Abstract
Transforming growth factor-β1 (TGF-β1) has been shown to down-regulate NO synthesis in a variety of normal cells. In the present study, we investigated the influence of TGF-β1 upon NO production in tumor cells and its consequences for tumor development. During the growth of PROb colon carcinoma cells intraperitoneally injected in syngeneic BDIX rats, intratumoral concentration of TGF-β1 increases while NO concentration stays very low. Tumor regression induced by intraperitoneal injections of a lipid A is associated with a decrease in TGF-β1 and an increase in NO intratumoral concentration. In these tumors, PROb tumor cells are the NO- and TGF-β1-secreting cells. Using PROb cells transfected with an expression vector coding for TGF-β1 antisense mRNA, we demonstrate in vitro that there is an inverse correlation between the amount of TGF-β1 secreted and the ability of PROb cells to secrete NO. As the same results were obtained in the presence of an anti-TGF-β type II receptor neutralizing antibody, and as exogenous TGF-β1 is without any effect on NO secretion by PROb cells, TGF-β1 apparently down-regulates NO synthesis in PROb cells by an intracellular mechanism. These results suggest that endogenous TGF-β1 constitutes a potential target in a search for new antitumoral agents.
Immunogenic tumors can suppress the antitumor immune response in order to grow in their immunocompetent syngeneic host. This immunosuppression can be mediated by tumor cells through the secretion of soluble suppressor factors. 1-9 One of the most well known immunosuppressive cytokines is transforming growth factor-β (TGF-β). In vitro, this cytokine acts negatively on nearly all the cells of the immune system. 5,8 In vivo, TGF-β has been involved in the immunosuppression established by gliomas, 5 hepatocellular carcinomas, 10 and colorectal cancers. 3,4,7,8 Among the immunomodulatory effects attributed to TGF-β is the suppression of the production of nitric oxide (NO) by activated macrophages. 5,9,11,12
NO is a cytotoxic molecule originally described as a mediator of macrophage cytotoxicity. 13,14 Many cell types, including tumor cells, 15-17 synthesize NO, the production of which in large quantities involves inducible NO synthase (iNOS; NOS type II). The NO induced has both immunosuppressive 15 and antitumor 14,17,18 properties. Therefore, NO synthesis must be under tight control. The iNOS expression appears to be regulated at several levels: induction, 19 transcriptional and posttranscriptional levels, 11,12 and posttranslational level. 12 TGF-β has emerged as one of the most potent NO down-regulatory cytokines described so far. Thus, autocrine or paracrine production of TGF-β may serve as a physiological counterbalance for iNOS expression, a mechanism which may be subverted by tumors for their own survival. A greater understanding of the mechanisms and consequences of NO and TGF-β production may lead to more effective therapeutic strategies in treating cancer.
Using a syngeneic model of peritoneal carcinomatosis induced in BDIX rats with PROb colon tumor cells, we previously showed the involvement of TGF-β1 in tumor-induced immunosuppression in vivo and the ability of an immunotherapeutic treatment to reverse TGF-β1-mediated immunosuppression. 3 In this model, an intraperitoneal treatment with a lipid A cures 30% of the rats. Tumor regression correlates with increased NO concentration in the lipid A-treated tumor nodules. In these nodules, tumor cells were identified as the NO-producing cells. 20 In the present study, we investigated the role of TGF-β1 in the regulation of NO synthesis in tumor nodules during tumor development and lipid A-mediated tumor regression.
Materials and Methods
Cells and Media
The tumor cell clone DHD/K12-PROb (named PROb) was isolated from a colon carcinoma induced by 1,2-dimethylhydrazine in a BDIX rat. 21 These cells as well as the PROb cells transfected with an expression vector coding (PROb Bulk) or not (PROb Neo) for TGF-β1 antisense mRNA and the two PROb cell clones (clone 3′ and clone 13) were cultivated in Ham F10 medium (BioWhittaker, Fontenay-sous-Bois, France) supplemented with 10% fetal bovine serum (FBS) from Gibco BRL (Cergy Pontoise, France), and 40 μg/ml gentamycin. The latter is referred to as complete medium. EC 219 cells, a rat endothelial established cell line 22 kindly given by Dr. L. Juillerat (Institute of Pathology, Lausanne, Switzerland), and mink lung epithelial cells (CCL 64, American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, BioWhittaker) supplemented with 10% FBS, 2 mmol/L L-glutamine (BioWhittaker) plus 40 μg/ml gentamycin. To adhere, EC 219 cells were cultured on collagen-coated flasks. 22 The cells were checked monthly and before each in vivo experiment to ensure they were free of mycoplasma contamination.
Peritoneal Carcinomatosis
Peritoneal tumors were induced in inbred BDIX rats (3- to 5-month-old males or females) purchased from IFFA Credo (l’Arbresles, France) by injection i.p. of 1 × 10 6 syngeneic PROb cells. Fourteen days later all control rats exhibited solid nodules of several millimeters and hemorrhagic ascites; they died of their tumors between weeks 8 and 12. 3,15,20
Immunotherapy
The immunostimulant OM 174 (OM Laboratories, Meyrin, Switzerland) is the sodium salt of a purified lipid A analog from Escherichia coli. It consists of a hydrophilic diphosphorylated β (1, 6) glucosamine disaccharide in which N-2 and N-2′ carry a hydrophobic group, respectively (R)-3-hydroxytetradecanoyl and (R)-3-dodecanoyloxytetradecanoyl.
The treatment started 14 days after the tumor cell injection (day 0), when all the rats exhibited tumor nodules 2–5 mm in diameter. It consisted of five i.p. injections at the dose of 1 mg/kg, administered biweekly in the treated rats. The control rats received the same number and frequency of i.p. injections of the vehicle used to inject OM 174 (NaCl 9 g/l). At 15, 22, 29, 35, and 42 days after the tumor cell injection, three rats from each group were killed and autopsied. Peritoneal nodules were collected and either put immediately into liquid nitrogen for 15 minutes and kept at −80°C for iNOS Western blotting and TGF-β1 dosages, or put in 10% buffered formalin for immunohistochemistry study.
Preparation of Tumor and PROb Cell Extracts
Tumor nodules collected at different times during the treatment were homogenized at 4°C with an ultra-turrax homogenizer in cold buffer A (50 mmol/L Tris-HCl, pH 7.4, 1 mmol/L EDTA, 10 mg/l antipain, 10 mg/l leupeptin, 10 mg/l soybean trypsin inhibitor, 10 mg/l pepstatin A, 10 mg/l chymostatin, 100 mg/l phenylmethylsulfonyl fluoride) using 4 ml of buffer per gram of tumor. The homogenate was centrifuged at 105,000 × g for 1 hour at 4°C and the supernatant (cytosol fraction) was collected and frozen at −20°C.
PROb cell extracts were prepared as follows: 4 × 10 6 cells were plated in 75-cm 2 flasks and cultured for 4 days in complete medium. Then the cells were washed 3 times with serum-free medium and left at 37°C for 24 hours. The medium was removed and the cells were detached with a mixture of trypsin (2.5 mg/ml) plus EDTA (10 mg/ml) and centrifuged 10 minutes at 100 × g. The lysis of the pellet was obtained by adding 500 μl of lysis buffer (0.2% triton X-100, 10% pepstatin A, 10% aprotinin, 10% antipain dihydrochloride in PBS). The pellet was homogenized and left in the lysis buffer at 4°C for 45 minutes, then centrifuged 10 minutes at 27,500 × g. The PROb cell extracts (cytosol fraction) were collected and frozen at −20°C. Before TGF-β1 determination, the protein content of tumor samples and PROb cell extracts were determined using the DC protein assay kit from Bio-Rad (Ivry sur Seine, France).
TGF-β1 Assay
Active TGF-β1 was measured using the enzyme-linked immunosorbent assay (ELISA) Quantikine human TGF-β1 kit (R & D Systems, Abingdon, UK). In some experiments, the samples were acidified by the addition of HCl 1 N to give a pH of 2.0 to activate latent TGF-β1, left 10 minutes at room temperature, and neutralized with 1.2 N NaOH/0.5 M HEPES. The kit does not detect TGF-β2 or TGF-β3 but allows the quantification of rat TGF-β1, as indicated in the manufacturer’s protocol.
Western Blotting
For iNOS detection, tumor extracts were separated in 7.5% SDS-PAGE gels and transferred onto nitrocellulose membranes (Bio-Rad). The membranes were blocked by overnight incubation at 4°C with 10% nonfat milk in PBS containing 0.1% Tween 20. Then they were incubated with a 1/500 dilution of rabbit anti-inducible or anti-constitutive NOS antiserum. 23 The membranes were washed and then incubated with anti-rabbit IgG conjugated with horseradish peroxidase and bands were revealed using an enhanced chemiluminescence (ECL) kit (Amersham, Les Ulis, France) and exposure to Hyperfilm at ambient temperature.
For TGF-β receptor detection, PROb cell extracts and electrophoresis were performed as previously described. 24 Marker proteins were Rainbow markers (Amersham). Western blotting of 10% polyacrylamide gels was done on Immobilon 0.45-μm membranes (Millipore, Saint Quentin, France) using a mini-protean II apparatus (Bio-Rad) at 100 volts for 1 hour at 4°C. The blots were processed as recommended by the antibody supplier and revealed with an ECL kit (Amersham) and Kodak films. Anti-TGF-β RI was generously supplied by Drs. Miyazono and Heldin from the Ludwig Institute (Uppsala, Sweden) 25 and the anti-TGF-β RII was from Santa Cruz Biotechnology (Wembley, UK). Primary antibody dilutions were 1:2500 for anti-TGF-β RI and 1:2000 for anti-TGF-β RII. Secondary antibody dilutions were respectively 1:10,000 and 1:5000. The CCL64 mink epithelial cell line was used as positive control.
TGF-β1 Receptor Affinity Labeling
For these experiments, cells were plated at 1 × 10 6 per 10 cm diameter dish in 8 ml of DMEM plus 5% FBS. The next day, cells were rinsed with PBS and incubated in serum-free DMEM for 3 hours at 37°C. Cells were affinity-labeled using 75 pmol/L 125I-TGF-β1 (NEN, Les Ulis, France) in presence or absence of 4 nmol/L unlabeled human recombinant TGF-β1 (R & D Systems) in PBS with 1 mg/ml BSA for 1 hour at 4°C. TGF-β1 binding sites were cross-linked to 125I-TGF-β1 using 0.2 mmol/L disuccinimidyl suberate (Pierce, Rockford, IL) in PBS for 20 minutes. Cells were collected by scraping, centrifuged in PBS, and solubilized in extraction buffer (10 mmol/L Tris-HCl, pH 7.2, 1 mmol/L EDTA, 1% Triton X-100, 1 mmol PMSF). Extracts were cleared by centrifugation and separated on 8% polyacrylamide gels under reducing conditions. For autoradiography, the dried gels were exposed to Kodak films.
Stimulation of the PROb and EC 219 Cells
The PROb cell suspension was adjusted to 0.5 × 10 6 cells/ml of complete medium and 100 μl/well were seeded in quadruplicate, in 96-well plates for 24 hours at 37°C. The cells were then washed twice with Ham F10 medium and 200 μl of fresh Ham F10 medium, either containing or not containing the cytokines, were added. The EC 219 cell suspension was adjusted to 0.15 × 10 6 cells/ml of complete medium and 1 ml/well were seeded in 24-well plates coated with collagen for 24 hours at 37°C. Then, the cells were washed twice with DMEM medium and 1 ml of fresh complete medium, either containing or not containing the cytokines, was added. The cytokines used were recombinant human IL-1β (1 ng/ml) kindly given by Dr. A. Minty from Sanofi (Labège, France), recombinant human TNF-α (50 ng/ml) given by Dainippon (Tokyo), rat IFN-γ (100 U/ml) generously given by Roussel Uclaf (Romainville, France) and recombinant human TGF-β1 (0.5 to 10 ng/ml) obtained from Genzyme (Paris). In some experiments, an anti-human TGF-β type II receptor neutralizing antibody (R & D Systems) or NO donors: glyceryl trinitrate (GTN, Merck, Nogent sur Marne, France) and the diethylamine NO complex (DEA/NO, Coger, Paris), were added. The plates were incubated at 37°C for either 48 hours (PROb cells) or 24 hours (EC 219 cells). Cell supernatants were collected and frozen at −20°C for subsequent NO dosage.
Quantitation of NO Production
NO was measured as the sum of the nitrite (NO2−) and nitrate (NO3−) content. Nitrate was reduced to nitrite using nitrate reductase (Boehringer Mannheim, Meylan, France) in the presence of NADPH. The samples were then assayed for NO2− content by the Griess assay 26 as described elsewhere. 15 In some experiments, 5 mmol/L of NG-monomethyl-l-arginine acetate (NMMA) purchased from Calbiochem (San Diego, CA) was added.
Immunohistological Studies
As previously described, 7 tumor sections (3 μm) were deparaffinized and endogenous peroxidase activities were quenched in 0.3% (v/v) hydrogen peroxide in absolute methanol for 30 minutes. Sections were incubated with an anti-TGF-β1 antibody (R & D Systems) at 4°C overnight. After washing in PBS, a peroxidase-conjugated antibody was added for 1 hour at 37°C. The peroxidase reaction was developed with the AEC kit (Vector Laboratories, Burlingame, CA). No immunostaining was detected when the slides were processed either in the absence of the primary antibody or in the presence of nonimmune serum. After hematoxylin counterstaining, the slides were permanently mounted in an aqueous medium (Aquatex) and analyzed for the presence and the distribution of the immunostaining. For the morphological study, sections were stained with hematoxylin-eosin-saffron (HES).
PROb Cell Transfection
The phCMV vector was kindly provided by Dr. R. Breathnach (INSERM U463, Nantes, France) and the pGEM-3Z vector was obtained from Promega (Charbonnières, France). The rat cDNA was transferred from BS KS pRTGF-β1 plasmid (No. 63197, TCC) to the phCMV plasmid in a two-step process. First, the cDNA was excised from pRTGF-β1 using HindIII and XbaI and ligated in the multicloning site of the pGEM-3Z plasmid. Then, the cDNA was excised from this plasmid using KpnI and HindIII and ligated, in an antisense orientation, in the multicloning site of the phCMV plasmid under control of the cytomegalovirus promoter. The phCMV plasmid contains the gene coding for geneticin resistance. Lipofectamine was used as recommended by the manufacturer (Gibco BRL) to transfer the phCMV TGF-β1-antisense plasmid in the PROb cell line. Selection of the transfected cells was performed in complete medium containing 200 μg/ml neomycin (Gibco BRL). Surviving clones were isolated using cloning rings.
Results
Lipid A Immunotherapy Increased iNOS Protein Expression in PROb Tumor Nodules
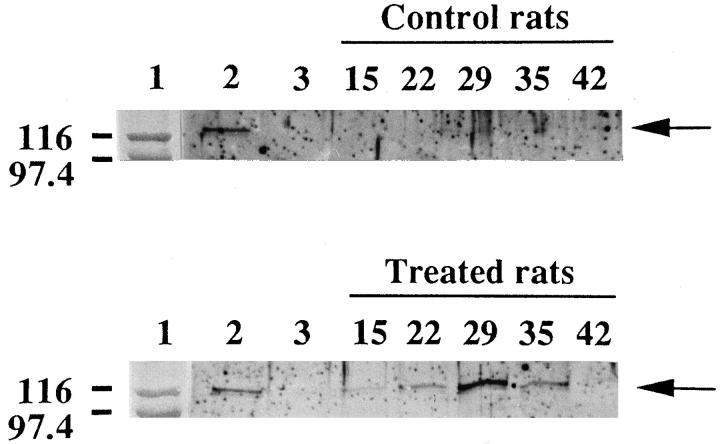
We have previously shown that NO concentration (NO2− and NO3−) increased up to fivefold above the basal level in PROb tumor nodules from lipid A-treated rats. 20 In the present study, we analyzed the expression of iNOS protein by Western blotting in tumor samples collected at different times during tumor growth and tumor regression induced by the lipid A treatment. Using an anti-iNOS antibody, virtually no immunoreactive band was observed at 125 kd in tumor nodules from untreated rats. Conversely, in tumor nodules from lipid A-treated rats, such an immunoreactive band was clearly visible in all the samples; its intensity increased from day 15 to day 29 of the treatment, then decreased after the last lipid A injection (Figure 1) ▶ . Western blot analysis of the same gel with anticonstitutive NOS antibody did not show any immunoreactive bands, whereas immunoreactive bands of comparable intensity were obtained for all the samples, with an anti-β actin antibody used as loading control (not shown).
Figure 1.
Western blot analysis of iNOS in tumor nodules from rats treated or not with the lipid A during tumor development. Rats were injected i.p. with PROb tumor cells at day 0 and treated or not with OM 174. Tumor nodules were taken from rats of both groups on days 15, 22, 29, 35, and 42 after the tumor cell injection. Lane 1: molecular weight markers; lane 2: positive control, extract of liver from a LPS-treated rat; Lane 3: negative control, brain extract from normal rat. Left margin indicates MW × 10−3. The arrows indicate the position of the iNOS protein. One of two representative experiments is shown.
Lipid A Immunotherapy Decreased TGF-β1 Concentration in PROb Tumor Nodules
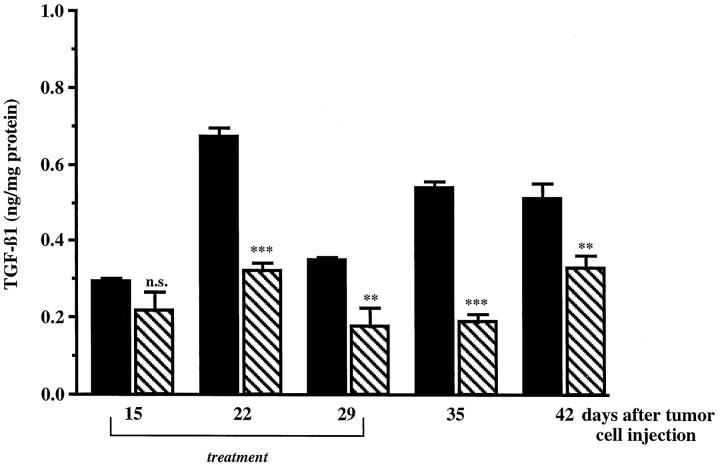
Because TGF-β1 was demonstrated to inhibit NO synthesis in a variety of normal cells, we investigated its expression in PROb tumor nodules. Total TGF-β1 concentration was measured by ELISA in peritoneal tumor nodules collected at different times during tumor growth and tumor regression induced by the lipid A treatment. After one week of lipid A treatment, TGF-β1 concentration was significantly lower in treated compared to untreated tumor nodules. Decreased TGF-β1 intratumoral level persisted 2 weeks after the last lipid A injection (Figure 2) ▶ . Comparable results were observed for active TGF-β1, but concentrations were lower.
Figure 2.
Concentration of TGF-β1 in tumor nodules from rats treated or not with the lipid A during tumor development. Rats were injected i.p. with PROb tumor cells at day 0 and treated (▧) or not (▪) with OM 174. Tumor nodules were taken from rats of both groups on days 15, 22, 29, 35, and 42 after the tumor cell injection, homogenized in an extraction buffer, and centrifuged. The total TGF-β1 concentration of the supernatants was measured by the ELISA method. The statistical analysis was performed using Student’s t-test (n.s., not significant; **, P ≤ 0.01; ***, P ≤ 0.001). One of three representative experiments is shown.
PROb Cells Were the TGF-β1-Producing Cells in PROb Tumors
Histological and immunohistological studies of treated and untreated tumor nodules collected 28 days after the tumor cell injection showed that untreated tumor nodules were made up mainly of epithelial neoplastic cells, associated with a few inflammatory cells, fibroblasts and macrophages. Some necrotic areas occurred in the center of the nodules (Figure 3A ▶ 1). In such nodules, 90–100% of the PROb tumor cells were strongly stained by the anti-TGF-β1 antibody, whereas macrophages, lymphocytes, and fibroblasts remained unstained (Figure 3A ▶ 2). In the lipid A-treated tumor nodules collected at day 28 (ie, after the last lipid A injection), both the size of the nodules and the number of tumor cells decreased. Furthermore, the tumor cells were localized only in the center of the nodules and were surrounded with an inner crown of fibroblasts and collagen and an outer crown of lymphocytes (Figure 3B ▶ 1). In these nodules, the PROb tumor cells were the only cells to be stained by the anti-TGF-β1 antibody, even though the staining was lighter than in untreated tumors and involved only half of the PROb cells (Figure 3B ▶ 2).
Figure 3.
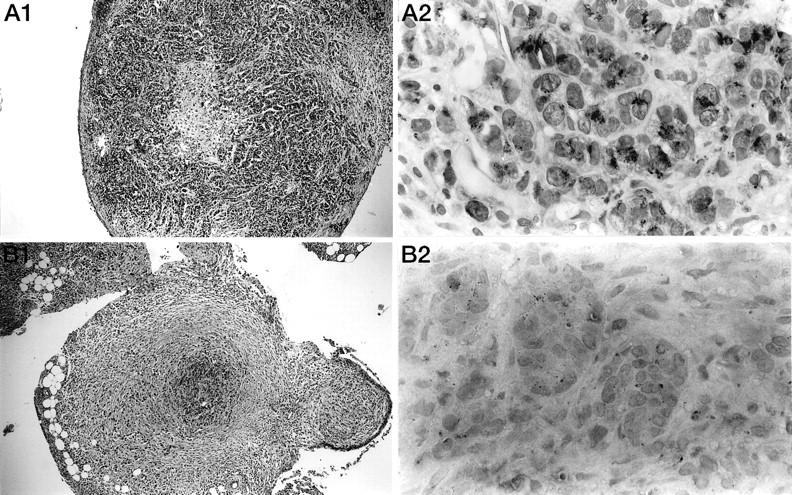
Morphological study and immunohistological detection of TGF-β1 in tumor nodules from rats treated or not with the lipid A. Histological sections of tumor nodules were taken on day 28 after tumor cell injection from mesentery of untreated control rats (A) or after the fifth injection of OM 174 in treated animals (B). First, the sections in both groups were morphologically studied (A1, B1; HES: × 100). Then, the sections were stained with an anti-TGF-β1 antibody (A2, B2; AEC: × 1000).
Exogenous TGF-β1 Did Not Modify NO Synthesis by Cultured PROb Cells
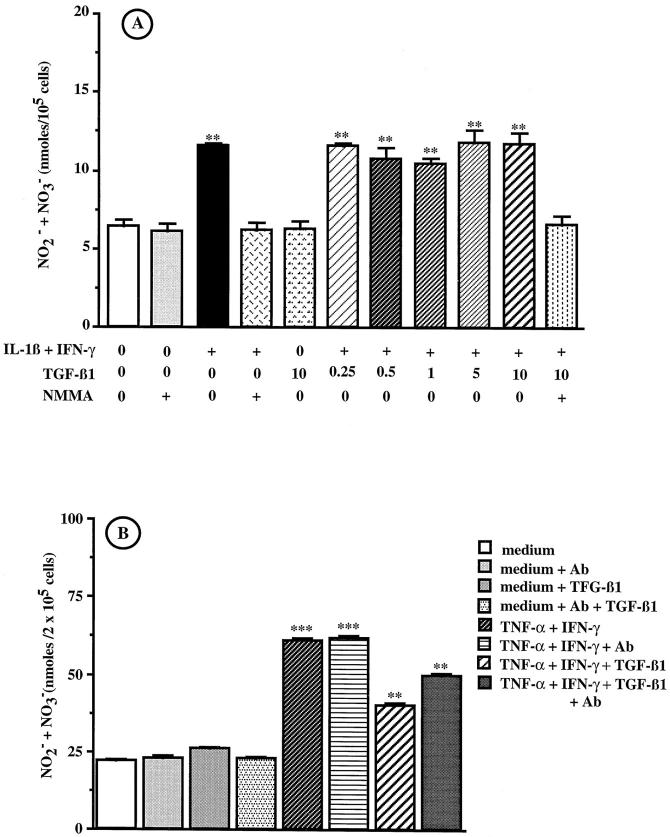
We have previously shown that the NO-producing cells in tumor nodules were the PROb tumor cells. 20 Constitutively, PROb cells cultured in vitro produced low amounts of NO (about 6 nmol/ml). The combination of IL-1β (1 ng/ml) and IFN-γ (100 U/ml) for 48 hours significantly increased NO secretion. This effect was totally inhibited in the presence of the NO synthase inhibitor NMMA (Figure 4A) ▶ . Addition of 0.1, 1, and 10 μg/ml of lipid A did not stimulate NO secretion by either unstimulated or cytokine-stimulated PROb cells. At concentrations ranging from 0.25–10 ng/ml, rhTGF-β1 did not influence NO production by either unstimulated or cytokine-stimulated PROb cells (Figure 4A) ▶ , whereas 0.25 ng/ml TGF-β1 inhibited NO production of EC 219 endothelial rat cells stimulated with 50 ng/ml TNF-α plus 100 U/ml IFN-γ (Figure 4B) ▶ . TGF-β1 remained without effect when added to cell cultures 24 hours before adding IL-1β plus IFN-γ for 48 hours, as well as when incubation time with the stimulatory cytokines and TGF-β1 was increased to 72 and 96 hours or when cytokine concentrations were increased to 25 ng/ml (IL-1β) and 250 U/ml (IFN-γ), respectively.
Figure 4.
Effect of exogenous TGF-β1 on NO secretion by PROb and EC 219 cells. A: PROb cells were incubated for 48 hours with either medium alone or containing 1 ng/ml IL-1β plus 100 U/ml IFN-γ to which 0, 0.25, 0.5, 1, 5, or 10 ng/ml TGF-β1 or 5 mmol/L NMMA were added. The supernatants were collected and tested for the presence of NO2− and NO3− by the Griess assay. 26 One of four representative experiments is shown. B: EC 219 cells were incubated for 24 hours with medium, either alone or containing 50 ng/ml TNF-α plus 100 U/ml IFN-γ, in the absence or in the presence of 0.25 ng/ml TGF-β1. An anti-TGF-β RII neutralizing antibody (20 μg/ml) was added or not (Ab). The supernatants were collected and tested for the presence of NO2− and NO3− by the Griess assay. One of two representative experiments is shown. The statistical analysis of NO secretion was performed using Student’s t-test (**, P ≤ 0.01; ***, P ≤ 0.001).
PROb Cells Expressed Both Type I and Type II TGF-β Receptors
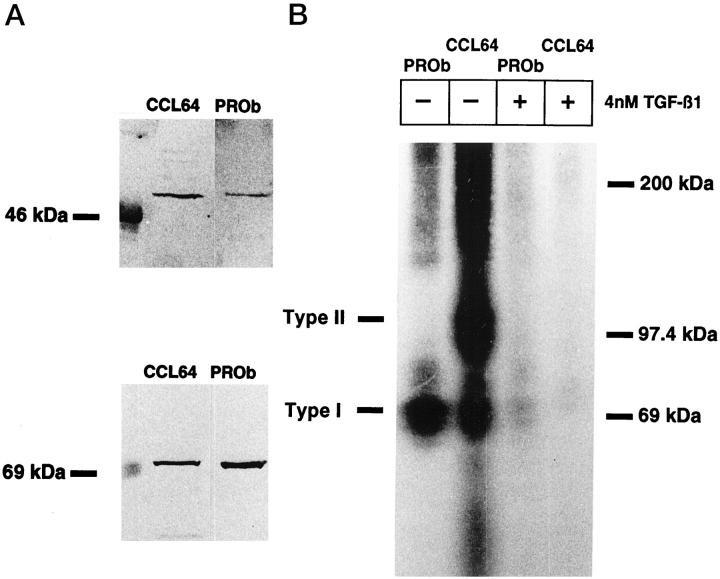
Using CCL64 cells as a positive control, we demonstrated the expression of TGF-β type I (RI) and TGF-β type II (RII) receptors on PROb cells by Western blotting experiments. Immunoreactive bands detected in PROb cells migrated at 53 kd and 75 kd with anti-TGF-β RI and anti-TGF-β RII antibodies, respectively (Figure 5A) ▶ . Because no inhibitory effect of TGF-β1 had been detected on cultured PROb cells, we performed cross-linking experiments to study the capacity of these receptors to bind 125I-TGF-β1. Whereas two bands were observed for CCL 64 cells, one band corresponding to TGF-β RI but no band corresponding to TGF-β RII was visible for PROb cells (Figure 5B) ▶ . The same results were obtained in the presence of an anti-TGF-β RII neutralizing antibody (not shown). Thus, PROb cells possess a nonfunctional type II receptor, which precludes normal signal transduction by exogenous TGF-β1. These results strongly suggest that in PROb tumors, the inverse correlation between NO and TGF-β1 is type II receptor-independent.
Figure 5.
Study of type I and type II TGF-β receptors on PROb cells. A: Type I and type II TGF-β receptors were detected by Western blot analysis. B: The functionality of both types of TGF-β receptors was studied by receptor cross-linking to 125I-TGF-β1 in the presence or absence of unlabeled TGF-β1 in excess. The CCL64 mink epithelial cells were used as a positive control. One of two representative experiments is shown.
Exogenous and Endogenous NO Did Not Modify TGF-β1 Synthesis by Cultured PROb Cells
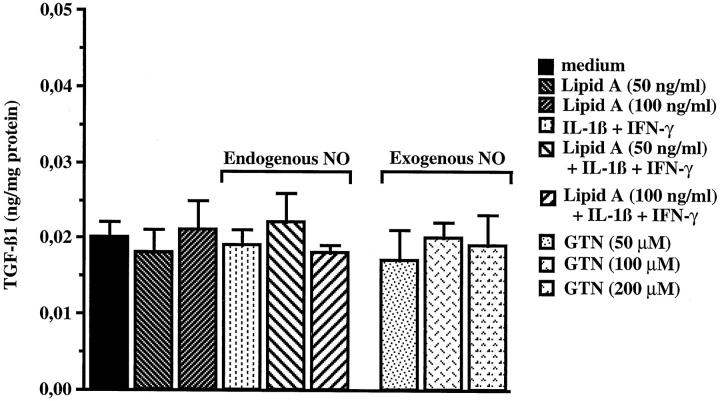
To check if the decrease in TGF-β1 concentration observed in lipid A-treated tumor nodules was the result of the increase in NO concentration, we measured the total TGF-β1 concentration in PROb cells after 48 hours incubation with lipid A, IL-1β plus IFN-γ, or NO donor glyceryl trinitrate (GTN). Figure 6 ▶ indicates that the lipid A alone or combined with the cytokines had no effect on TGF-β1 synthesis by PROb cells, nor did GTN at the concentrations tested. The same results were obtained with another NO donor: the diethylamine NO complex (DEA/NO) used at same concentrations as GTN. IL-1β or IFN-γ alone had no effect, either.
Figure 6.
Effect of exogenous and endogenous NO on TGF-β1 synthesis in PROb cells. The PROb cells were incubated for 48 hours with medium without FBS either alone or containing 50 or 100 ng/ml lipid A, in the presence or in the absence of IL-1β (1 ng/ml) plus IFN-γ (100 U/ml). The NO donor glyceryl trinitrate (GTN) was also added on PROb cells at 50, 100, and 200 μmol/L for 48 hours. After this incubation period, PROb cell extracts were prepared and the total TGF-β1 concentration was measured by ELISA after acidification and neutralization of the cell extracts to activate latent TGF-β1. One of four representative experiments is shown.
Down-Regulation of TGF-β1 Synthesis Correlated with an Increase in NO Secretion by PROb Cells
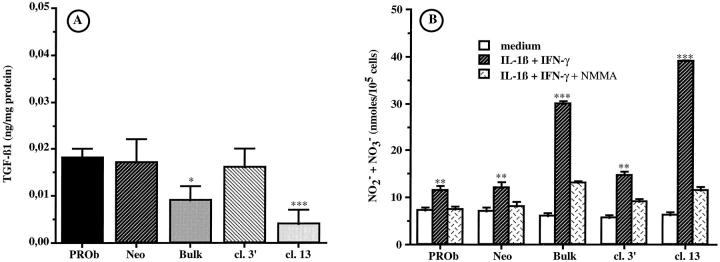
Because PROb cells are the NO- and TGF-β1-secreting cells in tumor nodules, we hypothesized that endogenous TGF-β1 could regulate NO secretion by PROb cells. To determine the role of endogenous TGF-β1, PROb cells were transfected with an expression vector coding for TGF-β1 antisense mRNA. As demonstrated in Figure 7A ▶ , the transfection successfully decreased the TGF-β1 secretion of PROb cells but did not modify their TGF-β RI and TGF-β RII expression (not shown). The TGF-β1 production of the uncloned transfected PROb cells (PROb Bulk) was 0.009 ± 0.003 ng/mg protein, compared to 0.018 ± 0.002 ng/mg protein for the nontransfected cells or 0.017 ± 0.005 ng/mg protein for the PROb cells transfected with the vector alone (PROb Neo). Among the different clones obtained from the PROb Bulk cells, two clones were selected: clone 3′, which secreted a comparable amount of TGF-β1 as the parental PROb cells (0.016 ± 0.004 ng/mg protein), and clone 13, which secreted barely detectable levels of TGF-β1 (0.004 ± 0.003 ng/mg protein).
Figure 7.
Effect of endogenous TGF-β1 on NO secretion by PROb cells stimulated with IL-1β plus IFN-γ. PROb cells were transfected with a vector containing (PROb Bulk) or not (PROb Neo) the TGF-β1 cDNA in an antisense orientation. Among the uncloned PROb Bulk cells, two clones were selected: clone 3′ (cl. 3′) and clone 13 (cl. 13). A: the capacity of the PROb cell clones to synthesize TGF-β1 was measured by ELISA after acidification and neutralization of the cell extracts to activate latent TGF-β1. B: the PROb cell clones were incubated for 48 hours with medium without FBS either alone or containing 1 ng/ml IL-1β plus 100 U/ml IFN-γ, in the absence or in the presence of 5 mmol/L of NMMA. The supernatants were collected and tested for the presence of NO2− and NO3− by the Griess assay. The statistical analysis of TGF-β1 and NO secretion was performed using Student’s t-test (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). One of three representative experiments is shown.
We then compared the NO secretion of the different PROb cell clones under stimulation with IL-1β plus IFN-γ in a medium without FBS. Figure 7B ▶ shows that there was an inverse correlation between the TGF-β1 secretion of the different PROb cell clones and their ability to secrete NO when stimulated. The PROb Bulk and the clone 13 cells secreted significantly (P ≤ 0.001) more NO when compared to the nontransfected PROb cells, the transfected clone 3′, and the PROb Neo cells. The NO secretion of the PROb Bulk and clone 13 cells was more than twice as high as that of the control PROb cells. This production was inhibited in the presence of NMMA, which had no effect by itself. Exogenous TGF-β1 (0.5, 1, or 5 ng/ml) had no effect on NO secretion by the four PROb cell clones.
TGF-β1 Acted as an Intracellular Mediator for NO Synthesis Inhibition in PROb Cells
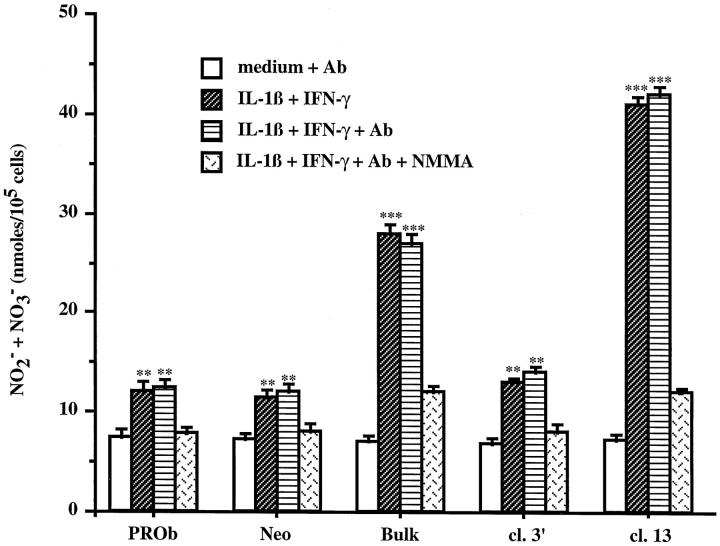
To study the mechanism by which TGF-β1 down-regulates NO secretion in PROb cells, we measured NO synthesis of the different PROb cell clones under stimulation with IL-1β plus IFN-γ in the presence or in the absence of an anti-TGF-β RII neutralizing antibody.
Because no commercial anti-rat TGF-β RII antibody was available, we used a human antibody to block TGF-β RII on PROb cells. To be sure of TGF-β RII inhibition, we first tested the cross-reactivity of the antibody on rat cells. Figure 4B ▶ shows that the rat endothelial EC 219 cells secreted NO when stimulated with TNF-α plus IFN-γ; this secretion was partially inhibited by 0.25 ng/ml TGF-β1. The addition of 20 μg/ml of the anti-human TGF-β RII antibody inhibited 50% of the TGF-β1 effect on NO synthesis by EC 219 rat cells, whereas the addition of 20 μg/ml of an unrelated human antibody had no effect. Since the parental PROb cells secrete about 0.018 ng/ml TGF-β1 (Figures 6 and 7A) ▶ ▶ , 20 μg/ml of the anti-TGF-β RII antibody should completely neutralize a potential autocrine effect of exogenous TGF-β1, supposing that the TGF-β RII is somehow functional despite the cross-linking data to the contrary.
Figure 8 ▶ indicates that in the presence of the anti-TGF-β RII antibody the basal and induced production of NO of the different PROb cell clones were not modified. Thus, in PROb cells, TGF-β1 appears to inhibit NO synthesis by an intracellular mechanism.
Figure 8.
Intracellular effect of TGF-β1 on NO secretion by PROb cells stimulated with IL-1β plus IFN-γ. PROb cell clones were incubated for 48 hours with either medium without FBS alone or containing 1 ng/ml IL-1β plus 100 U/ml IFN-γ, in the absence or in the presence of 20 μg/ml of an anti-TGF-β RII neutralizing antibody (Ab). The supernatants were collected and tested for the presence of NO2− and NO3− by the Griess assay. The statistical analysis of NO secretion was performed using Student’s t-test (**, P ≤ 0.01; ***, P ≤ 0.001). One of two representative experiments is shown.
Discussion
TGF-β1 has been demonstrated to be involved in tumor immunosuppression in various cancers including colon cancer. 3,4,7,8 Recently, it has been shown that plasma TGF-β1 levels might be used as a prognostic biomarker in colorectal adenocarcinoma because plasma TGF-β1 levels in colorectal patients were higher than in normal controls, reflecting overexpression of the gene in colon cancer tissues and association with disease progression. 4 One of the mechanisms by which TGF-β1 could trigger immunosuppression is its ability to inhibit NO secretion by activated macrophages. 5,9,11,12 In the present study, we demonstrate that endogenous TGF-β1 down-regulates NO synthesis in tumor cells by an intracellular mechanism.
We previously demonstrated the strong antitumor efficiency of a lipid A immunotherapy in the syngeneic model of peritoneal carcinomatosis induced by PROb colon carcinoma cells in BDIX rats. In vitro, lipid A shows neither antiproliferative nor cytotoxic activity on PROb cells. In vivo, tumor regression induced by the lipid A therapy is associated with an increased NO content in tumor nodules. 20 The present study demonstrates that constitutive NOS is not expressed in tumor nodules and that the increased NO content identified in tumor nodules regressing upon lipid A therapy is related to the accumulation of iNOS. In PROb tumors, the PROb tumor cells were identified as the cells responsible for NO production. Cultured PROb cells do not spontaneously secrete NO and lipid A has no direct effect on this production, but the combination of IL-1β and IFN-γ stimulates NO production by PROb cells in vitro. In vivo immunohistological studies showed an accumulation of IL-1β and IFN-γ in lipid A-treated tumor nodules compared to untreated nodules, suggesting a possible role for these cytokines in the increased intratumoral level of iNOS and NO in treated rats. 20 NO can be cytotoxic for tumor cells in vitro and demonstrates antitumor properties in vivo. 14,17,18 In vitro, we showed that NO secreted by PROb cells under IL-1β plus IFN-γ stimulation inhibits their proliferation. 20 Therefore, an increased NO intratumoral concentration appears to account for tumor regression observed in lipid A-treated animals. Nevertheless, until now, we had not been able to demonstrate this clearly, because the administration of NMMA or PROb cell transfection with an expression vector encoding for iNOS antisense mRNA did not allow a decision on this point.
TGF-β1 has been shown to inhibit NO production by a variety of normal cells 5,11,12,27,28 and TGF-β1 null mice demonstrated NO serum levels fourfold higher than control animals. 29 To our knowledge, the relationships between TGF-β1 and NO synthesis had never been analyzed in tumor cells. In PROb tumor nodules, we show that the intratumoral concentration of TGF-β1 is high during the growth of tumor nodules and decreases in nodules whose regression is induced by the lipid A therapy. Like many other colon cancer cell lines, 6,8 cultured PROb cells secrete TGF-β1. 2,3,7 In subcutaneous PROb tumors developed in rats 7 as well as in human colon tumors, 4 TGF-β1 was shown to be produced exclusively by tumor cells. Immunohistological studies performed on peritoneal PROb tumor nodules from either untreated or lipid A-treated rats confirmed that tumor cells were the only cells to be stained with the anti-TGF-β1 antibody. While nearly all the tumor cells were stained in tumor nodules from untreated rats, only half of them were stained in those from lipid A-treated animals. Craven et al 30 showed that NO generated exogenously by the NO donor S-nitroso-N-acetyl,D,L,-penicillamine or endogenously after exposure of mesangial cells to IL-1β suppressed bioactive TGF-β in these cells. In PROb cells, the lipid A alone or in combination with IL-1β and IFN-γ has no effect on TGF-β1 synthesis, nor have the NO donors GTN and DEA/NO. Therefore, the decreased TGF-β1 concentration observed in tumor nodules regressing upon lipid A therapy was not due to NO and might involve other cytokines secondarily induced in vivo. Thus, in peritoneal PROb tumor nodules, both NO and TGF-β1 were secreted by tumor cells but NO content was inversely related to TGF-β1 content during tumor growth and regression induced by the lipid A.
TGF-β1 is known to interact with cell surface receptors expressed on the plasma membrane of target cells. 6,8,25,31 Based on radioimmunoassay data, PROb cells bind TGF-β1. 2 The present Western blotting study shows that they express both type I and type II receptors, so that the inverse relationship between TGF-β1 and NO levels in PROb tumor nodules could have been explained if TGF-β1 secreted by tumor cells modulated NO synthesis in these cells by an autocrine or paracrine mechanism. In fact, exogenous TGF-β1 did not influence NO synthesis by cultured PROb cells, either unstimulated or stimulated by various combinations of IL-1β plus IFN-γ. Exogenous TGF-β1 did not influence PROb cell proliferation, either. 3 A similar loss of responsiveness to exogenous TGF-β1 was reported in some colon cancer cell lines as well as in advanced human colon cancers. 6,31 Three major types of TGF-β-binding proteins have been identified. TGF-β RIII may be involved in ligand presentation to TGF-β signaling receptor. 8,31 whereas molecular cloning and functional analyses have shown that both RI and RII receptors, are necessary for TGF-β signal transduction. 6,8,25,31 Here, cross-linking data indicated that the absence of any effect of exogenous TGF-β1 on PROb cells correlates with the fact that TGF-β RII does not bind 125I-TGF-β1, whereas RI does. A growing body of evidence suggests that some TGF-β1 biological effects would be type II receptor-dependent, whereas others would be type II receptor-independent. In a subclone of the MCF-7 cell line, cross-linking experiments showed that the TGF-β RII was not functional, yet the TGF-β RI remained capable of binding TGF-β1. The inactivation of the TGF-β RII has been shown to induce a specific abrogation of the TGF-β1 antiproliferative effect without affecting the TGF-β1 stimulation of its own transcription 32 or of the extracellular matrix. 33
TGF-β1 is known to be secreted by most cells in an inactive (latent) form but PROb cells, like several other colon tumor cell lines, 6 were shown to secrete a fraction of bioactive TGF-β1. 2 As the anti-TGF-β1 antibody used here for immunological studies specifically recognized active TGF-β1, PROb cells might contain intracellular active TGF-β1 as reported inside B cells and plasma cells. 34 A growing body of evidence indicates that some cytokines can act not only via cell surface receptors, but also within the cell. 35-38 Because the PROb cells were both the NO- and TGF-β1-secreting cells in tumors, we hypothesize that endogenous TGF-β1 could negatively regulate NO secretion in these cells. Transfection of PROb cells with an expression vector encoding TGF-β1 antisense mRNA significantly decreased their TGF-β1 synthesis. The absence of effect of exogenous TGF-β1 and the increased NO secretion in cytokine-stimulated PROb transfected cells, even in the presence of an anti-TGF-β RII neutralizing antibody, strongly argue for an intracellular effect of TGF-β1 on NO synthesis down-regulation in PROb tumor cells. Using microinjection technology, Smith et al demonstrated an intracellular role for IFN-γ and TNF-α. 35 Thus, an intracellular action of some cytokines may be fairly common.
Nuclear localization signals have been identified in several cytokines or their receptors. 36,38 Some cytokine-receptor complexes have been localized in the nucleus 36,37 but the role of these complexes within the nucleus is unknown. They could act as chaperones to accomplish a cytokine-specific nuclear translocation of signaling molecules that function within the nucleus. 36 Some of these complexes were demonstrated to have a DNA binding capacity, suggesting their implication in modulation of gene transcription. 37 In cell systems responding to exogenous TGF-β1, down-regulation of NO synthesis can occur at the transcriptional, posttranscriptional, and/or posttranslational level. 5,11,12 As PROb cells synthesize TGF-β RI able to bind TGF-β1, such complexes might act as chaperones for SMAD complexes, recently shown to be involved in TGF-β signaling and in the pathogenesis of colorectal cancer. 39 Nuclear factor-κB (NF-κB) is implicated in the regulation of the iNOS gene transcription. 40 Exogenous TGF-β1 was shown to inhibit NF-κB activity by increasing the transcription of I-κBα, a protein inhibitor of NF-κB. 41 It is possible that endogenous TGF-β1 linked to TGF-β RI increases this transcription. At the present time, endogenous TGF-β1-mediated regulation of iNOS expression remains to be fully explained.
To summarize, cytokines may exert their effect not only through surface receptor-driven events, but also through interactions with intracellular targets distinct from surface receptors. Our study supports for the first time an intracellular action of TGF-β1.
Acknowledgments
We thank Isabelle Barbieux of INSERM U 419 for technical help.
Footnotes
Address reprint requests to Patricia Lagadec, Faculty of Medicine Cancer Immunotherapy Research Lab, Ecole Pratique des Hautes Etudes, 7 Boulevard Jeanne d’Arc, 21033 Dijon cedex, France. E-mail: lagadecp@satie.u-bourgogne.fr.
Supported by research grants from the Conseil Regional de Bourgogne, OM Laboratories, the Ligue contre le Cancer de l’Yonne, de la Côte d’Or et de la Haute Marne, the Fondation Singer-Polignac, the Fondation de France, and l’Association pour la Recherche contre le Cancer (A.R.C.).
References
- 1.Ebert EC, Roberts AJ, Devereux D, Nagase H: Selective immunosuppressive action of a cofactor produced by colon cancer cells. Cancer Res 1990, 50:6158-6161 [PubMed] [Google Scholar]
- 2.Grégoire M, Garrigue L, Blottière HM, Denis MG, Meflah K: Possible involvement of TGF-β1 in the distinct tumorigenic properties of two rat colon carcinoma clones. Invasion Metastasis 1992, 12:185-196 [PubMed] [Google Scholar]
- 3.Lagadec P, Reveneau S, Lejeune P, Pinard D, Borman T, Bauer J, Jeannin JF: Immunomodulator OM 163-induced reversal of tumor-mediated immunosuppression and down-regulation of TGF-β1 in vivo. J Pharmacol Exp Ther 1996, 278:926-933 [PubMed] [Google Scholar]
- 4.Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, Imai Y, Shimomukai H, Nomura Y, Matsuda Y, Matsuzawa Y: High levels of transforming growth factor-β1 in patients with colorectal cancer: association with disease progression. Gastroenterology 1996, 110:375-382 [DOI] [PubMed] [Google Scholar]
- 5.Weller M, Fontana A: The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-β, T cell apoptosis, and the immune privilege of the brain. Brain Res Rev 1995, 21:128-151 [DOI] [PubMed] [Google Scholar]
- 6.Lahm H, Odartchenko N: Role of transforming growth factor-β in colo-rectal cancer. Growth Factors 1993, 9:1-9 [DOI] [PubMed] [Google Scholar]
- 7.Lieubeau B, Garrigue L, Barbieux I, Meflah K, Grégoire M: The role of transforming growth factor β1 in the fibroblastic reaction associated with rat colorectal tumor development. Cancer Res 1994, 54:6526-6532 [PubMed] [Google Scholar]
- 8.Wojtowicz-Praga S: Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother 1997, 20:165-177 [PubMed] [Google Scholar]
- 9.Alleva DG, Burger CJ, Elgert KD: Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-α production. Role of tumor-derived IL-10, TGF-β, and prostaglandin E2. J Immunol 1994, 153:1674-1686 [PubMed] [Google Scholar]
- 10.Ito N, Kawata S, Tamura S, Takaishi K, Shirai Y, Kiso S, Yabuuchi I, Matsuda Y, Wisioka M, Tarui S: Elevated levels of transforming growth factor β messenger RNA and its polypeptide in human hepatocellular carcinoma. Cancer Res 1996, 51:4080-4083 [PubMed] [Google Scholar]
- 11.Hausmann E, Hao SY, Pace J, Parmely M: Transforming growth factor-β1 and gamma-interferon provide opposing signals to lipopolysaccharide-activated mouse macrophage. Infect Immun 1994, 62:3625-3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C: Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor-β1. J Exp Med 1993, 178:605-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibbs JB, Taintor RR, Vavrin Z: Macrophage cytotoxicity: role for L-arginine deiminase and iminonitrogen oxidation to nitrite. Science 1987, 235:473-476 [DOI] [PubMed] [Google Scholar]
- 14.Farias-Eisner R, Sherman MP, Aeberhard E, Chaudhuri G: Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc Natl Acad Sci USA 1994, 91:9407-9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lejeune P, Lagadec P, Onier N, Pinard D, Oshima H, Jeannin JF: Nitric oxide involvement in tumor-induced immunosuppression. J Immunol 1994, 152:5077-5083 [PubMed] [Google Scholar]
- 16.Jenkins DC, Charles IG, Baylis SA, Lelchuk R, Ranomski MW, Moncada S: Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br J Cancer 1994, 70:847-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie KP, Dong ZY, Fidler IJ: Activation of nitric oxide synthase gene for inhibition of cancer metastasis. J Leukoc Biol 1996, 59:797-803 [DOI] [PubMed] [Google Scholar]
- 18.Xie K, Huang S, Dong Z, Juang SH, Wang Y, Fidler IJ: Destruction of bystander cells by tumor cells transfected with inducible nitric oxide (NO) synthase gene. J Natl Cancer Inst 1997, 89:421-427 [DOI] [PubMed] [Google Scholar]
- 19.Ding AH, Nathan CF, Stuehr DJ: Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol 1988, 141:2407-2412 [PubMed] [Google Scholar]
- 20.Onier-Cherix N, Hilpert S, Reveneau S, Arnould L, Saint-Giorgio V, Exbrayat JM, Jeannin JF: Expression of inducible nitric oxide synthase in tumors in relation with their regression induced by a lipid A in rats. Int J Cancer 1999, 81:755-760 [DOI] [PubMed] [Google Scholar]
- 21.Martin F, Caignard A, Jeannin JF, Leclerc A, Martin MS: Selection by trypsin of two sublines of rat colon cancer cells forming progressive or regressive tumors. Int J Cancer 1983, 32:623-627 [DOI] [PubMed] [Google Scholar]
- 22.Juillerat-Jeanneret L, Aguzzi A, Wiestler OD, Darekar P, Janzer RC: Dexamethasone selectively regulates the activity of enzymatic markers of cerebral endothelial cell lines. In Vitro Cell Dev Biol Anim 1992, 28A:537-543 [DOI] [PubMed] [Google Scholar]
- 23.Oshima H, Brouet IM, Brandaletova T, Adachi H, Oguchi S, Iida S, Kurashima Y, Morishita Y, Sugimura T, Esumi H: Polyclonal antibody against an inducible form of nitric oxide synthase from the liver of rats treated with propionibacterium acnes and lipopolysaccharides. Biochem Biophys Res Comm 1992, 187:1291-1297 [DOI] [PubMed] [Google Scholar]
- 24.Raynal S, Lawrence DA: Differential effects of transforming growth factor-β1 on protein levels of p21 WAF and cdk-2 and on cdk-2 kinase activity in human RD and CCL64 mink lung cells. Int J Oncol 1995, 7:337-341 [DOI] [PubMed] [Google Scholar]
- 25.Franzén P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin CH, Miyazono K: Cloning of a TGF-β type I receptor that forms a heterodimeric complex with the TGF-β type II receptor. Cell 1993, 75:681-692 [DOI] [PubMed] [Google Scholar]
- 26.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum JS: Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982, 126:131-138 [DOI] [PubMed] [Google Scholar]
- 27.Gilbert RS, Herschman HR: Transforming growth factor beta differentially modulates the inducible nitric oxide synthase gene in distinct cell types. Biomed Biophys Res Comm 1993, 195:380-384 [DOI] [PubMed] [Google Scholar]
- 28.Vodovotz Y, Letterio JJ, Geiser AG, Chesler L, Roberts AB, Sparrow J: Control of nitric oxide production by endogenous TGF-β1 and systemic nitric oxide in retinal pigment epithelial cells and peritoneal macrophages. J Leukoc Biol 1996, 60:261-270 [DOI] [PubMed] [Google Scholar]
- 29.Vodovotz Y, Geiser AG, Chesler L, Letterio JJ, Cambell A, Scott Lucia M, Sporn MB, Roberts AB: Spontaneously increased production of nitric oxide and aberrant expression of the inducible nitric oxide synthase in vivo in the transforming growth factor-β1 null mouse. J Exp Med 1996, 183:2337-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craven PA, Studer RK, Felder J, Phillips S, DeRubertis FR: Nitric oxide inhibition of transforming growth factor-beta and collagen synthesis in mesangial cells. Diabetes 1997, 46:671-681 [DOI] [PubMed] [Google Scholar]
- 31.Markowitz SD, Wang KJ, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV: Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 1995, 268:1336-1338 [DOI] [PubMed] [Google Scholar]
- 32.Mazars P, Barboule N, Baldin V, Vidal S, Ducommun B, Valette A: Effects of TGF-β1 (transforming growth factor-β1) on the cell cycle regulation of human breast adenocarcinoma (MCF-7) cells. FEBS Lett 1995, 362:295-300 [DOI] [PubMed] [Google Scholar]
- 33.Chen RH, Ebner R, Derynck R: Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-β activities. Science 1993, 260:1335-1338 [DOI] [PubMed] [Google Scholar]
- 34.Caver TE, O’Sullivan FX, Gold LI, Gresham HD: intracellular demonstration of active TGF-β1 in B cells and plasma cells of autoimmune mice. J Clin Invest 1996, 98:2496-2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MR, Muegge K, Keller JR, Kung HS, Young HA, Durum SK: Direct evidence for an intracellular role for IFN-γ. Microinjection of human IFN-γ induces Ia expression on murine macrophages. J Immunol 1990, 144:1777-1782 [PubMed] [Google Scholar]
- 36.Johnson HM, Torres BA, Green MM, Szente BE, Siler KI, Larkin J, Subramaniam PS: Cytokine-receptor complexes as chaperones for translocation of signal transducers. Biochem Biophys Res Comm 1998, 244:607-614 [DOI] [PubMed] [Google Scholar]
- 37.Weitzmann MN, Savage N: Nuclear internalisation and DNA binding activities of interleukin-1, interleukin-1 receptor and interleukin-1/receptor complexes. Biochem Biophys Res Comm 1992, 187:1166-1171 [DOI] [PubMed] [Google Scholar]
- 38.Zhan X, Hu X, Friedman S, Maciag T: Analysis of endogenous and exogenous nuclear translocation of fibroblast growth factor-1 in NIH 3T3 cells. Biochem Biophys Res Comm 1992, 188:982-991 [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y, Richardson JA, Parada LF, Graff JM: Smad 3 mutant mice develop metastatic colorectal cancer. Cell 1998, 94:703-714 [DOI] [PubMed] [Google Scholar]
- 40.Goldring C, Narayanan R, Lagadec P, Jeannin JF: Transcriptional inhibition of the inducible nitric oxide synthase gene by competitive binding of NF-κB/REL proteins. Biochem Biophys Res Commun 1995, 209:73-79 [DOI] [PubMed] [Google Scholar]
- 41.Arsura M, Wu M, Sonenshein GE: TGF-β1 inhibits NF-κB/REL activity inducing apoptosis of B cells: transcriptional activation of IκBα. Immunity 1996, 5:31-40 [DOI] [PubMed] [Google Scholar]