antibody binding was determined on sections incubated with the same concentration of an irrelevant antibody of the appropriate isotype. Normal tonsil and spleen were used as positive controls.
Intratumor-intraepithelial CD3+, CD4+, CD8+, GrB+, perforin+, TIA-1+, and CD56+ cells were counted in 10 randomly selected areas of tumor at ×400 using a Leica DMB microscope. Fields were chosen to contain the maximal amount of neoplastic cells with minimal stroma or necrotic debris. The number of intratumor CD4+ lymphocytes was also evaluated at ×200 in the neoplastic stroma and at the advancing edge of the tumor and scored as follows: 0, <10 CD4+ lymphocytes; +, <50 CD4+ lymphocytes; and ++, >50 CD4+ lymphocytes. Quantification of the relative number of positive cells was performed in all cases by using a video-assisted measuring system (MicroImage, SC Casti Imaging, Italy). All cases were evaluated independently by two pathologists (C. Doglioni and M. Guidoboni). Counts of labeled cells by the two observers did not vary by more than 1%.
Detection of Apoptosis
Apoptotic cells were identified by conventional morphological criteria on hematoxylin and eosin sections. Moreover, the TUNEL method was also used to identify DNA fragmentation in situ by the protocol derived from Gavrieli 32 as modified by Migheli. 33 All reagents for TUNEL reaction were obtained from Boehringer (Mannheim, Germany). Briefly, rehydrated sections were digested with proteinase K (Sigma) at 20 μg/ml in Tris/EDTA (TE) for 20 minutes at RT. After washing in TE and quenching of peroxidase activity with 3% H2O2 in distilled water for 10 minutes, slides were immersed in TdT buffer (25 mmol/L Tris/HCl, 0.2 mol/L sodium cacodylate, 2.5 mmol/L cobalt chloride, pH 6.6) for 5 minutes and then incubated in TdT mixture composed of 1 part enzyme solution (TdT) and 9 parts label solution (fluorescein-dUTP), diluted 1:4 with TdT buffer, for 2 hours at 37°C. After washing with 2X SSC and Tris-buffered saline, slides were immersed in a solution of Tris-buffered saline/Triton with 2% bovine serum albumin for 15 minutes at RT, followed by incubation with Converter POD (peroxidase-labeled anti-fluorescein antiserum) for 30 minutes at RT. After washing, peroxidase activity was visualized with DAB color reaction. Slides were counterstained with Harris’ hematoxylin, dehydrated, and mounted. Double labeling for GrB/TUNEL, CD8/TUNEL, and cytokeratin/TUNEL was performed on selected MSI+ CRCs by sequential TUNEL with DAB as chromogen giving a brown reaction product, followed by immunoalkaline phosphatase staining with a red (Fast Red) reaction product. For each case, the number of morphologically identifiable apoptotic cells and the TUNEL labeling index were determined by counting 1000 neoplastic cells in 10 consecutive fields at ×400, chosen randomly in non-necrotic areas of the tumors. Evaluation was performed with the same video-assisted method mentioned above.
Statistical Analysis
Possible relationships between immunophenotypic markers and clinicopathological parameters of CRCs were investigated by comparing mean cellular count values for different levels of clinicopathological variables. Data are expressed as mean ± SD. The Wilcoxon rank-sum test for unpaired data was performed for statistical evaluation of significant difference in the distribution of two groups. Mean counts for different immunophenotypic markers were also compared by analysis of covariance, introducing in the model age and gender. Variable frequency was compared using the χ2 and Fisher’s exact tests. A stepwise regression discriminant analysis was performed to identify which of the clinical and histopathological variables discriminated between subjects, after adjusting for age and gender, using cellular count values as a dependent variable. Starting from a full model with all variables included, nonsignificant variables were progressively deleted with a step-down procedure based on a likelihood ratio test. R2, which represents the percentage of variability of each variable considered, was also given from the regression model. The Pearson’s method for correlation analysis was used to compare TUNEL labeling index with the number of morphologically identifiable apoptotic cells and GrB+ cell counts, respectively.
Results
Clinicopathological Features of MSI+ CRCs
Overall, 18 of the 55 selected CRCs (33%) were classified as MSI+ and showed instability in two (six cases), three (three cases), four (four cases), five (four cases), and seven loci (one case). Microsatellite alterations at a single locus were found in only two samples. All loci investigated manifested replication errors, with variable frequencies of instability: 33% for L-myc, 31% for BAT26, 20% for BAT13 and D1S170, 14% for CA21, 13% for D3S1611, and 7% for DM. The observed mobility shifts resulted from both increases and decreases in fragment size. The MSI+ cases were also tested for the first panel of five microsatellite sequences previously proposed for the diagnosis of MSI in CRC. 34 High instability (≥40%) was confirmed in 17/18 MSI+ tumors, with the remaining one showing instability at 20% of the loci analyzed. These findings indicate that our panel of microsatellites and our MSI definition criteria were almost superimposable to those recently recommended for diagnostic use. 34,35
Five of the eighteen MSI+ tumors had developed in patients carrying germline MLH1 or MSH2 gene mutations. For the other 13 patients, the carrier status was excluded (n = 4), or not investigated (n = 9). The incidence of MSI differed substantially in relationship to some clinicopathological parameters (Table 1) ▶ . In particular, the patients with MSI+ tumors were significantly younger than those with MSI− tumors (P = 0.004), although this was mainly due to to the presence of true and putative HNPCC cases in our series. In fact, with hereditary cases removed (nine MSI+; mean age, 39.6 ± 12.2 years, and two MSI−; mean age, 34 ± 7.1 years), there was no evidence that sporadic MSI+ CRCs (nine cases; mean age, 63.2 ± 14.8 years) occurred in patients significantly younger than sporadic MSI− tumors (35 cases; mean age, 66.9 ± 10.1). In the MSI+ group, there was also a large excess of cancers located in the proximal region (15/18), whereas only 13/37 MSI− tumors were right-sided (P < 0.001). Moreover, the frequency of MSI proved to be significantly higher in poorly differentiated (9/12) than in medium and well differentiated (9/43) cancers (P = 0.002). All but one MSI+ (17/18) and 25/37 MSI− tumors showed the presence of peritumor lymphoid nodules (P = 0.04). All of three of the CRCs of the medullary type were MSI+. No differences related to sex, tumor stage, or mucinous differentiation were found between the two groups. Immunohistochemical analysis showed that cases with total or partial loss of either β2M or HLA class I heavy chain were similarly distributed among MSI+ and MSI− tumors (Table 1) ▶ .
Table 1.
Distribution of Several Clinicopathological Characteristics of CRCs According to the Presence of MSI
| Total | MSI− (n = 37) | MSI+ (n = 18) | p-value | |
|---|---|---|---|---|
| Age (mean± SD) | 65.1± 12.1 | 51.4± 17.9 | 0.004* | |
| Sex | ||||
| Males | 35 | 26 (70.3%) | 9 (50.0%) | |
| Females | 20 | 11 (29.7%) | 9 (50.0%) | 0.2† |
| Site | ||||
| Proximal | 28 | 13 (35.1%) | 15 (83.3%) | |
| Distal | 27 | 24 (64.9%) | 3 (16.7%) | <0.001† |
| Grading | ||||
| Well | 6 | 5 (13.5%) | 1 (5.6%) | |
| Moderate | 37 | 29 (78.4%) | 8 (44.4%) | |
| Poor | 12 | 3 (8.1%) | 9 (50.0%) | 0.002† |
| Duke’s | ||||
| A | 7 | 6 (16.2%) | 1 (5.6%) | |
| B | 29 | 18 (48.6%) | 11 (61.1%) | |
| C | 16 | 11 (29.7%) | 5 (27.8%) | |
| D | 3 | 2 (5.4%) | 1 (5.6%) | 0.7† |
| Mucinous histotype | ||||
| No | 42 | 31 (83.8%) | 11 (61.1%) | |
| Yes | 13 | 6 (16.2%) | 7 (38.9%) | 0.09† |
| Peritumor lymphoid nodules | ||||
| Absent | 13 | 12 (32.4%) | 1 (5.6%) | |
| Present | 42 | 25 (67.6%) | 17 (94.4%) | 0.04† |
| HLA class I heavy chain | ||||
| 0–25% | 2 | 1 (2.7%) | 1 (5.5%) | |
| 25–50% | 22 | 15 (40.5%) | 7 (38.9%) | |
| >50% | 31 | 21 (56.8%) | 10 (55.6%) | 0.9† |
| β2-microglobulin | ||||
| 0–25% | 3 | 1 (2.7%) | 2 (11.1%) | |
| 25–50% | 16 | 10 (27%) | 6 (33.3%) | |
| >50% | 36 | 26 (70.3%) | 10 (55.6%) | 0.2† |
*Wilcoxon rank sum test.
†Fisher’s exact test.
MSI Significantly Correlates with the Presence of Activated Cytotoxic Intraepithelial Lymphocytes (IELs)
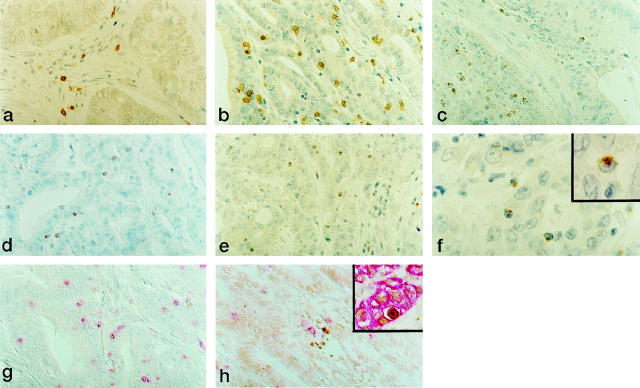
As observed in the majority of unselected colorectal tumors, 36 also in most of our MSI− cases the infiltration of mononuclear cells was generally restricted to the stromal areas with only few lymphocytes infiltrating between neoplastic cells (Figure 1a) ▶ . By contrast, MSI+ CRCs carried significantly higher numbers of CD3+ lymphocytes infiltrating either conserved neoplastic glandular structures or tumor areas characterized by deranged histological architecture (Table 2) ▶ . The majority of these IELs were potential cytotoxic effectors as revealed by the immunostaining for CD8 and TIA-1 (Table 2 ▶ and Figure 1, b and c ▶ ). Conversely, MSI− cases carried significantly fewer IELs positive for CD8 (Table 2) ▶ and TIA-1 (1.9 ± 1.7 versus 11.2 ± 6.5, P < 0.001). Of note, when compared with MSI− cases, MSI+ CRCs showed a strikingly higher percentage of IELs expressing perforin (Figure 1d ▶ and data not shown) and GrB (Figure 1e ▶ and Table 2 ▶ ). Consistently with recent findings, 37 these proteins with cytolytic function were usually not expressed by IELs or lamina propria lymphocytes from adjacent normal mucosa. Double-staining experiments showed that most of the GrB+ cells in MSI+ cases also expressed CD3 and CD8, confirming that these cells were activated CTLs (Figure 1g ▶ and data not shown). Natural killer CD56+ cells, which are known to express GrB and perforin, were detected in infiltrates only in minimal numbers (<1%). As shown in Table 2 ▶ , the GrB+/CD3+ and GrB+/CD8+ ratios were significantly higher in MSI+ CRCs, indicating that CTLs infiltrating these tumors were globally more activated than in MSI− cases. CD4+ IELs were absent or present in very low numbers in both MSI+ and MSI− cases. Moreover, these two groups of CRCs carried similar percentages of CD4+ lymphocytes located within the stroma or at the advancing edge of the tumor. These findings indicate that the recruitment, activation, and infiltration of lymphocytes within neoplastic epithelial structures of MSI+ cases are events that selectively involve CD8+ cytotoxic precursors.
Figure 1.
Characterization of intraepithelial lymphocytes in representative cases of MSI− (a) and MSI+ (b to g) CRCs. Activated CTLs infiltrating within neoplastic epithelial structures are present in significantly higher numbers in MSI+ CRCs. CD8 (a and b), TIA-1 (c), perforin (d), and GrB (e). Immunoperoxidase with hematoxylin counterstain. Original magnification, ×400. f: Polarized GrB immunolocalization in IELs infiltrating MSI+ CRCs. Original magnification, ×1000. g: Co-localization of CD8 (red) and GrB (brown) in IELs in MSI+ CRCs. Double immunolabeling with Fast Red and DAB as chromogens; original magnification, ×400. h: Apoptotic cell bodies (TUNEL+, brown) in close contact with CD8+ (red) intraepithelial lymphocytes in MSI+ CRCs. TUNEL developed with DAB, followed by immunostaining with anti-CD8 antibody developed with Fast Red; original magnification, ×400. Inset: Cytokeratin/TUNEL double labeling demonstrating that the large majority of TUNEL+ cells were neoplastic epithelial cells. TUNEL developed with DAB, followed by immunostaining with anti-cytokeratin antibodies developed with Fast Red; original magnification, ×400.
Table 2.
Immunophenotype of IELs According to the Presence of MSI and the Clinicopathological Characteristics of Patients with CRC
| n | CD3+ cell count | CD8+ cell count | GrB+ cell count | GrB+/CD3+ (%) | GrB+/CD8+ (%) | |
|---|---|---|---|---|---|---|
| MSI | ||||||
| Negative | 37 | 4.6 ± 4.1 | 3.7 ± 3.8 | 0.6 ± 1.3 | 7.4 ± 14.4 | 9.3 ± 16.6 |
| Positive | 18 | 15.1 ± 6.2 | 13 ± 6.4 | 5.3 ± 4.5 | 29.7 ± 17.6 | 36.8 ± 19.0 |
| P value* | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Age | ||||||
| <60 | 25 | 9.5 ± 6.7 | 7.6 ± 5.3 | 2.2 ± 2.7 | 16.5 ± 19.5 | 21.5 ± 22.3 |
| ≥60 | 30 | 6.4 ± 6.6 | 6.0 ± 7.3 | 2.1 ± 4.2 | 12.2 ± 17.7 | 16.1 ± 21.1 |
| P value* | 0.04 | 0.008 | 0.3 | 0.3 | 0.2 | |
| Site | ||||||
| Proximal | 28 | 9.9 ± 7.0 | 8.9 ± 6.9 | 3.3 ± 4.3 | 21.8 ± 21.0 | 27.4 ± 22.6 |
| Distal | 27 | 5.8 ± 6.0 | 4.5 ± 5.2 | 0.9 ± 2.0 | 6.7 ± 11.9 | 8.9 ± 16.0 |
| P value* | 0.01 | 0.005 | 0.002 | 0.002 | <0.001 | |
| Only proximal site | ||||||
| MSI− | 13 | 5.0 ± 2.6 | 4.2 ± 2.9 | 0.9 ± 1.5 | 13.5 ± 20.7 | 16.4 ± 21.8 |
| MSI+ | 15 | 14.8 ± 6.5 | 12.9 ± 6.8 | 5.5 ± 4.7 | 30.1 ± 18.5 | 37.0 ± 19.1 |
| P value* | <0.001 | <0.001 | <0.001 | 0.02 | 0.009 | |
| Only MSI− | ||||||
| Proximal site | 13 | 5.0 ± 2.6 | 4.2 ± 2.9 | 0.9 ± 1.5 | 13.5 ± 20.7 | 16.4 ± 21.8 |
| Distal site | 24 | 4.4 ± 4.7 | 3.4 ± 4.2 | 0.4 ± 1.2 | 4.1 ± 8.4 | 5.4 ± 11.5 |
| P value* | 0.2 | 0.1 | 0.09 | 0.06 | 0.05 | |
| Stage | ||||||
| A/B | 36 | 8.0 ± 7.2 | 7.2 ± 7.1 | 2.2 ± 4.0 | 15.8 ± 20.2 | 20.0 ± 22.2 |
| C/D | 19 | 7.4 ± 6.1 | 5.8 ± 5.0 | 1.5 ± 2.4 | 11.1 ± 14.9 | 15.6 ± 20.7 |
| P value* | 0.9 | 0.8 | 0.4 | 0.5 | 0.4 | |
| Mucinous histotype | ||||||
| No | 42 | 7.5 ± 6.8 | 6.6 ± 6.7 | 2.2 ± 3.8 | 13.8 ± 19.1 | 18.1 ± 22.4 |
| Yes | 13 | 8.8 ± 6.7 | 7.0 ± 5.7 | 2.0 ± 2.6 | 15.1 ± 17.1 | 19.7 ± 19.7 |
| P value* | 0.5 | 0.8 | 0.8 | 0.7 | 0.7 | |
| Grading | ||||||
| Well/Moderate | 43 | 5.9 ± 5.0 | 4.7 ± 4.3 | 1.2 ± 1.8 | 11.1 ± 16.8 | 14.6 ± 19.7 |
| Poor | 12 | 14.9 ± 7.9 | 13.8 ± 8.0 | 5.7 ± 5.5 | 25.5 ± 20.9 | 32.1 ± 23.6 |
| P value* | <0.001 | <0.001 | 0.002 | 0.02 | 0.02 | |
| Peritumor lymphoid nodules | ||||||
| Absent | 13 | 4.8 ± 4.6 | 3.7 ± 4.7 | 0.7 ± 1.9 | 6.4 ± 11.4 | 8.7 ± 15.3 |
| Present | 42 | 8.8 ± 7.1 | 7.7 ± 6.7 | 2.6 ± 3.8 | 16.6 ± 19.7 | 21.6 ± 22.5 |
| P value* | 0.1 | 0.05 | 0.04 | 0.09 | 0.04 |
GrB+/CD3+ and GrB+/CD8+ represent the ratios between the mean cell counts for GrB and CD3 and for GrB and CD8, respectively, expressed as percentage values.
*Wilcoxon rank sum test.
Infiltration by activated cytotoxic IELs was significantly correlated with the location of the tumor in the proximal colon, a poorly differentiated tumor phenotype and the presence of lymphoid nodules within peritumor stroma (Table 2) ▶ . Our findings seem also to rule out that the proximal site of the tumor may have any influence per se on the presence and activation of cytotoxic IELs. In fact, when the right-sided CRCs from our series were analyzed separately, only MSI+ tumors were significantly associated with the infiltration by activated cytotoxic IELs. Consistently, among MSI− cases, the presence of these cells did not show any relationship with the location of the tumor (Table 2) ▶ . Finally, the three MSI+ cases with distal location also showed a high content of IELs, with mean numbers of CD3+ and CD8+ cells similar to those detected in right-sided MSI+ CRCs (not shown). No correlation was found between the presence of activated cytotoxic IELs and sex, age, Duke’s stage, or mucinous differentiation (Table 2) ▶ . The possible influence of age on the presence of activated cytotoxic IELs was further investigated by using the analysis of covariance. Inclusion of age did not modify the results presented in Table 2 ▶ (not shown). Of note, multivariate statistical analysis showed that the MSI was the major predictor of the amount of activated cytotoxic IELs whereas grading, site, mucinous differentiation, and stage had only a modest impact on the infiltration of the tumor by these cells (Table 3) ▶ .
Table 3.
Variables Selected from Stepwise Procedure Linked with Different Phenotypic Markers of IELs Infiltrating CRCs
| R2 | |||||
|---|---|---|---|---|---|
| CD3+ | CD8+ | GrB+ | GrB+/CD3+ | GrB+/CD8+ | |
| MSI | 0.51 | 0.49 | 0.47 | 0.33 | 0.38 |
| Grading | 0.05 | 0 | 0.08 | * | * |
| Site | * | * | * | 0.04 | 0.04 |
| Mucinous histotype | * | * | 0.02 | 0.03 | 0.03 |
| Stage | * | * | 0.03 | * | * |
| Peritumor lymphoid nodules | * | * | * | * | * |
| R2 of model | 0.56 | 0.57 | 0.60 | 0.40 | 0.45 |
*Variables excluded from final model for not reaching the required significance level.
Increased Content of Activated Cytotoxic IELs in MSI+ CRCs Is Strictly Associated with Higher Numbers of Apoptotic Neoplastic Cells
Although GrB+ granules were dispersed throughout all of the cytoplasm of stromal lymphocytes from both MSI+ and MSI− tumors, a large fraction of GrB+ IELs in MSI+ cases showed a polar distribution of cytotoxic granules (Figure 1f) ▶ , a feature that characterizes cytotoxic effectors actively involved in killing their target cells. 15,17 Consistently, activated cytotoxic IELs from MSI+ cases were sometimes located in close proximity of structures morphologically identifiable as apoptotic bodies. To define whether the presence of activated cytotoxic IELs correlated with increased levels of apoptosis, MSI+ (n = 14) and MSI− (n = 20) CRCs were investigated for the frequency and distribution pattern of apoptotic cells. In both groups of CRCs, apoptotic elements identified by morphology and TUNEL+ cells were randomly distributed throughout the lesions. In the whole series, a strong linear correlation was found between the number of apoptotic cells assessed by morphology and the TUNEL labeling index (R 2 = 0.92; P = 0.01). Whereas MSI− CRCs showed apoptotic cell counts comparable to those reported by others in unselected CRCs 38,39 (2.1 ± 0.8 by morphology and 2.6 ± 1.1 by the TUNEL method), MSI+ cases exhibited significantly higher numbers of apoptotic cells (3.3 ± 2, P < 0.0001, and 4.1 ± 2.1, P < 0.0001, respectively). Among the TUNEL+ cells, apoptotic bodies predominated, but some cells with normal-looking nuclei were also detected. Cytokeratin/TUNEL double labeling indicated that most of TUNEL+ cells were neoplastic epithelial cells (Figure 1h) ▶ , whereas only occasional CD3+ lymphocytes also stained with TUNEL (not shown). Moreover, CD8+ and GrB+ IELs were often seen in close apposition to apoptotic (TUNEL+) neoplastic epithelial cells (Figure 1g ▶ and data not shown), suggesting a direct cytotoxic injury to cancer cell targets. Nevertheless, no evidence of linear correlation was found between the number of GrB+ IELs and TUNEL+ cells (data not shown).
Discussion
Accumulating evidence indicates that, unlike MSI− cases, most MSI+ CRCs are characterized by the presence of a pronounced intratumor inflammatory reaction, the nature of which, however, is still poorly defined. Elucidation of this issue appears of relevance not only to better understand the pathobiology of MSI+ CRCs but also to assess the possible influence of host immune responses on the apparently favorable clinical outcome displayed by these patients. 3,5,10-14 Similarly to what was recently reported by Jass et al, 40 also in our series of CRCs the number of CD8+ T lymphocytes infiltrating within neoplastic epithelial structures was significantly higher in MSI+ than in MSI− tumors. The results presented herein provide additional evidence indicating that the majority of CD8+ IELs infiltrating MSI+ CRCs are cytotoxic effectors (TIA-1+) characterized by a high degree of activation, as revealed by GrB and perforin expression. The observation that the number and distribution of infiltrating CD4+ lymphocytes were similar in MSI+ and MSI− tumors indicates that, in MSI+ CRCs, recruitment and activation of cytotoxic CD8+ T lymphocytes within cancer cell nests were probably specific events. Moreover, activated CD8+ effectors from MSI+ CRCs often showed polarization of cytotoxic granules and were located in close proximity of apoptotic bodies or cells with nuclear DNA fragmentation (TUNEL+), suggesting a possible cell-mediated killing of neoplastic cell targets. Similar findings were also observed in lymph node metastasis of MSI+ tumors, whereas the majority of MSI− CRCs displayed very few GrB+ CD8+ lymphocytes also in metastatic lymph nodes (not shown). These findings are consistent with the possible occurrence of cell-mediated immune responses against neoplastic cells in most of MSI+ CRCs. It is worth considering that, among the different clinicopathological parameters analyzed, MSI was the major determinant of the amount of activated cytotoxic IELs, as shown by multivariate analysis. These findings support the hypothesis that the genetic instability of MSI+ CRCs may lead to the production and heterogeneous expression of genetically altered proteins that may function as tumor-specific neoantigens able to elicit potentially effective anti-tumor immune responses. Nevertheless, besides enhancing tumor antigen production, the mutator phenotype that characterizes MSI+ CRCs could also favor the escape of tumor cells from immune control. In fact, it has been hypothesized that the increased frequency of mutations within the β2M gene observed in MSI+ CRCs may be responsible for the emergence of potentially immunoresistant neoplastic cell clones, able to escape tumor-specific CD8+ CTL recognition as a consequence of reduced or absent HLA class I expression. 41,42 Our immunohistochemical analysis, however, revealed that cases with total or partial loss of β2M or HLA class I heavy chain were similarly distributed among MSI+ and MSI− tumors. In particular, the total absence of these molecules was limited to a minority of cases in both groups of CRCs. In addition, reduced or absent expression of β2M or HLA class I heavy chain was never observed at the advancing edge of the tumor. These findings indicate that the possible selection for HLA-class-I-deficient clones is probably not a major phenomenon in MSI+ CRCs. Moreover, it remains to be established whether the emergence of these immunoresistant clones in MSI+ tumors is directly related to an increased rate of relapses and a worse prognosis.
It is worth considering that, besides the presence of a pronounced intratumor lymphoid infiltration, most MSI+ CRCs also show additional morphological evidence of tumor-associated immune responses, in the form of peritumor lymphoid nodules (the so-called Crohn’s-like lymphoid reaction). 8,9 Consistently with previous findings, 8,9 a strong correlation between MSI and the presence of lymphoid aggregates within peritumor stroma was found also in our series of CRCs. Nevertheless, the results of our multivariate analysis indicate that this particular peritumor infiltration has probably no direct influence on the recruitment and intratumor activation of cytotoxic effectors in MSI+ tumors. These findings suggest, therefore, that the presence of peritumor lymphoid nodules and increased content of activated cytotoxic IELs probably represent different aspects of a complex host immune response against the tumor. This may have a beneficial impact on the natural history of these tumors, as demonstrated by the observation that the presence of peritumor lymphoid aggregates was an independent indicator of a better prognosis, particularly for right-sided CRCs. 43,44 On these grounds, the findings that MSI+ CRCs are closely (and independently) correlated with both high numbers of activated cytotoxic IELs and the presence of peritumor lymphoid nodules strongly suggest that host immune responses may contribute to the apparent survival advantage of patients with MSI+ CRCs despite the poor histological differentiation usually displayed by these tumors.
The demonstration that apoptosis of neoplastic cells was significantly more frequent in MSI+ than in MSI− CRCs further supports the notion that these two groups of tumors are biologically different. The increase in the percentage of neoplastic cells undergoing apoptotic cell death observed in MSI+ tumors, although highly significant, was not very high. Nevertheless, considering that apoptosis is a rapid process with a transient phenotypic appearance, 45 this difference may be relevant in terms of tumor growth rates. In fact, it has been demonstrated that even low levels of apoptotic cell death (∼3%) can induce a tissue regression of 25% over several days if not balanced by proliferation. 46 Moreover, our findings may be of prognostic relevance as suggested by recent reports indicating that the frequency of apoptosis was inversely correlated with invasiveness and metastasization of CRCs and positively correlated with better survival rates in CRC patients aged 45 years and under. 47 The higher content of activated cytotoxic IELs observed in MSI+ CRCs probably contributes to the increased apoptotic cell death of neoplastic cells observed in these tumors. Nevertheless, the lack of linear correlation between the number of TUNEL+ and GrB+ cells suggests that this phenomenon is not entirely attributable to anti-tumor cytotoxic immune responses, being probably dependent, at least in part, on the intrinsic genetic instability of MSI+ CRCs. These tumors, in fact, as a consequence of their increased propensity to accumulate genetic alterations over time, may reach a mutation burden that is no longer compatible with cell survival, leading to higher rates of spontaneous apoptosis.
Besides being helpful in dissecting the clinicopathological heterogeneity of both HNPCC and sporadic CRCs, our findings may also have potentially relevant implications for the clinical management of CRCs. In fact, the identification of high numbers of activated CTLs in MSI+ CRCs suggests that patients with these tumors would probably benefit from therapeutic approaches that are less immunosuppressive than those conventionally used. Moreover, the possibility of treating patients with MSI+ CRCs with approaches aimed at potentiating host immune responses should be also considered to design new and perhaps more effective strategies for the management of this subset of tumors.
Acknowledgments
We thank Drs. C.A. Beltrami (Udine), A. Carbone (Aviano), A.P. Dei Tos (Treviso), D. Della Libera (Conegliano Veneto), S. Meli (Vicenza), G. Sacchi (S. Donà di Piave), and their institutions for providing tumor specimens. We are grateful to L. Della Puppa, E. Meggiolaro, and E. Rodeghiero for technical assistance. We also thank Dr. P. Tonel and Mrs. P. Pistello for help with the manuscript.
Footnotes
Address reprint requests to Dr. Mauro Boiocchi, Division of Experimental Oncology 1, Centro di Riferimento Oncologico, via Pedemontana Occidentale, 12–33081 Aviano (PN), Italy. E-mail: mboiocchi@ets.it.
Supported in part by Associazione Italiana per la Ricerca sul Cancro (AIRC). E. Capozzi is a recipient of an AIRC fellowship.
A. Russo’s present address: Epidemiology Unit, Centro per lo Studio E Prevenzione Oncologica, Florence, Italy.
References
- 1.Aaltonen LA, Peltomäki P, Leach FS, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, Powell SM, Jen J, Hamilton SR, Petersen GM, Kinzler KW, Vogelstein B, de la Chapelle A: Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260:812-816 [DOI] [PubMed] [Google Scholar]
- 2.Wu C, Akiyama Y, Imai K, Miyake S, Nagasaki H, Oto M, Okabe S, Iwama T, Mitamura K, Masumitsu H, Nomizu T, Baba S, Maruyama K, Yuasa Y: DNA alterations in cells from hereditary non-polyposis colorectal cancer patients. Oncogene 1994, 9:991-994 [PubMed] [Google Scholar]
- 3.Thibodeau SN, Bren G, Schaid D: Microsatellite instability in cancer of the proximal colon. Science 1993, 260:816-819 [DOI] [PubMed] [Google Scholar]
- 4.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M: Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363:558-561 [DOI] [PubMed] [Google Scholar]
- 5.Lothe RA, Peltomäki P, Meling GI, Aaltonen LA, Nyström Lahti M, Pylkkänen L, Heimdal K, Andersen TI, Moller P, Rognum TO, Fossa SD, Haldorsen T, Langmark F, Brogger A, de la Chapelle A, Borresen AL: Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 1993, 53:5849-5852 [PubMed] [Google Scholar]
- 6.Strand M, Prolla TA, Liskay RM, Petes TD: Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 1993, 365:274-276 [DOI] [PubMed] [Google Scholar]
- 7.Lynch HT, Smyrk MD: An update on Lynch syndrome. Curr Opin Oncol 1998, 10:349-356 [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Jen J, Vogelstein B, Hamilton SR: Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994, 145:148-156 [PMC free article] [PubMed] [Google Scholar]
- 9.Risio M, Reato G, di Celle PF, Fizzotti M, Rossini FP, Foa R: Microsatellite instability is associated with the histological features of the tumor in nonfamilial colorectal cancer. Cancer Res 1996, 56:5470-5474 [PubMed] [Google Scholar]
- 10.Albano WA, Recabaren JA, Lynch HT, Campbell AS, Mailliard JA, Organ CH, Lynch JF, Kimberling WJ: Natural history of hereditary cancer of the breast and colon. Cancer 1982, 50:360-363 [DOI] [PubMed] [Google Scholar]
- 11.Frei JV: Hereditary nonpolyposis colorectal cancer (Lynch syndrome II): diploid malignancies with prolonged survival. Cancer 1992, 69:1108-1111 [DOI] [PubMed] [Google Scholar]
- 12.Watson P, Lin KM, Rodriguez Bigas MA, Smyrk T, Lemon S, Shashidharan M, Franklin B, Karr B, Thorson A, Lynch HT: Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer 1998, 83:259-266 [PubMed] [Google Scholar]
- 13.Kee F, Collins BJ, Patterson CC: Prognosis in familial non-polyposis colorectal cancer. Gut 1991, 32:513-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sankila R, Aaltonen LA, Järvinen HJ, Mecklin JP: Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology 1996, 110:682-687 [DOI] [PubMed] [Google Scholar]
- 15.Kägi D, Ledermann B, Bürki K, Zinkernagel RM, Hengartner H: Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol 1996, 14:207-232 [DOI] [PubMed] [Google Scholar]
- 16.Liu CC, Walsh CM, Young JD: Perforin: structure and function. Immunol Today 1995, 16:194-201 [DOI] [PubMed] [Google Scholar]
- 17.Smyth MJ, Trapani JA: Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today 1995, 16:202-206 [DOI] [PubMed] [Google Scholar]
- 18.Anderson P, Nagler-Anderson C, O’Brien C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF: A monoclonal antibody reactive with a 15-kd cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol 1990, 144:574-582 [PubMed] [Google Scholar]
- 19.Tian Q, Streuli M, Saito H, Schlossman SF, Anderson P: A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 1991, 67:629-639 [DOI] [PubMed] [Google Scholar]
- 20.Liu CC, Rafii S, Granelli-Piperno A, Trapani JA, Young JD: Perforin and serine esterase gene expression in stimulated human T cells: kinetics, mitogen requirements and effects of cyclosporin A. J Exp Med 1989, 170:2105-2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viel A, Genuardi M, Capozzi E, Leonardi F, Bellacosa A, Paravatou Petsotas M, Pomponi MG, Fornasarig M, Percesepe A, Roncucci L, Tamassia MG, Benatti P, Ponz de Leon M, Valenti A, Covino M, Anti M, Foletto M, Boiocchi M, Neri G: Characterization of MSH2 and MLH1 mutations in Italian families with hereditary nonpolyposis colorectal cancer. Genes Chromosomes & Cancer 1997, 18:8-18 [DOI] [PubMed] [Google Scholar]
- 22.Genuardi M, Anti M, Capozzi E, Leonardi F, Fornasarig M, Novella E, Bellacosa A, Valenti A, Gasbarrini G, Roncucci L, Benatti P, Percesepe A: Ponz de Leon M, Coco C, De Paoli A, Valentini M, Boiocchi M, Neri G, Viel A: MLH1 and MSH2 constitutional mutations in colorectal cancer families not meeting the standard criteria for hereditary nonpolyposis colorectal cancer. Int J Cancer 1998, 75:835-839 [DOI] [PubMed] [Google Scholar]
- 23.Graham DM, Appelman HD: Crohn’s-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol 1990, 3:332-335 [PubMed] [Google Scholar]
- 24.Rüschoff J, Dietmaier W, Lüttges J, Seitz G, Bocker T, Zirngibl H, Schlegel J, Schackert HK, Jauch KW, Hofstaedter F: Poorly differentiated colonic adenocarcinoma medullary type: clinical phenotypic and molecular characteristics. Am J Pathol 1997, 150:1815-1825 [PMC free article] [PubMed] [Google Scholar]
- 25.Mäkelä TP, Hellsten E, Vesa J, Alitalo K, Peltonen L: An Alu variable polyA repeat polymorphism upstream of L-myc at 1p32. Hum Mol Genet 1992, 1:217. [DOI] [PubMed] [Google Scholar]
- 26.Wooster R, Cleton Jansen AM, Collins N, Mangion J, Cornelis RS, Cooper CS, Gusterson BA, Ponder BA, von Deimling A, Wiestler OD, Cornelisse CJ, Devilee P, Stratton MR: Instability of short tandem repeats (microsatellites) in human cancers. Nature Genet 1994, 6:152-156 [DOI] [PubMed] [Google Scholar]
- 27.Engelstein M, Hudson TJ, Lane JM, Lee MK, Leverone B, Landes GM, Peltonen L, Weber JL, Dracopoli NC: A PCR-based linkage map of human chromosome 1. Genomics 1993, 15:251-258 [DOI] [PubMed] [Google Scholar]
- 28.Hemminki A, Peltomäki P, Mecklin JP, Järvinen H, Salovaara R, Nyström Lahti M, de la Chapelle A, Aaltonen LA: Loss of the wild type MLH1 gene is a feature of hereditary nonpolyposis colorectal cancer. Nature Genet 1994, 8:405-410 [DOI] [PubMed] [Google Scholar]
- 29.Parsons R, Myeroff LL, Liu B, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B: Microsatellite instability and mutations of the transforming growth factor β type II receptor gene in colorectal cancer. Cancer Res 1995, 55:5548-5550 [PubMed] [Google Scholar]
- 30.Viel A, Dall’Agnese L, Canzonieri V, Sopracordevole F, Capozzi E, Carbone A, Visentin MC, Boiocchi M: Suppressive role of the metastasis-related nm23–H1 gene in human ovarian carcinomas: association of high messenger RNA expression with lack of lymph node metastasis. Cancer Res 1995, 55:2645-2650 [PubMed] [Google Scholar]
- 31.Maestro R, Gloghini A, Doglioni C, Piccinin S, Vukosavljevic T, Gasparotto D, Carbone A, Boiocchi M: Human non-Hodgkin’s lymphomas overexpress a wild-type form of p53 which is a functional transcriptional activator of the cyclin-dependent kinase inhibitor p21. Blood 1997, 89:2523-2528 [PubMed] [Google Scholar]
- 32.Gavrieli Y, Sherman Y, Ben Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migheli A, Cavalla P, Marino S, Schiffer D: A study of apoptosis in normal and pathologic nervous tissue after in situ end-labeling of DNA strand breaks. J Neuropathol Exp Neurol 1994, 53:606-616 [DOI] [PubMed] [Google Scholar]
- 34.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Rüschoff J: Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 1997, 57:4749-4756 [PubMed] [Google Scholar]
- 35.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S: A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998, 58:5248-5257 [PubMed] [Google Scholar]
- 36.Håkansson L, Adell G, Boeryd B, Sjögren F, Sjödahl R: Infiltration of mononuclear inflammatory cells into primary colorectal carcinomas: an immunohistological analysis. Br J Cancer 1997, 75:374-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chott A, Gerdes D, Spooner A, Mosberger I, Kummer JA, Ebert EC, Blumberg RS, Balk SP: Intraepithelial lymphocytes in normal human intestine do not express proteins associated with cytolytic function. Am J Pathol 1997, 151:435-442 [PMC free article] [PubMed] [Google Scholar]
- 38.Koike M: Significance of spontaneous apoptosis during colorectal tumorigenesis. J Surg Oncol 1996, 62:97-108 [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi Y, Dinjens WN, Bosman FT: Proliferation and apoptosis in proliferative lesions of the colon and rectum. Virchows Arch 1997, 431:111-117 [DOI] [PubMed] [Google Scholar]
- 40.Jass JR, Do K-A, Simms LA, Iino H, Winter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B: Morphology of sporadic colorectal cancer with DNA replication errors. Gut 1998, 42:673-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bicknell DC, Rowan A, Bodmer WF: Beta 2-microglobulin gene mutations: a study of established colorectal cell lines and fresh tumors. Proc Natl Acad Sci USA 1994, 91:4751-4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P: Selection for β2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol 1996, 6:1695-1697 [DOI] [PubMed] [Google Scholar]
- 43.Harrison JC, Dean PJ, el Zeky F, Vander Zwaag R: From Dukes through Jass: pathological prognostic indicators in rectal cancer. Hum Pathol 1994, 25:498-505 [DOI] [PubMed] [Google Scholar]
- 44.Harrison JC, Dean PJ, el Zeky F, Vander Zwaag R: Impact of the Crohn’s-like lymphoid reaction on staging of right-sided colon cancer: results of multivariate analysis. Hum Pathol 1995, 26:31-38 [DOI] [PubMed] [Google Scholar]
- 45.Potten CS: The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 1992, 11:179-195 [DOI] [PubMed] [Google Scholar]
- 46.Bursch W, Paffe S, Putz B, Barthel G, Schulte-Hermann R: Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis 1990, 11:847-853 [DOI] [PubMed] [Google Scholar]
- 47.Langlois NE, Lamb J, Eremin O, Heys SD: Apoptosis in colorectal carcinoma occurring in patients aged 45 years and under: relationship to prognosis mitosis and immunohistochemical demonstration of p53 c-myc and bcl-2 protein products. J Pathol 1997, 182:392-397 [DOI] [PubMed] [Google Scholar]