Abstract
Enlarged but nondysplastic crypts are frequently observed at the margins of colon tumors, forming what has been called a transitional epithelium. It is now thought that this is a reactive state and not a preneoplastic condition as previously suggested. We have used the mouse familial adenomatous polyposis model, ApcMin, to study these abnormal adenoma-associated crypts. We report that these nondysplastic crypts are enormous (as much as 10 times normal length) and branch more frequently than normal crypts. They express wild-type Apc protein and display the wild-type Apc allele. We conclude that the colossal crypts at adenoma margins have normal Apc gene function, consistent with the suggestion that their phenotype is a reactive state. The cause remains an open question, but the dramatic epithelial response hints at the presence of potent epithelial trophic factors in the vicinity of colon tumors.
It has long been recognized that the colonic epithelium at the margins of adenocarcinomas, although not neoplastic, is not normal. The crypts in this transitional region are enlarged and often branched. 1-5 However, as a similar state is observed at the margins of an array of lesions, it is now believed that it represents a reactive state and not a preneoplastic condition 6,7 and has since been largely ignored. We have noticed in studies of a mouse model of familial adenomatous polyposis 8 that although the dysplastic epithelium forming the bulk of adenomas is Apc negative, there is often a layer of Apc-positive nondysplastic crypts underlying the tumor. 9 These Apc-positive crypts are often cut in cross section when the tumor is sectioned orthogonally to the surface. This observation raised questions about the relationship of the Apc-positive crypts to the overlying tumor and to the surrounding normal epithelium. We show here that they represent greatly elongated crypts, with normal Apc gene function, that extend under the tumor from the margins.
Materials and Methods
Isolation of Intact Epithelium and Scanning Electron Microscopy
Fifteen week-old ApcMin mice (Jackson Laboratories, Bar Harbor, ME) were used. Intact epithelium was isolated, from small segments of colon each containing an adenoma, by intraventricular perfusion of 30 mmol/L EDTA in PBS followed by vibration into 4% paraformaldehyde in PBS. 10 After fixation for 30 minutes on ice, the isolated epithelium was washed in PBS and then rinsed and stored in methanol at −20°C. The colon adenomas and adjacent mucosa were dissected from the vibrated segments and fixed for 30 minutes in 2.5% glutaraldehyde in PBS. The adenomas were then processed for scanning electron microscopy (SEM) by post-fixing in 1% osmium tetroxide in 0.1 mol/L cacodylate HCl buffer, pH 7.4, and then dehydrated through a graded series of ethanol, critical-point dried, 11 and sputter coated with gold palladium.
Immunofluorescence
Colossal crypts were microdissected from the isolated epithelium, rinsed in 1% Nonidet P-40 (NP40) in PBS, freeze-thawed at −20°C to increase permeability, rinsed in 1% NP40, and blocked at room temperature for 1 hour in PBS containing 10% newborn calf serum and 1% NP40. The crypts were incubated overnight at 4°C in rabbit polyclonal antibodies specific for the carboxy terminus of Apc protein (Santa Cruz Biotechnology, Santa Cruz, CA), washed at room temperature with 1% NP40 for 3 hours, incubated overnight at 4°C with Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), and then washed with 1% NP40. Controls, which were negative, included omission of the primary anti-Apc antibody, omission of both antibodies, and Apc peptide competition.
Cryosections from colon adenomas were incubated with the anti-Apc antibodies and then stained with Cy3-conjugated goat anti-rabbit IgG as described. 9
Testing for Loss of Apc Heterozygosity by Polymerase Chain Reaction
Single colossal crypts were microdissected from the isolated epithelium. Small sheets of dysplastic epithelium were microdissected from the adenomas. The microdissected tissues were placed in individual polymerase chain reaction (PCR) tubes and digested overnight at 50°C in 10 μl of lysis buffer containing 1 mg/ml proteinase K (Boehringer Mannheim, Indianapolis, IN), 10 mmol/L Tris/HCL (pH 8), 50 mmol/L KCl, 0.45% NP40, and 0.45% Tween 20. The next morning the proteinase K was heat inactivated (10 minutes at 95°C), the tubes were centrifuged for 5 minutes, and the supernatant was transferred to new tubes. Loss of heterozygosity (LOH) of the Apc gene was checked using PCR. 12 Briefly, after digestion with HindIII the ApcMin allele PCR product generates a 144-bp fragment whereas the wild-type allele PCR product generates a 123-bp fragment. Thus, two DNA bands appear on gels (12% nondenaturing polyacrylamide gel electrophoresis) from phenotypically normal tissue from ApcMin mice. In contrast, as the vast majority of adenomas in ApcMin mice result from cells that have lost the wild-type allele, 9,12 the dysplastic epithelium from adenoma displays LOH, and only one band is evident from the ApcMin allele.
Branching Crypt Frequency
Crypts from the epithelium isolated from four of the ApcMin mice were randomly selected and mounted on slides. The frequency of crypts that were branching was determined using an oil immersion 100× objective. During the scoring, colossal crypts were identified by their size (usually 5 to 10 times longer than normal) and were recorded separately. The frequencies of branching normal and colossal crypts were compared with a two-tailed test for the equality of binomial means. 13
Results and Discussion
Sections through colon adenomas from ApcMin mice often display a layer of nondysplastic, Apc-expressing crypts underlying the dysplastic tissue, but the relationship of these Apc-positive crypts to surrounding normal tissue was unclear. Some sections suggest that the crypts bordering adenomas are enlarged and extend under and sometimes into the margins of the tumor (Figure 1, A and B) ▶ . When isolated, these crypts were found to be enormous, ranging from 2 to 10 times longer than normal crypts (Figure 1, C and D) ▶ . The branching frequency of these colossal crypts (11.8%; 12 of 102 crypts scored were branching) was significantly higher (P = 10−5) than that of normal crypts (2.4%; 99 of 4048 normal crypts scored were branching).
Figure 1.
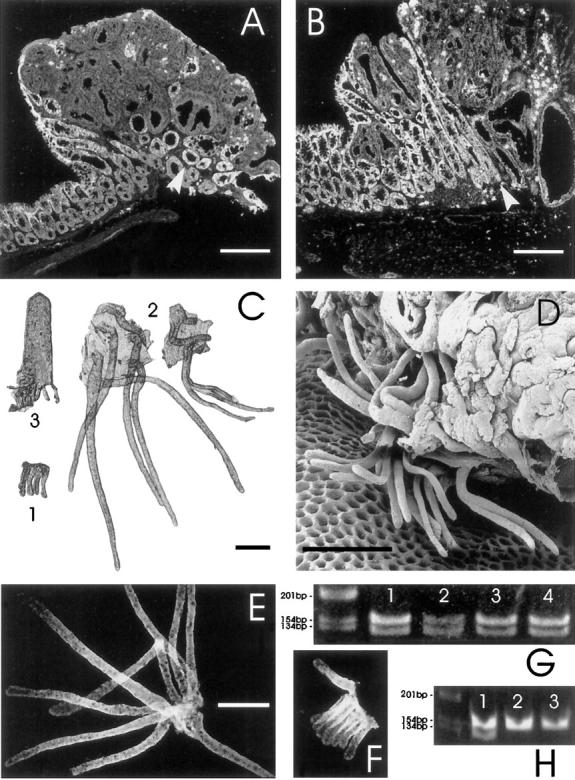
Demonstration of the presence of colossal crypts at the margin of colon adenomas from ApcMin mice and their Apc status. All scale bars represent 250 μm. A and B: Laser scanning confocal micrographs of frozen sections through colon adenomas from ApcMin mice. The sections have been stained with an antibody specific for the carboxy terminus of Apc. In A, note the absence of staining in the dysplastic tissue and the layer of Apc-positive crypts underlying the tumor (arrowhead). There are also some enlarged crypts, sectioned in a more longitudinal plane, at the left tumor margin. In B, note the large Apc-positive branching nondysplastic crypt at the tumor margin (arrowhead). C: Photomicrograph of intestinal epithelium isolated from an ApcMin mouse. A group of crypts from normal colon is labeled 1. Compare them with the two sets of colossal crypts from the margins of an adenoma, labeled 2. Note the branching colossal crypt on the right. A normal crypt-villus unit isolated from proximal jejunum, labeled 3, is shown for size comparison. D: Scanning electron micrograph showing the base of a large adenoma from the colon (note the honeycomb-like array of vacant pits in the lamina propria, from which crypts have been extracted by the isolation procedure). A few colossal crypts, which have been extracted from the margins of the tumor, remain attached to the tumor by surface epithelium. Many of the crypts are branched. The crypts shown are ∼500 μm long (approximately double the normal crypt height), although they appear smaller in this view due to foreshortening and partial obstruction of the crypt tops. Dysplastic epithelium is visible in the upper right corner. E and F: Fluorescence micrographs of isolated colossal (E) and normal crypts (F) stained for Apc protein with an antibody specific for the carboxy terminus. This demonstrates that the cells in colossal crypts are making full-length Apc. The figures are at the same magnification. G: Ethidium-bromide-stained gel showing PCR product from single colossal crypts (lanes 1 and 2) and groups of normal crypts (lanes 3 and 4). Note the presence of both wild-type (123-bp) and ApcMin (144-bp) alleles in both colossal and normal crypts. This confirms the antibody results presented in E and F. H: Demonstration of the loss of heterozygosity that occurs in dysplastic epithelium from ApcMin mice. Lane 1 shows PCR product from normal epithelium (with both Apc alleles) whereas lanes 2 and 3 show product from isolated dysplastic epithelium from adenomas (demonstrating only the ApcMin allele).
Crypt branching is the usual means of crypt replication with consequent epithelial growth. 14,15 The most appealing interpretation of the observation of an increased frequency of branching among the colossal crypts is that they are cycling faster than are normal crypts, potentially adding to tumor bulk with time. However, simply observing an increase in branching frequency is not sufficient to prove that the crypts are cycling faster. For example, if colossal crypts require a longer time to complete the branching process, due perhaps to their large size, then an increased proportion of branching crypts would be expected even if normal and colossal crypts have similar crypt cycle times.
Abnormalities in the frequency of branching non-neoplastic crypts have been reported in murine (ApcMin) and human (familial adenomatous polyposis) APC± colon. 16-18 It seems unlikely that this effect is directly related to the increased branching frequency among the colossal crypts found at tumor margins as the general effect is small and age dependent in mouse colon. 17,18 In humans, dysplastic crypts have a greatly increased branching frequency in comparison with non-neoplastic crypts. 16-18 Others have reported a 19-fold increase in branching rates of non-neoplastic crypts in these patients, 18 although we found no evidence of this 17 when obviously dysplastic crypts were excluded from counts (dysplastic crypts are frequent even when care is taken to sample only regions containing no macroscopic lesions).
Unusual tubular structures have also been observed near the margins of a wide variety of ulcers. 19 These highly contorted structures grow out from the base of neighboring crypts and are composed of a specialized nonproliferative cell lineage. 19 In contrast, the colossal crypts are independent structures with a well defined crypt base indicating that they are probably a distinct phenomenon.
Dysplastic epithelium from colon adenomas displays LOH of the Apc locus (Figure 1H) ▶ , as is usually the case in tumors from ApcMin mice. 9,12 The dysplastic epithelium also does not stain with the anti-Apc antibody specific for the carboxy terminus of Apc protein (Figure 1, A and B) ▶ . In contrast, the colossal crypts do stain (Figure 1, A, B, and E) ▶ and have wild-type Apc alleles (Figure 1G) ▶ indicating normal Apc gene function. Furthermore, the Apc staining pattern, which normally increases in intensity from crypt base to top (Figure 1F ▶ 20 ), appears normal in colossal crypts (Figure 1E) ▶ , suggesting a normal differentiation program in the colossal crypt cells.
As loss of function of the Apc gene, usually by LOH, 9,12 is the cause of dysplasia in this mouse model, our findings are consistent with previous conclusions that the nondysplastic colossal crypts found on the borders of tumors are indeed reactive and are not directly involved in tumorigenesis, 6,7 although they probably add to tumor bulk. This raises the question of the signal inducing the exceptional size and branching frequency of these crypts. Is it a growth factor originating from the tumor, or could it be a cytokine released as a result of immune interactions in the tumor? The response of the epithelium is so dramatic that the underlying cause should be investigated, as it likely involves potent epithelial trophic factors that may have clinical application, for example, in inducing epithelial repair.
Footnotes
Address reprint requests to Dr. Matthew Bjerknes, Department of Anatomy and Cell Biology, Medical Sciences Building, University of Toronto, Toronto, Ontario M5S 1A8, Canada. E-mail: bjerknes@crypt.med.utoronto.ca.
Supported by the Medical Research Council and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Filipe MI: Value of histochemical reactions for mucosubstances in the diagnosis of certain pathological conditions of the colon and rectum. Gut 1969, 10:577-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdette WJ: Carcinoma of the colon and antecedent epithelium. Cancer Res 1970, 30:253-256 [PubMed] [Google Scholar]
- 3.Dawson PA, Filipe MI: An ultrastructural and histochemical study of the mucous membrane adjacent to and remote from carcinoma of the colon. Cancer 1976, 37:2388-2398 [DOI] [PubMed] [Google Scholar]
- 4.Riddle RH, Levin B: Ultrastructure of the “transitional” mucosa adjacent to large bowel carcinoma. Cancer 1977, 40:2509-2522 [DOI] [PubMed] [Google Scholar]
- 5.Lawson MJ, White LM, Coyle P, Butler RN, Roberts-Thomson LC, Conyers RAJ: An assessment of proliferative and enzymatic activity in transitional mucosa adjacent to colonic cancer. Cancer 1989, 64:1061-1066 [DOI] [PubMed] [Google Scholar]
- 6.Isaacson P, Attwood PRA: Failure to demonstrate specificity of the morphological and histochemical changes in mucosa adjacent to colonic carcinoma (transitional mucosa). J Clin Pathol 1979, 32:214-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jass JR, Sobin LH: Histological Typing of Intestinal Tumours, ed 2. New York, Springer-Verlag, 1989, p 3
- 8.Su L-K, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF: Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992, 256:668-670 [DOI] [PubMed] [Google Scholar]
- 9.Reitmair AH, Cai J-C, Bjerknes M, Redston M, Cheng H, Pind MTL, Hay K, Mitri A, Bapat BV, Mak TW, Gallinger S: MSH2 deficiency contributes to accelerated APC mediated intestinal tumorigenesis. Cancer Res 1996, 56:2922-2926 [PubMed] [Google Scholar]
- 10.Bjerknes M, Cheng H: Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec 1981, 199:565-574 [DOI] [PubMed] [Google Scholar]
- 11.Anderson TF: Techniques for the preservation of three dimensional structure in preparing specimens for the electron microscope. Trans NY Acad Sci 1951, 13:130-134 [Google Scholar]
- 12.Luongo C, Moser AR, Gledhill S, Dove WF: Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res 1994, 54:5947-5952 [PubMed] [Google Scholar]
- 13.Lloyd E: Statistics, vol VI, part A: Handbook of Applicable Mathematics. Edited by Ledermann H. Toronto, John Wiley and Sons, 1984, pp 236–237
- 14.Bjerknes M: A test of the stochastic theory of stem cell differentiation. Biophys J 1986, 49:1223-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Totafurno J, Bjerknes M, Cheng H: The crypt cycle: crypt and villus production in the intestinal epithelium. Biophys J 1987, 52:279-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng H, Bjerknes M, Amar J, Gardiner G: Crypt production in normal and diseased human colonic epithelium. Anat Rec 1986, 216:44-48 [DOI] [PubMed] [Google Scholar]
- 17.Bjerknes M, Cheng H, Hay K, Gallinger S: APC mutation and the crypt cycle in murine and human intestine. Am J Pathol 1997, 150:833-839 [PMC free article] [PubMed] [Google Scholar]
- 18.Wasan HS, Park H-S, Liu KC, Mandir NK, Winnett A, Sasieni P, Bodmer WF, Goodlad RA, Wright NA: APC in the regulation of intestinal crypt fission. J Pathol 1998, 185:246-255 [DOI] [PubMed] [Google Scholar]
- 19.Wright NA, Pike C, Elia G: Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature 1990, 343:82-85 [DOI] [PubMed] [Google Scholar]
- 20.Smith KJ, Johnson KA, Bryan TM, Hill DE, Markowitz S, Willson JKV, Petersen GM, Hamilton SR, Vogelstein B, Kinzler KW: The APC gene product in normal and tumor cells. Proc Natl Acad Sci USA 1993, 90:2846-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]


