Abstract
Fas (Apo-1/CD95) is a cell-surface receptor involved in cell death signaling. The key role of the Fas system in negative growth regulation has been studied mostly within the immune system, and somatic mutations of Fas gene in cancer patients have been described solely in lymphoid-lineage malignancies. However, many nonlymphoid tumor cells have been found to be resistant to Fas-mediated apoptosis, which suggests that Fas mutations, one of the possible mechanisms for Fas resistance, may be involved in the pathogenesis of nonlymphoid malignancies as well. In this study, we have analyzed the entire coding region and all splice sites of the Fas gene for the detection of the gene mutations in 44 human malignant melanomas in skin by polymerase chain reaction, single-strand conformation polymorphism, and DNA sequencing. Overall, 3 tumors (6.8%) were found to have the Fas mutations, which were all missense variants and identified in the cytoplasmic region (death domain) known to be involved in the transduction of an apoptotic signal. The data presented here suggest that somatic alterations of the Fas gene might lead to the loss of its apoptotic function and contribute to the pathogenesis of some human malignant melanomas.
The Fas-Fas ligand (FasL) system has been recognized as a major pathway for the induction of apoptosis in cells and tissues. 1 Fas is a member of the death receptor subfamily of the tumor necrosis factor receptor superfamily. Fas has three cysteine-rich extracellular domains and an intracellular death domain essential for signaling. 2,3 Ligation of Fas by either agonistic antibody or its natural ligand transmits a death signal to the target cells, potentially triggering apoptosis. 3-8
Fas is widely expressed in normal and neoplastic cells 4 but the expression of this protein does not necessarily predict susceptibility to killing. 9 This can reflect the presence of inhibiting mechanisms of Fas-mediated apoptosis. Fas-mediated apoptosis can be blocked by several mechanisms, including the production of soluble Fas, 10 the lack of cell-surface Fas expression, 11-13 the overexpression of inhibitory proteins in signal transduction pathways such as Fas associated phosphatase-1 14 and FLICE-inhibitory protein (FLIP), 15 and the mutation of the primary structure of Fas. 16-25
The consequences of the Fas gene mutations have been well demonstrated in germline mutation models of this gene. 16-21 Mice bearing the Fas gene mutation (lpr) have an abnormality of mature T-cell deletion in the peripheral tissues, resulting in lymphadenopathy, splenomegaly, and systemic autoimmune disease. 16 Germline mutations of the Fas gene in human also results in autoimmune lymphoproliferative syndrome (ALPS), which is characterized by an increase in double-negative T cells and profound lymphadenopathy, 17-21 as observed in lpr mice. Most of the Fas mutations in ALPS were point mutations in the death domain, were heterozygous, and showed a dominant negative phenotype. 17-19 Interestingly, the lpr mice have been reported to have spontaneous development of plasmacytoid tumors 26 and some ALPS patients have been reported to have malignancies, 19-21 including multiple tumor development in one patient. 19 Although it is not clear if the tumors that occurred in ALPS patients arose as a result of Fas mutations, it is conceivable that Fas mutation might influence tumor development in these patients.
The key role of the Fas system in negative growth regulation has been studied mostly within the immune system 1 and somatic mutations of Fas gene in cancer patients have been described solely in lymphoid-lineage malignancies, including multiple myelomas, 22 childhood T-cell lymphoblastic leukemias, 23 adult T-cell leukemias, 24 and non-Hodgkin’s lymphomas. 25 Therefore, resistance against Fas-mediated apoptosis may lead to a longer survival of affected tumor cells and might contribute to tumorigenesis of these lymphoid-lineage malignancies.
There is mounting evidence that disruption of the Fas system occurs frequently in nonlymphoid malignancies as well. 9-12,27,28 To date, however, somatic mutations of Fas gene, one of the possible mechanisms that mediate the disruption of the Fas system, have not yet been reported in nonlymphoid malignancies, including cutaneous malignant melanoma (MM). In addition, previous loss of heterozygosity (LOH) studies have suggested that loss of one or more putative tumor suppressor genes at chromosome 10q may be involved in the development of cutaneous MM. 29-32 One of the candidate genes in this region is Fas located at chromosome 10q24.1. 3 In the present study, to characterize the potential apoptosis-resistant pathway of the Fas system in human cutaneous MM, we analyzed a series of 44 cutaneous MMs for somatic mutations of the Fas gene.
Materials and Methods
Tissue Samples and Microdissection
Paraffin-embedded tissues of human cutaneous MM were obtained from 44 surgically treated patients. All MMs analyzed showed vertical growth pattern. Diagnosis of each case had been confirmed by dermatopathologists morphologically and, if necessary, by immunohistochemistry and electron microscopy.
Malignant cells were selectively procured from hematoxylin and eosin-stained sections using a 30G1/2 hypodermic needle (Becton Dickinson, Franklin Lakes, NJ) affixed to a micromanipulator, as described previously. 33 We also microdissected infiltrating lymphocytes from the slides and used them for corresponding normal DNA. This microdissection technique used in this study has been proven to be precise and effective for procurement of tumor cells without normal cell contamination. 33
Single-Strand Conformation Polymorphism (SSCP) Analysis for Mutation and LOH
Genomic DNA each from normal lymphocytes or tumor cells was amplified with primer pairs covering the entire coding region and parts of the promoter region of Fas gene (Table 1) ▶ . Oligonucleotide primers were designed with the program Oligo (National Biosciences, Plymouth, MN) using sequences obtained from GenBank (accession No. M67454). Each polymerase chain reaction (PCR) was performed under standard conditions in a 10-μl reaction mixture containing 1 μl of template DNA, 0.5 μmol/L of each primer, 0.2 μmol/L of each deoxynucleotide triphosphate, 1.5 mmol/L MgCl2, 0.4 units of Taq polymerase, 0.5 μCi of [32P]dCTP (Amersham, Buckinghamshire, UK), and 1 μl of 10× buffer. The reaction mixture was denatured for 1 minute at 94°C and incubated for 40 cycles (denaturing for 40 seconds at 94°C, annealing for 40 seconds at variable temperatures as described in Table 1 ▶ , and extending for 40 seconds at 72°C). Final extension was continued for 5 minutes at 72°C. After amplification, PCR products were denatured 5 minutes at 95°C at a 1:1 dilution of sample buffer containing 98% formamide/5 mmol/L NaOH and were loaded onto a SSCP gel (FMC Mutation Detection Enhancement system, Intermountain Scientific, Kaysville, UT) with 10% glycerol. After electrophoresis, the gels were transferred to 3-mm Whatman paper and dried and autoradiography was performed with X-OMAT film (Eastman Kodak, Rochester, NY). For the detection of mutations, DNAs showing mobility shifts were cut out from the dried gel and reamplified for 35 cycles using the same primer set. Sequencing of the PCR products was carried out using the cyclic sequencing kit (Perkin-Elmer, Foster City, CA) according to the manufacturer’s recommendation.
Table 1.
Primers Used in PCR-SSCP Assay of Fas Gene
| Primer (site of PCR) | Sequence | Size of PCR product (bp) | Annealing temperature (°C) |
|---|---|---|---|
| PA-F (promoter) | 5′-ccatcctccttatcccacttcttt-3′ | ||
| 124 | 60 | ||
| PA-R | 5′-gcttgtctctgttccacctttca-3′ | ||
| PB-F (promoter) | 5′-ggcgcaacatctgtactttttcat-3′ | ||
| 145 | 58 | ||
| PB-R | 5′-agccttggctaattgctggagt-3′ | ||
| 1-F (exon 1) | 5′-ctcttctcccgcgggttggt-3′ | ||
| 171 | 61 | ||
| 1-R | 5′-cactttgcctatccccgggactaa-3′ | ||
| 2-F (exon 2) | 5′-gttgcttacttcagaaatcaataa-3′ | ||
| 249 | 53 | ||
| 2-R | 5′-actgtaatctctggatgttttgt-3′ | ||
| 3A-F (exon 3) | 5′-acttcccaccctgttacctg-3′ | ||
| 192 | 60 | ||
| 3A-R | 5′-catcacacaatctacatcttctgc-3′ | ||
| 3B-F (exon 3) | 5′-gtacacagacaaagcccatttttc-3′ | ||
| 171 | 57 | ||
| 3B-R | 5′-gtgtcaacatagcaccacagtagg-3′ | ||
| 4-F (exon 4) | 5′-cgcgataactaatagtttccaa-3′ | ||
| 228 | 55 | ||
| 4-R | 5′-ctctcagtcagtgttacttcccta-5′ | ||
| 5-F (exon 5) | 5′-aattattctgccaggcttttg-3′ | ||
| 180 | 53 | ||
| 5-R | 5′-gattggtttttcttcacatctttc-3′ | ||
| 6-F (exon 6) | 5′-tttcatataatatgccaatgttcc-3′ | ||
| 145 | 60 | ||
| 6-R | 5′-cttcccccaagttatttcaat-3′ | ||
| 7-F (exon 7) | 5′-ctacaaggctgagacctgagtt-3′ | ||
| 164 | 55 | ||
| 7-R | 5′-tttcaaggaaagctgatacctatt-3′ | ||
| 8-F (exon 8) | 5′-ttgtctttctctgcttccatt-3′ | ||
| 117 | 53 | ||
| 8-R | 5′-attggcctattactctaaaggatg-3′ | ||
| 9A-F (exon 9) | 5′-tgctggagtcatgacactaagt-3′ | ||
| 163 | 50 | ||
| 9A-R | 5′-caatgtgtcatacgcttctttc-3′ | ||
| 9B-F (exon 9) | 5′-taattggcatcaacttcat-3′ | ||
| 175 | 49 | ||
| 9B-R | 5′-gaatttgttgtttttcactcta-3′ | ||
| 9C-F (exon 9) | 5′-ggttttcactaatgggaatttcat-3′ | ||
| 191 | 50 | ||
| 9C-R | 5′-cttcattgacaccattctttcg-3′ |
F, forward primer; R, reverse primer.
Because it has been known that four biallelic polymorphisms at positions −1377 (promoter region), −670 (promoter region), 416 (exon 3), and 836 (exon 7) are located in Fas gene, 34,35 SSCP analysis at these polymorphic sites was used for the detection of both LOH and mutations. The PCR and SSCP conditions of LOH study were the same with the condition described above. Complete or nearly complete absence of one allele in tumor DNA of informative cases, as defined by direct visualization, was considered as LOH.
Immunohistochemistry
Rabbit antibody for human Fas (C-20, Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect Fas on paraffin-embedded tissue sections. Immunohistochemical procedures were performed as described previously. 36 Tumors were interpreted as positive for Fas by immunohistochemistry when at least weak to moderate cytoplasmic staining was seen in greater than 30% of the neoplastic cells. The Fas immunostaining was judged to be antibody-specific by several criteria, including use of normal rabbit sera at the same dilution produced no consistent immunostaining of any cells; intensity of the signal diminished as the dilution of antibody was increased; and preincubating antibody with blocking peptide of Fas (Santa Cruz Biotechnology) abrogated the positive immunostaining. The results were reviewed independently by three pathologists.
Results
Fas Gene Mutations
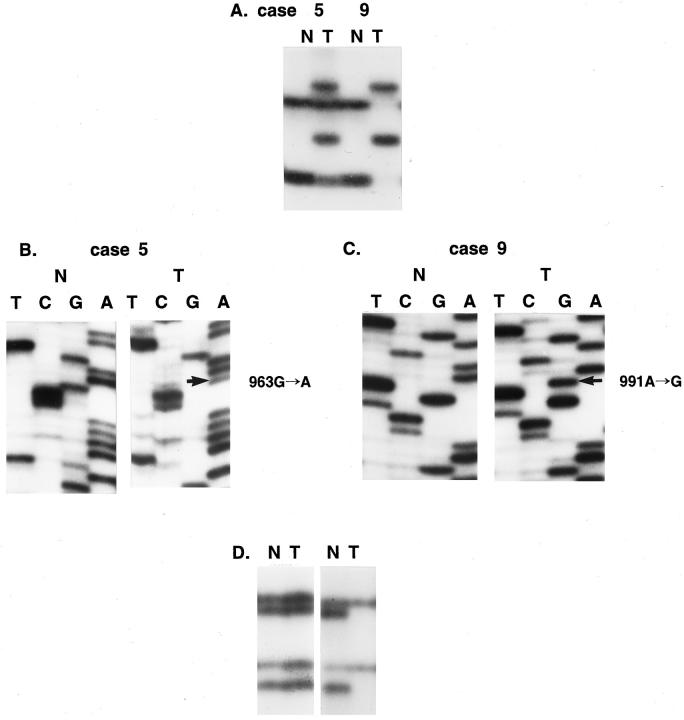
Using the microdissection technique we successfully procured tumor cells from histological sections of 44 MMs, as shown in Figure 1, A and B ▶ . Genomic DNA was isolated and analyzed for potential mutations in all nine exons of the Fas gene by PCR-SSCP analysis. Enrichment and direct sequence analysis of aberrantly migrating bands led to the identification of mutations in 3 of 44 samples (6.8%) (Table 2 ▶ and Figure 2A ▶ ). None of the normal samples showed evidence of mutations by SSCP (Figure 2A) ▶ , indicating the mutations detected in the MM specimens had risen somatically.
Figure 1.
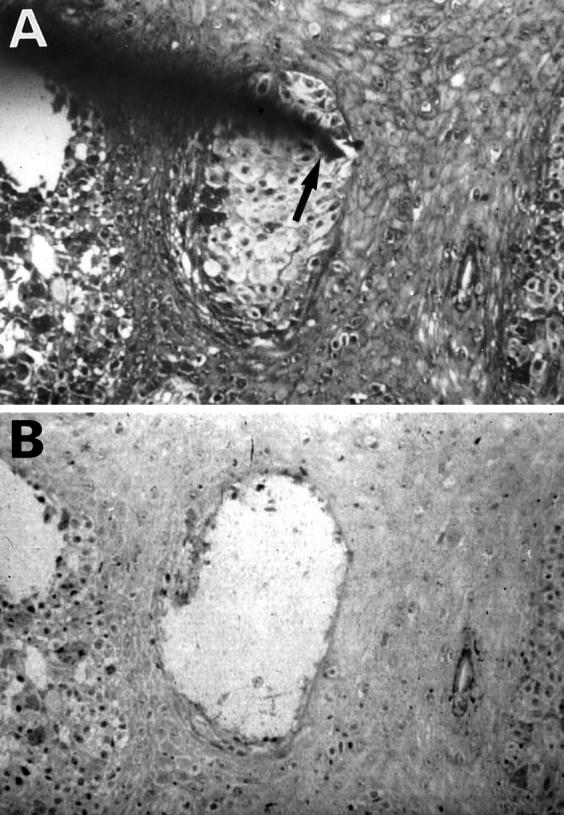
Microdissection of cutaneous MM. A: MM cells are arranged in irregularly shaped nests in the epidermis. The needle tip (arrow) is attached to a tumor cell nest. B: Tumor cell nest was dissected, leaving large holes behind. Original magnification, ×150
Table 2.
Mutations and LOH of Fas Gene in Malignant Melanomas
| Case No. | LOH analysis | Codon | Mutation site | Nucleotide change | Predictive effect | |||
|---|---|---|---|---|---|---|---|---|
| PA | PB | 3 | 7 | |||||
| 5 | NI | LOH | NI | NI | 241 | Exon 9 | 963 G to A | Ala → Thr |
| 9 | – | NI | NI | NI | 250 | Exon 9 | 991 A to G | Asn → Ser |
| 20 | NI | NI | NI | NI | 251 | Exon 9 | 993 G to A | Val → Ile |
| 11* | LOH | – | NI | NI | ||||
| 12* | LOH | NI | NI | NI | ||||
| 16* | LOH | NI | NI | NI | ||||
| 24* | LOH | NI | NI | NI | ||||
| 25* | LOH | NI | NI | NI | ||||
| 28* | LOH | LOH | NI | NI | ||||
| 29* | – | LOH | NI | NI | ||||
| 36* | LOH | – | NI | NI | ||||
| 37* | NI | NI | LOH | – | ||||
| 39* | LOH | – | NI | NI |
LOH, loss of heterozygosity; NI, not informative.
*Cases with LOH and without somatic mutation of Fas.
Figure 2.
Mutations and deletions of Fas gene in cutaneous MM. SSCP (A and D) and sequencing analysis (B and C) of DNA from tumors (Lane T) and normal tissues (Lane N). A: Part of exon 9 was amplified using primer set 9A. Left, SSCP of DNA from tumor (T) of case 5 shows wild-type bands and additional aberrant bands as compared to SSCP from normal lymphocytes (N). Right, SSCP of DNA from tumor (T) of case 9 shows only 2 aberrant bands without any wild-type bands as compared to SSCP from normal lymphocytes (N). B: Sequencing analysis from aberrant band of case 5. There is a G-to-A transition at nucleotide 963 (arrow) in tumor tissue as compared to normal tissue. C: Sequencing analysis from aberrant band of case 9. There is an A-to-G transition at nucleotide 991 (arrow) in tumor tissue as compared to normal tissue. D: Detection of allelic loss by amplifying a region encompassing the biallelic polymorphism, −670, in the Fas promoter. Left, representative SSCP showing retention of heterozygosity. Right, a representative pattern of LOH. Loss of two bands was observed in DNA from tumor cells (T) compared to the SSCP from normal cells (N).
All 3 mutations identified were missense variants (Figure 2, B and C ▶ , and Table 2 ▶ ) and were detected in exon 9, which encodes the death domain region of the Fas. 2,3 One of the mutations (case 5) showed a G-to-A transition at bp 963 (Figure 2B) ▶ . This mutation would result in the substitution of Ala to Thr at codon 241. An A-to-G transition in case 9 was identified at bp 991 (Figure 2C) ▶ , causing the substitution of Asn to Ser at codon 250. Case 20 showed a G-to-A transition at bp 993 (Table 2) ▶ , resulting in the substitution of Val to Ile at codon 251. We repeated the experiments three times, including tissue microdissection, PCR, SSCP, and sequencing analysis to ensure the specificity of the results, and found the data were consistent (data not shown).
Allelic Status
Because missense mutations in the death domain of Fas in patients with ALPS have been suggested to affect receptor function in a dominant-negative fashion, 17-19 we examined the allelic status of Fas in tumors carrying missense mutations. Overall, 31 of 44 cases (70%) were informative for at least one of the four polymorphic markers, and 11 of 31 (35%) informative cases showed LOH with one or more markers. The heterozygosity rates of the two polymorphic markers in exon 3 and 7 (primers 3A and 7) were too low for LOH study, whereas 30 of 44 (68%) cases showed heterozygosity with one or both of the two polymorphic markers in the promoter region (primers PA and PB) of the Fas gene.
In the three cases with the Fas gene mutations, one (case 5) showed LOH with marker PB (Figure 2D ▶ and Table 2 ▶ ). Another mutation case (case 20) was not informative for the polymorphic markers (Table 2) ▶ . The remaining mutation case (case 9) was heterozygous for marker PA, but did not show LOH (Table 2) ▶ . Interestingly, however, SSCP pattern of case 9 at the mutation sites (exon 9) showed only aberrant bands of mutant allele without those of the wild-type allele (Figure 2A) ▶ , and sequencing analysis also revealed only mutation sequence without wild-type one (Figure 2C) ▶ , indicating either homozygous mutation or hemizygous mutation with allelic loss. Therefore, although SSCP analysis at the polymorphic sites did not provide direct evidence of the second allele status in this mutation cases, the SSCP patterns at mutation sites and sequencing analysis suggested that the second allele of Fas in case 9 had been also altered. In cases without Fas mutation, 10 of 29 (34%) informative cases showed evidence of allelic loss (Table 2) ▶ .
Immunohistochemistry
We demonstrated Fas expressions in MMs by immunohistochemistry. The MMs analyzed showed immunoreactivity for Fas in 26 of 44 cases (59%). Fas immunostaining, when present, was cytoplasmic and along the cell membranes; nuclei were clearly negative (Figure 3) ▶ . All three MMs with Fas mutations showed positive immunostaining for Fas.
Figure 3.
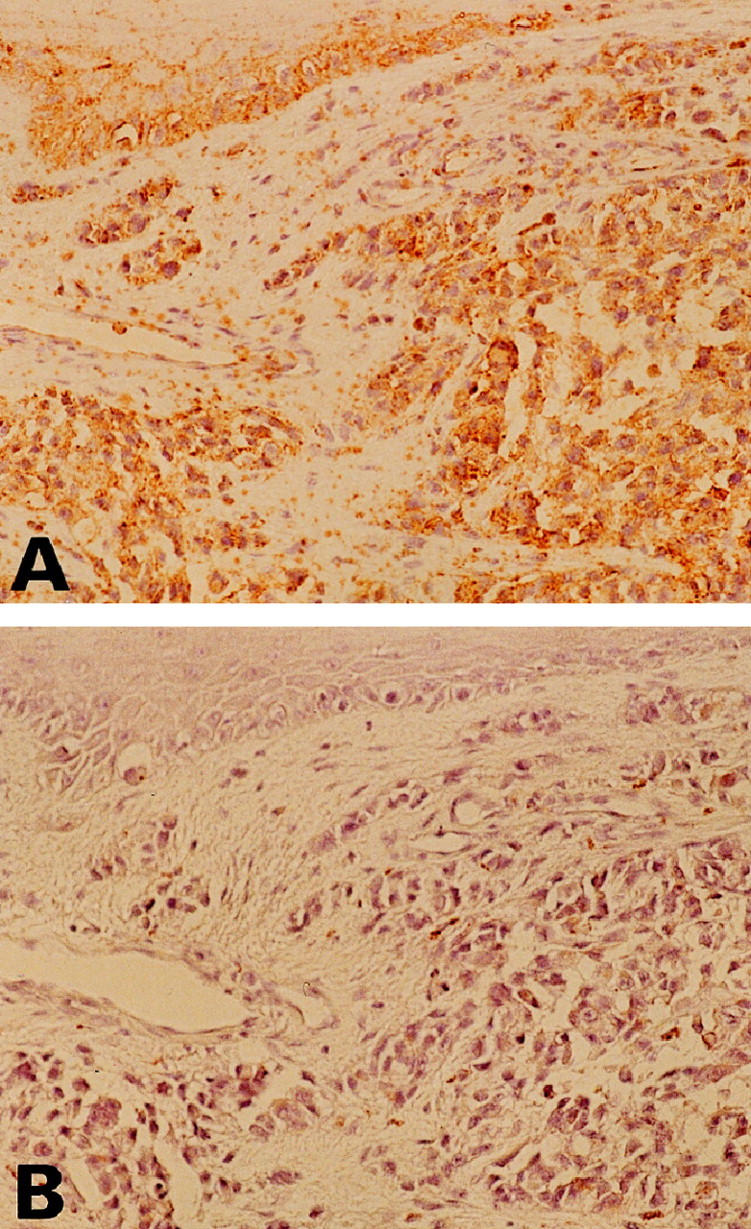
Visualization of Fas in cutaneous MM by immunohistochemistry. Antibodies were detected by a diaminobenzidine method that produces a brown color. Counterstaining of nuclei was with hematoxylin (blue). A: MM shows immunoreactivity for Fas. B: Negative control of Fas immunostaining. Fas antibody preincubation with the Fas peptide shows no detectable immunostaining in MM. (Original magnifications, ×100).
Discussion
The aim of this study was to detect Fas gene alterations, one of the possible mechanisms that may mediate Fas resistance in cutaneous MM in vivo. Although we do not actually know whether MM is resistant to Fas-mediated apoptosis in vivo, some data support the idea that MM may be resistant to Fas-mediated apoptosis. For example, some MM cell lines were reported to have resistance to Fas-mediated apoptosis, despite expressing Fas. 27,28 In the present study, we have systematically examined the Fas gene and documented somatic mutations in 3 of 44 MMs. These findings, together with the recent demonstration of a similar frequency of Fas mutations in lymphoid-lineage malignancies, suggest that Fas mutations may be involved in the development of different types of human malignancies, including MM.
Although functional studies have not yet been performed, some of the mutations identified in the present study are likely to disrupt or alter the normal function of Fas. To date, loss-of-function mutations of Fas in lpr mice, 16 ALPS patients, 17-21 and some lymphoid malignancies, 22-25 have been identified in the promoter and exons 2, 3, 4, 6, 7, 8, and 9. However, most of the mutations have been detected in exon 9, which encodes death domain. The death domain is evolutionarily highly conserved and has been shown to be necessary and sufficient for the transduction of an apoptotic signal. 1-3 In the current study, all three Fas mutations were identified in this conserved area, suggesting that the mutations might disrupt death signaling.
In multiple myeloma, Landowski et al 22 identified an identical point mutation in two different patients that generates an amino acid substitution at 253. Mutations of codon 248 and 256 of Fas were also identified in non-Hodgkin’s lymphomas. 25 Furthermore, two ALPS patients were reported to have a 2-bp deletion that generates an unrelated amino acid sequence beginning at residue 254. 18 Of the 3 Fas gene mutations in MMs, 2 mutations generated amino acid substitution at residues 250 and 251, which are close to the Fas mutations described above, indicating that this area may be a potential hot spot in the Fas coding sequence.
Most of the patients with ALPS carry a heterozygous mutation in the Fas gene. 17-19 In the ALPS, the affected Fas protein seemed to work in a dominant-negative fashion, and T lymphocytes from these patients did not die on activation. 17-19 Binding of FasL to Fas protein induces trimerization of Fas protein and FADD/MORT-1 and Daxx, the adapter proteins of Fas, bind to the trimerized Fas cytoplasmic region (death domain). Then, FADD/MORT-1 and Daxx transmit apoptotic signals. 37,38 In our study, one Fas mutation (case 20) seemed to have hemizygous mutations without allelic deletion (Table 2) ▶ . Therefore, in this case it is possible that the hemizygously mutated Fas protein(s) may bind with other normal Fas protein(s) to construct a structurally abnormal Fas trimer, which might have a defect in binding to the adapter proteins. In contrast, case 5 showed evidence of alterations of both alleles (Table 2) ▶ and case 9 showed only aberrant bands of mutant allele without those of the wild-type allele on SSCP of exon 9 (Figure 2A) ▶ , indicating potential biallelic inactivation of the Fas gene in these cases. These biallelic inactivations of the Fas gene may also lead to abnormal construction of Fas-trimer, but the functional difference between monoallelic and biallelic inactivations of Fas gene alterations in the tumorigenesis of MM remains unknown at this stage.
Previous reports have identified Fas protein expression in about half of primary MMs and cultured MM cell lines. 27,28,39 In agreement with these reports, we observed Fas protein expression in 26 of 44 MMs (59%). The Fas gene mutations in 3 MMs, all of which showed Fas expression by immunohistochemistry, might be involved in the mechanisms of Fas resistance of those tumors. Other MMs with positive Fas expression may have another strategies, including the expression of bcl-2 40 and FLIP, 15 to mediate the Fas resistance. In the MMs that were not shown to express Fas protein, loss or down-regulation of the protein may be another way to avoid Fas-mediated apoptosis.
Several lines of evidence suggest that the loss of Fas function can enhance lymphoid tumor development. For example, lymphomatogenesis driven by the Eμ-myc transgene was shown to be markedly accelerated in lpr mice compared to wild-type mice, confirming a causal, rather than correlative, role for Fas loss in tumor development. 41 Spontaneous development of B-cell lymphoid tumors in lpr mice also indicated that Fas gene mutation plays a key role in tumorigenesis. 26 Moreover, somatic Fas gene mutations in human cancers have been found exclusively in lymphoid malignancies. 22-25 These are well correlated with the facts that ALPS patients and lpr mice have shown phenotypical abnormalities only in the lymphoid system. 16-21 However, we were able to find somatic mutations of Fas gene in cutaneous MM, one of the nonlymphoid malignancies, potentially extending the concept of loss of Fas function to the pathogenesis of nonlymphoid malignancies as well and it is possible that Fas gene mutations may occur widely in nonlymphoid malignancies. Clearly, therefore, studies are now needed that attempt to find the potential Fas gene mutations in other nonlymphoid malignancies.
Footnotes
Address reprint requests to Sug Hyung Lee, Department of Pathology, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Socho-gu, Seoul 137–701, Korea. E-mail: suhulee@cmc.cuk.ac.kr.
Supported by a grant from the Basic Research Program of the Korea Science and Engineering Foundation (981-0709-073-2).
References
- 1.Nagata S: Apoptosis by death factor. Cell 1997, 88:355-365 [DOI] [PubMed] [Google Scholar]
- 2.Itoh N, Nagata S: A novel protein domain required for apoptosis: mutational analysis of human Fas antigen. J Biol Chem 1993, 268:10932-10937 [PubMed] [Google Scholar]
- 3.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S: The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66:233-243 [DOI] [PubMed] [Google Scholar]
- 4.Leithäser F, Dhein J, Mechtersheimer G, Koretz K, Brüderlein S, Henne C, Schmidt A, Debatin K-M, Krammer PH, Möller P: Constitutive and induced expression of APO-1, a new member of the new growth factor/tumor necrosis receptor superfamily, in normal and neoplastic cells. Lab Invest 1993, 69:415-429 [PubMed] [Google Scholar]
- 5.Suda T, Takahashi T, Golstein P, Nagata S: Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75:1169-1178 [DOI] [PubMed] [Google Scholar]
- 6.Suda T, Nagata S: Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med 1994, 179:873-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trauth BC, Klas C, Peters AMJ, Matzku S, Möller P, Falk W, Debatin KM, Krammer PH: Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989, 245:301-305 [DOI] [PubMed] [Google Scholar]
- 8.Yonehara S, Ishii A, Yonehara M: A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor J Exp Med 1989, 169:1747-1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen-Schaub LB, Radinsky R, Kruzel E, Berry K, Yonehara S: Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological responses. Cancer Res 1994, 54:1580-1586 [PubMed] [Google Scholar]
- 10.Natoli G, Ianni A, Costanzo A, Petrillo GD, Ilari I, Chirillo P, Balsano C, Levrero M: Resistance to Fas-mediated apoptosis in human hepatoma cells. Oncogene 1995, 11:1157-1164 [PubMed] [Google Scholar]
- 11.Hughes SJ, Nambu Y, Soldes OS, Hamstra D, Rehemtulla A, Iannettoni MD, Orringer MB, Beer DG: Fas/APO-1 (CD95) is not translocated to the cell membrane in esophageal adenocarcinomas. Cancer Res 1997, 57:5571-5578 [PubMed] [Google Scholar]
- 12.Nambu Y, Hughes SJ, Rehemtula A, Hamstra D, Orringer MB, Beer DG: Lack of cell surface Fas/APO-1 expression in pulmonary adenocarcinomas. J Clin Invest 1998, 101:1102-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett M, Macdonald K, Chan S-W, Luzio JP, Simari R, Weissberg P: Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 1998, 282:290-293 [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Irie S, Kituda S, Reed JC: FAP-1: a protein tyrosine phosphatase that associates with Fas. Science 1995, 268:411-415 [DOI] [PubMed] [Google Scholar]
- 15.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J: Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388:190-195 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S: Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992, 356:314-317 [DOI] [PubMed] [Google Scholar]
- 17.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM: Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 1995, 81:935-946 [DOI] [PubMed] [Google Scholar]
- 18.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IAG, Debatin KM, Fischer A, de Villarty JP: Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995, 268:1347-1349 [DOI] [PubMed] [Google Scholar]
- 19.Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB: Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med 1996, 335:1643-1649 [DOI] [PubMed] [Google Scholar]
- 20.Bettinardi A, Brugnoni B, Quiros-Roldan E, Malagoli A, Grutta SL, Correra A, Notarangelo LD: Missense mutations in the Fas gene resulting in autoimmune lymphoproliferative syndrome: a molecular and immunological analysis. Blood 1997, 89:902-909 [PubMed] [Google Scholar]
- 21.Infante AJ, Britton HA, DeNapoli T, Middelton LA, Lenardo MJ, Jackson CE, Wang J, Fleisher T, Straus SE, Puck JM: The clinical spectrum in a large kindred with autoimmune lymphoproliferative syndrome caused by a Fas mutation that impairs lymphocyte apoptosis. J Pediatr 1998, 133:629-633 [DOI] [PubMed] [Google Scholar]
- 22.Landowsky TH, Qu N, Buyuksal I, Painter JS, Dalton WS: Mutations in the Fas antigen in patients with multiple myeloma. Blood 1997, 90:4266-4270 [PubMed] [Google Scholar]
- 23.Beltinger C, Kurz E, Bohler T, Schrappe M, Ludwig WD, Debatin KM: CD 95 (APO-1/Fas) mutations in childhood T-lineage acute lymphoblastic leukemia. Blood 1998, 91:3943-3951 [PubMed] [Google Scholar]
- 24.Tamiya S, Etoh KI, Suzushima H, Takatsuki K, Matsuoka M: Mutation of CD 95 (Fas/APO-1) gene in adult T-cell leukemia cells. Blood 1998, 91:3935-3942 [PubMed] [Google Scholar]
- 25.Grønbæk K, Straten PT, Ralfkiaer E, Ahrenkiel V, Andersen MK, Hansen NE, Zeuthen J, Hou-Jensen K, Guldberg P: Somatic Fas mutations in non-Hodgkin’s lymphoma: association with extranodal disease and autoimmunity. Blood 1998, 92:3018-3024 [PubMed] [Google Scholar]
- 26.Davidson WF, Giese T, Fredrickson TN: Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. J Exp Med 1998, 187:1825-1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivoltini L, Radrizzani M, Accornero P, Squarcina P, Chiodoni C, Mazzocchi A, Castelli C, Tarsini P, Viggiano V, Belli F, Colombo MP, Parmiani G: Human melanoma-reactive CD4+ and CD8+ CTL clones resist Fas ligand-induced apoptosis and use Fas/Fas ligand- independent mechanisms for tumor killing. J Immunol 1998, 161:1220-1230 [PubMed] [Google Scholar]
- 28.Thomas WD, Hersey P: CD4 T cells kill melanoma cells by mechanisms that are independent of Fas (CD95). Int J Cancer 1998, 75:384-390 [DOI] [PubMed] [Google Scholar]
- 29.Healy E, Belgaid C, Takata M, Harrison D, Zhu NW, Burd DA, Rigby HS, Matthews JN, Rees JL: Prognostic significance of allelic losses in primary melanoma. Oncogene 1998, 16:2213-2218 [DOI] [PubMed] [Google Scholar]
- 30.Healy E, Belgaid CE, Takata M, Vahlquist A, Rehman I, Rigby H, Rees JL: Allelotypes of primary cutaneous melanoma and benign melanocytic nevi. Cancer Res 1996, 56:589-593 [PubMed] [Google Scholar]
- 31.Walker GJ, Palmer JM, Walters MK, Hayward NKA: Genetic model of melanoma tumorigenesis based on allelic losses. Genes Chromosomes Cancer 1995, 12:134-141 [DOI] [PubMed] [Google Scholar]
- 32.Isshiki K, Elder DE, Guerry D, Linnenbach AJ: Chromosome 10 allelic loss in malignant melanoma. Genes Chromosomes Cancer 1993, 8:178-184 [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Dong SM, Kim SY, Yoo NJ, Lee SH, Park WS: A simple, precise, and economical microdissection technique for analysis of genomic DNA from archival tissue sections. Virchows Arch 1998, 433:305-309 [DOI] [PubMed] [Google Scholar]
- 34.Fiucci G, Ruberti G: Detection of polymorphisms within the Fas cDNA gene sequence by GC-clamp denaturing gradient gel electrophoresis. Immunogenetics 1994, 39:437-439 [DOI] [PubMed] [Google Scholar]
- 35.Huang QR, Morris D, Manolios N: Identification and characterization of polymorphisms in the promoter region of the human Apo-1/fas (CD95) gene. Mol Immunol 1997, 34:577-582 [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Kim SY, Lee JY, Shin MS, Dong SM, Na EY, Park WS, Kim KM, Kim CS, Kim SH, Yoo NJ: Detection of soluble Fas using in situ reverse transcription-polymerase chain reaction. Lab Invest 1998, 78:453-459 [PubMed] [Google Scholar]
- 37.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM: FADD, a novel death domain- containing protein, interacts with the death domain of Fas, and initiates apoptosis. Cell 1995, 81:505-512 [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Khosravi-Far R, Chang HY, Baltimore D: Daxx, a novel Fas-binding protein that activates JNK, and apoptosis. Cell 1997, 89:1067-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oishi M, Maeda K, Sugiyama S: Distribution of apoptosis-mediating Fas antigen in human skin and effects of anti-Fas monoclonal antibody on human epidermal keratinocytes and squamous cell carcinoma cell lines. Arch Dermatol Res 1994, 286:396-407 [DOI] [PubMed] [Google Scholar]
- 40.Kanter-Lawensohn L, Hedblad MA, Wejde J, Larsson O: Immunohistochemical markers for distinguishing Spitz nevi from malignant melanomas. Mod Pathol 1997, 10:917-920 [PubMed] [Google Scholar]
- 41.Zörnig M, Grzeschiczek A, Kowalski M-B, Hartmann K-U, Möröy T: Loss of Fas/APO-1 receptor accelerates lymphomagenesis in Eμ L-MYC transgenic mice but not in animals infected with MoMuLV. Oncogene 1995, 10:2397-2401 [PubMed] [Google Scholar]