Abstract
Low grade B-cell lymphomas comprise several well defined, clinically and immunophenotypically distinct disease entities. Composite lymphomas showing phenotypic characteristics of more than one of these tumor subtypes in the same site are rare, and both common and separate clonal origins of the two tumor parts have been reported for cases studied by molecular methods. We describe the detailed immunohistochemical and molecular findings in three cases with features of composite low grade B-cell non-Hodgkin’s lymphoma (B-NHL). All three neoplasms contained morphologically distinct but interwoven compartments of different cell types, which exhibited discordant expression of several markers, including CD5, CD10, CD43, and cyclin D1. According to their morphology and phenotypes, they were classified as mantle cell lymphoma and follicular lymphoma (Case 1), follicular lymphoma and small lymphocytic lymphoma (Case 2), and mantle cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma (Case 3). PCR analysis of DNA obtained from whole tissue sections failed to reveal evidence for biclonality in any of the cases. We therefore isolated cell populations with different antigen expression patterns by laser capture microdissection and analyzed them by polymerase chain reaction amplification and sequencing of clonal immunoglobulin heavy chain gene rearrangements and oncogene rearrangements. Sequence analysis revealed unrelated clonal rearrangements in each of the two tumor parts in all three cases, suggesting distinct clonal origins. In addition, Case 1 showed a bcl-2 rearrangement present only in the follicular lymphoma part. Our findings suggest that low grade B-NHL with two distinct morphological and immunophenotypic patterns in the same anatomical site are frequently biclonal. This is in keeping with current classification schemes, which recognize subtypes of low grade B-NHL as separate disease entities. Furthermore, our analysis demonstrates the power of laser capture microdissection in revealing molecular microheterogeneity in complex neoplasms.
Malignant non-Hodgkin’s lymphomas are currently defined as clonal proliferations of B or T cells arrested at a specific stage of differentiation. Low grade B-NHL comprise several well defined disease entities including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), follicular lymphoma (FL), and marginal zone cell lymphoma (MZL). 1 These tumors are characterized and distinguished by a combination of clinical, morphological, and immunophenotypical features and distinctive genetic abnormalities. They show a range of cytologic differentiation and can transform into high grade neoplasms at variable frequency, but these phenomena usually represent an evolution of the same clonal process. 2-5 However, a number of so-called biphenotypic B-cell neoplasms with two phenotypically apparently unrelated malignant populations arising in a patient either synchronously or metachronously have been described. 6-19 When studied by molecular analysis, cases with evidence of true biclonality 11-14,16,19 as well as cases with a common origin from the same clonal progenitor cell 2,7-9,20-23 have been reported.
Among the biphenotypic neoplasms, composite non-Hodgkin’s lymphomas, ie, tumors with two morphologically and/or phenotypically different components in the same anatomical site, are rare, and some earlier reports lack molecular studies. 13,15,17-19,24,25
We present three cases of low grade B-NHL with two morphologically and immunophenotypically distinct tumor components occupying different but intimately interwoven microenvironments in the involved tissue. Molecular analysis of the two tumor components obtained by laser capture microdissection (LCM) revealed two unrelated clonal populations in all three cases despite their synchronous anatomical presentation.
Patients
Case 1
A 58-year-old female presented with small bowel obstruction, leading to resection of a stenosed segment of the small intestine and mesenteric lymph nodes. A diagnosis of malignant non-Hodgkin’s lymphoma was rendered. Clinical staging revealed no further manifestations of lymphoma and PB counts were in the normal range. Two bone marrow biopsies performed at 12 and 18 months were reported to show evidence of minimal, focal involvement by lymphoma. The patient received 22 cycles of polychemotherapy over a period of 2 years and remains in continuous complete remission 8 years after the primary manifestation.
Case 2
A 77-year-old male with a 1-year history of marked splenomegaly developed inguinal lymphadenopathy. A lymph node biopsy was performed and a diagnosis of lymphoma made. Flow cytometric immunophenotyping of a bone marrow aspirate showed a population of B cells with λ light chain restriction and coexpression of CD5 and partly CD23 and FMC7. Cytologic examination of the peripheral blood showed no involvement by lymphoma.
Case 3
A 69-year-old female underwent laryngectomy and bilateral neck dissection for a T3 squamous cell carcinoma of the vocal cords. The grossly enlarged lymph nodes showed no metastases of the carcinoma, but involvement by a malignant lymphoma. The peripheral blood showed 31,000 leukocytes/μl with 82.3% lymphocytes. Flow cytometry of the peripheral blood revealed a large B cell population coexpressing CD5 and CD23 and showing dim κ light chain expression. In addition, a small CD5+ B cell population with λ light chain restriction was found.
Materials and Methods
Histology and Immunohistochemistry
Only paraffin-embedded tissue was available from the diagnostic specimens of all patients. Immunophenotyping was performed with the antibodies listed in Table 1 ▶ using an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) according to the company’s protocols, with minor modifications. Heat-induced antigen retrieval was performed with a microwave pressure cooker as previously described. 26 Incubation was performed overnight for cyclin D1, p27, CD5, and CD10; the remaining primary antibodies were incubated for 32 minutes. The rest of the staining procedure was performed on the Ventana immunostainer.
Table 1.
Antibodies Used in this Study
| CD/name (clone) | Reactivity | Source | Dilution |
|---|---|---|---|
| CD3 polyclonal | T cells | Dako | 1:100 |
| CD5 (4C7) | T cells, B-sub | Novocastra | 1:50 |
| CD10 (56C6) | cALLA antigen | Novocastra | 1:40 |
| CD20 (L26) | B cells | Dako | 1:200 |
| CD21 (1F8) | FDC, B-sub | Dako | 1:20 |
| CD23 (Bu38) | B-sub, FDC | Binding Site | 1:200 |
| CD43 (Leu22) | T cells, B-sub | BD | 1:50 |
| IgD polyclonal | IgD | Dako | 1:400 |
| kappa polycl. | κ light chains | Dako | 1:25,000 |
| lambda polycl. | λ light chains | Dako | 1:25,000 |
| MIB1 | Ki67 antigen | Immunotech | 1:40 |
| bcl-2 (124) | bcl-2 protein | Dako | 1:20 |
| cyclin D1 (P2D11F11) | cyclin D1 protein | Novocastra | 1:10 |
| p53 (DO7) | p53 protein | Dako | 1:50 |
| p27 (Kip-1) | p27/kip1 protein | Transduction | 1:1000 |
BD, Becton Dickinson, Mountain View, CA; Binding Site, The Binding Site, Birmingham, UK; Dako, Dako Corp., Carpinteria, CA; Immunotech, Immunotech Inc., Westbrook, ME; Novocastra, Novocastra, Newcastle, UK; Transduction, Transduction Laboratories, Lexington, KY.
Laser Capture Microdissection
LCM was performed on routinely immunostained slides as described previously 27-29 using a PixCell laser capture microscope (Arcturus Engineering, Santa Clara, CA). In brief, the stained, dehydrated tissue section is overlaid with a thermoplastic film mounted on an optically transparent cap. The visually selected areas are bound to the membrane by short, low-energy laser pulses leading to focal melting of the polymer. Immunostains showing a positive reaction in only one of the two different tumor components were selected for LCM. Each component was microdissected separately at least three times. The cells were immersed in 50–100 μl Tris buffer, pH 8.0, containing 0.5 mmol/L EDTA and 400 μg/ml proteinase K and digested for 3 hours at 55°C. After digestion, the enzyme was heat-inactivated and the extract was used directly for PCR.
Polymerase Chain Reaction (PCR), Cloning, and Sequence Analysis
The amplification of rearranged immunoglobulin genes was achieved using previously published primers directed at common sequences of all JH region genes (JH∝, LJH, and VLJH) and against homologous sequences of either the framework 3 (VH) or the framework 2 (FR2A) regions of the immunoglobulin heavy chain (IgH) genes. 30,31 All PCRs were performed with a hot start technique using the TaqStart antibody (Clontech, Palo Alto, CA), which reversibly inactivates polymerase activity below 70°C. Five to 10 μl of the crude extract from microdissected tissue or 1 μg of DNA obtained from whole sections by standard phenol/chloroform extraction 32 were added as template. For the complementarity-determining-region III (CDRIII) PCR with primers VH and JH∝, 35 cycles of amplification consisting of denaturation at 94°C for 45 seconds, annealing at 56°C for 1 minute, and elongation at 74°C for 1 minute were performed with DNA from whole tissue sections, 40 cycles with microdissected material. Amplification with the primer FR2A was performed as a seminested procedure in conjunction with primer LJH for the first round of 35 cycles and primer VLJH for the 25 cycles of the second round. The CDRIII and FR2 PCR products were analyzed on 16% polyacrylamide or 3% Metaphor agarose (FMC Bioproducts, Rockland, ME) gels, respectively. Primers bcl-2 and JH∝ were used for the detection of t(14;18) translocations of follicular lymphoma involving the major breakpoint region. 33 For amplification of the mantle cell lymphoma-specific t(11;14) involving the major translocation cluster, primers bcl-1.1 and JH∝ were used. 34 The specificity of the bcl-2 amplification product was confirmed by Southern blotting and hybridization with a digoxigenin-labeled internal control oligonucleotide. Bound probe was detected with an alkaline phosphatase-labeled anti-DIG Fab fragment and CSPD as chemiluminescence substrate recommended by the manufacturer (Boehringer Mannheim, Indianapolis, IN). Results obtained from microdissected tissue were confirmed by repeat PCR at least once. Microdissected reactive follicles were used as polyclonal control. Negative controls included water, DNA extraction buffer, and unused LCM caps.
Clonal amplification products were extracted from polyacrylamide gel slices using the crush and soak technique. 32 The purified PCR products were reamplified with the same primers, ligated into the PCR 2.1 vector (TA Cloning Kit, Invitrogen, Carlsbad, CA), and cloned into INVαF′ bacteria according to the manufacturer’s instructions. Automated fluorescent sequencing of plasmids containing inserts was performed with the BigDye Terminator Cycle Sequencing Kit (PE Applied Biosystems, Foster City, CA). A minimum of 4 clones was sequenced for each amplified rearrangement. Comparison of sequences and identification of IG heavy chain gene usage was performed with the MacVector 5.0 software (Eastman Kodak, New Haven, CT).
Results
Histological and Immunohistochemical Findings
The immunohistochemical findings are summarized in Table 2 ▶ .
Table 2.
Immunophenotypical Findings
| Antibody | Case 1 | Case 2 | Case 3 | |||
|---|---|---|---|---|---|---|
| MCL | FL | SLL | FL | SLL | MCL | |
| CD5 | + | − | + | − | + | +/− |
| CD10 | − | + | − | + | − | − |
| CD20 | + | + | + | + | + | + |
| CD21 | − | − (FDC+) | − | − (FDC+) | − | − (FDC+) |
| CD23 | − | − (FDC+) | +/− | − (FDC+) | + | − (FDC+) |
| CD43 | + | − | + | − | + | + |
| IgD | n.e. | n.e. | + | + | −/+ | + |
| κ | − | − | − | − | − | − |
| λ | − | − | − | + | − | + |
| MIB1 | 1–2% | 2–10% | 1–2% | 20–30% | 1–5% | 3–10% |
| bcl-2 | + | + | + | − | + | + |
| cyclin D1 | − | − | − | − | − | + |
| p53 | − | − | − | − | − | − |
| p27 | − | + | + | + | + | − |
Differentially expressed antigens are in bold-face type. FDC, follicular dendritic cells; FL, follicular lymphoma; MCL, mantle cell lymphoma; SLL, small lymphocytic lymphoma; n.e., not evaluable.
Case 1
The tumor diffusely involved the mucosa and submucosa of the small intestine with occasional flat ulcerations and spread into the muscle and focally into the subserosal fat. On routinely stained sections, two tumor components of different morphology could be appreciated. The diffuse component consisted of a monotonous proliferation of small lymphoid cells with slightly irregular nuclei and inconspicuous nucleoli. Blasts were not identified. The second component was surrounded by the diffuse tumor component and consisted of moderately well defined, less densely packed follicular structures composed of centrocytes with irregular, cleaved nuclei and occasional centroblasts (Figure 1, A and B) ▶ . Lymphoepithelial lesions were absent. Regional lymph nodes showed involvement only by the diffuse component with a mantle zone growth pattern. Immunohistochemically, the two components could easily be distinguished by their distinct antigen expression patterns. In addition to CD20, the diffuse component co-expressed CD43 and CD5, whereas p27 was found only in rare cells probably representing reactive T cells. The follicular component stained strongly for p27 and CD10, but lacked CD5 and CD43 (Figure 1, C-E ▶ ). The cyclin-dependent kinase inhibitor p27 is not expressed by the majority of MCL, in contrast to FL and other low grade B-NHL. 26
Figure 1.
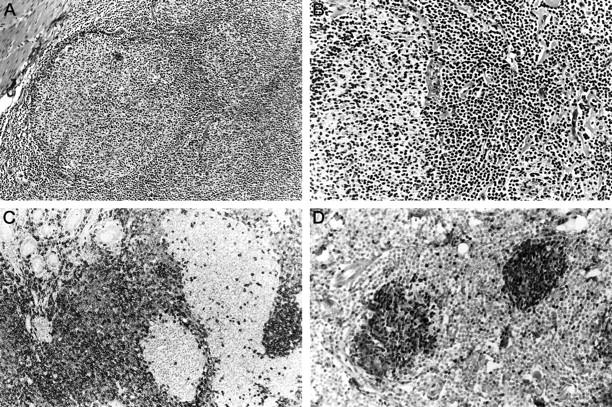
Case 1. A: Dense submucosal infiltrate with formation of irregular follicular structures and interspersed diffuse areas. H&E staining; original magnification, ×100. B: Two neoplastic follicles with an intervening diffuse area composed of small lymphoid cells with little nuclear irregularity. H&E; original magnification, ×200. C: The diffuse component shows expression of CD5, whereas the neoplastic follicles only contain rare, strongly positive cells, probably T cells. Immunoperoxidase; original magnification, ×200. D: The neoplastic follicles show strong nuclear staining for p27, whereas the MCL component is negative. Note the strong positivity in the intermingled reactive T cells. Immunoperoxidase; original magnification, ×200.
Case 2
The lymph node showed a proliferation of irregular follicles with absence of well defined mantle zones and a monotonous increase in small lymphocytes in the interfollicular area. The follicles contained a mixture of centrocytes and centroblasts, corresponding to a FL grade 2. The interfollicular proliferation consisted of small round lymphocytes without atypia and occasional paraimmunoblasts (Figure 2, A and B) ▶ . This diffuse component exhibited a phenotype consistent with SLL, showing co-expression of CD20 with CD5, CD23, and bcl-2. The neoplastic follicles showed only rare, strongly stained CD5- and CD43-positive cells, probably representing T cells, lacked bcl-2 expression and showed strong CD10 reactivity (Figure 2, C ▶ -E). The bone marrow showed focal involvement by aggregates of lymphoma cells coexpressing CD5 and CD20.
Figure 2.

Case 2. A: Replacement of the normal lymph node architecture by irregular follicular structures lacking well-defined mantle zones. The interfollicular areas appear monotonous. H&E; original magnification, ×40. B: At high power, the neoplastic follicles and a monomorphic population of small lymphocytes in the interfollicular space are clearly discernible. H&E; original magnification, ×200. C: The interfollicular cells express CD5, in addition to strongly stained cells representing T cells. The follicles are CD5−. Original magnification, ×200. D: A CD5-stained section after LCM of CD5-follicular areas. Original magnification, ×40. E: The neoplastic follicles express CD10, whereas the interfollicular regions are negative. Original magnification, ×40.
Case 3
Multiple lymph nodes showed replacement of the normal architecture by a monotonous proliferation of small lymphocytes and interspersed but clearly distinct nodular aggregates of medium-sized lymphoid cells with more irregular nuclei and open chromatin (Figure 3, A and B) ▶ . The nodular areas showed a stronger expression of CD20 and were positive for cyclin D1, strongly positive for IgD and λ light chains, but negative for p27 and CD23 and focally negative for CD5. In addition, they contained irregular meshworks of follicular dendritic cells stained by CD21. The diffuse part showed strong reactivity for p27 and expressed both CD5 and CD23, but lacked demonstrable cyclin D1 or Ig light chain expression, consistent with CLL/SLL (Figure 3, C-F ▶ ).
Figure 3.

Case 3. A: Nodular areas, sometimes resembling germinal centers with mantle zones, are surrounded by a diffuse lymphoid infiltrate. H&E; original magnification, ×40. B: At high magnification, the diffuse infiltrate is mainly composed of small lymphocytes with round nuclei, whereas the nodular areas show more nuclear irregularity with medium-sized cells with partly cleaved nuclei. H&E; original maginfication, ×400. C: The nodular areas strongly express IgD, and (D) lack p27, which is positive in the diffuse component. Immunoperoxidase; original magnification, ×100. E-F: Cyclin D1 is positive in the nodular areas. Note that some of the nodules are surrounded by a normal mantle zone area which are negative for cyclin D1, as well as the diffuse component. Immunoperoxidase; original magnifications, ×200 (E) and ×400 (F).
Molecular Findings
The molecular findings and the sequences of rearranged IgH genes are summarized in Tables 3 and 4 ▶ ▶ .
Table 3.
Molecular Findings on Microdissected Tissue
| Case 1 | Case 2 | Case 3 | ||||
|---|---|---|---|---|---|---|
| MCL | FL | SLL | FL | SLL | MCL | |
| CDRIII | R1 | R2 (R1) | R1 (R2) | R2 (R1) | (R1) | R1 |
| FR2 | n.a. | n.a. | R1 | n.a. | R2 | R1 (R2) |
| bcl-2 | − | R | −* | −* | −* | −* |
| bcl-1 | −* | −* | −* | −* | −* | −* |
FL, follicular lymphoma; MCL, mantle cell lymphoma; SLL, small lymphocytic lymphoma; R, clonal IgH or bcl-2 rearrangement, respectively. The numbers in parentheses indicate the presence of a weaker and inconsistent product identical in sequence to the rearrangement of the other tumor part.
n.a., not amplified.
*Performed on DNA extracted from whole, undissected sections.
Table 4.
Rearranged IgH Gene Sequences Obtained from Microdissected Tissues
VH primer sequences in bold. Mismatches with closest germline gene or primer sequence are underlined. Nucleotides showing intraclonal variation in lower case.
+, homologous to Case 3 MCL FR2 sequence.
#Only the 5′-ends of the sequences are shown.
*D region gene in reverse orientation.
Case 1
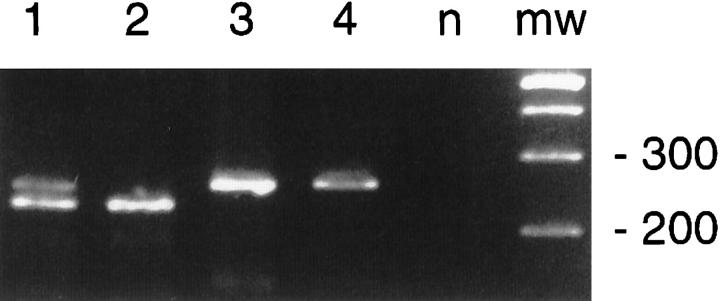
DNA extracted from whole tissue sections of the small bowel tumor showed a single clonal band by CDRIII PCR as well as a bcl-2 rearrangement. Multiple microdissections from the diffuse areas revealed an identical clonal band in the CDRIII PCR. In addition to a weak band of identical size, the microdissected follicular areas revealed a weaker but reproducible band of larger size not obtained from whole tumor sections (Figure 4A) ▶ . Sequencing of both CDRIII PCR products confirmed the presence of two unrelated clones. All microdissected follicular areas showed an identical bcl-2 rearrangement, which was confirmed by Southern blotting and sequencing. In contrast, only the largest of several microdissections from the diffuse component showed a weak signal for bcl-2, indicating the presence of contaminating FL cells (Figure 4B) ▶ . The findings were interpreted as composite MCL of the intestine with a clonally unrelated FL carrying a bcl-2 rearrangement.
Figure 4.
Case 1. A: CDRIII PCR of microdissected tissue obtained from a CD5-stained section. Lane mw: Molecular weight standard. Lanes 1 and 4: CD5+ MCL compartment. lanes 2 and 3: CD5-FL compartment. Lane n: negative control. Lane p: polyclonal control (microdissected reactive follicle). In addition to a weak band of the same size as the MCL compartment, the FL lanes show a second band of different size. Each lane represents a separate microdissection. B: Southern blot of bcl-2 PCR products obtained from paired microdissections with ∼30,000 cells (Lanes 1 and 2), 15,000 cells (Lanes 3 and 4), and 5000 cells (Lanes 5 and 6). Lanes 1, 3, and 5 show results obtained from the MCL compartment and Lanes 2, 4, and 6 those from the FL compartment. Lane w: PCR product from whole tissue section. Lane p: positive control. The weak signal in Lane 1 probably results from contaminating FL lymphoma cells.
Case 2
DNA obtained from whole lymph node sections as well as the microdissected, CD5+ tumor component rendered a strong clonal band of identical size. In addition to a weak band of the same size, microdissections from the CD5− follicular component revealed a strong band of different size, which was also identifiable as a minor product in the PCRs from the CD5+ component as well as from the whole section (Figure 5) ▶ . Sequencing of both bands revealed the presence of two different clonal rearrangements based on the differences in the D and N regions, although both rearrangements used the J5 gene segment. No bcl-2 or bcl-1 rearrangements were identified by PCR with DNA obtained from whole tissue sections. The case was interpreted as bcl-2-negative FL with a clonally unrelated interfollicular neoplasm consistent with SLL.
Figure 5.
Case 2 CDRIII PCR. Lane w: DNA extracted from whole tissue sections. Lanes 1 and 2: Microdissections from the CD5+ SLL component; Lanes 3 and 4: Microdissections from the CD5-FL component. Lane n: Negative control. The lower rearranged band derived from the FL component is barely visible in the whole tissue section PCR product.
Case 3
All microdissected areas from the nodular component obtained from two different lymph nodes rendered a clonal band of identical size and sequence by CDRIII PCR. A band of identical size and sequence but consistently lower intensity was amplified from the microdissections from the diffuse component (data not shown). In contrast, amplification with the FR2 primer set gave two different-sized clonal bands for the two tumor components. (Figure 6) ▶ . Sequence comparisons showed that the lower FR2 band, amplified from the cyclin D1+ nodular areas, resulted from the same clonal rearrangement as the CDRIII product, whereas the higher FR2 band, amplified predominantly from the CLL population, was clonally unrelated. This sequence showed mismatches with the CDRIII VH primer, probably accounting for its failure to amplify the CLL-specific rearrangement. No bcl-1 or bcl-2 rearrangements were detected by PCR with DNA obtained from whole tissue sections. In conjunction with the results of the immunohistochemistry and the flow cytometric immunophenotyping of the PB, the case was interpreted as composite MCL and CLL with peripheral blood involvement, predominantly by the CLL component.
Figure 6.
Case 3 seminested PCR with the FR2A primer set. Lanes 1 and 2: Microdissections from the MCL component. Lanes 3 and 4: Microdissections from the CLL component. One of the two PCR products derived from MCL areas show both rearranged bands, indicating contamination by CLL cells. 3% Metaphor gel.
Discussion
Our study demonstrates that composite low grade B-cell lymphomas containing two phenotypically distinct cell populations corresponding to separate B-NHL entities frequently represent a true collision tumor with two unrelated malignant clones, rather than divergent differentiation within a single neoplastic process. The biclonality was not evident from the molecular studies performed on DNA extracted from the whole tissue block in any of the cases and microdissection was crucial for establishing the complex nature of these neoplasms.
The current classification of low grade B-NHL is based on the concept that the different subtypes are characterized by neoplastic cells arrested at specific points during development, corresponding to various stages of normal B cell differentiation. Their distinct morphologies and immunophenotypes, the presence or absence of somatic hypermutation of the immunoglobulin genes, and characteristic genetic abnormalities define them as true disease entities. 1,35 CLL/SLL, MCL, FL, and MZL are the most common and best defined categories of the low grade B-NHL. In this context, composite lymphomas showing two phenotypically distinct cell populations with features of two of these B-NHL entities in the same anatomical location are of special interest despite their rarity, since they seem to challenge the conceptual separation of the low grade B-NHL.
The definition of composite lymphoma has evolved over time. Initially based on the presence of two morphologically distinct components in the same anatomical site, refined criteria required the demonstration of distinct immunophenotypes, and ultimately, molecular evidence of distinct clonal origins of the two tumor cell populations. 13,15,18,24,25,36 The most frequent occurrence of two different lymphoma morphologies presenting in the same patient is a high grade tumor arising in the setting of a concomitant or antecedent low grade neoplasm. This generally represents histological transformation of the low grade tumor, and a common clonal origin can be demonstrated in most, but not all, cases. 2,3,14,16,20,21,23,37
Composite B-cell neoplasms with two phenotypically different low grade components are infrequent; only a few reports contain sufficient immunophenotypic and molecular genetic information. 9,11,13,17-19,25,36
Although our three cases showed some unusual morphological features, only a detailed immunophenotypic analysis made a diagnosis of composite lymphoma possible. The differential expression of markers such as CD5, CD10, CD43, and cyclin D1 allowed us to identify the two cytological and architectural patterns as distinct tumor components and also to distinguish the FL infiltrates of Case 1 and the MCL nodules of Case 3 from residual germinal centers. Based on these findings, we chose to classify the two tumor components separately, following established criteria. Although the cases show some abnormalities of phenotype, such as the absence of bcl-2 expression in the FL of Case 2 or the lack of detectable cyclin D1 expression in the MCL component of Case 1, these variations are well described in otherwise typical examples of these entities. 34,38,39 The characteristic absence of p27 expression in the MCL components of Cases 1 and 3, in contrast to both FL and SLL, confirmed previous findings and demonstrates the utility of this antibody when used in combination with the established markers mentioned above. 26,40
Despite the convincing immunophenotypic evidence for the presence of composite lymphomas, biclonality could not be established by PCR amplification of DNA obtained from whole tissue sections in any of the cases. Only molecular analysis of microdissected tissue with comparison of rearranged immunoglobulin sequences allowed us to confirm the presence of two neoplasms of independent clonal origin in the same anatomical site. The lack of amplification of one of the two rearrangements in Cases 1 and 2 from DNA obtained from whole sections is most likely due to differences in amplification efficiency rather than only to the numerical predominance of one of the cell populations, because enrichments achievable with crude microdissection from hematoxylin and eosin (H&E)-stained sections failed to bring out the second clone in Case 1, which appeared only after high level enrichment attained with more precise LCM from immunostained sections. In Case 3, the CLL clone was amplifiable only with the FR2 primers. The failure to detect the CLL clone in the CDRIII PCR was probably a result of the mismatches at the VH primer binding site preventing the amplification of this rearrangement but allowing the detection of rare contaminating MCL cells present even in microdissected CLL populations.
Because biclonality and ongoing Ig gene alterations can occur in morphologically and phenotypically homogeneous B-NHL, mainly FL, it might be argued that the second rearrangement, found only in the microdissected tissues but not in the bulk tissue extracts, does not necessarily represent a distinct second neoplasm. 7,8,12,41-43 However, the reproducible linkage of these second clonal bands to the areas with profoundly different phenotypes is evidence against this possibility. Furthermore, the ongoing Ig gene alterations that occur in FL, generally consist of point mutations and occasionally small inserts or deletions that will not change the VDJ gene segment usage detected by PCR. In addition, bcl-2 rearrangements, as seen in Case 2, are stable markers of the neoplastic clone and are preserved, despite ongoing Ig gene alterations. 41 The failure to detect a bcl-2 rearrangement in the FL of Case 2 and of bcl-1 rearrangements in the MCL components of Cases 1 and 3 is not unusual, because only 35–50% of these rearrangements can be detected with the primer sets used in this study. The few cases of composite low grade B-NHL reported with sufficient phenotypic and molecular information to allow classification according to current criteria also seem to represent two clonally unrelated neoplasms. 13,17,19 Due to the apparent rarity of these cases, one can only speculate about the reasons for the simultaneous occurrence of two distinct low grade B-NHL. It should be noted, however, that none of our patients had a condition predisposing to B-cell lymphoma, such as autoimmune disease or immunosuppression.
The histological and immunophenotypic findings in our cases must be distinguished from the variable expression of antigens or changing morphology in different areas of the same tumor, which are probably due to a compartmentalization of neoplastic cells according to their functional or maturational status. Such zoning phenomena have been described in FL and especially MZL, a finding which initially led to the frequent description of composite lymphomas in this group. 44 However, cytogenetics or molecular analysis with or without microdissection has usually supported a common clonal origin of the two populations. 4,5,44,45
In summary, we present three cases of composite low grade B-cell lymphoma with two phenotypically distinct populations residing in separate but intimately interwoven compartments of the same anatomical site. The distinct clonal origin of the two compartments as revealed by molecular analysis of microdissected tissue confirmed the presence of two separate neoplasms and supports the current concepts of the classification of low grade B-NHL. The more frequent application of microdissection on lymphomas with unusual morphology and phenotype may reveal the existence of such cases more frequently. The ease and speed with which LCM can be performed on routinely immunostained tissue sections for standard PCR assays makes it a potentially useful tool for the analysis of molecular microheterogeneity of tumors.
Acknowledgments
We thank C. Harris and S. Delay for their expert help with immunohistochemistry, Drs. D. Hubner, J. Hampton, J. Clay, M. Vrana, S. Pearlman, and J. Saunders for referring the cases to us and providing clinical information and follow-up, and Dr. S. Pittaluga for helpful discussions.
Footnotes
Address reprint requests to Dr. Mark Raffeld, Hematopathology Section, Laboratory of Pathology, National Cancer Institute, NIH Building 10, Room 2N110, 9000 Rockville Pike, Bethesda MD 20892. E-mail: mraff@box-m.nih.gov.
Supported in part by grants from the Austrian Science Funds to F. F. (Erwin-Schroedinger Stipendium J1402 MED) and L. Q.-M. (Charlotte-Buehler Stipendium H00083).
References
- 1.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolff-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Müller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American Classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 2.de Jong D, Voetdijk BMH, Beverstock GC, van Ommen GJB, Willemze R, Kluin PM: Activation of the c-myc oncogene in a precursor B-cell blast crisis of follicular lymphoma, presenting as composite lymphoma. N Engl J Med 1988, 318:1373-1378 [DOI] [PubMed] [Google Scholar]
- 3.Zelenetz AD, Chen TT, Levy R: Histologic transformation of follicular lymphoma to diffuse lymphoma represents tumor progression by a single malignant B cell. J Exp Med 1991, 173:197-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dogan A, Du M-Q, Aiello A, Diss TC, Ye H-T, Pan L-X, Isaacson PG: Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. Blood 1998, 91:4708-4714 [PubMed] [Google Scholar]
- 5.Abou-Elella AA, Nathwani BN, Gascoyne R, Weisenburger DD, Velankar M, Greiner TC, Chan WC: The relationship between monocytoid B-cell (MBC) lymphoma and coexisting follicular lymphoma. Mod Pathol 1998, 11:124A [Google Scholar]
- 6.Brouet JC, Laurent FG, Grange MJ, Chevalier A, Jaquillat C, Seligmann M: The association of chronic lymphocytic leukaemia and multiple myeloma: a study of eleven patients. Brit J Haematol 1985, 59:55-66 [DOI] [PubMed] [Google Scholar]
- 7.Cleary ML, Galili N, Trela M, Levy R, Sklar J: Single cell origin of bigenotypic and biphenotypic B cell proliferations in human follicular lymphomas. J Exp Med 1988, 167:582-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong D, Voetdijk BMH, van Ommen GJB, Kluin PM: Alterations in immunoglobulin genes reveal the origin and evolution of monotypic and bitypic B cell lymphomas. Am J Pathol 1989, 134:1233-1242 [PMC free article] [PubMed] [Google Scholar]
- 9.Fermand JP, James JM, Herait P, Brouet JC: Associated chronic lymphocytic leukemia and multiple myeloma: origin from a single clone. Blood 1985, 66:291-293 [PubMed] [Google Scholar]
- 10.Fisher RI, Jones RB, DeVita VT, Simon RM, Garvin AJ, Berard CW, Young RC: Natural history of malignant lymphomas with divergent histologies at staging evaluation. Cancer 1981, 47:2022-2025 [DOI] [PubMed] [Google Scholar]
- 11.Siegelman MH, Cleary ML, Warnke R, Sklar J: Frequent biclonality and Ig gene alterations among B cell lymphomas that show multiple histologic forms. J Exp Med 1985, 161:850-863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sklar J, Cleary ML, Thielemans K, Gralow J, Warnke R, Levy R: Biclonal B-cell lymphoma. New Engl J Med 1984, 311:20-27 [DOI] [PubMed] [Google Scholar]
- 13.Sulak LE, Craig FE, Montiel MM, Banks PM: Biclonal composite lymphoma (letter). Arch Pathol Lab Med 1990, 114:638. [PubMed] [Google Scholar]
- 14.Trümper L, Matthaei-Maurer DU, Knauf W, Möller P: Centroblastic lymphoma of the thyroid supervening long-lasting chronic lymphocytic leukemia (B-CLL). Demonstration of biclonality by immunohistochemical and gene rearrangement analysis. Klin Wochenschr 1988, 66:736-742 [DOI] [PubMed] [Google Scholar]
- 15.York JC, Glick AD, Cousar JB, Collins RD: Changes in the appearance of hematopoietic and lymphoid neoplasms: clinical, pathologic, and biologic implications. Hum Pathol 1984, 15:11-38 [DOI] [PubMed] [Google Scholar]
- 16.van Dongen JJM, Hooijkaas H, Michiels JJ, Grosveld G, de Klein A, van der Kwast ThT, Prins MEF, Abels J, Hagemeijer A: Richter’s syndrome with different immunoglobulin light chains and different heavy chain rearrangements. Blood 1984, 64:571-575 [PubMed] [Google Scholar]
- 17.Duque RE, Everett ET, Iturraspe J: Biclonal composite lymphoma: a multiparameter flow cytometric analysis. Arch Pathol Lab Med 1990, 114:176-179 [PubMed] [Google Scholar]
- 18.Wolfe JA, Borowitz MJ: Composite lymphoma: a unique case with two immunologically distinct B-cell neoplasms. Am J Clin Pathol 1984, 81:526-529 [DOI] [PubMed] [Google Scholar]
- 19.Cachia AR, Diss TC, Isaacson PG: Composite mantle cell lymphoma and plasmacytoma. Hum Pathol 1997, 28:1291-1295 [DOI] [PubMed] [Google Scholar]
- 20.Miyamura K, Osada H, Yamauchi T, Itoh M, Kodera Y, Suchi T, Takahashi T, Ueda R: Single clonal origin of neoplastic B-cells with different immunoglobulin light chains in a patient with Richter’s syndrome. Cancer 1990, 66:140-144 [DOI] [PubMed] [Google Scholar]
- 21.Nakamine H, Masih AS, Sanger WG, Wickert RS, Mitchell DW, Armitage JO, Weisenburger DD: Richter’s syndrome with different immunoglobulin light chain types: molecular and cytogenetic features indicate a common clonal origin. Am J Clin Pathol 1992, 97:656-663 [DOI] [PubMed] [Google Scholar]
- 22.Saltman DL, Ross JA, Banks RE, Ross FM, Ford AM, Mackie MJ: Molecular evidence for a single clonal origin in biphenotypic concomitant chronic lymphocytic leukemia and multiple myeloma. Blood 1989, 74:2062-2065 [PubMed] [Google Scholar]
- 23.Schots R, Dehou M-F, Jochmans K, Heirman C, de Waele M, van Camp B, Thielemans K: Southern blot analysis in a case of Richter’s syndrome: evidence for a postrearrangement heavy chain gene deletion associated with the altered phenotype. Am J Clin Pathol 1991, 95:571-577 [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Hendrickson MR, Dorfman RF: Composite lymphoma. Cancer 1977, 33:959-976 [DOI] [PubMed] [Google Scholar]
- 25.Poon M-C, Flint A, Miles GL: Composite lymphoma: report of a unique case. Cancer 1980, 46:1676-1682 [DOI] [PubMed] [Google Scholar]
- 26.Quintanilla-Martinez L, Thieblemont C, Fend F, Kumar S, Pinyol M, Campo E, Jaffe ES, Raffeld M: Mantle cell lymphomas lack expression of p27/kip1, a cyclin-dependent kinase inhibitor. Am J Pathol 1998, 153:175-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui R, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA: Laser capture microdissection. Science 1996, 274:998-1001 [DOI] [PubMed] [Google Scholar]
- 28.Bonner RF, Emmert-Buck M, Cole K, Pohida T, Chuaqui R, Goldstein S, Liotta LA: Laser capture microdissection: molecular analysis of tissue. Science 1997, 278:1481-1483 [DOI] [PubMed] [Google Scholar]
- 29.Fend F, Emmert-Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, Raffeld M: Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol 1999, 154:61-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trainor KJ, Brisco MJ, Story CJ, Morley AA: Monoclonality in B-lymphoproliferative disorders detected at the DNA level. Blood 1990, 75:2220-2222 [PubMed] [Google Scholar]
- 31.Ramasamy I, Brisco M, Morley A: Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol 1992, 45:770-775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch EF, Maniatis T: Molecular cloning, a laboratory manual. 2d ed. 1989, Cold Spring Harbor Laboratory Press Cold Spring Harbor, NY
- 33.Stetler-Stevenson M, Raffeld M, Cohen P, Cossman J: Detection of occult follicular lymphoma by specific DNA amplification. Blood 1988, 72:1822-1825 [PubMed] [Google Scholar]
- 34.Kumar S, Krenacs L, Otsuki T, Kumar D, Harris CA, Wellmann A, Jaffe ES, Raffeld M: bcl-1 rearrangement and cyclin D1 protein expression in multiple lymphomatous polyposis. Am J Clin Pathol 1996, 105:737-743 [DOI] [PubMed] [Google Scholar]
- 35.Klein U, Goossens T, Fischer M, Kanzler H, Braeuninger A, Rajewsky K, Kuppers R: Somatic hypermutation in normal and transformed cells. Immunol Rev 1998, 162:261-280 [DOI] [PubMed] [Google Scholar]
- 36.Kim H: Composite lymphoma and related disorders. Am J Clin Pathol 1993, 99:445-451 [DOI] [PubMed] [Google Scholar]
- 37.Matolcsy A, Inghirami G, Knowles DM: Molecular genetic demonstration of the diverse evolution of Richter’s syndrome (chronic lymphocytic leukemia and subsequent large cell lymhpoma). Blood 1994, 83:1363-1372 [PubMed] [Google Scholar]
- 38.Gelb AB, Rouse R, Dorfman RF, Warnke RA: Detection of immunophenotypic abnormalities in paraffin-embedded B-lineage non-Hodgkin’s lymphoma. Am J Clin Pathol 1994, 102:825-834 [DOI] [PubMed] [Google Scholar]
- 39.Lai R, Arber DA, Chang KL, Wilson CS, Weiss LM: Frequency of bcl-2 expression in non-Hodgkin’s lymphoma: a study of 778 cases with comparison of marginal zone lymhpoma and monocytoid B-cell hyperplasia. Mod Pathol 1998, 11:864-869 [PubMed] [Google Scholar]
- 40.Sanchez-Beato M, Saez AI, Martinez-Montero JC, Mateo MS, Sanchez-Verde L, Villuendas R, Piris MA: Cyclin-dependent kinase inhibitor p27kip1 in lymphoid tissue: p27 kip1 expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 41.Raffeld M, Wright JJ, Lipford E, Cossman J, Longo DL, Bakhshi A, Korsmeyer SJ: Clonal evolution of t(14;18) follicular lymphomas demonstrated by immunoglobulin genes and the 18q21 major breakpoint region. Cancer Res 1987, 47:2537-2542 [PubMed] [Google Scholar]
- 42.Elenitoba-Johnson KSJ, Khorsand J, King TC: Splenic marginal zone cell lymphoma associated with clonal B-cell populations showing different immunoglobulin heavy chain sequences. Mod Pathol 1998, 11:905-913 [PubMed] [Google Scholar]
- 43.Fishleder A, Tubbs R, Hesse B, Levine H: Uniform detection of immunoglobulin-gene rearrangement in benign lymphoepithelial lesions. New Engl J Med 1987, 316:1118-1121 [DOI] [PubMed] [Google Scholar]
- 44.Ngan BY, Warnke RA, Wilson M, Tagaki K, Cleary ML, Dorfman RF: Monocytoid B-cell lymphoma: a study of 36 cases. Hum Pathol 1991, 22:409-421 [DOI] [PubMed] [Google Scholar]
- 45.Slovak ML, Weiss LM, Nathwani BN, Bernstein L, Levine AM: Cytogenetic studies of composite lymhpomas: monocytoid B-cell lymphoma and other B-cell non-Hodgkin’s lymphomas. Hum Pathol 1993, 24:1086-1094 [DOI] [PubMed] [Google Scholar]