Abstract
Progressive neuronal degeneration in brain regions involved in learning and memory processes is a common occurrence in patients infected with human immunodeficiency virus type 1 (HIV-1). We now report that levels of Par-4, a protein recently linked to neuronal apoptosis in Alzheimer’s disease, are increased in neurons in hippocampus of human patients with HIV encephalitis and in monkeys infected with a chimeric strain of HIV-1 and simian immunodeficiency virus. Par-4 levels increased rapidly in cultured hippocampal neurons following exposure to the neurotoxic HIV-1 protein Tat, and treatment of the cultures with a Par-4 antisense oligonucleotide protected the neurons against Tat-induced apoptosis. Additional findings show that Par-4 participates at an early stage of Tat-induced neuronal apoptosis before caspase activation, oxidative stress, and mitochondrial dysfunction. Our data suggest that Par-4 may be a mediator of neuronal apoptosis in HIV encephalitis and that therapeutic approaches targeting the Par-4 apoptotic cascade may prove beneficial in preventing neuronal degeneration and associated dementia in patients infected with HIV-1.
Many patients infected with human immunodeficiency virus type 1 (HIV-1) develop progressive cognitive deficits and histopathological alterations in the brain including glial reactivity and neuronal loss in hippocampus and frontal cortex. 1-4 Although the cellular and molecular mechanisms underlying this neurodegenerative process are unknown, increasing data suggest roles for neurotoxic HIV-1 proteins. HIV-1 appears to infect primarily nonneuronal cells in the brain, and it is therefore likely that the virus exerts its neurodegenerative actions indirectly. 1,5,6 Several proteins produced from the HIV-1 genome, including the coat proteins gp120 and gp41 and Tat, a 86–104 amino acid protein essential for viral replication, exhibit neurotoxic activities. 7-11 Tat mRNA levels are elevated in the brains of patients with HIV-1 infection 12,13 and both gp41 11 and Tat 14 have been detected in the brains of patients infected with HIV-1. Tat is known to be actively released from HIV-1-infected cells into the extracellular environment 15 and is released from infected microglial cells. 16 Tat was also detected in the brains of animals with encephalitis resulting from infection with a chimeric strain of HIV and simian immunodeficiency virus (SHIV). 17 Tat can interact directly with the neuronal plasma membrane and induce excitation 18 and can induce apoptosis of primary human and rodent cortical neurons by a mechanism involving calcium influx and mitochondrial dysfunction. 19-21
Apoptosis is an active form of cell death characterized by cell shrinkage, mitochondrial membrane depolarization, activation of proteases called caspases, and release of factors from mitochondria that induce nuclear DNA fragmentation. 22 Neuronal apoptosis is suspected in several different neurodegenerative conditions including Alzheimer’s disease 23 and AIDS dementia. 1,6 Although it has been proposed that so-called killer proteins mediate apoptosis, the identification of such proteins has been difficult. Prostate apoptosis response-4 (Par-4) is a leucine zipper- and death domain-containing protein whose expression increases in prostate cells undergoing apoptosis. 24 Par-4 was recently shown to play a pivotal role in apoptosis of cultured PC12 cells and primary hippocampal neurons following trophic factor withdrawal and exposure to oxidative insults. 25 In addition, analyses of postmortem brain tissue from Alzheimer’s patients revealed a striking increase in levels of Par-4 protein in vulnerable neuronal populations. 25 We now report that Par-4 levels are dramatically increased in hippocampal neurons from patients with HIV encephalitis and in monkeys infected with SHIV. Moreover, Tat induces Par-4 expression in rat hippocampal neurons in cell culture, and Par-4 antisense treatment protects neurons against Tat-induced cell death. These findings suggest that Par-4 may play an active role in the pathogenesis of AIDS dementia.
Materials and Methods
Brain Tissue and Immunohistochemistry
Analyses in the present study were performed on paraffin sections from the hippocampal formation of three patients with HIV encephalitis, two patients with no known neurological illness, three rhesus macaques (designated 16A, 23A, and 44O) infected with a neurovirulent chimeric SHIV, 26 and an uninfected monkey. The SHIV viral strain contains the tat gene of HIV-1HX132. Each infected monkey had encephalitis without any opportunistic infections in the nervous system; the neuropathological findings and the mode of infection of animals 16A and 23A have been reported previously. 26 Monkeys were perfused with normal saline and the right half of the brain was immediately immersion-fixed in formalin. Human hippocampal tissue was immersion-fixed in formalin.
For immunostaining, brain sections were deparaffinized, incubated for 1 hour in the presence of blocking serum (1% normal goat serum), and incubated overnight at 4°C in the presence of primary antibody in phosphate-buffered saline (PBS). Sections were then washed in PBS and incubated for 1 hour in the presence of biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA). Sections were washed in PBS and further processed by sequential incubations in ABC reagent (avidin-peroxidase complex) and diaminobenzidine tetrahyhdrochloride (Vector Laboratories) enhanced with NiCl2 for detection of Par-4. Sections were counterstained with hematoxylin, examined, and photographed under bright-field optics. The Par-4 antibody was a rabbit polyclonal used at a 1:4000 dilution, 25 and the Tat antibody was a rabbit polyclonal used at a dilution of 1:2000. No staining was observed with either antibody when the antibody was preadsorbed with the synthetic peptide used as the immunogen.
Hippocampal Cell Cultures and Experimental Treatments
Dissociated cell cultures of hippocampus were established from 18-day Sprague-Dawley rat embryos as described previously. 21 Cells were plated in 35-mm diameter plastic- or glass-bottomed dishes on a polyethyleneimine substrate in 0.8 ml of medium consisting of minimum essential medium with Earle’s salts supplemented 10% with heat-inactivated fetal bovine serum, 1 mmol/L L-glutamine, 1 mmol/L pyruvate, 20 mmol/L KCl, and 26 mmol/L sodium bicarbonate (pH 7.2). Following cell attachment, the culture medium was replaced with Neurobasal Medium with B27 supplements (Gibco BRL, Rockville, MD). Experiments were performed in 7- to 9-day-old cultures, a time period during which neurons express glutamate receptors and are vulnerable to excitotoxic and metabolic insults. 27 Recombinant Tat was produced and purified as described previously. 21,28 Briefly, the Tat gene encoding the first 72 amino acids of HIV-1BRU (obtained from Dr. Richard Gaynor through the AIDS repository at the National Institutes of Health) was inserted into an Escherichia coli vector Pin Point Xa-2 (Promega, Madison, WI). Tat was expressed with a fusion protein that was naturally biotinylated. Tat was purified on a column of soft release avidin resin. Tat protein was cleaved from the fusion protein using factor Xa and desalted on a PD10 column. The protein was >95% pure as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining and confirmed by Western blot analysis. Par-4 antisense oligodeoxynucleotide (5′-ATAGCCGCCGGTCGCCATGTT-3′) and a scrambled control oligodeoxynucleotide (5′-CCGTGTCTGATCTTCGTGCGT-3′) were prepared as 2 mmol/L stocks in sterile water. Immediately before experimental treatment the culture maintenance medium was replaced with Locke’s buffer (154 mmol/L NaCl, 5.6 mmol/L KCl, 2.3 mmol/L CaCl2, 1 mmol/L MgCl2, 3.6 mmol/L NaHCO3, 5 mmol/L glucose, 5 mmol/L HEPES; pH 7.2).
Western Blot Analysis and Immunostaining of Cultured Hippocampal Cells
The methods were essentially identical to those described previously. 25 For Western blot analysis, 50 μg of solubilized proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel), transferred to a nitrocellulose sheet, and incubated sequentially with Par-4 antibody (rabbit polyclonal 25 ), HRP-conjugated anti-rabbit secondary antibody, and chemiluminescence detection solution (Amersham, Arlington, IL). For immunostaining, cells were fixed in 4% paraformaldehyde/PBS and membranes were permeabilized by incubation in 0.2% Triton X-100 in PBS. Cells were then incubated sequentially in blocking serum (1% normal goat serum in PBS), Par-4 antibody, biotinylated goat anti-rabbit secondary antibody, and fluorescein-conjugated avidin (Vector Laboratories). Images of Par-4 immunofluorescence were acquired using a confocal laser scanning microscope, using the same laser intensity and photodetector gain to permit quantitative comparisons of relative levels of immunoreactivity in cells. The average pixel intensity per cell body was determined using the Imagespace software provided by the manufacturer (Molecular Probes, Eugene, OR). Between 15 and 25 neurons were analyzed per culture, and determinations were made in at least 4 separate cultures per treatment condition.
Quantification of Apoptosis
Apoptosis was assessed by counting cells with apoptotic nuclei using methods described previously. 21 Briefly, after experimental treatment, cells were fixed in 4% paraformaldehyde and were then stained with the fluorescent DNA-binding dye Hoechst 33342 (Molecular Probes). Hoechst-stained cells were visualized under epifluorescence illumination (340-nm excitation and 510-nm barrier filter) using a 40× oil immersion objective. The percentage of apoptotic cells (cells with condensed and fragmented DNA were considered apoptotic) in each culture was determined. Two hundred cells per culture were counted, and counts were made in at least 4 separate cultures per treatment condition. Analyses were performed without knowledge of the treatment history of the cultures.
Measurements of Mitochondrial Transmembrane Potential and Reactive Oxygen Species
The dye rhodamine 123 (Molecular Probes) was used as a measure of mitochondrial transmembrane potential using methods described previously. 27 Briefly, cells were incubated for 30 minutes in the presence of 10 μmol/L of the dye and then washed in Locke’s solution. Confocal laser scanning microscope images of cellular fluorescence were acquired with excitation at 488 nm and emission at 510 nm, and the average pixel intensity per cell body was determined. The dye dihydrorhodamine 123 (DHR) enters mitochondria and fluoresces when oxidized by reactive oxygen species (ROS) (principally peroxynitrite and hydroxyl radical) to the positively charged rhodamine 123 derivative. 29,30 After experimental treatment, cells were loaded with 10 μmol/L DHR 123 for 30 minutes at 37°C in a 5% CO2 incubator and washed with Locke’s solution. Confocal laser scanning microscope images of cellular fluorescence were acquired with excitation at 488 nm and emission at 510 nm, and the average pixel intensity per cell body was determined and data analyzed as described above for rhodamine 123 fluorescence.
Measurement of Caspase Activity
Caspase-3-like protease activity was assessed in individual cells by a method described previously, 31 that used DEVD-CHO, a pseudosubstrate and inhibitor of caspase-3. At designated time points following exposure of cultures to Tat, cells were exposed for 10 minutes to Locke’s solution containing 0.01% digitonin. Cells were then incubated for 20 minutes in the presence of 10 μg/ml DEVD-biotin (Calbiochem, San Diego, CA), washed three times with PBS (2 ml/wash), and fixed for 30 minutes in a cold solution of 4% paraformaldehyde in PBS. Cells were then incubated for 30 minutes in PBS containing 5 μg/ml Oregon Green-streptavidin (Molecular Probes) and washed twice with PBS. Images of cellular fluorescence, corresponding to conjugates of activated caspase-3 with DEVD-biotin, were acquired using a confocal laser scanning microscope and levels of fluorescence (average pixel intensity per cell) were quantified.
Results and Discussion
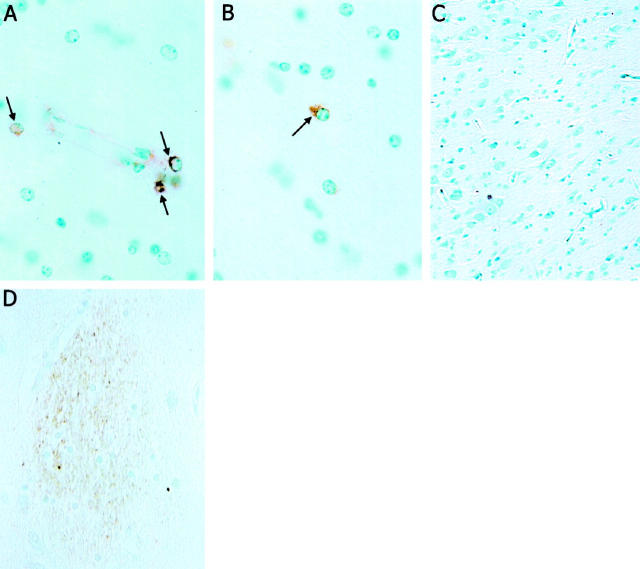
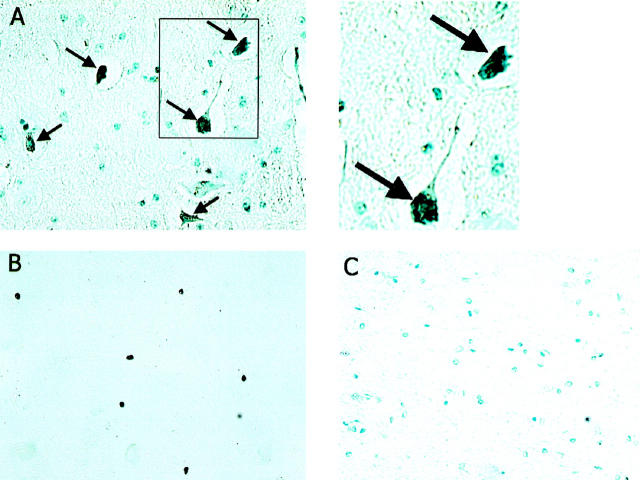
Hippocampal tissue from three patients with HIV encephalitis, two neurologically normal control human patients, three SHIV-infected monkeys, and two control monkeys were immunostained with Tat antibody. Tat-immunoreactive cells were present in perivascular regions and white matter of SHIV-infected monkeys, and scattered throughout the hippocampus of each HIV encephalitis patient (Figure 1) ▶ . In contrast, no Tat-immunoreactive cells were present in hippocampal tissue from control human or monkey brains (Figure 1 ▶ and data not shown). Par-4-immunoreactive neurons were observed in hippocampal tissue from each SHIV-infected macaque (Figure 2A) ▶ and each AIDS dementia patient (Figure 2B) ▶ . Par-4 immunoreactivity was present in the cell bodies and neurites of many neurons that appeared undamaged, as well as in neurons with a dystrophic appearance. In addition, Par-4-immunoreactive pyknotic neurons were also observed (Figure 2A) ▶ . No Par-4-immunoreactive cells were seen in uninfected normal monkey brain tissue (Figure 2C) ▶ , and only rare, faintly stained Par-4-immunoreactive cells were seen in hippocampal tissue from neurologically normal control human patients (data not shown).
Figure 1.
Presence of Tat immunoreactivity in neurons in the brains of SHIV-infected macaques and HIV encephalitis patients. Formalin-fixed paraffin sections from the hippocampal formation of a SHIV-infected macaque with encephalitis (A and B), an uninfected macaque (C), and a patient with HIV encephalitis (D) were immunostained with Tat antibody (see Methods). Several Tat-immunoreactive cells are present in perivascular cells (A, arrows) and in white matter (B, arrow) of the SHIV-infected macaque, whereas no Tat-immunoreactive cells are present in the uninfected macaque (C). Tat immunoreactivity is prominent in microglial nodules in the section from the AIDS dementia patient (D). Similar results were obtained in analyses of sections from three HIV encephalitis patients, three SHIV-infected macaques, two human control patients, and two uninfected monkeys.
Figure 2.
Par-4 is present in neurons in the brains of monkeys infected with a chimeric strain of HIV and simian immunodeficiency virus and in patients with HIV encephalitis. Formalin-fixed paraffin sections from the hippocampal formation of a SHIV-infected macaque with encephalitis (A), a patient with AIDS dementia (B), and an uninfected macaque (C) were immunostained with Par-4 antibody (see Methods). Many Par-4-immunoreactive cells with neuronal morphology are present in the tissue from the SHIV-infected monkey (black; this section was counterstained with hematoxylin) and the HIV encephalitis patient (this section was not counterstained), whereas no Par-4-immunoreactive cells are present in the hippocampal tissue from the uninfected control monkey. These sections are representative of results obtained in analyses of sections from three HIV encephalitis patients, three SHIV-infected macaques, two human control patients, and two uninfected monkeys.
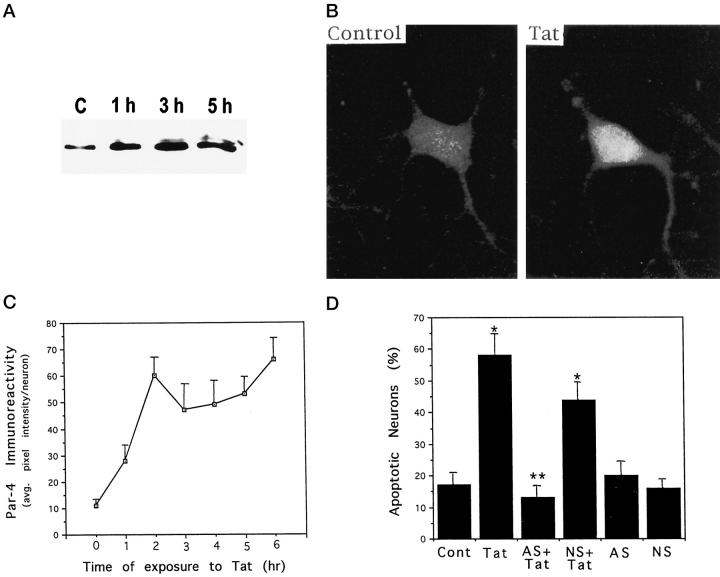
Recent findings suggest that Par-4 is a key mediator of cell death in prostate cancer cells exposed to chemotherapeutic agents 24 and in cultured neural cells subjected to trophic factor withdrawal or exposed to oxidative insults. 25 The HIV-1 derived protein Tat can induce apoptosis of cultured rat hippocampal neurons by a mechanism involving increased levels of oxidative stress and mitochondrial dysfunction. 21 When cultured rat hippocampal neurons were exposed to Tat, a rapid increase in levels of Par-4 protein was observed by Western blot analysis and semiquantitative analysis of immunostained cells (Figure 3) ▶ . Par-4 levels increased within 1 hour of exposure to Tat and remained elevated through 5 hours of exposure. Confocal images of Par-4 immunoreactivity in hippocampal neurons indicated that Par-4 was distributed throughout the cytoplasm (Figure 3B) ▶ . As documented in our previous studies, 21 hippocampal neurons underwent nuclear chromatin condensation and DNA fragmentation within 12 hours of exposure to Tat (Figure 3D) ▶ . To determine whether Par-4 played an active role in the cell death process induced by Tat, we pretreated cultures with a Par-4 antisense oligodeoxynucleotide under conditions which we previously demonstrated to selectively suppress Par-4 induction in hippocampal neurons exposed to apoptotic insults. 25 Par-4 antisense treatment completely prevented Tat-induced apoptosis, whereas the level of Tat-induced apoptosis was not significantly affected in cultures pretreated with a control oligodeoxynucleotide with a scrambled sequence (Figure 3D) ▶ .
Figure 3.
Evidence that Par-4 mediates Tat-induced apoptosis of cultured hippocampal neurons. A: Hippocampal cultures were exposed for the indicated time periods to 200 nmol/L Tat, and levels of Par-4 protein were assessed by Western blot analysis (50 μg protein/lane; see Methods). B: Confocal laser scanning microscope images of Par-4 immunoreactivity in a hippocampal neuron in a vehicle-treated control culture (left) and a culture that had been exposed for 4 hours to 200 nmol/L Tat (right). C: Cultures were exposed for the indicated time period to 200 nmol/L Tat, cells were immunostained with Par-4 antibody, and levels of Par-4 immunoreactivity in neurons were measured by confocal microscope analysis (see Methods). Values are the mean and SE of determinations made in 4–6 cultures (15–25 neurons analyzed in each culture). D: Cultures were pretreated for 2 hours with 20 μmol/L Par-4 antisense (AS) or non-sense (NS) oligonucleotides, and then exposed for 12 hours to vehicle or 200 nmol/L Tat. The percentage of neurons with apoptotic nuclei in each culture was quantified. Values are the mean and SE of determinations made in 4 cultures. *P < 0.01 compared to control value. **P < 0.01 compared to value for cultures exposed to Tat alone. Analysis of variance with Scheffé’s post hoc tests.
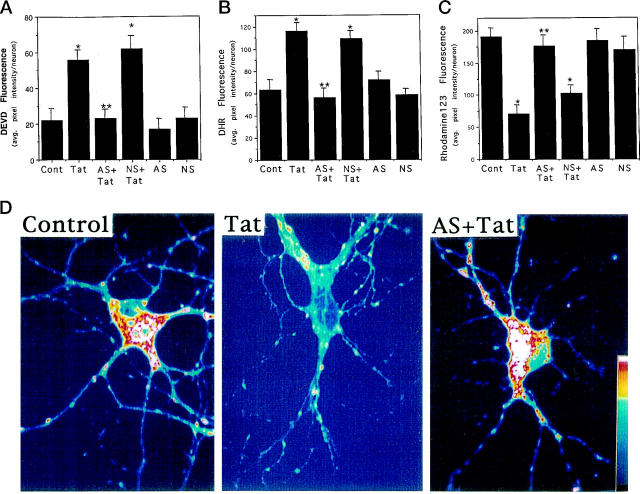
Because Par-4 protein levels increased rapidly in hippocampal neurons exposed to Tat, and because caspase activation, oxidative stress, and mitochondrial dysfunction play important roles in intermediate stages of Tat-induced apoptosis, 21 we determined whether Par-4 played a role in these important steps in the apoptotic cascade induced by Tat. Exposure of hippocampal neurons to Tat resulted in an increase in levels of caspase activity measured 6 hours later (Figure 4A) ▶ . Pretreatment of cultures with a Par-4 antisense oligonucleotide prevented caspase activation, whereas a control oligonucleotide was ineffective (Figure 4A) ▶ . Tat also induced an increase in levels of DHR fluorescence (a measure of mitochondrial ROS) within 6 hours of exposure, and pretreatment of cultures with Par-4 antisense oligonucleotide significantly attenuated the mitochondrial ROS production (Figure 4B) ▶ . Tat caused an impairment of mitochondrial function, detected as a decrease in levels of rhodamine 123 fluorescence, and Par-4 antisense treatment prevented the loss of mitochondrial function (Figure 4, C and D) ▶ . Collectively, these data suggest that Par-4 acts at an early stage of the apoptotic process before caspase activation, mitochondrial dysfunction, and ROS production.
Figure 4.
Evidence that Par-4 induction mediates Tat-induced caspase activation, oxidative stress, and mitochondrial dysfunction in cultured hippocampal neurons. (A-C): Cultures were pretreated for 2 hours with 20 μmol/L Par-4 antisense (AS) or non-sense (NS) oligonucleotides and were then exposed for 6 hours to vehicle or 200 nmol/L Tat. Levels of DEVD fluorescence (A, a measure of levels of activated caspase-3), DHR fluorescence (B, a measure of levels of oxidative stress), and rhodamine 123 fluorescence (C, a measure of mitochondrial function) were quantified. Values are the mean and SE of determinations made in 4–6 cultures (15–25 neurons analyzed in each culture). *P < 0.01 compared to control value. **P < 0.01 compared to value for cultures exposed to Tat alone. Analysis of variance with Scheffé’s post hoc tests. D: Confocal laser scanning microscope images of rhodamine 123 fluorescence in hippocampal neurons from an untreated control culture, a culture exposed to 200 nmol/L Tat for 6 hours, and a culture preincubated for 2 hours with 20 μmol/L Par-4 antisense (AS) and then exposed to 200 nmol/L Tat for 6 hours. Note that Tat caused a decrease in the level of rhodamine 123 fluorescence, and that Par-4 antisense largely prevented the effect of Tat.
Previous studies documented DNA damage and increased expression of apoptosis-related genes in cerebral cortical tissue from AIDS dementia patients. 32-34 Our data provide additional evidence that neuronal apoptosis occurs in HIV-1-infected patients and further suggest that Par-4 serves as a critical link in the biochemical chain of events leading to neuronal death in AIDS dementia. Essentially no Par-4-immunoreactive neurons were found in hippocampal neurons from neurologically normal humans, monkeys, or rats. In contrast, numerous Par-4-immunoreactive neurons and Tat-positive nonneuronal cells were present in hippocampus from patients and monkeys with HIV or SHIV encephalitis. Tat induced a rapid increase in Par-4 protein levels in cultured hippocampal neurons that occurred many hours before cell death. A cause-effect relationship between Par-4 expression and neuronal death was suggested by our data showing that Tat-induced apoptosis is prevented in hippocampal neurons treated with Par-4 antisense DNA.
In a recent study we established an association between Par-4 expression and neuronal degeneration in Alzheimer’s disease patients and showed that Par-4 plays a role in the apoptosis-inducing effects of amyloid β-peptide and the pro-apoptotic effects of presenilin-1 mutations linked to early-onset familial Alzheimer’s disease. 25 The dementia associated with both Alzheimer’s disease and HIV encephalitis is believed to result from synaptic degeneration and cell death. Although HIV encephalitis and Alzheimer’s disease have distinct causes, there appear to be several common features in the mechanisms leading to neuronal degeneration. Both Tat and amyloid β-peptide promote excitotoxicity 18,35 and can induce neuronal apoptosis. 21,23 Increased oxidative stress and disruption of cellular calcium homeostasis play central roles in the neurotoxic actions of both Tat and amyloid β-peptide. 21,35 Calcium influx 24 and oxidative stress 25 appear to be important triggers for induction of Par-4. Par-4 induction, in turn, appears to play a pivotal role in effecting several key steps in the apoptotic cascade including mitochondrial dysfunction and caspase activation. Three major neurotoxic HIV-1 proteins have been identified that may contribute to the neurodegenerative process in AIDS dementia. All three proteins (gp120, Tat, and gp41) have been shown to promote disruption of calcium homeostasis and oxidative stress in neurons, 1,6,7,9,10,21 and might thereby promote Par-4 induction and neuronal apoptosis. Taken together with the strong association between Par-4 expression and neuronal degeneration in both Alzheimer’s disease and HIV-1 encephalitis, the data suggest that Par-4 serves as a convergence point in the neurodegenerative process initiated by diverse environmental factors and genetic aberrancies. Thus, Par-4 appears to be a general (as opposed to a disease-specific) mediator of neuronal apoptosis.
HIV encephalitis is rapidly becoming a major problem in our society due to the increasing life expectancy of symptomatic HIV-1-infected patients. As with other forms of dementia, including Alzheimer’s disease, neuronal degeneration and death are responsible for cognitive deficits in AIDS dementia. Therapeutic approaches aimed at protecting neurons against the neurotoxic actions of HIV-1-derived proteins may therefore prove effective in preventing or attenuating AIDS dementia. Based largely on studies of the mechanisms whereby HIV-1-derived proteins such as Tat and gp120 kill neurons, several classes of therapeutic agents are being developed including glutamate receptor antagonists, calcium channel blockers, and antioxidants. 1 Our data indicate that suppression of Par-4 production can protect cultured hippocampal neurons against Tat-induced apoptosis, suggesting that Par-4 is a potential therapeutic target in patients infected with HIV-1. A better understanding of the mechanisms that induce Par-4 production and the steps in the apoptotic cascade engaged by Par-4 may reveal new strategies for the prevention and treatment of HIV encephalitis.
Acknowledgments
We thank Dr. O. Narayan of the University of Kansas for providing brain tissue of SHIV and control monkeys.
Footnotes
Address reprint requests to Mark P. Mattson, 211 Sanders-Brown Building, University of Kentucky, Lexington, KY 40536. E-mail: mmattson@aging.coa.uky.edu.
Supported by grants NS29001, AG14554, and AG05119 (to M. P. M.) and NS01941 (to W. F. M.) from the National Institutes of Health and by a grant to A. N. from the University of Kentucky Research Foundation.
References
- 1.Lipton SA: Neuropathogenesis of acquired immunodeficiency syndrome dementia. Curr Opin Neurol 1997, 10:247-253 [DOI] [PubMed] [Google Scholar]
- 2.Ketzler S, Weiss S, Haung H, Budka H: Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol 1990, 80:92-94 [DOI] [PubMed] [Google Scholar]
- 3.Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA: Cortical dendritic pathology in human deficincy virus encephalitis. Lab Invest 1992, 66:285-291 [PubMed] [Google Scholar]
- 4.Wiley C, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R: Neocortical damage during HIV infection. Ann Neurol 1991, 29:651-657 [DOI] [PubMed] [Google Scholar]
- 5.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO: Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA 1998, 95:3117-3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein LG, Gendelman HE: HIV-1 infection of the nervous system: pathogenic mechanisms. Ann Neurol 1993, 33:429-436 [DOI] [PubMed] [Google Scholar]
- 7.Nath A, Geiger J: Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol 1998, 54:19-33 [DOI] [PubMed] [Google Scholar]
- 8.Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB: Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal polypeptide. Nature 1988, 335:639-642 [DOI] [PubMed] [Google Scholar]
- 9.Dawson VI, Dawson TM, Uhl GR, Snyder SH: Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical neurons. Proc Natl Acad Sci USA 1993, 90:3256-3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB: Synergistic effects of HIV coat protein and NMDA receptor mediated neurotoxicity. Neuron 1991, 7:111-118 [DOI] [PubMed] [Google Scholar]
- 11.Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Chritov VI, Dawson TM, Dawson VL: Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science 1996, 274:1917-1921 [DOI] [PubMed] [Google Scholar]
- 12.Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE: Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol 1993, 33:576-582 [DOI] [PubMed] [Google Scholar]
- 13.Wiley CA, Baldwin M, Achim CL: Expression of regulatory and structural mRNA in the central nervous system. AIDS 1996, 10:843-847 [DOI] [PubMed] [Google Scholar]
- 14.Nath A, Geiger JD, Mattson MP, Magnuson DSK, Jones M, Berger JR: Role of viral proteins in neuropathogenesis of HIV infection with emphasis on Tat. NeuroAIDS 1998, 1: (www.sciencemag.org/NAIDS)
- 15.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B: HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 1997, 11:1421-1431 [DOI] [PubMed] [Google Scholar]
- 16.Tardieu M, Hery C, Peudenier S, Boespflug O, Montagnier L: Human immunodeficiency virus type 1-infected cells can destroy human neural cells after cell-to-cell adhesion. Ann Neurol 1992, 32:11-17 [DOI] [PubMed] [Google Scholar]
- 17.Everall IP, Hudson L, Liu J, Nath A, Male D, Narayan O: Detection of the human immunodeficiency virus regulatory gene Tat protein and mRNA in CNS tissues. J Neurovirol 1988, 4:349-355 [DOI] [PubMed] [Google Scholar]
- 18.Magnuson DKS, Brownstone RM, Nath A: HIV-1 Tat activates non-NMDA excitatory amino acid receptors and causes neurotoxicity. Ann Neurol 1995, 37:373-380 [DOI] [PubMed] [Google Scholar]
- 19.Haughey N, Nath A, Geiger JD: HIV-1 regulatory protein Tat and Tat fragments induce calcium in human brain cells. Can J Inf Dis 1995, 6:14-20 [Google Scholar]
- 20.New DR, Ma M, Epstein LG, Nath A, Gelbard HA: Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol 1997, 3:168-173 [DOI] [PubMed] [Google Scholar]
- 21.Kruman I, Nath A, Mattson MP: HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation and mitochondrial calcium overload. Exp Neurol 1998, 154:276-288 [DOI] [PubMed] [Google Scholar]
- 22.D’Mello SR: Molecular regulation of neuronal apoptosis. Curr Top Dev Biol 1998, 39:187-213 [DOI] [PubMed] [Google Scholar]
- 23.Mattson MP, Guo Q, Furukawa K, Pedersen WA: Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer’s disease. J Neurochem 1998, 70:1-14 [DOI] [PubMed] [Google Scholar]
- 24.Sells SF, Han S-S, Muthukkumar S, Maddiwar N, Johnstone R, Boghaert E, Gillis D, Liu G, Nair P, Monnig S, Collins P, Mattson MP, Sukhatme VP, Zimmer SG, Wood DP, McRoberts JW, Shi Y, Rangnekar VM: Expression and function of the leucine zipper protein Par-4 in apoptosis. Mol Cell Biol 1997, 17:3823-3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Q, Fu W, Xie J, Luo H, Sells SF, Geddes JW, Bondada V, Rangnekar VM, Mattson MP: Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer’s disease. Nat Med 1998, 4:957-962 [DOI] [PubMed] [Google Scholar]
- 26.Raghaven R, Stephens EB, Koag SV, Adany I, Pinson DM, Li Z, Jai F, Shani M, Wang C, Leung K, Foresman L, Narayan O: Neuropathogenesis of chimeric simian/human imunodeficiency virus infection in pig-tailed and rhesus macaques. Brain Pathol 1997, 7:851-861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson MP, Zhang Y, Bose S: Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Exp Neurol 1993, 121:1-13 [DOI] [PubMed] [Google Scholar]
- 28.Nath A, Psooy K, Martin C, Knudsen B, Magnuson DSK, Haughey N, Geiger JD: Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol 1996, 70:1475-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kooy NW, Royall JA, Ischoropoulos H, Beckman JS: Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 1994, 16:149-156 [DOI] [PubMed] [Google Scholar]
- 30.Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K: Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of Mn-SOD and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res 1997, 49:681-697 [DOI] [PubMed] [Google Scholar]
- 31.Mattson MP, Keller JN, Begley JG: Evidence for synaptic apoptosis. Exp Neurol 1998, 153:35-48 [DOI] [PubMed] [Google Scholar]
- 32.An SF, Giometto B, Scaravilli T, Tavolato B, Gray F, Scaravilli F: Programmed cell death in brains of HIV-1-positive AIDS and pre-AIDS patients. Acta Neuropathol 1996, 91:169-173 [DOI] [PubMed] [Google Scholar]
- 33.Krajewski S, James HJ, Ross J, Blumberg BM, Epstein LG, Gendelman HE, Gummuluru S, Dewhurst S, Sharer LR, Reed JC, Gelbard HA: Expression of pro- and anti-apoptosis gene products in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol 1997, 23:242-253 [PubMed] [Google Scholar]
- 34.Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D: Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest 1996, 98:1979-1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattson MP: Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev 1997, 77:1081-1132 [DOI] [PubMed] [Google Scholar]