Abstract
Pseudomyxoma peritonei (PMP) is a poorly understood condition characterized by mucinous ascites and multifocal peritoneal mucinous tumors. Women with PMP often have mucinous tumors involving both the appendix and the ovaries. Several previous histopathological and immunohistochemical studies of PMP have suggested that most, if not all, cases of PMP in women are derived from mucinous adenomas of the appendix rather than from primary ovarian tumors. A few studies of the molecular genetics of PMP have been recently reported. However, these studies analyzed only a small number of cases and some included a heterogeneous group of mucinous tumors, including both benign and malignant appendiceal and ovarian tumors. We analyzed K-ras mutations and allelic losses of chromosomes 18q, 17p, 5q, and 6q in a substantial number of morphologically uniform cases of PMP with synchronous ovarian and appendiceal tumors as well as in appendiceal mucinous adenomas (MAs) and ovarian mucinous tumors of low malignant potential (MLMPs) unassociated with PMP. Each of the 16 PMP cases (100%) analyzed demonstrated identical K-ras mutations in the appendiceal adenoma and corresponding synchronous ovarian tumor. K-ras mutations were identified in 11 of 16 (69%) appendiceal MAs unassociated with PMP and in 12 of 16 (75%) ovarian MLMPs unassociated with PMP. Two PMP cases showed identical allelic losses in the matched ovarian and appendiceal tumors. A discordant pattern of allelic loss between the ovarian and appendiceal tumors at one or two of the loci tested was observed in six PMP cases. In all but one instance, LOH was observed in the ovarian tumor, whereas both alleles were retained in the matched appendiceal lesion, suggesting tumor progression in a secondary (metastatic) site. Our findings strongly support the conclusion that mucinous tumors involving the appendix and ovaries in women with PMP are clonal and derived from a single site, most likely the appendix.
Pseudomyxoma peritonei (PMP) is a poorly understood condition characterized by mucinous ascites and multifocal peritoneal mucinous tumors. Women with PMP often have mucinous tumors involving both the appendix and ovaries, and there has been considerable debate regarding the origin of the tumor in such cases. 1-5 Often, the ovarian tumors in PMP cases are interpreted as the primary site of the disease and are diagnosed as mucinous low malignant potential tumors (MLMPs) or ovarian mucinous adenocarcinomas with extraovarian spread. However, based on careful evaluation of the clinical, gross, and microscopic features of PMP, we and others have concluded that most, if not all, cases of PMP in women are more likely derived from appendiceal mucinous adenomas (MAs). 1,2,4 This conclusion is further supported by recent immunohistochemical studies showing that the pattern of cytokeratin expression in mucinous tumors associated with PMP is similar to that of appendiceal MAs in the absence of PMP (CK20+ and usually CK7−) and different from that of primary ovarian mucinous tumors (CK7+ and usually CK20+). 6,7 However, based on clinicopathological or immunohistochemical features alone, it is impossible to definitively exclude the possibility that some ovarian and appendiceal mucinous tumors in PMP cases may have arisen as separate synchronous primaries.
A few recent cytogenetic and molecular genetic studies of PMP in women have attempted to resolve this issue. 8-11 Most, but not all, reached the conclusion that PMP is indeed a clonal process. However, in these studies only a small number of PMP cases were analyzed, and some were complicated by evaluation of a heterogeneous group of mucinous tumors that included both benign and malignant appendiceal and ovarian tumors.
The purpose of the current study was to analyze several molecular markers in a substantial number of well characterized cases of PMP in women with synchronous appendiceal and ovarian mucinous tumors and to compare the results to similar analyses of appendiceal MAs and ovarian MLMP tumors unassociated with PMP. We restricted our case selection to those characterized by the clinical syndrome of PMP in which there is mucinous ascites and a distinctive pathological lesion that we have termed disseminated peritoneal adenomucinosis. 12 The peritoneal tumors in adenomucinosis are composed of abundant extracellular mucin containing scant strips of nonstratified or focally tufted mucinous epithelium with minimal cytological atypia and mitotic activity. The ovarian mucinous tumors contain similar-appearing mucinous epithelium, often in association with abundant dissecting mucin in the ovarian stroma (pseudomyxoma ovarii). The appendiceal mucinous tumors demonstrate a wide spectrum of histological appearances, ranging from dilated cystadenomas with flattened epithelium, to lesions with features of colonic-type hyperplastic polyps, to very proliferative villous adenomas. We specifically excluded cases of peritoneal mucinous carcinomatosis in which the peritoneal, appendiceal, and ovarian tumors contained histologically malignant mucinous epithelium characterized by significant cytological atypia and proliferative activity. The molecular markers analyzed included K-ras gene mutation (common in both colorectal adenomas 13 and ovarian mucinous tumors), 14 losses of heterozygosity on chromosomes 5q, 17p, and 18q (common in colorectal tumors), 13 and losses of heterozygosity on chromosome 6q (common in ovarian epithelial tumors). 15,16 Through such a study, we hoped to definitively establish the monoclonality of PMP and to gain additional insights into the likely site of origin (ovary versus appendix) in women with this disease.
Materials and Methods
Case Selection
Seventeen cases of PMP with synchronous ovarian and appendiceal mucinous tumors were analyzed. The morphological and immunohistochemical features of these cases have been previously reported. 1,6,12 In addition, 16 mucinous adenomas (MAs) of the appendix and 16 ovarian mucinous tumors of low malignant potential (MLMPs) were obtained from the Surgical Pathology archives of The Johns Hopkins Hospital. All MAs and MLMPs were confined to the appendix and ovary, respectively, without other associated mucinous tumors.
DNA Extraction
Ten consecutive 5-μm formalin-fixed tissue sections were cut from each paraffin block and mounted onto glass slides. Lesional regions were carefully microdissected from weakly hematoxylin-stained sections with 22-gauge needles under a light microscope, using adjacent H&E-stained sections as dissection guides. Genomic DNA was extracted from microdissected tumor tissue as well as from matched non-neoplastic tissue using standard methods as we have previously described. 17
K-ras Mutation Analysis
A 190-bp segment of the K-ras gene encompassing codons 2 to 38 and the first 81 nucleotides of intron 1 was amplified using primers K-ras-F (5′-ACTGAATATAAACTTGTGGTAGTTGGAG) and K-ras-R (5′-TCATGAAAATGGTCAGAGAAACC). Polymerase chain reactions (PCRs) containing 1X PCR buffer (20 mmol/L Tris/HCl, pH 8.4, and 50 mmol/L KCl), 1.5 mmol/L MgCl2, 100 μmol/L dNTP’s, 0.4 μmol/L K-ras-F, 0.4 μmol/L K-ras-R, and 2.5 U of Taq polymerase (Life Technologies, Gaithersburg, MD) were performed using 35 cycles of 30 seconds at 94°C, 30 seconds at 56°C, and 60 seconds at 72°C. The resulting PCR products were evaluated on 2% ethidium-bromide-stained agarose gels. The confirmed PCR products were purified for direct sequencing by treatment with 10.0 U of exonuclease I and 2.0 U of shrimp alkaline phosphatase (Amersham, Arlington Heights, IL) at 37°C for 15 minutes followed by a brief incubation at 80°C for 15 minutes. The purified PCR products were directly sequenced using the ThermoSequenase radiolabeled terminator cycle sequencing kit (Amersham) according to the manufacturer’s instructions. Products of the cycle sequencing reactions were resolved on 6% acrylamide/8 mol/L urea denaturing gels. Gels were fixed in a solution of 5% methanol and 5% acetic acid, dried, and subjected to radiography. Results were confirmed by repeating each PCR and direct sequencing reaction.
Loss of Heterozygosity Analysis/PCR Amplification of Microsatellite Loci
DNA samples from tumor tissues were analyzed for losses of heterozygosity (LOH) at specific loci by comparison with DNA from matched normal tissues. The genotype at polymorphic microsatellite sequences was assessed by PCR amplification using the following MAP pair (Research Genetics, Huntsville, AL) primer sets: D5S592 (5q22.1), D6S474 (6q16.3–22.33), D6S1027 (6q27), D17S1303 (17p13.1–13.3), D18S51 (18q21.33), and D18S499 (18q21.32–21.33). The D18S51 locus was evaluated only in a subset of cases that were uninformative at the D18S499 locus. Each PCR reaction contained 37.5 mmol/L Tris, 2.2 mmol/L MgCl2, 200 μmol/L dATP, 200 μmol/L dGTP, 200 μmol/L dTTP, 25 μmol/L dCTP, 2 μCi (3000 Ci/mmol) of dCTP, 1 μmol/L of each primer, and 1.0 U of Taq polymerase. Target DNA sequences were amplified using an initial denaturation step at 95°C for 5 minutes followed by 30 cycles of 95°C for 30 seconds, 55°C for 1 minute, and 72°C for 1 minute and by a final extension step at 72°C for 5 minutes. PCR products were resolved by electrophoresis on 6% polyacrylamide/8 mol/L urea gels. After electrophoresis, gels were fixed in a solution containing 5% acetic acid and 5% methanol, dried, and subjected to autoradiography. LOH was scored when there was a relative decrease (>50%) in the intensity of the signal of one allele in the tumor as compared with the allele signals in matched normal DNA. Tumors were scored as uninformative when only one allele was present in DNA from matched normal tissue.
Results
A typical example of an appendiceal mucinous adenoma with an associated ovarian mucinous tumor from a case of PMP is shown in Figure 1 ▶ . The morphological features of the ovarian tumors associated with PMP that we believe distinguish these tumors from bona fide primary ovarian mucinous tumors are described in detail elsewhere. 1 Briefly, the ovarian mucinous tumors associated with PMP are often bilateral and relatively small, involve the surface and superficial cortex of the ovary, and contain scant strips of bland mucinous epithelium with abundant dissecting mucin (pseudomyxoma ovarii). Some ovarian tumors contain more abundant mucinous epithelium, as seen in Figure 1B ▶ , and mimic a primary ovarian mucinous tumor. In contrast, primary ovarian MLMPs are almost always unilateral and large, contain abundant proliferative mucinous epithelium with little or no dissecting mucin, and are confined to the stroma of the ovary without surface or superficial cortical nodules.
Figure 1.

A: Appendiceal mucinous adenoma associated with pseudomyxoma peritonei. H&E; magnification, ×65. B: Matched ovarian mucinous tumor in the same case of pseudomyxoma peritonei. H&E; magnification, ×50.
The results of the K-ras mutational analysis are presented in Tables 1 to 3 ▶ ▶ ▶ , and representative data are shown in Figure 2 ▶ . Identical K-ras mutations were identified in the synchronous ovarian and appendiceal tumors in each of the 16 PMP cases (100%) for which sufficient quantities of both tumors were available for analysis. Mutation of K-ras was also identified in the appendiceal tumor of the remaining PMP case. The corresponding ovarian tumor could not be analyzed because there was insufficient tissue available. Overall, 82% of the K-ras mutations in PMP cases affected codon 12, and 18% affected codon 13. K-ras mutations were identified in 11 of 16 (69%) MAs unassociated with PMP (82% at codon 12 and 18% at codon 13) and in 12 of 16 (75%) ovarian MLMP tumors unassociated with PMP (100% at codon 12).
Table 1.
K-ras Mutational Analysis of Synchronous Appendiceal and Ovarian Mucinous Tumors in PMP
| Case | K-ras mutation | Amino acid change | Status of mutations in both sites | |
|---|---|---|---|---|
| Appendiceal tumor | Ovarian tumor | |||
| PMP 1 | Codon 13 GGC→GTT | NA | GLY→VAL | NA |
| PMP 2 | Codon 12 GGT→GAT | Codon 12 GGT→GAT | GLY→ASP | Identical |
| PMP 3 | Codon 12 GGT→GAT | Codon 12 GGT→GAT | GLY→ASP | Identical |
| PMP 4 | Codon 12 GGT→GTT | Codon 12 GGT→GTT | GLY→VAL | Identical |
| PMP 5 | Codon 12 GGT→GTT | Codon 12 GGT→GTT | GLY→VAL | Identical |
| PMP 6 | Codon 12 GGT→GAT | Codon 12 GGT→GAT | GLY→ASP | Identical |
| PMP 7 | Codon 12 GGT→GTT | Codon 12 GGT→GTT | GLY→VAL | Identical |
| PMP 8 | Codon 12 GGT→GTT | Codon 12 GGT→GTT | GLY→VAL | Identical |
| PMP 9 | Codon 12 GGT→GTT | Codon 12 GGT→GTT | GLY→VAL | Identical |
| PMP 10 | Codon 12 GGT→TGT | Codon 12 GGT→TGT | GLY→CYS | Identical |
| PMP 11 | Codon 12 GGT→GAT | Codon 12 GGT→GAT | GLY→ASP | Identical |
| PMP 12 | Codon 12 GGT→GAT | Codon 12 GGT→GAT | GLY→ASP | Identical |
| PMP 13 | Codon 12 GGT→GAT | Codon 12 GGT→GAT | GLY→ASP | Identical |
| PMP 14 | Codon 13 GGC→GAC | Codon 13 GGC→GAC | GLY→ASP | Identical |
| PMP 15 | Codon 12 GGT→GAT | Codon 12 GGT→GAT | GLY→ASP | Identical |
| PMP 16 | Codon 13 GGC→GAC | Codon 13 GGC→GAC | GLY→ASP | Identical |
| PMP 17 | Codon 12 GGT→GTT | Codon 12 GGT→GTT | GLY→VAL | Identical |
NA, insufficient mucinous epithelium for analysis.
Table 2.
K-ras Mutational Analysis of Appendiceal Mucinous Adenomas
| Case | K-ras mutation | Amino acid change |
|---|---|---|
| APP 1 | Codon 13 GGC→GAC | GLY→ASP |
| APP 2 | Codon 12 GGT→AGT | GLY→SER |
| APP 3 | Codon 12 GGT→GAT | GLY→ASP |
| APP 4 | Wild type | None |
| APP 5 | Codon 12 GGT→GAT | GLY→ASP |
| APP 6 | Wild type | None |
| APP 7 | Codon 12 GGT→GTT | GLY→VAL |
| APP 8 | Codon 12 GGT→GTT | GLY→VAL |
| APP 9 | Codon 13 GGC→GAC | GLY→ASP |
| APP 10 | Codon 12 GGT→GAT | GLY→ASP |
| APP 11 | Wild type | None |
| APP 12 | Codon 12 GGT→GTT | GLY→VAL |
| APP 13 | Codon 12 GGT→GAT | GLY→ASP |
| APP 14 | Wild type | None |
| APP 15 | Codon 12 GGT→GTT | GLY→VAL |
| APP 16 | Wild type | None |
Table 3.
K-ras Mutational Analysis of Ovarian Mucinous Low Malignant Potential Tumors
| Case | K-ras mutation | Amino acid change |
|---|---|---|
| OV 1 | Codon 12 GGT→GTT | GLY→VAL |
| OV 2 | Wild type | None |
| OV 3 | Codon 12 GGT→TGT | GLY→CYS |
| OV 4 | Codon 12 GGT→TGT | GLY→CYS |
| OV 5 | Codon 12 GGT→TGT | GLY→CYS |
| OV 6 | Wild type | None |
| OV 7 | Codon 12 GGT→GTT | GLY→VAL |
| OV 8 | Wild type | None |
| OV 9 | Wild type | None |
| OV 10 | Codon 12 GGT→GAT | GLY→ASP |
| OV 11 | Codon 12 GGT→GAT | GLY→ASP |
| OV 12 | Codon 12 GGT→TGT | GLY→CYS |
| OV 13 | Codon 12 GGT→GAT | GLY→ASP |
| OV 14 | Codon 12 GGT→GTT | GLY→VAL |
| OV 15 | Codon 12 GGT→CGT | GLY→ARG |
| OV 16 | Codon 12 GGT→GAT | GLY→ASP |
Figure 2.
K-ras mutations in representative ovarian (PMP 6O) and appendiceal (PMP 6A) tumors associated with pseudomyxoma peritonei. Arrows indicate point mutation (GGT→GAT; antisense direction) in both tumors not present in matched normal DNA (PMP 6N).
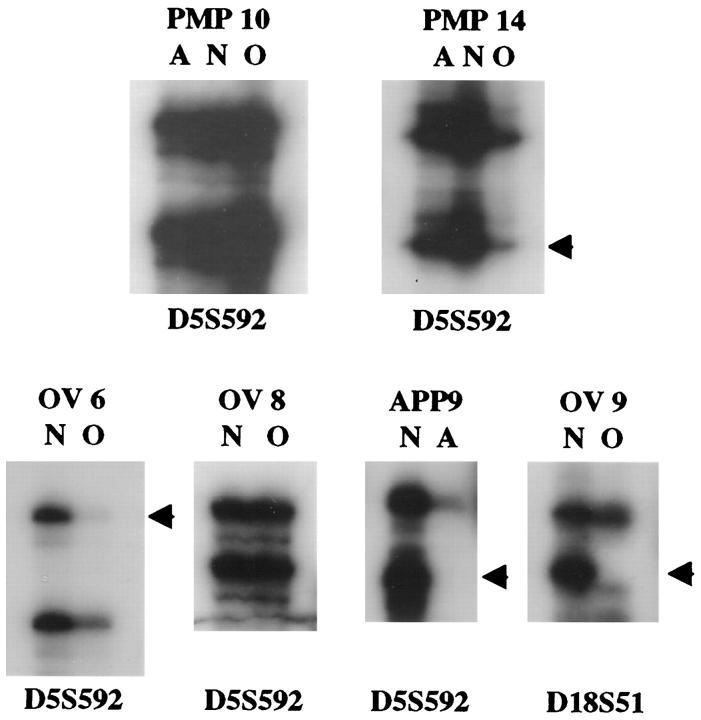
LOH analysis of polymorphic microsatellite loci on chromosomes 18q, 5q, 17p, and 6q demonstrated relatively few allelic losses in any of the tumors analyzed (Tables 4 to 6 ▶ ▶ ▶ and Figure 3 ▶ ). These chromosomal regions were chosen for analysis because frequent allelic losses of chromosomes 18q, 5q, and 17p have been identified in primary colorectal carcinomas 13 and frequent losses of 6q have been identified in primary ovarian epithelial tumors. 15,16 Of the 16 PMP cases evaluated, 8 lacked LOH at any of the informative loci tested. In two cases (PMP 5 and PMP 6), loss of the same allele at the 5q and/or 6q locus was observed in both the ovarian and appendiceal tumors. The remaining six cases showed discordant results between the ovarian and appendiceal tumors at one or two of the loci tested. In all but one instance, LOH was observed in the ovarian tumor although both alleles were retained in the matched appendiceal lesion, supporting the notion that the appendiceal tumor precedes spread to the ovary in PMP. LOH at the 5q marker was most frequently detected in the PMP cases (27% of informative cases). Allelic imbalance at a minimum of one tested locus was identified in 8 of 16 appendiceal adenomas unassociated with PMP. As in the PMP cases, LOH at the 5q marker was most frequently detected (36% of informative cases). Only 4 of the 16 ovarian MLMP tumors unassociated with PMP demonstrated LOH at any of the loci tested, and only 1 of 12 (8%) informative MLMP tumors showed LOH at the 5q marker.
Table 4.
Loss of Heterozygosity Analysis of PMP Cases
| Case | Loss of heterozygosity | |||||
|---|---|---|---|---|---|---|
| D5S592 | D6S474 | D6S1027 | D17S1303 | D18S51 | D18S499 | |
| PMP 1APP | * | * | * | * | * | * |
| PMP 1OV | * | * | * | * | * | * |
| PMP 2APP | − | − | − | Uninformative | * | − |
| PMP 2OV | − | − | − | Uninformative | * | − |
| PMP 3APP | − | − | − | − | * | − |
| PMP 3OV | LOH | − | − | − | * | − |
| PMP 4APP | − | − | Uninformative | − | * | − |
| PMP 4OV | − | LOH | Uninformative | * | * | LOH |
| PMP 5APP | LOH | Uninformative | LOH† | * | * | − |
| PMP 50V | LOH | Uninformative | LOH† | * | * | − |
| PMP 6APP | − | LOH | Uninformative | − | * | − |
| PMP 6OV | − | LOH | Uninformative | − | * | − |
| PMP 7APP | − | Uninformative | Uninformative | − | Uninformative | Uninformative |
| PMP 7OV | − | Uninformative | Uninformative | * | Uninformative | Uninformative |
| PMP 8APP | Uninformative | − | − | − | * | − |
| PMP 8OV | Uninformative | − | LOH | * | * | − |
| PMP 9APP | − | − | Uninformative | Uninformative | − | Uninformative |
| PMP 9OV | − | − | Uninformative | Uninformative | − | Uninformative |
| PMP 10APP | − | Uninformative | Uninformative | Uninformative | * | − |
| PMP 10OV | − | Uninformative | Uninformative | Uninformative | * | − |
| PMP 11APP | − | Uninformative | Uninformative | Uninformative | * | − |
| PMP 11OV | − | Uninformative | Uninformative | Uninformative | * | LOH |
| PMP 12APP | − | − | Uninformative | Uninformative | * | − |
| PMP 12OV | − | * | Uninformative | Uninformative | * | − |
| PMP 13APP | − | − | Uninformative | Uninformative | − | Uninformative |
| PMP 13OV | − | − | Uninformative | Uninformative | − | Uninformative |
| PMP 14APP | − | − | Uninformative | − | * | − |
| PMP 14OV | LOH | − | Uninformative | − | * | − |
| PMP 15APP | LOH | − | Uninformative | Uninformative | * | − |
| PMP 15OV | − | − | Uninformative | Uninformative | * | − |
| PMP 16APP | − | − | − | − | * | − |
| PMP 16OV | − | − | − | − | * | − |
| PMP 17APP | − | Uninformative | Uninformative | − | − | Uninformative |
| PMP 17OV | − | Uninformative | Uninformative | − | − | Uninformative |
*Not evaluated; −, no LOH.
†Retained allele exhibits microsatellite alteration (shift).
Table 5.
Loss of Heterozygosity Analysis of Appendiceal Mucinous Adenomas
| Case | Loss of heterozygosity | |||||
|---|---|---|---|---|---|---|
| D5S592 | D6S474 | D6S1027 | D17S1303 | D18S51 | D18S499 | |
| APP 1 | − | − | * | − | * | − |
| APP 2 | Uninformative | Uninformative | Uninformative | LOH | * | LOH |
| APP 3 | LOH | * | Uninformative | − | * | − |
| APP 4 | LOH | LOH | − | LOH | * | − |
| APP 5 | LOH | * | − | Uninformative | * | LOH |
| APP 6 | − | − | − | − | LOH | Uninformative |
| APP 7 | − | Uninformative | − | − | * | − |
| APP 8 | Uninformative | * | Uninformative | Uninformative | * | LOH |
| APP 9 | LOH | − | LOH | − | * | − |
| APP 10 | Uninformative | − | − | Uninformative | − | Uninformative |
| APP 11 | − | − | LOH | * | * | − |
| APP 12 | * | * | − | * | * | Uninformative |
| APP 13 | Uninformative | − | − | − | * | − |
| APP 14 | − | − | − | − | * | − |
| APP 15 | − | Uninformative | − | LOH | − | Uninformative |
| APP 16 | − | − | − | − | * | − |
*Not evaluated; −, no LOH.
Table 6.
Loss of Heterozygosity Analysis of Ovarian Mucinous Low Malignant Potential Tumors
| Case | Loss of heterozygosity | |||||
|---|---|---|---|---|---|---|
| D5S592 | D6S474 | D6S1027 | D17S1303 | D18S51 | D18S499 | |
| OV 1 | − | * | * | − | * | − |
| OV 2 | − | − | − | Uninformative | * | − |
| OV 3 | − | − | − | − | * | Uninformative |
| OV 4 | − | * | − | * | * | − |
| OV 5 | − | − | − | * | * | − |
| OV 6 | LOH | * | − | Uninformative | * | − |
| OV 7 | Uninformative | * | − | * | * | − |
| OV 8 | − | * | − | LOH | * | − |
| OV 9 | − | * | Uninformative | Uninformative | LOH | Uninformative |
| OV 10 | − | − | − | − | − | Uninformative |
| OV 11 | Uninformative | − | − | Uninformative | − | Uninformative |
| OV 12 | − | − | − | − | * | * |
| OV 13 | Uninformative | Uninformative | − | LOH | * | − |
| OV 14 | Uninformative | − | − | − | * | − |
| OV 15 | − | − | − | − | * | − |
| OV 16 | − | Uninformative | Uninformative | Uninformative | − | Uninformative |
*Not evaluated; −, no LOH.
Figure 3.
Loss of heterozygosity (LOH) analysis in representative tumors. LOH (arrows) is observed at D5S592 in the ovarian tumor (O) of a pseudomyxoma case (PMP 14) but not in the matched appendiceal tumor (A), in an ovarian MLMP unassociated with PMP (OV 6), and in an appendiceal adenoma unassociated with PMP (APP 9). LOH at D18S51 is shown in another ovarian MLMP (OV 9). Other PMP (PMP 10) and ovarian MLMP (OV 8) tumors are informative for the D5S592 marker but show retention of both alleles. DNA from matched normal tissue (N) was compared with that from tumor tissue in each case.
Discussion
The results of the K-ras mutational analysis presented in this study strongly support the conclusion that the mucinous tumors involving the appendix and ovaries in women with PMP are clonal and derived from one site. Given the limited spectrum of K-ras mutations that are encountered in human cancers (mutations of codons 12, 13, and 61), it is possible that two independent mucinous tumors could acquire the same mutation by chance. However, the likelihood of this occurring in all 16 PMP cases analyzed is virtually nil. It is notable that all appendiceal adenomas associated with PMP demonstrated K-ras mutations, whereas not all appendiceal adenomas unassociated with PMP had mutations. These findings suggest the possibility that K-ras mutation is necessary, but probably not sufficient, for an appendiceal adenoma to acquire the potential to spread as PMP.
Although less than definitive, the results of the LOH analysis support the hypothesis that PMP originates in an appendiceal mucinous adenoma and then spreads to other sites, including the ovary. The finding of LOH in the ovarian tumor, but not in the appendiceal tumor, in most PMP cases showing allelic losses, can be interpreted as evidence of tumor progression in a secondary (metastatic) site.
The two previous molecular genetic studies of synchronous appendiceal and ovarian mucinous tumors reported in the literature 8,9 were quite small and did not include comparison groups of mucinous appendiceal and ovarian tumors unassociated with PMP. Cuatrecasas and colleagues found identical K-ras mutations in the synchronous ovarian and appendiceal tumors of all five cases with detectable mutations, consistent with a common origin of the tumors. 9 The sixth case evaluated lacked K-ras mutation in either tumor. Chuaqui et al analyzed selected LOH in 12 cases of synchronous ovarian and appendiceal tumors. 8 K-ras mutations were not studied. Six cases lacked LOH at any of the tested loci, three cases showed identical patterns of LOH in tumors from both sites, and three cases showed discordant patterns of LOH between the ovarian and appendiceal lesions. These investigators concluded that the latter cases likely reflect origins from different sites. However, interpretation of their data is complicated by heterogeneity of the lesions studied, as both benign and malignant mucinous lesions were included.
We analyzed a morphologically homogeneous group of synchronous mucinous ovarian and appendiceal tumors associated with PMP. Our K-ras mutation data, along with that of Cuatrecasas et al, argue quite convincingly for a common origin of tumors at the different sites. Similar to the study of Chuaqui and colleagues, we also found a discordant pattern of LOH in some of our PMP cases. We believe that this discordance likely reflects tumor heterogeneity (ie, emergence of tumor subclones at different sites characterized by different genetic alterations) rather than multifocal origin.
Very little is known about the genetic alterations contributing to the development and/or progression of mucinous adenomas of the appendix. We have shown that mutations of the K-ras oncogene and allelic losses affecting chromosome 5q are common in these tumors. In this respect, adenomas of the appendix are similar to those affecting the colorectum.
In summary, PMP is a clinical syndrome of mucinous ascites associated with multiple peritoneal mucinous tumors that appear histologically benign (disseminated peritoneal adenomucinosis). When carefully searched for, a ruptured appendiceal mucinous adenoma (or a mass of mucinous tumor with fibrosis that has obliterated the appendix) is often found. Based on clinicopathological, immunohistochemical, and now molecular data, we conclude that PMP originates in the appendix and then spreads to secondarily involve other sites, including the ovary.
Footnotes
Address reprint requests to Dr. Kathleen R. Cho, University of Michigan, Department of Pathology, 4301 MSRBIII, Box 0638, 1150 West Medical Center Drive, Ann Arbor, MI 48109. E-mail: kathcho@umich.edu.
References
- 1.Ronnett BM, Kurman RJ, Zahn CM, Shmookler BM, Jablonski KA, Kass ME, Sugarbaker PH: Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol 1995, 26:509-524 [DOI] [PubMed] [Google Scholar]
- 2.Young R, Gilks C, Scully R: Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei: a clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol 1991, 15:415-429 [DOI] [PubMed] [Google Scholar]
- 3.Seidman J, Elsayed A, Sobin L, Tavassoli F: Association of mucinous tumors of the ovary and appendix: a clinicopathologic study of 25 cases. Am J Surg Pathol 1993, 17:22-34 [DOI] [PubMed] [Google Scholar]
- 4.Prayson R, Hart W, Petras R: Pseudomyxoma peritonei: a clinicopathologic study of 19 cases with emphasis on site of origin and nature of associated ovarian tumors. Am J Surg Pathol 1994, 18:591-603 [PubMed] [Google Scholar]
- 5.Sumithran E, Susil B: Concomitant mucinous tumors of appendix and ovary: result of a neoplastic field change? Cancer 1992, 70:2980-2983 [DOI] [PubMed] [Google Scholar]
- 6.Ronnett B, Shmookler B, Diener-West M, Sugarbaker P, Kurman R: Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol 1997, 16:1-9 [DOI] [PubMed] [Google Scholar]
- 7.Guerrieri C, Franlund B, Fristedt S, Gillooley JF, Boeryd B: Mucinous tumors of the vermiform appendix and ovary, and pseudomyxoma peritonei: histogenetic implications of cytokeratin 7 expression. Hum Pathol 1997, 28:1039-1045 [DOI] [PubMed] [Google Scholar]
- 8.Chuaqui R, Zhuang Z, Emmert-Buck M, Bryant B, Nogales F, Tavassoli F, Merino M: Genetic analysis of synchronous mucinous tumors of the ovary and appendix. Hum Pathol 1996, 27:165-171 [DOI] [PubMed] [Google Scholar]
- 9.Cuatrecasas M, Matias-Guiu X, Prat J: Synchronous mucinous tumors of the appendix and the ovary associated with pseudomyxoma peritonei. Am J Surg Pathol 1996, 20:739-746 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira M, Qvist H, Giercksky K, Bohler P, Heim S: Cytogenetic analysis of several pseudomyxoma peritonei lesions originating from a mucinous cystadenoma of the appendix. Cancer Genet Cytogenet 1997, 93:157-159 [DOI] [PubMed] [Google Scholar]
- 11.Noumoff J, LiVolsi V, Faruqi S: An insight into etiology of pseudomyxoma peritonei by chromosomal and immunohistochemical analysis. Gynecol Oncol 1993, 49:136 [Google Scholar]
- 12.Ronnett B, Zahn C, Kurman R, Kass M, Sugarbaker P, Shmookler B: Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: a clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei.” Am J Surg Pathol 1995, 19:1390-1408 [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Fearon E, Hamilton S, Kern S, Preisinger A, Leppert M, Nakamura Y, White R, Smits A, Bos J: Genetic alterations during colorectal-tumor development. N Engl J Med 1988, 319:525-532 [DOI] [PubMed] [Google Scholar]
- 14.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J: K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer 1997, 79:1581-1586 [DOI] [PubMed] [Google Scholar]
- 15.Cooke I, Shelling A, Le Meuth V, Charnock M, Ganesan T: Allele loss on chromosome arm 6q and fine mapping of the region at 6q27 in epithelial ovarian cancer. Genes Chromosomes & Cancer 1996, 15:223-233 [DOI] [PubMed] [Google Scholar]
- 16.Tibiletti M, Bernasconi B, Furlan D, Riva C, Trubia M, Buraggi G, Franchi M, Bolis P, Mariani A, Frigerio L, Capella C, Taramelli R: Early involvement of 6q in surface epithelial ovarian tumors. Cancer Res 1996, 56:4493-4498 [PubMed] [Google Scholar]
- 17.Kessis T, Silberman M, Sherman M, Hedrick L, Cho K: Rapid identification of patient specimens with microsatellite DNA markers. Mod Pathol 1996, 9:183-188 [PubMed] [Google Scholar]