Abstract
Psoriasis is an immunologically mediated skin disease linked to several different class I major histocompatibility complex alleles. However, the phenotype of the pathogenic lymphocyte and nature of the T cell activating event which triggers conversion of symptomless (PN) skin into psoriatic plaques (PP skin) is unknown. This study extends our previous observations in which autologous blood-derived immunocytes were injected into PN skin engrafted onto SCID mice to produce full-fledged PP lesions. The first question addressed is whether injected CD4+ T cells or CD8+ T cells were responsible for phenotypic conversion of PN to PP skin. In five different patients only CD4+ but not CD8+ T cell lines produced psoriatic lesions. Next, immunological events occurring within PN skin following injection of CD4+ T cells in grafts that had sufficient tissue available for detailed analysis was examined. In two patients, intraepidermal resident CD8+ T cells were induced to proliferate during lesion development, expressing acute activation markers CD25 and CD69. In another patient, injection of CD4+ T cells revealed CD69 expression by intraepidermal CD4+ as well as CD8+ T cells. To explore the molecular basis for local T cell activation and proliferation, we discovered that intraepidermal immunocytes, including both CD4 and CD8+ T cells, expressed surface receptors (ie, CD94, CD158a, CD158b) typically confined to natural killer cells (ie, natural killer receptors; NKRs) accumulated immediately before onset of acute lesions. The presence of NKR bearing immunocytes was also observed in 10 of 15 different biopsies of chronic plaques taken directly from patients, whereas PN skin (n = 8) or normal skin from healthy donors (n = 8), did not contain such NKR positive immunocytes. Of particular relevance to psoriasis is that these NKRs recognize various class I alleles including those typically inherited by psoriatic family members such as HLA-C and HLA-B allotypes. We conclude that injection of CD4+ T cells into PN skin triggers a series of local immunologically mediated stimulatory events that produce further T cell activation and appearance of both CD4 and CD8+ T cells that express NKRs.
Psoriasis, a common skin disease, results from the interplay between environmental and inherited triggering factors. 1 Many groups are examining the genetic transmission of this disease to elucidate the gene(s) responsible for causing the phenotypic conversion of symptomless (PN) skin into psoriatic plaques (PP skin). Although different groups have reached somewhat conflicting results regarding the chromosomal location for the putative psoriasis locus, the most consistent and striking linkage association in psoriasis appears to involve class I major histocompatibility complex (MHC) alleles. 2
Immunodermatologists familiar with this class I association have focused predominantly on CD8+ T cells, because such cells generally recognize processed peptides displayed by antigen presenting cells in the context of class I MHC cell surface molecules. 3 Many immunophenotypic studies of psoriasis have been performed, and most document the presence of CD8+ T cells in epidermis, whereas CD4+ T cells tend to predominate in the dermal compartment, with only rare incidental natural killer (NK) cells in dermis. 4 Even in psoriatic lesions that are only three days old, CD8+ T cells begin accumulating in the epidermis. 5 Furthermore, when peripheral blood-derived mixtures of CD4+/CD8+ T cells are injected into PN skin engrafted onto SCID mice, the induction of PP skin is characterized by preferential migration of CD8+ T cells into the hyperplastic epithelium. 6 The reports identifying a clonal population of intraepidermal CD8+ but not CD4+ T cells in PP skin, 7 as well as statistically significant reduction in the intraepidermal CD8+ T cell population after successful immunospecific therapy, 8 all support a key role for CD8+ T cells and their interaction with epidermal cells. However, to date it has not been possible to explain the exact role of the various MHC class I alleles in psoriasis, because no one has consistently identified any specific peptide antigen responsible for causing psoriasis. 9,10
Against this backdrop, it was unexpected to observe that when T cell cultures were established from the peripheral blood of five different patients, all of the CD4+ T cell lines (5/5), but none (0/5) of the CD8+ T cells lines, were capable of inducing PN-to-PP conversion using the SCID mouse model. 11 By examining engrafted skin injected with CD4 + T cells, only a small fraction of the overall mononuclear population expressed the T cell acute activation marker, CD69. 12 Further analysis in 2 of 5 patients revealed the majority of the CD69-positive cells were actually CD8+ cells, and these positive cells were associated with the epidermal compartment, as mid- and deep dermal lymphocytes were CD69-negative. This result suggested that, like allergic contact dermatitis reactions, which early psoriasis lesions can simulate, 13 CD4+ T cells were providing critically important help signals in a regulatory fashion, whereas the actual effector cells may be CD8+ immunocytes. 14 As we considered a possible role for class I MHC antigens in psoriasis, we began focusing on NK receptors (NKR) used by NK cells to interface with targets involving self/nonself determinants triggered via class I MHC recognition. 15 NK cells are one of the three lineages of lymphocytes, besides T cells and B cells, that are most clearly identified with the control of viral infections and tumor development. 16 Although previous investigators found only very rare NK cells in psoriatic plaques 4 and no difference in NK cell activity in the blood of patients with psoriasis compared to normal individuals, 17 we identified immunocytes in skin of psoriatic patients expressing NKRs. As T cell subsets in blood have been identified that express NKRs, the emphasis in other systems has been on defining the inhibitory role of these receptors, highlighting the notion that CD8+T cells bearing such inhibitory NKRs (killer inhibitory receptors) 18 would not lyse autologous normal class I MHC-bearing cells.
In this report, we present several findings, including i) CD4+ but not CD8+ T cell lines derived from peripheral blood are capable of converting PN skin engrafted onto SCID mice to PP skin after intradermal injections; ii) after injection of CD4+ T cells, local restimulation of indigenous CD8+ T cells as well as CD4+ T cells occurs as intraepidermal lymphocytes are induced to express CD69 and CD25; and iii) identification of immunocytes expressing various NKRs (ie, CD94, CD158a, CD158b) in both acute and chronic lesions of psoriasis.
Based on this data we suggest a novel hypothesis for psoriasis in which direct recognition and interaction of these NKRs with class I MHC alleles may play a role in the development of psoriasis. In essence we propose that various MHC class I alleles themselves (rather than exogenous peptide antigens) are responsible for directly triggering T cells bearing the activating forms of CD94/CD158a/CD158b. Upon examination of MHC class alleles most likely involved in psoriasis, such as Cw6, and comparison of this list to the types of class I alleles that interact with NKRs, a correlation between these ligand/receptors can be appreciated. 19 Taken together, these results suggest a new etiological and pathophysiological pathway in which immunocytes are postulated to be triggered to release cytokines and proliferate by using receptors typically confined to NK cells, thereby interfacing with class I MHC surface molecules on epidermal cells.
Materials and Methods
Patients
The psoriasis patients in this study were otherwise healthy. After obtaining their informed consent, keratome samples were obtained from symptomless (PN) skin (n = 5, 2 females, 3 males) or from untreated active psoriatic plaques (PP skin) (n = 15). Samples from normal volunteers without any skin disease (NN skin) (n = 8) were also obtained under local anesthesia. In addition, a separate set of PN skin biopsies (n = 8) was analyzed using immunohistochemical staining.
Human Skin/SCID Mouse Chimera and Tissue Processing
Human skin xenografts were orthotopically transplanted onto 7- to 8-week-old SCID mice (Taconic Farms, Germantown, NY) following previously described procedures. 6 Briefly, human skin xenografts measuring 1.5 × 1.5 × 0.5 cm were sutured to the flank of SCID mice with absorbable 5–0 Vicryl Rapide suture (Ethicon, Somerville, NJ) and covered with Xeroform dressings (Kendall Co., Mansfield, MA). Dressings were removed 1 week later and animals maintained pathogen-free throughout the study. Two to three weeks after transplantation, autologous cells or reagents diluted in sterile PBS were injected intradermally (300 ul vol) into the xenograft. Human skin/SCID mouse chimeras were killed within 2–3 weeks of the last intradermal injection, and 4-mm punch biopsies (Baker’s Biopsy Punch, Cummins Derm, Miami, FL) were obtained from each xenograft. Biopsies were fixed in neutral-buffered formalin for paraffin embedding and/or mounted on gum tragacanth (Sigma Chemical Co., St. Louis, MO), snap-frozen in liquid nitrogen-chilled isopentane, and stored at −80°C.
Immunostaining
Cryostat sections of skin were acetone-fixed and stained using either a highly sensitive avidin-biotin immunoperoxidase technique with 3 amino 4-ethylcarbazole to produce a positive red reaction product as described, 6 or an indirect immunofluorescence procedure was performed. For immunoperoxidase single antigen staining the following antibodies were purchased to detect their respective antigens: anti-CD3, CD4, CD8, CD16, CD25, CD57, CD69, and α/β TCR (Becton-Dickinson, Mountain View, CA), and anti-CD94 (clone Hp-3B1), CD158a (clone EB6), CD158b (clone GL183) were purchased from Coulter Corp. (Miami, FL). Anti-CD56 was purchased from PharMingen (San Diego, CA).
Two-color immunofluorescence staining was performed using several different protocols. In the first method, cryostat sections were incubated with mouse anti-human monoclonal antibodies against the following antigens: mouse anti-CD94, mouse anti-CD158a, mouse anti-CD158b (all Coulter Corp.), or mouse anti-CD25 or anti-CD69 (Becton-Dickinson), all at 10 ug/ml final concentration for 30 minutes at room temperature. After washing in FA buffer (Difco, Detroit, MI), a rhodamine-conjugated goat anti-mouse antibody was added (1:50 dilution; Biosource International, Camarillo, CA) for 30 minutes at room temperature. All of the cells exposing these antigens appeared red due to the rhodamine conjugate in the reaction. To saturate unoccupied binding sites, sections were then incubated with mouse serum (Chrompure mouse IgG, 10 ug/ml, Jackson Immunoresearch, West Grove, PA). To identify colabeling in the tissue sections, two different strategies were used. Biotinylated conjugated secondary antibodies were used to detect the following antigens: mouse anti-CD3 (Becton-Dickinson), mouse anti-CD8 (Coulter Corp.), and mouse anti-TCR α/β (Becton-Dickinson) were added at 10 ug/ml for 30 minutes at room temperature and washed with FA buffer. Next, streptavidin-FITC conjugate (1:50 dilution, PharMingen) was added for 30 minutes at room temperature, producing a green fluorescence product for these antigens. To visualize CD4, a second reaction scheme, in which a rabbit anti-human CD4 (Immunodiagnostics, Bedford, MA) (1:5 dilution) followed by the secondary ab, with a goat anti-rabbit FITC-conjugated ab was used at 1:25 dilution (Biosource International). For all these reactions, after the last wash in FA buffer, a mounting solution was applied (para-phenylenediamine in glycerol) before coverslipping. All slides were examined and photographed using an Olympus AX-80 microscope.
Cell Culture Studies
Autologous immunocytes were isolated from heparinized blood by Ficoll-Hypaque (Pharmacia LKB Biotechnology, Piscataway, NJ) density centrifugation. 1–2 × 10 6 peripheral blood mononuclear cells (PBMC)/ml were cultured on tissue culture dishes (Corning Glass Works, Corning, NY) in complete media containing 10% heat-inactivated autologous serum in RPMI-I640 containing 24 mmol/L HEPES (GIBCO BRL, Gaithersburg, MD) supplemented with 2 mmol/L L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin, and 50 ug/ml gentamicin (GIBCO BRL). Immunocytes were cultured in complete media with or without 1 ug/ml each of staphylococcal enterotoxins SEB and SEC2 (Toxin Technologies, Sarasota, FL) and 20 U/ml human interleukin (IL)-2 (Boehringer Mannheim, Indianapolis, IN) for 72 hours at 37°C in a humidified atmosphere containing 5% CO2. CD4+ and CD8+ T cell lines were produced by negative selection and continued exposure to IL-2. Such cells lines were >98% pure CD4+ or CD8+ T cells after the first several weeks in culture, as determined by flow cytometry. In addition, T cell lines were produced using immobilized anti-CD3 mAb and IL-2.
Results
Injection of CD4+ T Cell Lines but not CD8+ T Cell Lines Induced Psoriasis
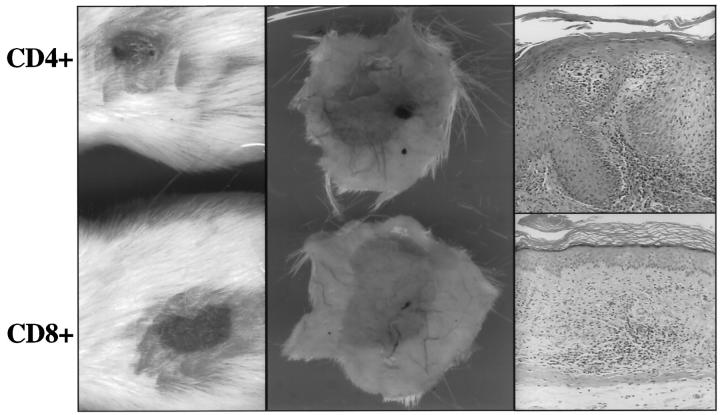
In preliminary experiments, while using unfractionated Ficoll-Hypaque interface-derived immunocytes, the removal of the CD4+ T cell population by magnetic bead selection immediately before injection abrogated the ability of the injected immunocytes to induce psoriasis. However, in these same two patients, removal of CD8+ T cells from the Ficoll-Hypaque interface mononuclear cells did not alter the conversion of PN to PP skin (data not shown). Based on these results we further studied an additional five patients to determine whether highly enriched specific T cell subsets were capable of inducing psoriasis without the other cell types typically present in the Ficoll-Hypaque interface, such as monocytes, macrophages, NK cells, B lymphocytes, or dendritic cells. T cell lines were generated from Ficoll-Hypaque interface cells stimulated 1–2 weeks with superantigens and IL-2. CD4+ or CD8+ T cell lines were expanded and maintained in IL-2 containing medium following negative selection. CD4+ and CD8+ T cells were injected into PN skin after being in culture for various durations (19, 21, 22, 25, and 35 days). Comparing the clinical and microscopic appearance of PN grafts injected with unfractionated/acutely activated Ficoll-Hypaque derived immunocytes (days 2–4) to PN skin injected with CD4+ T cell lines (but not CD8+ T cells), induction of psoriasis was seen to be similar between these populations of immunocytes (Figure 1) ▶ . The lower horizontal panels reveal the distribution of CD3+ T cells after injection of either CD8+ T cells (left side), CD4+ T cells (middle), or unfractionated Ficoll-Hypaque cells, including both CD4+ and CD8+ T cells (right side). Note that although T cells are seen at the dermal-epidermal interface in the left side panel, they do not trigger significant epidermal hyperplasia, but in the middle and right side panels, numerous T cells infiltrate the epidermis, accompanied by prominent keratinocyte hyperplasia.
Figure 1.
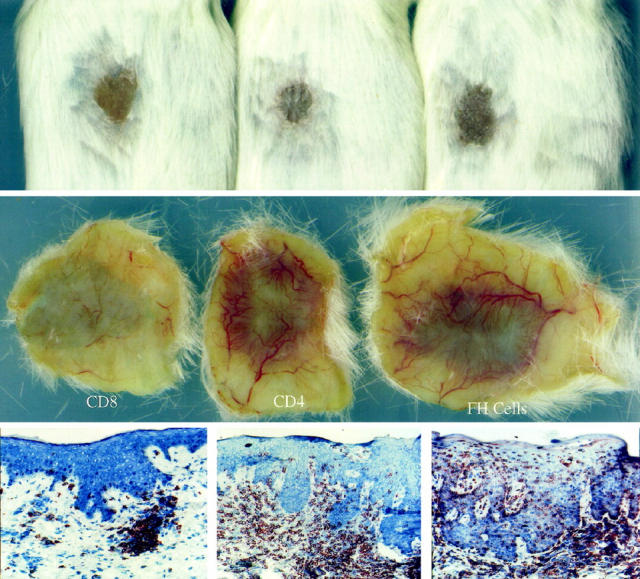
Compared to unfractionated Ficoll-Hypaque derived immunocytes (right side panels), injected CD4+ T cells also induce PN-to-PP conversion (middle panels) , but CD8+ T cells do not cause psoriasis lesions (left side panels). Upper and middle horizontal panels portray clinical features in which surface scale production and underlying dermal angiogenesis is seen for PN skin converted to PP skin. Lower horizontal panels reveal immunostaining to detect CD3 (pan T cell marker).
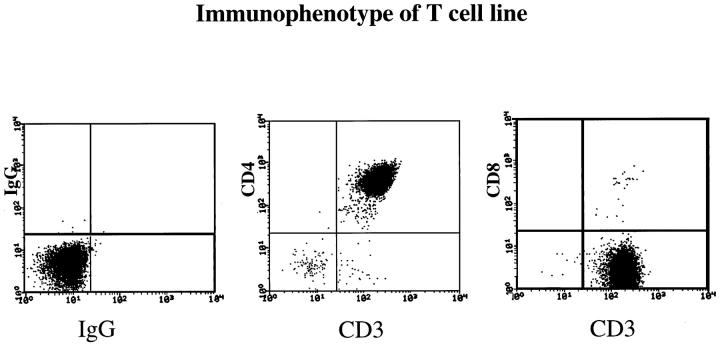
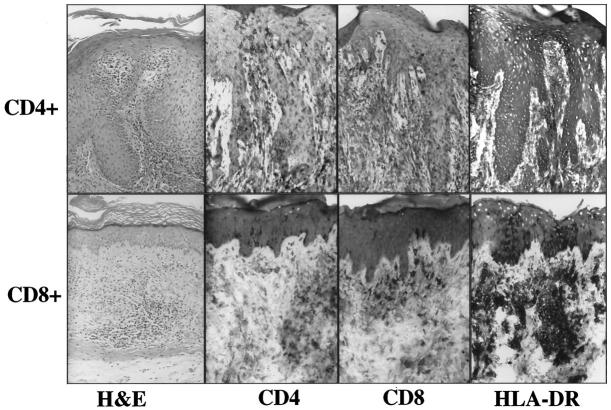
A representative flow cytometry profile of a CD4+ T cell line is presented in Figure 2 ▶ . Note that >95% of the CD3+ lymphocytes are CD4+, with rare CD8+ T cells among these cultured cells. In two of these five patients, we also used immobilized anti-CD3 mAb and IL-2 (rather than bacterial superantigens) to stimulate CD4+ and CD8+ T cells and observed that only injected CD4+ T cells, but not CD8+ T cells, induced PN-to-PP conversion (Figure 3) ▶ . The lack of PN-to-PP conversion by CD8+ T cells was not due to a lack of survival or absence of stimulated CD8+ T cells, as immunostaining of injected PN skin revealed scattered CD8+ T cells in the upper dermis and lower epidermis (Figure 4) ▶ . Moreover, using human leukocyte antigen-DR (HLA-DR) expression, the PN grafts injected with the CD8+ T cells had prominent HLA-DR staining of the lymphocytes in the upper dermis and even focal areas of keratinocyte expression, although much more intense and diffuse keratinocyte HLA-DR expression was seen after injection of CD4+ T cells. This latter finding strongly suggests the presence of γ interferon (IFN-γ) production by the activated dermal mononuclear cell population. Furthermore, when these CD4+ and CD8+ T cell lines were examined immediately before injection into PN skin, flow cytometric analysis revealed that 22% of CD8+ T cells versus 14% of CD4+ T cells expressed CD69, indicating that a significant fraction of CD8+ T cells was acutely activated. Taken together, all of these results indicated that highly purified and activated CD4+ T cells, but not CD8+ T cells, were capable of mediating PN-to-PP skin conversion. In the next section, we characterized the local immunological reactions occurring after injection of CD4+ T cells into PN skin.
Figure 2.
Flow cytometry profile of CD4+ T cell line (day 23 in culture) established from peripheral blood using bacterial superantigens and IL-2, followed by negative selection to remove all other cell types and continued propagation using IL-2. Note that compared to isotype controls, the cell population is CD3+ CD4+ T cells (>95%), with only rare CD8+ T cells.
Figure 3.
Using immobilized anti-CD3 mAb and IL-2, activated CD4+ T cells but not CD8+ T cells induce PN-to-PP conversion (thick, scaly skin with angiogenic tissue response, and epidermal hyperplasia). After CD4+ T cell injection, there is loss of granular cell layer, parakeratosis, elongation of rete pegs and influx of lymphocytes into epidermis (all histological hallmarks of psoriasis). In contrast, after CD8+ T cell injection, numerous lymphocytes are present in dermis, but with no change in the stratum corneum, granular cell layer, or epidermal thickness.
Figure 4.
Following intradermal injection of CD4+ T cell, numerous CD4+ T cells are present in the hyperplastic epidermis with only rare CD8+ T cells in the epidermis and diffuse keratinocyte HLA-DR expression. Following intradermal injection of CD8+ T cells, only rare scattered CD4+ T cells in the dermis are seen as well as CD4+ Langerhans cells in the epidermis. Upper dermal collections of CD8+ T cells include HLA-DR-positive mononuclear cells and focal epidermal keratinocyte HLA-DR expression.
Response of PN Skin to Injection of CD4+ T Cells
Besides the routine clinical and light microscopic findings noted above, an immunophenotypic analysis of the PN skin grafts injected with autologous CD4+ T cell lines was performed in samples from four of these patients. For one patient, PN grafts were harvested at 3 and 14 days after injection of CD4+ T cells; in the other three patients, skin grafts were harvested 2–3 weeks after injection. In one patient, we observed a striking increase in the number of intraepidermal CD8+ T cells 2 weeks after the injection of the CD4+ cell line (Figure 5) ▶ . Even though numerous CD4+ T cells were present in the upper dermis, there were more CD8+ T cells than CD4+ T cells in the epidermal compartment. In a second patient, CD8+ T cells appeared more abundant than routinely observed in PN skin, but not as prominent as was observed in the first patient. In the other two patients examined, there was no apparent increase in the number of CD8+ T cells in the epidermis. Thus, it should be noted that the number of intraepidermal CD8+ T cells after intradermal CD4+ T cell injection varied among grafts. The range in the density of intraepidermal CD8+ T cells went from <5 per high-power field to >50 per high-power field. These results indicated that an activation event involving resident CD8+ lymphocytes was occurring in some of the skin samples after the injection of the CD4+ T cells that extended beyond the response of the keratinocytes and endothelial cells. To determine which types of immunocytes were being activated after injection of CD4+ cells, cryostat sections were immunostained to detect acute T cell activation markers CD25 and CD69. Because these markers revealed similar staining profiles, only the CD69 results will be portrayed. In preliminary studies we documented that although CD69 is rapidly induced (4–6 hours) by bacterial superantigens, it is down-modulated in 6–8 days if no additional stimulus is provided. 20 Thus, because the CD4+ T cells are in PBS before injection and the grafts were harvested 2–3 weeks later, we reasoned that any CD69-positive T cells would signify a reactivation event occurring within the local microenvironment.
Figure 5.
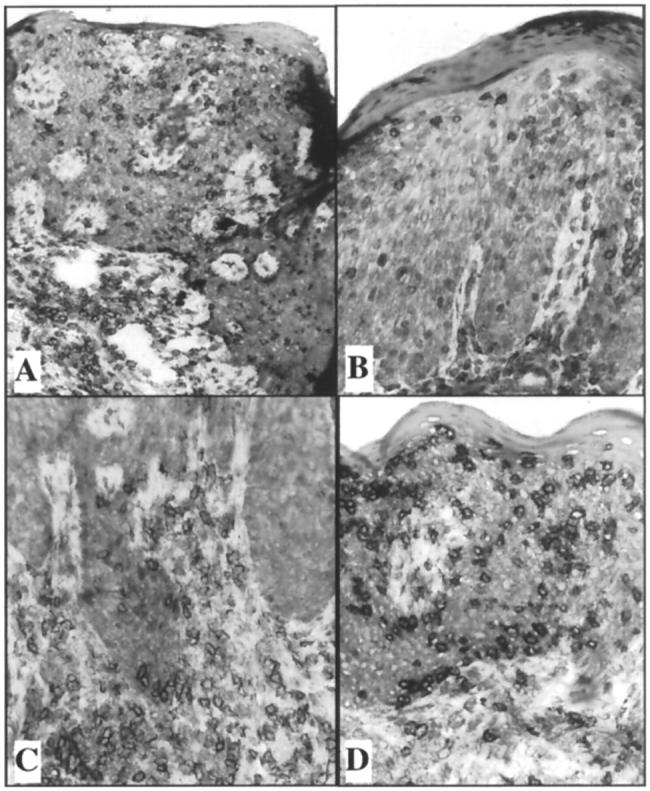
Appearance of CD8+ T cells in epidermis following dermal injection of highly purified CD4+ T cells. CD3 staining of injected CD4+ T cells (A). CD4+ staining demonstrating scattered lymphocytes in epidermis (B) and dermis (C). CD8+ staining demonstrating numerous intraepidemal CD8+ lymphocytes (D). Note even though only CD4+ T cells were injected into the dermis, CD8+ T cells outnumber CD4+ T cells in the epidermis.
Interestingly, in the two patients in whose tissue CD8+ T cells accumulated in the epidermis 2–3 weeks following intradermal injection of CD4+ T cells, two-color immunofluorescence staining revealed the majority of CD69-positive cells were CD8+, with only rare CD4+ T cells expressing this acute activation marker. In one of these two patients, harvesting of PN grafted skin only 3 days after injection of CD4+ T cells revealed the presence of CD8+ T cells expressing CD69 (Figure 6) ▶ . In the third patient examined, CD69-positive cells included both CD4+ as well as CD8+ T cells (Figure 7) ▶ . In the fourth patient, CD69 was identified primarily on CD4+ T cells with only a rare CD8+ T cell expressing CD69. It was also noted during these immunophenotyping studies that the number of CD8 α homodimer-positive cells was greater than the number of CD8 α/beta heterodimer-positive cells. Additional studies are in progress to further define the nature of this T cell subset that appears to express only homodimers of α chains rather than heterodimers of α/beta CD8 chains. These results indicated that resident CD8+ T cells were being acutely activated after injection of CD4+ T cells in some of the grafts examined immediately before lesion formation. Next we sought to further characterize the immunophenotype of the intraepidermal activated immunocytes.
Figure 6.
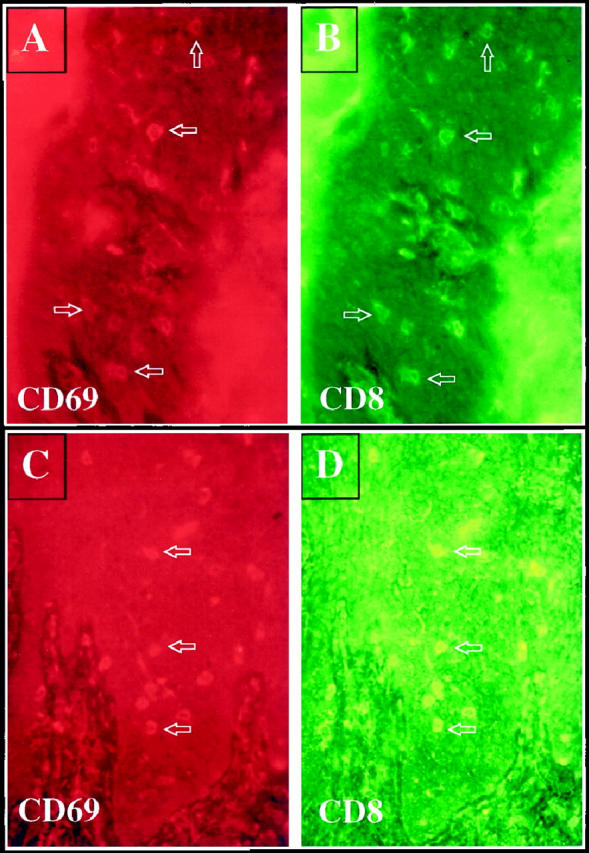
Following injection of CD4+ T cells into PN skin, psoriatic lesions developed that contained CD69 positive cells (A, 3 days after injection; C, 2 weeks after injection). Note scattered rhodamine-stained CD69 positive cells (red) are present in the epidermis at both time points (arrows). Double staining to detect CD8 (FITC-green) revealed that most CD69-positive cells were also expressing CD8 (B, 3 days after injection; D, 2 weeks after injection).
Figure 7.
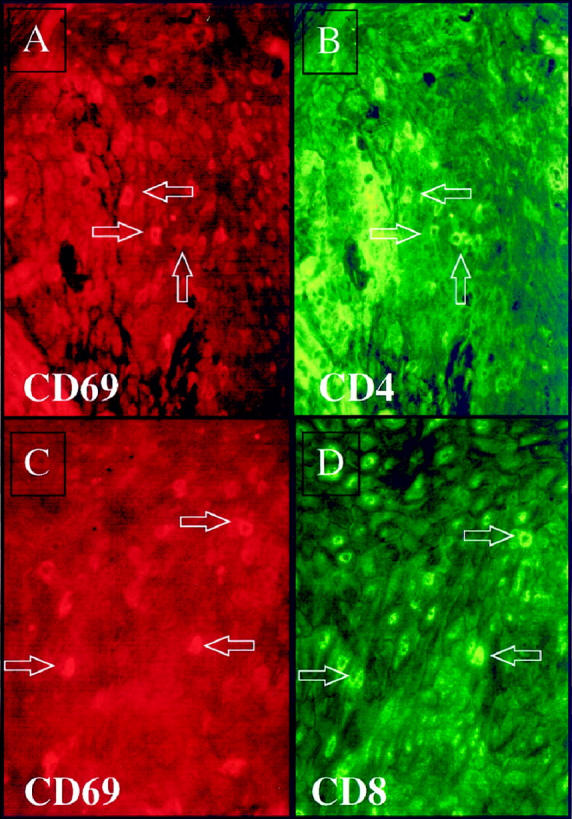
Twenty-one days after injection of a CD4+ T cell line into PN skin, scattered intraepidermal immunocytes express rhodamine-stained CD69 (A and C). FITC-labeled antibody staining revealed that both CD4+ T cells (B), as well as CD8+ T cells (D) were CD69-positive. Arrows indicate double-positive cells.
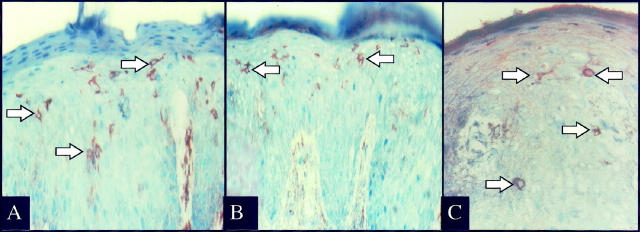
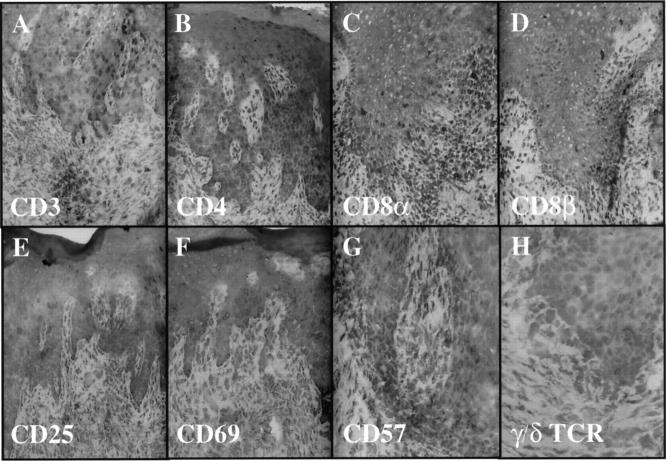
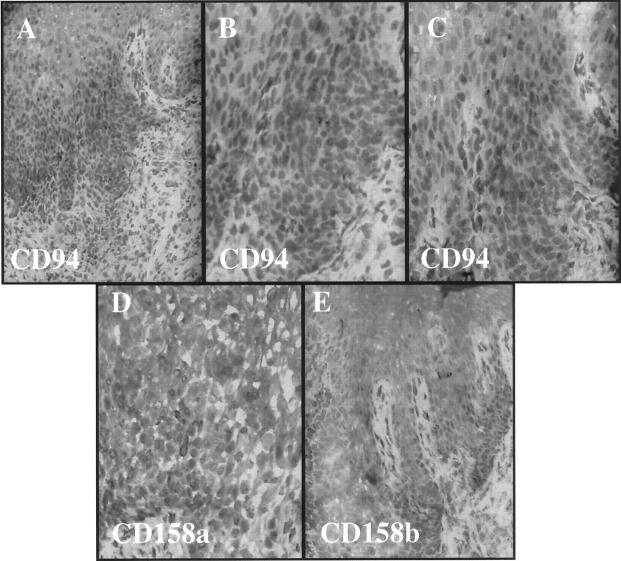
Given the rapid activation of resident CD8+ T cells, we explored the possibility that immunocytes involved in psoriasis lesion formation would express various cell surface molecules typically seen on NK cells. Whereas there were usually only rare or absent CD57-positive NK cells in the skin samples, scattered intraepidermal and dermal immunocytes expressing NKRs were identified, including the presence of CD94- (Figure 8A) ▶ , CD158a- (Figure 8B) ▶ , and CD158b- (Figure 8C) ▶ positive immunocytes in the PN skin injected with autologous CD4+ T cells. An immunophenotypic profile of the second patient revealed that 3 weeks after injection of highly purified CD4+ T cells into the dermis, numerous CD4+ and CD8α+ T cells (only rare CD8β+) are present in the epidermis (Figure 9) ▶ . These intraepidermal T cells express CD25 and CD69, but there are rare or no CD57 or γ/δ TCR + cells in this graft. Moreover, CD94+ cells are present in the epidermis accompanied by fewer CD158a- and CD158b-positive cells (Figure 10) ▶ . Based on their relative frequency, it appears that not all CD94+ T cells coexpress CD158a and/or CD158b.
Figure 8.
Fourteen days after injection of CD4+ T cells into PN skin many intraepidermal immunocytes express CD94 (A), CD158a (B), and CD158b (C). Note the presence of numerous cytoplasmic spikes extending from the positively stained cells (arrows).
Figure 9.
Twenty-one days after injection of CD4+ T cells into PN skin, induction of psoriasis is accompanied by influx of CD3-positive T cells into epidermis (A), including CD4 (B), CD8α (C), and rare CD8β (D) T cells. Acute activation markers CD25 (E) and CD69 (F) are present predominantly on intraepidermal lymphocytes with rare to no CD57 (G) or γ/δ TCR+ cells (H).
Figure 10.
Serial sections from Figure 9 ▶ reveal conspicuous CD94+ cells in epidermis (A-C) with occasional CD158a- (D) and CD158b- (E) positive cells in epidermis.
To compare and contrast the local tissue reaction of PN skin injected with CD4+ T cells, we examined the first three patients from whom tissue was available for study in which CD8+ T cells had been injected. Whereas only rare CD4+ T cells were present in PN skin injected with CD8+ T cells, there were scattered CD8+ T cells in PN skin, but there was no CD69+ T cells or CD94+ T cells in this graft that did not develop psoriasis (data not shown). Lack of CD94+ T cells in the PN graft injected with CD8+ T cells contrasted with the same patient’s PN grafts that converted into PP skin after CD4+ T cells were injected and accompanied by CD94+ cells in psoriatic epidermis (data not shown).
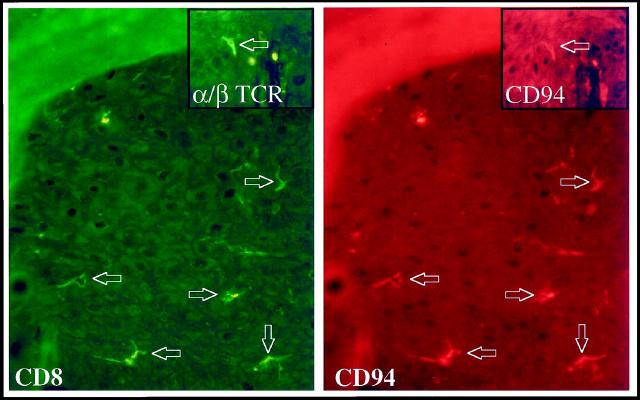
Based on these findings, we next determined the phenotype of the cells bearing NKRs. Because there were consistently more CD94-positive immunocytes than CD158a- and CD158b-positive cells, the two-color immunofluorescence results reported focus on the CD94-expressing cells in the epidermis. By two-color immunofluorescence staining, the majority of the CD94-positive cells were also expressing CD8 (Figure 11) ▶ , with occasional CD4+ T cells expressing CD94, CD158a, and CD158b (data not shown). Using antibody against the α/β chain of the TCR, CD94-positive cells were positive for this marker of T cells (Figure 11 ▶ , inset); and CD158a- and CD158b-positive cells were also observed to express the α/β T cell receptor (TCR) (data not shown). The use of markers other than CD57 for epidermal NK cells such as CD56 was not possible because of high reactivity with surrounding keratinocytes. Similar difficulty was encountered with CD16; because some NK cells do not express either CD56 or CD16, we cannot completely exclude the possibility that some NK cells were present. However, using CD57 only very rare positive NK cells were observed, and in many fields no CD57-positive cells were present (data not shown). Thus, because NK cells do not express TCR and only very rare CD57+ cells were detected, we believe the NKR+ TCR+ cells in this experimental system are not traditional NK cells.
Figure 11.
Three days after injection of CD4+ T cells into PN skin many CD8+ immunocytes (FITC-green stained) also express CD94 (rhodamine-red stained) (arrows). The inset demonstrates that CD94+ cells express the α/β TCR, thereby confirming these as T lymphocytes.
In another patient, CD94-positive cells included both CD4+ and CD8+ T cell subsets (Figure 12) ▶ . Given the overall staining profiles described above, we prefer simply to refer to these CD94/CD158a/CD158b-positive cells with an activated cytological appearance as immunocytes. It should be emphasized that T lymphocytes, particularly CD8+ cells, perform many immunological functions similar to those of NK cells and are derived from a common NK-T cell progenitor. To our knowledge, no one has previously demonstrated the presence of NKR-bearing lymphocytes in acute lesions of psoriatic epidermis as described above, or documented expression of NKRs by immunocytes in chronic psoriatic plaques as described below. Furthermore, when the CD4+ T cell lines were examined before injection into PN skin, only rare (<1%) of the cell population expressed either CD94, CD158a, or CD158b (data not shown). Studies are in progress to identify the stimulus for T cell up-regulation of these NKRs.
Figure 12.
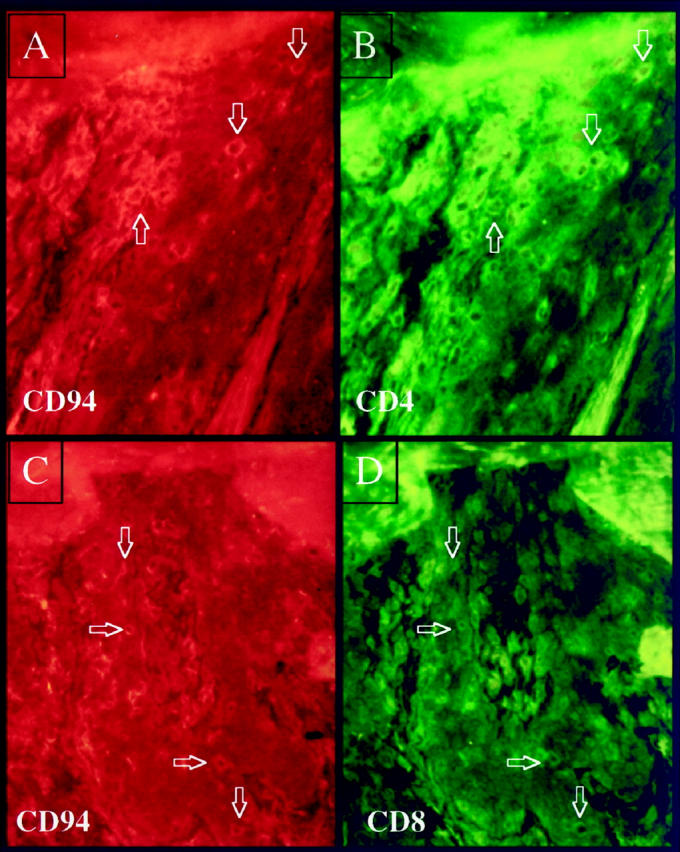
Twenty-one days after injection of CD4+ T cells into PN skin many intraepidermal CD94 positive immunocytes are present (rhodamine-red stained) as shown in panels A and C; double staining reveals that both CD4+ T cells (FITC-green) (B) and CD8+ T cells (FITC-green) (D) are also CD94+ (arrows).
Presence of CD94/CD158a/CD158b-Positive Immunocytes in Chronic Psoriatic Plaques
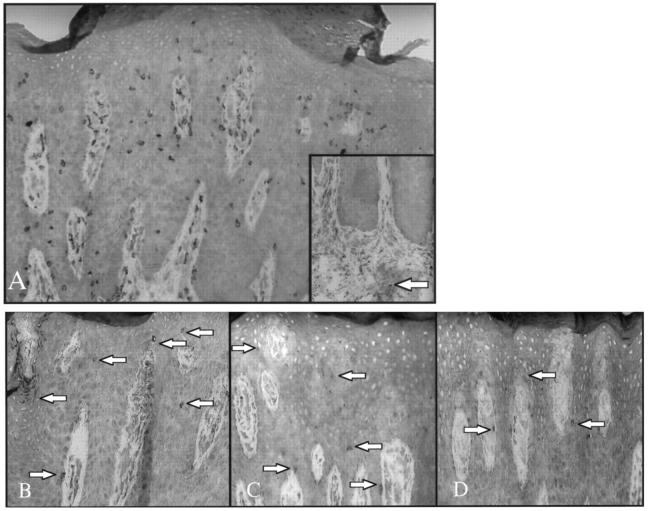
To compare these unexpected findings in the acute lesions of psoriasis using SCID mice, 15 different chronic plaques taken directly from patients were examined. Although numerous CD3+ T cells were abundant in the epidermis and dermis (Figure 13A) ▶ , scattered immunocytes in epidermis and dermis expressing CD94 (Figure 13B) ▶ , CD158a (Figure 13C) ▶ , and CD158b (Figure 13D) ▶ were present in 10 of the 15 plaques. In all positive cases, more NKR-positive cells were seen in the epidermis than in the dermis. Compared to the overall number of CD3+ T cells, the NKR-positive cells were generally <10% of the entire immunocyte population. The main difference in the appearance of these positively labeled cells observed in examining acute versus chronic lesions was that the immunocytes in the established plaques were generally less conspicuous in their overall density and had fewer cytoplasmic spikes or elongated processes than the acute lesions obtained from the SCID mice mentioned above. By two-color fluorescence staining, the majority of these NKR positive cells were CD8+, with only rare CD4+ cells double positive for these NKRs (data not shown). CD94-, CD158a-, CD158b-, and CD57-positive cells were rare to absent in either dermis or epidermis of the PN and NN skin samples examined. A rare dermal CD57-positive NK cell was present in PP skin (Figure 13A ▶ , inset).
Figure 13.
Chronic psoriatic plaque contains numerous CD3+ T cells in dermis and epidermis (A), with only a single CD57-positive NK cell identified in dermis (inset). Serial sections of identical plaque reveal scattered intraepidermal immunocytes (arrows) expressing CD94 (B), CD158a (C), and CD158b (D).
Discussion
Psoriasis is one of the most frequent immunologically-mediated diseases, affecting up to 3% of various populations worldwide, 21,22 yet its development has been an enigma for several decades. Although some progress has been made in determining the basis for the genetic susceptibility to psoriasis, in which linkage to MHC class I alleles was identified by several groups, 2 the precise immunological pathway by which the psoriatic phenotype becomes manifest in skin has been elusive. By taking advantage of the SCID mouse model, we have extended previous findings by ourselves and others, 6,11,12,23-25 establishing that highly purified blood-derived CD4+ T cells, but not CD8+ T cells, induce psoriasis when injected into PN skin. Characterization of the response of engrafted PN skin revealed that an acute immunological stimulatory event occurred when CD4+, but not CD8+, T cells were injected intradermally, as reflected by the appearance of T cells in the epidermis bearing acute activation markers (ie, CD69 and CD25).
These results using the SCID mouse model are in agreement with earlier reports documenting activated CD8+ T cells in epidermis of untreated psoriatic plaques 26 and determination that clones of CD8+ T cells are present in psoriatic epidermis. 7 The minimum number of intraepidermal CD8+ T cells necessary for psoriasis is currently unclear, although one study has documented that untreated psoriatic plaques had 13 CD8+ T cells per high-power field, which was reduced to <5 CD8+ T cells per high-power field with effective treatment. 27 The inability of the purified CD8+ T cell lines injected into PN skin to induce psoriasis presents a paradox in that the injected CD4+ T cells induced psoriasis and activation of indigenous CD8+ T cells. One attempt to resolve this paradox is the proposal that the highly purified blood-derived CD8+ T cells maintained in vitro using this experimental protocol did not include either directly pathogenic T cell subsets, and/or have the capacity to activate resident pathogenic T cells indirectly from their dormant state. In this scenario, it is possible to postulate that CD4+ T cells may on one hand provide appropriate help signals to CD8+ T cells, and on the other hand function more directly as effector cells, as has been documented by investigators examining other skin-related immune reactions. 14,28,29 Just as there are many different environmental triggering factors for psoriasis, both bacterial superantigens as well as immobilized anti-CD3 mAb could sufficiently activate the CD4+ T cells to induce psoriasis in this model system.
In addition to defining the response of engrafted PN skin with respect to CD4/CD8 and CD25/CD69 as mentioned above, we also observed that injection of highly purified CD4+ T cells led to appearance of epidermal immunocytes present immediately before and during emergence of psoriatic lesions expressing surface receptors typically confined to NK cells (ie, CD94/CD158a/CD158b). So far, we have not identified the skin-derived stimulus responsible for induction of these NKRs by such a prominent number of lymphocytes, particularly those infiltrating PN skin during lesion development. Because such induction of NKR expression was not seen when CD8+ T cells were injected into PN skin in the absence of psoriatic lesion formation, it appears that the microenvironment immediately preceding the genesis of a plaque is critically important to such expression. In another set of experiments in which allogenic activated immunocytes were injected into normal skin, the local psoriatic tissue reaction even spread from the human to the adjacent murine skin and included NKR-positive T cells. 30,31 The importance of the milieu of acute psoriatic lesions may also explain the absence of NKR-positive lymphocytes in some of the established or chronic lesions. There is a precedent for the importance of a local tissue environment on T cell phenotype in that in a murine system, injection of lymph node derived co-isogenic CD4+/CD45 RB high lymphocytes into SCID mice led to a transdifferentiation event in the small intestine, with these T cells acquiring CD8α but not CD8β surface receptors accompanied by induction of epithelial hyperplasia. 32 Interestingly, in our model we also noted a divergence in CD8α versus CD8β surface expression by CD4+ T cells in the acute lesion of psoriasis produced in SCID mice and work is in progress to define this population of T cells. It should be noted in humans that there is already a known skin-gut connection, as some psoriatic patients also suffer from Crohn’s disease.
Even though the overall number of NKR-positive immunocytes in the engrafted PN skin that converted to PP skin was generally less than 10% of the overall T cell population, NKR-bearing T cells may still be the final effector population responsible for causing psoriasis. As has been documented in allergic contact dermatitis, the frequency of the actual antigen-specific pathogenic T cell population is <1%. 33 The NKR-positive T cells may be triggered to become activated and release appropriate cytokines by recognition of various class I alleles expressed by epidermal cells. One key concept for this report is that epidermal immunocytes present during the emergence and maintenance of psoriasis express potentially activating receptors previously highlighted for their presence on NK cells. Indeed, although NK cells and T lymphocytes share a common progenitor cell and mediate similar immune reactions, 15 this report clearly documents the presence of CD94, CD158a, and CD158b on immunocytes in psoriasis. Using our SCID mouse model, it was possible to identify these immunocytes within days preceding lesion formation. Their continued presence in the majority of established plaques indicates not only that they are important for producing acute lesions, but also that may also play a role in the maintenance of chronic lesions. The importance of NKR-bearing immunocytes in creation of psoriasis is suggested by the pre-injection of blocking antibody against CD94 into PN skin, which prevented induction of psoriasis in this SCID mouse model (Nickoloff, unpublished observation).
The notion that immunocytes may be directly activated by recognition of class I alleles, without the need for any additional antigen (ie, exogenous or endogenous) highlights the truly autoimmune basis for the disease. 30,34 As the autoreactive NKR-positive immunocytes are triggered to release cytokines, they can recruit additional memory T cells that can amplify the initial stimulatory event and contribute to the cytokine cascade that mediates PN-to-PP phenotypic conversion. We postulate that the disease occurs because pathogenic T cells are not appropriately deleted during development because of incomplete negative selection and, when they localize to skin, become activated and recognize specific alleles such as HLA-Cw6, cytokines are released that ultimately are responsible for converting PN skin into PP skin. In contrast to previous studies that focused on the inhibitory nature of NKRs on T cells to explain why CD8+ T cells do not kill normal autologous cells, 16 our studies highlight the potential activating capability of NKRs on immunocytes. Work is in progress to determine the basis for the enhanced expression of the NK receptors such as CD94/CD158a/CD158b on immunocytes in psoriatic patients, and the relative activating potential of various class I alleles that they recognize.
It should be noted that there are an increasing assortment and lineage of class I genes that may be relevant to psoriasis and that could be responsible for triggering the activation of appropriate immunocytes in the skin of genetically susceptible individuals. 35-41 Just as previous studies established that skin and intestinal epithelial γδ+ T cells in the murine system can interact with keratinocytes and epithelial cells including MHC class I- related molecules, 42-44 we postulate that αβ+ T cells via these NKRs or other NKR-like surface receptors may be important in patients with psoriasis, with or without concomitant Crohn’s disease. Based on recent findings, 31 other relevant ligand/receptors such as CD161 and CD1d should also be investigated for their immunological and genetic links to psoriasis. 45-47 Finally, it will be of interest to determine whether these same NKRs on immunocytes in psoriatic patients are responsible for the lower than expected incidence/prevalence of viral infection and tumors present in psoriatic skin, 22 because such immunocytes may represent a bridge between innate and acquired immunity. 15,48 Indeed, the amazingly rapid response by which these immunocytes bearing NKRs become activated and trigger substantial epidermal hyperplasia and angiogenesis may not only be effective in immune surveillance of infections and neoplastic assaults but serve an important wound-healing function as well. 19 From this perspective, the relatively high prevalence of psoriasis worldwide may be better understood, as having such a subset of immunocytes may confer an advantage over the general population in combating such assaults and promptly repairing the skin to restore cutaneous homeostasis following injury.
Footnotes
Address reprint requests to Dr. Brian J. Nickoloff, Loyola University Medical Center, Skin Cancer Research Laboratories, Cardinal Bernardin Cancer Center, Room 301, 2160 South First Avenue, Maywood, Illinois 60153. E-mail: bnickol@luc.edu.
References
- 1.Farber EM, Reush MK, Glinski W, Karasek MA, Recht B, Nickoloff BJ: Perspectives on psoriasis. Farber EM Noll L Morhenn V Jacobs PH eds. Psoriasis: Proceedings of the Fourth International Symposium. 1987, :pp 3-9 Elsevier Press, New York [Google Scholar]
- 2.Elder JT, Nair RP, Guo S, Henseler T, Christophers E, Voorhees JJ: The genetics of psoriasis. Arch Dermatol 1996, 127:881-884 [PubMed] [Google Scholar]
- 3.Takada S, Engleman E: Evidence for an association between CD8 molecules and the T cell receptor complex on apoptotic T cells. J Immunol 1987, 139:3231-3235 [PubMed] [Google Scholar]
- 4.Bos JD, Hulsebosch HJ, Krieg SR, Baxter PM, Cormane RM: Immunocompetent cells in psoriasis. Arch Dermatol Res 1983, 275:181-189 [DOI] [PubMed] [Google Scholar]
- 5.Hanney H, Gu SQ, Johannesson A, Sundkrist KG, Biberfeld P: Subpopulations of mononuclear cells in microscopic lesions of psoriatic patients. Selective accumulation of suppressor/cytotoxic T cells in epidermis during the evolution of the lesion. J Invest Dermatol 1989, 83:416-420 [DOI] [PubMed] [Google Scholar]
- 6.Wrone-Smith T, Nickoloff BJ: Dermal injection of immunocytes induces psoriasis. J Clin Invest 1996, 98:1878-1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JC, Smith LR, Froning KJ, Schwabe BJ, Laxer JA, Caralli LL, Kurlaad HH, Karasek MA, Williamson DI, Brostoff SW: CD8+T cells in psoriatic lesions preferentially use T cell receptor Vβ3 and/or Vβ 13.1 genes. Proc Natl Acad Sci USA 1994, 91:9282-9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb SL, Gilleaudeau P, Johnson R, Estes L, Woodworth TG, Gottlieb AB, Krueger JG: Response of psoriasis to a lymphocyte-selective toxin suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med 1995, 1:442-497 [DOI] [PubMed] [Google Scholar]
- 9.Nickoloff BJ: Cytokine network in psoriasis. Arch Dermatol 1991, 127:871-884 [PubMed] [Google Scholar]
- 10.Valdimarsson H, Baker BS, Jonsdottir I, Powles A, Fry L: Psoriasis: a T cell mediated auto-immune disease induced by streptococcal superantigens? Immunol Today 1995, 16:145-149 [DOI] [PubMed] [Google Scholar]
- 11.Nickoloff BJ, Wrone-Smith T: Animal models of psoriasis. Nat Med 1997, 3:475-476 [DOI] [PubMed] [Google Scholar]
- 12.Nickoloff BJ, Wrone-Smith T: Superantigens, autoantigens, and pathogenic T cells in psoriasis. J Invest Dermatol 1998, 110:459-460 [DOI] [PubMed] [Google Scholar]
- 13.Braun-Falco O, Christophers E: Structural aspects of initial psoriatic lesions. Arch Dermatol Res 1974, 251:95-110 [DOI] [PubMed] [Google Scholar]
- 14.Gocinski BL, Tigelaar RE: Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody deletion. J Immunol 1990, 144:4121-4128 [PubMed] [Google Scholar]
- 15.Spits H, Lanier LL, Phillips JH: Development of human T and natural killer cells. Blood 1995, 85:2654-2670 [PubMed] [Google Scholar]
- 16.Trinchieri G: Biology of natural killer cells. Adv Immunol 1898, 47:187-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen CT, Viander M: Basic and interferon-augmented natural killer (NK) cell activity in psoriasis. Acta Derm Venereol 1983, 63:384-387 [PubMed] [Google Scholar]
- 18.Long EO, Colonna M, Lanier LL: Inhibitory MHC class I receptors on NK, and T cells: a standard nomenclature. Trends Cell Biol 1996, 17:100. [DOI] [PubMed] [Google Scholar]
- 19.Moretta A, Biassoni R, Bottino, Pende D, Vitale M, Poggi A, Mingari MC, Moretta L: Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol Rev 1997, 155:105-117 [DOI] [PubMed] [Google Scholar]
- 20.Carusso A, Licenziati S, Corulli M: Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry 1997, 24:71-76 [DOI] [PubMed] [Google Scholar]
- 21.Vyse TJ, Todd JA: Genetic analysis of autoimmune disease. Cell 1996, 85:311-316 [DOI] [PubMed] [Google Scholar]
- 22.Henseler T: The genetics of psoriasis. J Am Acad Dermatol 1997, 37(suppl):1s-11s [PubMed] [Google Scholar]
- 23.Nickoloff BJ, Kunkel SL, Burdick M, Strieter RM: Human skin-psoriatic skin chimeras: validation of a new animal model. Am J Pathol 1995, 146:580-588 [PMC free article] [PubMed] [Google Scholar]
- 24.Boehncke WH, Dressel D, Zollner TM, Kaufmann R: Pulling the trigger on psoriasis. Nature 1996, 379:777. [DOI] [PubMed] [Google Scholar]
- 25.Gilhar A, David M, Ullmann Y, Bertuski T, Kalish R: T-lymphocyte dependence of psoriatic pathology in human psoriatic skin grafted to SCID mice. J Invest Dermatol 1997, 109:283-288 [DOI] [PubMed] [Google Scholar]
- 26.Austin LM, Coven TR, Bhardwaj N, Steinman R, Krueger JG: Intraepidermal lymphocytes in psoriatic lesions are activated GMP-17 (TIA-1) + CD8+CD3+ CTLs as determined by phenotypic analysis. J Cutan Pathol 1998, 25:79-88 [DOI] [PubMed] [Google Scholar]
- 27.Krueger JG, Wolfe JT, Nabeya RT, Vallant VP, Gilleaudeau P, Heftler NS, Austin LM, Gottlieb AB: Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology, and by a selective depletion of intraepidermal T cells. J Exp Med 1995, 182:2057-2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H: Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med 1994, 179:865-872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valujskikh A, Matesic D, Gilliam A, Anthony D, Haggi TM, Heeger PS: T cells reactive to a single immunodominant self-restricted allopeptide induce skin rejection in mice. J Clin Invest 1998, 101:1398-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nickoloff BJ: Pathogenesis and immunointervention strategies for psoriasis. Mol Med Today 1998, 4:512-513 [DOI] [PubMed] [Google Scholar]
- 31.Nickoloff BJ, Wrone-Smith T, Bonish B, Porcelli SA: Response of murine and normal human skin to injection of allogenic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells including CD94, CD158, and CD161. Arch Dermatol 1999, 135:546-552 [DOI] [PubMed] [Google Scholar]
- 32.Morrissey PJ, Charrier K, Horovitz DA, Fletcher FA, Watson JD: Analysis of the intra-epithelial lymphocyte compartment in SCID mice that received co-isogenic CD4+ T cells. J Immunol 1995, 154:2678-2686 [PubMed] [Google Scholar]
- 33.Kalish R, Johnson KL: Enrichment and function of urushiol (poison ivy) specific T lymphocytes in lesions of allergic contact dermatitis to urushiol. J Immunol 1990, 145:3706-3713 [PubMed] [Google Scholar]
- 34.Steinman L: Escape from “horror autotoxicus”: pathogenesis and treatment of autoimmune disease. Cell 1996, 80:7-10 [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb AB, Krueger JG: HLA region genes and immune activation in the pathogenesis of psoriasis. Arch Dermatol 1990, 126:1083-1086 [PubMed] [Google Scholar]
- 36.Asahina A, Akazaki S, Nakagawa H, Kuwata S, Tokunaga K, Ishibashi Y, Juji T: Specific nucleotide sequence of HLA-C is strongly associated with psoriasis vulgaris. J Invest Dermatol 1991, 97:254-258 [DOI] [PubMed] [Google Scholar]
- 37.Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RDR, Frodsham A, Browne J, Barber R, Terwilliger J, Lathrop GM, Barker JNWN: Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet 1997, 6:813-820 [DOI] [PubMed] [Google Scholar]
- 38.Jenisch S, Henseler T, Nair RP, Guo SW, Westphal E, Stuart P, Kronke M, Vorhees JJ, Christophers E, Elder JT: Linkage analysis of human leukocyte antigen (HLA) markers in familial psoriasis: strong disequilibrium effects provide evidence for a major determinant in the HLA-B/-C region. Am J Hum Genet 1998, 63:191-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Chaplin DD: Identification on the HLA class I region of a gene expressed late in differentiation. Proc Natl Acad Sci USA 1993, 90:9470-9474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zwirner NW, Fernandez-Vina MA, Stastny P: MICA, a new polymorphic HLA-related antigen is expressed mainly by keratinocytes, endothelial cells, and monocytes. Immunogenetics 1998, 47:139-148 [DOI] [PubMed] [Google Scholar]
- 41.Bahram S, Bresnahan M, Geraghty DE, Spies T: A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci USA 1994, 91:6259-6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosmeru R, Havran WL: Modulation of epithelial cell growth by intraepidermal γδ T cells. Science 1994, 266:1253-1255 [DOI] [PubMed] [Google Scholar]
- 43.Mallick-Wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC: Conservation of T cell receptor conformation in epidermal cells with disrupted primary Vα gene usage. Science 1998, 279:1729-1733 [DOI] [PubMed] [Google Scholar]
- 44.Groh V, Steinle A, Bauer S, Spies T: Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 1998, 279:1737-174 [DOI] [PubMed] [Google Scholar]
- 45.Nickoloff BJ: The immunologic and genetic basis of psoriasis. Arch Dermatol 1999 (in press) [DOI] [PubMed]
- 46.Capon F, Novelli G, Semprini S, Clementi M, Nudo M, Vultaggio P, Mazzanti C, Gobello T, Botta A, Fabrizi G, Dallapiccola B: Searching for psoriasis susceptibility genes in Italy: genome scan and evidence for a new locus on chromosome 1. J Invest Dermatol 1999, 112:32-35 [DOI] [PubMed] [Google Scholar]
- 47.Davis DM, Mandelbuim O, Luque I, Baba E, Boyson J, Strominger JL: The transmembrane sequence of human histocompatibility leukocyte antigen (HLA)-C as a determinant in inhibition of a subset of natural killer cells. J Exp Med 1999, 189:1265-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metzhitov R, Janeway CA, Jr: Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 1997, 9:4-9 [DOI] [PubMed] [Google Scholar]