Abstract
Many studies have established that the Swi/Snf family of chromatin-remodeling complexes activate transcription. Recent reports have suggested the possibility that these complexes can also repress transcription. We now present chromatin immunoprecipitation evidence that the Swi/Snf complex of Saccharomyces cerevisiae directly represses transcription of the SER3 gene. Consistent with its role in nucleosome remodeling, Swi/Snf controls the chromatin structure of the SER3 promoter. However, in striking contrast to activation by Swi/Snf, which requires most Swi/Snf subunits, repression by Swi/Snf at SER3 is dependent primarily on one Swi/Snf component, Snf2. These results show distinct differences in the requirements for Swi/Snf components in transcriptional activation and repression.
Keywords: Swi/Snf, repression, chromatin remodeling, Saccharomyces cerevisiae
The Saccharomyces cerevisiae Swi/Snf complex is the founding member of a large family of ATP-dependent chromatin-remodeling complexes that have been well characterized as transcriptional activators (Kingston and Narlikar 1999; Sudarsanam and Winston 2000; Vignali et al. 2000; Narlikar et al. 2002). Genetic and biochemical studies from both yeast and humans have provided strong evidence that Swi/Snf complexes can be recruited to the promoters of specific genes (Peterson and Workman 2000). Once at a promoter, these complexes can remodel nucleosomes to facilitate the binding of transcription factors to their sites on nucleosomal DNA (Peterson and Workman 2000; Vignali et al. 2000).
In addition to their roles as transcriptional activators, several studies have suggested that Swi/Snf complexes serve as transcriptional repressors (Sudarsanam and Winston 2000; Urnov and Wolffe 2001). This idea arose both from studies of specific genes and from whole-genome expression analyses (for review, see Sudarsanam and Winston 2000; see also Angus-Hill et al. 2001). In addition, biochemical experiments have shown that Swi/Snf complexes can remodel nucleosomes in both directions between an inactive and a remodeled state (Lorch et al. 1998; Schnitzler et al. 1998). Although these reports support a role for Swi/Snf in repression of transcription, no experiments have tested whether Swi/Snf repression in vivo is direct or indirect, and if it involves the nucleosome-remodeling activity of Swi/Snf. Recent studies of two Swi/Snf-related complexes, Isw2 and RSC, have suggested that these complexes play direct roles in repression of transcription (Goldmark et al. 2000; Kent et al. 2001; Damelin et al. 2002; Ng et al. 2002).
The experiments presented in this paper investigate the repression of the S. cerevisiae SER3 gene by Swi/Snf. Our results strongly suggest a direct role for Swi/Snf in transcriptional repression via controlling chromatin structure. Surprisingly, and in contrast to Swi/Snf activation, Swi/Snf repression has a strong requirement for only one Swi/Snf component, the Snf2 ATPase.
Results and Discussion
Repression of SER3 is dependent primarily on the Snf2 ATPase
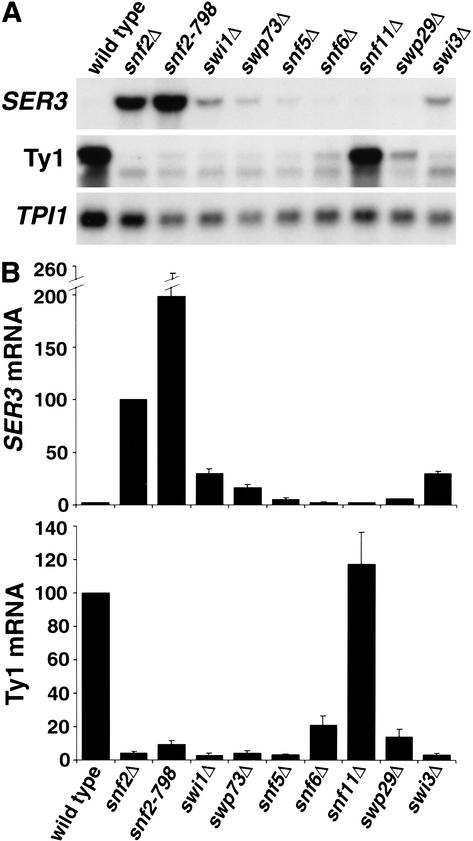
To investigate the role of Swi/Snf in transcriptional repression, we chose to study the S. cerevisiae SER3 gene, which encodes an enzyme required for serine biosynthesis (E. Albers, pers. comm.). Genome-wide expression analyses of snf2Δ and swi1Δ mutants identified SER3 as a gene strongly repressed by Swi/Snf in rich medium (Holstege et al. 1998; Sudarsanam et al. 2000). Surprisingly, repression of SER3 is three times more dependent on Snf2 than on Swi1 (Sudarsanam et al. 2000). This result contrasts with analyses of genes activated by Swi/Snf, including HO, SUC2, and Ty1 elements, which suggest an equal dependence on Snf2, Swi1, and most other Swi/Snf subunits (Winston and Carlson 1992). To investigate further the requirement for Swi/Snf components for both activation and repression, we tested eight mutants, each lacking a different Swi/Snf subunit, for the levels of Ty1 and SER3 mRNAs by Northern analysis (Fig. 1). As expected, seven of these swi/snf mutants had at least an 80% decrease in Ty1 mRNA levels, showing that these subunits are strongly required for activation of Ty1 transcription. Snf11 was not required for Ty1 activation, which is expected because Swi/Snf mutant phenotypes were not detected previously for a snf11Δ mutant (Treich et al. 1995). In striking contrast to Swi/Snf activation, only Snf2, the catalytic ATPase subunit, was strongly required for repression of SER3. Two snf2 mutations, snf2Δ and snf2-798 (an allele encoding a K to A change of amino acid 798 that no longer has ATPase activity; Khavari et al. 1993), caused a 50-fold or greater increase in SER3 mRNA levels. The derepression of SER3 in the snf2-798 mutant indicates that the ATPase activity of Snf2 is important for its role in repression. Among the remaining Swi/Snf subunits tested, swp73Δ, swi1Δ, and swi3Δ showed a moderate repression defect, whereas snf5Δ, snf6Δ, swp29Δ, and snf11Δ mutants had wild-type levels of repression. Therefore, repression of SER3 was dependent predominantly on a single subunit, Snf2. These results suggest a fundamental difference in the mechanisms by which Swi/Snf confers repression and activation.
Figure 1.
Repression of SER3 is strongly dependent on Snf2. (A) Northern analysis of SER3, Ty1, and TPI1 mRNAs. RNA was isolated from wild-type (FY2082), snf2Δ (FY2083), snf2-798 (FY2084), swi1Δ (FY1852), swp73Δ (FY1702), snf5Δ (FY1658), snf6Δ (FY2085), snf11Δ (FY2086), swp29Δ (FY2087), and swi3Δ (FY2088) strains grown in YPD to 1–2 × 107 cells/mL. (B) Quantitation of Northern analysis. The relative levels of the SER3 (top bar graph) and Ty1 (bottom bar graph) mRNAs were measured by PhosphorImager (Molecular Dynamics) and normalized to the level of TPI1 mRNA. The level of SER3 mRNA in the snf2Δ strain and Ty1 mRNA in the wild-type strain were set to 100. Each value represents the average and standard error of at least three independent experiments.
The lack of a role for Snf5 in repression of SER3 emphasizes the difference between Swi/Snf activation and repression, as several previous studies have shown that Snf5 plays important roles in Swi/Snf complex formation and chromatin-remodeling activity. First, in vitro studies of human Swi/Snf defined the Snf5 homolog INI1 as one of four core members of the complex that are sufficient to reconstitute a level of remodeling activity equivalent to that of the complete complex (Phelan et al. 1999). Second, in snf5Δ mutants the size of the remaining Snf2-containing complex is ∼700 kD, a significant decrease from the 2-MD Swi/Snf complex in wild-type cells (Peterson et al. 1994; Geng et al. 2001). Third, in studies of activation, a snf5 mutation has been identified that blocks nucleosome remodeling by Swi/Snf in vivo, but does not block complex formation (Geng et al. 2001). Therefore, Snf5 plays critical roles in many contexts; however, it does not appear to play any significant role in SER3 repression.
Swi1 and Swp73 play minor roles in SER3 repression
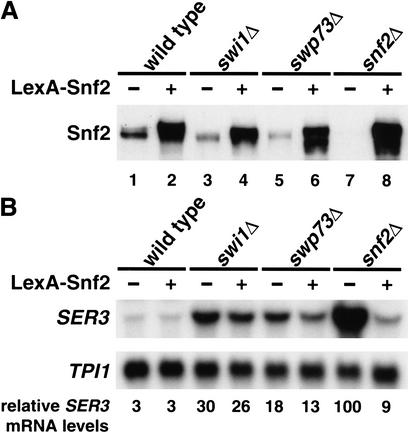
The modest requirement for some Swi/Snf subunits in SER3 repression could be due either to a direct effect on Swi/Snf's repression activity or to an indirect effect by a role in Snf2 stability. In support of the second possibility, we observed that Snf2 levels are decreased in swi1Δ and swp73Δ mutants (Fig. 2A, cf. lanes 1, 3, and 5), consistent with previous work that suggested that the stability of Swi/Snf components is mutually dependent (Peterson and Herskowitz 1992). Therefore, we tested whether the modest defect in SER3 repression observed in swi1Δ and swp73Δ mutants was caused by reduced Snf2 levels. To do this, we overexpressed a functional lexA–SNF2 fusion in swi1Δ, swp73Δ, and snf2Δ strains and assayed Snf2 protein levels (Fig. 2A) and SER3 mRNA levels (Fig. 2B). In all strains, overexpression of lexA–SNF2 resulted in a level of Snf2 greater than that of a wild-type strain (Fig. 2A). However, the elevated level of Snf2 failed to fully repress SER3 in either the swi1Δ or swp73Δ mutants (Fig. 2B). As expected, overexpression of lexA–SNF2 complemented the snf2Δ repression defect (Fig. 2B). We conclude from these results that Swi1 and Swp73 likely play direct, albeit minor roles in Swi/Snf repression of SER3.
Figure 2.
A subset of Swi/Snf subunits plays a minor role in repression. (A) Western analysis of wild-type (FY2089), swi1Δ (FY2091), swp73Δ (FY2093), and snf2Δ (FY2095) strains containing pJAM198 (no LexA–Snf2), or wild-type (FY2090), swi1Δ (FY2092), swp73Δ (FY2093), and snf2Δ (FY2096) strains containing pJAM200 (expressing LexA–Snf2). Strains were grown to 1–2 × 107 cells/mL in YPD medium supplemented with 200 μg/mL G418 to maintain selection for the plasmids. Equal amounts (50 μg) of whole-cell extracts were separated by SDS-PAGE and Western-blotted using an anti-Snf2 antibody. (B) Northern analysis of SER3 and TPI1 was performed on RNA isolated from the same cultures used in A. Each value represents the average level of SER3 mRNA normalized to TPI1 mRNA from two independent experiments.
Snf2 and Snf5 are physically present at the SER3 promoter
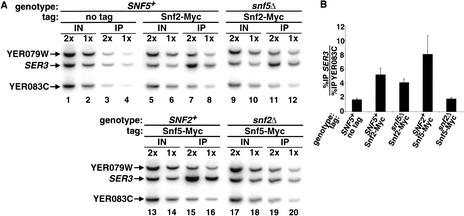
A major question regarding the function of Swi/Snf in transcriptional repression is whether its role is direct or indirect. To address this question, we used the method of chromatin immunoprecipitation to examine whether Snf2 is physically present at the SER3 promoter. To allow specific immunoprecipitation of Snf2, we used a version of Snf2 fused to 18 copies of the Myc epitope. The SNF2–Myc allele encodes a functional Snf2 protein as it fully complemented all phenotypes caused by a snf2Δ mutation (data not shown). Our results show that Snf2 was physically present at the SER3 promoter (Fig. 3, cf. lanes 3,4 and 7,8). Consistent with the observation that Snf5 was not required for SER3 repression (Fig. 1), Snf2 remained associated with the SER3 promoter in a snf5Δ strain (Fig. 3, lanes 11,12). The physical association of Snf2 with the SER3 promoter, taken together with the strong requirement for Snf2 in SER3 repression, strongly suggests that Snf2 is a direct repressor of SER3 transcription.
Figure 3.
Snf2 and Snf5 are recruited to the SER3 promoter. (A) Chromatin immunoprecipitations were performed on wild-type (FY2103) and snf5Δ (FY2102) strains expressing Snf2–Myc and on wild-type (FY2101) and snf2Δ (FY2104) strains expressing Snf5–Myc. An untagged strain (FY1338) was used as a negative control. Snf2–Myc and Snf5–Myc were immunoprecipitated with A14 anti-Myc antibody (Santa Cruz) from strains grown in YPD medium. The PCR products correspond to the promoter region of SER3 and the promoter regions of two flanking genes, YER079W and YER083C, which serve as negative controls. One set of PCR reactions from twofold dilutions of each chromatin sample is shown. (B) Quantitation of chromatin immunoprecipitation. The %IPs of SER3 and YER083C were calculated for each strain. Each value represents the average ratio of %IP SER3 to %IP YER083C with the standard error from three independent experiments.
We also used chromatin immunoprecipitation to test for the presence of Snf5, a Swi/Snf subunit not required for SER3 repression. As for Snf2, we used a functional SNF5–Myc fusion protein. These results show that Snf5–Myc was also present at the SER3 promoter (Fig. 3, lanes 15,16). Consistent with a loss of repression of SER3 observed in the snf2Δ mutant, the association of Snf5 with SER3 was dependent on Snf2 (Fig. 3, lanes 19,20). The presence of Snf5 suggests that the entire Snf/Swi complex associates with the SER3 promoter, although many of the subunits, including Snf5, are not required for SER3 repression.
Snf2 controls the chromatin structure at the SER3 promoter
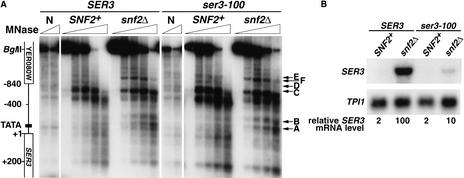
Previous in vivo and in vitro studies have established that activation by Swi/Snf occurs via an alteration of chromatin structure (Kingston and Narlikar 1999; Peterson and Workman 2000; Vignali et al. 2000; Narlikar et al. 2002). To test whether repression by Swi/Snf also involves chromatin changes, we compared the sensitivity of the SER3 promoter to cleavage by micrococcal nuclease (MNase) in SNF2+ (repressed) and snf2Δ (derepressed) strains. The results of indirect end-labeling experiments revealed two significant sets of differences in the SER3 MNase digestion pattern between the two strains (Fig. 4A). First, in the SER3 promoter region surrounding a consensus TATA site, there was little MNase cleavage in the SNF2+ strain; however, two sites (A and B) were hypersensitive to MNase digestion in the snf2Δ strain. Second, three sites further 5′ of the TATA were cleaved by MNase in SNF2+ chromatin (C,D, and E), but were protected in chromatin isolated from an snf2Δ strain. In addition, a new MNase cleavage site (F) appeared. To determine if the altered MNase cleavage pattern in the snf2Δ mutant is caused by the loss of Snf2 rather than the increased level of transcription, we assayed the MNase cleavage pattern of SNF2+ and snf2Δ strains in which transcription of SER3 is greatly reduced by a mutation in the SER3 TATA element (ser3-100). Although this TATA mutation caused a 10-fold reduction in SER3 mRNA levels in an snf2Δ mutant (Fig. 4B), the changes in the MNase cleavage pattern were identical to those observed at the wild-type SER3 promoter (Fig. 4A). These results strongly suggest that Snf2 is required to maintain a repressive chromatin structure over the SER3 promoter.
Figure 4.
Swi/Snf regulates chromatin structure over the SER3 promoter. (A) SNF2+ (FY2097), snf2Δ (FY2098), SNF2+ ser3-100 (FY2099), and snf2Δ ser3-100 (FY2100) strains were grown in YPD medium to 1–2 × 107 cells/mL. Spheroplasts were isolated and incubated with increasing amounts of MNase. DNA was isolated, digested with BglII, and subjected to indirect end-labeling analysis using a probe that anneals to +301 to +529 (+1 = ATG) in the coding sequence of SER3. All strains were deleted for the SER33 ORF to prevent cross-hybridization of the SER3 probe. The SER3 genomic region is diagramed on the left, and the approximate positions of altered MNase cleavage sites are marked with arrows on the right. N denotes the naked DNA controls. (B) Northern analysis of SER3 was performed on RNA isolated from the same strains listed in A. The level of SER3 mRNA was normalized to TPI1 and set to 100 for the snf2Δ strain. The average and standard error for SER3 mRNA levels from four independent experiments are reported as follows: SNF2+, 2.2 ± 0.7; snf2Δ, 100; SNF2+ ser3-100, 1.5 ± 0.3; snf2Δ ser3-100, 10 ± 2.
Our findings for Swi/Snf, taken together with recent reports that show the physical presence of RSC and Isw2 at the promoters of repressed genes (Goldmark et al. 2000; Kent et al. 2001; Damelin et al. 2002; Ng et al. 2002), provide strong evidence for nucleosome-remodeling complexes acting directly to repress transcription. Although we have shown that Swi/Snf associates with the SER3 promoter, the mechanism of Swi/Snf recruitment to this promoter remains unknown. Swi/Snf may be recruited through interaction with a DNA-binding protein, in a manner similar to Isw2 recruitment by the Ume6 repressor (Goldmark et al. 2000; Kent et al. 2001). Alternatively, Swi/Snf might have binding specificity for a particular chromatin structure at the SER3 promoter.
There are several possible mechanisms by which Swi/Snf could repress transcription. First, based on in vitro experiments showing that Swi/Snf can catalyze remodeling of nucleosomes in either direction between the inactive and remodeled states (Lorch et al. 1998; Schnitzler et al. 1998), Swi/Snf might create an inactive nucleosome conformation at SER3 that prevents transcription-factor access to the promoter. Second, Swi/Snf could facilitate the binding of a transcriptional repressor of SER3. Finally, Swi/Snf nucleosome remodeling could facilitate a subsequent step required for repression, such as histone modification. Recent studies have shown that some Swi/Snf-related complexes associate with histone deacetylase activity (Narlikar et al. 2002). Identification of the cis-acting elements and other trans-acting factors required for SER3 regulation should provide additional insights into the mechanism by which Swi/Snf represses transcription.
Materials and methods
S. cerevisiae strains and methods
All S. cerevisiae strains used in this study (Table 1) are derivatives of a GAL2+ S288C strain (Winston et al. 1995). Standard strain construction methods and media recipes were as described previously (Rose et al. 1990). The snf2Δ::LEU2 (Cairns et al. 1996), snf2-798 (K to A change of amino acid 798; Khavari et al. 1993), swp73Δ1::LEU2 (Cairns et al. 1996), swi1Δ1::LEU2 (Peterson and Herskowitz 1992), and snf5Δ2 (Sudarsanam et al. 1999) alleles have been described previously. Strains containing snf6Δ::kanMX and snf11Δ::kanMX were constructed through crosses with commercially available deletions (Research Genetics). The ser33Δ::kanMX, swp29Δ::kanMX, and swi3Δ::kanMX alleles were constructed by replacing the open reading frames with the kanMX marker (Baudin et al. 1993; Lorenz et al. 1995; Brachman et al. 1998). SER33, a gene with 82% identity to SER3, was deleted in strains FY2097, FY2098, FY2099, and FY2100 to prevent cross-hybridization to the SER3 probe used in our chromatin analysis experiments. Deletion of SER33 had no effect on SER3 mRNA levels (data not shown). The SNF2–C18Myc and SNF5–C18Myc alleles marked by K. lactis TRP1 were generated by introducing 18 copies of the Myc epitope at the C-terminal end of the SNF2 and SNF5 genes by PCR-mediated integration using plasmid pWZV88. Both strains were wild type for all phenotypes tested, including growth on glucose and raffinose and in the absence of inositol (data not shown). They also maintained complete repression of SER3 (data not shown). The ser3-100 mutants, which carry a TATAAA → CCTAGG mutation in a putative TATA box at −103 to −98 (+1 = ATG) within the SER3 promoter, were constructed by two-step gene replacement using plasmid pJAM196.
Table 1.
Saccharomyces cerevisiae strains
| Strain
|
Genotype
|
|---|---|
| FY1338 | MATαura3Δ0 trp1Δ63 his3Δ200 lys2Δ0 met15Δ0 leu2Δ0 |
| FY1658 | MATa ura3-52 his3Δ200 lys2-128δ snf5Δ2 |
| FY1702 | MATa ura3Δ0 his3Δ200 lys2Δ0 leu2Δ0 swp73Δ1∷LEU2 |
| FY1852 | MATa ura3Δ0 his3Δ200 ade8 met15Δ0 leu2Δ0 swi1Δ1∷LEU2 |
| FY2082 | MATa ura3Δ0 trp1Δ63 his3Δ200 lys2Δ0 met15Δ0 |
| FY2083 | MATa ura3Δ0 his3Δ200 lys2Δ0 met15Δ0 leu2Δ0 snf2Δ∷LEU2 |
| FY2084 | MATa ura3Δ0 snf2-798 |
| FY2085 | MATa ura3Δ0 his3Δ200 snf6Δ∷kanMX |
| FY2086 | MATa ura3Δ0 his3Δ200 snf11Δ∷kanMX |
| FY2087 | MATa ura3Δ0 his3Δ200 swp29Δ∷kanMX |
| FY2088 | MATa ura3Δ0 swi3Δ∷kanMX |
| FY2089 | MATa ura3Δ0 trp1Δ63 his3Δ200 lys2Δ0 met15Δ0 <pJAM198> |
| FY2090 | MATa ura3Δ0 trp1Δ63 his3Δ200 lys2Δ0 met15Δ0 <pJAM200> |
| FY2091 | MATa ura3Δ0 his3Δ200 ade8 met15Δ0 leu2Δ0 swi1Δ1∷LEU2 <pJAM198> |
| FY2092 | MATa ura3Δ0 his3Δ200 ade8 met15Δ0 leu2Δ0 swi1Δ1∷LEU2 <pJAM200> |
| FY2093 | MATa ura3Δ0 his3Δ200 leu2Δ0 swp73Δ1∷LEU2 <pJAM198> |
| FY2094 | MATα ura3Δ0 his3Δ200 lys2Δ0 leu2Δ0 swp73Δ1∷LEU2 <pJAM200> |
| FY2095 | MATa ura3Δ0 his3Δ200 lys2Δ0 met15Δ0 leu2Δ0 snf2Δ∷LEU2 <pJAM198> |
| FY2096 | MATa ura3Δ0 his3Δ200 lys2Δ0 met15Δ0 leu2Δ0 snf2Δ∷LEU2 <pJAM200> |
| FY2097 | MATa ura3Δ0 his3Δ200 lys2Δ0 leu2Δ0 ser33Δ∷kanMX |
| FY2098 | MATa ura3Δ0 his3Δ200 lys2Δ0 leu2Δ0 ser33Δ∷kanMX snf2Δ∷LEU2 |
| FY2099 | MATa ura3Δ0 his3Δ200 lys2Δ0 leu2Δ0 ser33Δ∷kanMX ser3-100 |
| FY2100 | MATa ura3Δ0 his3Δ200 lys2Δ0 leu2Δ0 ser33Δ∷kanMX snf2Δ∷LEU2 ser3-100 |
| FY2101 | MATα ura3Δ0 trp1Δ63 his3Δ200 lys2Δ0 met15Δ0 leu2Δ0 SNF5-C18MYC∷TRP1 |
| FY2102 | MATa ura3Δ0 his3Δ200 lys2-128δ snf5Δ2 SNF2-C18MYC∷TRP1 |
| FY2103 | MATα ura3Δ0 trp1Δ63 his3Δ200 lys2Δ0 met15Δ2 leu2Δ0 SNF2-C18MYC∷TRP1 |
| FY2104 | MATα ura3Δ0 or ura3-52 trp1Δ63 his3Δ200 lys2Δ0 met15Δ2 leu2Δ0 or leu2Δ1 SNF5-C18MYC∷TRP1 snf2Δ∷LEU2 |
Plasmid DNA construction and analysis
Plasmids were constructed and isolated from Escherichia coli by standard methods (Ausubel et al. 1988). pJAM196 was constructed by ligating an EcoRI DNA fragment containing SER3 sequence from −340 to +252 (+1 = ATG) into pRS406 (Christianson et al. 1992) and subsequently changing the sequence of a TATA box (TATAAA) to an AvrII site (CCTAGG) using the Stratagene QuikChange Site-Directed Mutagenesis Kit. pJAM198 and pJAM200 are derivatives of pRS423 (Christianson et al. 1992) and pLEXA-SNF2 (Laurent et al. 1991), in which the HIS3 marker has been replaced with the kanMX marker (Brachmann et al. 1998) by plasmid gap repair (Muhlrad et al. 1992).
Northern hybridization analysis
Cells were grown in YPD media to a concentration of 1–2 × 107 cells/mL. To maintain plasmids pJAM198 and pJAM200, YPD media was supplemented with 200 μg/mL of G418 (Invitrogen). Total yeast RNA was prepared and separated on a 1% agarose gel as described previously (Ausubel et al. 1988). A probe specific to SER3 was synthesized by PCR-amplification of DNA from +1378 to +1626 (+1 = ATG) and radiolabeled with [α-32P]dATP by random priming (Ausubel et al. 1988). The TPI1 and Ty1 probes have been described previously (Winston et al. 1987; Hirschhorn et al. 1992).
Western analysis
Yeast cultures were grown to 1–2 × 107 cells/mL, and whole-cell extracts were prepared by bead lysis. Protein concentrations were measured by Bradford assay (Bio-Rad). Equal amounts of extract were separated by SDS-PAGE and transferred to immobilon membrane (Millipore). Snf2 and LexA–Snf2 proteins were detected using an anti-Snf2 antibody (1:2000; Geng et al. 2001), followed by HRP-conjugated secondary antibody (1:5000) and chemiluminescence.
Chromatin immunoprecipitation
The procedure for chromatin immunoprecipitation was adapted from previously described methods (Dudley et al. 1999; Kuras and Struhl 1999). Briefly, cells from 200-mL YPD cultures were cross-linked by adding formaldehyde to a final concentration of 1%. Chromatin was prepared in FA lysis buffer containing 140 mM NaCl and no SDS. Cross-linked chromatin was sonicated to an average length of 500 bp with a size range from 200 to 1200 bp. Snf2–Myc and Snf5–Myc were immunoprecipitated from 1/10 of the cross-linked chromatin by a two-step method (Harlow and Lane 1999) using rabbit polyclonal anti-Myc A14 antibody (Santa Cruz) followed by IgG-sepharose beads (Pharmacia). Dilutions of input DNA (1/1000 and 1/2000) and immunoprecipitated DNA (1/2.5 and 1/5) were subjected to quantitative radioactive PCR as described (Larschan and Winston 2001), and the products were separated on a 7.5% nondenaturing polyacrylamide gel. The SER3 promoter region was detected using a primer set that amplifies a 302-bp product from −424 to −123 (+1 = ATG). Primer sets amplifying a 358-bp product of YER079W from −475 to −117 (+1 = ATG) that is 3.9 kb 5′ of SER3 and a 273-bp product of YER083C from −301 to −29 (+1 = ATG) that is 4.4 kb 3′ of SER3 were added to each PCR reaction as negative controls. The relative amount of each PCR product immunoprecipitated (%IP) was calculated as described previously (Larschan and Winston 2001). Specific binding of Snf2–Myc and Snf5–Myc to SER3 was evaluated by calculating the ratio of the %IP of SER3 to the %IP of YER083C for each strain.
Analysis of chromatin structure by MNase
Yeast strains were grown in YPD media to 1–2 × 107 cells/mL. Spheroplasts were isolated and subjected to micrococcal nuclease (MNase) digestion as adapted from previously described methods (Kent et al. 1993; Kent and Mellor 1995). Approximately 1.2 × 109 cells were incubated with 2 mg/mL zymolyase (ICN 100,000 units/g) for 2 min. Spheroplasts from 2 × 108 cells were aliquoted and digested with 0, 0.625, 1.25, 2.5, or 5 units of MNase at 37°C for 4 min. Purified genomic DNA from an equivalent amount of cells was digested using either 0.5 or 0.75 units of MNase at 37°C for 1 min to serve as naked DNA controls. MNase-treated DNA samples were digested completely with BglII, separated on a 1% agarose gel, and analyzed by indirect end-labeling (Hirschhorn et al. 1992). A 228-bp PCR product corresponding to base pairs +301 to +529 (+1 = ATG) of the SER3 open reading frame was synthesized by PCR, radiolabeled by random priming (Ausubel et al. 1988), and used as the probe to detect SER3 DNA. DNA fragments of lengths 1684, 1177, 868, and 228 bp, synthesized by PCR from the SER3 region, were used as size standards to calculate positions of MNase cleavage.
Acknowledgments
We thank Andrea Duina, Jenny Wu, and Robert Kingston for critical comments on the manuscript; Nicholas Kent for helpful advice on the MNase experiments; Brehon Laurent for providing the anti-Snf2 antibody and the pLexA–SNF2 plasmid; Craig Peterson for providing the snf2-798 allele; and Wolfgang Zachariae and Kim Nasmyth for providing the pWZV88 plasmid. This work was supported by grant GM32967 from the National Institutes of Health (to F.W.) and by Postdoctoral Fellowships from the Human Frontiers Science Program and the Canadian Institute for Health Research (to J.A.M).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL winston@rascal.med.harvard.edu; FAX (617) 432-3993.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1009902.
References
- Angus-Hill ML, Schlichter A, Roberts D, Erdjument-Bromage H, Tempst P, Cairns BR. A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol Cell. 2001;7:741–751. doi: 10.1016/s1097-2765(01)00219-2. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. New York, NY: Greene/Wiley-Interscience; 1988. [Google Scholar]
- Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Cairns BR, Levinson RS, Yamamoto KR, Kornberg RD. Essential role of Swp73p in the function of yeast Swi/Snf complex. Genes & Dev. 1996;10:2131–2144. doi: 10.1101/gad.10.17.2131. [DOI] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Damelin M, Simon I, Moy TI, Wilson B, Komili S, Tempst P, Roth FP, Young RA, Cairns BR, Silver PA. The Genome-wide localization of Rsc9, a component of the RSC chromatin- remodeling complex, changes in response to stress. Mol Cell. 2002;9:563–573. doi: 10.1016/s1097-2765(02)00475-6. [DOI] [PubMed] [Google Scholar]
- Dudley AM, Rougeulle C, Winston F. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes & Dev. 1999;13:2940–2945. doi: 10.1101/gad.13.22.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F, Cao Y, Laurent BC. Essential roles of Snf5p in Snf–Swi chromatin remodeling in vivo. Mol Cell Biol. 2001;21:4311–4320. doi: 10.1128/MCB.21.13.4311-4320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark JP, Fazzio TG, Estep PW, Church GM, Tsukiyama T. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell. 2000;103:423–433. doi: 10.1016/s0092-8674(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Using antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- Hirschhorn JN, Brown SA, Clark CD, Winston F. Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes & Dev. 1992;6:2288–2298. doi: 10.1101/gad.6.12a.2288. [DOI] [PubMed] [Google Scholar]
- Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- Kent NA, Mellor J. Chromatin structure snap-shots: Rapid nuclease digestion of chromatin in yeast. Nucleic Acids Res. 1995;23:3786–3787. doi: 10.1093/nar/23.18.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent NA, Bird LE, Mellor J. Chromatin analysis in yeast using NP-40 permeabilised sphaeroplasts. Nucleic Acids Res. 1993;21:4653–4654. doi: 10.1093/nar/21.19.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent NA, Karabetsou N, Politis PK, Mellor J. In vivo chromatin remodeling by yeast ISWI homologs Isw1p and Isw2p. Genes & Dev. 2001;15:619–626. doi: 10.1101/gad.190301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khavari PA, Peterson CL, Tamkun JW, Mendel DB, Crabtree GR. BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature. 1993;366:170–174. doi: 10.1038/366170a0. [DOI] [PubMed] [Google Scholar]
- Kingston RE, Narlikar GJ. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes & Dev. 1999;13:2339–2352. doi: 10.1101/gad.13.18.2339. [DOI] [PubMed] [Google Scholar]
- Kuras L, Struhl K. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature. 1999;399:609–613. doi: 10.1038/21239. [DOI] [PubMed] [Google Scholar]
- Larschan E, Winston F. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes & Dev. 2001;15:1946–1956. doi: 10.1101/gad.911501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent BC, Treitel MA, Carlson M. Functional interdependence of the yeast SNF2, SNF5, and SNF6 proteins in transcriptional activation. Proc Natl Acad Sci. 1991;88:2687–2691. doi: 10.1073/pnas.88.7.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch Y, Cairns BR, Zhang M, Kornberg RD. Activated RSC–nucleosome complex and persistently altered form of the nucleosome. Cell. 1998;94:29–34. doi: 10.1016/s0092-8674(00)81218-0. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, Muir RS, Lim E, McElver J, Weber SC, Heitman J. Gene disruption with PCR products in Saccharomyces cerevisiae. Gene. 1995;158:113–117. doi: 10.1016/0378-1119(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- Ng HH, Robert F, Young RA, Struhl K. Genome-wide location and regulated recruitment of the RSC nucleosome-remodeling complex. Genes & Dev. 2002;16:806–819. doi: 10.1101/gad.978902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CL, Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell. 1992;68:573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Workman JL. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr Opin Genet Dev. 2000;10:187–192. doi: 10.1016/s0959-437x(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Dingwall A, Scott MP. Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc Natl Acad Sci. 1994;91:2905–2908. doi: 10.1073/pnas.91.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol Cell. 1999;3:247–253. doi: 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in yeast genetics: A laboratory course manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Schnitzler G, Sif S, Kingston RE. Human SWI/SNF interconverts a nucleosome between its base state and a stable remodeled state. Cell. 1998;94:17–27. doi: 10.1016/s0092-8674(00)81217-9. [DOI] [PubMed] [Google Scholar]
- Sudarsanam P, Winston F. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 2000;16:345–351. doi: 10.1016/s0168-9525(00)02060-6. [DOI] [PubMed] [Google Scholar]
- Sudarsanam P, Cao Y, Wu L, Laurent BC, Winston F. The nucleosome remodeling complex, Snf/Swi, is required for the maintenance of transcription in vivo and is partially redundant with the histone acetyltransferase, Gcn5. EMBO J. 1999;18:3101–3106. doi: 10.1093/emboj/18.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsanam P, Iyer VR, Brown PO, Winston F. Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci. 2000;97:3364–3369. doi: 10.1073/pnas.050407197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treich I, Cairns BR, de los Santos T, Brewster E, Carlson M. SNF11, a new component of the yeast SNF–SWI complex that interacts with a conserved region of SNF2. Mol Cell Biol. 1995;15:4240–4248. doi: 10.1128/mcb.15.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov FD, Wolffe AP. Chromatin remodeling and transcriptional activation: The cast (in order of appearance) Oncogene. 2001;20:2991–3006. doi: 10.1038/sj.onc.1204323. [DOI] [PubMed] [Google Scholar]
- Vignali M, Hassan AH, Neely KE, Workman JL. ATP-dependent chromatin-remodeling complexes. Mol Cell Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F, Carlson M. Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet. 1992;8:387–391. doi: 10.1016/0168-9525(92)90300-s. [DOI] [PubMed] [Google Scholar]
- Winston F, Dollard C, Malone EA, Clare J, Kapakos JG, Farabaugh P, Minehart PL. Three genes are required for trans-activation of Ty transcription in yeast. Genetics. 1987;115:649–656. doi: 10.1093/genetics/115.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F, Dollard C, Ricupero-Hovasse SL. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]