Abstract
X-linked Alport syndrome is a progressive nephropathy associated with mutations in the COL4A5 gene. The kidney usually lacks the α3-α6 chains of collagen type IV, although each is coded by a separate gene. The molecular basis for this loss remains unclear. In canine X-linked hereditary nephritis, a model for X-linked Alport syndrome, a COL4A5 mutation results in reduced mRNA levels for the α3, α4, and α5 chains in the kidney, implying a mechanism coordinating the production of these 3 chains. To examine whether production of α6 chain is under the same control, we studied smooth muscle cells from this animal model. We determined the canine COL4A5 and COL4A6 genes are separated by 435 bp, with two first exons for COL4A6 separated by 978 bp. These two regions are ≥ 78% identical to the human sequences that have promoter activity. Despite this potential basis for coordinated transcription of the COL4A5 and COL4A6 genes, the α6 mRNA level remained normal in affected male dog smooth muscle while the α5 mRNA level was markedly reduced. However, both α5 and α6 chains were absent at the protein level. Our results suggest that production of the α6 chain is under a control mechanism separate from that coordinating the α3-α5 chains and that the lack of the α6 chain in Alport syndrome is related to a failure at the protein assembly level, raising the possibility that the α5 and α6 chains are present in the same network. The lack of the α6 chain does not obviously result in disease, in particular leiomyomatosis, as is seen in Alport patients with deletions involving the COL4A5 and COL4A6 genes.
Alport syndrome is a group of genetic disorders of collagen type IV with progressive glomerular disease 1,2 characterized by a multilaminar appearance of the glomerular basement membrane (GBM) by electron microscopy 3 and an abnormal GBM composition with respect to type IV collagen. 4-9 Rare patients with X-linked Alport syndrome also suffer from diffuse leiomyomatosis (DL) in which there is a diffuse or multinodular proliferation of smooth muscle in the esophagus and sometimes in the trachea, bronchial tree and genital area. 10-12
Type IV collagen is a family of triple helical isoforms comprised of six distinct chains, designated α1-α6 encoded by 6 different genes, COL4A1-COL4A6, respectively. 13 The α1 and α2 chains are present in all basement membranes; the α3-α6 chains have a more restricted distribution. In the kidney, the α3, α4, and α5 chains are present in the GBM, whereas the α6 chain colocalizes with the α1, α2, and α5 chains in Bowman’s capsule and basement membranes of vascular smooth muscle cells. 5,8,9 Outside of the kidney, the α6 chain is coexpressed with these same three chains in basement membranes of smooth muscle cells in the esophagus, uterus, bladder, some other parts of the GI tract, some blood vessels, as well as in the basement membranes of the epidermis and bronchial tree; all these basement membranes lack the α3 and α4 chains. 8,9,14 Curiously, the only site where all six chains colocalize in is seminiferous tubule basement membrane. 15
The COL4A5 and COL4A6 genes are paired in a head-to-head fashion on the X chromosome. 16-18 Over 200 mutations have been found in the COL4A5 gene in families with X-linked Alport syndrome. 19 In patients with both Alport syndrome and DL, deletions are present involving the 5′ ends of the COL4A5 and COL4A6 genes, and the deletions in COL4A6 do not extend beyond intron 2. 10-12,20 How collagen gene mutations alter the structure of type IV collagen remains undefined. In Alport syndrome, most COL4A5 mutations lead to absence of the α5 chain from the basement membranes, as well as the α3, α4, and α6 chains in the kidney 4-9 and the α6 chain in the skin. 21 This implies that a mechanism exists for the synthesis of certain basement membranes that links the assembly, at either the mRNA or protein level, of triple helical molecules containing the α5 chain with triple helical molecules containing the α3, α4, and/or α6 chains (depending on the specific basement membrane involved). The existence of an α1-α6 network and α3-α6 network in seminiferous tubule basement membrane 15 indicates a structural linkage between the α3-α6 chains and suggests that an α5 chain could be required at the protein level for the incorporation of these other chains into specific basement membranes. In Alport syndrome with DL, both the α5 and α6 chains are missing from the esophagus 22 Because the esophagus has never been studied in pure Alport syndrome patients (ie, those lacking DL), it is unknown whether the α6 chain is absent in the setting of mutation confined to the COL4A5 gene or whether loss of the α6 chain in this site is unique to Alport syndrome with DL. If the latter is true, then loss of the α6 chain could be important in the pathogenesis of DL.
The purpose of the present study was to determine the influence of a COL4A5 mutation on the expression of α6 chain at the mRNA and protein levels. This was accomplished using a unique family of Samoyed dogs with an X-linked form of Alport syndrome that closely resembles the human disease at the clinical, genetic, morphological, and immunohistochemical levels 23-27 and that is caused by a single base substitution in the COL4A5 gene that results in a premature stop codon. 28 Previously, we had shown in this model the existence of a mechanism coordinating the expression of the α3, α4, and α5 chains at the mRNA level. 29 The present study extends these results by showing this coordinating mechanism does not include the α6 chain, hence the loss of the α6 chain in Alport syndrome is a failure at the protein assembly level. Moreover, the loss of the α6 chain does not, on its own, result in DL.
Materials and Methods
Generation of COL4A5 and COL4A6 Promoter Regions
Pairs of synthetic oligonucleotide primers for were constructed based on sequences of the human α5 cDNA 30 and human α6 cDNA. 17 The sequences for the α5 primers were:
External: sense: 5′ AGA CTC AGG GCC AGT AAG A 3′
Internal: sense: same as external
The sequences for the α6 primers were from human exon 2:
External: antisense: 5′ TTC CTC GGT CAG GCA CAA 3′
Internal: antisense: 5′ AAC CAG GAG CAG CCA CAA 3′
The locations of the primers corresponded to bp 138–156 (sense) according to the sequence published by Sugimoto et al 30 and bp 23–40 (external antisense) and bp 2–19 (internal antisense) of exon 2 according to the sequence published by Oohashi et al. 17 The first round of PCR was performed using 600 ng of dog genomic DNA from kidney cortex prepared as previously described 28 as a template. To this were added 300 ng of both external primers in Perkin-Elmer PCR buffer containing 1.5 mmol/L MgCl2 and 2 μl 10 mmol/L dNTPs, and 0.5 μl of AmpliTaq Gold (2.5 U) to a total volume of 100 μl. Samples were denatured at 95°C for 10 minutes and 35 cycles were carried out in a Perkin-Elmer Cetus DNA thermal cycler. Each cycle consisted of denaturation at 95°C for 1 minute, annealing at 55°C for 1 minute and extension at 72°C for 2 minutes. For the second round of PCR, a 2-μl aliquot of the first PCR reaction mixture was amplified by the same method, except using the internal primers.
Nucleotide Sequencing
The amplified product was purified by QIAEX II Gel Extraction Kit (Qiagen, Santa Clarita, CA) according to the manufacturer’s instructions. The sequence was obtained by subcloning the PCR product into the TA cloning PCR vector (Invitrogen, San Diego, CA), then using a Thermal Sequenase Fluorescent-labeled Primer Cycle Sequencing kit (Amersham, Oakville, ON) and a Licor 4000L sequencer.
Preparation of RNA from Dog Bladder
Normal and affected dogs (paired littermates born to 2 different carrier females) were sacrificed at 1 or 3 months of age and samples of bladder tissue snap-frozen in liquid nitrogen. Total RNA was prepared from 0.5 g of bladder tissue by Trizol reagent (Gibco BRL, Burlington, ON) and the mRNA was recovered using the Straight A’s mRNA Isolation System (Novagen, Madison, WI).
Northern Blot Analysis and Densitometry
Samples (1–1.5 μg) of mRNA prepared from normal and affected male dog bladder were separated by electrophoresis and blotted as previously described. 28 The probes for the α1, α2, α5, and α6 mRNAs were cDNAs for the respective canine NC1 domains that were obtained using nested PCR reactions as previously described. 28,29 Probes for exon 1′ and exon 1 of COL4A6 were obtained from a PCR product that included both exons. The external and internal sense primers (which were identical) were based on canine DNA upstream of exon 1′ with the following sequence: 5′ GCG GAT GGG TCT TAG AAG 3′. The antisense primers were the same as those used to generate the COL4A5 and COL4A6 promoter regions described above. The PCR reactions were performed as mentioned above. A 1.58-kb PCR fragment was generated and then digested with RsaI, HhaI and TaqI. The 260-bp fragment from the 5′ end of the PCR product up to the first TaqI cut site included all of exon 1′. The 397 bp fragment between the second and third HhaI cut sites included all of exon 1. Both fragments were purified by the QIAEX II Gel Extraction Kit (Qiagen). All probes were labeled with 32P-dCTP by random primer synthesis. A probe for actin was used as a control for loading. Each gel was run in duplicate. Hybridization signals from the autoradiographs were quantitated using a Molecular Dynamics computing 300A densitometer. The signals from the α(IV) transcripts were quantitated relative to the signal from the actin transcript to control for variations in gel loading. All measurements were taken at two different exposure times.
Immunohistochemistry for the α1-α6 Chains of Collagen Type IV
For immunostaining, samples of bladder from 1- and 3-month-old dogs, skin from 1-month-old dogs, and lung and testis from 3-month-old dogs were embedded in OCT, snap-frozen in liquid nitrogen, sectioned at 5 μm, and fixed in acetone. The antibodies used were rat monoclonals raised against peptide sequences specific for each of the human α1-α6 chains of collagen type IV. Their specificity has been previously established. 8,21 These antibodies react with α1-α6 chains of dog glomeruli. 31 Sections were pretreated with an acid-KCl solution (pH 1.5) to expose epitopes and then blocked with 1.5% normal rabbit serum (Sigma, St. Louis, MO). An ABC immunoperoxidase technique was used: the primary antibody (1:100 dilution for 1.5 hours) was followed by a biotinylated rabbit anti-rat antibody (1:200 dilution for 1 hour) (Vector Laboratories, Burlingame, CA), then a peroxidase-conjugated avidin-biotin complex for 30 minutes (Santa Cruz Biotechnology, Santa Cruz, CA), with a 5-minute incubation in diaminobenzidine as a chromogen. Sections were then counterstained with hematoxylin.
Results
Comparison of Nucleotide Sequence of the Normal Canine and Human α5/α6 Promoter Region
The human COL4A6 gene has two first exons, with the exon closer to the COL4A5 gene designated exon 1′ and the more distal one exon 1. 30 The canine COL4A6 gene contains regions comparable to the human gene; analysis of the PCR fragments from canine genomic DNA provided the nucleotide sequence for 36 bp from the start of the translated region of the COL4A5 gene, the 217 bp of the 5′ untranslated region of the COL4A5 gene, the 435 bp separating the canine COL4A5 and COL4A6 genes, the 93 bp corresponding to exon 1′ of COL4A6, the 978 bp separating exon 1′ and exon 1 of COL4A6, the 239 bp corresponding to exon 1 of COL4A6 and the 197 bp of intron 1 of COL4A6. This sequence has been deposited in GenBank and is presented in part in Figure 1 ▶ . The degree of identity between dog and human sequences varied from 77–97%, 30 depending on the region: 97% for the translated portion of the COL4A5 gene, 82% for the untranslated portion of the COL4A5 gene, 84% for the region between the COL4A5 and COL4A6 genes, 78% for exon 1′ of the COL4A6 gene, 77% for the region between exon 1′ and exon 1 of COL4A6, 87% for exon 1 of the COL4A6 gene, and 86% for the part of intron 1 which could be compared (human sequence is not available for all of intron 1).
Figure 1.
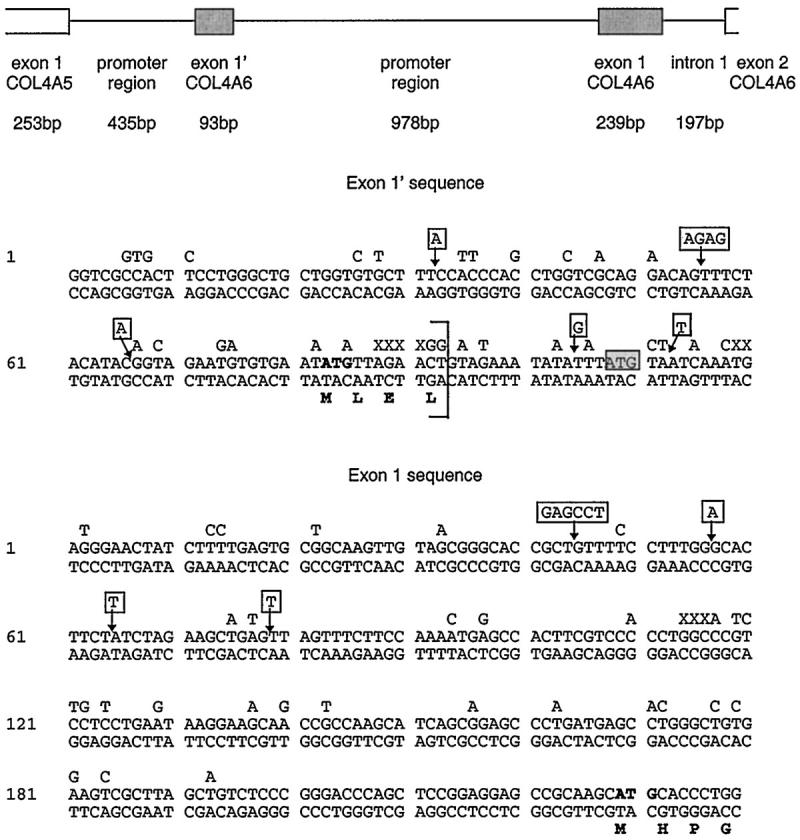
Genomic organization of the canine COL4A5/COL4A6 promoter region. Sequences for potential exons 1′ and 1 are given below. Base pairs above the sequence are given for the human sequence where it differs from the canine. Additional base pairs in the canine sequence are indicated by X. Sequences absent in the canine sequence but present in the human are boxed with an arrow indicating the site of deletion. Potential translated sequences are in bold. The start codon for the human exon 1′ is boxed in gray and lies outside the border of the canine exon 1′ (bracketed).
The canine COL4A5 and COL4A6 genes are in a head-to-head arrangement and are transcribed from opposite strands. The intergenic region is a possible promoter region. 30 Within this, no TATA box is found; instead there are 3 A+T-rich sequences which are conserved between the human and dog genomes. Potential motifs for transcription factors include three CCAAT sequences (CAT box) and three CCCTCCC sequences (CTC box); two of these are conserved between the human and dog genome. No GC boxes are present. The region between exon 1′ and exon 1 of COL4A6 is a second possible promoter region. In this sequence, there is also no TATA box but there are eight A+T-rich sequences, five of which are conserved between the human and dog genomes. There are also three CAT box sequences but no CTC box sequences. Only one of the CAT boxes in conserved in the human sequence; there is also a CAT box in the human sequence that is not conserved in the dog sequence.
For the designated exon 1′, the 3′ boundary for this exon was chosen in accord with the consensus rules for splice sites for exons. This region is poorly conserved between human and dog genomes, resulting in a difference in size of exon 1′ between the two species and a change in the signal peptide. The start of the canine translated region encodes four residues (compared to five in human exon 1′); only the first two residues are identical in both species. 30 In contrast, for the sequence designated as exon 1, the signal peptide is identical to that for the human exon 1.
Northern Blot Analysis of Canine α(IV) mRNAs
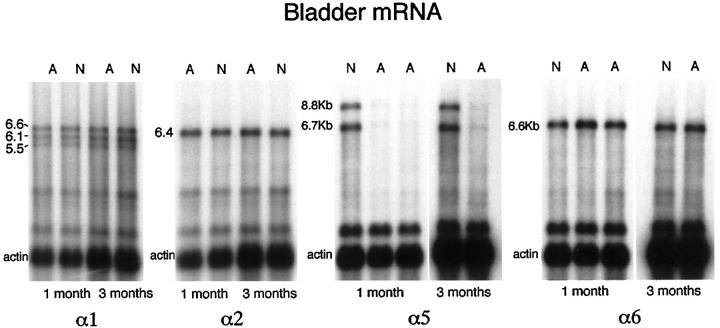
For the α1 mRNA, transcripts were present at 6.6, 6.1, and 5.5 kb in both normal and affected dog bladder in similar amounts (Figure 2) ▶ . For α2 mRNA, a single transcript was present at 6.4 kb in both normal and affected dog bladder, at similar levels. The sizes of the mRNAs matched what had been found previously in dog kidney. 29 As reported previously 28 for canine α5 mRNA, a minor transcript at ∼8.6 kb and a major transcript at ∼6.7 kb were identified in both normal and affected male dog bladder. As with kidney, the abundance of both α5 transcripts was decreased in affected dogs by 92% as determined by densitometry. The canine α6 mRNA only a single transcript 6.5 kb was detected in both normal and affected dog bladder. The level of this message was comparable in affected and normal dogs. There were no differences between the results obtained at the 1- and 3-month time points in this study. Probes specific for exon 1 and exon 1′ of COL4A6 were used to determine which of these two exons was used in mRNA from dog bladder smooth muscle. By Northern analysis, only the probe for exon 1 resulted in a signal (Figure 3) ▶ .
Figure 2.
Northern analysis comparing α(IV) transcripts in bladder of normal (N) and affected (A) male dogs with X-linked HN at both 1 and 3 months of age. A probe for actin was used to control for loading. For α1 mRNA there are three transcripts of ∼6.6, 6.1, and 5.5 kb. For α2 mRNA there is a single transcript of ∼6.4 kb. For α5 mRNA there are two transcripts of ∼8.6 and 6.7 kb in normal dogs, with almost no signal in affected male dogs. For α6 mRNA there is a single transcript of 6.5 kb.
Figure 3.
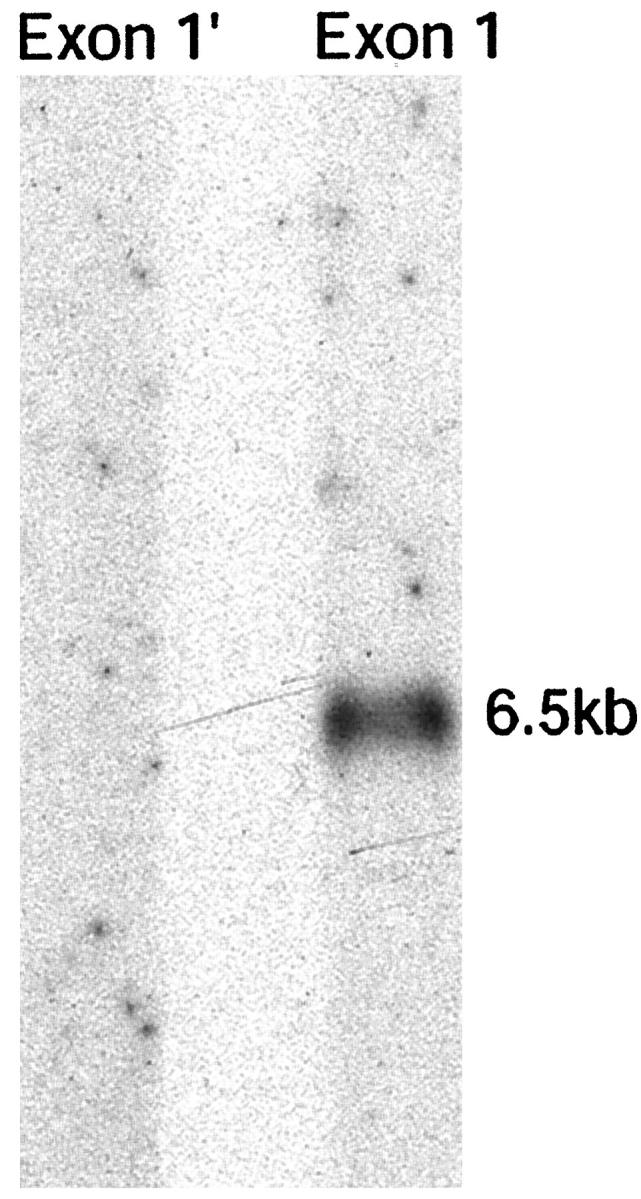
Northern blot analysis comparing use of exon 1 and exon 1′ in α6 mRNA from dog bladder. Only the lane probed for exon 1 results in a signal.
Immunohistochemical Studies
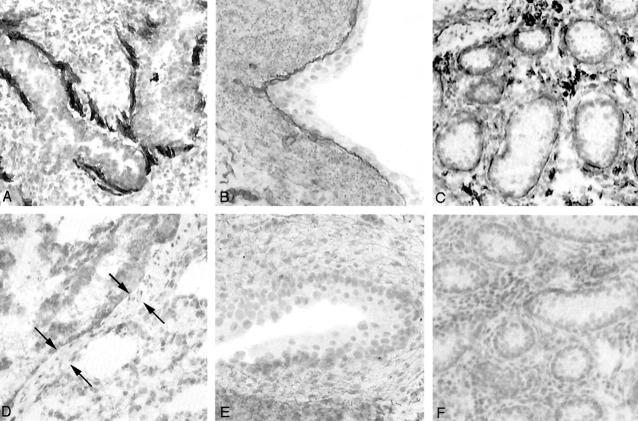
Basement membranes in the bladder are found underlying the mucosal lining, and around each smooth muscle cell in the muscularis propria layer and in vessels. In normal dogs, the basement membranes surrounding smooth muscle cells were positive for the α1, α2, α5, and α6 chains (Figure 4) ▶ . No basement membrane in the bladder was positive for either the α3 or α4 chains. In affected male dogs, all basement membranes were positive for the α1 and α2 chains only; no staining for the α3-α6 chains was seen. There was no apparent increase in the amount of staining for the α1 or α2 chains. Staining for the α6 chain was also examined in lung, skin, bladder mucosa and testis (Figure 5) ▶ . In normal dogs, the α6 chain was present in basement membranes surrounding smooth muscle of vessels and airways, underlying bronchial and bladder mucosal surfaces, at the dermal-epidermal junction and surrounding seminiferous tubules. None of these basement membranes was positive for the α6 chain in affected male dogs.
Figure 4.
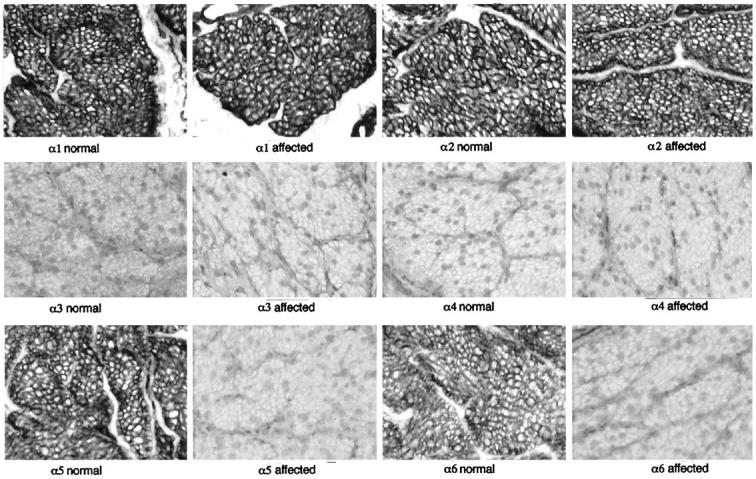
Immunoperoxidase staining of normal and affected male dog smooth muscle in bladder using monoclonal antibodies against the α1-α6 chains of collagen type IV. Only the α5 and α6 chains show a difference with absent staining in affected male dogs. There is no apparent increase in staining for the α1 and α2 chains. Original magnification, ×225.
Figure 5.
Immunoperoxidase staining of normal dog (A-C) and affected male dog (D-F) using a monoclonal antibody against the α6 chain of collagen type IV. In normal lung, there is staining of peribronchiolar smooth muscle (A); in normal bladder mucosa, there is staining of the epithelial basement membrane (B); and in normal testis, there is staining of the seminiferous tubule basement membrane (C). No staining of any basement membranes is seen in affected dog lung (D), skin (E), or testis (F). Original magnifications, ×160 (A, C, D, and F) and ×320 (B and E).
Discussion
To determine the influence of a COL4A5 mutation on the expression of the α6 chain at the mRNA and protein levels, we used the Samoyed dog model of Alport syndrome in which there is a premature stop codon in the COL4A5 gene and almost no α5 mRNA. 28 Previously, we had found this mutation also resulted in decreased message levels for the α3 and α4 chains in the kidney; 29 hence, we asked whether the same might be true for the α6 chain, given that the COL4A5 and COL4A6 genes are head-to-head in the human genome, separated by a bidirectional promoter. Our first step was to confirm that these two genes in dogs were in a similar arrangement. Next, we sought to determine the message and protein levels for the α5 and α6 chains. Previously, in kidney the level of α6 mRNA in affected dogs was 74–80% of normal, but there is so little α6 chain in kidney cortex that it was difficult to interpret whether this was a true reduction in message (implying coordinate expression of the α3-α6 chains) or just experimental variation. Because the α6 chain is most abundant in smooth muscle, bladder was finally chosen as a source for this chain. Although esophagus is involved in patients with DL and Alport syndrome, we found there is virtually no smooth muscle in canine esophagus, as noted previously. 32 Message levels were determined at two different time points in normal and affected male dogs using chain-specific cDNA probes. 28,29 The α1-α6 chains were assessed at the protein level using chain-specific antibodies. 8,21
The canine genomic sequence obtained confirmed that the COL4A5 and COL4A6 genes were in a similar head-to-head arrangement as in the human genome and shared a similar organization as much as can be predicted from sequence comparisons. Specifically, there are regions which correspond to the two first exons and two promoter regions for COL4A6 described for the human gene. 30 Whether such regions in the canine genome have true promoter activity remains to be determined experimentally. There are, however, features in these regions similar to the promoter region separating the COL4A1 and COL4A2 genes in the human, 33,34 rat, 35 and mouse 36 genomes. This region is actually two overlapping promoters that share common elements but are unidirectional and gene-specific. 37,38 Instead of a TATA box, which normally fixes the orientation of RNA polymerase, there are A+T-rich sequences 25–30 bp upstream of exon 1 of each gene, directing transcription downstream. DNA motifs that bind transcriptional activators have been identified in the COL4A1/COL4A2 promoter including CCAAT (CAT box) and CCCTCCC sequences (CTC box). 37-39 The two putative promoter regions for the canine COL4A6 gene contain these elements, and the majority were conserved between dog and human genomes, supporting the concept that these regions may have promoter activity.
The head-to-head arrangement of the canine COL4A5 and COL4A6 genes allows for a potential coregulation of expression of these two genes. There is support for this concept at the protein level; in the developing glomerulus, the α5 and α6 chains appear concurrently in the S phase, before the appearance of the α3 and α4 chains in human 9 and dog kidney. 31 In fact, the α5 and α6 chains colocalize in all sites except the GBM, which lacks the α6 chain, 8,9 and skeletal muscle, which lacks the α5 chain. 8 We had previously uncovered evidence for coregulation of expression for the α3, α4, and α5 chains 29 and the present study addresses whether this coregulation extends to the α6 chain as well. We found there was no reduction of α6 mRNA level in affected dogs at 1 or 3 months of age despite a consistent near absence of the α5 mRNA level, as had been noted previously for kidney. We conclude that transcription of the COL4A6 gene is unimpaired in the setting of the COL4A5 mutation in this model and hence production of the α6 chain is under a separate control mechanism from that coordinating the α3-α5 chains. Similarly, a recent study using cultured dermal fibroblasts from patients with X-linked Alport syndrome showed variably reduced levels of α5 mRNA and normal levels of α6 mRNA. 40 Lack of coordination of transcription of these two chains had been predicted from previous studies in which the ratio of α5 mRNA to α6 mRNA varied from tissue to tissue, attributed to either different regulatory sequences for different tissues, or modification by posttranslational events. 41 Furthermore, GBM development normally involves a switch from a baseline α1/α2 network to a specialized α3-α5 network, an event documented in human, rodent and dog kidney. 31,42,43 Because the GBM does not contain the α6 chain, one could also predict the switch would not involve coordinating the expression of the α6 chain with the α3-α5 chains.
There are two human COL4A6 transcripts which use different exon 1s spliced to a common exon 2 and differ in the sequence of the signal peptide. 30 By RT-PCR, both transcripts are expressed in kidney, lung, and placenta, whereas only one is expressed in keratinocytes, implying there is site-specific regulation of the expression of the COL4A6 gene by alternate promoters. 8 We found smooth muscle of the dog bladder uses only exon 1 in the α6 mRNA transcript by Northern blot analysis. The signal peptide for this exon is identical to that for the human exon 1, whereas the signal peptide for the canine exon 1′ is different in both sequence and length compared to human exon 1′. This finding suggests there may be evolutionary pressure to maintain the sequence of exon 1, and perhaps this is the preferred form of the α6 chain for cells which normally assemble this chain into their basement membranes. It is equally possible that this sequence in the canine genome is not even a true exon and only one form of the canine α6 chain exists.
Even though there were no changes in the message level for the α6 chain in the setting of a COL4A5 nonsense mutation, this chain was completely absent at the protein level in bladder smooth muscle of affected dogs. This would imply the loss of the α6 chain results from a failure at the protein assembly level, secondary to absence of the α5 chain. Examination of other basement membranes in affected dogs reinforces this concept since the α5 and α6 chains are absent from the epidermis, the epithelia of bladder and lung, peribronchial smooth muscle, seminiferous tubules and Bowman’s capsule. While this likely applies to human Alport syndrome as well, until these specific tissues are studied, the possibility remains that only certain mutations in the COL4A5 gene result in loss of both the α5 and α6 chains. Our results suggest that the α5 and α6 chains are contained within the same network, but may or may not be in the same triple helical collagen molecule. Should the α5 and α6 chains form heterotrimers, then an abnormal α5 chain could lead to faulty heterotrimer assembly resulting in absence of these chains in Alport syndrome. Should the α5 chains be in trimers distinct from those containing the α6 chain, then the α5 chain may be necessary for incorporation of α6 chain-containing trimers. At present, the α6 chain has been studied at the network level only in seminiferous tubule basement membrane in which there are two α6-chain containing networks, both of which also contain the α5 chain. 15 The exact composition of trimers in these two networks remains to be determined. As well, these two networks contain the α3 and α4 chains, both of which are absent from other α6-chain-containing basement membranes such as Bowman’s capsule, epidermal basement membrane and those of smooth muscle. 8 Hence, the network arrangement in these latter sites must differ from seminiferous tubule basement membrane, and could include an α5/α6 network and/or an α1/α2/α5/α6 network.
Absence of the α6 chain has been noted in the kidney 9,21,44 and skin 8,21 of human Alport syndrome patients. There is a general lack of availability of other tissues to study in this disease. Of the α6-containing basement membranes in the body, none of these sites is involved in Alport syndrome except in patient who also have DL, in which case the esophagus, the tracheo-bronchial tree and genital tract can show nodular proliferations of smooth muscle. In the esophagus, loss of both the α5 and α6 chains from the basement membrane of the myocytes has been noted. 11 DL patients consistently have mutations involving both the COL4A5 and COL4A6 genes; 10,11,20 whereas the state of the basement membrane of myocytes in these sites in patients having mutations confined to the COL4A5 gene is unknown. Should the α6 chain be present in pure Alport syndrome patients in these sites, then absence of the α6 chain could be an important pathogenetic event in producing DL. Our results using the canine model for Alport syndrome suggest this is not the case. We studied bladder (as a substitute for esophagus since canine esophagus lacks smooth muscle 32 ), lung (which may be involved in DL), skin and testis and found that the α6 chain is completely absent from all these sites, similar to previous results in canine kidney. 31 This family of dogs has never developed leiomyomata in any site. We conclude that absence of the α6 chain alone or in combination with absence of the α5 chain is not sufficient to result in the condition of DL and some other mechanism is operational in this disease. For example, it has been postulated that the deletions in Alport syndrome with DL could result in loss of a yet undiscovered gene in intron 2 10,11 or loss of a regulatory sequence controlling smooth muscle proliferation. 45
Finally, in the human kidney, it has been proposed that the conversion from an α1/α2 network to an α3/α4/α5 network in the GBM is fundamentally important to the long term stability of the GBM in its role as an ultrafilter and this switch may confer long term stability to GBM by protecting against proteolytic degradation. 42 There is also evidence at the protein level of accumulation of the α1 and α2 chains in the GBM in Alport syndrome. 5,46 It has been proposed that this is might be a compensatory change by glomeruli to deal with a lack of the α3/α4/α5 network, or this network may provide a signal which, when absent, is permissive to overproduction of the α1 and α2 chains. As much as smooth muscle cell basement membrane exemplifies an α6-containing basement membrane, none of the above mechanisms appears to be operational. There is no obvious disease which results from the absence of the α5 and α6 chains, nor is there any increase in the production of the α1 and α2 chains, either at the mRNA or the protein level. In other words, a mutation in the COL4A5 gene can disturb the composition of basement membranes in multiple organs, but the effect of the altered structure may vary from no apparent abnormality to progressive organ disease.
Footnotes
Address reprint requests to Dr. Paul Thorner, Division of Pathology, The Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada M5G 1X8.
Supported by a grant from the Medical Research Council of Canada (MT-1325 to P.T. and R.J.) and by Grant-in-Aid for International Scientific Research (Joint Research, 09044308 to Y. N.) of the Ministry of Education, Science, Sports, and Culture, Japan.
The sequence reported in this paper has been deposited in the GenBank database (accession number AF128530).
References
- 1.Grünfeld J-P: The clinical spectrum of hereditary nephritis. Kidney Int 1985, 27:83-92 [DOI] [PubMed] [Google Scholar]
- 2.Habib R, Gubler M-C, Hinglais N, Noël L-H, Droz D, Levy M, Mahieu P, Foidart J-M, Perrin D, Bois E, Grünfeld J-P: Alport’s syndrome: experience at Hôpital Necker. Kidney Int 1982, 21(Suppl 11):S20-S28 [PubMed] [Google Scholar]
- 3.Spear G, Slusser R: Alport’s syndrome: emphasizing electron microscopic studies of the glomerulus. Am J Pathol 1972, 69:213-224 [PMC free article] [PubMed] [Google Scholar]
- 4.Butkowski R, Wieslander J, Kleppel M, Michael A, Fish A: Basement membrane collagen in the kidney: regional localization of novel chains related to collagen IV. Kidney Int 1989, 35:1195-1202 [DOI] [PubMed] [Google Scholar]
- 5.Kashtan C, Kim Y: Distribution of the α1 and α2 chains of collagen type IV and of collagens V and VI in Alport syndrome. Kidney Int 42 1992, 42:115-126 [DOI] [PubMed] [Google Scholar]
- 6.Kleppel M, Fan W, Cheong H, Kashtan C, Michael A: Immunochemical studies of the Alport antigen. Kidney Int 1992, 41:1629-1637 [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi K, Yoshikawa N, Iijima K, Kitagawa K, Nakamura H, Ito H, Yoshioka K, Kagawa M, Sado Y: Immunohistochemical study of α1–5 chains of type IV collagen in hereditary nephritis. Kidney Int 1994, 46:1413-1421 [DOI] [PubMed] [Google Scholar]
- 8.Ninomiya Y, Kagawa M, Iyama K, Naito I, Kishiro Y, Seyer J, Sugimoto M, Oohashi T, Sado Y: Differential expression of two basement membrane collagen genes, COL4A6 and COL4A5, demonstrated by immunofluorescence staining using peptide-specific monoclonal antibodies. J Cell Biol 1995, 130:1219-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peissel B, Geng L, Kalluri R, Kashtan C, Rennke H, Gallo G, Yoshioka K, Sun M, Hudson B, Neilson E, Zhou J: Comparative distribution of the α1(IV), α5(IV), and α6(IV) collagen chains in normal human adult and fetal tissues and in kidneys from X-linked Alport syndrome patients. J Clin Invest 1995, 96:1948-1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antignac C, Zhou J, Sanak M, Cochat P, Rousell B, Deschênes G, Gros F, Knebelmann B, Hors-Cayla M-C, Tryggvason K, Gubler M-C: Alport syndrome and diffuse leiomyomatosis: deletions in the 5′ end of the COL4A5 collagen gene. Kidney Int 1992, 42:1178-1183 [DOI] [PubMed] [Google Scholar]
- 11.Heidet L, Dahan K, Zhou J, Xu Z, Cochat P, Gould J, Leppig K, Proesmans W, Guyot C, Guillot M, Roussel B, Tryggvason K, Grünfeld J-P, Gubler M-C, Antignac C: Deletions of both α5(IV) and α6(IV) collagen genes in Alport syndrome and in Alport syndrome associated with smooth muscle tumours. Hum Mol Genet 1995, 4:99-108 [DOI] [PubMed] [Google Scholar]
- 12.Heidet L, Cohen-Solal L, Boye E, Thorner P, Kemper M, David A, Larget Piet L, Zhou J, Flinter F, Zhang X, Gubler M, Antignac C: Novel COL4A5/COL4A6 deletions, and further characterization of the diffuse leiomyomatosis-Alport syndrome (DL-AS) locus define the DL critical region: Cytogenet Cell Genet 1997, 78:240–246 1997, 78:240–246 [DOI] [PubMed]
- 13.Hudson B, Reeders S, Tryggvason K: Type IV collagen: Structure, gene organization, and role in human diseases: Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. Minireview. J Biol Chem 1993, 268:26033–26036 [PubMed]
- 14.Seki T, Naito I, Oohashi T, Sado Y, Ninomiya Y: Differential expression of type IV collagen isoforms, α5(IV) and α6(IV) chains, in basement membranes surrounding smooth muscle cells. Histochem Cell Biol 1988, 110:359-366 [DOI] [PubMed] [Google Scholar]
- 15.Kahsai T, Enders G, Gunwar S, Brunmark C, Wieslander J, Kalluri R, Zhou J, Noelken M, Hudson B: Seminiferous tubule membrane: composition and organization of type IV collagen chains, and the linkage of α3(IV) and α5(IV) chains. J Biol Chem 1997, 272:17023-17032 [DOI] [PubMed] [Google Scholar]
- 16.Hostikka S, Eddy R, Byers M, Höyhtya M, Shows T, Tryggvason K: Identification of a distinct type IV collagen α chain with restricted kidney distribution and assignment of its gene to the locus of X chromosome-linked Alport syndrome. Proc Natl Acad Sci USA 1990, 87:1606-1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oohashi T, Sugimoto M, Mattei M-G, Ninomiya Y: Identification of a new collagen IV chain, α6(IV), by cDNA isolation and assignment of the gene to chromosome Xq22, which is the same locus for COL4A5. J Biol Chem 1994, 269:7520-7526 [PubMed] [Google Scholar]
- 18.Zhou J, Mochizuki T, Smeets H, Antignac C, Laurila P, de Paepe A, Tryggvason K, Reeders S: Deletion of the paired α5(IV) and α6(IV) collagen genes in inherited smooth muscle tumors. Science 1993, 261:1167-1169 [DOI] [PubMed] [Google Scholar]
- 19.Knebelmann B, Breillat C, Forestier L, Arrondel C, Jacassier D, Giatras I, Drouot L, Deschênes G, Grünfeld J-P, Broyer M, Gubler M-C, Antignac C: Spectrum of mutations in the COL4A5 collagen gene in X-linked Alport syndrome. Am J Hum Genet 1996, 59:1221-1232 [PMC free article] [PubMed] [Google Scholar]
- 20.Heidet L, Cai Y, Sado Y, Ninomiya Y, Thorner P, Guicharnaud L, Boye E, Chauvet V, Solal L, Beziau A, Torres R, Antignac C, Gubler M-C: Diffuse leiomyomatosis associated with X-linked Alport syndrome: extracellular matrix study using immunohistochemistry and in situ hybridization. Lab Invest 1997, 76:233-243 [PubMed] [Google Scholar]
- 21.Sado Y, Kagawa M, Kishiro Y, Sugihara K, Naito I, Seyer J, Sugimoto M, Oohashi T, Ninomiya Y: Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different α chains of human type IV collagen. Histochem Cell Biol 1995, 104:267-275 [DOI] [PubMed] [Google Scholar]
- 22.Heidet L, Boye E, Cai Y, Sado Y, Zhang X, Fléjou J-F, Fékété F, Ninomiya Y, Gubler M, Antignac C: Somatic deletion of the 5′ ends of both the COL4A5 and COL4A6 genes in a sporadic leiomyoma of the esophagus. Am J Pathol 1998, 152:673-678 [PMC free article] [PubMed] [Google Scholar]
- 23.Baumal R, Thorner P, Valli V, Marrano P, Binnington A, Bloedow A: Renal disease in carrier female dogs with X-linked hereditary nephritis: implications for female patients with this disease. Am J Pathol 1991, 139:751-764 [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen B, Tryphonas L, Wong J, Thorner P, Maxie M, Valli V, Baumal R, Basrur P: Mode of inheritance of Samoyed hereditary glomerulopathy: an animal model of hereditary nephritis in humans. J Lab Clin Med 1986, 107:551-555 [PubMed] [Google Scholar]
- 25.Jansen B, Thorner P, Baumal R, Valli V, Maxie M, Singh A: Samoyed hereditary glomerulopathy (SHG): evolution of splitting of glomerular capillary basement membranes. Am J Pathol 1986, 125:536-545 [PMC free article] [PubMed] [Google Scholar]
- 26.Thorner P, Jansen B, Baumal R, Valli V, Goldberger A: Samoyed hereditary glomerulopathy: immunohistochemical staining of basement membranes of kidney for laminin, collagen type IV, fibronectin, and Goodpasture antigen, and correlation with electron microscopy of glomerular capillary basement membranes. Lab Invest 1987, 56:435-443 [PubMed] [Google Scholar]
- 27.Thorner P, Baumal R, Binnington A, Valli V, Marrano P, Clarke H: The NC1 domain of collagen type IV in neonatal dog glomerular basement membranes: significance in Samoyed hereditary glomerulopathy. Am J Pathol 1989, 134:1047-1054 [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng K, Thorner P, Marrano P, Baumal R, McInnes R: Canine X chromosome-linked hereditary nephritis: a genetic model for human X-linked hereditary nephritis resulting from a single base mutation in the gene encoding the α5 chain of collagen type IV. Proc Acad Natl Sci USA 1994, 91:3989-3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorner P, Zheng K, Kalluri R, Jacobs R, Hudson B: Coordinate gene expression of the α3, α4 and α5 chains of collagen type IV: evidence from a canine model of X-linked nephritis with a COL4A5 gene mutation. J Biol Chem 1996, 271:13821-13828 [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto M, Oohashi T, Ninomiya Y: The genes COL4A5 and COL4A6, coding for basement membrane collagen chains α5(IV) and α6(IV), are located in head-to-head in close proximity on human chromosome Xq22 and COL4A6 is transcribed from two alternative promoters. Proc Natl Acad Sci USA 1994, 91:11679-11683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey S, Zheng K, Sado Y, Naito I, Ninomiya Y, Jacobs R, Hudson B, Thorner P: The role of distinct type IV collagen networks in glomerular development and function. Kidney Int 1998, 54:1857-1866 [DOI] [PubMed] [Google Scholar]
- 32.Evans H: Miller’s Anatomy of the Dog. 3rd edition. Philadelphia, WB Saunders Co, 1993, pp 422–425
- 33.Pöschl E, Pollner R, Kühn K: The genes for the α1(IV) and α2(IV) chains of human basement membrane collagen type IV are arranged head-to-head and separated by a bidirectional promoter of unique structure. EMBO J 1988, 7:2687-2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soininen R, Huotari M, Hostikka S, Prockop D, Tryggvason K: The structural genes for α1 and α2 chains of human type IV collagen are divergently encoded on opposite DNA strands and have an overlapping promoter region. J Biol Chem 1988, 263:17217-17220 [PubMed] [Google Scholar]
- 35.Grande J, Melder D, Kluge D, Wieben E: Structure of the rat collagen IV promoter. Biochim Biophys Acta 1996, 1309:85-88 [DOI] [PubMed] [Google Scholar]
- 36.Burbelo P, Martin G, Yamada Y: α1(IV), and α2(IV) collagen genes are regulated by a bidirectional promoter, a shared enhancer. Proc Natl Acad Sci USA 1988, 85:9679-9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heikkilä P, Soininen R, Tryggvason K: Directional regulatory activity of cis-acting elements in the bidirectional α1(IV) and α2(IV) collagen gene promoter. J Biol Chem 1993, 268:24677-24682 [PubMed] [Google Scholar]
- 38.Schmidt C, Fischer G, Kadner H, Genersch E, Kühn K, Pöschl E: Differential effects of DNA-binding proteins on bidirectional transcription from the common promoter region of human collagen type IV genes COL4A1 and COL4A2. Biochim Biophys Acta 1993, 1174:1-10 [DOI] [PubMed] [Google Scholar]
- 39.Fischer G, Schmidt C, Opitz J, Cully Z, Kühn K, Pöschl E: Identification of a novel sequence element in the common promoter region of human collagen type IV genes, involved in the regulation of divergent transcription. Biochem J 1993, 292:687-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki S, Zhou B, Fan W, Kim Y, Barker D, Denison J, Atkin C, Gregory M, Zhou J, Segal Y, Sado Y, Ninomiya Y, Michael A, Kashtan C: Expression of mRNA for type IV collagen α1, α5 and α6 chains by cultured dermal fibroblasts from patients with X-linked Alport syndrome. Matrix Biol 1998, 17:279-291 [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Ding M, Zhao Z, Reeders S: Complete primary structure of the sixth chain of human basement membrane collagen α6(IV): isolation of the cDNAs for α6(IV) and comparison with five other type IV collagen chains. J Biol Chem 1994, 269:13193-13199 [PubMed] [Google Scholar]
- 42.Kalluri R, Shield F, Todd P, Hudson B, Neilson E: Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest 1997, 99:2470-2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miner J, Sanes J: Collagen IV α3, α4, and α5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 1994, 127:879-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naito I, Kawai S, Nomura S, Sado Y, Osawa G, Network JA: Relationship between COL4A5 gene mutation and distribution of type IV collagen in male X-linked Alport syndrome. Kidney Int 1996, 50:304-311 [DOI] [PubMed] [Google Scholar]
- 45.Ueki Y, Naito I, Oohashi T, Sugimoto M, Seki T, Yoshioka H, Sado Y, Sato H, Sawai T, Sasaki F, Matsuoka M, Fukuda S, Ninomiya Y: Topoisomerase I, and II consensus sequences in a 17 kb deletion junction of the COL4A5, and COL4A6 genes, and immunohistochemical analysis of esophageal leiomyomatosis associated with Alport syndrome. Am J Hum Genet 1998, 62:253-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashtan C, Michael A: Alport syndrome. Kidney Int 1996, 50:1445-1463 [DOI] [PubMed] [Google Scholar]