Abstract
Extensive neuronal loss and aggregation of tau as cytoplasmic inclusions in neurons and glial cells in selected cortical and subcortical regions is the most striking characteristic of frontotemporal dementia and parkinsonism linked to chromosome 17, which is caused by exonic or intronic mutations in the tau gene. Here, we examined the effects of four exonic mutations in four-repeat tau using stably transfected Chinese hamster ovary cells. The proportion of polymerized tubulin was the largest in the P301L transfectant. G272V and P301L transfectants showed greater instability of microtubules in the presence of Colcemid than wild-type tau, V337M, or R406W transfectants. Thus no distinct phenotypes were shared by the mutant tau transfectants with regard to microtubule assembly and stability. Unexpectedly, R406W showed low and negligible levels of phosphorylation at Thr 231 and Ser 396, respectively, in the transfectant. This presents a sharp contrast to the observation that tau aggregates in R406W-affected brains are heavily phosphorylated at these two sites. This result suggests that hyperphosphorylation at these sites cannot occur in the tau R406W bound to microtubules, and thus that the hyperphosphorylated species of tau may be generated only after disruption of microtubules.
Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) constitutes a distinct group of neurodegenerative diseases characterized clinically by behavioral disorders and parkinsonism, genetically by autosomal dominant inheritance linked to chromosome 17, and neuropathologically by filamentous tau aggregates in neurons and glial cells, accompanied by neuronal loss. 1,2 Further studies using the candidate gene approach demonstrated six missense mutations and four intronic mutations in the tau gene in FTDP-17 families. 3-7 These findings clearly indicate that these tau mutations are necessary and sufficient for the tau aggregation and neuronal loss observed in FTDP-17.
Tau, a microtubule (MT)-associated protein (MAP), binds to tubulin at three or four repeats of 31 or 32 amino acids each, called the MT-binding domain, located in the carboxyl half of tau. In adult human brain, six isoforms containing three or four repeats are generated by alternative mRNA splicing from a single gene. Six known missense mutations in tau in FTDP-17 are located within or close to the MT-binding domain, and the intronic mutations are in the 5′ splice site of exon 10, and thought to be involved in the formation of a stem loop, resulting in an increased proportion of four-repeat tau. 4-6 Regarding exonic mutations, it is tempting to speculate that these mutations in tau interfere with its interaction with tubulin and thus decrease its ability to promote tubulin assembly. 3,4 Along this line of investigation, two groups recently reported independently that the exonic mutations cause significant defects in MT assembly in the cell-free system. 8,9 However, acute depletion of tau was reported to have no effects on MT dynamics in cultured neurons 10 and, further, tau-knockout mice showed remarkably subtle effects on neurogenesis and neuronal organization. 11 These results suggest that the tau mutation effects on MT assembly found in the cell-free system may not always be problematic within the cells or in vivo. Accordingly, it is too early to speculate about the pathogenesis of FTDP-17 based on the results from the cell-free system alone. Furthermore, the effects of phosphorylation, which is known to affect the ability of tau to promote MT assembly, cannot be assessed by those cell-free experiments using Escherichia coli-produced tau. 8,9 Thus, we have established Chinese hamster ovary (CHO) cells stably transfected with wild-type and exon-mutated tau cDNA to investigate the effects of the mutations within the cell.
Materials and Methods
Antibodies and Dye
The following antibodies were used: rat monoclonal antibody to tyrosinated α-tubulin, YL1/2 (Harlan Sela-Lab, Hillcrest, UK); mouse monoclonal antibody to α-tubulin, N356 (Amersham Pharmacia Biotech, Airlington Heights, IL); mouse monoclonal antibody to hamster lysosome-associated membrane protein 2 (LAMP2), UH3 (Developmental Studies Hybridoma Bank, Iowa City, IA); and mouse monoclonal and rabbit polyclonal antibodies to tau, tau 1 (Roche Diagnostics, Tokyo), 12,13 AT8 (Innogenetics, Kapelanielaan, Belgium), 14 5E2, 15 M4 and C5, 16,17 PHF 1, 18 and AP422. 19 MitoTracker Red was purchased from Molecular Probes, Inc. (Eugene, OR).
Construction for Expression Plasmids and Transfection
Unique EcoRI and BglII sites were introduced into the 5′ and 3′ noncoding regions, respectively, of four-repeat (0N4R) tau cDNAs without exons 2 and 3, using polymerase chain reaction. The EcoRI-BglII fragment was isolated from the amplified product and ligated with pCXN 20 digested with the same endonucleases. The resultant recombinant plasmid, pCXN-4Rtau-wild-type, was used for further studies. To generate tau cDNA harboring mutations (G272V, P301L, V337M, and R406W, numbered according to the longest human tau isoform), 21 the polymerase chain reaction/oligonucleotide primer-directed mutagenesis strategy was used. The entire nucleotide sequence was determined by the dideoxynucleotide termination method using a DNA sequencer (model 4000L; LI-Cor, Lincoln, NE). The resultant amplified fragment was digested with the appropriate endonucleases and then inserted into the same sites of pCXN. The derivatives were pCXN-4Rtau G272V, P301L, V337M, and R406W.
Vector alone, vectors containing 4R wild-type tau, and mutant tau were transfected into CHO cells using Lipofectamine (Life Technologies, Rockville, MD) according to the manufacturer’s instructions. Clones that survived in G418 (Wako Pure Chemical, Tokyo; 0.4 mg/ml) were isolated and maintained at 37°C in 5% CO2 in Ham’s F-12 medium (Life Technologies) supplemented with 10% fetal bovine serum containing G418 (0.1 mg/ml).
Biochemical Analysis
Cultured cells grown in 10-cm dishes were scraped into ice-cold Tris-buffered saline (50 mmol/L Tris-HCl, pH 7.4, 0.15 mol/L NaCl) with various protease inhibitors as described before. 22 The homogenates were solubilized with sodium dodecyl sulfate and the protein concentration in each homogenate was determined by bicinchoninic acid protein assay (Pierce, Rockford, IL). For quantitative blotting, the homogenates containing the same protein amounts were spun at 5000 rpm for 5 minutes and resultant supernatants were treated by heat. The heat-stable fractions containing tau were concentrated with saturated ammonium sulfate. 22 The alkaline phosphatase treatment was performed essentially as described elsewhere. 22 Aliquots of protein were subjected to Western blotting with tau 1 or N356. The expression levels of tau and tubulin in each of the transfectants were quantitated using a GS700 Imaging Densitometer (Bio-Rad, Hercules, CA) using authentic tau or tubulin within a linear range, and particular cell sublines containing similar levels of tau (< mean ± 100%) were selected for the following studies.
Subcellular Fractionation and Isolation of Tau
Transfected cells from each confluent 15-cm culture dish were fractionated as previously described. 23 Separation of polymerized tubulin and free tubulin was performed according to the protocol designed by Merrick et al. 24 Tau, bound or unbound to MT in cultured cells, was extracted as described. 25
Immunofluorescence Microscopy
Cells were fixed with cold methanol or 4% paraformaldehyde in MT-stabilizing buffer (80 mmol/L PIPES, pH 6.9, 1 mmol/L MgCl2, 1 mmol/L EGTA), followed by incubation in 0.3% Triton X-100 in phosphate-buffered saline for 5 minutes and incubation with antibodies essentially as described. 26 For the secondary antibodies, donkey anti-mouse IgG conjugated with rhodamine or fluorescein and donkey anti-rat IgG conjugated with rhodamine were used (Jackson ImmunoResearch Lab, West Grove, PA). Anti-mouse and anti-rat antibodies were preabsorbed with rat and mouse nonimmune serum, respectively, to remove cross-reactivity. To visualize mitochondria, MitoTracker Red was added at a concentration of 300 nmol/L 30 minutes before fixation. In some experiments, cells were treated with 0.1 μg/ml Colcemid (Wako Pure Chemical) for 2 hours or 20 μmol/L cytochalasin B (Wako Pure Chemical) for 20 minutes. Labeled cells were examined under a Zeiss Axioskop microscope (Carl Zeiss Co., Tokyo).
Results
Immunocytochemical Analysis
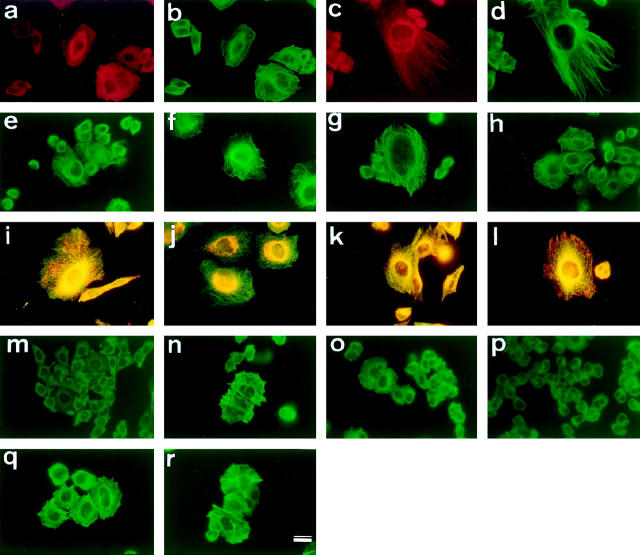
First, we examined by immunocytochemistry the intracellular distribution of tau in transfected CHO cells that do not normally express tau. When fixed with paraformaldehyde, the immunoreactivity of tau 1 was distributed diffusely in the cytoplasm, suggesting that a significant proportion of tau is unbound to MTs (Figure 1, a and b) ▶ , an observation consistent with a previous report. 25 To see the tau bound to MTs, the cells were fixed with cold methanol to wash away cytosolic tau. 26 With this fixation protocol, tau 1 immunoreactivity was almost completely colocalized with MTs (Figure 1, c and d) ▶ . In many cells, MT bundles were observable after tau transfection (Figure 1, a–h) ▶ 27 but no distinct difference in the incidence and localization of MT bundles was found among transfectants (Figure 1, c and e ▶ -h). As expected, mock-transfected cells were completely negative for tau (data not shown).
Figure 1.
CHO cells transfected with WT were fixed with paraformaldehyde (a, b) or methanol (c-r) and immunostained with tau 1 (a, c, e-h), YL1/2 (anti-tyrosinated α-tubulin) (b, d, i-l, m-r), MitoTracker Red (i, j), or UH3 (anti-LAMP2) (k, l). a, b: Tau is diffusely distributed in the cytoplasm rather than localized to MTs in the WT transfectant, when fixed with paraformaldehyde. c, d: Tau is apparently colocalized with MTs in the WT transfectant, when fixed with methanol. Some cells showed MT bundling. e-h: G272V (e), P301L (f), V337M (g), and R406W (h) transfectants were fixed with methanol and immunostained with tau 1. MT bundling is observed in all of the transfectants. No distinct distribution of tau is seen among mutant tau transfectants. i, j: Mitochondria were labeled with MitoTracker Red, and tubulin with YL1/2. Mitochondria are distributed throughout the cytoplasm of the mock transfectant, but confined around the nucleus in the WT transfectant (j), and the mutant tau transfectants (data not shown). k, l: Lysosomes were labeled with UH3, and tubulin with YL1/2. The diffuse distribution of lysosomes in the mock transfectant (k) is similar to that in the WT transfectant (l), and the mutant tau transfectants (data not shown). m-r: Transfectants were treated with 0.1 μg/ml Colcemid for 2 hours. MTs were almost completely depolymerized in the mock (m), G272V (o), and P301L transfectants (p), whereas in the WT, V337M and R406W transfectants, some MTs remained (n, q, r). Scale bar, 10 μm.
One of the physiological roles of tau within the cell is thought to be the stabilization of MTs. 27 It is possible that mutant tau molecules stabilize MTs to a lesser extent in the cell. To examine the possibility, each transfectant was treated with Colcemid, a MT-depolymerizing drug. 28 Treatment with 0.1 mg/ml Colcemid for 2 hours completely depolymerized MTs in mock-transfected cells (Figure 1m) ▶ . With the same treatment, a certain population of wild-type tau, tauV337M, and R406W (hereafter referred to as WT, V337M, and R406W, respectively) transfectants still showed MT staining (Figure 1, n, q, and r) ▶ , whereas such MT staining was very rare in tauG272V and P301L (hereafter referred to as G272V and P301L, respectively) transfectants (Figure 1, o and p) ▶ . To quantitate these results, the number of MT-positive cells per 1000 cells was counted under a fluorescence microscope. The proportion of MT-positive cells was 46% for WT, 48% for V337M, 37% for R406W, 14% for G272V, and 6.3% for P301L transfectants. Thus, G272V and P301L transfectants showed significantly greater vulnerability to Colcemide than WT transfectant (P < 0.01; Student’s t-test). This experiment was repeated four times with similar results.
It was recently reported that overexpression of tau affects kinesin-dependent trafficking of intracellular organelles. 29 To examine whether the mutations alter the intracellular distribution of organelles, mitochondria and lysosomes were labeled by MitoTracker Red and UH3 (anti-LAMP2 antibody), respectively. In mock-transfected cells, both organelles were distributed throughout the cytoplasm (Figure 1, i and k) ▶ . In contrast, in tau-transfected cells, mitochondria were confined to the perinuclear area (Figure 1j) ▶ , while lysosomes were scattered throughout the cytoplasm (Figure 1l) ▶ . These results were consistent with the previously reported result, 29 but again, no difference was observed between wild-type and mutant transfectants.
It is well known that disruption of the subcortical microfilament network induces process formation in cultured cells. 30 Because tau also binds to actin in several cell lines, 31 we examined whether tau mutants had any effect on process formation. Treatment of the transfected CHO cells with cytochalasin B induced the outgrowth of MT-containing processes from the cell surface. Although mock-transfected cells tended to give off a greater number of processes than WT and mutant tau-transfected cells, there were no significant differences in the number and length of processes among the transfectants (data not shown).
Biochemical Analysis
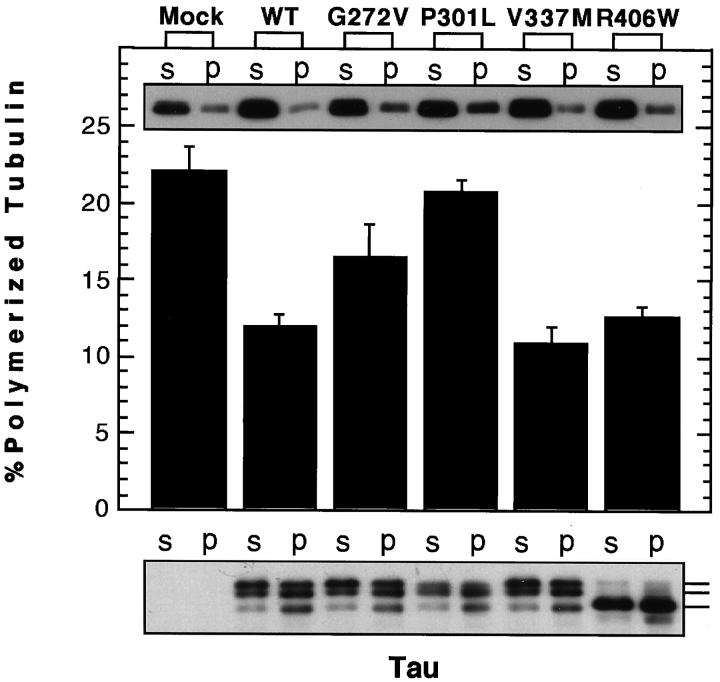
Quantitation of polymerized tubulin in each transfectant provided rather conflicting data. The expression levels of tubulin in tau transfectants appeared to be slightly increased compared to that in mock transfectants (Figures 2 and 3) ▶ ▶ . The proportion of polymerized tubulin was significantly decreased in WT, V337M, and R406W transfectants compared to that in mock transfectants (P < 0.05, Mann-Whitney U test) (Figure 2) ▶ . Furthermore, tubulin in G272V and P301L transfectants was polymerized to a greater extent than that in WT transfectants (P < 0.05, Mann-Whitney U test). These results apparently contradict the results on the ability of the mutants to promote MT assembly in the cell-free system. 8,9 Consistent with these results, tau appeared to be much more abundant in the supernatant of each transfectant homogenate (Figure 2 ▶ and legend). This probably agrees with the above-described immunocytochemical observations of tau transfectants showing diffuse cytoplasmic staining for tau when fixed with paraformaldehyde.
Figure 2.
Quantitation of polymerized tubulin and MT-bound tau. Aliquots from suspended pellets and corresponding supernatants were subjected to Western blotting for α-tubulin using N356 (top panel) and cytosolic and MT-bound tubulin were quantitated by densitometry. The results are shown as bars (middle panel) (m ± SE). For tau, each aliquot from the pellet was threefold greater in volume than that from the supernatant (bottom panel). Thus, the amount of tau in the supernatant and pellet cannot be directly compared on the blot. Three major tau 1-immunoreactive bands are discernible on the blot. The lowest band shows the same mobility as dephosphorylated four-repeat (0N4R) tau. Note that the proportion of the tau showing the fastest mobility is greater in the pellet compared to that in the supernatant, indicating that each nonphosphorylated counterpart has a higher affinity for MT (bottom panel). A weakly reactive band below the lowest tau band presumably represents a product of degradation.
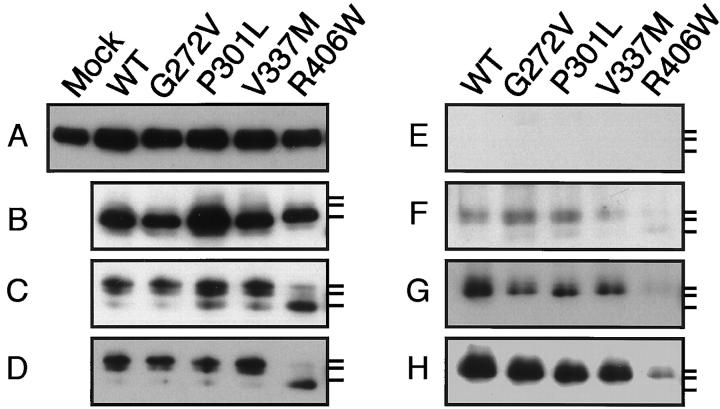
Figure 3.
Tubulin and tau in the stable transfectants. The same amount of protein was subjected to Western blotting with N356 (A), tau 1 (B and C), 5E2 (D), AT8 (E), M4 (F), C5 (G), or PHF1 (H). A: The levels of α-tubulin in all transfectants were slightly increased as compared with that in the mock transfectant. B: After alkaline phosphatase treatment, the mobility of all tau species corresponded to that of the fastest one indicated by the lowest bar on the right. C: Without alkaline phosphatase treatment, there are three reactive bands on the blot. In WT, G272V, P301L, and V337M-transfected cells, the top band was the most intense, followed by the second band, and the lowest band was very faint. In contrast, in the R406W transfectant, the lowest band was strongest. D: Labeling similar to that in C was observed with 5E2. E: No immunoreactivity for AT8 was found. F: WT, G272, P301, and V337M were phosphorylated at Thr 231 only to a slight extent, whereas this site in R406W was phosphorylated to a lesser extent. Note that only the top band is labeled for WT and all of the mutant species except R406W. G: C5 labeled all tau species but R406W, which showed almost undetectable reactivity. Note that only the top band was labeled. H: PHF 1 strongly labeled WT, G272, P301, and V337M, but labeled R406W only very weakly.
It was reported that a single tau cDNA transfected in L-cells provided multiple tau 1-immunoreactive bands on the Western blot, presumably generated by various degrees of phosphorylation. 27 In our hands, three bands were detected by tau 1 when samples were not treated with alkaline phosphatase (Figure 2) ▶ . The top band was stronger in intensity than the lower two bands in WT, G272V, P301L, and V337M-transfected cells. However, in R406W transfectants, the intensity of the lowest band was much stronger than that of the upper two bands (Figure 2) ▶ . After dephosphorylation, the mobilities of tau in all transfectants corresponded to that of the lowest band (Figure 2B) ▶ . Thus, the upper two bands and the lowest band represent differentially phosphorylated and nonphosphorylated species of tau, respectively. The three bands were probed with various phosphorylation-dependent and phosphorylation-independent antibodies including tau 1, 12,13 AT8, 14 5E2, 15 M4 and C5, 16,17 PHF 1, 18 and AP422. 19 Phosphorylation-dependent antibodies other than AT8 and AP422 (data not shown) labeled exclusively the top band except in the R406W transfectant. In this transfectant, only PHF 1 among phosphorylation-dependent antibodies weakly labeled the top band on the blot of its homogenate (Figure 3) ▶ . PHF 1 recognizes phosphorylation at both Ser 396 and Ser 404, 18 suggesting that either site is barely phosphorylated in R406W. To confirm this, C5, which is highly specific for the phosphorylation only at Ser 396, 17 was used and found to be unable to label the band. This indicates that Ser 396 is barely phosphorylated in R406W. Presumably, a lesser extent of the PHF 1 reactivity is due to lack of phosphorylation at Ser 396 and possibly at other sites, including Ser 400 and Thr 403. 19 The faint reactivity of M4 also indicates that R406W is less phosphorylated at Thr 231 (Figure 3F) ▶ . 16 Altogether, in the R406W transfectant phosphorylation of tau at Thr 231 and Ser 396 is profoundly reduced compared to that in other transfectants.
We also examined the subcellular localization of tau in the transfectants. 23 The relative amounts of tau in cytosolic, membrane, and mitochondrial fractions were quantitated by Western blotting using tau 1. In all transfectants, the fraction with the greatest abundance of tau was the membrane fraction, followed by the cytosolic fraction (data not shown). The proportion of tau in the mitochondrial fraction was significantly greater in the V337M transfectant (P < 0.05, Mann-Whitney U test). Its proportion in the membrane fraction was significantly lower in the P301L transfectant (P < 0.05, Mann-Whitney U test), whereas that in cytosolic fraction was significantly greater in the P301L transfectant (P < 0.05, Mann-Whitney U test).
Discussion
Because three of the mutations studied, namely G272V, P301L, and V337M, are located in the MT-binding domain and one mutation, R406W, is close to the domain, it has been reasonably speculated that these mutations could modify the effect of tau on MT assembly and stability. 4 Pursuing this line of investigation, two independent groups carried out functional assays on recombinant tau with these missense mutations and showed that each mutation, including G272V, P301L, V337M, or R406W, led to a significant reduction in the ability to promote MT assembly in the cell-free system. 8,9 These findings indicate that all of the missense mutations studied here share a common characteristic, reduction of the ability of tau to promote MT assembly, although the extent to which this characteristic has been reported for them differs significantly from one study to another. R406W has the least effect on MT assembly, according to one report, 8 whereas according to another it has the greatest effect. 9 Based on these results, both groups have claimed that this partial loss of function is significant in the pathogenesis of FTDP-17.
In the present study, we sought to examine the effects of the mutations on MT assembly in cultured cells, because there may be significant differences between cell-free and intracellular conditions. In fact, transfection with cDNA containing the MT-binding domain alone which worked in the cell-free system had no visible effects in cultured cells. 26 Small regions on both sides of MT-binding domain of tau were shown to be essential for its MT localization in cultured cells. 26 For this reason, we established stable cell lines expressing four-repeat (0N4R) tau without and with four missense mutations and examined the effects of these mutations within cells. In both wild-type and mutant transfectants, the proportion of polymerized tubulin was decreased compared to that in mock transfectants. In parallel with this, a much greater proportion of tau was recovered in the supernatant containing nonpolymerized tubulin (Figure 2) ▶ . We do not know the exact reason for this, but possible explanations include (i) increased levels of tubulin in the tau transfectants may reduce an apparent effect of tau, (ii) MTs in CHO cells may be almost saturated with MT-associated proteins other than tau so that only a fraction of tau can have an effect on MT assembly and stabilization, and (iii) it is possible that tau may interact with unknown proteins other than tubulin (see below), which may reduce the interaction between tau and tubulin. Thus, it is possible that the presence of tau (and tubulin) does not necessarily lead to MT assembly within the cell, an observation that contrasts with observations of the cell-free system.
The transfectants used for the present analyses exhibited similar levels of expression of tau (the maximal difference was within twofold; data not shown) and of tubulin (Figure 3) ▶ . Thus, any differences in the phenotype of CHO cells can probably be ascribed to the effects of mutation, although we cannot completely exclude the possibility that some of the effects of the mutants are caused by inherent differences in the expression levels of tau and tubulin. With this reservation, we carefully searched for any differences between wild-type and mutant tau and failed to find any effects consistently attributable to mutant tau in CHO cells. It is possible that loss of function of mutant tau is compensated by other MT-associated proteins in CHO cells that do not normally express tau. However, a fraction of transfected tau was colocalized with MTs and led to MT bundling (Figure 1) ▶ . Furthermore, wild-type and some mutant tau appeared to stabilize MTs to a greater extent against Colcemide when compared with the mock transfectant (Figure 1) ▶ . Thus, it is likely that a fraction of transfected tau has a discernible effect on the stabilization of MT in transfected CHO cells. The results on MT assembly in the cell-free system should reflect MT nucleation and growth, 8,9 but not saturation levels, and stabilization of MTs, which could be a more important role of tau within the cell 32 and presumably in vivo. Thus, our observations suggest that the reported partial loss of function of mutant tau 8,9 may not be critical for causing FTDP-17.
The Colcemid treatment allowed us to differentiate the three mutations in the MT-binding domain into two groups: G272V and P301L in one group and V337M in the other. G272V and P301L are located in the characteristic MT-binding motif, PGGG, whereas V337M is located two residues downstream of the carboxyl terminus of the third repeat. The neuropathological features reported in the affected brains harboring these mutations are quite different. 2,33 These findings may raise the possibility that the different pathologies caused by mutations within or outside the PGGG may be related to the MT stability following Colcemid treatment. However, biochemical quantitation of polymerized tubulin after Colcemid treatment did not support the view that G272V and P301L transfectants are more susceptible to Colcemid than V337M or WT transfectants (data not shown). This apparent contradiction may be related to the facts that immunofluorescence analysis here focused only on the MT-bound tau, not total tau (see above) and that only a minor proportion of tubulin is polymerized in the tau transfectants. The MTs remaining after Colcemid treatment could represent a very small proportion of tubulin, and thus may not become apparent by biochemical quantitation.
One mutation, R406W, is located close to key residues for phosphorylation, Ser 396, 400, and 404, and Thr 403, all of which are hyperphosphorylated in PHF-tau. 19 Thus, one can reasonably speculate that this mutation could have a profound effect on phosphorylation on these sites. 3,4 These sites may tend to be more easily phosphorylated and phosphorylated species may destabilize MTs, leading to neuronal degeneration. In fact, the phosphorylation at Ser 396 was previously shown to substantially decrease the affinity of tau for MTs. 25 In view of this, it is surprising to note that in the R406W transfectant, tau cannot be phosphorylated at this site. The greater resistance of the R406W transfectant to Colcemid may be also explained by the lesser extent of the phosphorylation at Ser 396, because S396A-transfected CHO cells showed greater resistance to nocodazole-induced MT depolymerization than cells expressing wild-type tau. 25 Indeed, in brains affected by the R406W mutation, tau filaments are formed and Ser 396 in the tau comprising those filaments is hyperphosphorylated. 34 Interestingly, this mutation also leads to decreased phosphorylation at Thr 231, which is also abnormally hyperphosphorylated in tau filaments in affected brains (L Reed, personal communication). 34 These results could be of particular importance for considering the significance of hyperphosphorylation of tau in filaments. Because in any transfected cell there is abundant unpolymerized tubulin in the cytoplasm, cytosolic tau is likely to bind to free (monomeric and oligomeric) tubulin; tau in the cytoplasm exists largely in the tubulin-bound form. It is possible that this tubulin-tau complex is the substrate for various protein kinases involved in tau phosphorylation. In the case of R406W, Ser 396 are not phosphorylated to any significant extent, possibly due to an aberrant conformation under normal conditions. Thus, for the two aforementioned sites of R406W to be fully phosphorylated as seen in tau aggregates in the brain with the R406W mutation, some profound derangement of MT including MT disruption in the cell would be required. The hyperphosphorylation at these sites could occur in MT-unbound tau instead of MT-bound tau, or in tubulin-stripped tau instead of tubulin-bound tau. Assuming that the neurodegeneration in FTDP-17 is due to an unknown function of mutant tau (see below), possibly due to its gain of function, the above result suggests that such hyperphosphorylation occurs in degenerating neurites and neuronal perikarya where MTs are depolymerized or tubulin is being lost. Tau could be left behind because of greater resistance to proteases and greater aggregation potential. 35 This points to the possibility that tau aggregation by itself is not the cause but a consequence of neuronal degeneration.
Tau was initially isolated as a factor that stimulated MT assembly in the cell-free system, 36 but now it is known that this protein appears to play many other roles by interacting with actin, 37 βPP, 38 PP2A, 39 PP1, 40 phospholipase Cγ, 41 presenilin 1, 42 and src-family nonreceptor tyrosine kinase 43 (for review see Ref. 44 ). As shown above, tau is not only a cytosolic protein but also exists in other compartments including the nucleolus, 45 nucleus, 46 and plasma membrane. 23 Thus, tau probably plays important roles other than MT assembly and stabilization in vivo, and much more attention should be paid to such functions of tau. This assumption may be consistent with a recent observation that N279K and S305N mutations do not affect the ability to promote MT assembly, but have potential effects through alteration of the splicing of exon 10. 47 Finally, it should be noted that the role of MT-associated proteins in MT dynamics depends on the cell type. 48 These cell type-specific roles of MT-associated proteins may explain why the present study was unable to uncover any abnormal phenotype shared by all mutant tau transfectants. In this context, studies using neuronal cells and transgenic animals are essential to an understanding of the significance of tau mutations leading to tau aggregation and neuronal loss in FTDP-17.
Acknowledgments
We thank Dr. J. Miyazaki for providing the expression vector pCNX and Drs. K. S. Kosik and S. Greenberg for providing monoclonal antibodies 5E2 and PHF 1, respectively. The mouse monoclonal antibody UH3 developed by Drs. B. L. Granger and S. Uthayakumar was obtained from the Developmental Studies Hybridoma Bank established under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa (Iowa City).
Footnotes
Address reprint requests to Yasuo Ihara, M.D., Department of Neuropathology, Faculty of Medicine, University of Tokyo, 7–3-1 Hongo, Bunkyo-ku, Tokyo 113. E-mail: yihara@m.u-tokyo.ac.jp.
References
- 1.Foster NL, Wilhelmsen K, Sima AAF, Jones MZ, D’Amato CJ, Gilman S, Spillantini MG, Lynch T, Mayeux RP, Gaskell JrPC, Hulette CM, Pericak-Vance MA, Welsh-Bohmer KA, Dickson DW, Heutik P, Kros J, van Swieten JC, Arwert F, Ghetti MB, Murrell J, Lannfelt L, Hutton M, Phelps CH, Snyder DS, Oliver E, Ball MJ, Cummings JL, Miller BL, Katzman R, Reed L, Schelper RL, Lanska DJ, Brun A, Fink JK, Khul DE, Knopman DS, Wszolek Z, Miller CA, Bird TD, Lendon C, Elechi C: Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Ann Neurol 1997, 41:706–715 [DOI] [PubMed]
- 2.Spillantini MG, Bird TD, Ghetti B: Frontotemporal dementia and parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol 1998, 8:387-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poorkaj P, Thomas DB, Wijisman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD: Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 1998, 43:815-825 [DOI] [PubMed] [Google Scholar]
- 4.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, deGraaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P: Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393:702-705 [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, Murrell JR, Goedart M, Farlow MR, Klug A, Ghetti B: Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 1998, 95:7737-7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B, Li D, Payami H, Awert F, Markkopoulou K, Andreadis A, Souza ID, Lee VM-Y, Reed L, Trojanowski JQ, Zhulareva V, Bird T, Schellenberg G, Wilhelmsen KC: Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci USA 1998, 95:13103-13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iijima M, Tabira T, Poorkaj P, Schellenberg GD, Trojanowski JQ, Lee VM-Y, Schmidt ML, Takahashi K, Nabika T, Matsumoto T, Yamashita Y, Yoshioka S, Ishino H: A distinct familial presenile dementia with a novel missense mutation in the tau gene. Neuro Report 1999, 10:497-501 [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa M, Smith MJ, Goedert M: Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 1998, 437:207-210 [DOI] [PubMed] [Google Scholar]
- 9.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, Mckeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM-Y: Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282:1914-1917 [DOI] [PubMed] [Google Scholar]
- 10.Tint I, Slaughter T, Fischer I, Black MM: Acute inactivation of tau has no effect on dynamics of microtubules in growing axons of cultured sympathetic neurons. J Neurosci 1998, 18:8660-8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N: Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 1994, 369:488-491 [DOI] [PubMed] [Google Scholar]
- 12.Liu W-K, Moore WT, Williams RT, Hall FL, Yen S-H: Application of synthetic phospho- and unphospho-peptides to identify phosphorylation sites in a subregion of the tau molecule, which is modified in Alzheimer’s disease. J Neurosci Res 1993, 34:371-376 [DOI] [PubMed] [Google Scholar]
- 13.Szendrei GI, Lee VM-Y, Otvos L, Jr: Recognition of the minimal epitope of monoclonal antibody tau-1 depends upon the presence of phosphate group but not its location. J Neurosci Res 1993, 34:243-249 [DOI] [PubMed] [Google Scholar]
- 14.Goedert M, Jakes R, Vanmechelen E: Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 1995, 189:167-1697624036 [Google Scholar]
- 15.Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM, Lee G: Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1988, 1:817-825 [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa M, Watanabe A, Takio K, Suzuki M, Arai T, Titani K, Ihara Y: Characterization of two distinct monoclonal antibodies to paired helical filaments (PHF): further evidence for fetal-type phosphorylation of the tau in PHF. J Neurochem 1993, 60:2068-2077 [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M, Jakes R, Crowther RA, Lee VM-Y, Ihara Y, Goedert M: Characterization of mA6 AP422, a novel phosphorylation-dependent monoclonalantibody against tau protein. FEBS Lett 1996, 384:25-30 [DOI] [PubMed] [Google Scholar]
- 18.Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM: Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res 1994, 39:669-673 [DOI] [PubMed] [Google Scholar]
- 19.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Titani K, Ihara Y: Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem 1995, 270:823-829 [DOI] [PubMed] [Google Scholar]
- 20.Niwa H, Yamamura K, Miyazaki J: Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108:192-200 [DOI] [PubMed] [Google Scholar]
- 21.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]
- 22.Gu T, Oyama F, Ihara Y: τ is widely expressed in rat tissues. J Neurochem 1996, 67:1235-1244 [DOI] [PubMed] [Google Scholar]
- 23.Brandt R, L 130 gar J, Lee G: Interaction of tau with the neutral plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol 1995, 131:1327–1340 [DOI] [PMC free article] [PubMed]
- 24.Merrick SE, Trojanowski JQ, Lee VM: Selective destruction of stable microtubules and axons by inhibitors of protein serine/threonine phosphatases in cultured human neurons. J Neurosci 1997, 17:5726-5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y: Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduce microtubule binding. Neuron 1993, 10:1089-1099 [DOI] [PubMed] [Google Scholar]
- 26.Lee G, Rook SL: Expression of tau protein in non-neuronal cells: microtubule binding and stabilization. J Cell Sci 1992, 102:227-237 [DOI] [PubMed] [Google Scholar]
- 27.Kanai Y, Takemura R, Oshima T, Mori H, Ihara Y, Yanagisawa M, Masaki T, Hirokawa N: Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol 1989, 109:1173-1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlow S, Gonzalez-Garay ML, West RR, Olmsted JB, Cabral F: Stable expression of heterologus MT-associated proteins (MAPs) in Chinese hamster ovary cells: evidence for differing roles of MAPs in MT organization. J Cell Biol 1994, 126:1017-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E-M: Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol 1998, 143:777-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edson K, Weisshaar B, Matus A: Actin depolymerisation induces process formation on MAP2-ransfected non-neuronal cells. Development 1993, 117:689-700 [DOI] [PubMed] [Google Scholar]
- 31.Cross D, Vial C, Maccioni RB: A tau-like protein interacts with stress fibers, and microtubules in human, and rodent cell lines J Cell Sci 1993, 105:51-60 [DOI] [PubMed] [Google Scholar]
- 32.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW: Modulation of the dynamic instability of tubuline assembly by the microtubule-associated protein tau. Mol Biol Cell 1992, 3:1141-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spillantini MG, Crowther RA, Kamphorst W, Heutink P, van Swieten JC: Tau pathology in two Dutch families with mutations in the microtubule-binding region of tau. Am J Pathol 1998, 153:1359-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van Hoesen GW, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ: Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol 1997, 42:564-572 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe A, Takio K, Ihara Y: Deamidation and isoaspartate formation in smeared tau in paired helical filaments: unusual properties of the microtubule-binding domain of tau. J Biol Chem 1999, 274:7368-7378 [DOI] [PubMed] [Google Scholar]
- 36.Weingarten MD, Lockwood AH, Hwo SY, Kirshner MW: A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 1975, 72:1858-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correas I, Padilla R, Avila J: The tubulin-binding sequence of brain microtubule-associated proteins, tau and MAP-2, is also involved in actin binding. Biochem J 1990, 269:61-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giaccone G, Pedrotti B, Migheli A, Verga L, Perez J, Racagni G, Smith MA, Perry G, De Gioia L, Selvaggini C, Salmona M, Ghiso J, Frangione B, Islam K, Bugiani O, Tagliavini F: βPP, and Tau interaction: A possible link between amyloid and neurofibrillary tangles in Alzheimer’s disease. Am J Pathol 1996, 148:79-87 [PMC free article] [PubMed] [Google Scholar]
- 39.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC: Regulation of the phosphorylation state and microtubule-binding activity of tau by protein phosphatase 2A. Neuron 1996, 17:1201-1207 [DOI] [PubMed] [Google Scholar]
- 40.Liao H, Li Y, Brautigan DL, Gundersen GG: Protein phosphatase 1 is targeted to microtubules by the microtubule-associated protein tau. J Biol Chem 1998, 273:21901-21908 [DOI] [PubMed] [Google Scholar]
- 41.Jenkins SM, Johnson GV: Tau complexes with phospholipase C-γ in situ. NeuroReport 1998, 9:67-71 [DOI] [PubMed] [Google Scholar]
- 42.Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S, Wolozin B: Presenilin 1 associates with glycogen synthase kinase-3β, and its substrate tau Proc Natl Acad Sci USA 1998, 95:9637-9641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G: Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci 1998, 111:3167-3177 [DOI] [PubMed] [Google Scholar]
- 44.Johnson GVW, Hartigan JA: Tau protein in normal and Alzheimer’s disease brain: an update. Alzheimer’s Dis Rev 1998, 3:125-141 [DOI] [PubMed] [Google Scholar]
- 45.Loomis PA, Howard TH, Castleberry RP, Binder LI: Identification of nuclear tau isoforms in human neuroblastoma cells. Proc Natl Acad Sci USA 1990, 87:8422-842645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brady RM, Zinkowski RP, Binder LI: Presence of tau in isolated nuclei from human brain. Neurobiol Aging 1995, 16:479-486 [DOI] [PubMed] [Google Scholar]
- 47.Hasegawa M, Smith MJ, Iijima M, Tabira T, Goedert M: FTDP-17 mutations N279K, and S305N in tau produce increased splicing of exon 10. FEBS Lett 1999, 443:93-96 [DOI] [PubMed] [Google Scholar]
- 48.Schelden E, Wadsworth P: Observation and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol 1993, 120:935-945 [DOI] [PMC free article] [PubMed] [Google Scholar]