Abstract
Fibrin or a fibrinous exudate can facilitate angiogenesis in many pathological conditions. In vitro, the outgrowth of capillary-like structures in fibrin can be mimicked by exposing human microvascular endothelial cells (hMVECs) to an angiogenic growth factor and tumor necrosis factor (TNF)-α. Urokinase-type plasminogen activator (u-PA) and plasmin activities are required for this angiogenic process. This study focuses on the role and localization of the u-PA receptor (u-PAR) in newly formed microvascular structures. The u-PAR-blocking monoclonal antibody (MAb) H-2 completely inhibited the formation of capillary-like tubular structures induced by exposure of hMVECs to basic fibroblast growth factor and TNF-α. This was accompanied by a several-fold increase in u-PA accumulation in the conditioned medium. The effect of MAb H-2 was not caused by blocking cellular activation by u-PA/u-PAR interaction, as the amino-terminal fragment (ATF) of u-PA, which also activates u-PAR, prevented tube formation. In addition, the inhibition by MAb H-2 was not due to an effect of the antibody on u-PAR-vitronectin binding. These data show that inhibition of tube formation can be caused not only by inhibition of u-PA or plasmin activities but also by unavailability of the u-PAR for cell-bound proteolysis. Immunohistochemical analysis showed that in in vitro angiogenesis u-PAR and u-PA were localized on the invading, tube-forming hMVECs and not on the endothelial cells that are located on top of the fibrin matrix. u-PAR and u-PA were also prominently expressed on endothelial cells of neovessels present in an atherosclerotic plaque. These data may give more insight into the role of u-PAR in repair-associated angiogenesis.
In the adult, angiogenesis is usually associated with pathological conditions. It is an important factor in the growth of many solid tumors and the proliferative in-growth of pannus tissue in rheumatoid arthritis, and it is the underlying cause of proliferative diabetic retinopathy. 1-3 Angiogenesis can also restore blood flow to ischemic tissue and recanalize a mural thrombus. 4,5 Four sequential steps can be distinguished: the degradation of the basement membrane and interstitial matrix, endothelial cell migration, endothelial cell proliferation, and the formation of tubular structures with a lumen and a new basement membrane. 6 Three of these steps critically depend on proteolytic activity, generated in particular by the plasminogen activator (PA)/plasmin system and the matrix metalloproteinases (MMPs). 7-9 Recent studies showed that several of these proteolytic activities are localized to specific cellular receptors, which control their activities in space and time. 10,11
In conditions in which neovascularization occurs in adults, frequently a fibrinous exudate is formed, which facilitates the angiogenesis process. 12 The formation of capillary-like structures can be mimicked in vitro in a model consisting of a three-dimensional fibrin matrix covered by microvascular endothelial cells (MVECs). It was shown to depend critically on the urokinase-type PA (u-PA)-mediated activation of plasminogen. 13,14 Although in bovine MVECs, angiogenic factors basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) by themselves are able to induce angiogenesis in vitro, 13,15 human (h)MVECs require the additional exposure to tumor necrosis factor (TNF)-α for the induction of u-PA 16 and the formation of capillary-like structures. 14 In both cell types, bFGF and VEGF induce the expression of a cellular u-PA receptor (u-PAR). 7,14,17 The u-PAR is a glycosyl phosphatidyl inositol-anchored glycoprotein. 18 It has been shown that u-PAR plays an essential role in endothelial cell migration 7 and in the invasion of tumor cells in tissues. 19-21 Several studies also have suggested that it plays a role in angiogenesis. 14,21,22
The u-PAR plays an important role in controling u-PA activity. The single-chain proenzyme u-PA can be activated on the u-PAR more efficiently. 19 In the cell environment, active two-chain u-PA is quickly inhibited by the plasminogen activator inhibitor-1 (PAI-1), 23 but bound to u-PAR u-PA displays a higher activity. 24 To ensure the continuous availability of free u-PAR, the u-PA:PAI-1 complex is internalized together with u-PAR. The u-PA:PAI-1 complex is degraded in the lysosomes, whereas the u-PAR is recycled to the cell surface. 25 This internalization process is aided by other receptors, in particular, the α2-macroglobulin receptor/low-density lipoprotein receptor-related protein (α2-MR/LRP), glycoprotein 330, and the very-low-density lipoprotein (VLDL) receptor, in various cell types, 26,27 but in endothelial cells other yet unknown mechanisms are also involved. 28
Beside a function in controling u-PA activity, u-PAR is also found to play a role in activating cell signaling pathways, including diacylglycerol formation, 29 activation of a serine kinase, 30 protein tyrosine phosphorylation, and the activation of the JAK/Stat pathway 31 and focal adhesion kinase. 32 Furthermore, it enforces cellular interaction with the extracellular matrix, in particular, by binding to the matrix protein vitronectin. 33,34
In this study we investigated to what extent the u-PAR controls the availability of u-PA and u-PA-dependent in vitro angiogenesis in fibrin matrices and whether u-PAR and u-PA are site-specifically localized in newly formed human microvascular structures.
Materials and Methods
Materials
Medium 199 (M199) supplemented with 20 mmol/L HEPES and penicillin/streptomycin were obtained from Biowhittaker (Verviers, Belgium); newborn calf serum (NBCS) was obtained from Gibco (Grand Island, NY). Tissue culture plastics were from Costar (Cambridge, MA), and l-glutamine was from ICN (Costa Mesa, CA). A crude preparation of endothelial cell growth factor (ECGF) was prepared from bovine hypothalamus as described by Maciag et al. 35 Human serum was obtained from a local blood bank and was prepared from freshly obtained blood from 10 to 20 healthy donors, pooled, and stored at 4°C. Trypsin was purchased from Gibco, heparin and thrombin from Leo Pharmaceutic Products (Weesp, The Netherlands), and human fibrinogen from Chromogenix AB (Mölndal, Sweden). Factor XIII was generously provided by Dr. H. Keuper (Centeon Pharma, Marburg, Germany). Human vitronectin came from Collaborative Research (Bedford, MA). bFGF was purchased from Pepro Tech EC (London, UK), and human recombinant TNF-α was a gift from Dr. J. Travernier (Biogent, Gent, Belgium) and contained 2.45 × 10 7 U/mg protein and less than 40 ng of lipopolysaccharide per mg of protein. Aprotinin was purchased from Pentapharm (Basel, Switzerland). Receptor-associated protein (RAP) was produced in Escherichia coli strain DH5α as a fusion protein with glutathione S-transferase (RAP-GST) and purified by gluthathione-Sepharose chromatography as described previously. 36 The amino-terminal fragment (ATF) from u-PA came from our laboratory; it was produced by Chinese hamster ovary (CHO) cells transfected with an adenoviral shuttle vector containing the ATF fragment of human u-PA, amino acids 1 to 138 and the 11 carboxyl-terminal amino acid residues of u-PA, which contain the stop codon. Rabbit polyclonal anti-u-PA antibodies were also prepared in our laboratory 37 as were anti-FITC monoclonal antibodies (MAbs). The monoclonal u-PAR-blocking antibody H-2 came from Boehringer Mannheim, Penzberg, Germany, 38 and LM609 was purchased from Chemicon, Temecula, CA. Horseradish peroxidase (HRP) conjugates of goat anti-rabbit IgG, rabbit anti-mouse IgG, and rabbit anti-von Willebrand factor IgG were from Dako (Glostrup, Denmark). Technovit 8100 was obtained from Heraeus Kulzer (Wehrheim, Germany).
Other materials used in the methods described below have been specified in detail in related references or in the text or were purchased from standard commercial sources.
Cell Culture
Human foreskin microvascular endothelial cells (hMVECs) were isolated, cultured, and characterized as previously described. 39,40 hMVECs were cultured on gelatin-coated dishes in M199 supplemented with 20 mmol/L HEPES (pH 7.3), 10% human serum, 10% heat-inactivated NBCS, 150 μg/ml crude ECGF, 2 mmol/L l-glutamine, 5 U/ml heparin, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C under 5% CO2/95% air atmosphere. Experiments were performed after they reached confluency (0.7 × 10 5 cells/cm2) and had been cultured without growth factor for at least 24 hours.
In Vitro Angiogenesis Model
Human fibrin matrices were prepared by the addition of 0.1 U/ml thrombin to a mixture of 5 U/ml factor XIII (final concentrations), 2 mg/ml fibrinogen, 2 mg/ml sodium citrate, 0.8 mg/ml NaCl, and 3 μg/ml plasminogen in M199, and 300-μl aliquots of this mixture were added to the wells of 48-well plates. After clotting at room temperature, the fibrin matrices were soaked with M199 supplemented with 10% (v/v) human serum and 10% (v/v) NBCS for 2 hours at 37°C to inactivate the thrombin. Highly confluent endothelial cells (0.7 × 10 5 cells/cm2) were detached and seeded in a 1.25:1 split ratio on the fibrin matrices and cultured for 24 hours in M199 supplemented with 10% human serum, 10% NBCS, and penicillin/streptomycin. The endothelial cells were then stimulated with the mediators for the time indicated. Every second day, the culture medium was collected and fresh medium was added. Invading cells and the formation of tubular structures of endothelial cells in the three-dimensional fibrin matrix were analyzed by phase contrast microscopy, and the total length of tube-like structures of six randomly chosen microscopic fields (7.3 mm2/field) was measured using a Nikon FXA microscope equipped with a monochrome CCD camera (MX5) connected to a computer with Optimas image analysis software and expressed as mm/cm2.
Cell Attachment Assay
Cell attachment assays were performed in bacteriological 96-well plates (Greiner, Frickenhausen, Germany, ELISA plates) coated with vitronectin or fibrin as described previously. 41 In brief, for vitronectin, 10 μl of vitronectin (100 μg/ml) was applied, resulting in a protein-coated area of 0.12 cm2. After 16 hours at 4°C, the fluid was removed, and 100 μl of 60% methanol was added to each well for 2 hours at 4°C. The methanol was removed, and wells were washed for 30 minutes at 4°C with blocking buffer (50 mmol/L Tris/HCl (pH 7.8), 110 mmol/L NaCl, 5 mmol/L CaCl2, 0.1 mmol/L phenylmethylsulfonyl fluoride, 1% bovine serum albumin, and 0.1 μmol/L sodium azide) to block unbound sites on the plastic. After the removal of blocking buffer, the wells were washed three times with M199 containing 0.03% human serum albumin.
For coating with fibrin, fibrin matrices were prepared as described above. In addition, 100 μl of fibrin was added to each well. Matrices were soaked with M199 supplemented with 10% (v/v) human serum and 10% (v/v) NBCS for 2 hours at 37°C before use. hMVECs (90% confluent) were precultured in M199 supplemented with 10% human serum, 10% NBCS, and penicillin/streptomycin. For attachment assays, hMVECs were detached, resuspended in M199 containing 0.03% human serum albumin, and seeded at a concentration of 5000 cells/150 μl on the matrix-coated 96-well plates. The 96-well dishes were incubated at 37°C under 5% CO2/95% air atmosphere for 90 minutes. Each well was washed three times with serum-free medium, and attached cells were fixed for 20 minutes with 80% methanol at 4°C. Cells attached to vitronectin were stained with amido black, and cells attached to fibrin were stained with toluidine blue. Attached cells were counted using a Nikon FXA microscope equipped with a monochrome CCD camera (MX5) connected to a computer with Optimas image analysis software.
Determination of Specific u-PA Binding and u-PA:PAI-1 Degradation
Diisopropylfluorophosphate-treated u-PA (Ukidan; DIP-u-PA) was radiolabeled using Na125I by using the lodogen procedure (Pierce Chemical Co., Rockford, IL). Binding of 125I-labeled DIP-u-PA to hMVECs was determined at 0°C. The cells were placed on melting ice and incubated for 10 minutes with 50 mmol/L glycine/HCl buffer (pH 3.0) to remove receptor-bound endogenous u-PA. Subsequently, the cells were washed twice with ice-cold M199 and incubated with 8 nmol/L 125I-labeled DIP-u-PA in endothelial-cell-conditioned medium (M199 supplemented with 1% human serum albumin, conditioned for 24 hours) for 3 hours. Incubation was performed in endothelial-cell-conditioned medium to exclude residual binding of u-PA to cell-associated PAI-1. In parallel incubations, a 50-fold excess of DIP-u-PA was included to assess non-specific binding. After the incubation period, unbound ligand was removed by extensive washing with ice-cold M199. Cell-bound ligand was solubilized with 0.3 mol/L NaOH, and the radioactivity was determined in a gamma counter (Cobra Auto gamma, Packard, Meriden, CT). Specific binding was calculated by subtraction of nonspecific binding from the total binding. For determining u-PA:PAI-1 degradation, hMVECs were incubated for 5 hours at 37°C in the presence of 8 nmol/L 125I-labeled u-PA in endothelial cell-conditioned medium. Because of the excess of PAI-1 present 125I-labeled u-PA was rapidly converted to the 125I-labeled u-PA:PAI-1 complex. After the incubation period, an aliquot of the supernatant fluid was taken and the degradation products were determined as the supernatant’s radioactivity obtained after protein precipitation by trichloroacetic acid (final concentration 10%) and corrected for trichloroacetic acid radioactivity in the initial preparation.
u-PA ELISA
u-PA antigen was measured as previously described. 14 In short, 96-well microtiter plates were coated with a mixture of two MAbs, UK 2.1 and UK 26.15, recognizing different epitopes on the u-PA antigen. These MAbs recognize single-chain u-PA, two-chain u-PA, and the u-PA:PAI-1 complex with comparable efficiency. The next day, the plates were blocked with casein, and serial dilutions of standard u-PA (UKIDAN (Serono, Aubonne, Switzerland), assuming that one unit, as determined by the manufacturer, is 10 ng of protein) or culture supernatants were added. Finally, an incubation was done with a HRP conjugate of anti-u-PA antibody (LMW 11.1), and then tetramethylbenzidine substrate was added to react. The reaction was stopped with H2SO4 after 15 min incubation at room temperature. The extinction at 450 nm was measured with a multichannel spectrophotometer (Titertek multiscan, Flow Labs, McLean, VA).
Biological Immunoassay (BIA) for Plasmin-Activatable sc-u-PA and tc-u-PA Activity
This assay was performed as described by Binnema et al. 42 In short, u-PA irrespective of its molecular form is extracted from the conditioned media by a mixture of two MAbs, UK 2.1 and UK 26.15, immobilized on microtiter plates. In the next step, the PA activity of the immuno-immobilized material is measured before (active tc-u-PA) and, in parallel wells, after a 30-minute activation step of the immuno-immobilized material with human plasmin (active tc-u-PA plus plasmin-activatable sc-u-PA). The PA activity was assayed in a two-step cascade with plasminogen and S-2251 as described. 42
Calculation and Assay of u-PA:PAI-1 Complex
The amount of u-PA:PAI-1 complex was calculated from the difference between total u-PA antigen and sc-u-PA antigen (no active tc-u-PA was detectable in the collected samples). The validity of this subtraction was verified by ELISA of the u-PA:PAI-1 complex using anti-PAI-1 IgG as catching antibody and anti-u-PA IgG as tagging antibody. Comparable values were obtained in the two assays.
Immunohistochemistry
Fibrin matrices were fixed at 4°C for 3 hours in 2% p-formaldehyde in phosphate-buffered saline (PBS, pH 7.4), washed for 3 hours in 6% sucrose, dehydrated in a graded series of ethanol, and finally embedded in Technovit 8100, according to the manufacturer’s recommendations (Heraeus Kulzer).
Serial sections were cut (4 μm) perpendicular to the surface of the matrix sheet. For morphological evaluation, the sections were stained with 0.25% phloxin. For immunohistochemistry, the sections were treated for 15 minutes with 0.1% trypsin (w/v) in 0.1% CaCl2 (w/v) in 0.1 mol/L Tris/HCl, pH 7.8, at 37°C and subsequently washed in PBS. Incubation with the anti-u-PA receptor MAb H-2 (10 μg/ml in PBS with 0.1% casein (w/v)) or polyclonal anti u-PA (0.04 μg/ml in PBS with 0.1% casein (w/v)) was done for 2 hours at 37°C. After four washes in PBS, HRP-conjugated rabbit anti-mouse or goat anti-rabbit antibodies (1:40 in PBS with 0.1% casein (w/v) and 0.1% normal human serum) were added and incubated for 1 hour at room temperature.
The peroxidase activity was developed with 3-amino-9-ethyl-carbazole as substrate (10 mg of 3-amino-9-ethyl-carbazole dissolved in 2.5 ml of dimethylformamide in 50 ml of 0.1 mol/L sodium acetate buffer, pH 5; after filtration, 50 μl of H2O2 was added). The sections were counterstained with Mayer’s hematoxylin and mounted in Kaiser’s glycerin (7 g of gelatin in 42 ml of distilled water).
Human atherosclerotic plaques were embedded in paraffin and cut in sections of 4 μm. The cross sections were prewashed in PBS containing 1% NaN3 and 1% of a 30% H2O2 solution. Subsequently, they were incubated for 15 minutes in a block buffer (150 mmol/L NaCl, 10 mmol/L Tris/HCl, 5 mmol/L EDTA, 0.25% (w/v) gelatin, 0.05% (v/v) Tween-20) to reduce background staining. After three wash steps in PBS, the first antibody (H-2, 6 μg/ml; anti-u-PA, 0.01 μg/ml; or anti-von Willebrand factor, 1.2 μg/ml) was added in PBS supplemented with 0.05% Tween-20 and 0.1% bovine serum albumin, followed by an overnight incubation at 4°C. The next day, the sections were washed in PBS and HRP-conjugated rat anti-mouse or goat anti-rabbit (1:300 in PBS supplemented with 0.05% Tween-20, 0.1% bovine serum albumin, and 0.1% normal human serum) was added and incubated for 1 hour at 37°C. Hereafter the sections were washed and developed as described above.
Data are expressed as the mean ± SD.
Results
The Anti-u-PAR MAb H-2 Inhibits u-PA Binding to Endothelial Cells
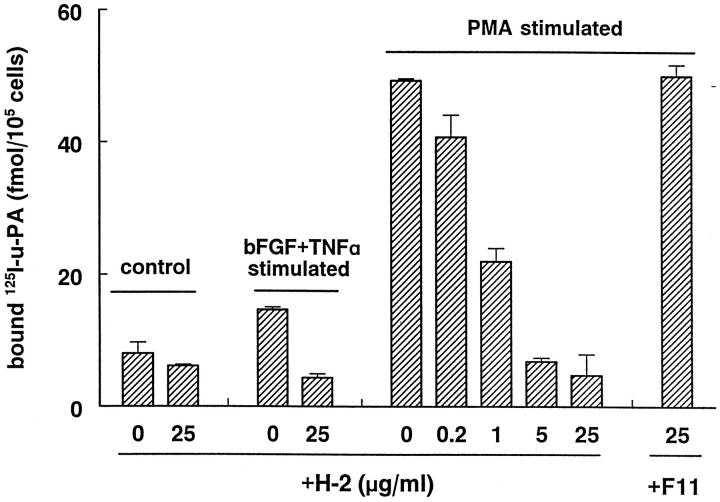
It has been shown before that the MAb H-2 specifically recognizes u-PAR on a Western blot of lysates of monocytes at a molecular mass of 55 kd. 38 We show here that MAb H-2 prevents u-PA binding to its receptor on endothelial cells. MAb H-2 completely inhibited the specific binding of 125I-labeled DIP-u-PA to hMVECs, in which u-PAR expression had been enhanced (1.8 ± 0.03-fold) by preincubation with bFGF and TNF-α or strongly enhanced (6.0 ± 0.005-fold) by preincubation with 10−8 mol/L phorbol myristate acetate for 24 hours (Figure 1) ▶ . This inhibition was detectable at 0.2 μg/ml and maximal at 5 μg/ml (Figure 1) ▶ . The nonblocking anti-u-PAR MAb F11 did not have an effect on specific binding of 125I-labeled DIP-u-PA to hMVECs. Similar results were obtained using human umbilical vein or iliac artery endothelial cells.
Figure 1.
Inhibition of u-PA/u-PAR interaction by MAb H-2. hMVECs were preincubated for 24 hours in M199 supplemented with 10% human serum with or without phorbol myristate acetate (10−8 mol/L) or bFGF and TNF-α (20 ng/ml each). Subsequently, the cells were cooled on ice, and the specific binding of 125I-labeled DIP-u-PA to hMVECs was determined in the presence of the antibody H-2 (0, 0.2, 1, 5, or 25 μg/ml) or the nonblocking antibody F11 (25 μg/ml) as described in Materials and Methods. The data represent mean ± SD of triplicate wells of a representative experiment. Similar results were obtained in two independent experiments.
The Anti-u-PAR MAb H-2 Prevents Formation of Tubular Structures by hMVECs in a Fibrin Matrix
The MAb H-2 was used to establish the involvement of the u-PAR in the formation of capillary-like tubular structures by hMVECs in a three-dimensional fibrin matrix. When hMVECs were cultured without growth factors or cytokines, the confluent monolayer on top of the fibrin matrix remained unaltered (Figure 2A) ▶ . Stimulation of the cells with the combination of bFGF and TNF-α (bFGF/TNF-α) induced the formation of tubular structures, which developed in a period of 3 to 8 days (Figure 2B) ▶ . Simultaneous incubation of the bFGF/TNF-α-stimulated cells with the MAb H-2 inhibited tube formation (Figure 2C) ▶ in a dose-dependent manner (Figure 3A) ▶ , up to 88 ± 4% at 5 μg/ml H-2. A similar extent of inhibition was observed when anti-u-PA IgG or the plasmin inhibitor aprotinin were used (Figure 3A) ▶ . The presence of MAb H-2 caused an increased u-PA accumulation in the conditioned medium of bFGF/TNF-α-stimulated hMVECs (Figure 3B) ▶ .
Figure 2.
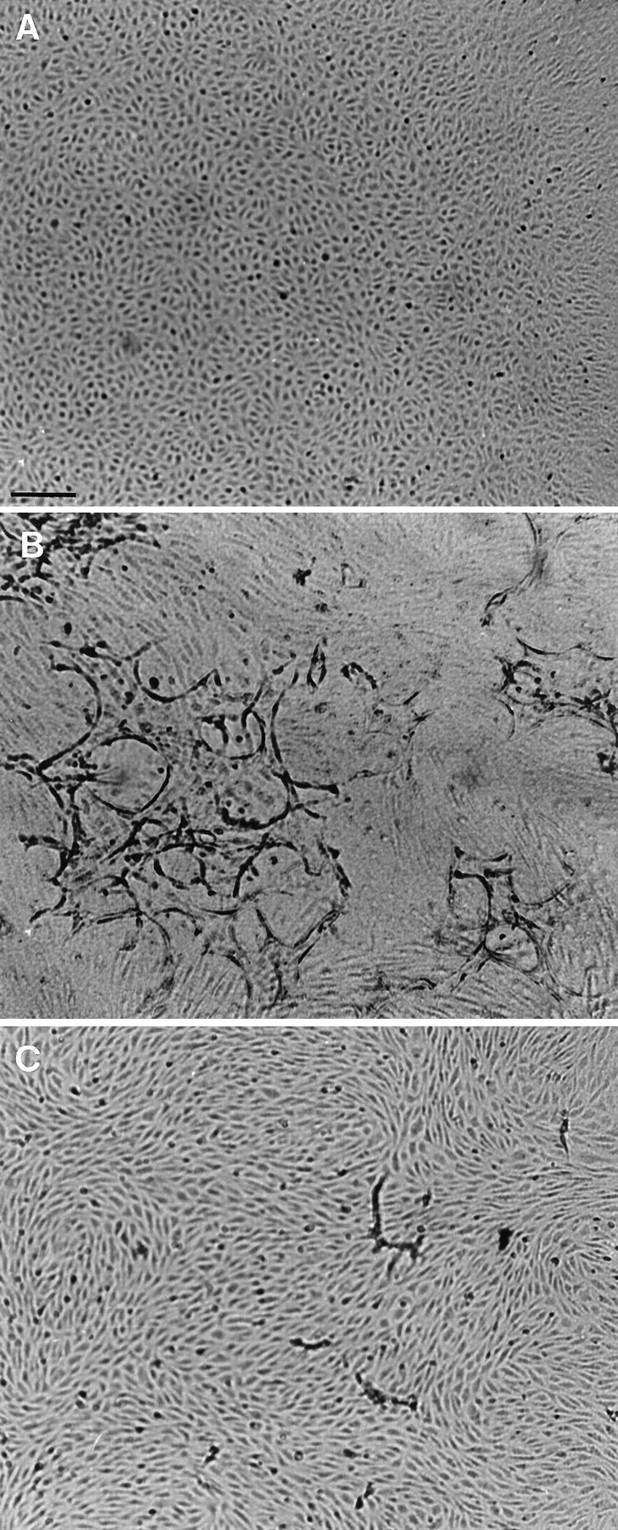
Capillary-like tube formation is inhibited by blocking u-PAR antibodies. hMVECs were cultured on top of a three-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and were not stimulated (A) or stimulated with 20 ng/ml bFGF and 20 ng/ml TNF-α (B) or with bFGF and TNF-α in combination with 5 μg/ml MAb H-2 (C). After 8 days of culture, non-phase photomicrographs were taken. Bar, 300 μm.
Figure 3.

Dose-dependent inhibition of capillary-like tube formation by blocking u-PAR antibodies. A: hMVECs were cultured on the surface of a three-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and not stimulated (control) or stimulated with 20 ng/ml bFGF and 20 ng/ml TNF-α in the presence of 0, 0.2, 1, 5, or 25 μg/ml MAb H-2, 25 μg/ml mouse IgG (α-FITC), 100 U/ml aprotinin (aprt.), or polyclonal anti-u-PA IgG (100 μg/ml). After 8 days of culturing, total tube length/cm 2 ± SD of triplicate wells was measured as described and the medium was collected. Similar results were obtained in three independent experiments. B: u-PA antigen levels in the collected media were determined by ELISA as described and expressed as cumulative production of u-PA (ng/10 5 cells). The data represent mean ± SD of triplicate wells. Similar results were obtained in three independent experiments.
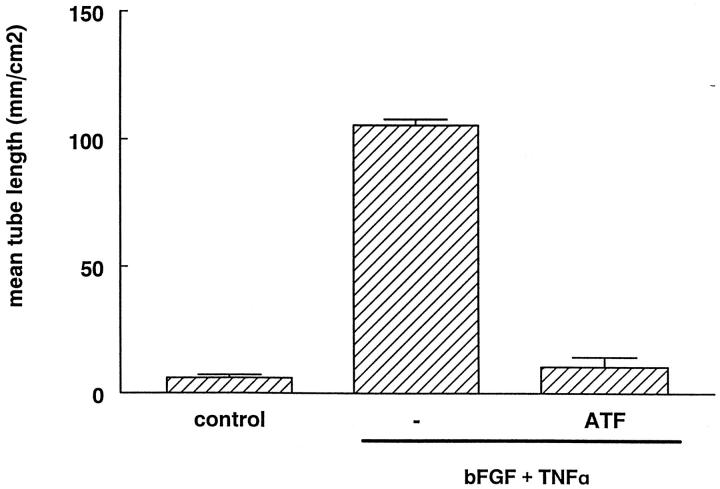
Interestingly, when the binding of u-PA to its receptor was competed by the amino-terminal fragment of u-PA (ATF) a complete prevention of the formation of tubular structures was observed (Figure 4) ▶ . The ATF lacks the proteolytically active domain but is still able to bind to the receptor and to activate u-PAR-dependent signal transduction. 32 Therefore, complete prevention of tube formation by MAb H-2 is unlikely primarily due to blockage of u-PAR-dependent cell signaling. An effect of MAb H-2 or ATF on cell proliferation is also highly unlikely. It was shown by staining with the proliferation marker bromodeoxyuridine that hardly any proliferation occurred in our model in the presence of TNF-α (data not shown).
Figure 4.
ATF inhibits capillary-like tube formation. hMVECs were cultured on the surface of a three-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and not stimulated (control) or stimulated with 20 ng/ml bFGF and 20 ng/ml TNF-α in the presence of conditioned media of CHO cells, which were mock transfected or were transfected with an ATF construct ([ATF] = 6.25 μg/ml). After 8 days of culturing, total tube length/cm 2 ± SD of triplicate wells was measured as described. Similar results were obtained in three independent experiments.
u-PAR-Vitronectin Interaction Is Not Required for Tube Formation in Fibrin
In addition to a role in localizing u-PA proteolytical activity to the cell surface, the u-PAR also interacts with vitronectin and thus facilitates cell-matrix interaction and cell spreading. When hMVECs were seeded in vitronectin-coated dishes, cell adherence was completely prevented by the MAb LM609 that blocks the vitronectin receptor αvβ3-integrin (Figure 5A) ▶ . The MAb H-2 reduced the adhesion of hMVECs to vitronectin-coated dishes by 52 ± 8%. When hMVECs were seeded in fibrin-coated dishes, neither MAb LM609 nor MAb H-2 reduced cell attachment (Figure 5A) ▶ . MAb LM609 also did not significantly reduce the formation of tubular structures or invasion of hMVECs in a fibrin matrix, whereas MAb H-2 completely inhibited the formation of tubular structures in parallel dishes (Figure 5B) ▶ . These data suggest that direct interaction of the endothelial cells with binding sites on fibrin dominated in the formation of tubular structures and that the action of MAb H-2 on this process was not due to the reduction of u-PAR-vitronectin interaction.
Figure 5.
MAb H-2 inhibits adhesion of hMVECs to vitronectin but not to fibrin. A: Cell adhesion assay on vitronectin or fibrin was performed as described in Materials and Methods. hMVECs were allowed to adhere to vitronectin (hatched bars) or fibrin (closed bars) for 90 minutes in the presence of control antibody anti-FITC (10 μg/ml), LM609 (10 μg/ml), or H-2 (10 μg/ml). Each condition was performed sixfold, and experiments were repeated two times. B: hMVECs were cultured on the surface of a three-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and not stimulated (control) or stimulated with 20 ng/ml bFGF and 20 ng/ml TNF-α alone (no Ab) or in the presence of LM609 (10 μg/ml) or H-2 (10 μg/ml). After 8 days of culturing, the mean total tube length/cm 2 ± SD of triplicate wells was measured as described. The effects of LM609 and H-2 are expressed as the mean percentage of control ± SD of three independent experiments.
Accumulation and Cellular Uptake of u-PA Is Modified by MAb H-2
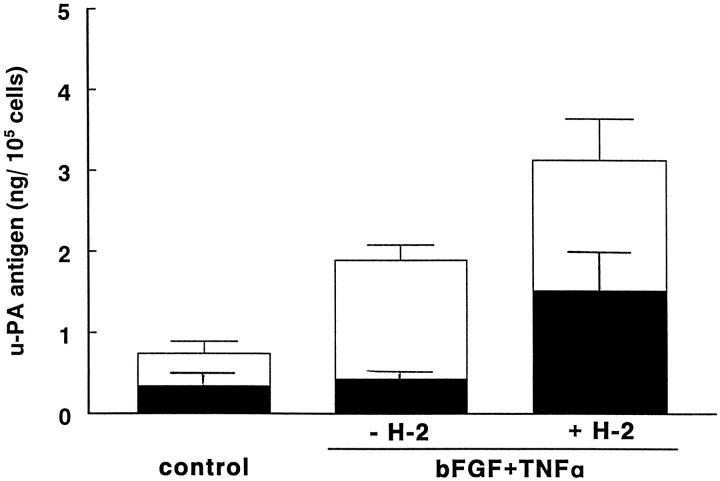
During incubation, u-PA is internalized as a u-PA:PAI-1 complex and degraded. MAb H-2 prevents this internalization by blocking the u-PA:u-PAR interaction. As a consequence, the amount of u-PA antigen that accumulated in the conditioned medium increased (Figure 6) ▶ . The accumulated u-PA antigen consisted of single-chain u-PA (sc-u-PA or pro-urokinase) and a complex of u-PA and PAI-1; no active u-PA was detectable in the conditioned media. When the amounts of sc-u-PA and u-PA:PAI-1 complexes were determined in bFGF/TNF-α-stimulated endothelial cells, we found that in the presence of MAb H-2 an equal amount of pro-urokinase (sc-u-PA) and u-PA:PAI-1 complex were produced (Figure 6) ▶ . In the absence of MAb H-2, the amount of sc-u-PA was considerably less, whereas that of u-PA:PAI-1 complex was unaltered. Because only the u-PA:PAI-1 complex is internalized, this suggests that the missing amount of sc-u-PA is activated on the uPAR and subsequently inactivated and internalized. The u-PA:PAI-1 encountered in the conditioned medium apparently derives from u-PA that is activated independent of receptor binding. Furthermore, the data indicate that prevention of tube formation by the presence of MAb H-2 is not due to a limitation of the amount of u-PA that is available in the conditioned medium but to a lack of focally exposed u-PA, ie, bound to and activated on the cellular u-PAR.
Figure 6.
MAb H-2 increases the amount of sc-u-PA compared with total u-PA in conditioned media of hMVECs. Confluent hMVECs were cultured for 24 hours in M199 medium supplemented with 10% human serum and stimulated without (control) or with bFGF in combination with TNF-α (20 ng/ml each) in the absence or presence of 5 μg/ml H-2 antibody. After incubation, conditioned media were collected and plasmin-activatable u-PA (▪) and total u-PA antigen (▪ + □) were assayed by BIA and ELISA, respectively, as described in Materials and Methods. In the samples, tc-u-PA activity (data not shown) was below the detection limit (0.1 ng/ml). The data represent the mean ± SD of three independent experiments.
RAP-GST Partly Reduces u-PAR Availability and Formation of Capillary-Like Tubular Structures
Internalization of the u-PA:PAI-1/u-PAR complex is needed to recover unoccupied u-PAR for the subsequent new binding and activation of u-PA. The u-PAR is a glycosyl phosphatidyl inositol-linked protein and needs accessory membrane proteins to become internalized. In other cell types, LRP and VLDL receptors, proteins of the LDL receptor class, have been identified as such accessory proteins. 26,27 These proteins are inhibited by the receptor-associated protein RAP.
To evaluate whether interference with u-PAR internalization by blocking the complexation of the occupied u-PAR to accessory membrane proteins reduced the formation of capillary-like tubular structures, RAP-GST was incubated with the cells. RAP-GST reduced the internalization of 125I-labeled DIP-u-PA:PAI-1 complex by endothelial cells with 34 ± 2% (Figure 7A) ▶ . This was accompanied by a reduction of 23 ± 5% of the formation of tubular structures (Figure 7B) ▶ . These data support the suggestion that a decreased availability of free u-PAR reduces the formation of capillary-like structures.
Figure 7.
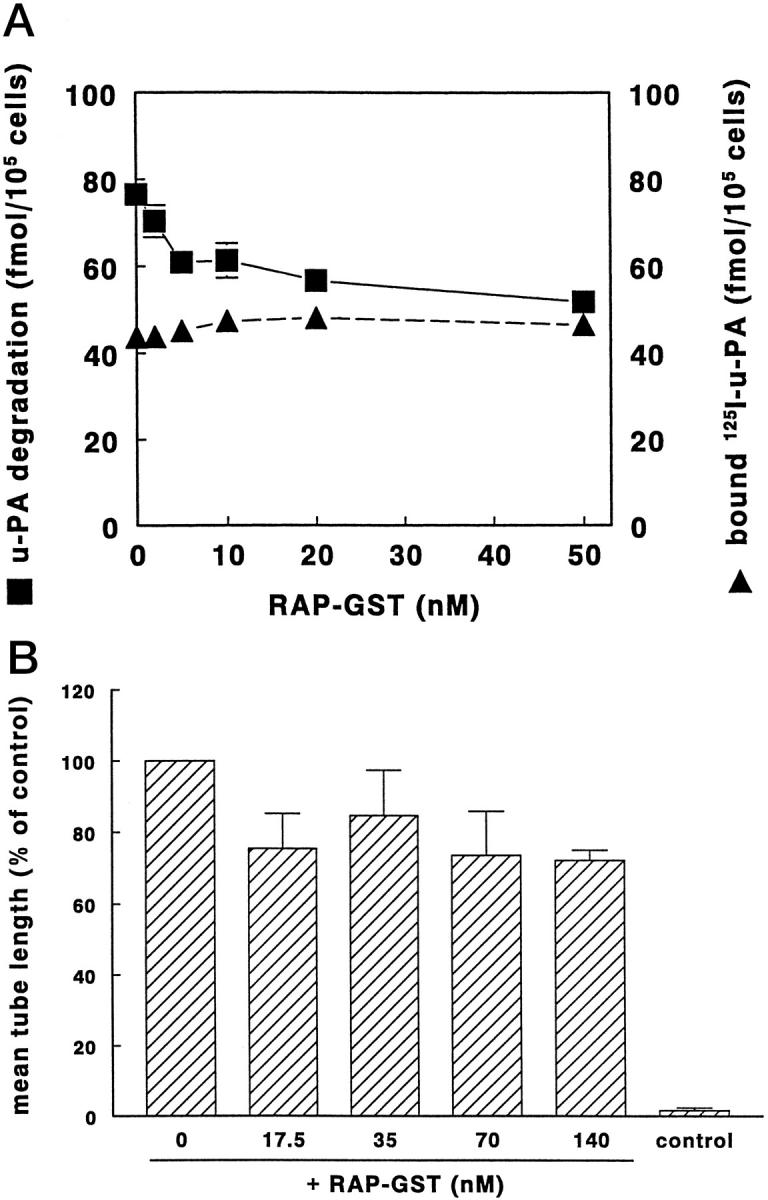
Effect of RAP on u-PA:PAI-1 degradation and capillary-like tube formation in hMVECs. A: Confluent hMVECs were cultured for 24 hours in M199 medium supplemented with 10% human serum and stimulated with phorbol myristate acetate (10−8 mol/L). Subsequently, cells were cooled on ice, and the specific binding of 125I-labeled DIP-u-PA was determined in the presence of 0, 2, 5, 10, 20, and 50 nmol/L RAP-GST or cells were incubated for 5 hours at 37°C in the presence of 125I-labeled u-PA:PAI-1 and 0, 2, 5, 10, 20, and 50 nmol/L RAP-GST. u-PA:PAI-1 degradation was determined as described in Materials and Methods. The data represent the mean ± SD of triplicate wells. B: hMVECs were cultured on the surface of a three-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and not stimulated (control) or stimulated with 20 ng/ml bFGF and 20 ng/ml TNF-α in the presence of 0, 17.5, 35, 70, or 140 nmol/L RAP-GST. After 8 days of culturing, the mean total tube length/cm 2 ± SD of triplicate wells was measured as described. The effect of RAP is expressed as the mean percentage of control ± SD of four independent experiments.
Localization of the u-PA Receptor and u-PA in Capillary-Like Tubular Structures
To investigate whether the u-PAR and its ligand are localized at specific sites in our in vitro angiogenesis model, we studied the localization of the expression of these factors in cross sections of the fibrin matrix by immunohistochemistry.
Immunostaining for u-PAR showed that under control conditions the u-PAR could be detected on only a few distinct cells in the monolayer (Figure 8B) ▶ . When the hMVECs were incubated in the presence of bFGF and TNF-α for 8 days, tubular structures were clearly visible underneath the endothelial monolayer (compare Figure 8A ▶ with 8, C and F). These capillary structures consisted of hMVECs surrounding a lumen. The u-PAR is prominently expressed on the endothelial cells of the tubular structures (Figure 8, D and G) ▶ . u-PA was also almost exclusively localized on endothelial cells forming the tubular structures, in particular, on cells in the deepest and probably most recently formed tubular structures (Figure 8, E and H) ▶ . Under control conditions (unstimulated cells) no positive immunostaining for u-PA was detectable (data not shown). Negative controls (same staining procedure but without the primary antibody) showed no immunostaining.
Figure 8.
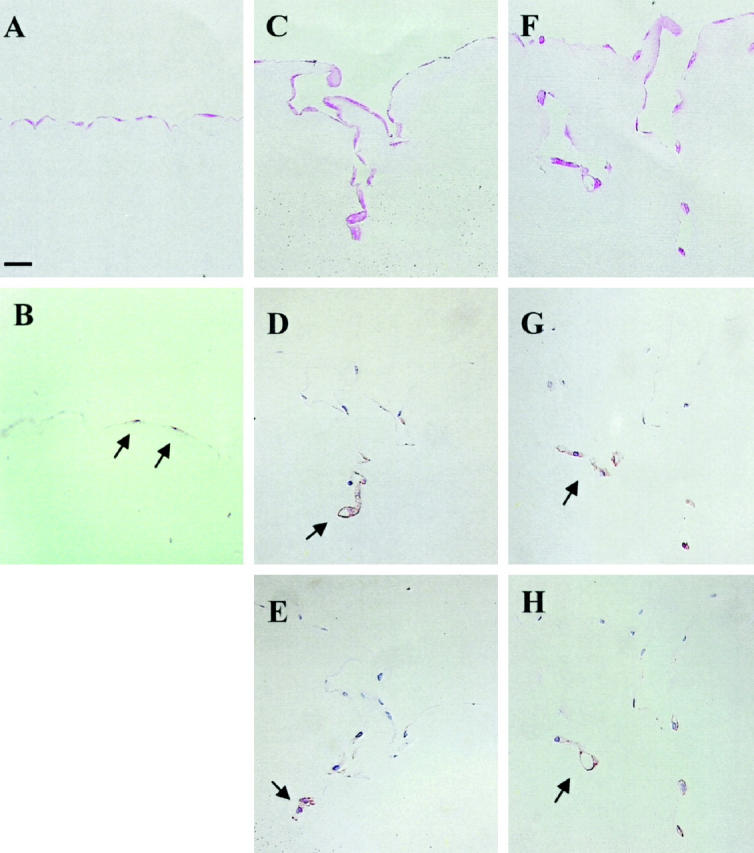
Localization of u-PAR and u-PA in capillary-like tubular structures of hMVECs in fibrin matrices. hMVECs were cultured on the surface of a three-dimensional fibrin matrix in M199 supplemented with 10% human serum and 10% NBCS and stimulated without or with 20 ng/ml bFGF and 20 ng/ml TNF-α. After 8 days of culture, the fibrin matrices were fixed and embedded as described, and 4-μm cross sections perpendicular to the matrix surface were cut. Immunohistochemistry was performed as described. A, C, and F: Phloxin staining of unstimulated (A) and of stimulated (C and F) cells. B, D, and G: Immunostaining for u-PAR on unstimulated cells (B) and immunostaining for u-PAR on stimulated cells (D and G). E and H: Immunostaining for u-PA on stimulated cells. Positive cells are indicated by a red end product (arrowheads). Bar, 50 μm.
Localization of the u-PAR and u-PA in an Atherosclerotic Plaque
To compare our in vitro model with an in vivo condition, sections of atherosclerotic plaques with organized thrombi were studied. The neointima with incorporated thrombus contained new vascular structures, which could be visualized by immunohistochemistry using antibodies against von Willebrand factor (Figure 9A) ▶ . Staining with antibodies against u-PAR revealed that the endothelial cells of the neovessels expressed high levels of u-PAR (Figure 9B) ▶ . This anti-u-PAR staining was more prominent than that in the endothelial cells lining the original vessel wall (data not shown). In addition to the endothelial cells, smooth muscle cells also stained positive for the u-PAR (Figure 9B) ▶ . Immunostaining for u-PA was found on part of the endothelial cells of the newly formed vessels. A few smooth muscle cells of the neointima were also positive for u-PA antigen but to a lesser extent than for u-PAR antigen (Figure 9C) ▶ . Negative controls (same staining procedure but without the primary antibody) showed no positive staining.
Figure 9.

Localization of u-PAR and u-PA in neovessels formed in a human atherosclerotic plaque. Immunohistochemistry was performed on paraffin sections of an atherosclerotic plaque that had incorporated a mural thrombus, as described in Materials and Methods. A: The endothelial cells of the newly formed microvessels are visible after immunostaining for von Willebrand factor. B and C: Immunostaining for u-PAR (B) and immunostaining for u-PA (C). Black arrows indicate examples of positive endothelial cells; white arrowheads indicate examples of positive smooth muscle cells. Bar, 50 μm. Results are representative for organized plaques of two patients.
Discussion
In this report we have provided additional evidence for the involvement of the u-PAR in the formation of capillary-like tubular structures by hMVECs in a fibrin matrix and have shown the specific localization of the u-PAR and u-PA in the endothelial lining of newly formed tubular structures in vitro and microvessels in vivo.
The u-PA/u-PAR system enables endothelial cells to degrade the basement membrane directly or indirectly and to invade the fibrin matrix. In previous experiments it was shown that scavenging u-PA by anti-u-PA antibodies or soluble u-PAR inhibits tube formation in fibrin matrices almost completely. 14 We have extended these studies and showed that specific blockage of the u-PAR also prevented the formation of tubular structures by hMVECs in a fibrin matrix, despite the fact that sufficient u-PA was present in the conditioned medium around the cells.
On the basis of present knowledge, three mechanisms can be anticipated that may contribute to the requirement of u-PA:u-PAR interaction in angiogenesis. First, the u-PAR localizes u-PA activity to specific sites on the cell surface. On these sites proteolysis of matrix proteins occurs, which is kept in balance with the formation of new cell-matrix interactions. This mechanism fits well with the previous observations that in vitro angiogenesis is also inhibited by blocking u-PA and plasmin activities. 14,43
Second, binding of u-PA to its receptor causes activation of a signal transduction pathway in the cell. 29,30 This cellular activation by u-PAR is also mediated by the binding of the amino-terminal fragment (ATF) of u-PA, 32 which lacks the catalytic domain but binds to the u-PAR similarly to u-PA. However, in contrast to u-PA, ATF is unable to stimulate tube formation in our model. 14,44 Furthermore, it can block tube formation, probably by competing with u-PA for binding to its receptor (our own unpublished data). Therefore, it is unlikely that u-PAR-dependent signal transduction plays an important role in the formation of tubular structures by hMVECs in fibrin matrices. A role for u-PAR in cell proliferation as mentioned by Fibbi et al 45 is also unlikely, because the proliferation rate of hMVECs is extremely low in the conditions in which we assayed the formation of tubular structures, due to the continuous presence of TNF-α (checked by the proliferation marker bromodeoxyuridine).
Finally, the u-PAR affects integrin function 46 and interacts directly with the matrix protein vitronectin. 33,34 The interaction between vitronectin and u-PAR is enhanced by binding of u-PA and reduced by PAI-1. 47-49 Because vitronectin associates with fibrin, 50 one may suggest that the u-PAR:vitronectin interaction may be involved in endothelial migration in a fibrin matrix. In our study the attachment and cell spreading of hMVECs to vitronectin was inhibited partially by MAb H-2, but these processes were unaffected when hMVECs were seeded on fibrin-coated dishes. This suggests that the fibrin matrix itself contains sufficient interaction sites for hMVECs. In particular, the RGD sequence in the α-chains and possibly the Bβ chains participate in this interaction. 51-53 Also, when hMVECs formed tubular structures in the fibrin matrix, an effective αvβ3-blocking antibody, LM609, did not affect the outgrowth of tubular structures. It should be noted that in our in vitro model a pure fibrin matrix has been used, whereas a fibrinous exudate in vivo also contains other plasma proteins. Because vitronectin may not penetrate sufficiently into the pure fibrin matrix, it is possible that in our model the potential contribution of vitronectin is underestimated. Nevertheless, the data indicate that in our experimental conditions the reduction of angiogenesis by inhibition of u-PA binding to its receptor is not primarily caused by affecting the interaction between vitronectin and hMVECs.
Taken together, our data show that inhibition of tube formation can be caused not only by inhibition of u-PA or plasmin activities but also by the unavailability of u-PAR to localize cell-bound proteolysis. This observation is in accordance with the finding that angiogenesis occurring in certain tumors in vivo can be reduced by administration of a catalytic inactive u-PA, which retains receptor binding and thus competes for binding of native u-PA. 21,22
The concomitant binding of u-PA and plasminogen to the cell surface accelerates the plasminogen activation cascade. 24,54 Because u-PA activity is rapidly inactivated by PAI-1, the continuous regeneration of unoccupied u-PAR from the u-PA:PAI-1-receptor complex is needed. Our data show that a reduction of the extent of internalization of these complexes causes a reduction of available u-PAR on the cell surface and is accompanied by a decrease in the extent of tube formation by hMVECs.
If u-PA and u-PAR are critically involved in in vitro angiogenesis in a fibrin matrix, one would expect that these proteins are present in the invading capillary-like structures. Indeed, it was found that the u-PAR is strongly expressed on the endothelial cells of the tubular structures. The localization of u-PA was confined to the cells located deep in the fibrin matrix. These cells probably represent the structures that have been formed most recently, and probably are proteolytically most active. Although the localization of u-PA does not necessarily mean that u-PA is synthesized by the cells to which it is allocated (compare Ref. 55 ), the more general expression of the u-PAR as compared with u-PA suggests that u-PA is produced in higher amounts at the sites where it is encountered. The difference in u-PAR expression between the cells remaining on top of the fibrin matrix and those lining the tubular structures is striking. Apparently, cells that are invading the fibrin gel express a different gene repertoire, which includes u-PAR and probably the αvβ3-integrin. 56 Alternatively, the fact that a few cells in the unstimulated monolayer are positive for u-PAR may reflect the existence of a subpopulation of cells that are more able to invade the fibrin matrix if adequate stimuli, which induce u-PA expression, are provided. Evidence for heterogeneous populations of endothelial cells has also been proposed by Asahara et al. 57 These investigators isolated putative progenitor endothelial cells for angiogenesis on the basis of their specific surface expression of CD34.
It should be noted that our study is confined to neovascularization in a fibrin matrix. The condition of the in vitro system used in this study can best be compared with pathological conditions in which neovascularization occurs upon fibrin degradation. 12 Recanalization of a mural thrombus associated with an atherosclerotic plaque is such a condition. Indeed, the u-PAR was clearly expressed in the neovessels present in an organized fibrin-rich neointima. In some of the endothelial cells of such neovessels, u-PA was also present. Our findings are in agreement with the co-localization of factors of the plasminogen activator system as observed in other studies on atherosclerotic plaques. 58,59 The confined localization and expression of u-PAR and u-PA in endothelial cells actively involved in angiogenesis may explain how these cells are capable of realizing a localized fibrinolytic activity that is sufficiently limited to prevent complete lysis of the scaffold matrix and cell detachment. Understanding the balance between detachments and the formation of new cell matrix attachment sites may provide leads as to how angiogenesis can selectively be influenced in pathological conditions.
Acknowledgments
We thank Bea van der Vecht, Erna Peters, and Mario Vermeer for their excellent technical assistance and Ellen Braat for advice on the biological immunoassay.
Footnotes
Address reprint requests to Dr. Victor W. M. van Hinsbergh, Gaubius Laboratory TNO-PG, PO Box 2215, 2301 CE Leiden, The Netherlands. E-mail: vwm.vanhinsbergh@pg.tno.nl.
Supported by the Dutch Heart Foundation grant 95.193 and The Dutch Cancer Society grant TNOP 97-1511.
References
- 1.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nature Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64:327-336 [DOI] [PubMed] [Google Scholar]
- 3.Battegay EJ: Angiogenesis: mechanistic insights, neovascular diseases and therapeutic prospects. J Mol Med 1995, 73:333-346 [DOI] [PubMed] [Google Scholar]
- 4.Isner JM: Angiogenesis for revascularization of ischemic tissues. Eur Heart J 1997, 18:1-2 [DOI] [PubMed] [Google Scholar]
- 5.O’Brien ER, Garvin MR, Dev R, Stewart DK, Hinohara T, Simpson JB, Schwartz SM: Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol 1994, 145:883-894 [PMC free article] [PubMed] [Google Scholar]
- 6.Folkman J: How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes Memorial Award Lecture. Cancer Res 1986, 46:467-473 [PubMed] [Google Scholar]
- 7.Pepper MS, Sappino AP, Stocklin R, Montesano R, Orci L, Vassalli JD: Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol 1993, 122:673-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacarach E, Itin A, Keshet E: In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiological angiogenesis. Proc Natl Acad Sci USA 1992, 89:10686-10690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscatelli D, Rifkin DB: Membrane and matrix localization of proteases: a common theme in tumor invasion and angiogenesis. Biochim Biophys Acta 1988, 948:67-85 [DOI] [PubMed] [Google Scholar]
- 10.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloprotease MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 1996, 85:683-693 [DOI] [PubMed] [Google Scholar]
- 11.Yebra M, Parry GCN, Stromblad S, Mackman N, Rosenberg S, Mueller BM, Cheresh DA: Requirement of receptor-bound urokinase-type plasminogen activator for integrin αvβ5-directed cell migration. J Biol Chem 1996, 271:29393-29399 [DOI] [PubMed] [Google Scholar]
- 12.Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM: Fibrin containing gels induce angiogenesis: implications for stroma generation and wound healing. Lab Invest 1987, 57:673-686 [PubMed] [Google Scholar]
- 13.Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD: Transforming growth factor-β1 modulates basic fibroblast growth factor-induced proteolitic and angiogenic properties of endothelial cells in vitro. J Cell Biol 1990, 111:743-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koolwijk P, Erck van M, de Vree W, Vermeer MA, Weich HA, Hanemaaijer R, van Hinsbergh VWM: Cooperative effect of TNFα, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix: role of urokinase activity. J Cell Biol 1996, 132:1177-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepper MS, Ferrara L, Orci L, Montesano R: Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun 1992, 189:824-831 [DOI] [PubMed] [Google Scholar]
- 16.Van Hinsbergh VWM, van den Berg EA, Fiers W, Dooijewaard G: Tumor necrosis factor induces the production of urokinase-type plasminogen activator by human endothelial cells. Blood 1990, 10:1991-1998 [PubMed] [Google Scholar]
- 17.Mignatti P, Mazzieri R, Rifkin DB: Expression of the urokinase receptor in vascular endothelial cells is stimulated by basic fibroblast growth factor. J Cell Biol 1991, 113:1193-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploug M, Rønne E, Berhendt N, Jensen AL, Blasi F, Danø KL: Cellular receptor for urokinase plasminogen activator: carboxy terminal processes and membrane anchoring. J Biol Chem 1991, 266:1926-1933 [PubMed] [Google Scholar]
- 19.Quax PHA, Pederson N, Masucci MT, Weening-Verhoeff EJ, Danø K, Verheijen JH, Blasi F: Complementation between urokinase-producing and receptor-producing cells in extracellular-matrix degradation. Cell Regul 1991, 2:793-803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Hagege J, Doublet JD, Callard P, Sraer JD, Ronne E, Rondeau E: Endothelial and macrophage upregulation of urokinase receptor expression in human renal cell carcinoma. Hum Pathol 1997, 28:206-213 [DOI] [PubMed] [Google Scholar]
- 21.Min HY, Doyle LV, Vitt CR, Zandonella CL, Stratton-Thomas JR, Shuman MA, Rosenberg S: Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res 1996, 56:2428-2433 [PubMed] [Google Scholar]
- 22.Evans CP, Elfman F, Parangi S, Conn M, Cunha G, Shuman MA: Inhibition of prostate cancer neovascularization and growth by urokinase-plasminogen activator receptor blockade. Cancer Res 1997, 57:3594-3599 [PubMed] [Google Scholar]
- 23.Loskutoff DJ: Regulation of PAI-1 gene expression. Fibrinolysis 1991, 5:197-206 [Google Scholar]
- 24.Ellis V, Behrendt N, Danø K: Plasminogen activation by receptor bound urokinase: a kinetic study with both cell-associated and isolated receptor. J Biol Chem 1991, 266:12752-12758 [PubMed] [Google Scholar]
- 25.Nykjær A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F: Recycling of the urokinase receptor upon internalization of the uPA: serpin complexes. EMBO J 1997, 16:2610-2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreasen PA, Sottrup-Jensen L, Kjøller L, Nykjær A, Moestrup SK, Petersen CM, Gliemann J: Receptor mediated endocytosis of plasminogen activators and activor/inhibitor complexes. FEBS Lett 1994, 338:239-245 [DOI] [PubMed] [Google Scholar]
- 27.Heegaard CW, Simonsen ACW, Oka K, Kjøller L, Christensen A, Madsen B, Ellgaard L, Chan L, Andreasen PA: Very low density lipoprotein receptor binds and mediates endocytosis of urokinase-type plasminogen activator-type-1 plasminogen activator inhibitor complex. J Biol Chem 1995, 270:20855-20861 [DOI] [PubMed] [Google Scholar]
- 28.Argraves KM, Battey FD, MacCalmans CD, McCrae KR, Gåfvels M, Kozarsky KF, Chappell DA, Straus JF, Strickland DK: The very low density lipoprotein receptor mediates the cellular catabolism of lipoprotein lipase and urokinase-plasminogen activator inhibitor type I complexes. J Biol Chem 1995, 270:26550-26557 [DOI] [PubMed] [Google Scholar]
- 29.Del Rosso M, Anichini E, Pedersen N, Blasi F, Fibbi G, Pucci M, Ruggiero M: Urokinase-urokinase receptor interaction: non-mitogenic signal transduction in human epidermal cells. Biochem Biophys Res Commun 1993, 2:347-352 [DOI] [PubMed] [Google Scholar]
- 30.Busso N, Masur SK, Lazega D, Waxman S, Ossowski L: Induction of cell migration by pro-urokinase binding to its receptor: possible mechanism for signal transduction in human epithelial cells. J Cell Biol 1994, 126:259-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumler I, Weis A, Mayboroda OA, Maasch C, Jerke U, Haller H, Gulba DC: The JAK/Stat pathway and urokinase receptor signaling in human aortic vascular smooth muscle cells. J Biol Chem 1998, 273:315-321 [DOI] [PubMed] [Google Scholar]
- 32.Tang H, Kerins DM, Hao Q, Inagami T, Vaughan DE: The urokinase-type plasminogen activator receptor mediates tyrosine phosphorylation of focal adhesion proteins and activation of mitogen-activated protein kinase in cultured endothelial cells. J Biol Chem 1998, 273:18268-18272 [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA: Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem 1994, 269:32380-32388 [PubMed] [Google Scholar]
- 34.Rao NK, Shi GP, Chapman HA: Urokinase receptor is a multifunctional protein: influence of receptor occupancy on macrophage gene expression. J Clin Invest 1995, 96:465-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maciag T, Cerundolo J, Ilsley PR, Fornad R: An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci USA 1979, 76:5674-5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK: Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J 1988, 7:4119-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wijgaards G, Kluft C, Groeneveld E: Demonstration of urokinase-related fibrinolytic activity in human plasma. Br J Haemotol 1982, 51:165. [DOI] [PubMed] [Google Scholar]
- 38.Bohuslav J, Horejsí V, Hansmann C, Stöckl J, Weidle UH, Majdic O, Bartke I, Knapp W, Stockinger H: Urokinase plasminogen activator receptor, β2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med 1995, 181:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Defilippi P, van Hinsbergh VWM, Bertolotto A, Rossino P, Silengo L, Tarone G: Differential distribution and modulation of expression of α1/β1 integrin on human endothelial cells. J Cell Biol 1991, 114:855-863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Hinsbergh VWM, Sprengers ED, Kooistra EA: Effect of thrombin on the production of plasminogen activators and PA inhibitor-1 by human foreskin microvascular endothelial cells. Thromb Haemost 1987, 57:148-153 [PubMed] [Google Scholar]
- 41.van der Pluijm G, Vloedgraven HJM, Ivanov B, Robey FA, Grzesik WJ, Robey PG, Papapoulos SE, Löwik CWGM: Bone sialoprotein peptides are potent inhibitors of breast cancer cell adhesion to bone. Cancer Res 1996, 56:1948-1955 [PubMed] [Google Scholar]
- 42.Binnema DJ, Dooijewaard G, Turion PN: An analysis of the activators of single-chain urokinase-type plasminogen activator (scu-PA) in the dextran sulphate euglobulin fraction of normal plasma and of plasmas deficient in factor XII and prekallikrein. Thromb Haemost 1991, 12:144-148 [PubMed] [Google Scholar]
- 43.Yasunaga C, Nakashima Y, Sueishi K: A role of fibrinolytic activity in angiogenesis. Lab Invest 1989, 61:698-704 [PubMed] [Google Scholar]
- 44.Lansink M, Koolwijk P, van Hinsbergh V, Kooistra T: Effect of steroid hormones and retinoids on the formation of capillary-like tubular structures of human microvascular endothelial cells in fibrin matrices is related to urokinase expression. Blood 1998, 92:927-938 [PubMed] [Google Scholar]
- 45.Fibbi G, Caldini R, Chevanne M, Pucci M, Schiavone N, Morbidelli L, Parenti A, Granger HJ, Del Rosso M, Ziche M: Urokinase-dependent angiogenesis in vitro and diacylglycerol production are blocked by antisense oligonucleotide against the urokinase receptor. Lab Invest 1998, 78:1109-1119 [PubMed] [Google Scholar]
- 46.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA: Regulation of integrin function by the urokinase receptor. Science 1996, 273:1551-1555 [DOI] [PubMed] [Google Scholar]
- 47.Kanse SM, Kost C, Wilhelm OG, Andreasen PA, Preissner KT: The urokinase receptor is a major vitronectin-binding protein on endothelial cells. Exp Cell Res 1996, 224:344-353 [DOI] [PubMed] [Google Scholar]
- 48.Waltz DA, Natkin LR, Fujita RM, Wei Y, Chapman HA: Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J Clin Invest 1997, 100:58-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang AW, Kuo A, Barnathan ES, Okada SS: Urokinase receptor-dependent upregulation of smooth muscle cell adhesion to vitronectin by urokinase. Arterioscler Thromb Vasc Biol 1998, 18:1855-1860 [DOI] [PubMed] [Google Scholar]
- 50.Preissner KT, Jenne D: Vitronectin: a new molecular connection in haemostasis. Thromb Haemost 1991, 66:189-194 [PubMed] [Google Scholar]
- 51.Chang MC, Wang BR, Huang TF: Characterization of endothelial cell differential attachment to fibrin and fibrinogen and its inhibitor by Arg-Gly-Asp-containing peptides. Thromb Haemost 1995, 74:764-769 [PubMed] [Google Scholar]
- 52.Thiagarajan P, Rippon AJ, Farrel DH: Alternative adhesion sites in human fibrinogen for vascular endothelial cells. Biochemistry 1996, 35:4169-4175 [DOI] [PubMed] [Google Scholar]
- 53.Francis CW, Bunce LA, Sporn LA: Endothelial cell responses to fibrin mediated by FPB cleavage and the amino terminus of the beta chain. Blood Cells 1993, 19:291-306 [PubMed] [Google Scholar]
- 54.Stephens RW, Pøllänen J, Tapiovaara H, Leung KC, Sim PS, Salonen EM, Rønne E, Saksela O, Moscatelli D, Rifkin DB: Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol 1989, 108:1987-1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner SN, Atkinson MJ, Wagner C, Hofler H, Schmitt M, Wilhelm O: Sites of urokinase-type plasminogen activator expression and distribution of its receptor in the normal kidney. Histochem Cell Biol 1996, 105:53-60 [DOI] [PubMed] [Google Scholar]
- 56.Christofidou-Solomidou MC, Bridges M, Murphy GF, Albelda SM, DeLisser HM: Expression and function of endothelial cell αV integrin receptors in wound-induced human angiogenesis in human skin/SCID mice chimeras. Am J Pathol 1990, 151:975-983 [PMC free article] [PubMed] [Google Scholar]
- 57.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatterman, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275:964–967 [DOI] [PubMed]
- 58.Raghunath PN, Tomaszewski JE, Brady ST, Caron RJ, Okada SS, Barnathan ES: Plasminogen activator system in human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 1995, 15:1432-1443 [DOI] [PubMed] [Google Scholar]
- 59.Lupu F, Heim DA, Bachmann F, Hurni M, Kakkar VV, Kruithof EKO: Plasminogen activator expression in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol 1995, 13:1444-1455 [DOI] [PubMed] [Google Scholar]