Abstract
The metastasis suppressor gene KAI1 was identified by its ability to inhibit the formation of pulmonary metastases in experimental models for prostatic carcinoma. Down-regulation of this gene may be correlated with the invasive phenotype in melanomas and colon and bladder carcinomas and with the metastatic phenotype in carcinomas of the lung, breast, prostate, and pancreas. The goal of our study was to establish an immunohistochemical method to detect KAI1 expression in archival tissues. Using cell lines with known KAI1 levels and paraffin-embedded KAI1 positive tissues as controls, we observed strong membrane staining in lymphoid follicular centers and squamous epithelia. We then demonstrated the utility of our assay by studying KAI1 expression in 34 lymphoid and 57 squamous lesions. All eight reactive lymph nodes were KAI1 positive. In contrast, three of 13 follicular small cleaved and five of 13 diffuse large cell lymphomas were KAI1 negative. Seventy-nine percent (37 of 47) of invasive squamous cell carcinomas from the lung (n = 15), head and neck (n = 18), and cervix (n = 14) showed extensive KAI1 down-regulation. Loss of KAI1 expression was also found in a subset of 10 high-grade cervical dysplasias. Our data show that (i) immunohistochemistry is a suitable technique for evaluating KAI1 expression in archival tissues; (ii) KAI1 was not expressed in a subset of both low-grade and high-grade lymphomas; and (iii) there was extensive down-regulation of KAI1 in squamous cell carcinomas, suggestive of an important role of the gene in the suppression of invasion in these malignancies.
The formation and progression of a malignant neoplasm is a multistep process that involves abrogation of growth control, extension of cellular life span, invasion of surrounding tissues, and metastasis. The molecular mechanisms that determine the metastatic phenotype are largely unknown. However, recent evidence suggests that abnormal expression of one or more cell surface molecules of the transmembrane 4 superfamily (TM4SF) may contribute to invasion and metastasis. 1-7 One member of this group, KAI1 (also known as R2, IA4, C33 antigen, and CD82) is a highly glycosylated protein encoded by the KAI1 gene on chromosome 11p11.2. 4 This gene was found to suppress the metastatic potential of prostate cancer and melanoma cells. 4,8 While normally expressed in many different tissues, 3 KAI1 is down-regulated in several common types of human malignancies, including carcinomas of the prostate, lung, breast, bladder, and colon, and this down-regulation is associated with more aggressive behavior, such as metastasis. 1,3,5,6,9-13 As with any suppressor gene product, analysis of KAI1 in tumors is complicated by the presence of the normal protein in many admixed nonneoplastic cells. Thus, in situ techniques, such as immunohistochemistry or in situ hybridization, are employed to selectively evaluate the cell population of interest in tissue sections. Anti-KAI1 antibodies have been used successfully in immunohistochemical assays to detect expression of the protein in frozen tissue sections. 1-3,8,13,14 However, to study the biological and clinical relevance of aberrant KAI1 expression, investigators often have to rely on cohorts of fixed rather than frozen tumors. The current study was prompted by the need to establish a reliable immunohistochemical assay that would allow the detection of KAI1 in archival tissues. During the course of assay optimization, we noted a high level of expression in squamous epithelia and follicular centers in control tissues such as tonsils. We then demonstrated the utility of our assay by identifying substantial down-regulation in paraffin-embedded malignant neoplasms derived from these cell types.
Materials and Methods
Cell Lines, Tissues, and Reagents
To establish the immunohistochemical KAI1 assay, we used the following formalin-fixed and paraffin-embedded cell lines as controls: rat prostate cancer cell line AT6.1, 15 with and without the transfected KAI1 gene or transferred human chromosome 1116,17; human colon cancer cell lines SW480, SW620, RK0, and DLD-1; human primary rectal carcinoma cell lines SW837 and SW1463; and the androgen-independent human prostate cancer cell line PC-3. The AT6.1 cell line was a kind gift from Dr. J. Isaacs (Johns Hopkins University, Baltimore, MD). The human colorectal cancer cell lines were obtained from the American Type Tissue Collection (Manassas, VA). RKO was provided by Dr. Bert Vogelstein (Johns Hopkins University). The cell lines were grown in media recommended by the providers of the cell lines, in a humidified incubator with 5% CO2 at 37°C. All of the cell lines tested negative for Mycoplasma contamination at the National Institute of Environmental Health Sciences. In addition, we used several excess formalin-fixed, paraffin-embedded tissues from the Surgical Pathology Service at the University of North Carolina (UNC) Hospitals, including tonsil, colon, and breast. To study KAI1 expression in squamous and lymphoid neoplasms, we retrieved 18 archival head and neck squamous cell carcinomas (SqCCas) from the files of the UNC Surgical Pathology Service; 15 bronchogenic SqCCa from the Prince Charles Hospital in Queensland; 14 invasive and 10 intraepithelial squamous neoplasms of the cervix from the National Naval Medical Center in Bethesda, MD and from the Walter Reed Army Medical Center in Washington, DC; eight reactive lymph nodes, 13 follicular small cleaved (FSC), and 13 diffuse large cell (DLC) lymphomas from the files of the Armed Forces Institute of Pathology. The majority of these tumors had been fixed in formalin, although a subset of lymphoid lesions had been fixed in B-5. The mouse hybridoma cell line producing the anti-KAI1 antibody C33 was generously provided by Dr. Osamu Yoshie (Shionogi Institute for Medical Science, Osaka, Japan). The hybridoma supernatant was partially purified on a column. The stock antibody concentration of combined fractions 1 and 2 was 0.294 mg/ml. The immunohistochemical detection reaction utilized the Elite ABC kit from Vector (Burlingame, CA).
Western Analysis
The Western blot protocol used was based on a previously reported method. 18 Cell monolayers were washed twice in cold phosphate-buffered saline (PBS). Then, 5 ml of cold PBS were added to the monolayer, and the cells were scraped into a 15-ml conical tube (Sarstedt, Newton, NC). The cells were centrifuged at 4°C for 5 minutes at 1200 rpm. The supernatant was aspirated, and the pellet was gently vortexed to briefly disperse the cells. Cell proteins were solubilized in 150–400 μl of lysis buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 3 mM MgCl2, and 0.5% NP-40, 2 mM phenylmethylsulfonyl fluoride) for 10 minutes on ice. The lysates were then centrifuged at 14,000 rpm at 4°C for 10 minutes. The supernatant was removed, and the protein concentration was determined by the Bradford method. An equal volume of Laemmli’s sample buffer without 2-mercaptoethanol was added to the soluble protein and boiled for 5 minutes. The proteins were size fractionated by 17.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon-P membrane (Millipore), and incubated with the KAI1 C33 hybridoma supernatant at a 1:100 dilution. Bound antibody was measured by the ECL Western blotting analysis system (Amersham Life Sciences, Buckinghamshire, England).
To ensure equal loading of protein, actin control experiments were performed as above, with the following modifications. Solubilized proteins were mixed with an equal volume of Laemmli’s sample buffer supplemented with 10 mM dithiothreitol. The proteins were separated on a 10% SDS-PAGE gel, transferred to a nylon membrane, and incubated with an anti-actin monoclonal antibody (Sigma, St. Louis, MO).
Immunohistochemistry
Paraffin sections (4 μm) were placed on “Probe-on plus” slides (Fisher Scientific, Pittsburgh, PA), dewaxed in xylene, rehydrated, and heated in 0.01 M citrate buffer (pH 6) to 95–100°C in a steamer for 30 minutes. After washing in buffer, the slides were reacted with 0.2% trypsin (pH 7.8) for 3 minutes at room temperature (RT). After washing and quenching of endogenous peroxide, the sections were reacted with the C33 antibody (negative controls: nonspecific mouse IgG) overnight at RT. For cell blocks, the antibody was diluted 1:100; for tissues, the titer was 1:30. These conditions were determined after we tested several antigen retrieval methods, singly and in combination, a range of primary incubation times (1 hour to overnight), and a range of primary antibody concentrations (1:20 to 1:1000). The detection reaction followed the Vectastain recommended protocol (30-minute incubations). Several modifications to the latter, including a signal amplification step and use of streptavidin, did not improve the staining results. Diaminobenzidine was used for color development, and hematoxylin was used as the counterstain.
Results
Development of the Immunohistochemical Assay
Previous studies indicated that KAI1 was expressed at high levels in several cell types, including activated lymphocytes, and frozen section immunohistochemistry (IHC) demonstrated that the antigen was localized to the cell membrane. 1-3,19 Thus, we used archival tissues with abundant lymphoid cells, including tonsils, as positive controls to develop an immunohistochemical assay for KAI1 in paraffin sections. In addition, we utilized formalin-fixed and paraffin-embedded cell lines that had defined KAI1 levels by Western blot analysis (Table 1) ▶ . Compared to frozen tissues, paraffin sections required a higher concentration of antibody and a longer incubation time to achieve comparable staining results. The optimum titer was 1:100 for cell lines and 1:30 for tissues. Moreover, a combination of two antigen retrieval methods, heating in near-boiling citrate buffer and digestion with trypsin, was required to detect the antigen (see Materials and Methods). We determined the level of KAI1 protein expression by Western blotting in 10 cell lines (Figure 1A) ▶ . There was a good correlation between these data and the immunocytochemical staining pattern of formalin-fixed, paraffin-embedded pellets of the same cell lines (Table 1 ▶ , Figure 1 ▶ ). In KAI1 negative cell lines such as AT6.1, weak to moderate, nonspecific cytoplasmic reactivity, but no membrane staining, was observed (Figure 1B) ▶ . A culture of AT6.1 cells transfected with a KAI1 expression vector showed strong membrane reactivity and Golgi staining in the majority of the cells (Figure 1C) ▶ . Human rectal adenocarcinoma cells with a moderate to high level of KAI1 (SW837) displayed distinct membrane staining in some, but not all, cells (Figure 1D) ▶ . Among the control tissues, human tonsil consistently showed the strongest reactivity, with intense membrane staining in both the squamous epithelium and the follicular centers (Figure 2A) ▶ . Because of the strong reactivity of these two tissues, we proceeded to study the expression of KAI1 in tumors derived from them. To assess KAI1 expression in a lesion of interest, we always expected to see preserved reactivity in a subset of admixed lymphocytes and/or macrophages or in nonneoplastic squamous epithelium, when present. 2,3,17-19
Table 1.
KAI1 Expression by Cell Lines Determined by Western Blotting and Immunocytochemistry
| Cell line | Western | Immunocytochemistry |
|---|---|---|
| PC-3 | − | − |
| RKO | − | − |
| DLD-1 | − | − |
| SW620 | − | − |
| AT6.1 | − | − |
| AT6.1/KAI1 | + | + |
| AT6.1/chr.11 | + | + |
| SW480 | + | + |
| SW837 | + | + |
| SW 1463 | + | + |
Figure 1.
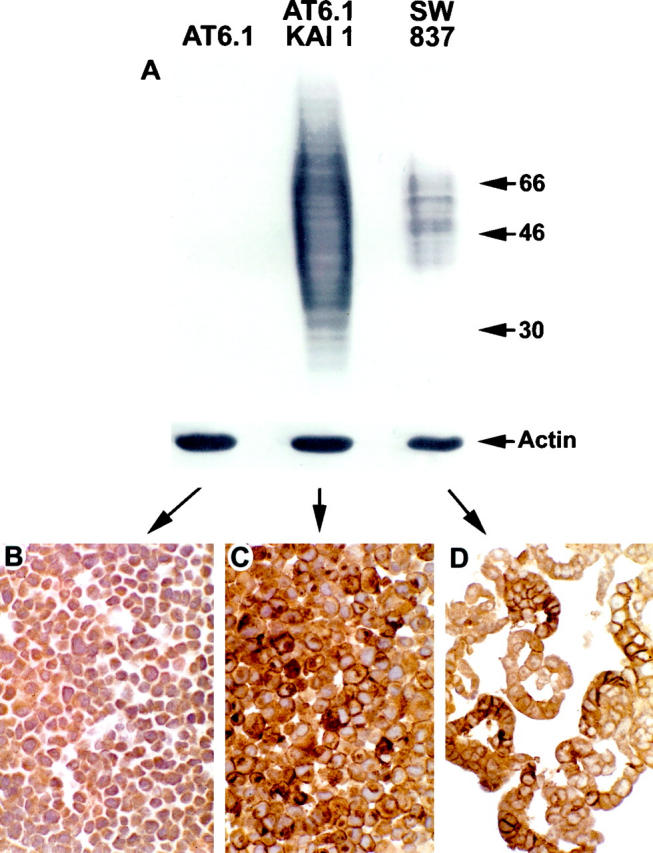
KAI1 immunocytochemical staining patterns of formalin-fixed and paraffin-embedded cell pellets: correlation with Western blotting. (A) Western blot of three cell lines. The parental line AT6.1 is negative for KAI1. A derivative cell line transfected with a KAI1 expression vector is strongly positive (center lane). Note the broadness of the band, suggestive of complex glycosylation. Cell line SW837 is also positive for KAI1, with a narrower banding pattern. Molecular mass markers (kd) are on the right. Actin control bands at the bottom of the blot. (B–D) Immunocytochemical stains of cell blocks. (B) Highly metastatic rat prostate cancer cell line AT6.1. Note moderate cytoplasmic background but lack of specific membrane staining. (C) AT6.1 cells transfected with a KAI1 expression vector. Note moderate to strong membrane and Golgi staining in the majority of the cells. (D) Human primary rectal cancer cell line SW837. Note moderate to strong membrane staining in about half of the cells. Original magnifications ×400 (B–D).
Figure 2.
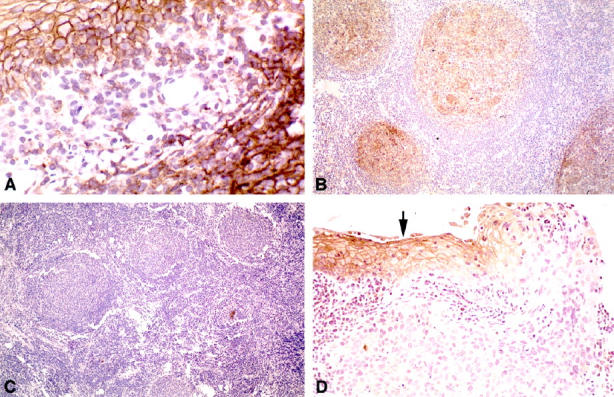
KAI1 immunohistochemical staining patterns of formalin-fixed and paraffin-embedded human tissues. A: Tonsil (positive control). Note strong cell membrane reactivity in both the squamous epithelium (upper left) and the subjacent lymphoid follicle (lower right); in the intervening stroma, some mononuclear inflammatory cells are also positive. B: KAI1-positive FSC lymphoma. The neoplastic follicles are highlighted. C: KAI1-negative FSC lymphoma. There is no membrane staining throughout the tumor. D: Squamous cell carcinoma from the head and neck area. The dysplastic epithelium adjacent to the tumor shows strong membrane reactivity (arrow). In contrast, the in situ and invasive carcinoma displays no membrane staining. Original magnifications ×400 (A), ×100 (B, C), ×200 (D).
Expression of KAI1 in Lymphoid Lesions
Initially, we observed strong KAI1 staining in follicular centers in several control tissues. We then studied lymphoid lesions from a set of tumors that had previously been characterized for abnormalities in the RB/p16 pathway (Table 2) ▶ . 20 All eight lymph nodes with reactive changes were KAI1 positive. Ten of 13 FSC (77%) and eight of 13 DLC (62%) lymphomas also showed distinct membrane reactivity (Figure 2B) ▶ . Conversely, three of 13 (23%) low-grade and five of 13 (38%) high-grade lymphomas were negative for KAI1 (Figure 2C) ▶ ; there was preserved reactivity in benign lymphocytes and macrophages.
Table 2.
KAI1 Expression in Lymphoid Tissues
| Lesion | n | KAI1 negative |
|---|---|---|
| Reactive lymph nodes | 8 | 0 (0%) |
| FSC lymphomas | 13 | 3 (23%) |
| DLC lymphomas | 13 | 5 (38%) |
Expression of KAI1 in Squamous Cell Carcinomas
We studied 47 archival tumors from three different anatomic sites (head and neck, lung, and cervix). Approximately 80% of the SqCCa showed extensive loss of KAI1 staining, with relatively normal levels in the remaining 20% (Table 3) ▶ . In 26 of 37 tumors displaying KAI1 down-regulation, no definite membrane staining was noted within the lesion. In 11 carcinomas, KAI1 expression was confined to small nests of tumor cells (less than 10% of total). In some cases, loss of KAI1 appeared to occur at the in situ stage, with preserved membrane staining in adjacent dysplastic epithelium (Figure 2D) ▶ . Morphologically normal squamous epithelium, when present, served as an internal positive control.
Table 3.
KAI1 Expression in Invasive Squamous Cell Carcinomas
| Anatomical site of tumor | n | KAI1-positive tumor cells | ||
|---|---|---|---|---|
| 0% | 1–10% | >10% | ||
| Lung | 15 | 10 | 1 | 4 |
| Head and neck | 18 | 9 | 5 | 4 |
| Cervix | 14 | 7 | 5 | 2 |
| Total | 47 | 26 | 11 | 10 |
Expression of KAI1 in Squamous Intraepithelial Lesions of the Cervix
To determine if loss of KAI1 may occur in preinvasive squamous neoplasia, as our observations in some tissues adjacent to invasive SqCCa suggested (see above), we evaluated 10 cases of cervical high-grade squamous intraepithelial lesion (HGSIL) that were not associated with invasive carcinoma. Seven lesions were diffusely and strongly positive, and two lesions were largely negative. In one case, there was strong staining superficially, but loss of reactivity in the deeper aspects. The latter staining pattern was unlikely to be artifactual, because inflammatory cells in the subjacent stroma stained positively.
Correlation of KAI1 Expression and Tumor Suppressor Gene Abnormalities
Because p53 purportedly activates KAI1 expression, 21 we studied a group of lung cancers that had previously been analyzed for p53 abnormalities. 22 In our limited number of cases (n = 15), there was no correlation between KAI1 down-regulation and aberrant p53 status, as determined by IHC, loss of heterozygosity (LOH) at 17p13, or single-strand conformational polymorphism (SSCP) abnormalities in exons 5–8 (data not shown). The downstream signaling pathways for KAI1 are unknown; therefore we were interested in the relationship between KAI1 expression and p16 and pRB, two gene products that are critical in the regulation of cell cycle progression. The same group of lung cancers, along with series of head and neck cancers and lymphomas, had previously been characterized for the expression patterns of p16 and pRB (n = 57). 20,22 We did not find an association between the absence of p16 or pRB and down-regulation of KAI1 (data not shown).
Discussion
KAI1 is expressed in many cell types, including various epithelia. 3,16,18 It encodes a glycoprotein that belongs to the family of TM4SF proteins. These cell membrane proteins are thought to play a role in cell-cell and cell-matrix adhesion, but their functional significance has not been elucidated in detail. 1,23 Dong et al first described the capacity of KAI1 to suppress the metastatic potential of rat prostate cancer cells. 16 Subsequently, down-regulation of this gene has been associated with the metastatic phenotype of several common human malignancies, including carcinomas of the lung, breast, prostate, pancreas, bladder, and colon. 3,6,9,13,14,24,25 Moreover, the gene has recently been implicated in the inhibition of cellular motility and invasion. 4,6 Thus, there is increasing evidence that down-regulation of KAI1 may play an important role in the progression of some malignant neoplasms.
Several methodological approaches have been described to determine the level of KAI1 expression in human tissues, including RT-PCR and Northern and Western analysis. 9,10,16 Interpretation of such analyses is rendered difficult by the invariable presence of nonneoplastic cells that may express KAI1 at high levels. To circumvent this problem, other investigators have employed in situ hybridization 5,25 or immunohistochemistry. 2,3,13,26 The obvious advantage of these techniques is that they allow the evaluation of KAI1 expression specifically in the cell population(s) of interest.
The anti-KAI1 antibody we used, C33, is among several antibodies that have been used successfully for analysis of KAI1 by frozen section immunohistochemistry. 18,19 Strong membrane staining in benign prostatic epithelial cells and down-regulation in a large proportion of prostatic adenocarcinomas were described by two groups. 1,26 Huang et al found a high frequency of reduced KAI1 expression in carcinomas of the lung, breast, colon, and stomach. 3 However, several laboratories reported difficulties with the immunohistochemical analysis of archival tissues. 1,3 We are aware of very few previous studies that analyzed KAI1 levels in paraffin-embedded tumors. In one series, there was loss of KAI1 expression in some archival prostatic adenomacarcinomas, but about half of the cases were nonreactive, and the tissues were significantly less reactive compared to frozen sections. 17 In a small series of 14 paraffin-embedded colonic adenocarcinomas, down-regulation of KAI1 was found in a subset of them. 6 Friess et al found a correlation of KAI1 expression by IHC, ISH, and Northern blotting in pancreatic carcinomas. 25
An important goal of our study was to establish a reliable immunohistochemical assay for KAI1 in paraffin-embedded tissues, which would enable us to correlate KAI1 levels with the metastatic phenotype and other biological and clinical features of archival tumors. We are confident that our assay has adequate specificity, because cell lines known to lack KAI1 did not show the expected staining pattern (Table 1 ▶ , Figure 1B ▶ ). When KAI1 was expressed, it was associated with a specific membrane staining pattern (Figure 1C,D) ▶ . In cell lines transfected with KAI1 or transferred with chromosome 11, immunocytochemistry showed the presence of expressed protein in a subset of the cells (Figure 1C) ▶ . Interestingly, in KAI1-positive cell lines, not all cells showed membrane reactivity, and furthermore, the staining intensity was variable (Figure 1D) ▶ , suggesting that expression of the glycoprotein may be subject to yet unknown regulatory mechanisms. Overall, there was excellent qualitative agreement between protein expression determined by Western analysis and by immunocytochemistry (Table 1) ▶ . Like other investigators, we noted that fixation and processing greatly reduced KAI1 antigenicity, which could be partially restored with rather stringent antigen retrieval methods. The combination of techniques described here has not been reported previously. An adverse side effect of this treatment is the partial destruction of some tissue sections, which may require restaining or use of alternative paraffin blocks.
Although we could convincingly demonstrate the marked down-regulation of KAI1 in lymphoid and squamous neoplasms (see below), we cannot rule out the possibility that low levels of this glycoprotein still exist in the tumor cells. The mechanism of KAI1 down-regulation is not known. The 5′ promoter region of the gene contains a CpG island, 27 raising the possibility of gene silencing by promoter methylation. Mutations in the gene appear to be uncommon, 1 and it is unclear how they might affect the immunohistochemical reactivity of the protein. The KAI1 gene is located on chromosome 11p11.2. 8,16 Although allelic loss at this site may be observed in prostate cancer, 8,16 there is no mutation of the remaining allele. Whatever the mechanism, IHC is a suitable modality for demonstrating the relative or absolute lack of KAI1 in neoplastic tissues. One additional limitation of this technique, however, is that it is unlikely to detect aberrant KAI1 glycosylation. The latter has been observed in several types of tumors, although the functional significance of this type of posttranslational modification remains unclear. 9,18,28 However, it was previously demonstrated that highly branched asparagine- (N-) linked glycosylation of glycoproteins was correlated with the malignant phenotype in rodent and human models. 29
During the establishment of the immunohistochemical KAI1 assay, we noted that strong membrane staining was present in germinal centers. This staining pattern was previously described by others 17 and is consistent with earlier reports of high KAI1 levels in activated lymphocytes. 18,19,30 To test the hypothesis that KAI1 down-regulation may occur in lymphoid neoplasia, we stained eight reactive lymph nodes as well as 13 FSC (low-grade) and 13 DLC (high-grade) lymphomas. As expected, all benign lymph nodes were KAI1 positive. In contrast, about one-quarter of FSC and over one third of DLC lymphomas were KAI1 negative, with preserved staining in admixed nonneoplastic cells (Table 2 ▶ , Figure 2C ▶ ). Whether loss of KAI1 in lymphomas is associated with a more aggressive clinical phenotype remains to be determined.
We also found strong membrane staining in squamous epithelia, consistent with previous reports. 2 We observed extensive down-regulation in 37 of 47 squamous cell carcinomas from the lung, head and neck, and cervix, and in the majority of down-regulated cases there was no membrane staining in the invasive portion of the tumor (Figure 2D) ▶ . In an IHC study on skin frozen sections, Okochi et al reported marked down-regulation of KAI1 in basal cell carcinomas. 2 Because the latter rarely metastasize, it was suggested that KAI1 loss may be important for stromal invasion in this tumor type. Our data support the hypothesis that down-regulation of KAI1 may be an important event in the progression of squamous cell carcinomas from the in situ to the invasive stage. There is previous immunohistochemical evidence that KAI1 may be reduced or absent in up to two-thirds of squamous cell carcinomas of the lung, 14 which correlated well with an earlier study using the RT-PCR technique. 11 Those data are in agreement with our observation that KAI1 was down-regulated in 11 of 15 (73%) bronchogenic SqCCa. Finally, White et al reported that 10 of 11 non-small-cell lung cancer cell lines were KAI1 negative. 28
There are few data on KAI1 expression in preinvasive neoplasia. Okochi et al did not find evidence of loss of KAI1 expression in cutaneous squamous cell carcinomas in situ. 2 We observed KAI1 down-regulation in some precursor lesions associated with invasive squamous cell carcinomas. We studied 10 additional high-grade dysplasias from the cervix, which showed several staining patterns. Seven were diffusely positive, two were largely negative, and one case displayed loss of KAI1 staining at the base of the lesion. Therefore, KAI1 down-regulation can occur in preinvasive squamous neoplasms, but it is unknown whether it increases the likelihood of stromal invasion. Larger studies with clinical follow-up data are required to address this issue.
It was recently suggested that expression of KAI1 is controlled, in part, by p53. 21 Among the lesions we studied, only the small number of bronchogenic SqCCa had previously been tested for p53 abnormalities. No correlation between the latter and KAI1 expression was found, but the statistical power was limited because of small numbers. As far as we know, the relationship between KAI1 status and cell cycle regulation has not been studied. We had previously determined the presence or absence of two important cell cycle regulatory proteins, p16 and pRB, in 57 of the 73 tumors included in this study. We found no correlation between KAI1 and p16 or pRB expression. This observation is of some interest because a significant subset of tumors show p16 inactivation by promoter hypermethylation. 31 KAI1 contains CpG islands in the 5′ portion of the gene, 27 and it is tempting to speculate that methylation of the promoter may be a mode of down-regulation of this gene as well.
In conclusion, we describe a method for detecting aberrant KAI1 expression in archival tissues by immunohistochemistry. Although markedly reduced KAI1 reactivity is considered abnormal, a normal staining pattern does not necessarily imply a normally functioning gene product. The technique may not be suitable for detecting abnormal glycosylation patterns that may be functionally important in some tumors. 9,18 Loss of KAI1 occurred in a significant subset of lymphomas. The frequent down-regulation of the gene in squamous cell carcinomas, and its loss in some preinvasive squamous neoplasms suggest an important role of KAI1 in the progression to the invasive phenotype. It is hoped that the technique described here may facilitate studies on archival tissues that further define the role of KAI1 in the development of human tumors and their metastases.
Acknowledgments
We are indebted to Dr. Osamu Yoshie, Shionogi Institute for Medical Science in Osaka, for providing us with the C33 antibody. We thank Linda Summerville for expert secretarial assistance. We also thank Maribel Bruno and Pattie Lamb for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Joseph Geradts, Nuffield Department of Pathology and Bacteriology, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DU, England. E-mail: joseph.geradts@ndp.ox.ac.uk.
D.P.L. was supported by a grant from the National Institutes of Health (MO1 RR00046-38S2).
References
- 1.Dong J-T, Suzuki H, Pin SS, Bova S, Schalken JA, Isaacs WB, Barrett JC, Isaacs JT: Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res 1996, 56:4387-4390 [PubMed] [Google Scholar]
- 2.Okochi H, Kato M, Nashiro K, Yoshie O, Miyazono K, Furue M: Expression of tetra-spans transmembrane family (CD9, CD37, CD53, CD63, CD81 and CD82) in normal and neoplastic human keratinocytes: an association of CD9 with α3β1 integrin. Br J Dermatol 1997, 137:856-863 [PubMed] [Google Scholar]
- 3.Huang C-L, Taki T, Adachi M, Yagita M, Sawada S, Takabayashi A, Inufusa H, Yoshie O, Miyake M: MRP-1/CD9 and KAI1/CD82 expression in normal and various cancer tissues. Int J Oncol 1997, 11:1045–1051. [DOI] [PubMed]
- 4.Takaoka A, Hinoda Y, Sato S, Itoh F, Adachi M, Hareyama M, Imai K: Reduced invasive and metastatic potentials of KAI1-transfected melanoma cells. Jpn J Cancer Res 1998, 89:397-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, Yang J-L, Markovic B, Jackson P, Yardley G, Barrett J, Russell PJ: Loss of KAI1 messenger RNA expression in both high-grade and invasive human bladder cancers. Clin Cancer Res 1997, 3:1045-1049 [PubMed] [Google Scholar]
- 6.Takaoka A, Hinoda Y, Satoh S, Adachi Y, Itoh F, Adachi M, Imai K: Suppression of invasive properties of colon cancer cells by a metastasis suppressor KAI1 gene. Oncogene 1998, 16:1443-1453 [DOI] [PubMed] [Google Scholar]
- 7.Maecker HT, Todd SC, Levy S: The tetraspanin superfamily: molecular facilitators. FASEB J 1997, 11:428-442 [PubMed] [Google Scholar]
- 8.Kawana Y, Komiya A, Ueda T, Nihei N, Kuramochi H, Suzuki H, Yatani R, Imai T, Dong J-T, Imai T, Yoshie O, Barrett JC, Isaacs JT, Shimazaki J, Ito H, Ichikawa T: Location of KAI1 on the short arm of human chromosome 11 and frequency of allelic loss in advanced human prostate cancer. Prostate 1997, 32:205-213 [DOI] [PubMed] [Google Scholar]
- 9.Adachi M, Taki T, Ieki Y, Huang C-I, Higashiyama M, Miyake M: Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res 1996, 56:1751-1755 [PubMed] [Google Scholar]
- 10.Yang X, Welch DR, Phillips KK, Weissman BE, Wei LL: KAI1, a putative marker for metastatic potential in human breast cancer. Cancer Lett 1997, 119:149-155 [DOI] [PubMed] [Google Scholar]
- 11.Adachi M, Taki T, Konishi T, Huang C-I, Higashiyama M, Miyake M: Novel staging protocol for non-small-cell lung cancers according to MRP-1/CD9 and KAI1/CD82 gene expression. J Clin Oncol 1998, 16:1397-1406 [DOI] [PubMed] [Google Scholar]
- 12.Huang C-I, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M: Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol 1998, 153:973-983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sho M, Adachi M, Taki T, Hashida H, Konishi T, Huang C-I, Ikeda N, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M: Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer (Pred Oncol) 1998, 79:509-516 [DOI] [PubMed] [Google Scholar]
- 14.Higashiyama M, Kodama K, Yokouchi H, Takami K, Adachi M, Taki T, Ishiguro S, Nakamori S, Yoshie O, Miyake M: KAI1/CD82 expression in nonsmall cell lung carcinoma is a novel, favourable prognostic factor. Cancer 1998, 83:466-474 [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa T, Ichikawa Y, Dong J-T, Hawkins AL, Griffin CA, Isaacs WB, Oshimura M, Barrett JC, Isaacs JT: Localization of metastasis suppressor gene(s) for prostatic cancer to the short arm of human chromosome 11. Cancer Res 1992, 52:3486-3490 [PubMed] [Google Scholar]
- 16.Dong J-T, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC: KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995, 268:884-886 [DOI] [PubMed] [Google Scholar]
- 17.Ward JM, Konishi N, Ohshima M, Lamb PW, Jorcyk CL, Barrett JC: Expression of KAI1 in paraffin-embedded normal, hyperplastic and neoplastic prostate and prostate carcinoma cell lines. Pathol Int 1998, 48:87-92 [DOI] [PubMed] [Google Scholar]
- 18.Fukudome K, Furuse M, Imai T, Nishimura M, Takagi S, Hinuma Y, Yoshie O: Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV-1)-induced syncytium formation: altered glycosylation of C33 antigen in HTLV-1-positive T cells. J Virol 1992, 66:1394-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai T, Fukudome K, Takagi S, Nagira M, Furuse M, Fukuhara N, Nishimura M, Hinuma Y, Yoshie O: C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J Immunol 1992, 149:2879-2886 [PubMed] [Google Scholar]
- 20.Geradts J, Andriko JW, Abbondanzo SL: Loss of tumor suppressor gene expression in high-grade but not low-grade non-Hodgkin’s lymphomas. Am J Clin Pathol 1998, 109:669-674 [DOI] [PubMed] [Google Scholar]
- 21.Mashimo T, Watabe M, Hirota S, Hosobe S, Miura K, Tegtmeyer PJ, Rinker-Shaeffer CW, Watabe K: The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc Natl Acad Sci USA 1998, 95:11307-11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geradts J, Fong KM, Zimmerman PV, Maynard R, Minna JD: Correlation of abnormalities of RB, p16, and p53 expression with 3p loss of heterozygosity, other genetic abnormalities and clinical features in 103 primary non-small cell lung cancers. Clin Cancer Res 1999, 5:791-800 [PubMed] [Google Scholar]
- 23.Hemler ME, Mannion BA, Berditchevski F: Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta 1996, 1287:67-71 [DOI] [PubMed] [Google Scholar]
- 24.Dong J-T, Rinker-Schaeffer CW, Ichikawa T, Barrett JC, Isaacs JT: Prostate cancer—biology of metastasis and its clinical implications. World J Urol 1996, 14:182-189 [DOI] [PubMed] [Google Scholar]
- 25.Friess H, Guo X-Z, Berberat P, Graver HU, Zimmermann A, Korc M, Büchler MW: Reduced KAI1 expression in pancreatic cancer is associated with lymph node and distant metastases. Int J Cancer (Pred Oncol) 1998, 79:349-355 [DOI] [PubMed] [Google Scholar]
- 26.Ueda T, Ichikawa T, Tamaru J-i, Mikata A, Akakura K, Akimoto S, Imai T, Yoshie O, Shiraishi T, Yatani R, Ito H, Shimazaki J: Expression of the KAI1 protein in benign prostatic hyperplasia and prostate cancer. Am J Pathol 1996, 149:1435-1440 [PMC free article] [PubMed] [Google Scholar]
- 27.Dong J-T, Isaacs WB, Barrett JC, Isaacs JT: Genomic organization of the human KAI1 metastasis-suppressor gene. Genomics 1997, 41:25-32 [DOI] [PubMed] [Google Scholar]
- 28.White A, Lamb PW, Barrett JC: Frequent downregulation of the KAI1 (CD82) metastasis suppressor protein in human cancer cell lines. Oncogene 1998, 16:3143-3149 [DOI] [PubMed] [Google Scholar]
- 29.Asada M, Furukawa K, Segawa K, Endo T, Kobata A: Increased expression of highly branched N-glycans at cell surface is correlated with the malignant phenotypes of mouse tumor cells. Cancer Res 1997, 57:1073-1080 [PubMed] [Google Scholar]
- 30.Lebel-Binay S, Gil ML, Lagaudriere C, Miloux B, Marchiol-Fournigault C, Quillet-Mary A, Lopez M, Fradelizi D, Conjeaud H: Further characterization of CD82/IA4 antigen (type III surface protein): an activation/differentiation marker of mononuclear cells. Cell Immunol 1994, 154:468-483 [DOI] [PubMed] [Google Scholar]
- 31.Herman JG, Merlo A, Mao L, Lapidus RG, Issa J-PJ, Davidson NE, Sidransky D, Baylin SB: Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 1995, 55:4525-4530 [PubMed] [Google Scholar]


