Abstract
Peutz-Jeghers syndrome (PJS) is an autosomal-dominant disorder characterized by hamartomatous polyps in the gastrointestinal tract and by pigmented macules of the lips, buccal mucosa, and digits. Less appreciated is the fact that PJS also predisposes patients to an increased risk of gastrointestinal cancer, and pancreatic cancer has been reported in many PJS patients. It was recently shown that germline mutations of the STK11/LKB1 gene are responsible for PJS. We investigated the role of STK11/LKB1 in the development of pancreatic and biliary cancer in patients with and without the PJS. In a PJS patient having a germline splice site mutation in the STK11/LKB1 gene, sequencing analysis of an intestinal polyp and pancreatic cancer from this patient revealed loss of the wild-type allele of the STK11/LKB1 gene in the cancer. Inactivation of STK11/LKB1, by homozygous deletions or somatic sequence mutations coupled with loss of heterozygosity, was also demonstrated in 4–6% of 127 sporadic pancreatic and biliary adenocarcinomas. Our results demonstrate that germline and somatic genetic alterations of the STK11/LKB1 gene may play a causal role in carcinogenesis and that the same gene contributes to the development of both sporadic and familial forms of cancer.
Peutz-Jeghers syndrome (PJS) is an autosomal-dominant disease characterized by nonneoplastic hamartomas of the gastrointestinal tract and by mucocutaneous melanin macules. 1 PJS has also been associated with an increased risk of developing cancers. 2-5 Gastrointestinal, gynecologic, lung, breast, thyroid, basal cell, prostate, and pancreatic cancers have been reported in PJS patients. 2-5 It was recently shown that germline mutations of the STK11/LKB1 gene on distal chromosome 19p, which encoded a novel serine/threonine kinase, were responsible for PJS. 6-9 The increased risk for cancer among PJS patients would suggest that STK11/LKB1 is a candidate tumor-suppressor gene, 10 but the role of STK11/LKB1 gene inactivation in neoplasia has not been conclusively demonstrated. 11-14
Pancreatic cancer is an attractive neoplasm to examine for inactivation of STK11/LKB1, because it is one of the more common neoplasms to develop in PJS patients. Of the 53 PJS patients reported in four independent studies, six (11%) were diagnosed with pancreatic adenocarcinoma. 2-5 The demonstration that the STK11/LKB1 is inactivated in the pancreatic cancer of a PJS patient and in sporadic pancreatic cancers would strongly support a causal link between these mutations and the development of pancreatic cancers and would help establish the tumor-suppressor role of STK11/LKB1 in neoplasia.
Materials and Methods
PJS Patient and DNA Analysis
Patient PJS1 was an affected family member of a well-followed kindred with PJS. 15 She had biopsy-proven Peutz-Jeghers polyps of the duodenum (Figure 1A) ▶ and was diagnosed with adenocarcinoma at the age of 35 on biopsy of a peripancreatic lymph node, thought originally and on review to be most consistent with a pancreatic origin on the basis of histological features (Figure 1B) ▶ . DNA was prepared from microdissected histological sections of her surgically biopsied cancer and Peutz-Jeghers polyps. Microdissected samples were incubated overnight at 37°C in 0.04% proteinase K, 10 mmol/L Tris-HCl (pH 8.0), 1 mmol/L EDTA, and 1% Tween-20. Proteinase K was inactivated at 95°C for 8 minutes before DNA analysis.
Figure 1.
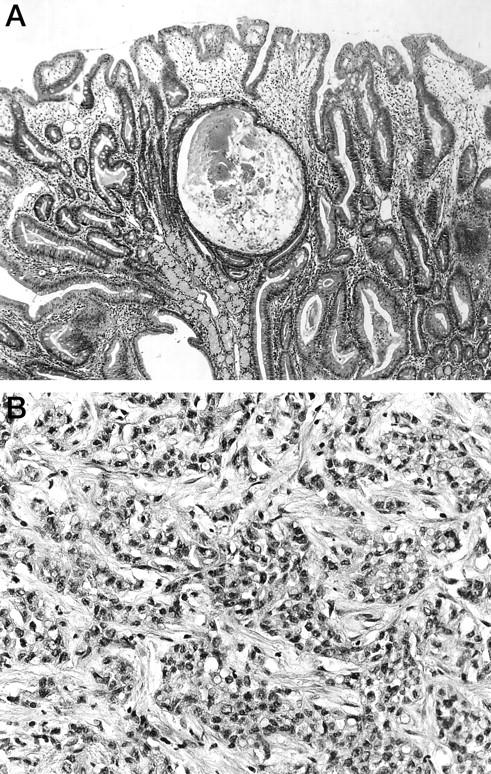
Histological findings of patient PJS1. A: The Peutz-Jeghers polyp of the duodenum (original magnification, ×50). B: Poorly differentiated adenocarcinoma of the pancreas (original magnification, ×250). Hematoxylin & eosin staining.
Tissue Samples and Cell Lines
Cancers of the pancreas and distal common bile duct resected at The Johns Hopkins Hospital between 1992 and 1997 were xenografted as described. 16 In addition, at the time of the surgery, resected normal duodenal mucosa was frozen and stored at −80°C. The pancreatic cell lines Su86.86, CFPAC1, AsPC1, Capan1, Capan2, Panc1, MiaPaCa2, BxPc3, and Hs766T were purchased from American Type Culture Collection (Manassas, VA) and COLO357 from European Collection of Animal Cell Cultures (Salisbury, Wiltshire, UK). Pancreatic cell line PL45 was established in our laboratory. 16
Homozygous Deletion Analysis
Genomic DNA samples (40 ng per sample) were screened for homozygous deletions using PCR analysis as previously described. 16,17 The primers used to amplify exon 1, 4/5, and 9 of STK11/LKB1 were as reported previously. 8 Duplex PCR analyses were performed with pairs of internal control primers and STK11/LKB1-specific primers. Amplification of integrin-β−4 or MKK4 was used as a positive internal control. Primers are as listed in Table 1 ▶ .
Table 1.
Sequences of Primers Used for Amplification and Sequencing of STK11/LKB1
| Primer | Sequence |
|---|---|
| STK11-E1-F2 | 5′-gaa ggg ttt ttc cct tcc-3′ |
| STK11-E1-R2 | 5′-cct tca cct tgc cgt aag ag-3′ |
| STK11-E3-F1 | 5′-tcc aga gcc cct ttt ctg-3′ |
| STK11-E3-R1 | 5′-aat gaa tat cag gac aag cag-3′ |
| STK11-E3-F3 | 5′-gta tat ggt gat gga gta ctg |
| STK11-E3-SEQ-F2 | 5′-ccg tgc tcc ctg ggc ctg tga gtg-3′ |
| STK11-E3-SEQ-F3 | 5′-cgt gtg tgg cat gca gga aat gct g-3′ |
| STK11-E3-SEQ-R | 5′-tgt ggc ctc acg gaa agg agc ctg-3′ |
| STK11-E9-F2 | 5′-tcc cag aag agg agg cca g-3′ |
| STK11-E9-R2 | 5′-cgg tca cca tga ctg act ag-3′ |
| STK11-E1-SEQ-F | 5′-gga agt cgg aac aca agg aag gac-3′ |
| STK11-E1-SEQ-F2 | 5′-cag ccg cgc cgc aag cgg gcc aag-3′ |
| STK11-E2-SEQ-R | 5′-gcc tcg agg cag gcg gac cca ggg-3′ |
| STK11-E3-SEQ-F2 | 5′-ccg tgc tcc ctg ggc ctg tga gtg-3′ |
| STK11-E3-SEQ-R | 5′-tgt ggc ctc acg gaa agg agc ctg-3′ |
| STK11-E4/5-SEQ-F2 | 5′-agc tgc aaa ggg gac ccc tgt gag-3′ |
| STK11-E5-SEQ-F | 5′-tag gca cgt gct agg ggg ggc cct g-3′ |
| STK11-E6-SEQ-R | 5′-ctg aca ccc cca acc cta cat ttc-3′ |
| STK11-E7-SEQ-F2 | 5′-cgg gga cgg ttg gtg ggg tct cag-3′ |
| STK11-E8-SEQ-F2 | 5′-ccg ccc tgg tgc cag cct gac agg-3′ |
| STK11-E9-SEQ-F2 | 5′-ggg gcc agc cag gtc cct gtg gct-3′ |
| INTB4-A-F | 5′-gtg tcc gtg tgg ata agg ac-3′ |
| INTB4-A-R | 5′-tcc atg acc acg atg ctc tc-3′ |
| MKK4-E-F | 5′-tgg gga aaa ttg gct tta act ac-3′ |
| MKK4-E-R | 5′-cga gac cat tat gac cta ttg tg-3′ |
Loss of Heterozygosity and Sequence Analyses
Loss of heterozygosity (LOH) was determined using three polymorphic markers, D19S886, D19S565, and D19S216 (Research Genetics, Huntsville, AL). LOH was considered to be conclusive only when analysis of the neoplastic DNA showed the complete loss of one of the two alleles present in the patient’s corresponding normal DNA. When a normal DNA sample was unavailable, LOH status was presumptively shown by the unambiguous presence of only a single allele size at all three polymorphic markers evaluated. All samples which displayed conclusive or presumptive LOH were subject to sequencing. Each exon was amplified by PCR from genomic DNA, treated with exonuclease I and shrimp alkaline phosphatase (USB, Cleveland, OH), and subjected to cycle-sequencing (ThermoSequenase, Amersham, Arlington Heights, IL). The majority of the PCR primers have been reported previously. 8 Additional primers are listed in Table 1 ▶ .
Results
Germline Mutation of STK11/LKB1 and Tumorigenesis
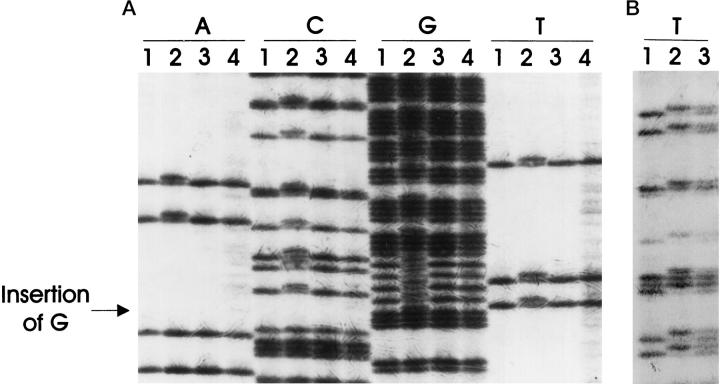
To determine the genetic basis for the increased risk of cancer among PJS patients, we examined the status of the STK11/LKB1 gene in cancer tissues obtained from a patient diagnosed with PJS. In patient PJS1, the known germline mutation of this family at the splice donor site of intron 3 of STK11/LKB1 15 was demonstrated in nonneoplastic tissue (Table 3) ▶ (Figure 2A) ▶ . DNA from this patient’s microdissected adenocarcinoma and epithelium of a Peutz-Jeghers intestinal polyp were then sequenced and the second allele of STK11/LKB1 was lost (>80% decrease in allele intensity by densitometry) in the pancreatic cancer, but not in the intestinal polyp (Figure 2B) ▶ . Due to the limited amount of archival material, only limited sequencing was performed. Because LOH is not the only mechanism of gene inactivation, it is possible that the second allele of STK11-LKB1 in the polyp could be inactivated by methylation, small deletions, or point mutation outside of intron 3. The germline mutation is predicted to affect splicing of the STK11/LKB1 transcript.
Table 3.
Mutations of the STK11/LKB1 Gene Identified in Pancreatic and Biliary Cancers
| Tumor type | Sample | Allele loss | Position of gene alteration* | Gene alteration† | Predicted product | Origin of gene alteration |
|---|---|---|---|---|---|---|
| Pancreatic | PJS1 | LOH | Nucleotide +2 Intron 3 | CGG gtg to CGG ggtg | Insertion, altered splicing | Germline |
| Pancreatic | PX30 | LOH | Exons 1 to 9 | Homozygous deletion | Absence | Somatic |
| Pancreatic | PX68 | LOH | Codon 36 Exon 1 | TAC CAG to TAA CAG | Tyrosine to Stop | Somatic |
| Pancreatic | PX104 | LOH | Codon 217 Exon 5 | CCG GCT T to CGG CTT | Deletion, frameshift | Somatic |
| Pancreatic | PX289 | LOH | Codon 312 Exon 8 | AAA CAT C to AAC ATC | Deletion, frameshift | Somatic |
| Biliary | PX115 | LOH | Exon 1 | Homozygous deletion | Absence | Somatic |
*Codons, nucleotides, and exons are numbered according to GDB entries AF032984, AF032985, and AF032986.
†The underlined nucleotides are either deleted or inserted. Exonic sequences are in capital letters and intronic sequences are in lower case. The spaces between trinucleotides denote codon structure.
Figure 2.
Germline mutation of STK11/LKB1 in patient PJS1 and loss of the wild-type allele of STK11/LKB1 in the pancreatic cancer of the same patient. A: The germline mutation of STK11/LKB1 in the family of patient PJS1 was confirmed (lanes 2) ( Ref. 15 ). CFPAC1 (lanes 1) and two pancreatic cancer xenografts (lanes 3 and 4) exhibited normal sequences. The samples were sequenced with a forward primer, STK11-E3-SEQ-F2. The arrow indicates the site of the nucleotide insertion mutation. B: DNA from normal tissue of a non-Peutz-Jeghers patient (lane 1), cancer of patient PJS1 (lane 2), and polyps of patient PJS1 (lane 3) were PCR-amplified and sequenced with a reverse primer, STK11-E3-SEQ-R. The cancer, but not the polyp, of patient PJS1 showed loss of the remaining allele of STK11/LKB1.
Somatic Inactivation of STK11/LKB1 in Pancreatic and Biliary Cancers
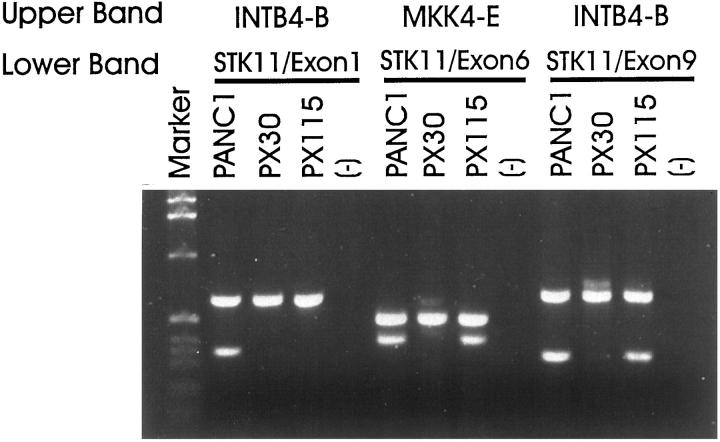
To further validate STK11/LKB1 as a tumor-suppressor gene, we evaluated the role of somatic mutation in STK11/LKB1 in sporadic pancreatic cancer. Using primers specific for exon 1, 4/5, and 9 of STK11/LKB1, we screened for homozygous deletions among a panel of 100 xenografts of primary pancreatic ductal adenocarcinomas, 16 xenografts of primary distal common bile duct adenocarcinomas, 19 xenografts of other primary carcinomas of the periampullary region (predominantly duodenal and ampullary cancer), and 11 pancreatic cancer cell lines (Table 2) ▶ . One pancreatic (PX30) and one distal common bile duct (PX115) adenocarcinoma exhibited homozygous deletions of STK11/LKB1 (Figure 3) ▶ . The entire genomic sequence of STK11/LKB1 was deleted from PX30, whereas only exon 1 of STK11/LKB1 was deleted in PX115. Both homozygous deletions were confirmed by duplex PCR (Figure 3) ▶ and verified in parallel xenografts derived from the same primary tumor samples (data not shown). In PX115, adequate DNA was available for Southern blot analysis, which confirmed the absence of STK11/LKB1 sequences (data not shown). The homozygously deleted regions in PX30 and PX115 did not extend to the closest available neighboring markers, D19S886 and D19S565. These markers were originally used to define the distal and proximal boundaries in maps of the PJS gene localization. 6,7
Table 2.
Sporadic Neoplasms Analyzed for STK11/LKB1 Mutations
| Tumor type | Total number of samples | Number of samples analyzed in | Number of mutated samples | ||
|---|---|---|---|---|---|
| Homozygous deletion screening | LOH study | Sequencing | |||
| Pancreatic adenocarcinoma | 100 | 100 | 92 | 30 | 4 |
| Biliary adenocarcinoma | 16 | 16 | 0 | 0 | 1 |
| Pancreatic cell lines | 11 | 11 | 11 | 9 | 0 |
| Other carcinomas* | 19 | 19 | 0 | 0 | 0 |
*Primary carcinomas of the periampullary region, predominantly duodenal and ampullary cancers.
Figure 3.
Duplex PCR analysis of homozygous deletions in pancreatic and biliary cancers. Detection of homozygous deletions in the genomic DNAs of pancreatic cancer xenograft PX30 and biliary cancer xenograft PX115 by duplex PCR using pairs of internal control primers (INTB4-B or MKK4-E) and STK11/LKB1-specific primers. The entire coding region of STK11/LKB1 was deleted in PX30. Only exon 1 of STK11/LKB1 was deleted in PX115.
LOH frequency at the STK11/LKB1 locus in pancreatic cancer was determined with the highly polymorphic markers D19S886, D19S565, and D19S216 (heterozygosity index = 0.61, 0.81, and 0.76, respectively). Conclusive LOH was found in 22 of the 69 pancreatic cancers for which normal DNA was available (32%) and presumptive LOH was inferred in 8 of the 23 pancreatic cancers (35%). Presumptive LOH of 19p at the STK11/LKB1 locus was seen in 9 of the 11 pancreatic cancer cell lines (82%). In addition, four cancers harbored LOH breakpoints between D19S886 and D19S565. The localization of these breakpoints to the STK11/LKB1 locus further suggests that STK11/LKB1 is the target of the allelic loss observed. All coding sequences and splice junctions of the STK11/LKB1 gene amplified from the genomic DNA of the 39 selected pancreatic xenografts and cancer cell lines exhibiting conclusive or presumptive LOH were sequenced (Table 2) ▶ . One non-sense and two frameshift mutations were detected (3 of 103 (3%) studied for LOH) (Figure 4, A and B ▶ , and Table 3 ▶ ) and confirmed in independent PCR products amplified from the samples. One mutation was in exon 1 and one in exon 5, and both of these were within the catalytic kinase domain of STK11/LKB1 (codons 37–314). 8 The third mutation was in exon 8, and it potentially would affect the function of the regulatory domain of STK11/LKB1 that comprises the 119 residues at the carboxyl-terminus. 8 Two of the three xenografts in which somatic mutations were identified also had primary tumor and/or parallel xenografts available for study, and both mutations were confirmed in these samples (Figure 4, A and B) ▶ . Two somatic sequence alterations, which were judged unlikely to produce a functional impairment, were also observed (A to G at −6 in intron 5 and C to T at −51 in intron 5). In addition, five intronic polymorphisms were noted in intron 1 (+36, G to T), intron 2 (+24, G to T), intron 3 (+16, GGG insertion, and +47, GGG insertion), and intron 7 (+7, G to C); three of these have been reported in other studies. 13
Figure 4.
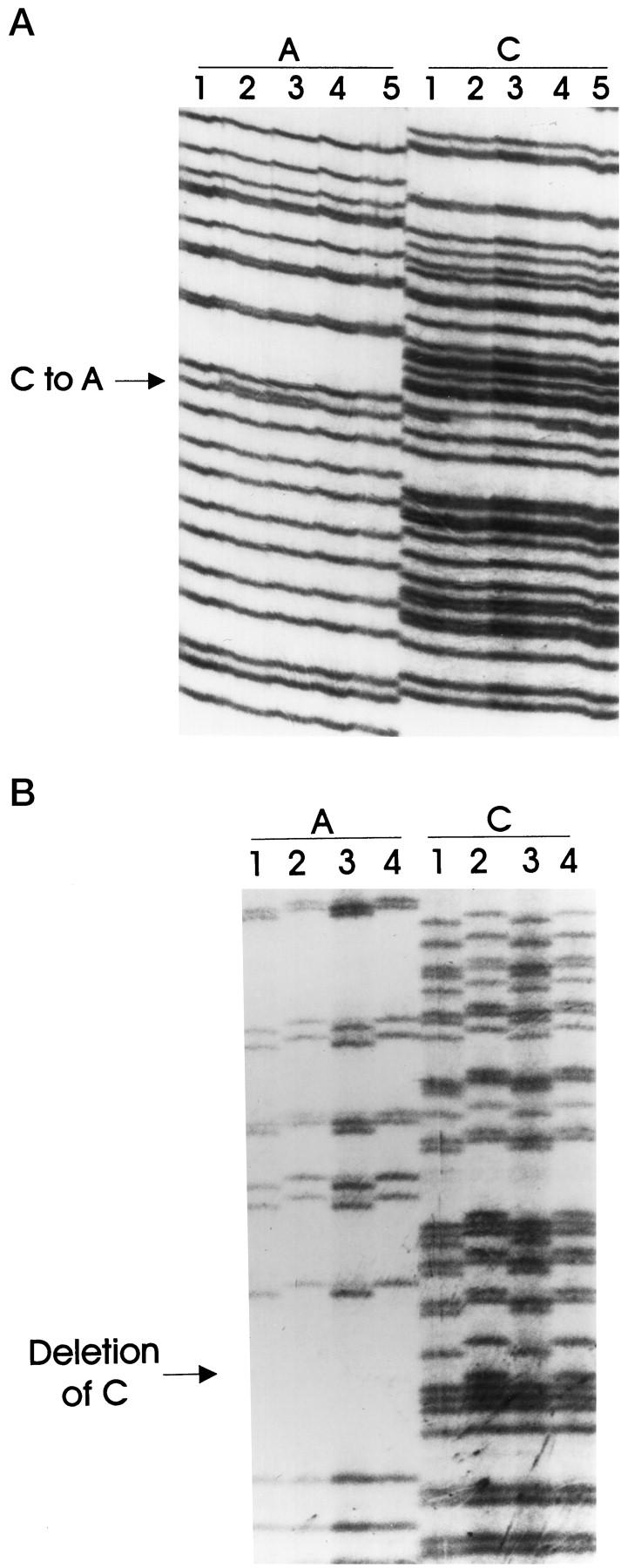
Somatic mutations in the exonic sequences of STK11/LKB1. A: PX68 (lanes 2) and PX68-1A (lanes 3) are two parallel xenografts of the same pancreatic cancer, and both exhibited the same somatic nucleotide substitution that created a stop codon (a non-sense mutation). The mutation was not detected in the normal tissue of the patient (PN68, lanes 1 and 4). Lanes 5 belong to another pancreatic tumor xenograft, which did not harbor a somatic mutation in STK11/LKB1. The arrow points to the site of the mutation. B: Xenograft tumor PX104 (lanes 1) and its corresponding primary cancer, PC104 (lanes 3), harbored the same nucleotide deletion, which was not detected in the normal tissue of the patient (PN104, lanes 2) or in another pancreatic xenograft (lanes 4). The arrow indicates the site of the mutation.
Although deletion mutations and LOH analysis could not themselves support the conclusion that the STK11/LKB1 gene was the mutation target, subsequent findings of nonsense and frameshift mutations within the functional domains of the gene strongly indicate the STK11/LKB1 gene as the tumor suppressor target.
Discussion
PJS predisposes affected family members to the development of cancer. 2,4,5 Four independent studies have shown that the risk of death from gastrointestinal cancer among PJS patients is 13- to 30-fold greater than the risk in the general population. 4,5 The average age at which cancer is diagnosed in patients with PJS ranges from 38–50 years, and there is a reported 20–25 years of latency from the time of PJS diagnosis. 2-5 There have been several reports of LOH on 19p in breast, colorectal, and pancreatic cancers; however, since the STK11/LKB1 gene has been cloned, several efforts have failed to show biallelic somatic inactivation of STK11/LKB1 in colorectal, testicular, and breast cancers. 12-14,18 Here, we provide the genetic evidence to support the epidemiological clues that the PJS gene, STK11/LKB1, is a classic tumor-suppressor gene involved in pancreatic and biliary neoplasia. Furthermore, this gene appears to play a role in the development of both sporadic and familial (PJS) pancreatic and biliary cancers. In sporadic cancers, STK11/LKB1 was somatically inactivated in 4% of the pancreatic cancers and in at least 6% of biliary cancers examined. The patient with a familial (PJS) pancreatic cancer inherited a mutated copy of the STK11/LKB1 gene and had somatic loss of the remaining wild-type allele. Indeed, the first kindred described in the seminal report by Jeghers, McKusick, and Katz 1 included a patient who died of pancreatic cancer who could now, with the new understanding of the causal link between PJS and pancreatic cancer, be inferred to be the obligatory mutation carrier. These observations conform to the Knudson model, wherein the same genes are inactivated in both familial and sporadic forms of a cancer. 10
The xenografted series of pancreatic and biliary cancers, in which we demonstrated the inactivation of STK11/LKB1, have been well characterized genetically, providing additional opportunities to examine the tumor-suppressor role of STK11/LKB1. 16,17,19-22 For example, it would be unusual for two genes in the same pathway to be inactivated in a cancer. 22 We can therefore infer that the STK11/LKB1 suppressive pathway is distinct from the p53, p16, and DPC4 pathways; genetic inactivations of the p53 and p16 genes are known to coexist in tumor PX68, and DPC4 is homozygously deleted from tumors PX30 and PX115. 16,19,20 K-ras, which is mutated in 95% of pancreatic cancer cases, 19 is also mutated in tumors PX30, PX68, and PX104.
In summary, we demonstrated the biallelic inactivation of STK11/LKB1 in a pancreatic cancer of a patient with the PJS and in 4–6% of sporadic pancreatic and biliary adenocarcinomas, illustrating the role of this gene in familial and sporadic cancer development.
Acknowledgments
We thank Susan Booker, Francis Giardiello, Johan Offerhaus, and Paul Wilson for their support. To learn more about pancreatic cancer visit our web site, http://path.jhu.edu/pancreas.
Footnotes
Address reprint requests to Dr. Scott E. Kern, Department of Oncology, Ross Building 632, 720 Rutland Avenue, The Johns Hopkins University School of Medicine, Baltimore, MD 21205-2196. E-mail: sk@jhmi.edu.
Supported by the National Institutes of Health Specialized Program of Research Excellence in Gastrointestinal Cancer grant CA-62924.
References
- 1.Jeghers H, McKusick VA, Katz KH: Generalized intestinal polyposis and melanin spots of the oral mucosa, lip and digits: a syndrome of diagnostic significance. N Engl J Med 1949, 241:1031-1036 [DOI] [PubMed] [Google Scholar]
- 2.Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJA, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD: Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med 1987, 316:1511-1514 [DOI] [PubMed] [Google Scholar]
- 3.Hizawa K, Iida M, Matsumoto T, Kohrogi N, Kinoshita H, Yao T, Fujishima M: Cancer in Peutz-Jeghers syndrome. Cancer 1993, 72:2777-2781 [DOI] [PubMed] [Google Scholar]
- 4.Boardman LA, Thibodeau SN, Schaid DJ, Lindor NM, McDonnell SK, Burgart LJ, Ahlquist DA, Podratz KC, Pittelkow M, Hartmann LC: Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med 1998, 128:896-899 [DOI] [PubMed] [Google Scholar]
- 5.Spigelman AD, Murday V, Phillips RKS: Cancer and the Peutz-Jeghers syndrome. Gut 1989, 30:1588-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemminki A, Tomlinson I, Markie D, Jarvinen H, Sistonen P, Bjorkqvist A-M, Knuutila S, Salovaara R, Bodmer W, Shibata D, de la Chapelle A, Aaltonen LA: Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet 1997, 15:87-90 [DOI] [PubMed] [Google Scholar]
- 7.Amos CI, Bali D, Thiel TJ, Anderson JP, Gourley I, Frazier ML, Lynch PM, Luchtefeld MA, Young A, McGarrity TJ, Seldin MF: Fine mapping of a genetic locus for Peutz-Jeghers syndrome on chromosome 19p. Cancer Res 1997, 57:3653-3656 [PubMed] [Google Scholar]
- 8.Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M: Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 1998, 18:38-43 [DOI] [PubMed] [Google Scholar]
- 9.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H: A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391:184-187 [DOI] [PubMed] [Google Scholar]
- 10.Knudson AG, Jr: Hereditary cancer, oncogenes, and antioncogenes. Cancer Res 1985, 45:1437-1443 [PubMed] [Google Scholar]
- 11.Dong SM, Kim KM, Kim SY, Shin MS, Y. NE, Lee SH, Park WS, Yoo NJ, Jang JJ, Yoon CY, Kim JW, Kim SY, Yang YM, Kim SH, Kim CS, Lee JY: Frequent somatic mutation in serine/threonine kinase 11/Peutz-Jeghers syndrome gene left-sided colon cancer. Cancer Res 1998, 58:3787-3790 [PubMed] [Google Scholar]
- 12.Wang Z-J, Taylor F, Churchman M, Norbury G, Tomlinson I: Genetic pathways of colorectal carcinogenesis rarely involve the PTEN and LKB1 genes outside the inherited hamartoma syndromes. Am J Pathol 1998, 153:363-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avizienyte E, Roth S, Loukola A, Hemminki A, Lothe RA, Stenwig AE, Fossa SD, Salovaara R, Aaltonen LA: Somatic mutations in LKB1 are rare in sporadic colorectal and testicular tumors. Cancer Res 1998, 58:2087-2090 [PubMed] [Google Scholar]
- 14.Bignell GR, Barfoot R, Seal S, Collins N, Warren W, Stratton MR: Low frequency of somatic mutations in the LKB/Peutz-Jeghers syndrome gene in sporadic breast cancer. Cancer Res 1998, 58:1384-1386 [PubMed] [Google Scholar]
- 15.Westerman AM, Entius MM, Boor PPC, Koole H, de Baar E, Offerhaus GJA, Lindhout D. Halley DJJ, de Rooij FWM, Wilson JHP: Novel mutation in the LKBI/STKII gene in Dutch Peutz-Jeghers families. Hum Mutat 1999 (in press) [DOI] [PubMed]
- 16.Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE: Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet 1994, 8:27-31 [DOI] [PubMed] [Google Scholar]
- 17.Schutte M, da Costa LT, Hahn SA, Moskaluk C, Hoque ATMS, Rozenblum E, Weinstein CL, Bittner M, Meltzer PS, Trent JM, Yeo CJ, Hruban RH, Kern SE: Identified by representational difference analysis of a homozygous deletion in pancreatic carcinoma that lies within the BRCA2 region. Proc Natl Acad Sci USA 1995, 92:5950-5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoglund M, Gorunova L, Andren-Sandberg A, Dawiskiba S, Mitelman F, Johansson B: Cytogenetic and fluorescence in situ hybridization analyses of chromosome 19 aberrations in pancreatic carcinomas: frequent loss of 19p13.3 and gain of 19q13.1–13.2. Genes Chromosomes Cancer 1998, 21:8-16 [PubMed] [Google Scholar]
- 19.Rozenblum E, Schutte M, Goggins M, Hahn SA, Lu J, Panzer S, Zahurak M, Goodman SN, Hruban RH, Yeo CJ, Kern SE: Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res 1997, 57:1731-1734 [PubMed] [Google Scholar]
- 20.Hahn SA, Schutte M, Hoque ATMS, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE: DPC4, a candidate tumor-suppressor gene at 18q21.1. Science 1996, 271:350-353 [DOI] [PubMed] [Google Scholar]
- 21.Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE: Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res 1994, 54:3568-3573 [PubMed] [Google Scholar]
- 22.Su GH, Hilgers W, Shekher MC, Tang DJ, Yeo CJ, Hruban RH, Kern SE: Alterations in pancreatic, biliary, and breast carcinomas support MKK4 as a genetically targeted tumor suppressor gene. Cancer Res 1998, 58:2339-2342 [PubMed] [Google Scholar]