Abstract
The amyloid cascade hypothesis of Alzheimer’s disease postulates that accumulation of amyloid β-protein (Aβ) precedes neurofibrillary tangle formation or neuronal loss in the cortex. Although this temporal profile has been proved in the neocortex by silver staining and immunocytochemical methods, CA1 of the hippocampus exhibits a distinct temporal profile during normal aging: the formation of neurofibrillary tangles precedes senile plaque formation. This temporal profile has been further confirmed by two-site enzyme immunoassay (EIA) quantitation of sodium dodecyl sulfate (SDS)-dissociable Aβ42; neurofibrillary tangles are already present despite undetectable levels of SDS-dissociable Aβ42. However, when the same specimens were subjected to Western blotting, many cases with or without neurofibrillary tangles showed some accumulation of SDS-stable Aβ dimers that cannot be detected by EIA. Thus, the temporal profile prerequisite for the hypothesis is still valid in CA1, and this finding also suggests that SDS-stable Aβ dimers have some significant effects on CA1 pyramidal neurons, which are most vulnerable to neurofibrillary tangle formation.
Among the hypotheses that have been proposed thus far on the pathogenesis of Alzheimer’s disease (AD), the most dominant is the so-called amyloid cascade hypothesis, which states that accumulation of amyloid β-protein (Aβ), the major component of senile plaques (SPs), leads to the formation of neurofibrillary tangles (NFTs) in susceptible neurons, eventually causing their death, through its neurotoxicity. 1 Two major Aβ species are defined by their different carboxyl-terminal lengths: Aβ40 terminating at Val-40 and Aβ42 terminating at Ala-42. The former accounts for ∼90% of the secreted Aβ, 2 but the latter exhibits a much higher aggregation potential than the former in vitro 3 and is by far the predominant species of SPs. 4
The hypothesis is supported by several findings: 1) in the brains from Down’s syndrome patients of various ages, the earliest lesion in the neocortex is found to be diffuse plaques consisting exclusively of Aβ42, 5 2) rare forms of familial AD are caused by mutations of β-amyloid precursor protein (APP), 1 and 3) mutations of presenilin 1 and 2 as well as of APP cause enhanced secretion of Aβ42. 1,2 Along this line of investigation, there is now a large collection of reports describing the neurotoxicity of Aβ. Its neurotoxicity is observed in vitro under well controlled conditions; fibrillar, but not amorphous, Aβ aggregates are believed to exert toxic effects on cultured neurons. 6,7 These toxic effects may be mediated through oxidative stress, 8 although other routes of mediation are also possible.
However, there are some important, but often neglected, observations refuting in vivo neurotoxicity of Aβ. The most remarkable is that in the hippocampus CA1 and entorhinal cortex, a great number of NFTs often occur without SPs during normal aging 9-12 and presumably in the initial stage of AD, 13 as has been repeatedly confirmed by several groups using silver staining or immunocytochemistry. In addition, two lines of transgenic mice exhibiting Aβ deposits did not show NFT formation or neuronal loss, suggesting that Aβ accumulation detected as extracellular Aβ deposits is not sufficient for NFT formation or neuronal loss. 14,15 These findings led us to believe that the amyloid cascade hypothesis of AD is not valid in CA1 and thus may not be so in the neocortex. However, neuropathological findings alone cannot invalidate the amyloid cascade hypothesis, because one cannot exclude the possibility that Aβ can accumulate to significant levels in CA1 without forming immunocytochemically detectable SPs, and that this invisible but significant accumulation could lead to NFT formation in a subset of neurons and/or their loss in CA1.
In this study, we sought to examine 1) whether NFTs appear in CA1/T4 (medial occipitotemporal cortex) without the accumulation of SDS-dissociable Aβ42 as quantitated by the sensitive two-site enzyme immunoassay (EIA) 16 and 2) whether there is a similar temporal relationship of NFT with SDS-stable Aβ dimers that are detected by Western blotting but not EIA, using specimens obtained from consecutive autopsy cases with ages ranging from 24 to 92 years. 17
Materials and Methods
Subjects
The present study is based on autopsies performed (n = 74; 56 men and 18 women) at the Tokyo Medical Examiner’s Office (Otsuka, Tokyo). 17 The ages at death of the 74 subjects ranged from 24 to 92 years (3 at 20 to 29 years of age, 4 at 30 to 39 years of age, 17 at 40 to 49 years of age, 18 at 50 to 59 years of age, 13 at 60 to 69 years of age, 10 at 70 to 79 years of age, 8 at 80 to 89 years of age, and 1 at 92 years of age). Postmortem delay ranged from 2 to 24 hours. AD was diagnosed based on both clinical and neuropathological criteria. 18,19
Tissue Preparation and Extraction
Cortical pieces, ∼80 to 110 mg each, of CA1 and T4 at the level of the lateral geniculate body were sampled from fresh brains at autopsy and stored at −80°C until use. The attached leptomeninges and vessels were carefully dissected out. Cortical blocks from adjacent sites and/or from the same locations on the contralateral side were fixed in 10% buffered formalin and processed for histological and immunocytochemical examination.
Each of the sampled pieces was homogenized as described elsewhere. 17 Five microliters from each homogenate was smeared on polylysine-coated glass slides for tau immunostaining. The remaining part of each homogenate was further homogenized and centrifuged at 100,000 × g for 15 minutes. The supernatant was used for quantitation of Aβ (soluble Aβ); the pellet (insoluble fraction) was homogenized in 70% formic acid and the resultant suspension was centrifuged as described above. The supernatant was neutralized with NaOH and trizma base and subjected to the EIA (insoluble Aβ). 20
Enzyme Immunoassay
The two-site EIA for Aβ consisted of Aβ monoclonal antibodies BNT77, BA27, and BC05. BNT77 was used as the capture antibody, and BA27 and BC05 were used as the detector antibodies. 20 BNT77, the epitope of which is thought to be located in Aβ11-16, is considered to capture all Aβ species truncated up to position 10, but not p3, which starts at Aβ17. 20 The BNT77-based EIA employed in this study detects only Aβ species that run as monomers on SDS-polyacrylamide gel electrophoresis. 16
Western Blotting
Each aliquot of the formic acid extract of insoluble fraction derived from 2.5 mg of tissue was dried by Speed Vac (Savant Instruments, Framingdale, NY) and solubilized with the SDS sample buffer (50 mmol/L Tris HCl (pH 6.8), 12% glycerol, 2% SDS, 2.5% mercaptoethanol, 4 mol/L urea). These samples were subjected to Tris/tricine gel electrophoresis, and the separated proteins were blotted onto a nitrocellulose membrane (pore size 0.22 μm; Schleicher & Schuell, Dassel, Germany). The blot, after heat treatment, 21 was incubated with appropriately diluted BA27 or BC05. After washing with Tris/saline-based buffer, the blot was further incubated with horseradish-peroxidase-conjugated goat anti-mouse IgG (Transduction Laboratories, Lexington, KY). Bound antibodies were visualized using the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Little Chalfont, UK). This modified version of Western blotting reproducibly detected as little as 20 pg (5 fmol) of Aβ1-42 or Aβ1-40 per lane, which is equal to 2 pmol/g wet weight.
Besides specimens, synthetic Aβ1-40 or 1-42 (10, 20, 50, and 100 pg; Bachem, Torrance, CA) was also loaded onto each gel for Western blot quantitation of Aβ. SDS-stable Aβ dimers were quantitated using a standard curve for synthetic Aβ40 or 42 (SDS-dissociable Aβ40 or 42), and the concentration was expressed as the Aβ40 or 42 monomer equivalent. Thus, it was postulated that the blotting efficiency and reactivity of SDS-stable dimers with BA27 or BC05 are the same as those of SDS-dissociable Aβ40 or 42. 16 It should be also noted that Aβ oligomers other than Aβ dimers are not taken into account.
Semiquantitative Immunocytochemistry for the Abundance of NFTs
The formalin-fixed, paraffin-embedded tissue blocks were cut into 6-μm-thick sections. These sections and smeared aliquots from the brain homogenates were immunostained with anti-human tau by the avidin-biotin method (Vectastain Elite, Vector Laboratories, Burlingame, CA). The abundance of NFTs was assessed by manually counting them on anti-human tau-immunostained sections in five unselected fields under ×200 magnification, and the numbers were averaged. The abundance was rated as follows: −, none; +, 1 to 5; ++, 6 to 10; +++, more than 10 per one ×200 field. The NFT abundance estimated on smears excellently agreed with that obtained from immunostained tissue sections.
Apolipoprotein E Genotyping
Typing of the apolipoprotein E (ApoE) genotype was performed using the polymerase chain reaction (PCR) as described previously. 17
Results
NFTs Appear without Accumulation of SDS-Dissociable Aβ42 in CA1
In the present series, there were 27 cases showing NFTs but no SPs in CA1, as judged by immunocytochemistry, a result that is in good agreement with the data reported by other groups. 9-13 In contrast, there was no case showing SPs but not NFTs in CA1. We examined whether there was a temporal relationship between the appearance of NFTs and the accumulation of SDS-dissociable Aβ that is quantitated by BNT77-based EIA. 16 Consistent with the immunocytochemical observations, NFTs occurred without accumulation of SDS-dissociable Aβ42 in the insoluble fraction of CA1 homogenates in 22 cases (Figure 1, A and B) ▶ . Because EIA is approximately 30-fold more sensitive than immunocytochemical detection of SPs, 17 it seemed that the EIA quantitation may have further confirmed the immunocytochemical data, that the formation of NFTs precedes that of SPs in CA1.
Figure 1.
The EIA level of insoluble Aβ42 (corresponding to Aβ42 monomers on the Western blot) in CA1 (A) and T4 (C) versus age and the abundance of NFTs in CA1 (B) and T4 (D), based on the consecutive autopsies (age range at death, 24 to 92 years). The detection limit for insoluble Aβ42 (- - -) by EIA was 12 pmol/g wet weight. Among a total of 74 cases, 40 cases did not show NFTs (◊) and 29 cases did (⧫) in CA1 (A); and 63 cases did not show NFTs (◊) and 6 cases did (⧫) in T4 (C), and 5 were AD cases (•). ○, non-AD cases in B and D. P < 0.05 for indicated comparisons in B and D.
Among the non-AD cases (69 cases), the abundance of NFTs was not correlated with the extent of Aβ42 accumulation (Figure 1B ▶ ; Mann-Whitney’s U test, not significant). Likewise, the appearance of NFTs was independent of the levels of SDS-dissociable Aβ42 in the soluble fraction or those of SDS-dissociable Aβ40 in the soluble and insoluble fractions of CA1 homogenates (data not shown). However, the formation of NFTs appeared to be related to the age at death of the subjects: none of the patients (24 cases) who died under the age of 50 years exhibited NFTs in CA1. For ages of 50 to 64 years and the age of 65 years and above, the incidence of NFTs increased to 9 of 22 (41%) and 25 of 28 (89%), respectively. NFTs were found in 22 EIA-negative cases in which SDS-dissociable Aβ42 levels were below the detection limit (<12 pmol/g; Figure 1A ▶ ).
Accumulation of SDS-Dissociable Aβ42 Precedes the Appearance of NFTs in T4
In contrast to CA1, there were only a small number of cases showing NFT formation in T4 (Figure 1, C and D) ▶ . There were nine cases showing SPs but no NFTs, whereas three cases showed NFTs but no SPs in T4 (data not shown). Twenty-one cases contained detectable levels of SDS-dissociable Aβ42, but no NFTs (Figure 1D) ▶ . The incidence of NFTs was significantly correlated with the extent of Aβ42 accumulation in T4 (Figure 1D ▶ ; Mann-Whitney’s U test, P < 0.05). Furthermore, there was no apparent relationship between the incidence of NFTs and the age of death of the subjects in T4 (Figure 1C) ▶ .
Presence of SDS-Stable Aβ Dimers in the Presence or Absence of NFTs in CA1
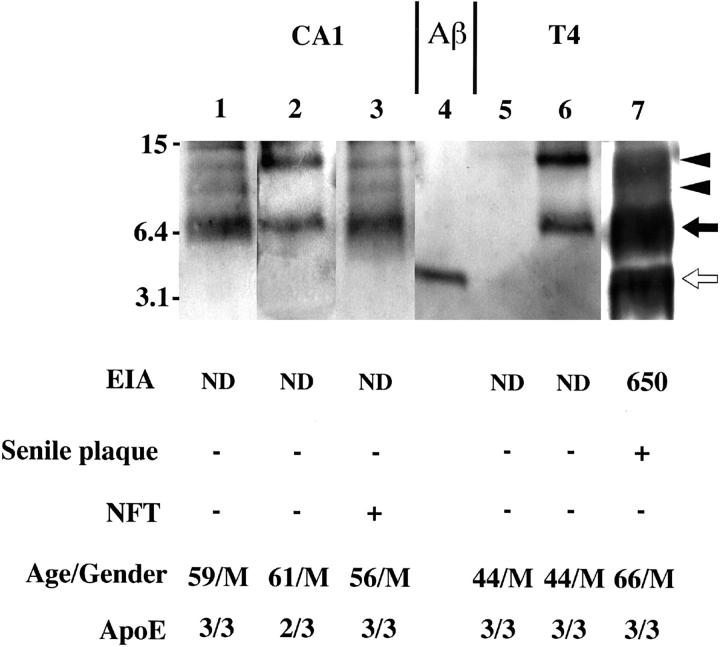
We further assessed by an improved version of Western blotting 21 the levels of SDS-stable Aβ dimer that cannot be detected by EIA. 16 Representative Western blots of CA1 specimens with BC05, together with BNT77-based EIA values and immunocytochemical findings for SPs and NFTs, are given in Figure 2 ▶ . Lanes 1, 2, and 3 are CA1 specimens from a 59-year-old man (ApoE genotype: ɛ3/ɛ3), a 61-year-old man (ɛ2/ɛ3), and a 56-year-old man (ɛ3/ɛ3), respectively, in all of whom accumulation of Aβ42 dimers but not SDS-dissociable Aβ was revealed by Western blotting, whereas negligible levels of SDS-dissociable Aβ42 were detected by EIA. Whereas the first two were free from NFTs, the last showed some NFT formation.
Figure 2.
Representative Western blots of the insoluble fraction of hippocampus CA1 (lanes 1 to 3) and T4 (lanes 5 to 7) homogenates. The formic acid extract (representing Aβ in the insoluble fraction) was subjected to Western blotting with BC05. Immunocytochemical scores regarding NFTs and SPs, insoluble Aβ42 levels quantitated by BNT77-based EIA (pmol/g wet weight), age/gender, and ApoE genotypes are also given. BNT77-quantitated Aβ corresponds to Aβ monomers on the Western blot. Lane 4, 50 pg of synthetic Aβ 1-42. ND, not detected (below the detection limit). The numbers in the left indicate molecular weights of marker proteins in kilodaltons. Open arrow , closed arrow, and arrowheads in the right indicate Aβ monomer, Aβ dimers, and putative Aβ oligomers, respectively.
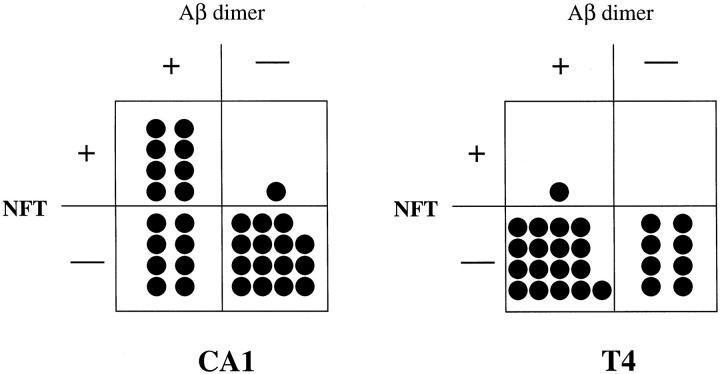
Among 32 cases showing negligible levels of SDS-dissociable Aβ by EIA in CA1, 8 showed accumulation of SDS-stable Aβ dimers (Aβ42 dimers, 3 cases; Aβ40 dimers, 1 case; both Aβ42 and Aβ40 dimers, 4 cases) and the presence of NFTs (Figure 3) ▶ . Eight cases showed accumulation of SDS-stable Aβ dimers (Aβ42 dimers, 4 cases; Aβ40 dimers, 3 cases; both Aβ42 and Aβ40 dimers, 1 case) but no NFTs. Fifteen cases showed neither SDS-stable Aβ dimers nor NFTs. Only one case showed NFT formation without SDS-stable Aβ dimer accumulation. Thus, SDS-stable Aβ dimers accumulated without NFT formation in CA1 (Figure 3 ▶ , Fisher’s exact method, P < 0.01), suggesting that SDS-stable Aβ dimers accumulated before NFT formation.
Figure 3.
The presence or absence of Aβ dimers as detected by Western blotting versus NFTs in CA1 (left) and T4 (right) among EIA-negative cases.
Representative Western blots of T4 specimens are given in Figure 2 ▶ ; lanes 5 and 6 are specimens from a 44-year-old man (ɛ3/ɛ3) and another 44-year-old man (ɛ3/ɛ3), respectively, both of whom exhibited no SPs. One showed no accumulation of Aβ monomers or Aβ dimers (lane 5), whereas the other showed accumulation of Aβ dimers but not Aβ monomers (lane 6). The third case (66-year-old man; ɛ3/ɛ3) showed significant accumulation of both dissociable Aβ42 and SDS-stable Aβ42 dimers (lane 7) and SPs but not NFTs.
Among 26 cases showing negligible levels of dissociable Aβ by EIA in T4, 17 showed accumulation of SDS-stable Aβ dimers (Aβ42 dimers, 4 cases; Aβ40 dimers, 9 cases; both Aβ42 and Aβ40 dimers, 4 cases) but no NFTs (Figure 3) ▶ . 16 There was no statistically significant difference in the incidence of SDS-stable dimers between CA1 and T4 (χ 2 test). Eight cases showed neither accumulation of SDS-stable Aβ dimers nor NFTs (Figure 3) ▶ . Only one case showed both SDS-stable Aβ dimer accumulation (Aβ42 dimers) and NFTs (Figure 3) ▶ . Thus, SDS-stable Aβ dimers accumulated without accompanying NFTs in T4, as in CA1.
With regard to the effect of ApoE alleles on NFT formation, 9 of 13 ɛ3/ɛ4 carriers showed NFTs in CA1, whereas 5 of them showed NFTs in T4. Of 58 ɛ3/ɛ3 carriers, 25 showed NFTs in CA1, and 6 of them showed NFTs in T4. There was a higher incidence of NFT in T4 of ɛ3/ɛ4 carriers than ɛ3/ɛ3 carriers (Fisher’s exact method, P < 0.05, data not shown), but not in CA1 (Fisher’s exact method, P = 0.13, data not shown).
Discussion
The present study clearly showed that many specimens of CA1 showing NFTs had undetectable levels of SDS-dissociable Aβ42. Apparently this result strengthens the view obtained from previous histochemical or immunocytochemical studies 9-13 that the amyloid cascade hypothesis is no longer valid in CA1 of the hippocampus. The detection limits of EIA for Aβ42 in the soluble and the insoluble fractions are 0.1 pmol/g wet weight and 12 pmol/g wet weight, respectively. 17,20 The latter value indicates that EIA is approximately 30-fold more sensitive than immunocytochemical detection of Aβ deposits. 17 From the above, some assumptions (see below) lead us to the following figures by simple calculation: that less than 0.88 nmol/L soluble Aβ42 and 105 nmol/L insoluble Aβ42 can accumulate in the extracellular space of CA1 showing NFT formation. The Aβ42 levels may have been underestimated due to proteolytic degradation during the postmortem period, but this may not be the case with the Aβ42 in the insoluble fraction, which appears not to be degraded even up to 18 hours postmortem, in contrast to up to a 50% decrease in Aβ42 levels in the soluble fraction during the similar period (Nakabayashi J, Morishima-Kawashima M, Ihara Y, unpublished data). Thus, the Aβ concentrations in the extracellular space of CA1 showing NFT formation are orders of magnitude lower than those employed for in vitro neurotoxicity experiments; neurotoxicity of Aβ is not observed up to 2.5 μmol/L in culture medium for dissociated cultured neurons 8 and 25 μmol/L for organotypic hippocampal cultures. 22 Thus, the CA1 pyramidal perikarya and their dendrites may be bathed in very low, if any, concentrations of soluble or insoluble Aβ42. It may be argued that their axon terminals, the most vulnerable portion of the neuron, are located in an area containing higher concentrations of Aβ, and therefore their neuronal perikarya may suffer the dying-back process. 23 CA1 neurons innervate the subiculum, where subicular neurons in turn project their axons through the fornix to the precommissural areas and mamillary body and to the entorhinal cortex. Although we did not quantitate Aβ in the subiculum, only one case that showed NFT formation without detectable Aβ accumulation showed immunocytochemically detectable SPs in the subiculum (data not shown). Obviously, these data alone may have led us to conclude that Aβ neurotoxicity alone cannot account for NFT formation in CA1.
Because of the undetectability of SDS-stable Aβ dimers by the most commonly used EIA, 16 we undertook the assessment of Aβ monomers (SDS-dissociable Aβ) and dimers in these specimens by an improved version of Western blotting. Most unexpectedly, many EIA-negative specimens showed some accumulation of SDS-stable Aβ40 or Aβ42 dimers but not of SDS-dissociable Aβ42. 16 This may suggest that SDS-stable Aβ dimers appear earlier than SDS-dissociable Aβ42 in CA1 and T4. 16 These SDS-stable Aβ dimers were detected both in CA1 and T4 in approximately one-half of the cases at the age of 40 to 60 years showing negligible Aβ42 levels by EIA. 16 Thus, it is likely that a much larger amount of Aβ dimers relative to SDS-dissociable Aβ42 exists in the earliest stage of β-amyloidogenesis.
There were significant differences in the extent of SDS-dissociable Aβ42 accumulation between CA1 and T4; even when certain cases showed significant accumulation of SDS-dissociable Aβ42 in T4, they often showed negligible levels of such Aβ42 in CA1. 17 Thus, it was quite unexpected that the majority of CA1 specimens showing NFTs contained Aβ dimers, and further that many cases without NFTs also contained some Aβ dimers (Figure 3) ▶ . The incidence of Aβ dimer-positive cases among EIA-negative cases in CA1 is similar to that in T4 (not significantly different, χ 2 test). These results suggest that 1) SDS-stable Aβ dimers may accumulate earlier than NFTs in CA1 (Figures 1 and 3) ▶ ▶ and 2) such Aβ dimers start to accumulate in CA1 and T4 at a similar time, whereas accumulation of SDS-dissociable Aβ42 appears to be significantly delayed in CA1 compared with T4. 17 Taken together, the temporal profile of Aβ accumulation and NFTs in CA1 may be as follows: SDS-stable Aβ dimers start to accumulate, followed by NFTs, and SDS-dissociable Aβ accumulation follows, and SPs appear at the end of this sequence. In contrast to CA1, there were many cases showing accumulation of SDS-dissociable Aβ42 and SPs but no NFTs in T4 (Figure 1) ▶ . Thus, in T4, SDS-stable Aβ dimers may accumulate first, followed by SDS-dissociable Aβ and SPs. NFTs appear at the end of the sequence. These findings suggest that CA1 may be particularly resistant to the accumulation of SDS-dissociable Aβ42 for unknown reasons and, furthermore, that SDS-stable Aβ dimers have a distinct metabolic pathway from SDS-dissociable Aβ in CA1.
Given that the mechanism underlying NFT formation during aging is the same in both CA1 and T4, SDS-stable Aβ dimers may be a candidate. More than 2 pmol/g wet weight of SDS-stable Aβ dimers (expressed by Aβ monomer equivalent; see Materials and Methods) may have already accumulated in CA1 showing no NFTs. It is reported that the stratum pyramidale of CA1 exhibits an exceptionally low extracellular volume fraction of 0.12 in rat brain. 24 If 1) this can be applied to human CA1, 2) the specific gravity of gray matter is assumed to be 1.0356, 25 and 3) SDS-stable Aβ dimers accumulate exclusively in the extracellular space, 17 nmol/L SDS-stable Aβ dimers would accumulate in the extracellular space of CA1 showing no NFTs. Thus, the concentrations of SDS-stable Aβ dimers in CA1 without NFTs are comparable to those employed for in vitro neurotoxicity experiments; diffusible, nonfibrillar Aβ1-42 oligomers exert neurotoxicity at nanomolar concentrations through an as yet unidentified cell surface receptor and the activation of fyn. 26 One of the most notable characteristics of SDS-stable Aβ dimers or oligomers is its inability to form fibrils. 26,27 This presents a sharp contrast to SDS-dissociable Aβ molecules that easily polymerize into fibrils and the amounts are well correlated with amyloid burden. 17 SDS-dissociable Aβ may be essential for SP formation, but not NFT formation.
Currently, we do not know whether the detected SDS-stable Aβ dimers exist in the extracellular space as postulated above or represent the Aβ species in the intracellular compartment. It is possible that these dimers are generated from secreted SDS-dissociable Aβ in the extracellular space. 28-30 Another possibility is that SDS-stable Aβ dimers accumulate in a certain intracellular compartment of neurons. Consistent with this, SDS-stable Aβ dimers appear to be located in the detergent-insoluble fraction of neuroblastoma cells. 31 Furthermore, our preliminary data show that the detergent-insoluble, low-density membrane fraction obtained from human brains contains these Aβ dimers. Thus, it is also possible that SDS-stable Aβ dimers detected in EIA-negative specimens represent the Aβ species bound to this particular membrane domain of neural cells.
The origin of the dimers and putative oligomers (see Figure 2 ▶ ) remains to be elucidated. SDS-stable Aβ dimers migrate usually at ∼6 kd, a little faster than the dimers generated from synthetic Aβ1-40 or 1-42. A fraction of the dimers is labeled with BAN50 (the epitope in Aβ1-10), suggesting that their amino termini are not largely deleted. Currently we are not certain as to whether faster migration of the SDS-stable dimers (Figure 2) ▶ represent amino-terminal truncation or an aberrant conformation by misfolding, as suggested by others. 30 We also do not know whether these dimers and oligomers are covalently linked or noncovalently linked. These molecules are not dissociated into monomers with SDS or other harsh denaturants, including urea and guanidine hydrochloride, raising the possibility of nondisulfide cross-linking. However, these properties do not necessarily indicate that these molecules are covalently linked. For example, the microtubule-binding domain of the tau in paired helical filaments shows similar aggregation that is highly resistant to SDS and guanidine hydrochloride, but appears not to be covalently linked. 32 In this case, deamidation and isoaspartate formation in the selected Asn and Asp residues likely have a significant role in SDS-stable oligomerization. 32 Possibly, similar post-translational modifications may be at work for the formation of SDS-stable Aβ dimers and oligomers.
One important question remains: which of the Aβ dimers, Aβ40 or 42, is more important in the initial phase of Aβ dimer deposition? We cannot completely exclude the possibility that Aβ40 dimers detected in autopsied brains are generated from Aβ42 dimers by partial proteolysis during the postmortem period. This may be possible because among brains with short postmortem delays there was no Aβ40 dimer-only brain. 16 However, to confirm this, careful examination of many autopsied cases with short postmortem delays is required. From the previous data alone, the appearance of Aβ42 dimers, but not Aβ40 dimers, is age dependent in CA1 and T4. 16 This raises the possibility that Aβ42 dimers are the species mainly involved in the deposition, although we cannot exclude the possibility that Aβ40 dimers have some important role in the initial stage of Aβ deposition.
In summary, SDS-stable Aβ dimers may be a missing link that can resolve an apparent contradiction of the amyloid cascade hypothesis in CA1.
Acknowledgments
We thank Ms. J. Saishoji and Ms. N. Naoi for tissue preparation and Ms. M. Anzai for the manuscript preparation.
Footnotes
Address reprint requests to Dr. Yasuo Ihara, Department of Neuropathology, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033. E-mail: yihara@m.u-tokyo.ac.jp.
References
- 1.Hardy J: Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci 1997, 20:154-159 [DOI] [PubMed] [Google Scholar]
- 2.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S: Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 1996, 2:864-870 [DOI] [PubMed] [Google Scholar]
- 3.Jarrett JT, Berger EP, Lansbury PT, Jr: The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32:4693-4697 [DOI] [PubMed] [Google Scholar]
- 4.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y: Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron 1994, 13:45-53 [DOI] [PubMed] [Google Scholar]
- 5.Iwatsubo T, Mann DMA, Odaka A, Suzuki N, Ihara Y: Amyloid β-protein (Aβ) deposition: Aβ42(43) precedes Aβ40 in Down’s syndrome. Ann Neurol 1995, 37:294-299 [DOI] [PubMed] [Google Scholar]
- 6.Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW: Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci 1993, 13:1676-1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenzo A, Yankner BA: β-Amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc Natl Acad Sci USA 1994, 91:12243-12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behl C, Davis JB, Lesley R, Schubert D: Hydrogen peroxide mediates amyloid β protein toxicity. Cell 1994, 77:817-827 [DOI] [PubMed] [Google Scholar]
- 9.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK: Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 1992, 13:179-189 [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Braak E: Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991, 82:239-259 [DOI] [PubMed] [Google Scholar]
- 11.Langui D, Probst A, Ulrich J: Alzheimer’s changes in non-demented and demented patients: a statistical approach to their relationships. Acta Neuropathol 1995, 89:57-62 [DOI] [PubMed] [Google Scholar]
- 12.Jellinger KA: Alzheimer’s changes in non-demented and demented patients. Acta Neuropathol 1995, 89:112-113 [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT: Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 1996, 16:4491-4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT: Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci 1997, 17:7053-7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT: APPSW transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol 1997, 56:965-973 [DOI] [PubMed] [Google Scholar]
- 16.Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, Games D, Schenk D, Sugihara S, Yamaguchi H, Ihara Y: Appearance of sodium dodecyl sulfate-stable amyloid β-protein (Aβ) dimer in the cortex during aging. Am J Pathol 1999, 154:271-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, Namekata K, Okeda R, Ihara Y: Quantitation of amyloid β-protein (Aβ) in the cortex during aging and in Alzheimer’s disease. Am J Pathol 1998, 152:1633-1640 [PMC free article] [PubMed] [Google Scholar]
- 18.Tierney MC, Fisher H, Lewis AJ: The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer’s disease: clinicopathological study of 57 cases. Neurology 1988, 38:356-364 [DOI] [PubMed] [Google Scholar]
- 19.Mirra SS, Heyman A, McKee D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L, : participating CERAD neuropathologists: The consortium to establish a registry for Alzheimer’s disease (CERAD). II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 42:1681-1688 [DOI] [PubMed] [Google Scholar]
- 20.Shinkai Y, Yoshimura M, Morishima-Kawashima M, Ito Y, Shimada H, Yanagisawa K, Ihara Y: Amyloid β-protein deposition in the leptomeninges and cerebral cortex. Ann Neurol 1997, 42:899-908 [DOI] [PubMed] [Google Scholar]
- 21.Ida N, Hartmann T, Pantel J, Schroeder J, Zerfass R, Foerstl H, Sandbrink R, Masters CL, Beyreuther K: Analysis of heterogenous βA4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem 1996, 271:22908-22914 [DOI] [PubMed] [Google Scholar]
- 22.Bruce AJ, Malfroy B, Baudry M: β-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci USA 1996, 93:2312-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vickers JC: A cellular mechanisms for the neuronal changes underlying Alzheimer’s disease. Neuroscience 1997, 78:629-639 [DOI] [PubMed] [Google Scholar]
- 24.McBain CJ, Traynelis SF, Dingledine R: Regional variation of extracellular space in the hippocampus. Science 1990, 249:674-677 [DOI] [PubMed] [Google Scholar]
- 25.Takagi H, Shapiro K, Marmarou A, Wisoff H: Microgravimetric analysis of human brain tissue: correlation with computerized tomography scanning. J Neurosurg 1981, 54:797-801 [DOI] [PubMed] [Google Scholar]
- 26.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL: Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 1998, 95:6448-6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo YM, Webster S, Emmerling MR, De Lima N, Roher AE: Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of Aβ peptides of Alzheimer’s disease. Biochim Biophys Acta 1998, 1406:291-298 [DOI] [PubMed] [Google Scholar]
- 28.Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ: Aggregation of secreted amyloid β-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem 1995, 270:9564-9570 [DOI] [PubMed] [Google Scholar]
- 29.Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ: Enhanced production and oligomerization of the 42-residue amyloid β-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem 1997, 272:7977-7982 [DOI] [PubMed] [Google Scholar]
- 30.Podlisny MB, Walsh DM, Amarante P, Ostaszewski BL, Stimson ER, Maggio JE, Teplow DB, Selkoe DJ: Oligomerization of endogenous and synthetic amyloid β-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry 1998, 37:3602-3611 [DOI] [PubMed] [Google Scholar]
- 31.Morishima-Kawashima M, Ihara Y: The presence of amyloid β-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry 1998, 37:15247-15253 [DOI] [PubMed] [Google Scholar]
- 32.Watanabe A, Takio K, Ihara Y: Deamidation and isoaspartate formation in smeared tau in paired helical filaments: unusual properties of the microtubule-binding domain of tau. J Biol Chem 1999, 274:7368-7378 [DOI] [PubMed] [Google Scholar]