Tumorigenesis can be viewed as a process of cellular evolution in which individual preneoplastic or tumor cells acquire mutations that increase proliferative capacity and thus confer a selective advantage. This evolutionary process permits tumor cells to escape the restrictions that limit growth of normal cells, such as the restraints imposed by the immune system or adverse metabolic conditions. The iterative process of mutations and selections underlying tumor evolution may be dependent on the ability of cells to mutate.
Normal cells possess multiple mechanisms that prevent mutations from occurring at both the nucleotide sequence level and the chromosome level. These mechanisms include enzymes that repair damaged DNA and signal transduction pathways (checkpoints) that induce cell cycle arrest or apoptosis when individual stages in the cell cycle are not appropriately completed. Normal cells are thus prevented from undergoing DNA replication until DNA damage is repaired, and are prevented from undergoing mitosis until chromosomes are properly attached to the mitotic spindles. 1,2 In contrast to normal cells, cancer cells are characterized by multiple mutations and, not surprisingly, are increasingly being found to harbor mutations in genes that control genomic stability. In fact, mutations in genes that ensure the faithful transmission of genetic information from one cell generation to the next could be early, critical events in tumor formation. 3 In their paper in this issue of The American Journal of Pathology, Willenbucher et al document two forms of genetic instability, namely microsatellite instability and chromosomal aberrations, that occur during tumorigenesis associated with ulcerative colitis. 4 Importantly, these authors observed genomic instability in histologically nondysplatic tissue. Although their findings cannot prove causality, they are consistent with the view that intrinsic genomic instability is required for tumorigenesis in ulcerative colitis.
Mutator Phenotype
The conception of cancer as a mutator phenotype is based on the rarity of mutations in normal cells and the high frequency of mutations in malignant cells. 5,6 This discrepancy led to the mutator phenotype hypothesis, which postulates that the mutation rate in the early stages of tumor development must be significantly greater than that of normal somatic cells. The spontaneous mutation rate of human cells is approximately 1.4 × 10−10 per base pair per cell generation. 7 Based on the number of cell divisions during an average life span, this rate cannot account for the large number of mutations found in many tumors. The mutator phenotype hypothesis proposes that the intrinsic genetic instability of cancer cells drives tumorigenesis by producing a pool of mutations, some of which confer a selective advantage, allowing cells to proliferate under adverse conditions. Others have suggested, however, that increased mutagenesis is not required to produce the multiple mutations observed in cancers, and hypothesize that selection pressures and clonal expansion are the major driving forces in tumorigenesis. 8 The key question is whether the large number of mutations observed in tumors is rate-limiting for tumor growth or whether the mutations are merely associated with tumorigenesis in a noncausal way. In reality, development of most cancers probably requires both intrinsic, genetically based genomic instability and clonal expansion driven by selective advantage. It is difficult to isolate the contribution of each. Nonetheless, several recent studies, including the report by Willenbucher et al in this issue of the Journal, provide evidence that genetic instability is an early characteristic of premalignant lesions and have begun to focus on molecular pathways involved in the genetic changes that arise during tumorigenesis. 4,9
Microsatellite Instability
Support for the mutator phenotype hypothesis derives from studies on a form of familial colon cancer known as hereditary nonpolyposis colon cancer (HNPCC). In HNPCC, mutation in one allele of the genes involved in DNA mismatch repair is inherited. 10,11 Loss of the second, wild-type allele occurs with high frequency, resulting in the development of colorectal carcinoma. Identification of the defective DNA repair pathway was based on studies in Escherichia coli in which cells harboring mutations in the mismatch repair genes mutS or mutL displayed an increased mutation frequency during genetic selection. 12 Mutants of the homologous genes in Saccharomyces cerevisiae were found to exhibit microsatellite instability, ie, alterations in the length of short repetitive sequences (microsatellites). 13 Simultaneously, it was reported that colon cancers exhibit instability of microsatellite sequences located throughout their genomes. 14 Based on the number of probes used to monitor these sequences, the authors calculated that, if a similar level of instability were present throughout the genome, cancer cells could contain as many as 10 5 mutations. The microsatellite instability phenotype detected in a small fraction of sporadic colon cancers was subsequently shown to be a common trait in HNPCC. The hypothesis that HNPCC is associated with a defective DNA mismatch repair pathway was confirmed by genetic linkage studies that showed mutations in hMSH2, 10,15 encoded on chromosome 2, or hMLH1, 16 encoded on chromosome 3, were present in the germlines of HNPCC kindred.
Microsatellite instability has now been reported in a wide variety of sporadic cancers including endometrial, pancreatic, colorectal, gastric, and ovarian neoplasms. What may not be fully appreciated is that the number of microsatellites in the human genome is enormous and that a very large number of mutations are present in these tumors. Moreover, although instability is routinely assayed at microsatellite markers in noncoding DNA, instability has also been observed in mononucleotide repeats within exons, causing inactivation of genes that control growth (eg, TGF-bRII17) and apoptosis (eg, BAX18). These observations support the premise that microsatellite instability is a manifestation of mutagenic mechanism(s) that can drive sporadic carcinogenesis. Notably, mutations in the genes that encode the mismatch repair enzymes have not been identified in all instances, raising the possibility that mutations in other, still unidentified pathways may generate microsatellite instability. There is also correlation between the methylation status of mismatch repair genes, their expression level, and microsatellite instability, indicating that mutations that affect gene expression can also result in a mutator phenotype. 19
The majority of sporadic tumors of any given type do not display high levels of microsatellite instability, suggesting that high levels of genetic instability may be a characteristic of only a select subgroup of tumors. However, since gel assays for detecting microsatellite instability are difficult to quantitate due to a significant background, this phenomenon may be more general than currently appreciated. Microsatellite instability is not limited to the process of tumorigenesis; several recent studies have demonstrated the occurrence of microsatellite instability in the inflammatory conditions of pancreatitis and ulcerative colitis. 20 It has been proposed that inflammation results in an increase in DNA damage, saturating the ability of the cell to repair the damage prior to replication. Mutations may be the initiating events in tumorigenesis that arise from an inflammatory background or the continuous production of mutations may be required for tumor progression.
Chromosomal Instability
Genetic instability is also manifested at the level of chromatin maintenance and segregation. In fact, the molecular pathways that preserve genomic information can be subdivided into those that maintain the integrity of genomic sequences, including the DNA mismatch repair pathway discussed above, and those that direct the segregation of genetic material during cell division. Numerous mechanisms that disrupt these pathways have been proposed to result in genetic instability or a mutator phenotype. 21,22
A change in chromosome number, or aneuploidy, is a defining characteristic of many types of cancer. Defects in one of multiple pathways can probably generate this phenotype. The loss or gain of whole chromosomes presumably results from defective mitosis involving chromosomal nondisjunction in which sister chromatids are not properly segregated to the daughter cells. The aberrant pathways that lead to chromosome mis-segregation have not been well defined but probably involve defects in the mitotic apparatus or spindle assembly checkpoint. 1 The other common chromosomal abnormalities identified in cancers include translocations, deletions, and amplifications. Translocations and deletions can be generated during the repair of double-strand breaks in DNA or as a result of premature mitosis involving damaged DNA. 23 In addition, the repair of double-strand breaks can result in dicentric or acentric chromosomes, which may be lost or fragmented during mitosis. Amplification of individual loci on chromosomes is another form of genetic instability that results in the overexpression of proteins linked with tumorigenesis or resistance to chemotherapeutic agents. 24
One mechanism by which tumors develop chromosomal copy number abnormalities was recently identified by Cahill et al. 25 These authors showed that a subset of colorectal carcinoma cell lines that exhibit whole chromosome instability (CIN) are defective in the mitotic spindle assembly checkpoint. The mitotic or spindle assembly checkpoint is a regulatory stage in the cell cycle that monitors the assembly of mitotic spindles and their attachment to kinetochores. It has been shown that the presence of unattached kinetochores 26 or kinetochores with improper tension before mitosis 27 results in delayed mitosis. This transient cell cycle arrest is postulated to allow for proper spindle assembly or repair of damaged spindles. 28 Based on the response of aneuploid colorectal carcinoma cells lines to spindle-disrupting agents, Cahill et al predicted that these cells may harbor mutations in key regulatory proteins involved in the spindle checkpoint; these authors identified a heterozygous mutant allele of the human BUB1 gene in 2 of 19 colorectal tumor cell lines. Inactivation of the BUB1 gene (budding uninhibited by benzimidazole) in budding yeast has been shown to disrupt the mitotic checkpoint and sensitize the cells to spindle inhibitors. 29 Since heterozygosity at the human locus results in a chromosome instability phenotype, Cahill et al further predicted that the mutation may function in a dominant negative fashion to inactivate the mitotic checkpoint.
Genomic Instability in Ulcerative Colitis
In the accompanying article in this issue, Willenbucher et al examine the frequencies of microsatellite instability and chromosomal alterations in colonic epithelial tissue from patients with long histories of ulcerative colitis. 4 Chromosomal alterations in tumors and adjacent dysplastic and histologically nondysplastic tissues were assessed by comparative genomic hybridization (CGH). Short probes generated from total genomic DNA extracted from colonic samples and normal cells were differentially labeled with fluorochromes and simultaneously hybridized to a normal metaphase spread. 30 The ratio of the colors along the chromosomes provides a quantitative map and relative copy number of DNA sequences in the test sample compared with normal genomic DNA. This method provides quantitative information on chromosomal alterations including the loss or gain of chromosomal fragments or whole chromosomes. Chromosomal translocations in which there is no copy number abnormality are not detected.
The authors found that most (85%) dysplastic epithelial tissues and tumor samples exhibited chromosomal abnormalities. However, they observed significant microsatellite instability in only 2 of the 14 cases (14%). These results are in accord with the studies of Lengauer et al, 22 who found a dramatic increase in whole chromosome instability in most colorectal tumor cell lines and significant microsatellite instability in only a few cell lines. The magnitude of the chromosomal instability identified by Lengauer et al suggested a gain or loss of at least 10−2 chromosomes per generation. Chromosomal instability was present exclusively in aneuploid cell lines, suggesting that this form of genetic instability is characteristic of a later stage of tumor progression during which aneuploidy frequently develops. However, Willenbucher et al identified significant chromosomal alterations in dysplastic and nondysplastic cells in addition to tumor cells, suggesting that chromosomal alterations may occur early among cells evolving in the setting of ulcerative colitis. 4
In both studies, the authors observed that microsatellite instability and chromosomal instability appear to be mutually exclusive. Tumor cells exhibiting microsatellite instability were less likely to exhibit chromosome alterations, whereas cells with chromosomal alterations did not necessarily exhibit significant microsatellite instability, suggesting that either form of genetic instability enhances mutation sufficiently to drive tumorigenesis. These observations are corroborated by two additional studies that used CGH analysis. 31,32 It can be argued that the concurrent expression of multiple forms of genetic instability may be deleterious, at least in some circumstances. It seems reasonable that moderate genetic instability may provide an adaptive advantage, whereas prolonged, severe genetic instability may be lethal.
Willenbucher et al found chromosomal alterations in nondysplastic tissue specimens from 5 of 14 patients (36%), but no significant microsatellite instability. 4 The absence of microsatellite instability in these nondysplastic tissues contrasts with the findings of other groups, a discrepancy the authors attribute to differences in detection methodology and to their own, more stringent definition of microsatellite instability (≥4 altered microsatellite loci among the 10 loci examined). In similar analyses of colonic tissue extracted from patients with ulcerative colitis, Brentnall et al 20 identified significant microsatellite instability in 50% of the nondysplastic tissue specimens by using a lower diagnostic threshold (instability at >2 of 7 loci examined). Subsequent reports have confirmed these observations. 33,34 Thus, the frequency and diagnostic threshold of microsatellite instability in nondysplastic specimens are still controversial matters.
Chromosomal Instability versus Microsatellite Instability in Tumorigenesis
The studies reported in this issue by Willenbucher et al 4 and previous studies by Lengauer et al 22 suggest that the predominant form of genetic instability during tumorigenesis stems from events involving either fragments of chromosomes or whole chromosomes, rather than sequence changes involving a few nucleotides. To account for the increased frequency of chromosomal instability relative to microsatellite instability, Lengauer et al 35 suggested that mutations in any of a large number of proteins required for proper chromosome segregation may result in chromosome instability. The dominant nature of the chromosome instability phenotype observed from cell fusion experiments 22 and following transfection with the mutant BUB1 gene 25 suggests that a single genetic modification can result in a significant defect. In contrast, alterations in genes involved in DNA mismatch repair are recessive, requiring two separate genetic events, one in each allele, to generate detectable microsatellite instability. 36
It is possible that differences in detection methods contribute to the observed frequencies of microsatellite instability and chromosomal instability. Assays of microsatellite instability by gel electrophoresis are relatively sensitive; they are subject to artifacts due to slippage of DNA polymerase during polymerase chain reaction amplification. 37 To overcome this difficulty, some authors have used a very stringent criterion of microsatellite instability, as did Willenbucher et al, who required the detection of alterations in at least 4 loci among the 10 loci examined. 4 This threshold may have limited the detection of microsatellite instability by these authors. In contrast, the detection of chromosomal alterations is not subject to similar restrictions. The frequent occurrence of chromosomal abnormalities in tumor cells as well as the relative ease of detecting them have helped pathologists to diagnose malignant cells for many decades. The recent introduction of both fluorescent in situ hybridization (FISH) and CGH has added new highly sensitive and easily quantifiable assays.
Origins of Genomic Instability in Ulcerative Colitis
Ulcerative colitis, a relapsing form of chronic inflammatory bowel disease, is associated with a high risk of colorectal carcinoma. 38 Patients frequently undergo colectomy for uncontrolled symptoms and to prevent the subsequent development of adenocarcinoma. Colonic tissue resected from these patients has revealed multiple stages in the progression of this disease. Cancer arises from the inflammatory background via a multistep process involving the progressive accumulation of mutations similar to those seen in sporadic colorectal carcinoma. 39 However, unlike sporadic colorectal carcinoma, tumors associated with ulcerative colitis usually develop from flat dysplastic epithelium rather than from adenomas. Diagnosis of dysplastic precursor lesions is difficult because no obvious changes are detected during endoscopy. The diagnosis is frequently based on multiple routine random biopsies that identify dysplastic epithelium.
By what mechanisms might genomic instability arise in ulcerative colitis? The inflammatory field in chronic active ulcerative colitis generates abundant reactive oxygen species, such as hydroxyl radicals, hydrogen peroxide, and superoxide radicals, that can damage DNA. The amount of damage may exceed the capacity of the DNA repair machinery, resulting in mutations including those in genes that effect genetic instability. In support of this idea, recent experiments have demonstrated that exposure of DNA to reactive oxygen species in vitro results in microsatellite instability when the DNA is replicated in E. coli. 40 Reactive oxygen species have also been shown to generate a wide spectrum of other DNA lesions including double-strand breaks. Under normal conditions, the presence of damaged DNA is monitored by the G2/M checkpoint, resulting in either arrest of the cell cycle until the damage has been repaired or apoptosis if the damage is not repairable. 41 Cells deficient for the G2/M checkpoint enter mitosis with damaged DNA, which may result in the loss or gain of large regions of chromatin similar to that observed by Willenbucher et al. 4 The gain or loss of whole chromosomes seen by these authors suggests that cells from ulcerative colitis tissues may be deficient for proteins that direct spindle assembly or mediate the mitotic checkpoint, such as the BUB1 protein identified by Cahill et al. 25 Widespread atypical mitosis has also been identified in the epithelium of patients with long-standing ulcerative colitis, further supporting the possibility that defective mitosis contributes to chromosomal instability. 42
Perhaps the most significant finding reported by Willenbucher et al is the presence of chromosomal alterations in both dysplastic and nondysplastic epithelium. This finding is supported by previous results in which aneuploid cell populations were occasionally identified in nondysplastic epithelium from colectomy specimens containing cancer. 9 That similar chromosomal abnormalities were found in the dysplastic and cancerous tissue suggests that the chromosomal changes precede the development of overt cancer. The fact that no significant chromosomal aberrations were detected in epithelia from low-risk patients with disease duration less than 8 years, or in normal diverticulitis specimens, further suggests that the chromosomal changes resulted from long-standing, persistent inflammation 4 and were not a direct consequence of acute inflammation. At least in the setting of ulcerative colitis, chromosomal alterations are an early characteristic of predysplastic lesions and may be useful as a diagnostic supplement to enhance the early detection of predysplastic lesions before histologic dysplastic morphology is apparent.
Is the presence of chromosomal instability in nondysplastic tissue a general feature of cells prior to the development of cancer? Or do the chromosomal alterations result from long-standing inflammation out of which tumors arise? Inflammation may produce a variety of changes in DNA sequences, some of which predispose to genetic instability. These questions may be difficult to answer because the inflammatory field present in ulcerative colitis provides a milieu effect from which tumors can arise throughout the affected tissue. Similar studies using comparative genomic hybridization with spontaneous colorectal carcinoma 43 have failed to identify chromosomal copy number abnormalities in the adjacent normal tissue. However, nondysplastic cells adjacent to the tumors may not represent the predysplastic cells from which the tumor has evolved. Tissues from other pathogenic processes such as Barrett’s esophagus or adenomatous polyposis coli that present similar field effects should be examined for similar chromosomal abnormalities in nondysplastic tissues.
Conclusion
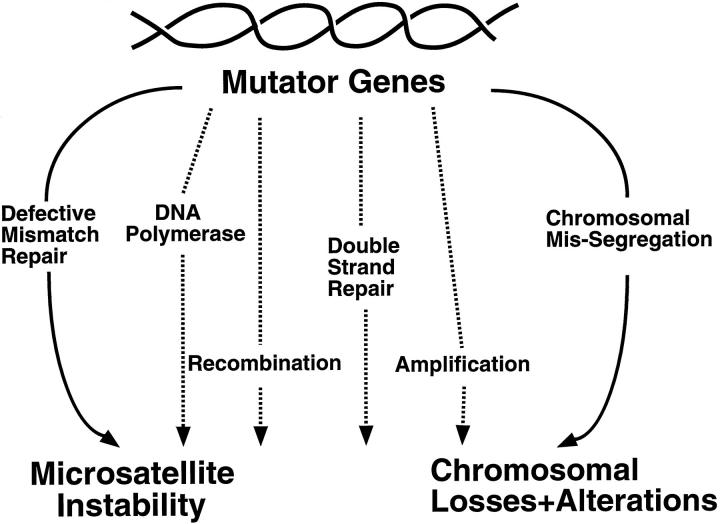
Reports are accumulating that describe the occurrence of microsatellite instability and chromosomal abnormalities early in tumorigenesis, lending support to the mutator phenotype hypothesis. It is apparent that numerous pathways are involved in maintaining genetic stability at both the nucleotide sequence and chromosomal levels, and that mutations in the many genes comprising these pathways are likely to generate a mutator phenotype (Figure 1) ▶ . It seems plausible that the microsatellite instability and chromosomal aberrations observed in cancer cells represent the most readily assayed expressions of intrinsic genomic instability, because they involve alterations in repetitive sequences that are hot-spots for mutations, or they involve karyotypic changes comprising millions of nucleotides, and thus are easy to detect. As we develop new methods to clone and sequence random mutations throughout the genome, we are likely to unearth constellations of alterations in tumor cells that are the end products of successive rounds of mutagenesis and selection.
Figure 1.
Mutations in multiple pathways in tumor cells can result in a mutator phenotype.
The identification of genetic instability as a primary characteristic of cancer cells suggests therapeutic strategies that target the instability. Because the basal level of instability is higher in cancer cells, these cells may be more sensitive to agents that further increase genetic instability. An example is the heightened sensitivity of tumors to spindle inhibitors such as Taxol. In addition, synthetic lethal analyses performed on yeast cells deficient for mismatch repair have shown that coexpression of both a defective mismatch repair pathway and mutations in genes involved in DNA replication, such as an error-prone DNA polymerase, results in a lethal phenotype (S.H. Friend and E.S. Kroll, unpublished results). Therefore, enhancing the basal mutation rate of tumors may be an effective therapeutic strategy.
Footnotes
Address reprint requests to Keith R. Loeb, Department of Molecular Pharmacology, DE-551, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109. E-mail: krloeb@u.washington.edu.
References
- 1.Hartwell LH, Kastan MB: Cell cycle control and cancer. Science 1994, 266:1821-1827 [DOI] [PubMed] [Google Scholar]
- 2.Skibbens RV, Hieter P: Kinetochores and the checkpoint mechanism that monitors for defects in the chromosome segregation machinery. Annu Rev Genet 1998, 32:307-337 [DOI] [PubMed] [Google Scholar]
- 3.Loeb LA: Mutator phenotype may be required for multistage carcinogenesis. Cancer Res 1991, 51:3075-3079 [PubMed] [Google Scholar]
- 4.Willenbucher RF, Aust DE, Chang CG, Zelman SJ, Ferrell LD, Moore DH, Waldman FM: Genomic instability is an early event during the progression pathway of ulcerative colitis-related neoplasia. Am J Pathol 1999, 154:1825-1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeb LA, Springgate CF, Battula N: Errors in DNA replication as a basis of malignant change. Cancer Res 1974, 34:2311-2321 [PubMed] [Google Scholar]
- 6.Nowell PC: The clonal evolution of tumor cell populations. Science 1976, 194:23-28 [DOI] [PubMed] [Google Scholar]
- 7.DeMars R, Held KR: The spontaneous azaguanine-resistant mutants of diploid human fibroblasts. Humangenetik 1972, 16:87-110 [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson I, Bodmer W: Selection, the mutation rate and cancer: ensuring that the tail does not wag the dog. Nat Med 1999, 5:11-12 [DOI] [PubMed] [Google Scholar]
- 9.Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS: DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 1992, 103:1611-1620 [DOI] [PubMed] [Google Scholar]
- 10.Fishel R, Lescoe MK, Rao MRS, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R: The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993, 75:1027-1038 [DOI] [PubMed] [Google Scholar]
- 11.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin J-P, Jarvinen H, Powell SM, Jin J, Hamilton SR, Petersen GM, Kinzler KW, Vogelstein B, de la Chapelle A: Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260:812-816 [DOI] [PubMed] [Google Scholar]
- 12.Modrich P: Mismatch repair, genetic stability, and tumor avoidance. Philosoph Transact Royal Soc 1995, 347:89-95 [DOI] [PubMed] [Google Scholar]
- 13.Strand M, Prolla TA, Liskay RM, Petes TD: Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 1993, 365:274-276 [DOI] [PubMed] [Google Scholar]
- 14.Ionov Y, Peinado MA, Malkhosyan S, Shibata S, Perucho M: Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363:558-561 [DOI] [PubMed] [Google Scholar]
- 15.Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, Peltomaki R, Sistonen P, Aaltonen LA, Nystrom-Lahti M, Guan X-Y, Zhang J, Meltzer PS, Yu J-W, Kao F-T, Chen DJ, Cerosaletti KM, Fournier REK, Todd S, Lewis T, Leach RJ, Naylor SL, Weissenbach J, Mecklin J-P, Jarvinen H, Petersen GM, Hamilton SR, Green J, Jass J, Watson P, Lynch HT, Trent JM, de la Chapelle A, Kinzler KW, Vogelstein B: Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993, 75:1215-1225 [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos N, Nicolaides NC, Wei Y-F, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD, Venter JC, Hamilton SR, Petersen GM, Watson P, Lynch HT, Peltomaki P, Mecklin J-P, de la Chapelle A, Kinzler KW, Vogelstein B: Mutation of a mutL homolog in hereditary colon cancer. Science 1994, 263:1625-1629 [DOI] [PubMed] [Google Scholar]
- 17.Markowitz S, Wang J, Myeroff L, Parsons R, Sun LZ, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV: Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 1995, 268:1336-1338 [DOI] [PubMed] [Google Scholar]
- 18.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M: Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 1997, 275:967-969 [DOI] [PubMed] [Google Scholar]
- 19.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB: Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 1998, 95:6870-6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brentnall TA, Crispin DA, Bronner MP, Cherian SP, Hueffed M, Rabinovitch PS, Rubin CE, Haggitt RC, Boland CR: Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res 1996, 56:1237-1240 [PubMed] [Google Scholar]
- 21.Loeb LA: Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res 1994, 54:5059-5063 [PubMed] [Google Scholar]
- 22.Lengauer C, Kinzler KW, Vogelstein B: Genetic instability in colorectal cancers. Nature 1997, 386:623-627 [DOI] [PubMed] [Google Scholar]
- 23.Paulovich AG, Toczyski DP, Hartwell LH: When checkpoints fail. Cell 1997, 88:315-321 [DOI] [PubMed] [Google Scholar]
- 24.Tlsty TD, Margolin BH, Lum K: Differences in the rates of gene amplification in nontumorigenic and tumorigenic cell lines as measured by Luria-Delbruck fluctuation analysis. Proc Natl Acad Sci USA 1989, 86:9441-9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill D, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B: Mutations of mitotic checkpoint genes in human cancers. Nature 1998, 392:300-303 [DOI] [PubMed] [Google Scholar]
- 26.Rieder CL, Schultz A, Cole R, Sluder G: Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol 1994, 127:1301-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Nicklas RB: Mitotic forces control a cell-cycle checkpoint. Nature 1995, 373:630-632 [DOI] [PubMed] [Google Scholar]
- 28.Hardwick KG: The spindle checkpoint. Trends Genet 1998, 14:1-4 [DOI] [PubMed] [Google Scholar]
- 29.Hoyt MA, Totis L, Roberts BT: S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 1991, 66:507-517 [DOI] [PubMed] [Google Scholar]
- 30.Iwabuchi H, Sakamoto M, Sakunaga H, Ma YY: Genetic analysis of benign, Low-grade, and high-grade ovarian tumors. Cancer Res 1995, 55:6172-6180 [PubMed] [Google Scholar]
- 31.Schlegel J, Stumm G, Scherthan H, Bocker T, Zirngibl H, Ruschoff J, Hofstadter F: Comparative genomic in situ hybridization of colon carcinomas with replication error. Cancer Res 1995, 55:6002-6005 [PubMed] [Google Scholar]
- 32.Muleris M, Dutrillaux AM, Olschwang S, Salmon RJ, Dutrillaux B: Predominance of normal karyotype in colorectal tumors from hereditary non-polyposis colorectal cancer patients. Genes Chromosomes Cancer 1995, 14:223-226 [DOI] [PubMed] [Google Scholar]
- 33.Park WS, Pham T, Wang C, Pack S, Mueller E, Mueller J, Vortmeyer A, Zhuang Z, Fogt F: Loss of heterozygosity and microsatellite instability in non-neoplastic mucosa from patients with chronic ulcerative colitis. Int J Mol Med 1998, 2:221-224 [PubMed] [Google Scholar]
- 34.Cravo ML, Albuquerque CM, Salazar de Sousa L, Gloria LM, Chaves P, Dias Pereira A, Nobre Leitao C, Quina MG, Costa Mira F: Microsatellite instability in non-neoplastic mucosa of patients with ulcerative colitis: effect of folate supplementation. Am J Gastroenterol 1998, 93:2060-2064 [DOI] [PubMed] [Google Scholar]
- 35.Lengauer C, Kinzler KW, Vogelstein B: Genetic instabilities in human cancers. Nature 1998, 396:643-649 [DOI] [PubMed] [Google Scholar]
- 36.Parsons R, Li G-M, Longley MJ, Fang W-h, Papadopoulos N, Jen J, de la Chapelle A, Kinzler KW, Vogelstein B, Modrich P: Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell 1993, 75:1227-1236 [DOI] [PubMed] [Google Scholar]
- 37.Hite JM, Eckert KA, Cheng KC: Factors affecting fidelity of DNA synthesis during PCR amplification of d(C-A)n .d(G-T)n microsatellite repeats. Nucleic Acids Res 1996, 24:2429-2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekbom A, Helmick C, Zack M, Adami HO: Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 1990, 323:1228-1233 [DOI] [PubMed] [Google Scholar]
- 39.Vogelstein B, Fearon ER, Kern SE, Hamilton SR, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AMM, Bos JL: Genetic alterations during colorectal-tumor development. N Engl J Med 1988, 319:525-532 [DOI] [PubMed] [Google Scholar]
- 40.Jackson AL, Loeb LA: The mutation rate and cancer. Genetics 1998, 148:1483-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elledge SJ: Cell cycle checkpoints: preventing an identity crisis. Science 1996, 274:1664-1672 [DOI] [PubMed] [Google Scholar]
- 42.Rubio CA, Befrits R: Atypical mitoses in colectomy specimens from patients with long standing ulcerative colitis. Anticancer Res 1997, 17:2721-2726 [PubMed] [Google Scholar]
- 43.Ried T, Knutzen R, Steinbeck R, Blegen H, Schrock E, Heselmeyer K, du Manoir S, Auer G: Comparative genomic hybridization reveals a specific pattern of chromosomal gains and losses during the genesis of colorectal tumors. Genes Chromosomes Cancer 1996, 15:234-245 [DOI] [PubMed] [Google Scholar]