Abstract
To investigate the mechanism by which presenilin (PS) overexpression induces apoptosis, we studied the effects of these proteins on cell cycle progression. Transiently transfected HeLa cells were bromodeoxyuridine (BrdU) labeled to visualize DNA synthesis by immunofluorescence and stained with propidium iodide to measure DNA content by fluorescence-activated cell sorting (FACS). BrdU labeling was decreased in cells expressing presenilin-1 (PS1), presenilin-2 (PS2), an Alzheimer’s disease-associated missense mutation PS2(N141I), and the carboxyl-terminally deleted PS2 construct PS2(166aa), compared with mock and neurofilament-light (NF-L) transfected cells. Analysis of BrdU incorporation in mitotically synchronized HeLa cells suggested that cells were arresting in the G1 phase of the cell cycle, and this was confirmed by FACS analysis. Interestingly, cell cycle progression was more inhibited by the expression of PS2(N141I) compared with wild-type PS2. In addition, ATM, the gene product mutated in ataxia-telangiectasia, does not appear to be a downstream effector of PS-induced cell cycle arrest as transfection of PS constructs into an ataxia-telangiectasia cell line also resulted in cell cycle inhibition. Quantitative immunoblotting of whole-cell lysates from PS-transfected cells did not reveal increases or decreases in the steady-state levels of p21, p27, p53, pRb, or c-myc, suggesting that the presenilins mediate cell cycle arrest by mechanisms other than simple changes in the steady-state levels of these cell-cycle-related proteins.
Although only a small percentage (∼5%) of Alzheimer’s disease (AD) cases are familial with autosomal dominant transmission patterns, three separate genes containing mutations that cause disease have been identified through linkage studies: the amyloid precursor protein (APP), presenilin-1 (PS1), and PS2 (reviewed in Ref. 1 ). Although the hallmarks of the end stages of disease, the senile plaque and the neurofibrillary tangle, have been well characterized, genetic studies are proving to be invaluable in efforts to understand the events precipitating neurodegeneration.
Mammalian PS1 and PS2 are homologous proteins with 67% amino acid similarity. PS1 null mice are nonviable 2,3 but can be rescued by PS1 AD-related missense mutations. 4,5 Both PS1 and PS2 are able to functionally replace SEL-12, a Caenorhabditis elegans PS homolog that is involved in Notch signaling. 6 However, presenilins harboring AD-associated mutations are unable to fully rescue the SEL-12 mutant phenotype. 7,8
In a functional screen to identify genes involved in apoptosis, the carboxyl-terminal fragment of PS2 rescued T cells from T-cell-receptor and Fas-induced apoptosis in a dominant-negative fashion. 9,10 It has since been demonstrated that the overexpression of full-length and amino-terminal fragments of PS2 induces apoptosis and that cells expressing AD-associated mutations in either PS1 or PS2 die faster than cells expressing wild-type presenilins. 11-17
After reporting that PS2 overexpression induces apoptosis in HeLa cells, 14 we began to investigate whether the cell death was related to cell cycle progression. HeLa cells expressing a carboxyl-terminally deleted PS2 construct consisting of the 166 amino-terminal amino acids of PS2, PS2(166aa), displayed a distinct nuclear phenotype before dying, reminiscent of DNA condensation at mitosis. Apoptosis has been linked to both the induction as well as the suppression of cell cycle proteins, and progression to and arrest in specific cell cycle phases have been associated with increased apoptotic sensitivity. 18 To characterize the mechanism of cell death induced by PS overexpression, we attempted to connect the initiation of apoptosis to a specific cell cycle phase, a link that could be important in understanding the role of these proteins in degenerative processes.
Evaluating bromodeoxyuridine (BrdU) incorporation in PS-overexpressing cells revealed that DNA synthesis was inhibited. BrdU labeling in mitotically synchronized HeLa cells and FACS analysis suggested that the presenilins arrest cells in the G1 phase of the cell cycle. In addition, the percentage of BrdU-positive cells decreased in cells expressing the familial AD (FAD) PS2(N141I) mutant compared with wild-type PS2. p21, p27, p53, c-myc, and pRb protein levels were neither increased nor decreased in PS-transfected cell lysates, indicating that arrest is due to mechanisms other than those that alter the steady-state levels of these proteins.
Materials and Methods
Plasmids
The cloning of wild-type PS2, PS2 (N141I), and PS2(166aa) into a cytomegalovirus (CMV) expression plasmid was previously described. 14 Full-length PS1 (gift of Dr. S. Sisodia, University of Chicago) was polymerase chain reaction (PCR) amplified with a 5′ primer, (5′CTAGTACTCTCCGCGGCCACCATGACAGAGTTACCTGC3′), that introduced a SacII site upstream of the ATG start site and a 3′ primer, (5′TATCGCTTAAGTCGACCTAGATATAAAATTGATG3′), that engineered a SalI site after the stop codon. The double-digested PCR product was cloned into the above mentioned CMV expression plasmid. Mouse NF-L was expressed using pCMV-NF-L (gift of Dr. M. Lee, Johns Hopkins University). 19
Cell Lines, Cell Culture, and Transfection
HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 20 and an ataxia-telangiectasia (AT) transformed cell line (GMO9607A, NIGMS Human Genetic Mutant Cell Repository, Camden, NJ) was grown in MEM supplemented with 10% fetal bovine serum. Exponentially growing and mitotically synchronized cells were transfected with 15 to 20 μg of CaPO4-precipitated DNA.
Transfection and BrdU Staining of Mitotically Synchronized HeLa Cells
HeLa cells were mitotically synchronized by shake-off. 20 Mitotic cells were separated from exponentially growing populations by gently tapping flasks and collecting the loose cells in the media. Cells were plated on glass coverslips, and 1 hour after shake-off, CaPO4-precipitated DNA was added followed by a glycerol shock 2 hours later. BrdU was added to the media immediately after the glycerol shock or at various times after the shock. The cells were fixed in 50 mmol/L glycine/70% ethanol at −20°C for 20 minutes or overnight, rehydrated in PBS, blocked in 0.8% bovine serum albumin, and stained for expressed proteins. Cells were next stained for BrdU incorporation (5-bromo-2′-deoxy-uridine labeling and detection kit I, Boehringer Mannheim, Indianapolis, IN) and with 1 μg/ml 4′6-diamidino-2-phenylindole (DAPI) as previously described. 14
Antibodies used for the PS staining were a goat polyclonal anti-PS1 generated against the amino-terminal 19 amino acids (Santa Cruz Biotechnology, Santa Cruz, CA) and an anti-PS2 rabbit polyclonal generated against a GST-fusion protein 14 consisting of the first 86 amino acids of PS2. NF-L was detected with a rabbit polyclonal antibody generated against recombinant mouse NF-L. Secondary antibodies were affinity-purified rhodamine-conjugated donkey anti-goat and donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Images were captured using a SenSys camera attached to a Leica DRB microscope and manipulated with IPLab Spectrum software (Scanalytics, Fairfax, VA) on a Power Macintosh.
The percentages of BrdU-positive cells were determined by scanning slides for transfected cells and then examining them for BrdU incorporation. At least three populations of cells on independent coverslips were counted for each construct evaluated. The number of BrdU-positive transfected cells was divided by the total number of transfected cells. P values were calculated by comparing means in an unpaired two-tailed t-test with the Statveiw 512 program (Abacus Concepts, Berkeley, CA).
Fluorescence-Activated Cell Sorting (FACS)
HeLa cells transfected with PS constructs were trypsinized, resuspended in media, spun down for 5 minutes at 200 × g, washed two times in PBS, and fixed in 1% paraformaldahyde on ice for 5 minutes at a final concentration of 10 6 cells/ml. While vortexing, cells were resuspended in 70% ethanol and left at 4°C overnight before staining.
Cells were stained at room temperature for 2 to 3 hours in 50 μg/ml propidium iodide and 100 Kunitz U/ml RNAse A in PBS. Cells were counted on a FACSCalibur cell sorter using CellQuest software (Beckton Dickinson, Mountain View, CA), and the percentages of cells in the G1, S, and G2/M phases of the cell cycle were determined using ModFit LT software (Verity Software House, Topsham, ME).
Protein Preparation, SDS Electrophoresis, and Immunoblotting
Cells were lysed in a buffer containing protease inhibitors, 20 and protein concentrations were determined by the BCA protein assay (Pierce, Rockford, IL). For immunoblot analysis of PS expression, lysates were mixed with Laemmli buffer containing 15 mmol/L dithiothreitol and electrophoresed unboiled on 8.5% SDS-polyacrylamide gels. Lysates used for analysis of p21, p27, p53, c-myc, and pRb levels were boiled in Laemmli buffer and electrophoresed on either 8.5% or 10% gels. The fractionated proteins were transferred onto 0.2-μm nitrocellulose membranes by electroblotting and processed as described previously. 14
Antibodies used were the following: a mouse monoclonal anti-p53 (DO-1), a rabbit polyclonal to residues 408–421 of c-myc, and goat polyclonals to the 19 carboxyl-terminal residues of p27, the 19 carboxyl-terminal residues of p21, and the 15 carboxyl-terminal residues of pRB (all from Santa Cruz Biotechnology).
Results
Transient Expression of PS1 and PS2 Blocks Cell Cycle Progression
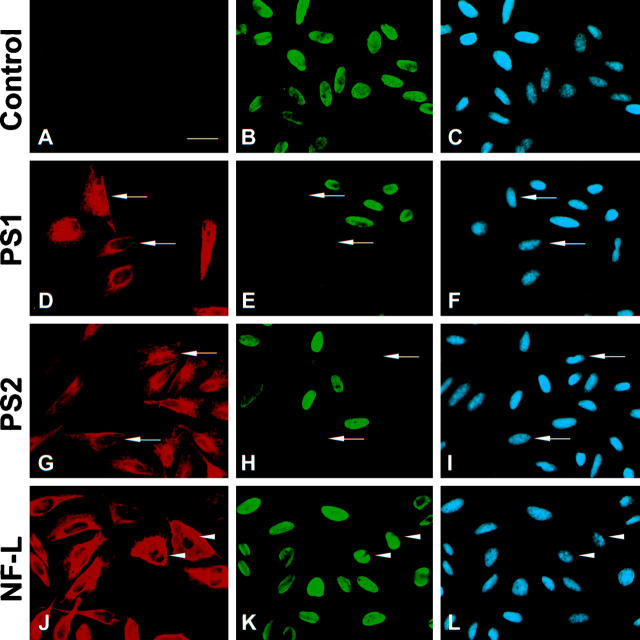
To begin to understand when during the cell cycle death is occurring in HeLa cells overexpressing PS, BrdU was added to the media of exponentially growing HeLa cells at the time of transfection, and the cells were fixed and double stained for PS and BrdU 18 hours later. In addition, NF-L, a protein not associated with cell cycle arrest, 21 was used as a control to test for the effects of protein overexpression on cell cycle progression. Many PS1- and PS2-transfected cells (Figure 1, D–I) ▶ did not incorporate BrdU into their genomes and thus had not passed through S-phase since the time of transfection as compared with surrounding nontransfected cells, mock transfected control cells (Figure 1, A–C) ▶ , and NF-L-expressing cells (Figure 1, J–L) ▶ , which were not impeded.
Figure 1.
Overexpression of PS1 and PS2 in HeLa cells inhibits cell cycle progression. HeLa cells were mock transfected (A to C) or transiently transfected with PS1 (D to F), PS2 (G to I), and NF-L (J and K) and evaluated for BrdU incorporation. Left panels show transfected protein, middle panels are BrdU labeling, and right panels are DAPI DNA images. None of the cells expressing PS entered S phase whereas NF-L overexpression was not inhibitory (J to L). Bar, 10 μm.
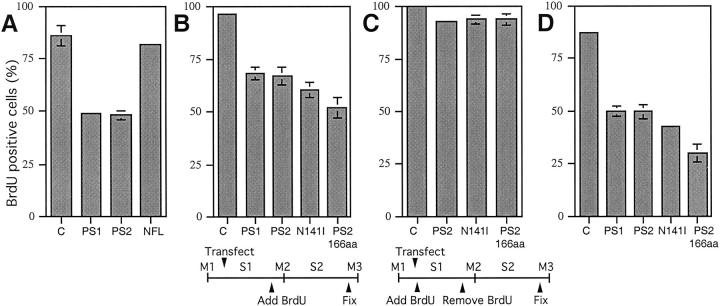
The percentages of cells positive for BrdU incorporation were next quantitated in these exponentially growing HeLa cells, and Figure 2A ▶ shows that BrdU labeling was significantly reduced in PS1- and PS2-expressing cells (49.0 ± 1.3% and 48.1 ± 1.9%, respectively) compared with untransfected control cells (85.7 ± 4.7%). Despite high levels of expression (Figure 1, J–L ▶ , arrowheads), NF-L (81.5 ± 1.3%) did not dramatically reduce BrdU labeling compared with control cells (85.7 ± 4.7%). Only cells with massive overexpression of NF-L resulting in protein aggregation around the nucleus failed to incorporate BrdU (data not shown). The NF-L aggregates could have either produced cellular stress resulting in a cell cycle block or prevented access of antibody to the nuclear BrdU antigens during the staining procedure. However, the reduction in the percentage of BrdU-positive cells due to NF-L expression was minor compared with PS-expressing cells. In addition, numerous examples of arrested cells expressing low levels of PS protein were found (see Figure 1 ▶ ), suggesting that cell cycle inhibition results from an inherent property of the presenilins and is not due simply to protein overexpression. Moreover, as described below, cell cycle arrest was increased by the overexpression of PS2 containing an AD-associated mutation signifying that the mutation acts to potentiate an intrinsic function of the protein.
Figure 2.
Quantification of BrdU incorporation in exponentially growing and mitotically synchronized cells. A: PS1, PS2, and NF-L were transiently transfected into exponentially growing HeLa cells. BrdU was added to the media at the time of transfection, and cells were fixed 18 hours after transfection. PS1 and PS2 reduced BrdU incorporation significantly (49.0 ± 1.3%, n = 3; 48.1 ± 1.9%, n = 3, respectively) compared with mock transfected control cells (85.7 ± 4.7%; n = 3; P < 0.0005 in a two-tailed unpaired t-test), and 81.5 ± 1.3% (n = 3) of cells expressing NF-L were BrdU positive. B and C: HeLa cells were mitotically synchronized by shake-off, plated, and transiently transfected with PS constructs. B: Cells passed through the first DNA synthesis (S1) before BrdU was added to the media; cells were fixed after the subsequent DNA synthesis (S2). PS1 (68 ± 3%, n = 3) and PS2 (67 ± 4.4%, n = 8) reduced BrdU incorporation significantly compared with mock transfected control cells (96.3 ± 1.2%, n = 3; P = 0.0001). PS2(N141I) (60.1 ± 3.6%, n = 7) was more inhibitory than PS2 (67 ± 4.4%, n = 8, P = 0.0058), and PS2(166aa) (52 ± 4.8%, n = 5) reduced DNA synthesis the most compared with PS2 (P = 0.0001). C: BrdU was added to the media at the time of transfection but removed after the first DNA synthesis (S1); cells were fixed after S2. BrdU incorporation in cells expressing PS2 (93 ± 1.6%, n = 3), PS2(N141I) (93.8 ± 2.2%, n = 3), and PS2(166aa) (93.8 ± 2.9%, n = 3) did not differ dramatically from the mock transfected controls (100%, n = 3). Therefore, the majority of unlabeled cells in B were not blocked before S1. D: The PS constructs were transiently transfected into the AT cell line GM09607A. Compared with mock transfected control cells (87.5 ± 0.9%, n = 3), BrdU labeling was significantly reduced by PS1 (49.7 ± 2.5%, n = 3) and PS2 (49.6 ± 3.5%, n = 4) expression (P < 0.0001). PS2(N141I) was more inhibitory than PS2 (42.5 ± 1%, n = 3; 49.6 ± 3.5%, n = 4; respectively; P = 0.019), whereas PS2(166aa) reduced DNA synthesis the most (29.8 ± 4.8%, n = 6; P = 0.00001) compared with PS2.
BrdU Incorporation in Mitotically Synchronized HeLa Cells Indicates G1 Arrest
To determine the cell cycle phase in which the presenilins induce arrest, we transfected PS1, PS2, the AD-associated missense mutant PS2(N141I), and the carboxyl-terminally truncated PS2(166aa) into mitotically synchronized HeLa cells and fixed them at specific times during the cell cycle. Previous analysis revealed that HeLa cells complete a cell cycle approximately every 23 hours with peak DNA synthesis occurring between 10 and 19 hours after mitotic shake-off. 20 This information helped us to establish a time table for the experiments described below.
Initially, BrdU incorporation was evaluated after the first DNA synthesis (S1) after shake-off. DNA precipitates were added to the plated mitotic cells 1 hour after shake-off, and the transfection was completed and BrdU added to the media 2 hours later. Cells were then fixed and stained for BrdU incorporation and PS expression 19 hours after shake-off (after S1). However, at this time point, PS expression levels were not high enough to detect large enough populations of PS-positive cells to produce statistically reliable results (data not shown). Therefore, we studied BrdU incorporation in these transfected synchronized cells after the second round of DNA synthesis (S2) when PS expression levels were higher (Figure 2B) ▶ . Perhaps the time required for protein expression was considerably longer due to the shake-off procedure, thus precluding analysis at S1.
In Figure 2B ▶ , synchronized HeLa cells were transfected 3 hours after shake-off, but BrdU was not added to the media until after S1 (Figure 2B ▶ time line). Consistent with results seen in exponentially growing cultures, expression of PS1 (68 ± 3%) and PS2 (67 ± 4.4%) inhibited DNA synthesis compared with mock transfected control cells (96.3 ± 1.2%). Moreover, expression of PS2(N141I) (60.1% ± 3.6%) reduced BrdU incorporation significantly (∼7.1%, ([67 − 60.1])/96.3) × 100%) compared with wild-type PS2. DNA synthesis was the most inhibited by PS2(166aa) (52 ± 4.8%).
In exponentially growing HeLa cells (Figure 2A) ▶ , the percentage of BrdU-positive cells was reduced by ∼44% compared with mock transfected control cells (1 − [48.1%/85.7%] × 100) whereas in synchronized HeLa cells a ∼30% (Figure 2B) ▶ reduction was observed. As PS expression was low to undetectable in the synchronized cells at the time point after S1, it is conceivable that the shake-off procedure delayed the expression of the presenilins, allowing compensatory mechanisms to be up-regulated. Exponential cultures may be more sensitive to arrest because they express high levels of PS only 18 hours after transfection. Furthermore, sensitivity to PS-induced arrest may correlate with exposure to high PS protein during specific cell cycle phases that may be better represented in exponential cultures that contain cells in all phases of the cell cycle.
To determine whether the PS-expressing cells arrested in Figure 2B ▶ were blocked before the first DNA synthesis (S1), we transfected synchronized cells, added BrdU to the media at the time of transfection, removed the BrdU after S1, and fixed and stained after S2 (see Figure 2C ▶ time line; same fixation time point as in Figure 2B ▶ ). If the reduction in BrdU labeling caused by PS expression in Figure 2B ▶ resulted from cell cycle arrest before S1, then the percentages of BrdU-positive cells in Figure 2C ▶ should be comparable to the values in Figure 2B ▶ . However, the PS-expressing cells in Figure 2C ▶ incorporated BrdU at levels similar to untransfected control cells (∼93% and 100%, respectively), indicating that the cells in Figure 2B ▶ were arrested between S1 and S2.
To test whether the PS-expressing cells arrested in Figure 2B ▶ were blocked at the G2/M restriction point that follows S1, synchronized transfected cells exposed to BrdU were shaken off at M2 (same method as initial synchronization), replated on coverslips, and stained 3 hours later for BrdU incorporation and PS expression. Many pairs of PS-expressing cells positive for BrdU could be seen (data not shown), suggesting that if PS-expressing cells synthesize their DNA, progression into cell division is not inhibited. Moreover, we found numerous examples of PS1- and PS2-expressing cells at different stages of mitosis (Figure 3, A and B) ▶ , providing additional evidence that PS expression does not impede cell cycle progression at G2/M.
Figure 3.

Merged fluorescent images of PS2 expression (green) and DAPI staining of DNA (blue) in HeLa cells. A and B: PS-overexpressing cells at various stages of mitosis. Bar, 10 μm. C to F: Cells transfected with PS2 constructs from cultures used for FACS analysis in Figure 4 ▶ showing 60% to 70% transfection efficiencies: mock transfected control cells (C), PS2 (D), PS2(N141I) (E), and PS2(166aa) (F). Bar, 100 μm.
FACS Analysis Confirms G1 Arrest
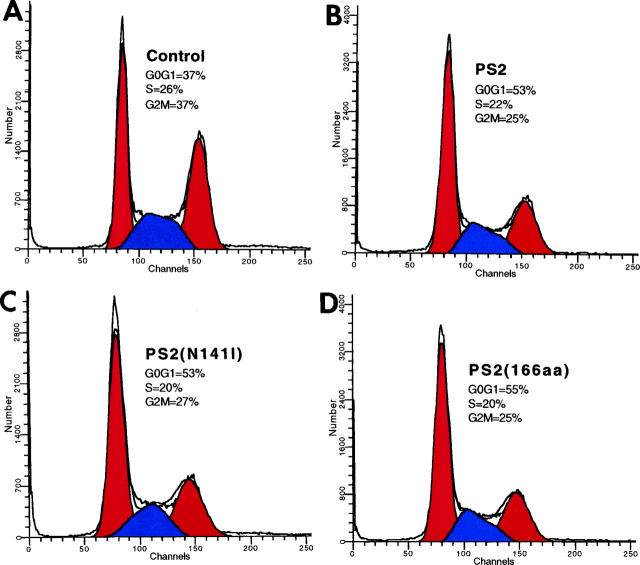
To confirm that PS expression induces arrest in the G1 phase of the cell cycle, transfected exponentially growing HeLa cells were stained with propidium iodide, and DNA content was measured by FACS analysis to determine the proportion of cells in the G0/G1, S, and G2/M phases of the cell cycle. Compared with control cells (Figure 4A) ▶ , expression of the PS2 constructs (Figure 4, B–D) ▶ increased the percentages of cells in G0/G1 by ∼43% to 48% ([53 − 37]/37 × 100%), which correlated with concomitant decreases in the percentages of cells in G2/M and S phase. The high transfection efficiencies obtained (∼60% to 70%; see Figure 3, C–F ▶ ) in these experiments support the conclusion that the increases in the proportions of cells in G0/G1 are due to the overexpression of the PS2 constructs.
Figure 4.
FACS analysis of PS2-expressing cells. Exponentially growing HeLa cells were mock transfected (A) or transiently transfected with PS2 (B), PS2(N141I) (C), or PS2(166aa) (D), fixed, and stained with propidium iodide. DNA content was measured by FACS analysis. Increases in the percentages of cells in the G0/G1 phase of the cell cycle are seen in all cell populations expressing PS2 constructs with concomitant decreases in G2/M and S phase percentages compared with control cells.
PS Expression Impedes Cell Cycle Progression in an AT Cell Line
A number of tumor suppressor genes with roles in halting cell cycle progression and initiating apoptosis have been identified by studying cancer predisposition syndromes. 22,23 ATM (ataxia-telangiectasia mutated) is the gene defective in the autosomal recessive disorder ataxia-telangiectasia (AT). Exposure to ionizing radiation does not induce p53 in AT cells, which consequently do not arrest at cell cycle checkpoints in response to DNA damage. 24
Therefore, we expressed our PS constructs in an AT cell line (GM09607A) to see whether ATM might be acting downstream of PS to inhibit cell cycle progression. If this hypothesis were correct, when ATM is missing, progression into S phase should proceed when PS protein is overexpressed. However, PS expression also caused cell cycle arrest in this cell line, ruling out ATM as a downstream effector of PS signaling (Figure 2D) ▶ . Cells transfected with full-length wild-type PS1 and PS2 were BrdU labeled at comparable levels (49.7 ± 2.5% and 49.6 ± 3.5%, respectively) with DNA synthesis significantly inhibited compared with mock transfected control cells (87.5 ± 0.9%). A difference in the percentage of cells incorporating BrdU was observed between PS2(N141I) (42.5 ± 1%) and wild-type PS2 (49.6 ± 3.5%) with PS2(N141I), again showing a ∼7.8% greater propensity for inhibition relative to the control ([42.5 − 49.6]/87.5 × 100%). BrdU labeling was reduced the most in cells expressing PS2(166aa) with only 29.8 ± 4.8% of the cells positive for BrdU incorporation.
p53, p21, p27, c-myc, and pRb Levels Do Not Change in PS-Transfected Cells
As PS expression arrests cells in the G1 phase of the cell cycle, we next looked for changes in the levels of the cell cycle inhibitory proteins p21, p27, and p53 and the cell cycle regulatory proteins c-myc and pRb in PS-transfected cell lysates. PS1 and PS2 proteins are highly expressed (Figure 5A ▶ ; see also Figure 3, C–F ▶ : 60% to 70% of the cells are transfected) in our preparations making it likely that if PS expression effects the levels of these cell cycle regulatory proteins (compared with mock transfected controls), differences would be detected. Full-length PS1 (lane 2) runs at 43 to 45 kd, PS2 and PS2(N141I) (lanes 4 and 5) at 50 to 54 kd, and PS2(166aa) (lane 6) at 31 to 33 kd, as shown previously. 14 Interestingly, higher molecular weight PS-immunoreactive complexes were also present in all PS-transfected cell lysates (lanes 2 to 6).
Figure 5.

Immunoblot analysis of PS-transfected cell lysates. A: PS expression in HeLa cells: lanes 1 and 3, mock transfected; lane 2, PS1; lane 4, PS2; lane 5, PS2(N141I); lane 6, PS2(166aa). B: Immunoblots of p21, p27, pRb, and c-myc levels in PS-transfected HeLa cells: lane 1, mock transfected; lane 2 , PS1; lane 3, PS2; lane 4, PS2(N141I); lane 5, PS2(166aa). p53 levels were evaluated in both HeLa and AT cells.
Because p53 is one of the best studied tumor suppressor protein and increases in its expression are closely associated with cell cycle arrest and the induction of apoptosis, 25 we examined the levels of p53 and its target gene, p21, which directly inhibits cell cycle kinases, 26 by immunoblotting PS transfected cell lysates. In addition, we evaluated levels of p27, a cdk inhibitor, c-myc, a proto-oncogene, and pRb, a cell cycle regulator controlling the G1/S transition. 27 Although all were easily detected in our lysates, their levels did not significantly change when the presenilins were overexpressed (Figure 5B ▶ , Lanes 2–5) .
Discussion
PS overexpression has been shown to induce apoptosis with cell death increased by the expression of AD-associated mutants. 11,13,14 Here we show that PS overexpression arrests cells in the G1 phase of the cell cycle, an effect that likely precedes the apoptosis. Cell cycle progression was more inhibited (∼7.1% in HeLa cells and ∼7.8% in AT cells) by the FAD-associated PS2(N141I) mutant compared with wild-type PS2, a difference that might be related to the increased cell death caused by the mutants. In addition, NF-L overexpression did not dramatically affect cell cycle progression, suggesting that the PS-induced cell cycle arrest is due to some intrinsic property of the presenilins and is not simply a result of protein overexpression.
Previously, we quantified cell death by counting the number of dead floating cells in cell cultures transfected with either wild-type PS2 or the FAD PS2(N141I) mutant. 14 Assaying BrdU incorporation in PS-transfected cells is an independent method of evaluating differences between the effects of mutant and wild-type proteins. Unlike counting dead floating cells, evaluating BrdU labeling is a direct assessment of the effects of PS overexpression as only cells expressing PS protein were counted. Thus, errors inherent in normalizing transfection efficiencies were avoided.
Two important questions emerge regarding PS-induced cell cycle arrest. First, how much overexpression is required to produce the effect? And second, why does the missense PS2 AD mutation (N141I) potentiate arrest compared with wild-type PS2? At this point, the answers to these questions are unknown. High PS expression did not always correlate with cell cycle arrest as examples of arrested cells with low expression by immunofluorescence were found although some high-expressing cells were still able to synthesize their DNA (data not shown). Although it is difficult to measure and compare protein expression levels in individually transiently transfected cells, comparable numbers of both wild-type and PS2(N141I)-expressing cells were detected in this assay with expression levels, as expected, ranging from low to high. Additionally, immunoblot analysis (Figure 5A ▶ , lanes 4 and 5) detected similar amounts of both proteins without significant proteolytic cleavage, suggesting that the potentiation of arrest by the FAD PS2(N141I) cannot be explained by a difference in either expression levels or proteolytic processing.
It is interesting that the expression of PS2(166aa), a construct consisting of the hydrophilic amino terminus and the first two transmembrane domains of PS2, inhibited DNA synthesis the most compared with the other PS constructs tested. We had previously shown that this sequence is sufficient for endoplasmic reticulum localization and the induction of apoptosis. 14 Expression of the carboxyl-terminal portion of PS2 has been shown to prevent apoptosis in a dominant negative fashion 10 ; therefore, it is possible that the deletion of the carboxy terminus from PS2(166aa) removes regions that regulate PS signaling. As the N141I mutant also decreased DNA synthesis compared with wild-type PS2, the point mutation in the second transmembrane domain could be altering the regulatory functions of the carboxyl-terminal domains. Another possibility is that as this construct is associated with a decreased rate of cell death at early time points after transfection, 14 the increased rate of cell cycle arrest may reflect an increased propensity of these cells to survive in an arrested state before dying.
Attempting to link the presenilins to signaling networks that cause both cell cycle arrest and apoptosis immediately raised p53 as a candidate effector. Increases in p53 expression, however, were not detected in either PS-transfected HeLa or AT cells. In fact, cell cycle progression was also inhibited by PS expression in the absence of ATM, a known inducer of p53 in response to genotoxic stress. 24 It was recently reported that PS1 RNA and protein levels decrease in response to high p53 and p21 expression, and antisense inhibition of PS1 was also shown to inhibit growth and increase the number of cells undergoing apoptosis. 28 As we detected neither increased nor decreased p53 in response to either PS1 or PS2 overexpression, a converse relationship between PS and p53 expression does not seem to exist. However, it is clear that the pathway in which the presenilins function is acutely sensitive to changes in PS protein levels as both overexpression and underexpression arrest growth and kill cells.
Changes in p21, p27, c-myc, and pRb were also not detected in PS-transfected cell lysates, which will make it necessary for us to evaluate the effects of the presenilins on other signaling pathways involved in cell cycle arrest and apoptosis. Although significant changes in the steady-state levels of these cell-cycle-regulated proteins were not detected in PS-arrested cells, participation of these proteins in cell cycle inhibition cannot be entirely excluded as we did not measure the stability, phosphorylation states, cellular localization, or interactions of these proteins with their known signaling effectors. We believe that careful examination of such properties as well as the identification of proteins that interact with the presenilins will be important in efforts to link these proteins to their functional pathways.
The presenilins have already been reported to interact with components of the Notch 29 and Wingless/Wnt pathways, including β-catenin, 30 δ-catenin, a novel member of the catenin family, 31 and glycogen synthase kinase-3β (GSK-3β). 32 In addition, mutant PS1 has been shown to destabilize β-catenin leading to increased apoptosis. 17 Interestingly, a study of cell cycle coordination in the cells of the developing Drosophila wing showed that Notch activity arrests cells in the G1 phase of the cell cycle by preventing Wingless/Wnt-induced G2 arrest. 33
It is unclear at this time whether the mutations in the presenilins directly initiate reexpression of cell cycle proteins in postmitotic neurons or whether their effects produce unrelated consequences. The reinitiation of cell cycle events, however, has been proposed to be a trigger of neuronal degeneration because elevations of cell cycle and apoptotic proteins, not present in age-matched controls, have been detected in histological examinations of AD brains. 34-41 The up-regulation of these proteins could be in response to inappropriate growth and differentiation signals, oxidative insult from metabolic stress, or regenerative attempts in responses to deterioration caused by the aging process. If the theory that the reinitiation of cell cycle events in AD neurons causes their demise is correct, then one might expect the level of cell cycle arrest induced by PS2(N141I) to be lower compared with wild-type PS2. However, just the opposite was observed. Therefore, we are unable to say at this time whether differences in the cell cycle regulatory functions of wild-type PS2 and PS2(N141I) are related in any way to the reports of cell cycle genes being reexpressed in AD neurons. Comprehensive studies evaluating the expression of cell cycle proteins in the brains of FAD PS and APP patients will be helpful in addressing this issue.
Studies of PHF-tau, APP cleavage, and histological examination of AD brain have all implicated cell cycle misregulation in AD (reviewed in Ref. 42 ). It is possible that neuronal attempts to regenerate in response to aging reinitiate cell cycle signaling events producing the downstream accumulations of the Aβ peptide and tau hyperphosphorylation. Our work provides one of the first connections between a genetic cause of AD and cell cycle regulation. First, it is possible that FAD PS2 mutations prevent dividing cells in the brain, possibly neural precursor cells or glia, from progressing through the cell cycle with deleterious effects on postmitotic neurons. Another possibility is that presenilin and APP mutations, which have been shown to induce pro-apoptotic cascades, up-regulate cyclin-dependent kinases, which appear to play dual roles in controlling proliferation and death signaling (see Ref. 43 ). The induction of cyclin-dependent kinase inhibitors, such as p16, p19, p21, and p27, in AD may reflect a protective response elicited by the cells to these death stimuli. There is good evidence for the up-regulation of cyclin-dependent kinases in cultured neurons upon insult by a number of apoptotic inducing agents (reviewed in Ref. 43 ). Interestingly, p16, p21, and p27, which are inhibitors of CDK4 and CDK6 kinases, protect neurons from these apoptotic insults, 43 and many of these same proteins are reexpressed in AD neurons. 37,38,41
Cell cycle regulation by cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors has been well characterized, 26 but how cell cycle events are regulated by cell fate patterning cues, differentiation signals, and genomic surveillance mechanisms is not as well understood. Dynamic signaling networks must integrate information concerning growth stimuli, developmental programs, and the status of the genome to direct the cell to either continue cycling, exit the cell cycle (G0), or arrest at a restriction point (G1/S or G2/M). A role for the presenilins in cell cycle control may have relevance to both apoptotic and developmental mechanisms due to the interrelationship of cell death, growth, and differentiation.
Acknowledgments
We thank Marielle Cavrois for help with the FACS analysis and Dr. Ann Pluta for critical comments on the manuscript.
Footnotes
Address reprint requests to Dr. Mervyn J. Monteiro, Medical Biotechnology Center, Room N352, 725 West Lombard Street, Baltimore, MD 21201. E-mail: monteiro@umbi.umd.edu.
Supported in part by NIH grant AG11386 to M.J. Monteiro.
References
- 1.Hardy J: Amyloid, the presenilins, and Alzheimer’s disease. Trends Neurosci 1997, 4:154-159 [DOI] [PubMed] [Google Scholar]
- 2.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S: Skeletal and CNS defects in presenilin-1-deficient mice. Cell 1997, 89:629-639 [DOI] [PubMed] [Google Scholar]
- 3.Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS: Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 1997, 387:288-292 [DOI] [PubMed] [Google Scholar]
- 4.Davis JA, Naruse S, Chen H, Eckman C, Younkin S, Price DL, Borchelt DR, Sisodia SS, Wong PC: An Alzheimer’s disease-linked PS1 variant rescues the developmental abnormalities of PS1-deficient embryos. Neuron 1998, 20:603-609 [DOI] [PubMed] [Google Scholar]
- 5.Qian S, Jiang P, Guan XM, Singh G, Trumbauer ME, Yu H, Chen HY, Van de Ploeg LH, Zheng H: Mutant human presenilin 1 protects presenilin 1 null mouse against embryonic lethality and elevates Abeta1–42/43 expression. Neuron 1998, 20:611-617 [DOI] [PubMed] [Google Scholar]
- 6.Levitan D, Greenwald I: Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer’s disease gene. Nature 1995, 377:351-354 [DOI] [PubMed] [Google Scholar]
- 7.Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I: Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci USA 1996, 93:14940-14944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumeister R, Leimer U, Zweckbronner I, Jakubek C, Grunberg J, Haass C: Human presenilin-1, but not familial Alzheimer’s disease (FAD) mutants, facilitate Caenorhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct 1997, 2:149-159 [DOI] [PubMed] [Google Scholar]
- 9.Vito P, Lacana E, D’Adamio L: Interfering with apoptosis: Ca(2+)-binding protein ALG-2 and Alzheimer’s disease gene ALG-3. Science 1996, 271:521-525 [DOI] [PubMed] [Google Scholar]
- 10.Vito P, Wolozin B, Ganjei JK, Iwasaki K, Lacana E, D’Adamio L: Requirement of the familial Alzheimer’s disease gene PS2 for apoptosis: opposing effect of ALG-3. J Biol Chem 1996, 271:31025-31028 [DOI] [PubMed] [Google Scholar]
- 11.Wolozin B, Iwasaki K, Vito P, Ganjei JK, Lacana E, Sunderland T, Zhao B, Kusiak JW, Wasco W, D’Adamio L: Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science 1996, 274:1710-1713 [DOI] [PubMed] [Google Scholar]
- 12.Deng G, Pike CJ, Cotman CW: Alzheimer-associated presenilin-2 confers increased sensitivity to apoptosis in PC12 cells. FEBS Lett 1996, 397:50-54 [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Furukawa K, Sopher BL, Pham DG, Xie J, Robinson N, Martin GM, Mattson MP: Alzheimer’s PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid β-peptide. Neuroreport 1996, 8:379-383 [DOI] [PubMed] [Google Scholar]
- 14.Janicki S, Monteiro MJ: Increased apoptosis arising from increased expression of the Alzheimer’s disease-associated presenilin-2 mutation (N141I). J Cell Biol 1997, 139:485-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP: Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid β-peptide: involvement of calcium and oxyradicals. J Neurosci 1997, 11:4212-4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolozin B, Alexander P, Palacino J: Regulation of apoptosis by presenilin 1. Neurobiol Aging 1998, 19:S23-S27 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner BA: Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 1998, 395:698-702 [DOI] [PubMed] [Google Scholar]
- 18.Meikrantz W, Schlegel R: Apoptosis and the cell cycle. J Cell Biochem 1995, 58:160-174 [DOI] [PubMed] [Google Scholar]
- 19.Lee MK, Xu Z, Wong PC, Cleveland DW: Neurofilaments are obligate heteropolymers in vivo. J Cell Biol 1993, 122:1337-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteiro MJ, Mical TI: Resolution of kinase activities during the HeLa cell cycle: identification of kinases with cyclic activities. Exp Cell Res 1996, 223:443-451 [DOI] [PubMed] [Google Scholar]
- 21.Monteiro MJ, Cleveland DW: Expression of NF-L and NF-M in fibroblasts reveals coassembly of neurofilament and vimentin subunits. J Cell Biol 1989, 108:579-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elledge SJ: Cell cycle checkpoints: preventing an identity crisis. Science 1996, 274:1664-1672 [DOI] [PubMed] [Google Scholar]
- 23.Paulovich AG, Toczyski DP, Hartwell LH: When checkpoints fail. Cell 1997, 88:315-321 [DOI] [PubMed] [Google Scholar]
- 24.Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJJ: A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992, 71:587-597 [DOI] [PubMed] [Google Scholar]
- 25.Levine AJ: p53, the cellular gatekeeper for growth and division. Cell 1997, 88:323-331 [DOI] [PubMed] [Google Scholar]
- 26.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 27.Evan G, Littlewood T: A matter of life and cell death. Science 1998, 281:1317-1322 [DOI] [PubMed] [Google Scholar]
- 28.Ray WJ, Yao M, Nowotny P, Mumm J, Zhang W, Wu JY, Kopan R, Goate AM: Evidence for a physical interaction between presenilin and Notch. Proc Natl Acad Sci USA 1999, 96:3263-3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roperch JP, Alvaro V, Prieur S, Tuynder M, Nemani M, Lethrosne F, Piouffre L, Gendron MC, Israeli D, Dausset J, Oren M, Amson R, Telerman A: Inhibition of presenilin 1 expression is promoted by p53 and p21WAF-1 and results in apoptosis and tumor suppression. Nature Med 1998, 7:835-838 [DOI] [PubMed] [Google Scholar]
- 30.Murayama M, Tanaka S, Palacino J, Murayama O, Honda T, Sun X, Yasutake K, Nihonmatsu N, Wolozin B, Takashima A: Direct association of presenilin-1 with β-catenin. FEBS Lett 1998, 433:73-77 [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Liyanage U, Medina M, Ho C, Simmons AD, Lovett M, Kosik KS: Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport 1997, 8:1489-1494 [DOI] [PubMed] [Google Scholar]
- 32.Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S, Wolozin B: Presenilin 1 associates with glycogen synthase kinase-3β and its substrate tau. Proc Natl Acad Sci USA 1998, 95:9637-9641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston LA, Edgar BA: Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature 1998, 394:82-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masliah E, Mallory M, Alford M, Hansen LA, Saitoh T: Immunoreactivity of the nuclear antigen p105 is associated with plaques and tangles in Alzheimer’s disease. Lab Invest 1993, 69:562-569 [PubMed] [Google Scholar]
- 35.Smith TW, Lippa CF: Ki-67 immunoreactivity in Alzheimer’s disease and other neurodegenerative disorders. J Neuropathol Exp Neurol 1995, 54:297-303 [DOI] [PubMed] [Google Scholar]
- 36.Vincent I, Rosado M, Davies P: Mitotic mechanisms in Alzheimer’s disease? J Cell Biol 1996, 132:413-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McShea A, Harris PL, Webster KR, Wahl AF, Smith MA: Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am J Pathol 1997, 150:1933-1939 [PMC free article] [PubMed] [Google Scholar]
- 38.Nagy Z, Esiri MM, Cato AM, Smith AD: Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol 1997, 94:6-15 [DOI] [PubMed] [Google Scholar]
- 39.Vincent I, Jicha G, Rosado M, Dickson DW: Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer’s disease brain. J Neurosci 1997, 17:3588-3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busser J, Geldmacher DS, Herrup K: Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci 1998, 18:2801-2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arendt T, Holzer M, Gartner U: Neuronal expression of cyclin dependent kinase inhibitors of the INK4 family in Alzheimer’s disease. J Neural Transm 1998, 105:949-960 [DOI] [PubMed] [Google Scholar]
- 42.Raina AK, Monteiro MJ, McShea A, Smith MA: The role of cell cycle-mediated events in Alzheimer’s Disease. Int J Exp Pathol 1999, 80:71-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park DS, Morris EJ, Padmanabhan J, Shelanski ML, Geller HM, Greene LA: Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J Cell Biol 1998, 143:457-467 [DOI] [PMC free article] [PubMed] [Google Scholar]