Abstract
Mre11, Rad50, and Nbs1 function in a protein complex that is central to the metabolism of chromosome breaks. Null mutants of each are inviable. We demonstrate here that hypomorphic Rad50 mutant mice (Rad50S/S mice) exhibited growth defects and cancer predisposition. Rad50S/S mice died with complete bone marrow depletion as a result of progressive hematopoietic stem cell failure. Similar attrition occurred in spermatogenic cells. In both contexts, attrition was substantially mitigated by p53 deficiency, whereas the tumor latency of p53−/− and p53+/− animals was reduced by Rad50S/S. Indices of genotoxic stress and chromosomal rearrangements were evident in Rad50S/S cultured cells, as well as in Rad50S/S and p53−/−Rad50S/S lymphomas, suggesting that the Rad50S/S phenotype was attributable to chromosomal instability. These outcomes were not associated with overt defects in the Mre11 complex's previously established double strand break repair and cell cycle checkpoint regulation functions. The data indicate that even subtle perturbation of Mre11 complex functions results in severe genotoxic stress, and that the complex is critically important for homeostasis of proliferative tissues.
Keywords: Mre11 complex, Rad50S, double strand breaks, hematopoiesis
The Mre11 complex, consisting of the highly conserved Mre11 and Rad50 proteins and Nbs1 (or Xrs2 in yeast), mediates diverse functions in double strand break (DSB) metabolism. In both mammals and Saccharomyces cerevisiae, the complex functions as a sensor of DSBs and influences the induction of DNA damage-dependent cell cycle checkpoints (Petrini 2000a; D'Amours and Jackson 2001; Grenon et al. 2001; Mirzoeva and Petrini 2001; Usui et al. 2001). Mre11 complex deficiency in S. cerevisiae profoundly impairs recombinational DNA repair as well as telomere maintenance and meiotic recombination (Haber 1998). In vertebrates, null mutants in each member of the Mre11 complex are inviable (Xiao and Weaver 1997; Luo et al. 1999; Yamaguchi-Iwai et al. 1999; Zhu et al. 2001).
The Mre11 complex specifies ssDNA endonuclease, 3′-to-5′ exonuclease, and DNA unwinding activities in vitro. Accordingly, it has been implicated in DNA end processing events in vivo. Spo11, the topoisomerase II-like enzyme that forms DSBs to initiate meiotic recombination, remains bound to the DSB termini in a group of hypomorphic RAD50 and MRE11 mutants, called S mutants (Alani et al. 1990; Usui et al. 1998). This suggests that the Spo11-DNA intermediate is cleaved by the Mre11 complex in wild-type cells.
In addition, rad50S and nuclease-deficient mre11 mutants are inviable in the absence of Rad27, a nuclease required for the completion of lagging strand DNA synthesis, raising the possibility that the Mre11 complex participates in the nucleolytic processing of DNA replication intermediates (Harrington and Lieber 1994; Moreau et al. 1999; Debrauwere et al. 2001). It appears that Mre11 complex nuclease activities are not critical to its DSB repair functions because rad50S, mre11S, and nuclease-deficient mre11 mutants exhibit only minor sensitivity to clastogens, and resection of DSBs generated by the HO endonuclease is normal (Alani et al. 1990; Nairz and Klein 1997; Bressan et al. 1998, 1999; Tsubouchi and Ogawa 1998; Usui et al. 1998; Moreau et al. 1999; Lee et al. 2002).
As suggested by the functional interaction with Rad27, the Mre11 complex appears to be particularly important during DNA replication (Petrini 2000b). γ-irradiation of synchronous cultures revealed that the extreme clastogen sensitivity of S. cerevisiae mre11Δ mutants is largely attributable to the inability of those cells to utilize sister chromatids in DNA repair, and that this effect is most pronounced in S phase (Ivanov et al. 1992; Bressan et al. 1999). Further, SbcCD, the bacterial Mre11 complex ortholog, appears to be required for processing aberrant DNA structures that arise at replication forks (Leach 1994; Connelly et al. 1998). In bacteriophage T4, recombination-dependent DNA replication and restart of stalled replication forks requires gp46/47, the T4 orthologs of Mre11/Rad50 (George et al. 2001; Stohr and Kreuzer 2001).
Several lines of evidence support a similar role for the Mre11 complex in vertebrates. First, Mre11-depleted DT40 chicken cells die with chromosomal damage suggestive of failure to resolve DSBs arising during DNA replication (Yamaguchi-Iwai et al. 1999). Second, DNA replication in Mre11-depleted Xenopus laevis extracts is associated with the accumulation of chromosome breaks (Costanzo et al. 2001). Finally, the human Mre11 complex becomes avidly associated with chromatin during S phase (Maser et al. 2001). These studies underscore the role of the Mre11 complex in DNA replication, and define a likely basis for the inviability of Mre11 complex-deficient vertebrate cells.
Mre11 complex functions at telomeres may also account for the inviability of Mre11 complex-deficient mammalian cells. The complex functions at telomeres in S. cerevisiae as well as in mammals, although the extent to which Mre11 complex telomere functions are conserved in yeast and mammalian cells is unknown. The human Mre11 complex is situated at telomeres via physical association with the telomere protection protein, TRF2 (Zhu et al. 2000). Given its association with TRF2, the Mre11 complex may function in telomere protection, and would be required for cellular viability via a role in preventing telomere failure (de Lange and Petrini 2000; Lombard and Guarente 2000; Zhu et al. 2000).
In this study, mice harboring a Rad50 allele analogous to the S. cerevisiae rad50S allele rad50-R20M (Alani et al. 1990) were derived. Rad50S/S mice were viable, but exhibited partial embryonic lethality, growth defects, and cancer predisposition. These pathologic outcomes were associated with progressive failure of Rad50S/S hematopoietic and germline cells which was partially mitigated by p53 deficiency. Primary and transformed cell cultures and tumor lines established from Rad50S/S mice were characterized by increased spontaneous apoptosis and chromosomal instability. We conclude that this effect of the Rad50K22M allele constitutes the mechanistic basis of hematopoietic and spermatogenic depletion in Rad50S/S mice. These findings underscore the importance of the Mre11 complex for maintenance of chromosome stability and in homeostasis of proliferative tissues.
Results
Derivation of Rad50S/S mice
Null Rad50 mutations were lethal at the cellular and organismal levels, precluding conventional gene inactivation as a means to analyze the Mre11 complex in vivo (Luo et al. 1999). Three hypomorphic Rad50 mutants, Rad50K6E, Rad50K22M, and Rad50R83I, corresponding to S. cerevisiae rad50S alleles were tested for their ability to support cell viability. mRad50Brdm1/mRad50Brdc1 embryonic stem (ES) cells harbor a null Rad50 allele (mRad50Brdm1) and a Rad50 allele that is inactivated upon expression of Cre recombinase (mRad50Brdc1). Because Rad50 is required for viability, Cre expression in mRad50Brdm1/mRad50Brdc1 cells is lethal unless a functional Rad50 cDNA is ectopically expressed (Luo et al. 1999). These cells were cotransfected with a Cre expression vector (pPGKhygro-Cre) and a wild-type or Rad50S cDNA expression construct. Wild-type and pRad50K22M, but not pRad50R83I or pRad50K6E, were sufficient for viability. On this basis, the Rad50K22M mutation, which corresponds to the rad50S allele rad50-R20M in yeast (Alani et al. 1990), was chosen for the derivation of mutant mice.
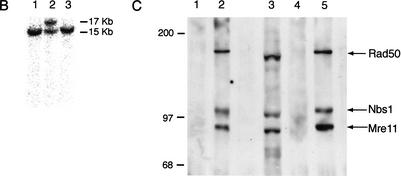
pkin-24ploxp contains exons 1, 2, and 3 of the Rad50 locus with an AAG to ATG change in exon 1, resulting in K→M at amino acid residue 22 (Fig. 1A; Dolganov et al. 1996). Homologous integration of pkin-24ploxp in ES cells was assessed by Southern blotting (Fig. 1B), and the ensuing targeted ES cells were used to derive mutant animals. Rad50S/S mice were susceptible to partial embryonic lethality at embryonic day 14–16 (E14–E16; P = 0.002), and were significantly underrepresented among 3-wk-old animals (P = 0.00001). These data indicated that the Rad50K22Mallele is hypomorphic.
Figure 1.
Derivation of Rad50K22M mutants. (A) Schematic of Rad50 locus. 5′ region and exons 1–3 (black boxes) are shown. Homologous integration of the targeting vector pk-in-24ploxp with the K22M mutation encoded in exon 1 (*) inserts a loxP-flanked (triangles) puromyocin-resistance gene (Puro) antisense and 5′ of Rad50 and creates the Rad50K22M allele. Cre-mediated deletion of Puro creates the Rad50K22Mcre allele. N, NcoI; 5P, 5′ probe. (B) Southern blot analysis of NcoI-digested DNA hybridized with the 5′ probe shown in A. The Rad50 and Rad50K22M alleles yield 15 and 17 Kb fragments, respectively. (Lane 2) Targeted ES cell clone. (Lanes 1,3) Untargeted clones. (C) Rad50+/+ (lanes 1,2) or Rad50S/S (lanes 4,5) immunoprecipitations performed with preimmune (lanes 1,4) or Nbs1 antisera (lanes 2,5). Whole-cell extract of Rad50S/S MEFs (lane 3) included as control. Samples were immunoblotted sequentially with Nbs1, Mre11, and Rad50 antisera. Size markers (KD) are shown.
Rad50S/S MEFs were not sensitive to ionizing radiation (IR), mitomycin C, hydroxyurea, or etoposide, nor did they exhibit growth defects. The formation of Mre11 IR-induced foci (IRIF) and the intra-S phase checkpoint were also unaffected (data not shown). In addition, wild-type levels of Mre11, Nbs1, and Rad50SK22M protein were present in extracts of Rad50S/S murine embryonic fibroblasts (MEFs), and their ability to assemble in complex was not overtly affected (Fig. 1C).
Although the Rad50K22M allele exerted a minimal cellular phenotype, it had a profound impact at the organismal level. Rad50S/S mice weighed 60% of wild-type littermates, and by 4–8 wk of age, most Rad50S/S animals had a gray, wasted appearance suggestive of anemia. The mean age of death of Rad50S/S mice was 2.6 ± 1.8 mo (N = 166). Whereas most Rad50S/S mice died tumor-free by 4 mo of age, 20% survived 4–7 mo. In this group of long-lived Rad50S/S mice, 20% died with metastatic thymic lymphomas, four exhibited splenic hyperplasia, and one mouse developed myeloid leukemia.
Meiotic progression in Rad50S/S mice
In S. cerevisiae rad50S mutants, meiotic DSBs are not processed due to the covalent attachment of Spo11 (Alani et al. 1990; Keeney and Kleckner 1995; Keeney et al. 1997). Because the function of Spo11 appears to be conserved in mice (Baudat et al. 2000; Romanienko and Camerini-Otero 2000), we analyzed Rad50S/S ovaries and testes to determine whether murine Rad50K22M led to an analogous block in meiotic progression.
Rad50S/S females were fertile and had normal ovaries (not shown). Testicular development was normal in prepubescent Rad50S/S males. Despite decreased cellularity in the seminiferous tubules of 4-wk-old Rad50S/S mice (Fig. 2A), older mice were also fertile.
Figure 2.
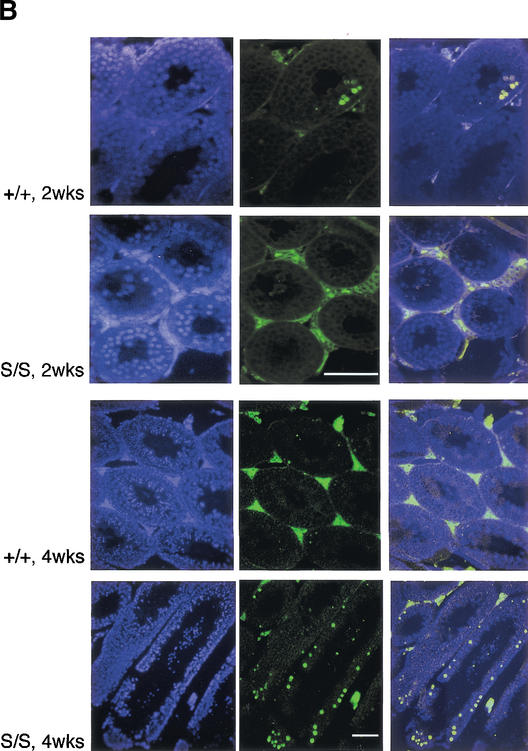
Progressive degeneration in Rad50S/S testes is not associated with meiotic recombination defects. (A) Hematoxylin and eosin (H&E) staining of testes sections from 2- and 4-wk-old Rad50+/+ and Rad50S/S mice. Bar, 100 μm; magnification, 400×. (B) TUNEL-staining of apoptotic cells (FITC; green; middle) and nuclear counterstaining with DAPI (blue; left) in testes sections. Two-channel overlay of TUNEL-FITC and DAPI (right). Two-week-old sections, magnification 400×. Four-week-old sections, magnification 100×. Bars, 100 μm. (C) Surface-spread spermatocyte nuclei were stained with Cor1 antiserum, which recognizes Scp3 protein (red). Images of Rad50+/+ and Rad50S/S spermatocyte nuclei in pachytene demonstrate normal chromosome pairing. Chromosomes from a second meiotic cell are seen in the lower right corner of the Rad50S/S image. Bar, 10 μm; magnification, 1000×. Graph, meiotic progression in ≥200 Rad50+/+ (white bars) and Rad50S/S (black bars) nuclei determined by Cor1 staining patterns and chromosomal structure.
Meiotic recombination defects in Spo11−/− mice result in depletion of germ cell precursors (Baudat et al. 2000; Romanienko and Camerini-Otero 2000). We reasoned that the cellular attrition in Rad50S/S testes might be the result of a similar, though less severe meiotic defect. TUNEL analysis of 4-wk-old Rad50S/S testes, unlike those from 2-wk-old Rad50S/S mice, revealed a 3–5-fold increase in the number of apoptotic tubules (Fig. 2B). However, cytologic analysis of meiotic progression did not indicate a stage-specific arrest, nor were aberrant chromosomal structures noted (Fig. 2C). These data suggest that the Rad50K22M allele did not impair meiotic recombination, and that cell death in Rad50S/S testes is attributable to a different source of genotoxic stress.
Hematopoietic stem cell failure in Rad50S/S mice
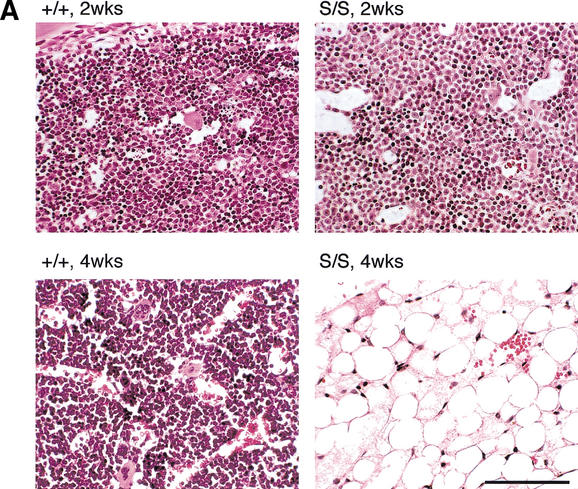
The premature death of Rad50S/S mice was associated with severe anemia. Two-wk-old Rad50S/S mice did not exhibit differences in the abundance of hematopoietic cells. However, by 4–8 wk of age, the bone marrow of most Rad50S/S mice was virtually devoid of hematopoietic cells and was largely composed of adipocytes (Fig. 3A). Spleen and thymus architecture was also grossly abnormal, with few lymphocytes present (data not shown). Long-lived Rad50S/S mice that did not develop thymic lymphomas died with bone marrow attrition identical to that seen in 4–8-wk-old mutants.
Figure 3.
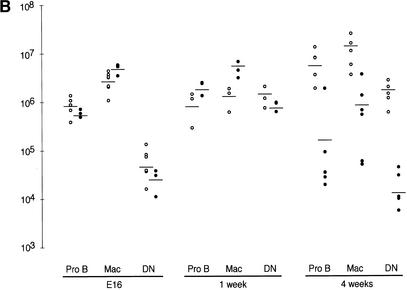
Rad50S/S progressive hematopoietic depletion is intrinsic to stem cells. (A) H&E staining of bone marrow sections from Rad50+/+ and Rad50S/S. Bar, 100 μm; magnification, 400×. (B) FACS analysis of hematopoietic tissues from E16, 1-wk-old, and 4-wk-old mice. The number of Pro B cells, macrophages (Mac), and double negative T cells (DN) in Rad50+/+ (○) and Rad50S/S (●) mice is shown. Each symbol represents data for one mouse, and lines show the mean within each group. (C) FACS analysis of fetal liver cell transfer recipients 4–6 wk posttransfer. As in B; Rad50+/+ recipients of Rad50+/+ fetal liver cells (○), Rad50S/S recipients of Rad50+/+ cells (□), Rad50+/+ recipients of a 1:1 mixture of Rad50+/+ and Rad50S/S cells (▵), and Rad50+/+ recipients of Rad50S/S cells (●). Control Rad50+/+ mice that did not receive transfers died within 2 wk after irradiation.
Fluorescence-activated cell sorting (FACS) analysis was used to correlate abundance of hematopoietic components with age. Double negative (CD4−,CD8−, DN) T cells, pro B cells (B220+,CD43+), and macrophages (B220−,CD43+) were present at wild-type levels in E16 Rad50S/S embryos (Fig. 3B). By 1 wk of age, pre B (B220+,CD43−) and immature B (B220+,IgM+) cells in Rad50S/S mice were 1.5–3-fold reduced, whereas pro B cells and macrophages were 2–3-fold increased. Thymocytes were unaffected at this age, but splenic B and T cells were decreased to 4.8%–12% and 7%–49% of wild-type, respectively (Fig. 3B; data not shown). By 4 wk of age, lymphocytes, macrophages, red blood cells and platelets were severely depleted (0.1%–18% wild-type, Fig. 3B, data not shown). These results demonstrated that progressive hematopoietic failure in Rad50S/S mice affected all hematopoietic lineages.
Cell transfer experiments were performed to determine whether Rad50S/S pathology was intrinsic to hematopoietic cells. Irradiated Rad50S/S mice were injected with wild-type fetal liver cells to assess the ability of Rad50S/S bone marrow stroma to support hematopoietic differentiation. Reconstitution of hematopoietic cells in Rad50S/S recipients of wild-type fetal liver was comparable to that in control recipients (Fig. 3C). PCR-based genotyping confirmed that wild-type cells were responsible for reconstitution of Rad50S/S mice (data not shown). In contrast, wild-type recipients of Rad50S/S fetal liver had 10–1000-fold fewer hematopoietic cells than controls at 6 wk after transfer (Fig. 3C). These data suggested that Rad50S/S bone marrow failure was attributable to hematopoietic failure independent of stromal defects.
To confirm that interpretation, we mixed wild-type and Rad50S/S fetal liver cells in a 1:1 ratio and transferred them into wild-type recipients. In this setting, Rad50S/S cells would be supported by the wild-type hematopoietic milieu during differentiation. Full reconstitution was evident 4 wk after transfer, and genotypic analysis showed that wild-type, but not Rad50S/S cells, were present in hematopoietic tissues of reconstituted animals (Fig. 3C; data not shown). Thus, the presence of wild-type hematopoietic cells and stroma did not increase the ability of Rad50S/S stem cells to reconstitute hematopoietic compartments. These experiments indicated that progressive bone marrow depletion in Rad50S/S mice was primarily attributable to hematopoietic stem cell failure.
Normal V(D)J recombination in Rad50S/S mice
DNA hairpins are intermediates in the site-specific recombination process required for antigen receptor gene assembly, V(D)J recombination (Roth et al. 1992; McBlane et al. 1995). Recent in vitro experiments suggest that the artemis/DNAPK complex may open V(D)J hairpins (Ma et al. 2002). However, in light of in vitro and in vivo data, the possibility remains that the Mre11 complex may also influence this process (Paull and Gellert 1998, 1999; Lobachev et al. 2002). We used ligation-mediated PCR to assess whether hairpin opening was defective in Rad50S/S mice (Zhu and Roth 1995). TCR Dδ2 hairpins were readily detected in thymus DNA from 1-wk-old scid mice; however none were detected in Rad50S/S samples (Fig. 4).
Figure 4.
Normal hairpin resolution in Rad50S/S thymocytes. Hairpin coding ends (CE), oligonucleotides were ligated to the D2/J1 blunt signal ends (SE) and the open CEs. Ends were PCR amplified using Dδ2– and oligospecific primers (arrows), and products were detected using the probe shown (line with a circle). Expected sizes of PCR products are indicated. Blot, LMPCR of 1 μg of MBN-treated thymic DNA from scid and Rad50S/S mice. M, radiolabeled size marker; sizes (bp) shown on left.
Quantitative PCR showed that in vivo V to DJ and D to J rearrangement frequencies, as well as the structure of the joints formed, were normal in E16 and in 4-wk-old Rad50S/S mice (data not shown). Transient V(D)J recombination assays indicated that coding and signal joints were formed at comparable frequencies in wild-type and Rad50S/S MEFs (recombination frequency for signal joints: 0.12% in wild-type, 0.29% in Rad50S/S; coding joints: 0.5% in wild-type, 1.2% in Rad50S/S), and like Rad50S/S in vivo joints, did not exhibit abnormal structures (data not shown). These data indicate that V(D)J recombination was normal in Rad50S/S mice, and suggest that hairpin opening during V(D)J recombination is not impaired by the Rad50K22M mutation.
p53 deficiency mitigates the Rad50S/S phenotype
The correlation of age with malignancy, together with the observation that testes and bone marrow showed age-dependent cellular attrition, suggested that the Rad50K22M mutation caused chronic genotoxic stress. Because p53 profoundly influences genotoxic stress responses (Prives and Hall 1999), we hypothesized that p53 deficiency would mitigate the Rad50S/S phenotype. Conversely, because potentially oncogenic genetic alterations would accumulate without inducing apoptosis in p53-deficient Rad50S/S mice, we expected Rad50S to reduce the tumor latency of p53−/− animals.
Indeed, reduced p53 dosage partially rescued Rad50S-dependent depletion in the hematopoietic compartment, and the Rad50K22M mutation accelerated p53+/− and p53−/− tumorigenesis (Fig. 5A,B). B cell numbers were increased 3–10-fold and 5–20-fold above Rad50S/S levels in p53+/− Rad50S/S and p53−/− Rad50S/S mice, respectively. Macrophages were threefold increased and T cells were 3–20-fold increased in both p53+/− Rad50S/S and p53−/− Rad50S/S mice (cf. Figs. 3B and 5A). The partial rescue of hematopoietic cell survival in p53+/− Rad50S/S and p53−/− Rad50S/S mice was associated with increased lifespan (Fig. 5B). Rad50K22M significantly altered p53+/− and p53−/− tumor susceptibility. p53+/− control mice in our colony developed osteosarcomas after 12 mo of age (N = 34), whereas p53+/− Rad50S/S mice died from thymic lymphomas at an average of 5 mo (N = 17; P = 0.002). p53−/− Rad50S/S animals (N = 7, average age of death 3.3 mo; P = 0.05) died earlier than p53−/− mice (N = 17, average age of death 4.5 mo) without changes in p53−/− tumor spectrum (Jacks et al. 1994). In contrast to Rad50S/S animals, the double mutants succumbed to lymphoma and did not show overt signs of anemia.
Figure 5.
p53 deficiency partially rescues the Rad50S/S phenotype. (A) FACS analysis of 4-wk-old 129/SvEv Rad50+/+ (○), p53+/− Rad50S/S (▴), and p53−/−Rad50S/S (▪) mice; depicted as in Fig. 3B. (B) Mouse survival. Each data point represents the fraction of surviving mice at a given age in months. N, total number of mice for each genotype. Only autopsied mice are included. The average age of death in months (mo) is shown for each genotype with the P-value compared to the relevant control determined by the Wilcoxon rank sum test. (C) Results from TUNEL staining of testes sections from 4-mo-old mice. The % apoptotic tubules is the average fraction of tubules with ≥4 apoptotic cells in serial sections from two mice of each genotype.
p53 deficiency also suppressed the attrition of cells in the seminiferous tubules, correlated with abrogation of testicular apoptosis in both p53+/− Rad50S/S and p53−/− Rad50S/S mice (Fig. 5C). The suppression of testicular apoptosis raised the possibility that defects in meiotic recombination, obscured by apoptotic attrition in p53-proficient cells, would be evident in double mutants. However, p53+/− Rad50S/S and p53−/− Rad50S/S males did not exhibit an arrest in meiotic progression, nor were aberrant chromosomal structures detected (data not shown). These data indicate that apoptosis in Rad50S/S testes was not associated with meiotic recombination defects.
Chronic genotoxic stress in cells from Rad50S/S mice
The rescue of Rad50S/S hematopoietic and testicular attrition and the reduced tumor latency in p53+/− Rad50S/S and p53−/− Rad50S/S mice supported the interpretation that chronic genotoxic stress was the underlying cause of the Rad50S/S phenotype. Indices of genotoxic stress were assessed in primary cell cultures. First, the apoptotic indices of wild-type and Rad50S/S MEFs were analyzed by FACS. Compared to wild-type controls, unirradiated Rad50S/S MEFs exhibited a 1.4–3.4-fold increase in the fraction of cells with sub-G1 DNA content. A similar increase in annexinV staining was also found in unirradiated Rad50S/S MEFs. The increase in apoptotic cells after irradiation (IR) was similar in Rad50S/S and control cells, indicating that the apoptotic response to IR was not compromised (Table 1).
Table 1.
Genotoxic stress in Rad50S/S cells
| Sample
|
Genotype
|
Cytology
|
FACS
|
||||
|---|---|---|---|---|---|---|---|
| % γ-H2AX+ O Gy
|
% γ-H2AX+ 0 Gy
|
% γ-H2AX+ 12 Gy
|
% sub G1 0 Gy
|
% Annexin+ 0 Gy
|
% Annexin+ 12 Gy
|
||
| 1960-3,5 | Rad50+/+ | 23, 25 | 0.58 | 0.73 | 1.00 | 0.5 | 1.7 |
| 1960-8 | Rad50S/S | 33, 43 | 1.27 | 3.14 | 1.40 | 0.9 | 2.5 |
| folda | +1.6 | +2.2 | +4.3 | +1.4 | +1.7 | +1.4 | |
| 193-11 | Rad50+/+ | 39, 34 | 0.45 | 0.79 | 1.34 | ND | ND |
| 9033-3,8 | Rad50S/S | 47, 51 | 0.67 | 1.19 | 2.54 | ND | ND |
| fold | +1.5 | +1.5 | +1.5 | +1.9 | |||
| 12317-5,6 | Rad50+/+ | ND | 1.1 | ND | 2.5 | 6 | 8 |
| 14205-6,7 | Rad50S/S | ND | 1.9 | ND | 8.5 | 12 | 14 |
| fold | +1.7 | +3.4 | +2.0 | +1.8 | |||
For cytology, passage 2 (p2) MEFs were grown on cover slips, extracted, fixed, and stained with antisera to γ-H2AX. Data are from two independent cultures from which ≥150 cells were analyzed. % γ-H2AX+ represents the fraction of cells with ≥3 nuclear γ-H2AX foci or complete nuclear staining.
For FACS, mock-treated or irradiated p2 MEFs were fixed after 1 h and stained with antisera to γ-H2AX or propidium iodide (PI) and unfixed cells were stained 24 h later for annexin V. Data are the average of three independent cultures from which 10,000 cells each were analyzed. % γ-H2AX+ shown is the % of cells with the most γ-H2AX+ staining determined by fluorescence intensity. % sub G1 represents cells with less than 2N DNA content determined by PI staining.
The fold increase in unirradiated Rad50S/Scells vs. Rad50+/+ cells (fold) was significant (p < 0.05) for each experiment as determined by Fisher's exact test. ND, not determined.
Second, the presence of phosphorylated histone H2AX (γ-H2AX), which is correlated with the presence of DNA damage (Rogakou et al. 1999; Paull et al. 2000; Ward and Chen 2001), was examined in MEFs by immunofluorescence and flow cytometry with γ-H2AX antiserum. The percentage of γ-H2AX-positive cells was increased in unirradiated Rad50S/S cells to levels comparable to that in wild-type irradiated cells (Table 1).
Finally, since the mammalian Mre11 complex localizes to sites of DNA replication (Maser et al. 2001), sites of DNA damage (Nelms et al. 1998; Mirzoeva and Petrini 2001), and to human telomeres (Zhu et al. 2000), we reasoned that attenuation of Mre11 complex function at any one of these venues could induce chromosome aberrations in Rad50S/S cells. To address this possibility, spectral karyotype (SKY) and conventional karyotypic analyses were performed. Increased levels of chromosome breaks were evident in ear fibroblast cultures established from Rad50S/S and p53−/− Rad50S/S mice (Table 2; Fig. 6A). Increases in chromosome breaks and rearrangements were also detected in SV40-transformed Rad50S/S ear fibroblasts by conventional analysis of Giemsa-stained metaphase cells (Table 2). These data were consistent with the interpretation that the Rad50K22M mutation exerted an impact on chromosome stability.
Table 2.
Karyotypic analysis of ear fibroblast cultures
| Samplea
|
Genotype
|
Chr
|
+/−
|
|
Breaks
|
Rearrangements
|
N
|
|---|---|---|---|---|---|---|---|
| 12221 p3 | +/+ | 40 | 0/0 | 0 | 8 | ||
| 41 | 1/0 |
|
0
|
2
|
|||
| total | 0 | 0 | 10 | ||||
| 12053 p3 | Rad50S/S | 40 | 0/0 | 0 | 9 | ||
| 38 | 0/1 |
|
1
|
1
|
|||
| total | 12 | 1 | 10 | ||||
| 11195 p3 | p53−/− | 40 | 0/0 | 0 | 10 | ||
| 40 | 1/1 | 0 | 1 | ||||
| total | 0 | 0 | 11 | ||||
| 11195p10 | p53−/− | 47-77 | 1/19 | 0 | 8 | ||
| 47-77 | 1/19 | 1 | 1 | ||||
| 47-77 | 1/19 | 2 | 1 | ||||
| total | 0 | 3 | 10 | ||||
| 6415p10 | p53−/− Rad50S/S | 80 | 0/0 | 0 | 1 | ||
| 35-82 | 1-4/1-9 | 0 | 10 | ||||
| 35 | 1/6 | 1 | 1 | ||||
| 107 | 28/0 | 0 | 1 | ||||
| total | 2 | 1 | 13 |
| Sampleb
|
Genotype
|
Chr
|
+/−
|
Breaks
|
Rearrangementsc
|
N
|
|---|---|---|---|---|---|---|
| 11683 | SV40 +/+ | ND | ND | 7 | 5 | 115 |
| 11685 | SV40 S/S | ND | ND | 17 | 22 | 124 |
Spectral karyotyping was performed on metaphase cells from primary ear fibroblast cultures at passage numbers indicated (p). Breaks were nonclonal and are shown as a total from all metaphases of each sample.
Chromosome aberrations were scored on Giemsa-stained metaphase spreads from nonclonal SV40-transformed ear fibroblast populations.
Rearrangements reflect the sum of exchanges and chromosome fusions.
Chr, chromosome number; +/−, number of chromosomes gained (+) or lost (−); N, number of cells examined; ND, not determined.
Figure 6.
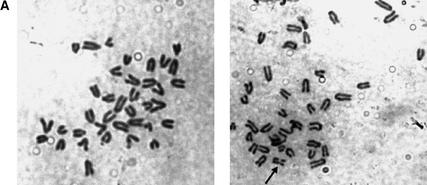
Chromosome instability in Rad50S/S cells. (A) Images of Giemsa-stained metaphase cells. (Left) a normal metaphase spread from a Rad50S/S ear fibroblast. (Right) Rad50S/S ear fibroblast metaphase with one broken chromosome (arrow). Magnification, 1000×. (B) SKY analysis of thymic lymphoma cells. Metaphase cells from p53−/−Rad50S/S (upper panel, tumor 5390) and Rad50S/S (lower panel, tumor 5587) tumors. (Left) Inverted image of the metaphase cells counterstained with DAPI. (Right) Spectral image of the metaphase cells. The structurally rearranged chromosomes are identified with arrows, and include short-arm fusions [der(13;14) and der(1;13)], an isodicentric fusion [idic(9)], and a small marker chromosome of unknown origin [mar] in the p53−/−Rad50S/S tumor, as well as dicentric and isodicentric rearrangements [dic(2;4) and idic(11)] in the Rad50S/S tumor. (C) (Right) Two-channel image of a representative metaphase spread from a p53−/−Rad50S/S lymphoma subjected to FISH using an FITC-conjugated telomeric probe (green) and counterstained with DAPI (blue). (Left) Inverted DAPI image of the metaphase cell. Bar, 10 μm; magnification, 1000×.
Because the events that lead to depletion of hematopoietic cells also predispose malignancy, the mechanistic basis of Rad50S/S pathology should be evident in tumors from these mice. Rad50S/S and p53−/− Rad50S/S thymic lymphoma cells exhibited increased chromosomal instability in SKY analysis (Table 3; Fig. 6B). Compared to p53−/− tumors, most Rad50S/S and p53−/− Rad50S/S tumors had more chromosome rearrangements and some exhibited an increased frequency of nonclonal karyotypes, suggesting that events leading to instability were ongoing (Table 3).
Table 3.
Spectral karyotype analysis of thymic lymphomas
| Sample
|
Genotype
|
Chr
|
+/−
|
|
Rearrangements
|
N
|
|---|---|---|---|---|---|---|
| AN1 | p53−/− | 61 | 21/0 | 0 | 9 | |
| 46 | 6/0 | 0 | 1 | |||
| 49 | 9/0 | 0
|
1
|
|||
| total | 0 | 11 | ||||
| 6141 | p53−/− Rad50+/S | 76 | 5/9 | total | 1 | 8 |
| 5492 | Rad50S/S | 42 | 3/1 | 0 | 7 | |
| 42 | 4/2 | 0 | 2 | |||
| 44 | 5/1 | 1 | 1 | |||
| 37 | 3/6 | 2 | 1 | |||
| 42 | 5/3 | 0 | 1 | |||
| 49 | 12/3 | 0 | 1 | |||
| 43 | 4/1 | 0 | 1 | |||
| total | 3 | 14 | ||||
| 5587 | Rad50S/S | 78 | 6/6 | 2 | 9 | |
| 40 | 0/0 | 0 | 1 | |||
| total | 18 | 10 | ||||
| 6175 | Rad50S/S | 41 | 1/0 | 0 | 3 | |
| 80 | 0/0 | 0 | 4 | |||
| 40 | 0/0 | 0 | 4 | |||
| total | 0 | 11 | ||||
| 5390 | p53−/− | 41 | 2/0 | 2 | 2 | |
| Rad50S/S | 39–40 | 1/1 | 3 | 3 | ||
| 40–43 | 3/1 | 2 | 3 | |||
| 42 | 3/0 | 1 | 1 | |||
| 42 | 5/1 | 2 | 1 | |||
| total | 22 | 10 |
The number of chromosome aberrations in tumor cultures determined by spectral karyotyping is shown. Chr, chromosome number; +/−, number of chromosomes gained (+) or lost (−); N, number of cells examined.
Giemsa-stained chromosomes from tumors revealed similar karyotypic instability. Notable was a significant increase in short-arm chromosome fusions (Table 4). Fluorescent in situ hybridization (FISH) with a CCCTAA probe was performed to determine whether fusions in Rad50S/S cells resulted from joining of telomeric or interstitial DNA. The majority of short-arm fusions in Rad50S/S and p53−/− Rad50S/S tumors contained two intense telomeric signals at the join, indicating that telomeric DNA was retained in these fusions (Fig. 6C). Fusions containing telomeric DNA were detected by FISH in all Rad50S/S and p53−/− Rad50S/S tumors, but not in p53−/− tumors (Table 4). These data raise the possibility that telomere failure was among the chromosomal phenotypes associated with the Rad50K22M mutation, and collectively support the interpretation that Rad50S/S cells are subject to chronic genotoxic stress.
Table 4.
Chromosome aberrations in thymic lymphomasa
| Tumor
|
Genotype
|
Fragments
|
Breaks
|
Exchangesb
|
End-to-endc
|
Short armd
|
Ne
|
Telomeric fusionf
|
|---|---|---|---|---|---|---|---|---|
| 8200 | p53−/− | 1.0 | 0 | 0.3 | 0 | 0.1 | 50 (80) | ND |
| AN1 | p53−/− | 0.1 | 0.1 | 0.4 | 0.1 | 0.2 | 51 (43) | ND |
| 6141 | p53−/− Rad50+/S | 3.5 | 0.2 | 0.5 | 0 | 0.2 | 54 (100) | 0 |
| 5492 | Rad50S/S | 0.4 | 0.1 | 0.2 | 0.1 | 0.1 | 60 (43) | 0.2 |
| 5587 | Rad50S/S | 0.1 | 0.1 | 0.3 | 0 | 0.3 | 60 (38) | 0.1 |
| 6175 | Rad50S/S | 0.6 | 0.3 | 0.4 | 0.1 | 0 | 50 (62) | 0.1 |
| 5390 | p53−/− Rad50S/S | 0.2 | 0 | 0.2 | 0.1 | 3.1g | 54 (100) | 2.1g |
| 6256 | p53−/− Rad50S/S | 0.3 | 0 | 0.2 | 0.2 | 1.1g | 48 (79) | 0.7g |
| 6531 | p53−/− Rad50S/S | 0.2 | 0.1 | 0.1 | 0 | 0.4g | 50 (40) | 0.2g |
Determined by phase contrast analysis.
Long-arm chromatid fusions.
Long-arm chromosome fusions.
Short-arm chromosome fusions.
Number of metaphase cells examined (% with at least one aberration).
Fusions (including exchanges, end-to-end, and short-arm fusions) per metaphase with telomeric DNA retained; determined in a separate experiment in which FISH was performed with a telomeric repeat probe. ND, not done.
p < 0.05 when compared to p53−/− tumors using the Wilcoxon rank sum test.
Discussion
The Mre11 complex is integral to a diverse spectrum of functions that bear upon chromosome stability. Genetic analyses in S. cerevisiae establish that the complex's influence can be generally attributed to either structural roles, which primarily influence the DNA recombination functions of the complex, or enzymatic (nucleolytic) roles, which appear to influence DSB end processing or degradation of certain DNA structures. The complex's structural role has been invoked to account for the inability of null Mre11 complex mutants to utilize sister chromatids during recombinational DNA repair, whereas nuclease-deficient mutants do not exhibit this property (Ivanov et al. 1992; Moore and Haber 1996; Bressan et al. 1999). A molecular basis for such a role is suggested by electron microscopic studies of the human Mre11 complex and structural analysis of the Pyrococcus furiosis Mre11 complex. Those data indicate that the complex may bridge DNA ends or sister chromatids via the coiled coil regions of Rad50 (de Jager et al. 2001; Hopfner et al. 2002). The in vivo role of the complex's nuclease functions have been inferred from the S. cerevisiae S alleles, in which the initial processing of meiotic DSBs is impaired, and from the synthetic lethality of nuclease-deficient mutants with Rad27 deficiency (Symington 1998; Debrauwere et al. 2001).
We determined previously that a null Rad50 mouse mutant, in which the structural role of the Mre11 complex is presumably abrogated, is inviable (Luo et al. 1999). Therefore, we established a Rad50S/S mouse mutant in which the Mre11 complex is intact, but functionally hypomorphic. In contrast to Rad50-deficient mice, Rad50S/S mice exhibit only partial embryonic lethality. Rad50S/S mice have shortened lifespan associated with progressive loss of cells in the male germ line and in hematopoietic lineages, and die with hematopoietic failure by 3 mo of age. Sporadically appearing longer-lived Rad50S/S animals are highly predisposed to malignancy. Underlying these phenotypic outcomes is the accumulation of chromosome aberrations. In p53-proficient mice, these events precipitate the cellular attrition observed, whereas in the context of p53 deficiency, abrogation of apoptosis diminishes cellular attrition but dramatically enhances tumorigenesis.
Progressive attrition of hematopoietic and spermatogenic lineages in Rad50S/S mice
Rad50S/S pathology is largely restricted to hematopoietic and germ cell lineages. Fetal liver cell transfer experiments demonstrated that the progressive hematopoietic failure observed is correlated with depletion of stem cells. An analogous progenitor depletion may underlie the progressive attrition of cells in the seminiferous tubules, because meiotic progression is not impaired by Rad50S. In this regard, the Rad50S/S mice are similar to late-generation telomerase-deficient (mTR−/−) mice, which experience telomere failure as a sequela to telomere shortening, and exhibit relatively selective depletion of hematopoietic and spermatogenic cells (Lee et al. 1998; Herrera et al. 1999).
Chronic genotoxic stress associated with the Rad50K22M allele would differentially affect hematopoietic and spermatogenic tissues for two reasons. First, in contrast to other proliferative tissues such as intestine or skin, they arise from extensive proliferation of a relatively small number of stem cells and committed precursors (Weissman 2000). Therefore, for each stem or precursor cell depleted, a relatively large proportion of the tissue—arising from geometric expansion from the depleted stem cell—would be lost. Second, decreased cellularity, as a primary outcome of stem cell depletion and the ensuing reduction in differentiated cells, imposes increased proliferative demand on the residual population. Whereas mutations that result in gross chromosomal instability would have broad pathological outcomes, less severe mutations would more specifically affect tissues that rely upon limited precursor populations.
The selective effect of Rad50K22M on hematopoietic and spermatogenic tissue is thus consistent with the observation that the magnitude of chromosomal instability in Rad50S/S mice was relatively low. The chromosomal aberrations seen in Rad50S/S and p53−/− Rad50S/S lymphomas were not seen in p53−/− tumors, and were largely clonal, indicating that these chromosomal aberrations are unique to the Rad50K22M allele, and further, that they accumulate at a relatively low rate. Were Rad50S/S mice longer-lived, a broader spectrum of pathology would likely emerge. By analogy, whereas hematopoietic and spermatogenic depletion are seen first in late-generation mTR−/− mice, an aged cohort of these mice exhibited much broader pathology, including skin and intestine defects (Herrera et al. 1999; Rudolph et al. 1999).
Murine Rad50S alleles
The inability of Rad50K6E and Rad50R83I to support cell viability was not predicted from the behavior of the corresponding S. cerevisiae mutants. Given the conservation of Mre11 and Rad50, it is unlikely that the enzymatic functions impaired by the murine S mutations differ from those in S. cerevisiae. Assuming that the three S mutations examined impair the same functions, the inviability of Rad50K6E and Rad50R83I could simply reflect a more severe form of the Rad50K22M phenotype, in which cell death ensues from greater increase in the levels of chromosome instability. It is also conceivable that increased severity could result from defects in processing a more diverse spectrum of substrates in Rad50K6E- and Rad50R83I-expressing cells.
The lack of meiotic recombination and V(D)J recombination defects in Rad50K22M mutants, which was also unexpected, could be explained by the same argument. S mutations that exert a profound effect on meiotic progression would be inviable. Although a meiotic arrest was not clearly evident in the Rad50K22M mice, a subtle defect might not have been detected by our analysis.
The Mre11 complex: Diverse mechanisms in maintaining genomic integrity
Mutations in MRE11 and NBS1 are associated with cancer predisposition, chromosome instability, and cell cycle checkpoint defects in A-TLD and NBS (Carney et al. 1998; Stewart et al. 1999). Despite their severe clinical and cellular phenotypes, those mutations do not confer overt recombinational DNA repair defects, as was true in Rad50S/S mice. In contrast, null Mre11 complex mutations, which cause profound DNA recombination defects in S. cerevisiae, are lethal in vertebrates. These outcomes support the possibility that essential and nonessential Mre11 complex functions are mechanistically distinct. The P. furiosis Mre11/Rad50 complex suggests a molecular basis for distinct mechanisms. This complex contains a bifunctional DNA binding domain in which the active site of the Mre11 nuclease is capable of binding ssDNA, whereas an adjacent site accommodates double-stranded DNA. Occupancy of the sites is mutually exclusive, suggesting that a given interaction with DNA facilitates enzymatic (in the ssDNA binding domain) or structural (in the dsDNA binding domain) functions (Hopfner et al. 2001). The data presented here argue that the essential functions of the complex cannot be clearly divided according to its enzymatic or structural roles.
Finally, the data clearly indicate that the Mre11 complex exerts a profound influence on homeostasis in mammalian tissues even when its checkpoint and DNA recombination functions are not overtly impaired. The Rad50S/S mouse therefore establishes that subtle hypomorphism in a DNA damage response pathway is sufficient to induce severe pathology. This observation has important implications for understanding the mechanistic basis of disease related to chromosomal metabolism.
Materials and methods
Analysis of Rad50S alleles
Missense amplification of Rad50 cDNA from pmRad50 (Luo et al. 1999) using the primers mR50–24S (5′-GATAGAGGATAA AGATATGCAGATTATCTCTTTC-3′) and mR50–24A (5′-GA AAGAGATAATCTGCATATCTTTATCCTCTATC-3′), mR50–26S (5′-CGGGCCCAGATTATCTTGCAGTTTCGAGATG-3′) and mR50–26A (5′-CATCTCGAAACTGCAAGATAATCTGG GCCCG-3′), or mR50–27S (5′-TCCCGGATCGAAGAGATGA GCATTC-3′) and mR50–27A (5′-GAATGCTCATCTCTTC GATCCGGGA-3′) resulted in pRad50K22M, pRad50R83I, and pRad50K6E, respectively. pPGKhygro-Cre was created by ligation of the pBS185 (GIBCO-BRL) HindIII Cre fragment into pPGKhyg (Mortensen et al. 1991). Hygromycin- and gancyclovir-resistant clones from mRad50Brdm1/ mRad50Brdc1 ES cells coelectroporated with 30 μg of KpnI-linearized pPGKhygro-Cre and 200 μg of an AflII-linearized Rad50 cDNA were analyzed by Southern blotting as described (Luo et al. 1999).
Derivation of mutant mice
Details of the pkin-24ploxp targeting vector construction will be furnished upon request. Following transfection of Ab2.2 ES cells and selection, targeted clones were identified by Southern blot. Blastocyst injections and derivation of chimeric mice were done by standard methods. Chimeras were mated with C57Bl6 mice (Harlan Sprague Dawley) or 129/SvEv mice (Taconic) for 129/SvEv. Rad50S/S mice in mixed and pure 129/SvEv backgrounds had the same phenotype. Tgn CMV-Cre transgenic mice (Nagy et al. 1998) were mated with Rad50+/K22M mice to derive Rad50+K22Mcre/K22Mcre mice. p53-deficient mice (Jacks et al. 1994) and double mutants were maintained on a 129/SvEv background. Details of genotyping Rad50S/S mice are available upon request.
Cell derivation and culture
E14.5 MEFs were maintained in DMEM/10% fetal bovine serum (FBS)/L-glutamine (GIBCO) with routine 1:3 passages. Ear fibroblasts obtained from the dorsal portion of one ear were minced, rinsed twice with PBS containing kanamycin (100 μg/mL), incubated at 37°C for 45 min in collagenase D/dispase neural protease from Bacillus polymyxa grade II (4 mg/mL each in DMEM, Boehringer Mannheim), and resuspended in DMEM/10% FBS with 5× PenStrep overnight. Filtered cells were plated and reached confluence before continued maintenance with 1:2 passages. Fugene 6 (Roche) was used to transfect cells with a variant of the SV40 DNA pX-8 (Fromm and Berg 1982) containing an XhoI linker in the origin palindrome to derive transformed cells. Thymic lymphoma single cell suspensions were cultured in 90% RPMI 1640 (GIBCO)/10% FBS/1 ng/mL recombinant mouse interleukin-2 and interleukin-7 (Sigma)/PenStrep.
Protein analysis
Immunologic methods were as described (Dolganov et al. 1996) with Nbs1 (93–3; Williams et al. 2002).
DNA damage sensitivity assays
Sensivity was determined by colony formation essentially as described (Williams et al. 2002). Five thousand cells were treated for 2–3 h with etoposide (0–10 μg/mL), mitomycin C (0–3 μg/mL), or hydroxyurea (0–50 mM). The surviving fraction was determined after 7 d by colony forming ability. The radioresistant DNA synthesis assay was as described (Lim et al. 2000) except that MEFs were pulsed for 30 min with [3H]thymidine 1 h post-IR with 10 Gy. Ionizing radiation-induced foci assays were as described (Maser et al. 1997). H2AX staining for immunofluorescence was performed on passage 2 MEFs using γ-H2AX-specific antiserum (Upstate Biotechnology; 1:1000) following in situ fractionation (Mirzoeva and Petrini 2001). For FACS, passage 2 MEFs were prepared 1 h after 0 or 12 Gy IR using the Cytofix/Cytoperm Staining Kit according to the manufacturer's directions (Pharmingen) and stained with γ-H2AX-specific antiserum (Upstate Biotechnology; 1:400) and anti-rabbit-FITC (Jackson Labs; 1:100) as described (J. Karlseder, O.K. Mirzoeva, B.R. Williams, J.H.J. Petrini, and T. de Lange, in prep.). Sub-G1 DNA content was determined for passage 2 MEFs fixed 1 h after 0 or 12 Gy IR using the Cytofix/Cytoperm Staining Kit according to the manufacturer's directions (Pharmingen), and stained with γ-H2AX-specific antiserum (Upstate Biotechnology; 1:1400) and anti-rabbit-FITC (Jackson Labs; 1:100; J. Karlseder, O.K. Mirzoeva, B.R. Williams, J.H.J. Petrini, and T. de Lange, in prep.) AnnexinV-FITC staining on unfixed passage 2 MEFs 24 h after 0 or 12 Gy was performed according to the manufacturer's directions (Sigma). All FACS data were analyzed with CellQuest software.
Histological analyses
Ten percent buffered formalin-fixed tissues were processed at the University of Wisconsin Veterinary School Histopathology Lab or the McArdle Histology Lab. Paraffin-embedded 5–10-μm sections were stained with hematoxylin and eosin, and pathological diagnoses were determined by two independent investigators. Color images were captured using a Pixera digital camera and processed using Adobe Photoshop.
Terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) using TdT and dUTP-FITC TUNEL labeling was per the manufacturer's directions (Roche). Images were captured as described (Mirzoeva and Petrini 2001).
Meiotic spreads
Preparation, staining, and analyses of meiotic spreads from 1- to 4-mo-old testes using mouse Cor1 and human CREST antisera were as described (Dobson et al. 1994).
Hematopoietic cell preparations and analyses
Single-cell suspensions of lymphoid tissues were depleted of red blood cells by hypotonic lysis and maintained in PBS/3% FBS. Biotin- and fluorochrome-labeled antibodies specific for B220 (r-PE), CD43 (biotin), IgM (FITC), CD4 (r-PE), CD8 (FITC), and Streptavidin (FITC) were from Pharmingen. Dead cells were excluded by staining with 7-AAD (Molecular Probes) or propidium iodide. FACS analyses were performed on FACSCaliber instruments (Becton Dickinson).
Peripheral blood was collected in heparinized capillary tubes and transferred to EDTA-coated microtubes. Blood cell counts were done with an Advia 120 Hematology System (Bayer) at the University of Wisconsin Veterinary School Clinical Pathology Laboratory.
V(D)J recombination assays
Semiquantitative PCR and Southern blot analysis of immunoglobulin and TCRβ gene segment rearrangements were as described (Schlissel et al. 1991; Sikes et al. 1999). Thymus DNA from three 1-wk-old Rad50+/+, Rad50S/S, and scid mice was prepared and pooled for use in the ligation-mediated PCR assay as described (Zhu and Roth 1995). Transient recombination assays in transformed MEFs were as described (Errami et al. 1998) using pMS127 (Rag1) and pMS216E (Rag2) expression vectors. JH290 rearrangements were amplified and sequenced as described (Taccioli et al. 1993).
Cell transfers
Four-week-old recipient mice were irradiated with 9–12 Gy from a Mark I 137Cs source at 2.5 Gy/min, and retro-orbitally injected 2 h later with 5 million E16 fetal liver cells. FACS of hematopoietic tissues was done 4–6 wk posttransfer. DNA extracts (Schlissel et al. 1991) from reconstituting cells were genotyped by PCR.
Chromosome analyses
Metaphase cells prepared as described (van Steensel et al. 1998) were used for FISH as described (Lansdorp et al. 1996) using an FITC-conjugated peptide nucleic acid (CCCTAA)3 probe or stained 15 min in 5% Giemsa (Sigma) for phase contrast analyses. For SKY analysis, metaphase cells were prepared using standard cytogenetic techniques (Roulston and Le Beau 1997) with the following modifications. Following mitotic arrest (2 h in 0.05μg/mL Colcemid, Invitrogen Life Technologies), cells were incubated 8 min in 0.075M KCl (37°C), and 2 mL fixative (3:1 absolute methanol:glacial acetic acid) was added dropwise. Cells were mixed, centrifuged, and resuspended in fresh fixative; the fixative step was repeated 3–6×, and air-dried slides were prepared. Differences in abundance of chromosome aberrations between genotypes were not correlated to culture duration.
Spectral karyotype (SKY) analysis
The SKY analysis was performed using the Applied Spectral Imaging (ASI) SkyPaint kit for mouse chromosomes. Slides were aged at room temperature for 1–14 days, pretreated for 15 min in 2×SSC (pH 7.0, 37°C), and washed 2× for 5 min each in PBS and 1× for 5 min in PBS/0.05M MgCl2. Slides were then incubated 10 min in 1% formaldehyde in PBS/0.05 M MgCl2, washed 5 min in PBS, and dehydrated in a graded ethanol series. Chromosomal DNA was denatured in 70% formamide/2×SSC (pH 7.0, 72°C, 2 min), and dehydrated in a graded ethanol series. The SKY probe mixture was denatured for 7 min at 75°C, incubated 1–2 h at 37°C, and then 10 μL of probe was applied to each slide. Slides were covered, sealed, and incubated 48 h (37°C). Unhybridized probe was removed by washing 3× for, 5 min each in 50% formamide/2×SSC (pH 7.0, 45°C,) and 2× for 5 min each in 1×SSC (45°C). Probe detection using SkyPaint; reagents was per the manufacturer's recommendations. Images were acquired using an SD-200 Spectracube (ASI) mounted on a Zeiss Axioplan 2. Analysis was performed using ASI image capturing (SI 2.2) and analysis software (SkyView 1.6). At least 10 metaphase cells were analyzed per lymphoma. Mouse chromosomes were classified according to the standardized karyotype refined by Cowell (1984).
Acknowledgments
We thank Santos Franco, Michelle Yao, Sheila Bitts, and Elizabeth M. Davis for technical contributions; Jennifer Salna, Mark Hughes, and Eric Michaels for animal care and management; Titia de Lange and the members of her lab for advice and reagents, especially Jan Karlseder and Agata Smogorzewska; Nadine Kolas, Peter Moens, and Nagendra Singh for providing invaluable technical instruction; members of the Petrini Lab, Wes Dunnick, and Andy Koff for critical comments on the manuscript; and William Morgan for help with chromosome analysis. C.F.B. was supported by an NSF Graduate Research Fellowship and a Wisconsin Alumni Research Foundation fellowship. R.S. was supported by NIH grant RR00144-01A1. L.E.H. was supported by an NIH predoctoral fellowship (T32-AI07495). M.M.L. was supported by NIH grant PHS U01 CA84221. D.B.R. is an Assistant Investigator of the Howard Hughes Medical Institute, and this work is supported in part by a grant from the NIH to D.B.R. (AI-36420). M.L.S. and E.O. are supported by NIH grants AI36944 and AI01412. J.H.J.P. is supported by an HFSPO grant and NIH grant GM56888. This is manuscript #3590 from the University of Wisconsin-Madison Laboratory of Genetics.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL petrinij@mskcc.org; FAX (646) 422-2062.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1007902.
References
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–998. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Bressan DA, Olivares HA, Nelms BE, Petrini JHJ. Alteration of N-terminal phosphoesterase signature motifs inactivates Saccharomyces cerevisiae Mre11. Genetics. 1998;150:592–600. doi: 10.1093/genetics/150.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan DA, Baxter BK, Petrini JHJ. The Mre11/Rad50/Xrs2 protein complex facilitates homologous recombination-based double strand break repair in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR, 3rd, Hays L, Morgan WF, Petrini JHJ. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: Linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Connelly JC, Kirkham LA, Leach DR. The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA. Proc Natl Acad Sci. 1998;95:7969–7974. doi: 10.1073/pnas.95.14.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo V, Robertson K, Bibikova M, Kim E, Grieco D, Gottesman M, Carroll D, Gautier J. Responses to DNA damage in Xenopus: Mre11 protein complex prevents double-strand break accumulation during chromosomal DNA replication. Mol Cell. 2001;8:137–147. doi: 10.1016/s1097-2765(01)00294-5. [DOI] [PubMed] [Google Scholar]
- Cowell JK. A photographic representation of the variability in the G-banded structure of the chromosomes in the mouse karyotype. A guide to the identification of the individual chromosomes. Chromosoma. 1984;89:294–320. doi: 10.1007/BF00292478. [DOI] [PubMed] [Google Scholar]
- D'Amours D, Jackson SP. The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes & Dev. 2001;15:2238–2249. doi: 10.1101/gad.208701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager M, van Noort J, van Gent DC, Dekker C, Kanaar R, Wyman C. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol Cell. 2001;8:1129–1135. doi: 10.1016/s1097-2765(01)00381-1. [DOI] [PubMed] [Google Scholar]
- de Lange T, Petrini JHJ. A new connection at human telomeres: Association of the Mre11 complex with TRF2. Cold Spring Harbor Symposium Quantitative Biology. 2000;65:265–274. doi: 10.1101/sqb.2000.65.265. [DOI] [PubMed] [Google Scholar]
- Debrauwere H, Loeillet S, Lin W, Lopes J, Nicolas A. Links between replication and recombination in Saccharomyces cerevisiae: A hypersensitive requirement for homologous recombination in the absence of Rad27 activity. Proc Natl Acad Sci. 2001;98:8263–8269. doi: 10.1073/pnas.121075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB. Synaptonemal complex proteins: Occurrence, epitope mapping and chromosome disjunction. J Cell Sci. 1994;107:2749–2760. doi: 10.1242/jcs.107.10.2749. [DOI] [PubMed] [Google Scholar]
- Dolganov GM, Maser RS, Novikov A, Tosto L, Chong S, Bressan DA, Petrini JHJ. Human Rad50 is physically associated with hMre11: Identification of a conserved multiprotein complex implicated in recombinational DNA repair. Mol Cell Biol. 1996;16:4832–4841. doi: 10.1128/mcb.16.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errami A, He DM, Friedl AA, Overkamp WJ, Morolli B, Hendrickson EA, Eckardt-Schupp F, Oshimura M, Lohman PH, Jackson SP, Zdzienicka MZ. XR-C1, a new CHO cell mutant which is defective in DNA-PKcs, is impaired in both V(D)J coding and signal joint formation. Nucleic Acids Res. 1998;26:3146–3153. doi: 10.1093/nar/26.13.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M, Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1:457–481. [PubMed] [Google Scholar]
- Furuse M, Nagase Y, Tsubouchi H, Murakami-Murofushi K, Shibata T, Ohta K. Distinct roles of two separable in vitro activities of yeast mre11 in mitotic and meiotic recombination. EMBO J. 1998;17:6412–6425. doi: 10.1093/emboj/17.21.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JW, Stohr BA, Tomso DJ, Kreuzer KN. The tight linkage between DNA replication and double-strand break repair in bacteriophage T4. Proc Natl Acad Sci. 2001;98:8290–8297. doi: 10.1073/pnas.131007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenon M, Gilbert C, Lowndes NF. Checkpoint activation in response to double-strand breaks requires the Mre11/Rad50/Xrs2 complex. Nat Cell Biol. 2001;3:844–847. doi: 10.1038/ncb0901-844. [DOI] [PubMed] [Google Scholar]
- Haber JE. The many interfaces of Mre11. Cell. 1998;95:583–586. doi: 10.1016/s0092-8674(00)81626-8. [DOI] [PubMed] [Google Scholar]
- Harrington JJ, Lieber MR. The characterization of a mammalian DNA structure-specific endonuclease. EMBO J. 1994;13:1235–1246. doi: 10.1002/j.1460-2075.1994.tb06373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfner KP, Karcher A, Craig L, Woo TT, Carney JP, Tainer JA. Structural biochemistry and interaction architecture of the DNA double- strand break repair Mre11 nuclease and Rad50-ATPase. Cell. 2001;105:473–485. doi: 10.1016/s0092-8674(01)00335-x. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BAL, Karcher A, Henderson B, Bodmer J, McMurray CT, Carney JP, Petrini JHJ, Tainer JA. Rad50 Zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;I418:562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- Ivanov EL, Korolev VG, Fabre F. XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics. 1992;132:651–664. doi: 10.1093/genetics/132.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Keeney S, Kleckner N. Covalent protein-DNA complexes at the 5′ strand termini of meiosis-specific double-strand breaks in yeast. Proc Natl Acad Sci. 1995;92:11274–11278. doi: 10.1073/pnas.92.24.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, Raap AK, Tanke HJ. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- Leach DR. Long DNA palindromes, cruciform structures, genetic instability and secondary structure repair. Bioessays. 1994;16:893–900. doi: 10.1002/bies.950161207. [DOI] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, II, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Lee SE, Bressan DA, Petrini JH, Haber JE. Complementation between N-terminal Saccharomyces cerevisiae mre11 alleles in DNA repair and telomere length maintenance. DNA Repair. 2002;1:27–40. doi: 10.1016/s1568-7864(01)00003-9. [DOI] [PubMed] [Google Scholar]
- Lim DS, Kim ST, Xu B, Maser RS, Lin J, Petrini JHJ, Kastan MB. ATM phosphorylates p95/Nbs1 in an S-phase checkpoint pathway. Nature. 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- Lobachev KS, Gordenin DA, Resnick MA. The Mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell. 2002;108:183–193. doi: 10.1016/s0092-8674(02)00614-1. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Guarente L. Nijmegen breakage syndrome disease protein and MRE11 at PML nuclear bodies and meiotic telomeres. Cancer Res. 2000;60:2331–2334. [PubMed] [Google Scholar]
- Luo G, Yao MS, Bender CF, Bladl AR, Bradley A, Petrini JHJ. Disruption of mRad50 causes ES cell lethality, abnormal embryonic development and sensitivity to ionizing radiation. Proc Natl Acad Sci. 1999;96:7376–7381. doi: 10.1073/pnas.96.13.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–794. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- Maser RS, Monsen KJ, Nelms BE, Petrini JHJ. hMre11 and hRad50 nuclear foci are induced during the normal cellular response to DNA double strand breaks. Mol Cell Biol. 1997;17:6087–6096. doi: 10.1128/mcb.17.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Mirzoeva OK, Wells J, Olivares H, Williams BR, Zinkel RA, Farnham PJ, Petrini JH. Mre11 complex and DNA replication: Linkage to E2F and sites of DNA synthesis. Mol Cell Biol. 2001;21:6006–6016. doi: 10.1128/MCB.21.17.6006-6016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBlane JF, van Gent DC, Ramsden DA, Romeo C, Cuomo CA, Gellert M, Oettinger MA. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- Mirzoeva OK, Petrini JH. DNA damage-dependent nuclear dynamics of the mre11 complex. Mol Cell Biol. 2001;21:281–288. doi: 10.1128/MCB.21.1.281-288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Haber JE. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S, Furgeson JR, Symington LS. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen RM, Zubiaur M, Neer EJ, Seidman JG. Embryonic stem cells lacking a functional inhibitory G-protein subunit (alpha i2) produced by gene targeting of both alleles. Proc Natl Acad Sci. 1991;88:7036–7040. doi: 10.1073/pnas.88.16.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Moens C, Ivanyi E, Pawling J, Gertsenstein M, Hadjantonakis AK, Pirity M, Rossant J. Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr Biol. 1998;8:661–664. doi: 10.1016/s0960-9822(98)70254-4. [DOI] [PubMed] [Google Scholar]
- Nairz K, Klein F. mre11S—a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes & Dev. 1997;11:2272–2290. doi: 10.1101/gad.11.17.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms BE, Maser RS, MacKay JF, Lagally MG, Petrini JHJ. In situ visualization of DNA double-strand break repair in human fibroblasts. Science. 1998;280:590–592. doi: 10.1126/science.280.5363.590. [DOI] [PubMed] [Google Scholar]
- Paull TT, Gellert M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- ————— Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes & Dev. 1999;13:1276–188. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Petrini JH. The Mre11 complex and ATM: Collaborating to navigate S phase. Curr Opin Cell Biol. 2000a;12:293–296. doi: 10.1016/s0955-0674(00)00091-0. [DOI] [PubMed] [Google Scholar]
- Petrini JHJ. S phase functions of the Mre11 complex. Cold Spring Harb Symp Quant Biol. 2000b;65:405–412. doi: 10.1101/sqb.2000.65.405. [DOI] [PubMed] [Google Scholar]
- Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6:975–987. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Roth DB, Menetski JP, Nakajima PB, Bosma MJ, Gellert M. V(D)J recombination: Broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992;70:983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Roulston D, Le Beau MM. Cytogenetic analysis of hematologic malignant disease. In: Barch MJ, Knutsen T, Spurbeck J, editors. The AGT cytogenetics laboratory manual. Philadelphia, PA: Lippincott-Raven; 1997. pp. 325–374. [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Schlissel MS, Corcoran LM, Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J Exp Med. 1991;173:711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes ML, Suarez CC, Oltz EM. Regulation of V(D)J recombination by transcriptional promoters. Mol Cell Biol. 1999;19:2773–2781. doi: 10.1128/mcb.19.4.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NGJ, Byrd PJ, Petrini JHJ, Taylor AMR. The DNA double strand break repair gene hMre11, is mutated in individuals with a new ataxia telangiectasia like disorder (ATLD) Cell. 1999;99:577–587. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- Stohr BA, Kreuzer KN. Repair of topoisomerase-mediated DNA damage in bacteriophage T4. Genetics. 2001;158:19–28. doi: 10.1093/genetics/158.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington LS. Homologous recombination is required for the viability of rad27 mutants. Nucleic Acids Res. 1998;26:5589–5595. doi: 10.1093/nar/26.24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccioli GE, Rathbun G, Oltz E, Stamato T, Jeggo PA, Alt FW. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993;260:207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Tsubouchi H, Ogawa H. A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis. Mol Cell Biol. 1998;18:260–268. doi: 10.1128/mcb.18.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Ohta T, Oshiumi H, Tomizawa J, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- Usui T, Ogawa H, Petrini JH. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol Cell. 2001;7:1255–1266. doi: 10.1016/s1097-2765(01)00270-2. [DOI] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Williams BR, Mirzoeva OK, Morgan WF, Lin J, Dunnick W, Petrini JH. A murine model of nijmegen breakage syndrome. Curr Biol. 2002;12:648–653. doi: 10.1016/s0960-9822(02)00763-7. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Weaver DT. Conditional gene targeted deletion by Cre recombinase demonstrates the requirement for the double-strand break repair Mre11 protein in murine embryonic stem cells. Nucleic Acids Res. 1997;25:2985–2991. doi: 10.1093/nar/25.15.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Sonoda E, Sasaki MS, Morrison C, Haraguchi T, Hiraoka Y, Yamashita YM, Yagi T, Takata M, Price, et al. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 1999;18:6619–6629. doi: 10.1093/emboj/18.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Roth DB. Characterization of coding ends in thymocytes of scid mice: Implications for the mechanism of V(D)J recombination. Immunity. 1995;2:101–112. doi: 10.1016/1074-7613(95)90082-9. [DOI] [PubMed] [Google Scholar]
- Zhu X-D, Kuster B, Mann M, Petrini JHJ, de Lange T. Cell cycle regulated association of Rad50/Mre11/Nbs1 with TRF2 and human telomeres. Nat Genet. 2000;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
- Zhu J, Petersen S, Tessarollo L, Nussenzweig A. Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice. Curr Biol. 2001;11:105–109. doi: 10.1016/s0960-9822(01)00019-7. [DOI] [PubMed] [Google Scholar]