Abstract
Microsatellite instability (MSI) is observed in 13–44% of gastric carcinoma. The etiology of MSI in gastric carcinoma has not been clearly defined. To assess the role of mismatch repair in the development of MSI in gastric cancer, expression of hMSH2 and hMLH1 was explored. We examined 117 gastric carcinomas for MSI and observed instability at one or more loci in 19 (16%) of these tumors. Of the 19 tumors with MSI, nine exhibited low-rate MSI (MSI-L) with instability at <17% of loci, whereas the remaining 10 exhibited high-rate MSI (MSI-H) with instability at >33% of loci examined. Immunohistochemical staining for hMLH1 and hMSH2 was performed on eight of the tumors with MSI-H, five with MSI-L, and 15 tumors without MSI. All eight tumors with MSI-H showed loss of staining for either hMLH1 (n = 5) or hMSH2 (n = 3). In contrast, tumors with MSI-L or without MSI all showed normal hMSH2 and hMLH1 protein expression patterns. Moreover, all eight of the tumors with MSI-H also showed instability at BAT-26, whereas none of the MSI-L tumors or tumors without instability showed instability at BAT-26. These findings suggest that the majority of high-level MSI in gastric cancer is associated with defects of the mismatch repair pathway. Although larger studies are needed, BAT-26 appears to be a sensitive and specific marker for the MSI-H phenotype in gastric carcinoma.
Microsatellite instability (MSI) is a form of genetic instability observed in virtually all tumors from patients with hereditary nonpolyposis colorectal cancer (HNPCC) and in a subset of various sporadic tumors, including colorectal, gastric and endometrial cancer. 1-17 The majority of HNPCC patients have germline mutations of one of several DNA mismatch repair (MMR) genes, most frequently hMSH2 or hMLH1. 18-21 Somatic mutations, which inactivate the remaining wild-type allele, lead to defective MMR and a form of genomic instability known as microsatellite instability. Defective MMR is thought to promote tumorigenesis by accelerating the accumulation of mutations in oncogenes and tumor suppressor genes. 22-24
MSI has been observed in a subset of gastric carcinomas ranging from 13% to 44%, depending on the group of cases studied and the type and number of markers examined. 5,25 Interestingly, mutations of hMSH2 and hMLH1, germline or somatic, are infrequent in sporadic tumors with MSI, including gastric carcinoma. 26,27 Studies of MSI+ sporadic colorectal cancer observed a frequent absence of hMLH1 expression, despite the lack of identifiable germline or somatic mutations of the hMLH1 gene. 28,29 More recent studies have shown that hypermethylation of the hMLH1 promoter rather than inactivating germline/somatic mutations appear to underlie the loss of hMLH1 expression. 30,31 In this study, immunohistochemical stains for hMLH1 and hMSH2 were performed on gastric carcinoma with high-level (MSI-H), low-level (MSI-L), or no MSI (MSS). Our results shed further light on the origin of high-level MSI in gastric carcinoma.
Materials and Methods
Sample Collection and Processing
One hundred seventeen surgically resected primary gastric adenocarcinoma specimens were collected and stored at −80°C over the past decade from hospitals in the United States and the Tuscany region of Italy. Normal tissue or peripheral blood samples were obtained from these patients as well. Sample collections were performed according to internal review board–approved protocols. Tumor, node, metastasis (TNM) staging of resected cancers was assessed according to the consensus criteria adopted by the American Joint Committee on Cancer. 32 Histopathology was assessed by our gastrointestinal pathologist (CAM), who was blinded to the MSI and immunohistochemistry results. Tumor infiltrating lymphocytes (TILs) and neutrophils (TINs) were scored on a scale of 0–4, with 1 indicating that <25% of cells present were of the given type, 2 indicating 25–50%, 3 indicating 50–75%, and 4 indicating >75%. The presence of necrosis was scored as minimal if <10% of cells were part of necrosis and overt if >10% of cells present were part of necrosis. Cryostat sectioning and microdissection of gastric cancer specimens to enrich for greater than 70% neoplastic cells were performed as described previously. 33 High-molecular-weight DNA was extracted from the tumor and normal samples by established organic methods. 33
Microsatellite Analyses
Primers for 32 microsatellite marker analysis were obtained from Research Genetics (Huntsville, Alabama). The primer pairs used in this study were D2S1384, D3S2402, D5S816, D6S1017, D7S817, D8S1179, D9S934, D11S1999, D15S657, D15S643, D17S974, D22S683, D1S1589, D21S1440, D3S1284, D4S1551, D4S1601, D4S43, D6S305, D6S404, D8S261, D9S171, D10S541, D12S104, D13S154, D13S159, D16S402, D17S784, D20S851, BAT-26, BAT-25, and TGF-β RII. One of the paired primers was end-labeled with [γ-32P] ATP in a standard tyrosine kinase reaction and used for polymerase chain reaction amplification in 10-μl reaction volumes according to established protocols. Amplification was performed on each tumor and normal DNA sample pair and subsequently electrophoresed on 7% acrylamide gels for autoradiographic analysis. Microsatellite instability was scored as present when a novel, abnormal sized band occurred in the tumor sample when compared to the corresponding normal DNA sample.
Immunohistochemistry
Immunohistochemical staining for hMLH1 and hMSH2 was performed as previously described. 28 The antibody to hMSH2 (Clone FE11, 0.5 μg/ml; Oncogene Science) is a mouse monoclonal antibody generated with a carboxy-terminal fragment of the hMSH2 protein, whereas the hMLH1 antibody (clone G168-728, 1 μg/ml; Pharmingen) is a mouse monoclonal antibody that was prepared with full-length hMLH1 protein. Lymphocytes and normal epithelium exhibit strong nuclear staining for hMSH2 and hMLH1 and thus served as positive internal controls for staining of these proteins.
Statistical Analysis
Chi-square tests were used to test for associations between MSI and BAT-26 and clinicopathologic features. The Wilcoxon rank-sum test was used to test for differences in age between the MSI categories. Because of the limited number of cases determined to be unstable, all associations were considered to be exploratory. Associations with a P value of <0.05 were interpreted to denote potentially meaningful associations.
Results
Nineteen (16%) of 117 gastric carcinomas demonstrated microsatellite instability at one or more loci of 10 markers initially analyzed, including BAT-26. Representative examples of tumors with MSI are shown in Figure 1 ▶ . Twenty-two additional microsatellite markers of various repeat lengths located throughout the genome were utilized to obtain a more detailed profile of instability in these 19 instable cases (see Table 1 ▶ ). A total of 12 tetra-, two tri-, 15 di-, and three mononucleotide microsatellite markers were analyzed for these instable cases. Nine of the 19 tumors demonstrated MSI at <17% of loci analyzed (MSI-L), whereas the other 10 demonstrated MSI at >33% of the loci (MSI-H), ranging from 10 to 24 instable loci.
Figure 1.
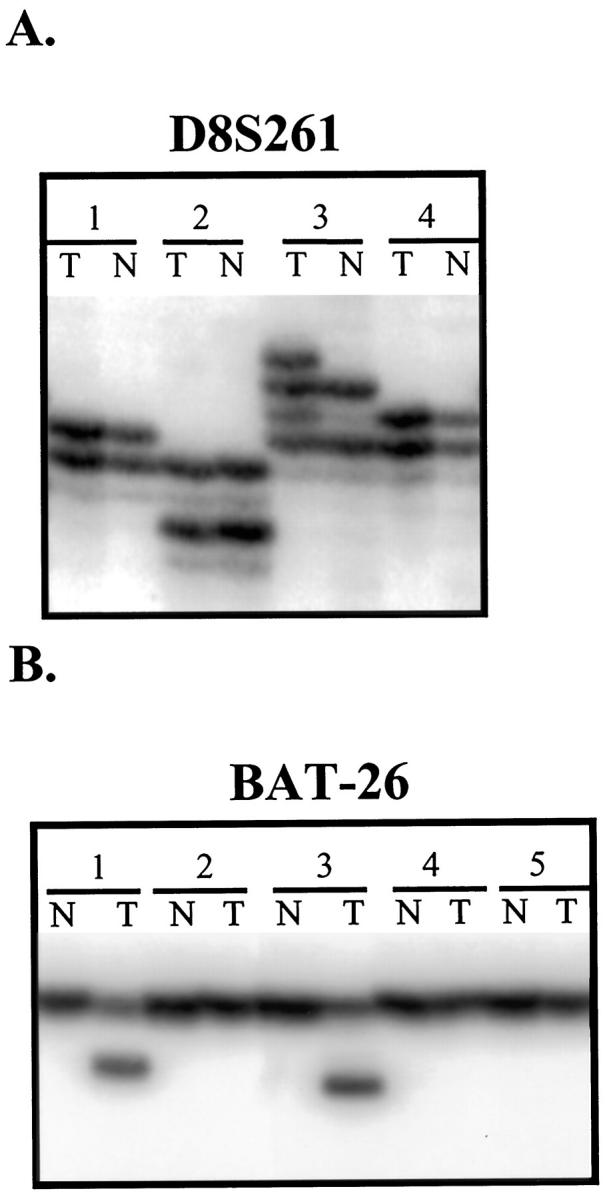
Representative examples of microsatellite instability observed in our panel of primary gastric carcinomas. On analysis of marker D8S261 (A), case 3 exhibits abnormal sized alleles in the tumor DNA (lane T) compared to its corresponding normal DNA (lane N). The relatively monomorphic BAT-26 marker (B) displays instability in cases 1 and 3, with abnormally small sized alleles in the tumor DNA compared to the paired normal DNA.
Table 1.
Profile of Microsatellite Instable Gastric Cancers
| Case | Markers* | Total no. of MSI loci | Rate of MSI (%)‡ | |||
|---|---|---|---|---|---|---|
| Mono (3)† | Tetra (12) | Tri (2) | Di (15) | |||
| G6 | 2§ | 4 | 1 | 4 | 11 | 34 |
| G15 | 2§ | 5 | 1 | 6 | 14 | 44 |
| G22 | 3§ | 9 | 2 | 11 | 25 | 78 |
| G30 | 0 | 2 | 0 | 2 | 4 | 13 |
| G38 | 3§ | 5 | 1 | 3 | 13 | 41 |
| G49 | 0 | 2 | 0 | 1 | 3 | 9 |
| G52 | 0 | 0 | 0 | 1 | 1 | 3 |
| G56 | 0 | 2 | 0 | 1 | 3 | 9 |
| G59 | 0 | 2 | 0 | 3 | 5 | 16 |
| G61 | 3§ | 2 | 1 | 9 | 15 | 47 |
| G63 | 1 | 2 | 0 | 0 | 3 | 9 |
| G65 | 3§ | 6 | 1 | 7 | 17 | 53 |
| G99 | 1 | 0 | 0 | 2 | 3 | 9 |
| G106 | 3§ | 7 | 0 | 4 | 14 | 44 |
| G111 | 0 | 0 | 0 | 2 | 2 | 6 |
| G119 | 0 | 2 | 0 | 2 | 4 | 13 |
| G134 | 3§ | 7 | 1 | 7 | 18 | 56 |
| G135 | 3§ | 6 | 0 | 9 | 18 | 56 |
| G165 | 3§ | 8 | 1 | 8 | 20 | 63 |
*Number of microsatellite markers analyzed in parentheses. Tetra, Tetranucleotide repeat; Tri, trinucleotide repeat; Di, dinucleotide repeat; Mono, mononucleotide.
†BAT-26, BAT-25, and TGF-beta RII mononucleotide repeats analyzed.
‡The rate of MSI is the proportion of instable loci observed compared to the total number of loci examined (n = 32).
§Bat-26 locus instability.
Strikingly, all 10 MSI-H tumors demonstrated instability at the mononucleotide marker BAT-26, whereas no MSI-L tumors or tumors without microsatellite instability exhibited instability at BAT-26. BAT-25 exhibited instability in all 10 MSI-H tumors and in two of nine MSI-L tumors. TGFBRII was instable in eight of 10 MSI-H tumors and none of nine MSI-L tumors. In the MSI-H tumors, the rate of MSI for individual markers ranged from 11% to 77% for the dinucleotide markers and from 0% to 77% for the tetranucleotide repeats. There was no apparent correlation between the “complexity” of the repeat and the rate of MSI with that marker.
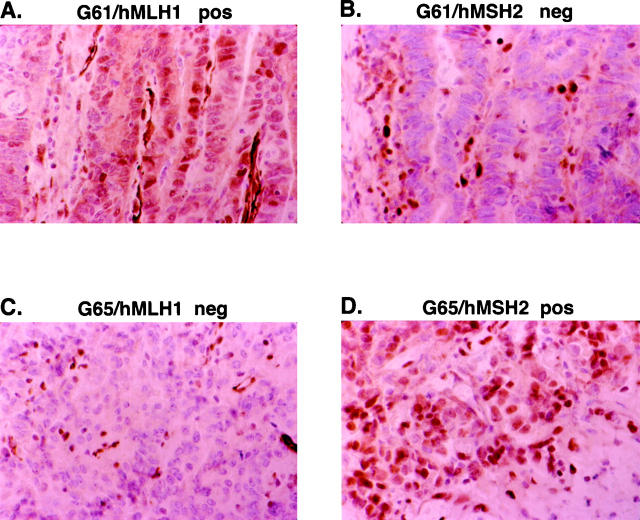
Thirteen of the MSI+ tumors (eight MSI-H and five MSI-L) and 15 MSS tumors with an absence of MSI were subsequently evaluated for expression of the mismatch repair (MMR) proteins hMSH2 and hMLH1. Paraffin-embedded tumor was not available for one MSI-H and five MSI-L tumors. The neoplastic cells of all eight MSI-H tumors showed a loss of protein staining for either hMLH1 (n = 5) or hMSH2 (n = 3) (Figure 2) ▶ . No tumor exhibited a loss of both MMR proteins. In contrast, the five MSI-L tumors and all 15 tumors without MSI had normal hMLH1 and hMSH2 protein expression patterns. Loss of MMR protein expression correlated perfectly with BAT-26 instability observed in these tumors (see Table 2 ▶ ).
Figure 2.
Representative examples of loss of hMSH2 or hMLH1 expression in two gastric carcinomas (G61 and G65) with high-level instability (MSI-H). The neoplastic cells in the tumor of case G61 show a loss of hMSH2 (B) expression but normal hMLH1 (A) protein expression in the neoplastic cells. The neoplastic cells in the tumor of case G65 display a loss of hMLH1 (C) but normal hMSH2 (D) protein expression in the neoplastic cells. Lymphocytes that show strong nuclear staining for hMSH2 and hMLH1 serve as positive internal controls (B and C).
Table 2.
Correlation of MSI with hMLH1 and hMSH2 Expression
| Case | MSI status* | BAT-26 Status† | IHC‡ |
|---|---|---|---|
| G15 | MSI-H | I | hMLH1 loss |
| G65 | MSI-H | I | hMLH1 loss |
| G134 | MSI-H | I | hMLH1 loss |
| G135 | MSI-H | I | hMLH1 loss |
| G165 | MSI-H | I | hMLH1 loss |
| G38 | MSI-H | I | hMSH2 loss |
| G61 | MSI-H | I | hMSH2 loss |
| G106 | MSI-H | I | hMSH2 loss |
| G30 | MSI-L | S | No loss |
| G56 | MSI-L | S | No loss |
| G59 | MSI-L | S | No loss |
| G99 | MSI-L | S | No loss |
| G119 | MSI-L | S | No loss |
*MSI status was categorized as MSI-H for cases demonstrating >33% of loci instable and MSI-L for cases demonstrating <17% of loci instable.
†I, Instability observed in the tumor DNA; S, no instability observed at this mononucleotide repeat marker.
‡Immunohistochemical staining results: No loss, positive nuclear staining of neoplastic cells for hMHL1 and hMSH2 observed; loss, absence of nuclear neoplastic cell staining for hMLH1 or hMSH2.
The clinicopathological characteristics of our gastric carcinomas are summarized in Table 3 ▶ . From medical records available to us, no case met the criteria for HNPCC. The sister of case G106 had been diagnosed with “esophageal cancer,” and the father of patient G38 had been diagnosed with colon cancer; however, further details were not obtainable.
Table 3.
Associations of MSI with Clinicopathological Features
| Feature | Status | MSS | MSI-L | MSI-H | P value* |
|---|---|---|---|---|---|
| (n = 98) | (n = 9) | (n = 10) | |||
| BAT-26 | Stable | 98 | 9 | 0 | <0.001 |
| Instable | 0 | 0 | 10 | ||
| Age | Median (range) | 66 (44–80) | 70 (51–77) | 70 (53–88) | 0.436† |
| Gender | Male | 71 | 8 | 6 | 0.348 |
| Female | 27 | 1 | 4 | ||
| Stage‡ | I/II | 37 | 2 | 7 | 0.038 |
| III/IV | 61 | 7 | 3 | ||
| Grade | Good | 10 | 3 | 0 | 0.151 |
| Moderate | 34 | 0 | 6 | ||
| Poor | 54 | 6 | 4 | ||
| Histology§ | I | 71 | 8 | 9 | 0.523 |
| D | 26 | 1 | 1 | ||
| Mixed | 1 | 0 | 0 | ||
| Location | Proximal | 34 | 4 | 0 | 0.022 |
| Mid/distal | 64 | 5 | 10 | ||
| Region¶ | N.A. | 68 | 9 | 4 | 0.036 |
| Italy | 30 | 0 | 6 | ||
| Pathological features∥ | (n = 15) | (n = 5) | (n = 8) | ||
| Mucin/intra** | Neg | 11 | 3 | 8 | 0.081 |
| Pos | 4 | 2 | 0 | ||
| Mucin/extra†† | Neg | 12 | 5 | 6 | 0.533 |
| Pos | 3 | 0 | 2 | ||
| Crohn’s-like reaction‡‡ | Neg | 8 | 2 | 2 | 0.111 |
| Pos | 3 | 1 | 4 | ||
| N/A | 4 | 2 | 2 | ||
| Growth pattern | Cribiform | 3 | 1 | 0 | 0.393 |
| Solid | 6 | 2 | 4 | ||
| Glandular | 6 | 2 | 4 | ||
| TIL‡‡ | 1 | 12 | 5 | 3 | 0.016 |
| 2 | 1 | 0 | 4 | ||
| 3 | 2 | 0 | 1 | ||
| TIN§§ | 1 | 13 | 4 | 6 | 0.273 |
| 2 | 2 | 1 | 1 | ||
| 3 | 0 | 0 | 1 | ||
| Necrosis | Minimal | 12 | 4 | 6 | 0.771 |
| Overt | 3 | 1 | 2 |
*Chi-square test for MSS + MSI-L versus MSI-H.
†Wilcoxon rank-sum test.
‡TNM stage according to AJCC criteria.32
§Histopathology according to Lauren’s classification: I = intestinal, D = diffuse.
¶Region of sample collection. N.A., North America.
∥The cases studied for IHC were also analyzed for these pathological features.
**Mucin production, either intracellularly (Intra) and/or extracellularly (Extra).
††Crohn’s like reaction presence was scored as present (Pos) or absent (Neg) or not able to be determined from sample analyzed (N/A). This p-value excludes N/A cases.
‡‡Tumor infiltrating lymphocytes (TIL) were scored on a scale of 0–4.
§§Tumor infiltrating neutrophiles (TIN) were scored on a scale of 0–4.
BAT-26 instability was found to be significantly (P < 0.001) associated with MSI-H and mismatch repair loss of protein expression (ie, hMLH1 or hMSH2). Exploratory analyses found a mid or distal location, higher scores of tumor infiltrating lymphocytes, and early stage to be associated with MSI-H and BAT-26 instability. Interestingly, an association of MSI-H was also noted with cases from the endemic region of Italy. Moreover, all nine MSI-L cases were from the nonendemic region of North America, although this finding may be a function of the small sample sizes. No other associations were considered noteworthy.
Discussion
MSI+ gastric carcinomas in this study could be divided into two groups, those with high-level instability (ie, MSI at ≥33% of loci) and those with low-level instability (ie, MSI at ≤17% of loci). All MSI-H tumors available for immunostaining exhibited a loss of either hMLH1 (n = 5) or hMSH2 (n = 3), whereas all available MSI-L (n = 5) and 15 MSS tumors showed normal hMLH1 and hMSH2 expression. These findings strongly suggest that defective mismatch repair due to loss of hMLH1 or hMSH2 expression underlies the MSI phenotype in MSI-H gastric tumors, but not MSI-L tumors. High-level MSI was associated with loss of hMLH1 or hMSH2 expression in all of the tumors studied, suggesting that other defects of alternative MMR genes will infrequently be the cause of MSI-H in gastric carcinoma.
Somatic or germline mutations of hMSH2 and hMLH1 have been infrequently observed in sporadic gastric cancers. Keller et al found that only one of 30 patients with varying degrees of family history of gastric cancer had a germline missense hMLH1 mutation. 26 Another study found three somatic hMLH1 mutations but no germline hMSH2 or hMLH1 mutations in 18 RER+ gastric tumors. 27 Wu et al found a single hMSH2 somatic missense mutations in 12 MSI gastric cancer patients. 34 Recent studies suggest that silencing of the hMLH1 gene through hypermethylation of the hMLH1 promoter may account for the majority of defective MMR and MSI observed in sporadic colorectal, endometrial, and gastric cancers. 30,31,35-37
Recent studies, although small in number of patients analyzed, indicate that the majority of tumors with loss of hMSH2 expression have germline hMSH2 mutations. 28,30 If this trend holds, it would suggest that most patients whose tumors show loss of hMSH2 actually have the HNPCC trait. Intriguingly, the father of patient G38 had colon cancer and the sister of G106 had a history of an “esophageal cancer.” Furthermore, patient G38, who had a stage IIIb gastric carcinoma resected in 1994, is still alive, consistent with the unusually good prognosis that is sometimes observed for malignancies in HNPCC patients. 38-40 Unfortunately, the lack of a detailed family history for our three patients demonstrating loss of hMSH2 protein expression in their gastric tumor (G38, G61, G106) does not allow further determination of HNPCC status in these kindred cases. Studies are under way to assess whether these three patients have hMSH2 germline mutations.
Although the number of tumors with MSI-H was relatively small, it appears that certain di- and tetranucleotide repeats are more likely to exhibit MSI in MSI-H tumors than other di- and tetranucleotide repeats. For example, the dinucleotide D12S104 exhibited MSI in seven of nine tumors, whereas dinucleotides D8S261, D9S171, and D10S541 exhibited MSI in only one of the nine tumors. Thibodeau et al have also observed that different dinucleotide repeats show different rates of MSI in MSI-H tumors. 41 The likelihood that a microsatellite repeat will be susceptible to instability may relate to the inherent mutation rate at that locus. Studies have shown variability in the mutation rates of di-, tri-, and tetranucleotide repeats in CEPH families. 42 Dietmaier et al 43 found that pure dinucleotide repeats were less likely to exhibit MSI in MSI-H tumors than complex dinucleotide repeats. However, we did not observe an association between the complexity of the di- or tetranucleotide repeat and the frequency of MSI observed in the MSI-H tumors.
In this study we find that the MSI-H phenotype shows statistically significant associations with tumor location (distal and mid-stomach), early stage, tumor infiltrating lymphocytes, and geographic region of occurrence. These findings lend further support to the hypothesis that MSI-H gastric tumors exhibit distinct clinicopathological characteristics. Other groups have consistently noted an association of the MSI-H phenotype with intestinal subtype, distal location (eg, antral), and more favorable prognosis. 8-10,14,44-49 Furthermore, some but not all studies have noted associations between the MSI-H phenotype and less frequent lymph node metastasis, 8,10,49 greater depth of invasion, 8 near-diploid DNA content, 10 and tumoral lymphoid infiltration. 8,10,48,49 A possible explanation for the unique clinicopathological phenotype observed in MSI-H gastric tumors may be the occurrence of mutations in a distinct set of cancer-related genes differing from those in tumors with no or low-level MSI. Tumor suppressor genes that have been shown to be critical targets of defective MMR in MSI-H tumors include TGF-β RII, IGFIIR, BAX, hMSH6, and hMSH3 genes. 8,46,49-60 These same genes are infrequently mutated in MSI-L or MSS tumors. 49,51 In this study we found instability of the poly A tract of the TGF-β RII gene in eight of the 10 MSI-H tumors but none of the MSI-L tumors. This provides further evidence for a role of TGF-β RII inactivation in MSI-H tumors.
Because MSI-H gastric carcinomas appear to be clinicopathologically distinct, it may prove valuable to have markers that identify this subgroup of gastric cancers. Markers with high sensitivity and specificity for the identification of MSI-H tumors would reduce the number of markers needed to identify this phenotype and increase the clinical feasibility of such testing. Although larger studies should be conducted, our findings suggest that BAT-26 instability may be a sensitive and specific marker of the MSI-H phenotype in gastric carcinomas. An additional advantage of utilizing BAT-26 for analysis is that there is not an absolute requirement for matching normal DNA. 61
In summary, the results of this study suggest that defective expression of hMLH1 or hMSH2 accounts for the defective MMR observed in most gastric cancers with high-level MSI. Our study is the first to demonstrate a significant correlation between BAT-26 instability and loss of hMSH2 or hMLH1 protein expression in MSI-H gastric tumors. These findings have important implications for the characterization and clinical stratification of patients with gastric cancer.
Footnotes
Address reprint requests to Dr. Steven M. Powell, University of Virginia Health Sciences Center, Box 10013, Charlottesville, VA 22906-0013. E-mail: smp8n@virginia.edu.
This study was supported by National Institutes of Health grant CAG7900-04 (SMP).
References
- 1.Aaltonen LA, Peltomaki P, Mecklin JP, Jarvinen H, Jass JR, Green JS, Lynch HT, Watson P, Tallqvist G, Juhola M: Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res 1994, 54:1645-1648 [PubMed] [Google Scholar]
- 2.Peltomaki P, Lothe RA, Aaltonen LA, Pylkkanen L, Nystrom-Lahti M, Seruca R, David L, Holm R, Ryberg D, Haugen A: Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res 1993, 53:5853-5855 [PubMed] [Google Scholar]
- 3.Thibodeau SN, Bren G, Schaid D: Microsatellite instability in cancer of the proximal colon. Science 1993, 260:816-819 [DOI] [PubMed] [Google Scholar]
- 4.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M: Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363:558-561 [DOI] [PubMed] [Google Scholar]
- 5.Wirtz HC, Muller W, Noguchi T, Scheven M, Ruschoff J, Hommel G, Gabbert HE: Prognostic value and clinicopathological profile of microsatellite instability in gastric cancer. Clin Cancer Res 1998, 4:1749-1754 [PubMed] [Google Scholar]
- 6.Hayden JD, Cawkwell L, Quirke P, Dixon MF, Goldstone AR, Sue-Ling H, Johnston D, Martin IG: Prognostic significance of microsatellite instability in patients with gastric carcinoma. Eur J Cancer 1997, 33:2342-2346 [DOI] [PubMed] [Google Scholar]
- 7.Keller G, Rudelius M, Vogelsang H, Grimm V, Wilhelm MG, Mueller J, Siewert JR, Hofler H: Microsatellite instability and loss of heterozygosity in gastric carcinoma in comparison to family history. Am J Pathol 1998, 152:1281-1289 [PMC free article] [PubMed] [Google Scholar]
- 8.Wu MS, Lee CW, Shun CT, Wang HP, Lee WJ, Sheu JC, Lin JT: Clinicopathological significance of altered loci of replication error and microsatellite instability-associated mutations in gastric cancer. Cancer Res 1998, 58:1494-1497 [PubMed] [Google Scholar]
- 9.Ottini L, Palli D, Falchetti M, D’Amico C, Amorosi A, Saieva C, Calzolari A, Cimoli F, Tatarelli C, De Marchis L, Masala G, Mariani-Costantini R, Cama A: Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res 1997, 57:4523-4529 [PubMed] [Google Scholar]
- 10.dos Santos NR, Seruca R, Constancia M, Seixas M, Sobrinho-Simoes M: Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology 1996, 110:38-44 [DOI] [PubMed] [Google Scholar]
- 11.Schneider BG, Pulitzer DR, Brown RD, Prihoda TJ, Bostwick DG, Saldivar V, Rodriguez-Martinez HA, Gutierrez-Diaz ME, O’Connell P: Allelic imbalance in gastric cancer: an affected site on chromosome arm 3p. Genes Chromosom Cancer 1995, 13:263-271 [DOI] [PubMed] [Google Scholar]
- 12.Chong JM, Fukayama M, Hayashi Y, Takizawa T, Koike M, Konishi M, Kikuchi-Yanoshita R, Miyaki M: Microsatellite instability in the progression of gastric carcinoma. Cancer Res 1994, 54:4595-4597 [PubMed] [Google Scholar]
- 13.Mironov NM, Aguelon MA, Potapova GI, Omori Y, Gorbunov OV, Klimenkov AA, Yamasaki H: Alterations of (CA)n DNA repeats and tumor suppressor genes in human gastric cancer. Cancer Res 1994, 54:41-44 [PubMed] [Google Scholar]
- 14.Strickler JG, Zheng J, Shu Q, Burgart LJ, Alberts SR, Shibata D: p53 mutations and microsatellite instability in sporadic gastric cancer: when guardians fail. Cancer Res 1994, 54:4750-4755 [PubMed] [Google Scholar]
- 15.Rhyu MG, Park WS, Meltzer SJ: Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene 1994, 9:29-32 [PubMed] [Google Scholar]
- 16.Duggan BD, Felix JC, Muderspach LI, Tourgeman D, Zheng J, Shibata D: Microsatellite instability in sporadic endometrial carcinoma. J Natl Cancer Inst 1994, 86:1216-1221 [DOI] [PubMed] [Google Scholar]
- 17.Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J: Genetic instability of microsatellites in endometrial carcinoma. Cancer Res 1993, 53:5100-5103 [PubMed] [Google Scholar]
- 18.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R: The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993, 75:1027-1038(erratum: Cell 1994 77:167) [DOI] [PubMed] [Google Scholar]
- 19.Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A: Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 1994, 368:258-261 [DOI] [PubMed] [Google Scholar]
- 20.Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD: Mutation of a mutL homolog in hereditary colon cancer. Science 1994, 263:1625-1629 [DOI] [PubMed] [Google Scholar]
- 21.Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, Peltomaki P, Sistonen P, Aaltonen LA, Nystrom-Lahti M: Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993, 75:1215-1225 [DOI] [PubMed] [Google Scholar]
- 22.Loeb LA: Many mutations in cancers. Cancer Surv 1996, 28:329-342 [PubMed] [Google Scholar]
- 23.Loeb LA: Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res 1994, 54:5059-5063 [PubMed] [Google Scholar]
- 24.Honchel R, Halling KC, Thibodeau SN: Genomic instability in neoplasia. Semin Cell Biol 1995, 6:45-52 [DOI] [PubMed] [Google Scholar]
- 25.Moskaluk CA, Rumpel CA: Allelic deletion in 11p15 is a common occurrence in esophageal and gastric adenocarcinoma. Cancer 1998, 83:232-239 [PubMed] [Google Scholar]
- 26.Keller G, Grimm V, Vogelsang H, Bischoff P, Mueller J, Siewert JR, Hofler H: Analysis for microsatellite instability and mutations of the DNA mismatch repair gene hMLH1 in familial gastric cancer. Int J Cancer 1996, 68:571-576 [DOI] [PubMed] [Google Scholar]
- 27.Semba S, Yokozaki H, Yasui W, Tahara E: Frequent microsatellite instability and loss of heterozygosity in the region including BRCA1 (17q21) in young patients with gastric cancer. Int J Oncol 1998, 12:1245-1251 [DOI] [PubMed] [Google Scholar]
- 28.Thibodeau SN, French AJ, Roche PC, Cunningham JM, Tester DJ, Lindor NM, Moslein G, Baker SM, Liskay RM, Burgart LJ, Honchel R, Halling KC: Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res 1996, 56:4836-4840 [PubMed] [Google Scholar]
- 29.Moslein G, Tester DJ, Lindor NM, Honchel R, Cunningham JM, French AJ, Halling KC, Schwab M, Goretzki P, Thibodeau SN: Microsatellite instability and mutation analysis of hMSH2 and hMLH1 in patients with sporadic, familial and hereditary colorectal cancer. Hum Mol Genet 1996, 5:1245-1252 [DOI] [PubMed] [Google Scholar]
- 30.Cunningham JM, Christensen ER, Tester DJ, Kim C-Y, Roche PC, Burgart LJ, Thibodeau SN: Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998, 58:3455-3460 [PubMed] [Google Scholar]
- 31.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB: Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 1998, 6870–6875 [DOI] [PMC free article] [PubMed]
- 32.American Joint Committee on Cancer. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual, ed 5. Philadelphia, Lippincott-Raven, 1998.
- 33.Powell SM, Harper JC, Hamilton SR, Robinson CR, Cummings OW: Inactivation of Smad4 in gastric carcinomas. Cancer Res 1997, 57:4221-4224 [PubMed] [Google Scholar]
- 34.Wu MS, Sheu JC, Shun CT, Lee WJ, Wang JT, Wang TH, Cheng AL, Lin JT: Infrequent hMSH2 mutations in sporadic gastric adenocarcinoma with microsatellite instability. Cancer Lett 1997, 112:161-166 [DOI] [PubMed] [Google Scholar]
- 35.Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R: Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 1997, 57:808-811 [PubMed] [Google Scholar]
- 36.Esteller M, Levine R, Baylin SB, Ellenson LH, Herzog TJ: MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene 1998, 17:2413-2417 [DOI] [PubMed] [Google Scholar]
- 37.Leung SY, Yuen ST, Chung LP, Chu KM, Chan ASY, Ho JCI: hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res 1999, 59:159-164 [PubMed] [Google Scholar]
- 38.Love RR: Small bowel cancers, B-cell lymphatic leukemia, and six primary cancers with metastases and prolonged survival in the cancer family syndrome of Lynch. Cancer 1985, 55:499-502 [DOI] [PubMed] [Google Scholar]
- 39.Frei JV: Hereditary nonpolyposis colorectal cancer (Lynch syndrome II). Diploid malignancies with prolonged survival. Cancer 1992, 69:1108-1111 [DOI] [PubMed] [Google Scholar]
- 40.Honchel R, Halling KC, Schaid DJ, Pittelkow M, Thibodeau SN: Microsatellite instability in Muir-Torre syndrome. Cancer Res 1994, 54:1159-1163 [PubMed] [Google Scholar]
- 41.Thibodeau SN, French AJ, Cunningham JM, Tester D, Burgart LJ, Roche PC, McDonnell SK, Schaid DJ, Vockley CW, Michels VV, Farr GH, O’Connell MJ: Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res 1998, 58:1713-1718 [PubMed] [Google Scholar]
- 42.Weber JL, Wong C: Mutation of human short tandem repeats. Hum Mol Genet 1993, 2:1123-1128 [DOI] [PubMed] [Google Scholar]
- 43.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J: Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 1997, 57:4749-4756 [PubMed] [Google Scholar]
- 44.Luinetti O, Fiocca R, Villani L, Alberizzi P, Ranzani GN, Solcia E: Genetic pattern, histological structure, and cellular phenotype in early and advanced gastric cancers: evidence for structure-related genetic subsets and for loss of glandular structure during progression of some tumors. Hum Pathol 1998, 29:702-709 [DOI] [PubMed] [Google Scholar]
- 45.Buonsanti G, Calistri D, Padovan L, Luinetti O, Fiocca R, Solcia E, Ranzani GN: Microsatellite instability in intestinal- and diffuse-type gastric carcinoma. J Pathol 1997, 182:167-173 [DOI] [PubMed] [Google Scholar]
- 46.Chung YJ, Song JM, Lee JY, Jung YT, Seo EJ, Choi SW, Rhyu MG: Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res 1996, 56:4662-4665 [PubMed] [Google Scholar]
- 47.Gleeson CM, Sloan JM, McGuigan JA, Ritchie AJ, Weber JL, Russell SE: Widespread microsatellite instability occurs infrequently in adenocarcinoma of the gastric cardia. Oncogene 1996, 12:1653-1662 [PubMed] [Google Scholar]
- 48.Seruca R, Santos NR, David L, Constancia M, Barroca H, Carneiro F, Seixas M, Peltomaki P, Lothe R, Sobrinho-Simoes M: Sporadic gastric carcinomas with microsatellite instability display a particular clinicopathologic profile. Int J Cancer 1995, 64:32-36 [DOI] [PubMed] [Google Scholar]
- 49.Oliveira C, Seruca R, Seixas M, Sobrinho-Simoes M: The clinicopathological features of gastric carcinomas with microsatellite instability may be mediated by mutations of different “target genes”: a study of the TGFβ RII, IGFII R, and BAX genes. Am J Pathol 1998, 153:1211-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo RJ, Wang Y, Kaneko E, Wang DY, Arai H, Hanai H, Takenoshita S, Hagiwara K, Harris CC, Sugimura H: Analyses of mutation and loss of heterozygosity of coding sequences of the entire transforming growth factor beta type II receptor gene in sporadic human gastric cancer. Carcinogenesis 1998, 19:1539-1544 [DOI] [PubMed] [Google Scholar]
- 51.Ottini L, Falchetti M, D’Amico C, Amorosi A, Saieva C, Masala G, Frati L, Cama A, Palli D, Mariani-Costantini R: Mutations at coding mononucleotide repeats in gastric cancer with the microsatellite mutator phenotype. Oncogene 1998, 16:2767-2772 [DOI] [PubMed] [Google Scholar]
- 52.Shinmura K, Tani M, Isogaki J, Wang Y, Sugimura H, Yokota J: RER phenotype and its associated mutations in familial gastric cancer. Carcinogenesis 1998, 19:247-251 [DOI] [PubMed] [Google Scholar]
- 53.Yin J, Kong D, Wang S, Zou TT, Souza RF, Smolinski KN, Lynch PM, Hamilton SR, Sugimura H, Powell SM, Young J, Abraham JM, Meltzer SJ: Mutation of hMSH3 and hMSH6 mismatch repair genes in genetically unstable human colorectal and gastric carcinomas. Hum Mutat 1997, 10:474-478 [DOI] [PubMed] [Google Scholar]
- 54.Chung YJ, Park SW, Song JM, Lee KY, Seo EJ, Choi SW, Rhyu MG: Evidence of genetic progression in human gastric carcinomas with microsatellite instability. Oncogene 1997, 15:1719-1726 [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto H, Sawai H, Perucho M: Frameshift somatic mutations in gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res 1997, 57:4420-4426 [PubMed] [Google Scholar]
- 56.Renault B, Calistri D, Buonsanti G, Nanni O, Amadori D, Ranzani GN: Microsatellite instability and mutations of p53 and TGF-beta RII genes in gastric cancer. Hum Genet 1996, 98:601-607 [DOI] [PubMed] [Google Scholar]
- 57.Ouyang H, Furukawa T, Abe T, Kato Y, Horii A: The BAX gene, the promoter of apoptosis, is mutated in genetically unstable cancers of the colorectum, stomach, and endometrium. Clin Cancer Res 1998, 4:1071-1074 [PubMed] [Google Scholar]
- 58.Ouyang H, Shiwaku HO, Hagiwara H, Miura K, Abe T, Kato Y, Ohtani H, Shiiba K, Souza RF, Meltzer SJ, Horii A: The insulin-like growth factor II receptor gene is mutated in genetically unstable cancers of the endometrium, stomach, and colorectum. Cancer Res 1997, 57:1851-1854 [PubMed] [Google Scholar]
- 59.Ohue M, Tomita N, Monden T, Miyoshi Y, Ohnishi T, Izawa H, Kawabata Y, Sasaki M, Sekimoto M, Nishisho I, Shiozaki H, Monden M: Mutations of the transforming growth factor beta type II receptor gene and microsatellite instability in gastric cancer. Int J Cancer 1996, 68:203-206 [DOI] [PubMed] [Google Scholar]
- 60.Myeroff LL, Parsons R, Kim SJ, Hedrick L, Cho KR, Orth K, Mathis M, Kinzler KW, Lutterbaugh J, Park K: A transforming growth factor beta receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res 1995, 55:5545-5547 [PubMed] [Google Scholar]
- 61.Zhou XP, Hoang JM, Li YJ, Seruca R, Carneiro F, Sobrinho-Simoes M, Lothe RA, Gleeson CM, Russell SE, Muzeau F, Flejou JF, Hoang-Xuan K, Lidereau R, Thomas G, Hamelin R: Determination of the replication error phenotype in human tumors without the requirement for matching normal DNA by analysis of mononucleotide repeat microsatellites. Genes Chromosom Cancer 1998, 21:101-107 [DOI] [PubMed] [Google Scholar]