Abstract
We have previously shown in transgenic mice that transforming growth factor (TGF)-α dramatically enhances c-myc-induced hepatocarcinogenesis by promoting proliferation and survival of hepatocellular carcinoma (HCC) cells. As transgenic livers display increased levels of mature TGF-β1 from the early stages of hepatocarcinogenesis, we have now assessed whether impairment of TGF-β1 signaling contributes to the deregulation of cell cycle progression and apoptosis observed during this process. Focal preneoplastic lesions lacking expression of TGF-β receptor type II (TβRII) were detected in c-myc/TGF-α but not in c-myc livers. In c-myc/TGF-α mice, 40% (2/5) of adenomas and 90% (27/30) of HCCs showed down-regulation of TβRII expression in comparison with 11% (2/18) of adenomas and 47% (14/30) of HCCs in c-myc mice. Down-regulation of the TGF-β1-inducible p15INK4B mRNA and reduced apoptotic rates in TβRII-negative HCCs further indicated the disruption of TGF-β1 signaling. Furthermore, both TβRII-negative and -positive c-myc TGF-α HCCs, but not c-myc HCCs, were characterized by decreased levels of the cell cycle inhibitor p27. These results suggest 1) an inverse correlation of decreased p27 expression with the particularly strong expression of TGF-α in these lesions, consistent with the capacity of TGF-α signaling to post-transcriptionally regulate p27, and 2) the presence of alternative, downstream defects of TGF-β1 signaling in c-myc/TGF-α HCCs that may impair the growth-inhibitory response to TGF-β1. Thus, the accelerated neoplastic development in c-myc/TGF-α mice is associated with an early and frequent occurrence of TβRII-negative lesions and with reduced levels of p27 in HCC cells, indicating that disruption of TGF-β1 responsiveness may play a crucial role in the enhancement of c-myc-induced hepatocarcinogenesis by TGF-α.
The frequent co-expression of transforming growth factor (TGF)-α and c-myc in human hepatocellular carcinoma (HCC) 1 strongly suggests an important role for these genes in malignant growth of the liver. Consistent with this notion, we have demonstrated in transgenic mouse models that sustained c-myc overexpression results in persistent proliferation of hepatocytes and increased occurrence of HCC development 2,3 and that co-expression of TGF-α dramatically enhances c-myc-induced hepatocarcinogenesis. 2,4 We have further shown that this synergistic effect is largely due to 1) an unrestrained cell cycle progression of neoplastic hepatocytes via disruption of the cyclin D-pRb-E2F pathway 5 and 2) a TGF-α-dependent inhibition of apoptosis in cancer cells in the transgenic livers. 2,5 In contrast, the mitotic activity of peritumorous dysplastic hepatocytes drastically declines, and many of them ultimately apoptose, predominantly as a result of progressive up-regulation of mature TGF-β1. 2,5 Thus, overexpression of mature TGF-β1, a potent growth inhibitor and apoptosis inducer for hepatocytes, 6,7 may also provide a selective milieu in which (pre)neoplastic cells with reduced sensitivity to this cytokine will progress more rapidly toward a malignant phenotype. Consistent with this idea, constitutive expression of active TGF-β1 transgene in mouse liver predisposed to both spontaneous and chemically induced hepatocarcinogenesis. 8 Furthermore, co-expression of c-myc and mature TGF-β1 in the liver accelerates hepatocarcinogenesis that is associated with reduced TGF-β receptor type II (TβRII) expression. 8 These observations suggest that decreased responsiveness to TGF-β1 and c-myc up-regulation contribute to HCC progression.
The data summarized above provide indirect evidence for a progressive loss of sensitivity to TGF-β1 during hepatocarcinogenesis in c-myc and c-myc/TGF-α mice. In epithelial cells, including hepatocytes, TGF-β1 inhibition of cell cycle progression is due to transcriptional down-regulation of proliferation-associated genes 9,10 and to inhibition of cyclin-dependent kinase (CDK) activities by induction/regulation of the CDK inhibitors (CDKIs) p15, p27, and p21. 10-15 These mechanisms contribute to maintaining the retinoblastoma protein (pRb) in hypophosphorylated form, a process critical for the growth-suppressive function of TGF-β1. 16 However, pRb was hyperphosphorylated in c-myc and c-myc/TGF-α HCCs. 5 In addition, many of the genes transcriptionally inhibited by TGF-β1, such as endogenous c-myc, 9 TGF-α, 17 cyclins D1 and A, 9,10,18 and E2F-1 and -2 19 were induced, 2,5 indicating that the increased levels of TGF-β1 2 were unable to block proliferation in the transformed cells. This interpretation is consistent with the observations that deregulated expression of c-myc, cyclin D1, or E2F is able to overcome TGF-β1-mediated growth inhibition. 9,19,20
Given the presence of increased levels of mature TGF-β1 from the early stages of hepatocarcinogenesis in mice co-expressing c-myc and TGF-α transgenes, 2 we have investigated whether impairment of TGF-β1-mediated growth-suppressive pathways may constitute a part of the multistage process of hepatocarcinogenesis in this transgenic model. 5
Materials and Methods
Transgenic Mice and Tissue Preparation
Generation of the Alb-c-myc (c-myc) single-transgenic and Alb-c-myc/MT-TGF-α (c-myc/TGF-α) double-transgenic mice, transgene expression pattern, and pathological changes were as reported before. 2,4 In the current study, only male mice were investigated due to a more rapid neoplastic development culminating in appearance of HCC after a latency of 4 months in c-myc/TGF-α mice and of more than 12 months in c-myc mice. 2 No tumors were observed in age-matched (C57BL/6J × CBA/J) × CD1 wild-type (wt) mice used as controls. Nontumorous liver lobes, individual tumors ≥5 mm, and separately microdissected peritumorous tissues were snap-frozen in liquid nitrogen and stored at −80°C until further use. Animal housing and care were in accordance with NIH guidelines.
Northern Blot Analysis
Ten micrograms of poly(A)+ RNA isolated from nontumorous, tumorous, and corresponding peritumorous tissues, was electrophoresed and blotted as described previously. 2,5 Blots were probed with 32P-labeled cDNA probes, including a 1.3-kb fragment of mouse p15INK4B, 21 kindly provided by Dr. C.J. Sherr (St. Jude Children’s Research Hospital, Memphis, TN), and a PCR-generated 0.35-kb fragment of mouse ribosomal protein L7 (rpL7) used to normalize RNA expression levels. The latter were visualized and quantified by PhosphorImager scanning and ImageQuant software (Molecular Dynamics, Sunnyvale, CA) as described. 5 Differences (mean ± SE) were analyzed by unpaired t-test and considered significant when the two-tailed P value was <0.05.
Western Blot Analysis
This was performed essentially as reported before. 5 Briefly, 0.3 g of non-neoplastic, neoplastic, and corresponding perineoplastic liver samples were homogenized in ice-cold lysis buffer containing 30 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol, 5 mmol/L EDTA, 1 mmol/L Na3 VO4, 20 mmol/L inorganic pyrophosphate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. After an incubation of 15 minutes on ice, homogenates were sonicated three times for 10 seconds each and centrifuged to eliminate insoluble debris. Protein concentrations in the clarified supernatants were measured with Bio-Rad protein assay kit (Bio-Rad, Hercules, CA), 100 μg of total lysate proteins solubilized in boiling Laemmli buffer containing β-mercaptoethanol were separated by 12% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and reacted with 1 μg/ml rabbit polyclonal anti-p27 antibody (Ab N-20; Santa Cruz Biotechnology, Santa Cruz, CA). The specificity of the reaction was tested by preincubating the primary Ab with the corresponding p27 control peptide (1:20 w/w; Santa Cruz). Equal loading conditions were confirmed by staining the membranes with Ponceau-S.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded 5-μm sections from non-neoplastic liver lobes, from livers containing foci and benign tumors, and from 30 c-myc and 30 c-myc/TGF-α HCCs and their corresponding adjacent tissues. Immunostainings for TβRII and I using Abs against the cytoplasmic kinase domain of either receptor (L-21 and V-22, respectively; Santa Cruz) have been reported previously. 3,8 Immunoreactivity was revealed with the Vectastain ABC Elite kit followed by Vector VIP peroxidase substrate (Vector Laboratories, Burlingame, CA), a reddish chromogen. Sections were then lightly counterstained with hematoxylin. In each run, sections reacted with primary Abs preadsorbed to corresponding immunogen peptides (Santa Cruz) showed no evidence of staining, thereby verifying the reaction specificity. Twenty random fields/section were examined with a ×200 magnification by two pathologists and immunostainings arbitrarily scored as follows: −, when absent; +, when <5% of cells in the section were positive; ++, when 5% to 50% of cells were positive; +++, when >50% were stained.
Results
Overexpression of TGF-β1 and Down-Regulation of TβRs during Hepatocarcinogenesis in c-myc and c-myc/TGF-α Transgenic Mice
Our previous in situ hybridization and IHC studies showed that hepatocarcinogenesis in c-myc and c-myc/TGF-α mice is characterized by strong autocrine up-regulation of mature TGF-β1 associated with growth inhibition and apoptosis of dysplastic hepatocytes. 2,5
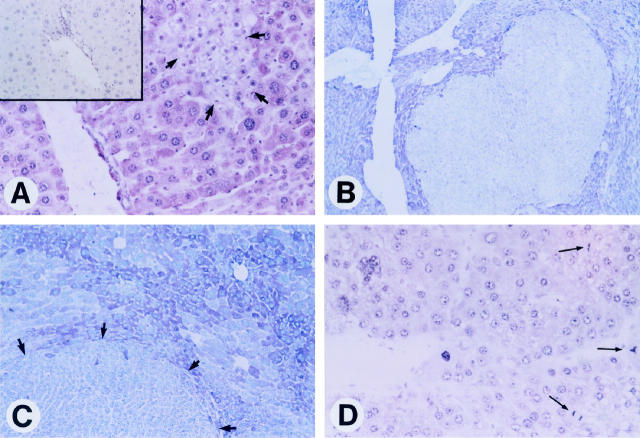
To address the possibility that these conditions may favor the selection of cells unresponsive to TGF-β1, we analyzed the expression of the transmembrane TβRII during hepatocarcinogenesis in the two transgenic lines. Upon ligand binding, the serine-threonine kinase TβRII recruits and phosphorylates TβRI, which consequently becomes active and propagates TGF-β1 signals to downstream effectors. 22,23 To study the expression pattern of TβRs we employed an immunohistochemical procedure previously used in other transgenic mouse hepatocarcinogenesis models. 3,8 Nontumorous and peritumorous transgenic tissues showed a more intense, albeit nonuniform, TβRII immunostaining than age-matched wt livers (data not shown). However, in c-myc/TGF-α livers not yet populated by frank tumors, we observed small focal lesions with decreased expression of TβRII (Figure 1A) ▶ . These early TβRII-negative cell clusters consisted of cells, apparently diploid, smaller than dysplastic hepatocytes and with a basophilic or basophilic-clear cell phenotype 2 and represented only a subset (15/80, 19%) of the preneoplastic foci analyzed in the double-transgenic livers. Interestingly, these foci were characterized by a much lower apoptotic activity and increased mitosis when compared with TβRII-positive foci, which were composed of large, polymorphic, eosinophilic cells. 2,5,8 In addition, in c-myc/TGF-α mice, two of five (40%) hepatocellular adenomas (HCAs) examined, both of basophilic phenotype, showed reduced TβRII expression. The extremely rapid HCC development in the double-transgenic mice 2 prevented us from staining a more informative number of benign tumors. Importantly, however, the vast majority (27/30, 90%) of c-myc/TGF-α HCCs displayed down-regulation of TβRII expression as compared with adjacent parenchyma (Figure 1, B–D ▶ ; Table 1 ▶ ), implying the possibility of a selection and expansion of TβRII-negative cells during tumor progression. We did not detect TβRII-negative preneoplastic cell clusters in c-myc livers. Decreased TβRII immunoreactivity was observed in 11% (2/19) of c-myc HCAs and in 47% (14/30) of c-myc HCCs (Table 1) ▶ , indicating that loss of TβRII during neoplastic development in c-myc transgenic livers is a slower and less frequent event. Notably, a significant number of HCCs in c-myc/TGF-α but not in c-myc mice, exhibited down-regulation of both TβRII and TβRI immunostaining (Table 1) ▶ , consistent with c-myc/TGF-α HCCs being biologically more aggressive than c-myc HCCs. 2 Indeed, all of the TβRI-negative HCCs were poorly differentiated and highly invasive lesions composed of anaplastic cells and neovascular structures that totally replaced the hepatic parenchyma and infiltrated adjacent organs. Also remarkable was that HCCs with loss of TβRs were particularly rich in mitotic figures (Figure 1D) ▶ and, according to our previous observations, 5 were characterized by apoptotic rates ∼10-fold lower than those in TβRII-positive HCCs. In keeping with this, TβRII-negative HCCs were bigger than their TβRII-positive counterparts (average size 2.2 × 1.9 ± 0.6 × 0.8 cm versus 1.3 × 1 ± 0.2 × 0.3 cm) and appeared more vascularized due to sinousoid dilation, proliferation of neocapillaries, and presence of unaccompanied arteries. These data support the notion that escape from TGF-β1 inhibitory effects may represent a major growth advantage for transformed cells in the transgenic livers.
Figure 1.
Expression patterns of TβRII during hepatocarcinogenesis in c-myc/TGF-α transgenic mice. Immunostaining was performed with an Ab against TβRII and VIP peroxidase substrate, a dark reddish chromogen, followed by light counterstaining with hematoxylin. A: Representative immunostaining of a 3-month-old c-myc/TGF-α preneoplastic liver showing a small TβRII-negative cell cluster marked by arrows. Note that the cells composing the cluster are all of similar small size and apparently diploid as opposed to the intensely reddish (TβRII-positive), large, and polymorphic dysplastic hepatocytes in surrounding parenchyma. Magnification, ×200. Serial section reacted with the primary Ab preincubated with a control peptide (inset) shows no immunostaining. B to D: Examples of TβRII down-regulation in neoplastic lesions. Tumor cells show no staining or very weak staining, in contrast to the prominent immunoreactivity of nontumorous surrounding parenchyma in a 6-month-old mouse (B; ×60). Peritumorous cells show intense, albeit heterogeneous, TβRII immunostaining as compared with the weak immunoreactivity of cells in a tumor marked by arrows (C; ×125). A HCC showing total lack of TβRII immunostaining and several mitotic figures (thin arrows) in an 8-month-old mouse (D; ×200).
Table 1.
Differential Expression of TGF-β1, TβRI, and TβRII in Peritumorous Tissues (PT), Preneoplastic Foci, HCAs and HCCs of c-myc and c-myc/TGF-α Transgenic Mice As Assessed by Immunohistochemical Staining
| Staining (pattern) | c-myc | c-myc/TGF-α | ||||||
|---|---|---|---|---|---|---|---|---|
| PT | Foci (n = 92) | HCA (n = 19) | HCC (n = 30) | PT | Foci (n = 80) | HCA (n = 5) | HCC (n = 30) | |
| TGF-β1 (cytoplasmic) | ++/+++ | ++ | ++ | ++ | +++ | +++ | +++ | ++/+++ |
| TβRI (cytoplasmic)* | +++ | +++ | +++ | 2 (7%)+† | +++ | +++ | +++ | 10 (33%)−/+† |
| TβRII (cytoplasmic)* | +++ | +++ | 2 (11%)+† | 14 (47%)−/+† | +++ | 15 (19%)−/+† | 2 (40%)−/+† | 27 (90%)−/+† |
Data on TGF-β1 IHC are from Santoni-Rugiu et al. 2 IHC was scored as described in the Materials and Methods.
*Significant difference between c-myc and c-myc/TGF-α HCAs or HCCs (P < 0.0001).
†Intensity of immunostaining in the remaining samples was comparable with that in PT.
Expression of p15INK4B mRNA Correlates with TβRII Status in Transgenic Livers
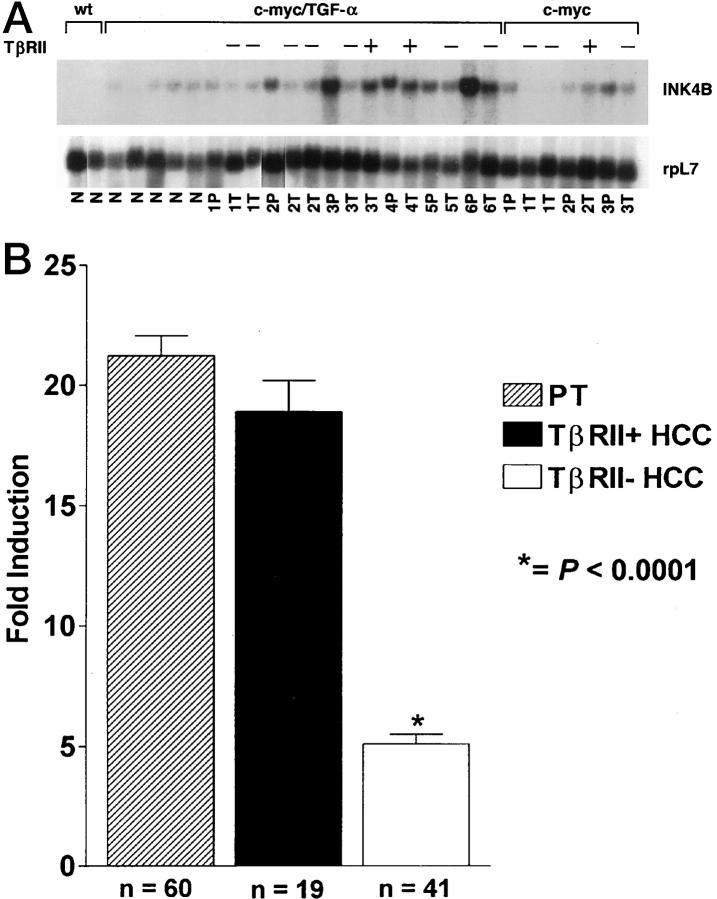
To better assess the impact of TβRII down-modulation on TGF-β1-regulated growth-inhibitory pathways, we analyzed mRNA expression of p15INK4B, a TGF-β1-inducible gene encoding a specific CDK4/6 inhibitor that causes G1 arrest in human and mouse epithelial cells. 11,21 p15INK4B mRNA expression levels correlated with TβRII immunoreactivity during transgene-induced hepatocarcinogenesis (Figure 2) ▶ . The steady-state level of p15INK4B transcript was higher in transgenic than age-matched wt livers (Figure 2) ▶ , consistent with the induction of TGF-β1 and TβRII in transgenic mice. More importantly, the c-myc and c-myc/TGF-α HCCs that retained TβRII expression displayed levels of p15INK4B mRNA comparable to those in the corresponding peritumorous tissues. In contrast, HCCs with significant reduction or total loss of TβRII showed significant down-regulation of p15INK4B mRNA as compared with the adjacent tissues (Figure 2) ▶ .
Figure 2.
Northern blot analysis of p15INK4B mRNA expression and its correlation with TβRII status during hepatocarcinogenesis in c-myc/TGF-α and c-myc transgenic mice. A: Representative example of steady-state p15INK4B mRNA levels in transgenic mice. Poly(A)+ RNA was obtained from wt liver (wt) and from nontumorous (N), peritumorous (P), and tumorous (T) tissues in c-myc/TGF-α and c-myc livers. +, HCCs expressing TβRII at levels comparable to those in the corresponding peritumorous tissues when assessed by IHC; −, HCCs with reduced or absent TβRII expression. Note, as an interesting example, the differential p15INK4B mRNA expression in a TβRII-negative HCC and in a TβRII-positive HCC isolated from the same c-myc/TGF-α mouse, labeled as 3. B: Quantitation of PhosphorImager-scanned Northern blots for p15INK4B mRNA expression in peritumorous tissue (PT, hatched bars), TβRII-positive HCCs (black bars), and TβRII-negative HCCs (white bars) of c-myc and c-myc/TGF-α mice. Scans were normalized to rpL7 and quantitated by ImageQuant software.
Selective Reduction of p27 Protein in c-myc/TGF-α HCCs
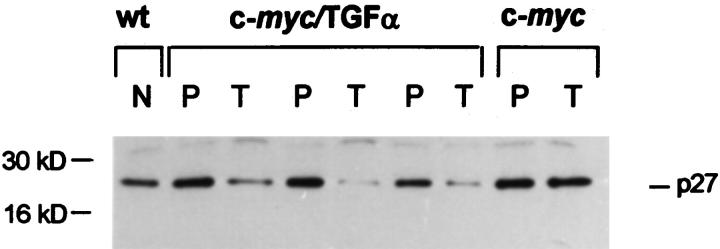
We next compared nontumorous and tumorous tissues with respect to the abundance of another important mediator of the growth-inhibitory response to TGF-β1, the CDKI p27. 12-15 Indeed, once induced by TGF-β1 in epithelial cells, p15 binds to and inhibits cyclin-D-dependent kinases, thereby promoting the redistribution of p27 from cyclin D-CDK4/6 complexes to cyclin E- and cyclin A-CDK2, resulting in inhibition of their kinase activity. 15 As illustrated in Figure 3 ▶ , immunoblot analysis of liver homogenate proteins showed higher levels of p27 in nontumorous transgenic tissues than in wt livers. This conceivably reflects the TGF-β1 up-regulation in nontumorous tissues, as TGF-β1 is capable of increasing p27 abundance in certain cell types. 12-15 However, c-myc/TGF-α HCCs displayed a remarkable down-modulation of p27 expression as compared with the corresponding adjacent tissues and with c-myc HCCs (Figure 3) ▶ . This decrease of p27 abundance in c-myc/TGF-α HCCs was unrelated to the TβRII status in the lesions, as it was also detected in the few TβRII-positive HCCs of double-transgenic mice. Conversely, it was not detected in TβRII-negative HCCs of c-myc mice. These findings suggest that factors such as the very strong expression of TGF-α in c-myc/TGF-α HCCs and their poor differentiation grade 2 may modulate p27 expression. These results also raise the possibility that neoplastic cells with reduced levels of p27 may be less responsive to TGF-β1-mediated cell cycle arrest even when they retain expression of TβRs.
Figure 3.
Representative Western blot showing p27 down-regulation in c-myc/TGF-α HCCs but not in c-myc HCCs. Total liver protein was extracted and used for Western analysis as described in Materials and Methods. N, nontumorous tissue in wt liver; P, peritumorous tissue; T, tumor. Samples in lanes 3 and 9 were obtained from TβRII-positive HCCs whereas those in lanes 5 and 7 were from TβRII-negative HCCs.
Discussion
We have shown here that the enhancement of c-myc-induced hepatocarcinogenesis by co-expression of TGF-α is associated with early and frequent occurrence of TβRII-negative preneoplastic and neoplastic lesions and with reduced levels of p27 in HCC cells. Disruption of TGF-β1 growth-suppressive pathways may therefore account, at least in part, for the synergistic effect of c-myc and TGF-α in hepatocarcinogenesis. Our data also support the general model of liver tumor promotion in which transformed cells gain a selective growth advantage in the presence of persistent inhibitory signals. In our transgenic mice the autocrine production of active TGF-β1 by the hepatocytes 2 creates a selective milieu in which transformed cells that down-modulate TβII expression may escape the growth-inhibitory effects of this cytokine and progress towards a more malignant phenotype. The high number of mitotic cells, the reduced apoptotic rates, the more prominent size, and histological aggressiveness observed in HCCs with TβRII down-regulation support this notion. Moreover, loss of TβRII expression correlates with tumor progression, as the vast majority of HCCs in c-myc/TGF-α mice showed reduced or absent immunostaining for the receptor, which, by contrast, was expressed in adjacent nontumorous tissues and in most of preneoplastic and benign lesions. These findings are in agreement with previous studies showing that certain human cancers, including HCC, can become refractory to TGF-β1 by reducing TβRII expression and/or function 24-26 and that expression of a dominant-negative TβRII transgene promotes chemical carcinogenesis in mouse epithelial tissues. 27,28 Re-introduction of the receptor in cells from TβRII-negative-cancers restores sensitivity to TGF-β1 and reduces their tumorigenicity. 29,30 Diminished expression of TβRI, an event rarer than loss of TβRII, has also been reported to correlate with the malignant phenotype in some human and rodent cancer cells. 31,32 Loss of TβRs is considered a late event in human tumorigenesis, mainly related to malignancy. 33 Consistent with this, c-myc HCCs, which have been described as histologically more differentiated and less aggressive than their c-myc/TGF-α counterparts, 2 displayed reduced TβRII immunostaining in less than 50% of the cases and virtually no reduction of TβRI expression level. On the other hand, TβRI expression was reduced in a subset of particularly undifferentiated c-myc/TGF-α HCCs that were also TβRII negative. These data suggest that TβRI down-regulation is not part of the early stages of hepatocarcinogenesis and is mainly associated with loss of differentiation and acquisition of high-grade malignancy. Yet, loss of TβRII was detected in a subpopulation of c-myc/TGF-α preneoplastic lesions composed of cells phenotypically different from TβRII-positive cells, such as the large, dysplastic hepatocytes and the cells forming eosinophilic foci. 2 Notably, these TβRII-positive cells were frequently apoptotic, 2,5,8 consistent with the idea that the intactness of TβRII signaling is instrumental in ensuring an efficacious antineoplastic response. Therefore, as 90% of c-myc/TGF-α HCCs displayed TβRII down-regulation, the TβRII-negative preneoplastic foci may potentially represent tumor precursors with high capability of malignant progression, due to being refractory to antiproliferative and apoptotic effects of TGF-β1. In line with this, we show here for the first time that the expression of the TGF-β1-inducible CDK4/6 inhibitor p15INK4B gene in HCC correlates with the TβRII status. HCCs characterized by reduced or absent TβRII expression down-regulate the transcript for p15, an essential post-receptor target, indicating that the functionality of TGF-β1 signaling is impaired in these lesions. Reduced levels of p15 in neoplastic cells makes the previously observed transgene-induced up-regulation of cyclin D1 5 even more likely to inactivate the suppressor function of pRb through CDK4/6 hyperactivity. Indeed, despite the intactness of p16 expression, pRb is extensively hyperphosphorylated in the HCCs of our transgenic mice. 5 Finally, TβRII-negative HCCs displayed reduced apoptotic activity as compared with HCCs that retained the receptor. Therefore, the down-regulation of TβRII in the vast majority of c-myc/TGF-α HCCs may be one of the factors accounting for the low apoptotic rates measured in these lesions. 5
TβRII-negative preneoplastic cell clusters were not detected in c-myc livers, whereas TβRII down-regulation was observed in only 11% and 47% of c-myc HCAs and HCCs, respectively. Thus, selection and expansion of cells that become insensitive to TGF-β1 by losing the expression of TβRII occur less frequently and at later stages during c-myc-induced hepatocarcinogenesis. This observation is consistent with the much slower tumor development in c-myc mice as compared with that in c-myc/TGF-α mice 2 and with the concept that loss of TGF-β1 responsiveness contributes to malignant progression. However, the emergence of TβRII-positive tumors in single- and, to a lesser extent, double-transgenic mice suggests the possible involvement of alternative mechanisms of TGF-β1 resistance in these lesions. In this respect, mutations of the insulin-like growth factor II receptor (IGFIIR), involved in TGF-β activation, 34-36 or mutations of the SMAD genes encoding the transducers of TβR kinase activity, 22,23 or overexpression of proteins that disrupt TGF-β-mediated cell cycle control by interacting with pRb, 37 have recently been described in human and experimental tumors, including HCCs. 35-37 Whether one or more of these mechanisms is/are present during the neoplastic process in the transgenic livers is currently a matter of investigation in our laboratory. It is also plausible that the constitutive expression of the c-myc transgene may represent itself a potential mechanism of TGF-β resistance as c-myc is one of the main transcriptional targets for TGF-β. 9 In any event, our current data imply that co-expression of TGF-α and c-myc results in early inactivation of TβRII during neoplastic development. This notion is supported by the frequency of HCCs showing TβRII down-modulation that increases from <50% in c-myc/TGF-β1 8 and c-myc (this report) transgenic mice, in which HCCs appear after a latency of 12 months, 2,3,8 to 90% in c-myc/TGF-α mice (this report), which develop HCCs after 4 months. 2 Thus, in addition to TGF-β1 up-regulation, co-expression of c-myc and TGF-α may provide cooperating signals resulting in selection of transformed cells with reduced TβRII expression and in enhanced tumor progression in the liver. Notably, overexpression of cyclin D1, a striking feature of c-myc/TGF-α HCCs, 5 was shown to decrease TβRII expression. 20
The selective reduction of p27 expression in c-myc/TGF-α HCCs reported in this study may also correlate with loss of response to TGF-β1, as inhibition of epithelial cell cycle progression by this cytokine relies, in part, on p27 activities. 12-15 It has been recently shown in mice that p27 may act as an inhibitor of CDK2 activity in the regenerating liver 38 and that the p27Kipl gene is haplo-insufficient for tumor suppression. 39 This implies that reduced levels of p27 protein may predispose to abnormal cell cycle and tumor progression, particularly when p27 function could be already counteracted, at least in part, by the overexpression of c-myc, 40 cyclins, 39 and E2F occurring during c-myc/TGF-α-induced hepatocarcinogenesis. 2,5 Our hypothesis is supported by the findings that p27 heterozygous mice are predisposed to physically, chemically, or spontaneously generated tumors 39 and that in several human epithelial cancers the amount of p27 protein expression inversely correlates with tumor aggressiveness and patient mortality. 41-43 Despite unaltered p27Kipl mRNA expression, the most aggressive tumors displayed high p27 proteolytic degradation, 42,43 consistent with the evidence that cellular abundance of this CDKI is controlled post-transcriptionally. 44,45 That p27 levels may be analogously regulated in c-myc/TGF-α HCCs is suggested by the preserved expression of p27Kipl mRNA in these lesions (E. Santoni-Rugiu, M.R. Jensen, and S.S. Thorgeirsson, unpublished) and by the high aggressiveness and poor differentiation of these tumors compared with HCCs in c-myc 2 or other transgenic mice (reviewed in Santoni-Rugiu and Thorgeirsson46). Indeed, activation of the Ras/mitogen-activated protein kinase (MAPK) pathway by several growth factors, including TGF-α, 47 can cause reduction of p27 levels by decreasing translation and stability of the protein (reviewed by Lloyd48). We cannot exclude that down-regulation of p27 may also represent a survival advantage for c-myc/TGF-α HCCs, as excessive overexpression of p27 was reported to induce apoptosis in normal and neoplastic cells. 49,50
It is noteworthy that the reduction of p27 abundance was independent of TβRII levels in the HCCs, as it was also detected in the few TβRII-positive HCCs of c-myc/TGF-α mice and not detected in TβRII-negative HCCs of c-myc mice. Although this supports the notion that the very strong TGF-α expression and the poor differentiation grade of c-myc/TGF-α HCCs 2 may play the most important role in modulating p27 expression in these lesions, it also suggests that p27 may have other functions in modulating cell proliferation on its own. This is in line with the fact that cells from p27Kipl nullizygous mice are still partially responsive to TGF-β-induced growth arrest. 51 Therefore, inactivation of TβRII represents a more effective way for circumventing TGF-β growth-suppressive function and consequently is frequently selected for in c-myc/TGF-α-induced hepatocarcinogenesis.
In conclusion, we have shown that the acceleration of c-myc-induced hepatocarcinogenesis by co-expression of TGF-α is associated with an earlier and more frequent occurrence of TβRII-negative lesions and with reduced levels of p27 in HCC cells, indicating that disruption of TGF-β1 signaling may play a crucial role in the promotion and progression of liver cancer.
Acknowledgments
We are grateful to Dr. G. Merlino for providing the TGF-α transgenic mice, Dr. C. J. Sherr for the gift of p15INK4B cDNA, Dr. H. C. Bisgaard for helpful suggestions, and N. Sanderson and A. Ton for excellent technical assistance. Financial support from the Danish Cancer Society to M. R. Jensen is gratefully acknowledged.
Footnotes
Address reprint requests to Dr. Snorri S. Thorgeirsson, National Cancer Institute, Bldg. 37, Room 3C28, 37 Convent Drive MSC4255, Bethesda, MD 20892-4255. E-mail: snorri_thorgeirsson@nih.gov
Present address for E. Santoni-Rugiu: Danish Cancer Society, Department of Cell Cycle and Cancer, Strandboulevarden 49, 2100 Copenhagen Ø, Denmark.
References
- 1.Grisham JW: Interspecies comparison of liver carcinogenesis: implications for cancer risk assessment. Carcinogenesis 1997, 18:59-81 [DOI] [PubMed] [Google Scholar]
- 2.Santoni-Rugiu E, Nagy P, Jensen MR, Factor VM, Thorgeirsson SS: Evolution of neoplastic development in the liver of transgenic mice co-expressing c-myc and transforming growth factor-α. Am J Pathol 1996, 149:407-428 [PMC free article] [PubMed] [Google Scholar]
- 3.Santoni-Rugiu E, Preisegger KH, Kiss A, Audolfsson T, Shiota G, Schmidt EV, Thorgeirsson SS: Inhibition of neoplastic development in the liver by hepatocyte growth factor in a transgenic mouse model. Proc Natl Acad Sci USA 1996, 93:9577-9582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami H, Sanderson N, Nagy P, Marino P, Merlino GT, Thorgeirsson SS: Transgenic mouse model for synergistic effects of nuclear oncogenes and growth factors in tumorigenesis: interaction of c-myc and transforming growth factor α in hepatic oncogenesis. Cancer Res 1993, 53:1719-1723 [PubMed] [Google Scholar]
- 5.Santoni-Rugiu E, Jensen MR, Thorgeirsson SS: Disruption of the pRb/E2F pathway and inhibition of apoptosis are major oncogenic events in liver constitutively expressing c-myc and transforming growth factor α. Cancer Res 1998, 58:123-134 [PubMed] [Google Scholar]
- 6.Fausto N, Laird AD, Webber EM: Role of growth factors and cytokines in hepatic regeneration. FASEB J 1995, 9:1527-1536 [DOI] [PubMed] [Google Scholar]
- 7.Schulte-Hermann R, Grausl-Krapp B, Bursch W: Apoptosis and hepatocarcinogenesis. Jirtle RL eds. Liver Regeneration and Carcinogenesis: Cellular and Molecular Mechanisms. 1995, :pp 141-178 Academic Press, New York [Google Scholar]
- 8.Factor VM, Kao C-Y, Santoni-Rugiu E, Woitach JT, Jensen MR, Thorgeirsson SS: Constitutive expression of mature transforming growth factor β1 in the liver accelerates hepatocarcinogenesis in transgenic mice. Cancer Res 1997, 57:2089-2095 [PubMed] [Google Scholar]
- 9.Alexandrow MG, Moses HL: Transforming growth factor β and cell cycle regulation. Cancer Res 1995, 55:1452-1457 [PubMed] [Google Scholar]
- 10.Sugiyama A, Nagaki M, Shidoji Y, Moriwaki H, Muto Y: Regulation of cell cycle-related genes in rat hepatocytes by transforming growth factor β1. Biochem Biophys Res Commun 1997, 238:539-543 [DOI] [PubMed] [Google Scholar]
- 11.Hannon GJ, Beach D: p15INK4b is a potential effector of cell cycle arrest mediated by TGF-β. Nature 1994, 371:257-261 [DOI] [PubMed] [Google Scholar]
- 12.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massagué J, Roberts JM, Koff A: p27Kipl, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 13.Polyak K, Lee M, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagué J: Cloning of p27Kipl, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78:59-66 [DOI] [PubMed] [Google Scholar]
- 14.Slingerland JM, Hengst L, Pan CH, Alexander D, Stampfer MR, Reed SI: A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor β-arrested epithelial cells. Mol Cell Biol 1994, 14:3683-3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynisdottir I, Polyak K, Iavarone A, Massagué J: Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev 1995, 9:1831-1845 [DOI] [PubMed] [Google Scholar]
- 16.Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massagué J: Growth inhibition by TGF-β linked to suppression of retinoblastoma protein phosphorylation. Cell 1990, 62:175-185 [DOI] [PubMed] [Google Scholar]
- 17.Mulder KM, Zhong Q, Choi HG, Humphrey LE, Brattain MG: Inhibitory effects of transforming growth factor β1 on mitogenic response, transforming growth factor α, and c-myc in quiescent, well-differentiated colon carcinoma cells. Cancer Res 1990, 50:7581-7586 [PubMed] [Google Scholar]
- 18.Ko TC, Sheng HM, Reisman D, Thompson EA, Beauchamp RD: Transforming growth factor β1 inhibits cyclin D1 expression in intestinal epithelial cells. Oncogene 1995, 10:177-184 [PubMed] [Google Scholar]
- 19.Schwarz JK, Bassing CH, Kovesdi I, Datto MB, Blazing M, Gorge S, Wang XF, Nevins JR: Expression of the E2F1 transcription factor overcomes type β transforming growth factor-mediated growth suppression. Proc Natl Acad Sci USA 1995, 92:483-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto A, Jiang W, Kim S-J, Spillare EA, Stoner GD, Weinstein IB, Harris CC: Overexpression of human cyclin D1 reduces the transforming growth factor β (TGF-β) type II receptor and growth inhibition by TGF-β1 in an immortalized human esophageal epithelial cell line. Proc Natl Acad Sci USA 1994, 91:11576-11580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quelle DE, Ashum RA, Hannon GJ, Rehberger PA, Trono D, Richter KH, Walker C, Beach D, Sherr CJ, Serrano M: Cloning and characterization of murine p16INK4A and p15INK4B genes. Oncogene 1995, 11:635-645 [PubMed] [Google Scholar]
- 22.Heldin CH, Miyazona K, ten Dijke P: TGFβ signaling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390:465-471 [DOI] [PubMed] [Google Scholar]
- 23.Massagué J: TGF-β signal transduction. Annu Rev Biochem 1998, 67:753-791 [DOI] [PubMed] [Google Scholar]
- 24.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV: Inactivation of the type II TGFR-β receptor in colon cancer cells with microsatellite instability. Science 1995, 268:1336-1338 [DOI] [PubMed] [Google Scholar]
- 25.Bedossa P, Peltier E, Terris B, Franco D, Poynard T: Transforming growth factor-β1 (TGF-β1) and TGF-β1 receptors in normal, cirrhotic, and neoplastic human livers. Hepatology 1995, 21:760-766 [PubMed] [Google Scholar]
- 26.Kiss A, Wang N-J, Xie J-P, Thorgeirsson SS: Analysis of transforming growth factor (TGF)-α/epidermal growth factor receptor, hepatocyte growth factor/c-met, TGF-β receptor type II, and p53 expression in human hepatocellular carcinomas. Clin Cancer Res 1997, 3:1059-1066 [PubMed] [Google Scholar]
- 27.Böttinger EP, Jakubczak JL, Haines DC, Bagnall K, Wakefield LM: Transgenic mice overexpressing a dominant-negative mutant type II transforming growth factor β receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7,12-dimethylbenz[a]anthracene. Cancer Res 1997, 57:5564-5570 [PubMed] [Google Scholar]
- 28.Amendt C, Schirmacher P, Weber H, Blessing M: Expression of dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and acceleration of carcinoma development. Oncogene 1998, 17:25-34 [DOI] [PubMed] [Google Scholar]
- 29.Inagaki M, Moustakas A, Lin HY, Lodish HF, Carr BI: Growth inhibition by transforming growth factor-β (TGF-β) type I is restored in TGF-β-resistant hepatoma cells after expression of TGF-β receptor type II cDNA. Proc Natl Acad Sci USA 1993, 90:5359-5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang J, Park K, Bang Y-J, Kim WS, Kim D, Kim S-J: Expression of transforming growth factor β type II receptor reduces tumorigenicity in human gastric cancer cells. Cancer Res 1997, 57:2856-2859 [PubMed] [Google Scholar]
- 31.Kim IY, Ahn H-J, Zelner DJ, Shaw JW, Lang S, Kato M, Oefelein MG, Miyazono K, Nemeth JA, Kozlowski JM, Lee C: Loss of expression of transforming growth factor β type I and type II receptors correlates with tumor grade in human prostate cancer tissues. Clin Cancer Res 1996, 2:1255-1261 [PubMed] [Google Scholar]
- 32.Okamoto M, Oyasu R: Overexpression of transforming growth factor β type I receptor abolishes malignant phenotype of a rat bladder carcinoma cell line. Cell Growth Differ 1997, 8:921-926 [PubMed] [Google Scholar]
- 33.Grady WM, Rajput A, Myeroff L, Liu DF, Kwon K, Willis J, Markowitz S: Mutation of the type II transforming growth factor-β receptor is coincident with the transformation of human colon adenomas to malignant carcinomas. Cancer Res 1998, 58:3101-3104 [PubMed] [Google Scholar]
- 34.Wang S, Souza RF, Kong D, Yin J, Smolinski KN, Zou T-T, Frank T, Young J, Flanders KC, Sugimura H, Abraham JM, Meltzer SJ: Deficient transforming growth factor-β1 activation and excessive insulin-like growth factor II (IGFII) expression in IGFII receptor-mutant tumors. Cancer Res 1997, 57:2543-2546 [PubMed] [Google Scholar]
- 35.Yamada T, De Souza A, Finkelstein S, Jirtle RL: Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci USA 1997, 94:10351-10355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills JJ, Falls JG, De Souza A, Jirtle RL: Imprinted M6p/Igf2 receptor is mutated in rat liver tumors. Oncogene 1998, 16:2797-2802 [DOI] [PubMed] [Google Scholar]
- 37.Woitach JT, Zhang M, Niu CH, Thorgeirsson SS: A retinoblastoma-binding protein that affects cell-cycle control and confers transforming ability. Nature Genet 1998, 19:371-374 [DOI] [PubMed] [Google Scholar]
- 38.Albrecht JH, Poon RYC, Ahonen CL, Rieland BM, Deng C, Crary GS: Involvement of p21 and p27 in the regulation of CDK activity and cell-cycle progression in the regenerating liver. Oncogene 1998, 16:2141-2150 [DOI] [PubMed] [Google Scholar]
- 39.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ: The murine gene p27Kipl is haplo-insufficient for tumor suppression. Nature 1998, 396:1177-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B: Growth arrest by the cyclin-dependent kinase inhibitor p27Kipl is abrogated by c-Myc. EMBO J 1996, 15:6595-6604 [PMC free article] [PubMed] [Google Scholar]
- 41.Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Pretzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM: Decreased levels of the cell-cycle inhibitor p27Kipl protein: prognostic implications in primary breast cancer. Nature Med 1997, 3:227-230 [DOI] [PubMed] [Google Scholar]
- 42.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nature Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 43.Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A: Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 1997, 57:3381-3385 [PubMed] [Google Scholar]
- 44.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 45.Hengst L, Reed SI: Translational control of p27Kipl accumulation during the cell cycle. Science 1996, 271:1861-1864 [DOI] [PubMed] [Google Scholar]
- 46.Santoni-Rugiu E, Thorgeirsson SS: Transgenic animals as models for hepatocarcinogenesis. Strain A Diehl AM eds. Liver Growth and Repair. 1998, :pp 100-142 Chapman and Hall, New York [Google Scholar]
- 47.Croix BS, Sheehan C, Rak JW, Flørenes VA, Slingerland JM, Kerbel RS: E-cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27Kipl. J Cell Biol 1998, 142:557-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lloyd AC: Ras versus cyclin-dependent kinase inhibitors. Curr Opin Genet Dev 1998, 8:43-48 [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Gorospe M, Huang Y, Holbrook NJ: p27Kipl overexpression causes apoptotic death of mammalian cells. Oncogene 1997, 15:2991-2997 [DOI] [PubMed] [Google Scholar]
- 50.Katayose Y, Kim M, Rakkar ANS, Cowan KH, Seth P: Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res 1997, 57:5441-5445 [PubMed] [Google Scholar]
- 51.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY: Mice lacking p27(Kipl) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85:707-720 [DOI] [PubMed] [Google Scholar]