Abstract
Human 15S-lipoxygenase-2 (15-LOX-2) is a recently identified lipoxygenase that has approximately 40% sequence identity to the known human 5S-, 12S-, and 15S-lipoxygenases. 15-LOX-2 has a limited tissue distribution, with mRNA detected in prostate, lung, skin, and cornea, but not in numerous other tissues, including peripheral blood leukocytes. In the current study, we have characterized the distribution of 15-LOX-2 in the human prostate by immunohistochemistry, demonstrated the ability of benign prostate tissue to form 15S-hydroxyeicosatetraenoic acid (15S-HETE) from exogenous arachidonic acid (AA), and begun characterizing possible alterations in 15-LOX-2 in prostate adenocarcinoma. Incubation of benign prostate tissue with [14C]AA resulted in formation of [14C]15-HETE, as determined by reverse- and straight-phase high-performance liquid chromatography. 15-HETE was the major AA metabolite formed. By immunohistochemistry, 15-LOX-2 is located in secretory cells of peripheral zone glands and large prostatic ducts and somewhat less uniformly in apical cells of transition and central zone glands. 15-LOX-2 was not detected in the basal cell layer, stroma, ejaculatory ducts, seminal vesicles, or transitional epithelium. Immunostaining of 18 radical prostatectomy specimens showed a loss of 15-LOX-2 in the majority of prostate adenocarcinomas; 14 of 18 cases showed loss of 15-LOX-2 in >25% of the tumor (mean, 74.9% negative for 15-LOX-2; range, 38.9% to 100%). Incubation of paired pure benign and pure malignant prostate tissue from the same radical prostatectomies showed that 15-HETE formation was markedly reduced (>90%) or undetectable in incubations of prostate adenocarcinoma. 15-LOX-2 is a novel human lipoxygenase with a limited tissue distribution that is strongly expressed in benign prostate glandular epithelium and lost to a variable degree in the majority of prostate adenocarcinomas.
Arachidonic acid (AA) metabolites are important mediators of a variety of physiological processes and inflammatory reactions. In addition, alterations in AA metabolism may potentially mediate key steps in certain neoplastic processes. 1-3 AA is metabolized via cyclooxygenase to prostaglandins, prostacyclin, and thromboxane, 4 and via lipoxygenases (LOX) to hydroxyeicosatetraenoic acids (HETEs) or leukotrienes (5-LOX pathway). 5,6 Until recently, three lipoxygenases were recognized in humans: a 5S-LOX found in leukocytes, a 12S-LOX found in platelets and certain epithelia, and a 15S-LOX in reticulocytes, eosinophils, macrophages, and skin. 7 Recently, in studying lipoxygenase expression in human skin, Brash et al 8 discovered a second 15S-lipoxygenase (herein referred to as 15-LOX-2). The cDNA-derived amino acid sequence of 15-LOX-2 showed only 44% identity to 5-LOX and 38% to 39% identity to 12-LOX and the reticulocyte type of 15-LOX (herein referred to as 15-LOX-1).
In addition to structural differences, 15-LOX-2 exhibits differences in certain enzymatic characteristics. In contrast to the reticulocyte type of 15S-lipoxygenase (15-LOX-1), 15-LOX-2 converts AA exclusively to 15S-hydroperoxyeicosatetraenoic acid (15-HPETE); this hydroperoxide is reduced by cellular peroxidases to 15S-hydroxyeicosatetraenoic acid (15-HETE). Also unlike 15-LOX-1, this newly characterized enzyme metabolizes linoleic acid poorly compared with AA. 8 The 15-LOX-2 cDNA was cloned from human hair roots. 8 In addition, 15-LOX-2 mRNA was detected in a limited number of tissues, including prostate, lung, and cornea. In contrast to the distribution of 15-LOX-1, 15-LOX-2 was not detected in peripheral blood leukocytes, nor was it detected in liver, kidney, spleen, thymus, testis, ovary, skeletal muscle, heart, brain, or intestinal tissue. 8
The limited tissue distribution of 15-LOX-2 suggests a possible role in regulation of organ-specific functions or differentiation or possible alterations in disease states. In continuing efforts to elucidate potentially important molecular alterations in prostate cancer development or progression, a limited number of recent investigations using both human tissues and prostate carcinoma cell lines have begun focusing on AA-metabolizing enzymes, including cyclooxygenase, 9 12-LOX, 10 5-LOX, 11 and 15-LOX-1. 12 In the current study, we have characterized the expression of 15-LOX-2 in benign prostate by immunohistochemistry, demonstrated the ability of benign prostate to form the enzymatic product of 15-LOX-2 (15-HETE) from AA, and begun exploring the possible altered expression of 15-LOX-2 and 15-HETE formation in prostate adenocarcinoma.
Materials and Methods
Case Selection and Histology
Fresh tissue was procured from radical prostatectomy or cystoprostatectomy specimens accessioned in the Surgical Pathology Laboratory at the Vanderbilt University Medical Center. For prostatectomies, the external aspects (surgical margins) were inked according to standard protocols and whole cross sections (perpendicular to the apex-base axis) were made. Based on gross examination, possible benign and tumor regions were separately excised in such a manner as to not compromise surgical pathological evaluation of the specimen. Fresh tissues were placed in liquid nitrogen or immediately placed in a −80°C freezer. Before utilization in enzyme assays, thin sections parallel to the longest axis of the tissue were removed, and frozen or routine formalin-fixed paraffin-embedded H&E-stained sections were prepared. The samples were assessed as to percentage of benign glandular, stomal, and adenocarcinoma tissue present. Although numerous samples of benign tissue were thus generated, owing to the known difficulty of recognizing prostate carcinoma grossly, only a subset of presumably malignant tissues so procured turned out to be 100% tumor. For enzyme assays, frozen normal tissue samples and pairs of pure benign glands and pure carcinoma admixed with stroma were used according to availability.
Whole-mount sections were processed for histology and subjected to standard surgical pathology evaluation. Cases that were used for AA incubations were also used for 15-LOX-2 immunohistochemistry. Additional random cases were selected to give a representative mixture of tumor grades with various combinations of organ-confined disease and extra-capsular extension (ECE) with or without seminal vesicle (SV) and margin involvement: five cases of Gleason grade 5 or 6 without ECE or SV involvement, three cases of Gleason grade 6 with ECE or SV involvement, four cases of Gleason grade 7 without ECE or SV involvement, three cases of Gleason grade 7 with ECE or SV involvement, and three cases of Gleason grade 8 to 10 with ECE or SV involvement. Numerous foci of well differentiated carcinoma (Gleason patterns 1 and 2) were also included (generally incidental in transition zone).
Preparation of 15-LOX-2 for Antibody Production
cDNA encoding the open reading frame of 15-LOX-2 was cloned into the pET3a vector and expressed in phage-induced Escherichia coli (HMS174 cells). Ten milliliters of overnight culture of HMS174 cells transformed with pET3a/15-LOX-2 in LB medium containing 50 μg/ml ampicillin was used to inoculate a fresh 500-ml culture of LB/ampicillin. Incubation was continued at 37°C until the absorbance at 600 nm reached 0.9 A. One hundred milliliters of phage culture (CE6, expressing T7 RNA polymerase) was then added together with 5 ml of 1 mol/L MgSO4, and incubation was continued for 3 hours at 37°C. Under these incubation conditions, overexpression of the 15-LOX-2 protein gave a catalytically inactive lipoxygenase, comprising 25% to 50% of the cellular protein. The bacterial pellets were collected by centrifugation, washed with phosphate-buffered saline (PBS), and resuspended in 40 ml of PBS, and the cells were disrupted by sonication. The insoluble fraction containing the majority of the lipoxygenase protein was collected by centrifugation and washed once with 1.5 mol/L NaCl followed by a wash with 1% Triton X-100 in PBS. The pellet was again washed in PBS, dissolved in 8 ml of loading buffer, and subjected to semipreparative sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% polyacrylamide gels with 4% stacking gels. The clearly visible band of 15-LOX-2 protein was cut from Coomassie-blue-stained gels, placed in a dialysis bag, and electroeluted. The protein was lyophilized, and an aliquot was quantified by its appearance on SDS-PAGE using bovine serum albumin as standard.
Preparation and Characterization of Polyclonal Antibody to 15-LOX-2
Rabbits were injected at six sites of back skin using an initial 0.1 mg of 15-LOX-2 per animal in 1:1 PBS/Freund’s complete adjuvant. A booster injection of 0.1 mg using PBS/Freund’s incomplete adjuvant was given 19 days later, and blood serum was collected beginning 14 days later. High-titer bleeds from a single rabbit were used for the Western analyses and immunohistochemistry reported herein.
The specificity of the rabbit antibody for 15-LOX-2 and possible cross-reactivity to other human lipoxygenases was examined by Western blotting using five human lipoxygenases. 15-LOX-1, 15-LOX-2, and 12R-LOX 13 were prepared by bacterial expression, each including an amino-terminal histidine tag. The proteins were recovered using a nickel affinity column (Qiagen, Chatsworth, CA) essentially according to the manufacturer’s instructions. Purified human 5-lipoxygenase was a generous gift from Dr. Denis Reindeau (Merck-Frosst). Aliquots of these four lipoxygenase proteins were run on SDS-PAGE, and after staining with Ponceau S, the samples were quantified by visual inspection relative to dilutions of a standard of bovine serum albumin. Aliquots of 50 or 5 ng were used for Western analysis. A partially purified preparation of human platelet 12-lipoxygenase from Oxford Biomedical Research (Oxford, MI) was used as a source of human 12S-LOX (platelet 12-LOX). For Western blots employing 5 ng of purified lipoxygenases, 8 μg of protein of the partially purified 12S-LOX was used, which, assuming the 12-LOX comprises at least 0.1% of the extract, corresponds to at least 8 ng of 12S-LOX protein. Adequate protein loading of this sample was further confirmed by staining of gels and visual inspection. Proteins were separated by 12% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes using standard conditions.
The Western analyses were carried out using 15-LOX-2 polyclonal antibody (1:6000) and an alkaline-phosphatase-conjugated affinity-purified donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA; 1:7500 in bovine serum albumin/Tris-buffered saline/0.1% Tween-20) and Tris-buffered levamisole (Sigma Chemical Co., St. Louis, MO) followed by nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate for development. 14
Immunohistochemistry and Quantitation of 15-LOX-2 Immunostaining
Whole-mount sections that contained adequate benign tissue and tumor typical of the overall specimen were selected. In general, both right and left halves of the respective whole-mount sections were recut onto separate immuno-slides for subsequent immunohistochemical staining. In some cases, multiple whole-mount blocks were used for immunostaining. Paraffin immunoperoxidase studies were performed using a 1:2500 dilution of either primary rabbit antisera for 15-LOX-2 or preimmune sera on an automated immunostainer (Ventana 320 automated immunohistochemistry system, Ventana Medical Systems, Tucson, AZ) with an avidin-biotin complex method without antigen retrieval techniques. 15 15-LOX-2 immunostaining in benign prostate tissue was uniform and is expressed descriptively or semiquantitatively as 0 to 4+: 0, no staining; 1+, focal weak staining; 2+, weak to moderate intensity staining in up to one-half of the cells in most glands or most cells in up to one-half of the glands; 3+, moderate to intense staining in greater than one-half of the cells or one-half of the glands but less than strong, uniform staining; 4+, strong uniform staining in essentially all cells. 15-LOX-2 immunostaining was quantitated in tumor portions by carefully mapping out with ink on the glass slides the areas of tumor that were positive and negative and then by making enlarged photocopies of such outlines. Areas were determined using a digitized graphics tablet (WACOM) and NIH Image Analysis software. Results are expressed as the percentage of tumor negative for 15-LOX-2 immunostaining.
Tissue Incubations and HPLC Analysis
A 50- to 100-mg amount of benign or tumorous prostate tissue was homogenized in 4 vol of buffer (50 mmol/L Tris with 100 mmol/L NaCl and 100 μmol/L CaCl2, pH 7.4). Incubations were begun by addition of 50 μmol/L [1-14C]arachidonic acid (final concentration; New England Nuclear, Boston, MA) in ⩽1% (final v/v) ethanol. Samples were incubated for 1 hour at 37°C with continual agitation and terminated by addition of 2.5 vol of cold methanol. After addition of 1.25 vol of dichloromethane, the sample was centrifuged to remove the protein precipitate, and the products were recovered in the mixed phase of methanol/water/dichloromethane. The sample was evaporated under a stream of nitrogen to remove most of the dichloromethane and methanol, water was added, and the products were recovered by C18 Sep-Pak extraction. 8 The extracts were analyzed by reversed-phase HPLC using a Beckman Ultrasphere 5-μm ODS column (25 × 0.46 cm) with a solvent of methanol/water/glacial acetic acid, either 90:10:0.01 (by volume) at a flow rate of 1.1 ml/minute (retention time of 15-HETE was approximately 5 minutes) or 80:20:0.01 at 1.1 ml/minute (retention time of 15-HETE approximately 15 minutes). Unlabeled HETEs (5-, 8-, 9-, 11-, 12-, and 15-HETEs) were added to each sample before HPLC analysis; this permitted an exact determination of the retention times of each HETE product within each individual chromatographic run. Ultraviolet (UV) spectra and the profiles at 205, 220, 235, and 270 nm were recorded using a Hewlett-Packard 1040A diode array detector, and radioactivity was monitored on-line using a Radiomatic Instruments Flo-One detector. Product formation in some incubations of benign and malignant prostate tissue pairs was also analyzed in more detail using reverse-phase HPLC with a solvent system of methanol/water/glacial acetic acid (75:25:0.01, by volume) at a flow rate of 1.01 ml/minute (retention time of 15-HETE approximately 31.5 minutes), with collection of 0.5-minute fractions and 10-minute scintillation counting of all fractions. In some samples, the main radiolabeled peaks were further characterized by normal-phase HPLC using a Beckman Ultrasphere 5-μm silica column (25 × 0.46 cm) and a solvent of hexane/isopropyl alcohol/glacial acetic acid 100:1:0.1 (by volume) at a flow rate of 1.1 ml/minute with UV detection using the diode array detector and on-line radioactive detection.
Results
15-HETE Formation by Benign Prostate Tissue
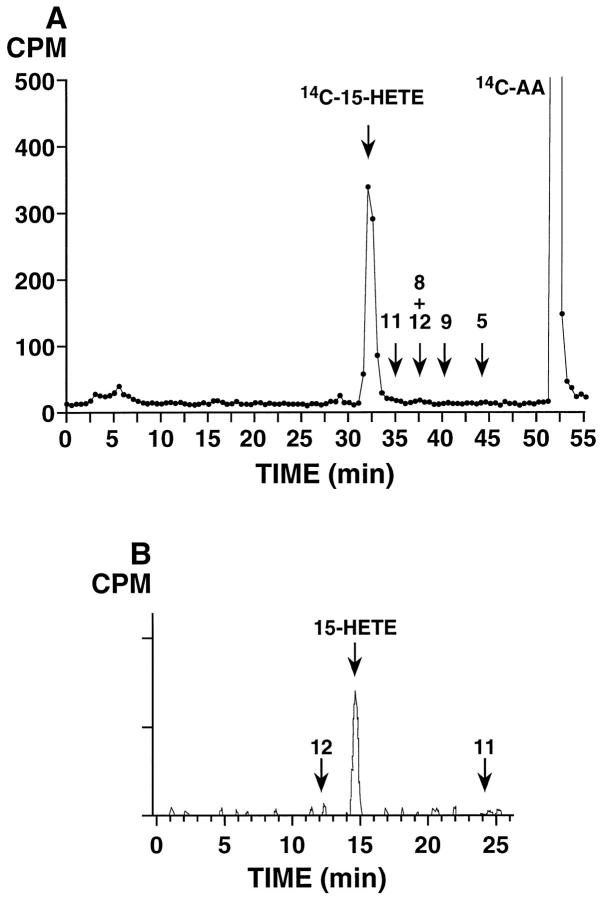
Incubations of benign prostate tissue homogenates with [14C]AA resulted in the formation of 15-HETE in all cases examined (n = 8; Figure 1 ▶ ). Identity of this product was demonstrated by its precise co-elution on reverse-phase HPLC (and on straight-phase HPLC in selected samples) with co-injected unlabeled 15-HETE (see Materials and Methods). The addition of a mixture of unlabeled HETEs to each sample allowed an exact definition of the retention times within each chromatographic run. The identity of the radiolabeled product as 15-HETE was further confirmed by the characteristic UV spectrum of the mixture of [14C]15-HETE and the co-injected authentic standard 16 ; the UV absorbance of the 15-HETE peak was distinctly elevated by the contribution from the [14C]15-HETE. The amount of 15-HETE produced under these incubation conditions was variable. However, in all cases, 15-HETE was the major product detected, with variably sized peaks in the prostaglandin region (Figures 1 and 3) ▶ ▶ . From analysis of these eight samples of benign prostate, minor amounts of other hydroxyeicosatetraenoates were occasionally detected; the most prominent of these additional HETE peaks, present in only one sample, was the one, tentatively identified as 12-HETE, eluting at 36 minutes in Figure 3B ▶ .
Figure 1.
[14C]AA metabolism in benign prostate tissue, showing prominent formation of [14C]15-HETE. A: Reverse-phase HPLC analysis using a Beckman Ultrasphere 5-μm ODS column (25 × 0.46 cm) with a solvent of methanol/water/glacial acetic acid (75:25:0.01, by volume) at a flow rate of 1.01 ml/minute switched to 100% methanol at 40 minutes; 0.5-minute fractions were collected and subjected to scintillation counting. Arrows show retention times of unlabeled HETE standards co-injected with the 14C sample. The experiment shown is representative of eight individual benign specimens analyzed. B: Normal-phase HPLC analysis of fractions 63 through 66 (31.5 to 33 minutes) from reverse-phase analysis in A, using a Beckman Ultrasphere 5-μm silica column (25 × 0.46 cm), a solvent system of hexane/isopropanol/glacial acetic acid (100:1:0.1, by volume) with a flow rate of 1.1 ml/minute and on-line detection of radiolabeled products using a Packard Flo-One Radiomatic detector. Arrows indicate retention times of co-injected unlabeled HETE standards.
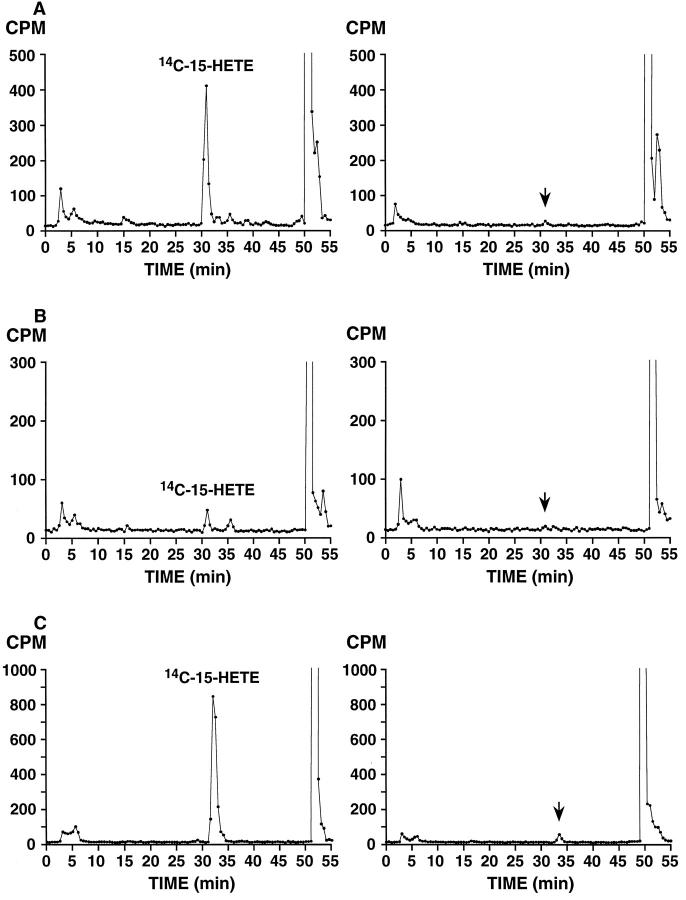
Figure 3.
15-HETE formation by benign (left) and malignant (right) prostate tissue from three different radical prostatectomy specimens. A: Benign and Gleason grade 3 + 4 = 7, with extra-capsular extension; B: Benign and Gleason grade 4 + 3 = 7, confined to prostate; C: Benign and Gleason grade 3 + 2 = 5, confined to prostate (with incubated tumor tissue from Gleason pattern 3 tumor in peripheral zone). Incubation with [14C]AA and extraction were performed as described in Materials and Methods. Product analysis was by reverse-phase HPLC using a Beckman Ultrasphere 5-μm ODS column (25 × 0.46 cm) with a solvent of methanol/water/glacial acetic acid (75:25:0.01, by volume) at a flow rate of 1.01 ml/minute switched to 100% methanol at 40 minutes; 0.5-minute fractions were collected and subjected to scintillation counting. [14C]15-HETE peaks are indicated in benign incubations and arrows corresponding to substantially reduced or undetected [14C]15-HETE in tumor incubations based on retention time of unlabeled HETE standards co-injected with 14C samples.
15-LOX-2 Immunostaining in Benign Prostate
The polyclonal antibody prepared against purified 15-LOX-2 was demonstrated to be specific for 15-LOX-2 and to not cross-react with other pertinent lipoxygenases by Western blotting. Whereas a strong immunoreactive band was obtained with purified 15-LOX-2 protein (5 ng), no immunoreactivity was seen with purified 15-LOX-1 or 5-LOX (5 or 50 ng) or a sample of partially purified 12S-LOX (not shown). Weak immunostaining was seen with a purified preparation of a recently identified 12R-LOX, 13 which may be expected, given the greater percentage of identity between 15-LOX-2 and 12R-LOX (approximately 50%) than between 15-LOX-2 and 5-LOX, 12S-LOX, and 15-LOX-1 (approximately 40%). 8,13 However, unlike 12S-LOX, which has been reported in prostate (see Discussion), 12R-LOX has not been identified in prostate. Hence, minimal cross-reactivity of a 15-LOX-2 antibody with a 12R-LOX is not an issue in prostate immunostaining, as benign prostate makes large amounts of 15-HETE (as reported herein), and prostate has not been observed to make 12R-HETE.
The cellular location of 15-LOX-2 was identified as ductal and acinar epithelium by immunohistochemistry. Benign prostatic epithelium consistently stained strongly and uniformly with the polyclonal antiserum against human 15-LOX-2 by paraffin immunoperoxidase (Figure 2A) ▶ . Stromal, vascular, and inflammatory cells were uniformly negative (Figure 2B) ▶ . The distribution of immunostaining was carefully examined in the benign portions of prostate sections from radical prostatectomies performed for prostate adenocarcinoma. There was strong uniform staining of apical (secretory) cells in peripheral zone glands, with essentially all cells staining in all glands (Figure 2, A and C) ▶ . The pattern of staining was predominantly cytoplasmic granular, with frequent nuclear staining also evident (Figure 2C) ▶ . Occasional detached cells in glandular and ductal lumens showed immunostaining, but corpora amylacea were negative. Basal cells did not stain (Figure 2C) ▶ ; this lack of staining was evident also in areas of basilar hyperplasia (not shown). Strong uniform 15-LOX-2 immunostaining was present in columnar secretory cells of large periurethral ducts and secondary prostatic ducts (Figure 2, D and E) ▶ . In contrast, transitional epithelium of the prostatic urethra, periurethral ducts, and areas of transitional metaplasia was completely negative (Figure 2E) ▶ .
Figure 2.
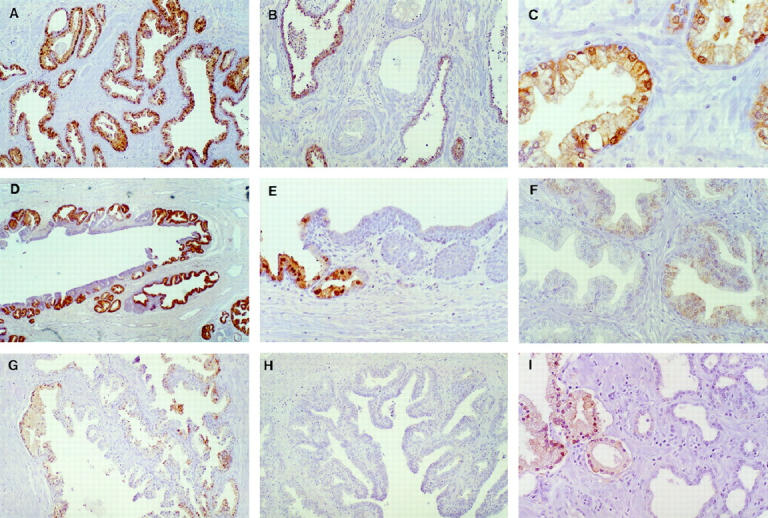
15-LOX-2 immunostaining in benign prostate. A: Uniform strong staining of glandular epithelium (original magnification, ×200). B: Absent immunostaining in vein and artery (middle), fibromuscular stroma, and admixed chronic inflammatory cells. Strong staining of glands in right and left of field (×100). C: Higher magnification showing cytoplasmic granular and focal nuclear staining of apical (secretory) epithelial cells (×400). D: Staining of secretory cells in large prostatic duct (×100). E: Higher magnification showing positive staining in columnar secretory cells (left) and lack of staining in transitional epithelium (right) of prostatic duct (×200). F: Non-uniform and reduced intensity staining in hyperplastic glands in transition zone (×200). G: Focal staining of glands in central zone (region around ejaculatory ducts toward base of gland; ×100). H: Complete absence of staining of ejaculatory duct (lumen toward lower right; ×100). I: Absent staining in atrophic glands (right). Compare with positive immunostaining of more normal-appearing glands, with ample cytoplasm, in left of photomicrograph (×200). Paraffin immunoperoxidase staining using rabbit polyclonal anti-15-LOX-2, 1:2500.
Some variability in the intensity and extent of prostate glandular epithelial staining was noted in different anatomical zones and benign histological alterations. Although most glands in the transition zone showed moderately intense staining in the majority of cells, staining was appreciably less uniform and less intense than seen in peripheral zone glands (Figure 2F) ▶ . This slightly reduced 15-LOX-2 immunostaining was noted in variably prominent nodules of transition zone glandular hyperplasia (the histological correlate of benign prostatic hyperplasia, or BPH), including in cystically dilated hyperplastic glands. In general, staining was stronger in glands in the periphery of such nodules, with lesser staining, including negative glands, in the center of hyperplastic nodules. Compared with the completely uniform strong staining in essentially all peripheral zone glands, benign glands in the central zone (surrounding the ejaculatory ducts) also showed slightly reduced intensity and frequency of 15-LOX-2 immunostaining (Figure 2G) ▶ . Ejaculatory ducts and seminal vesicles were uniformly negative (Figure 2H) ▶ . Reduced and even absent immunostaining was also noted in atrophic glands in the peripheral zone (Figure 2I) ▶ , with intermediate staining intensity occasionally noted in partially atrophic glands. No immunostaining was detected in any glandular elements (or any other tissue compartment) with negative preimmune serum controls (n = 5; data not shown). In addition, staining of benign prostate glands was eliminated by preincubation of antisera with purified 15-LOX-2 protein 8 (not shown). A summary of the semiquantitative 15-LOX-2 immunohistochemical staining in benign prostate is shown in Table 1 ▶ .
Table 1.
15-LOX-2 Immunostaining in Benign Prostate
| Prostate compartment | 15-LOX-2 Immunostaining (0–4+) | Number of informative cases |
|---|---|---|
| Peripheral zone glands | 4+ | 18 |
| Atrophic glands | 0–2+ | 9 |
| Transition zone glands (BPH) | 2+ | 7 |
| Central zone glands | 2–3+ | 4 |
| Periurethral/prostatic ducts* | 4+ | 7 |
| Stroma and inflammatory cells | 0 | 18 |
| Transitional epithelium; ejaculatory ducts; seminal vesicles | 0; 0; 0 | 6; 4; 5 |
*Columnar/secretory (not transitional) epithelial cells.
Decreased or Absent 15-LOX-2 Enzyme Activity in Prostate Adenocarcinoma
15-LOX-2 activity was assessed in benign prostate tissue and prostate adenocarcinoma frozen from the same radical prostatectomy specimens. In all benign samples, incubation with [14C]AA resulted in formation of 15-HETE as detected by reverse-phase HPLC analysis. In contrast, markedly reduced or no 15-HETE production was detected in simultaneously incubated samples of prostate adenocarcinoma (n = 3; Figure 3 ▶ ).
Reduced 15-LOX-2 in Prostate Adenocarcinoma by Immunohistochemistry
In contrast to the strong, uniform 15-LOX-2 immunostaining in benign prostate glands (particularly in the peripheral zone), 15-LOX-2 immunostaining was markedly reduced in prostate adenocarcinoma (Figure 4) ▶ . 15-LOX-2 immunostaining was at least focally absent in essentially all tumors, with the vast majority showing substantial portions of tumor that were negative on immunostaining. In 14 of 18 cases, 15-LOX-2 was absent in more than 25% of the tumor examined by immunohistochemistry. In these cases, 15-LOX-2 immunostaining was negative in 74.9 ± 24.4% of the tumor. 15-LOX-2 was absent in >50% of the tumor in 10 of 18 cases and essentially completely absent in 8 of 18 of the tumors.
Figure 4.
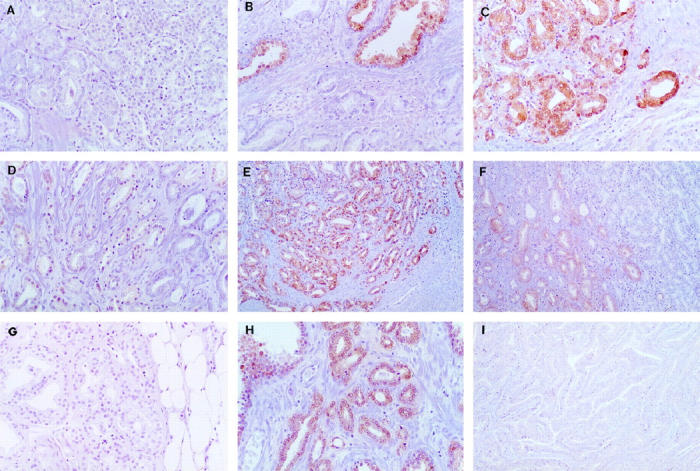
Reduced 15-LOX-2 immunostaining in prostate adenocarcinoma. A: Absent immunostaining in adenocarcinoma, from tumor that was overall 39% negative for 15-LOX-2 immunostaining (original magnification, ×200). B: Absent 15-LOX-2 immunostaining in adenocarcinoma, bottom, compared with strong staining in adjacent benign glands (top), from a case in which most of the tumor was higher grade than that illustrated and in which the tumor was overall 94% 15-LOX-2 negative (×200). C: Strong 15-LOX-2 immunostaining in Gleason pattern 2 tumor in transition zone (×200). D: Markedly reduced and absent 15-LOX-2 immunostaining in Gleason pattern 3 adenocarcinoma from same case as C, which was overall 58.8% 15-LOX-2 tumor negative (×200). E: Positive 15-LOX-2 immunostaining in transition zone Gleason pattern 2 adenocarcinoma (×100). F: Transition between focal weakly positive adenocarcinoma, left, and 15-LOX-2 negative tumor, right, in Gleason pattern 3 and transition to area of Gleason pattern 4, respectively, from same case as E (×100). G: 15-LOX-2-negative adenocarcinoma in area of extracapsular extension, from same case as in E and F, which was overall Gleason 3 + 4 and 44.2% 15-LOX-2 tumor negative (×200). H: Positive 15-LOX-2 immunostaining in acinar adenocarcinoma from case of mixed ductal and acinar tumor (×200). I: Negative 15-LOX-2 immunostaining in ductal adenocarcinoma, same case as in H, which was overall 45% 15-LOX-2 tumor negative (×100).
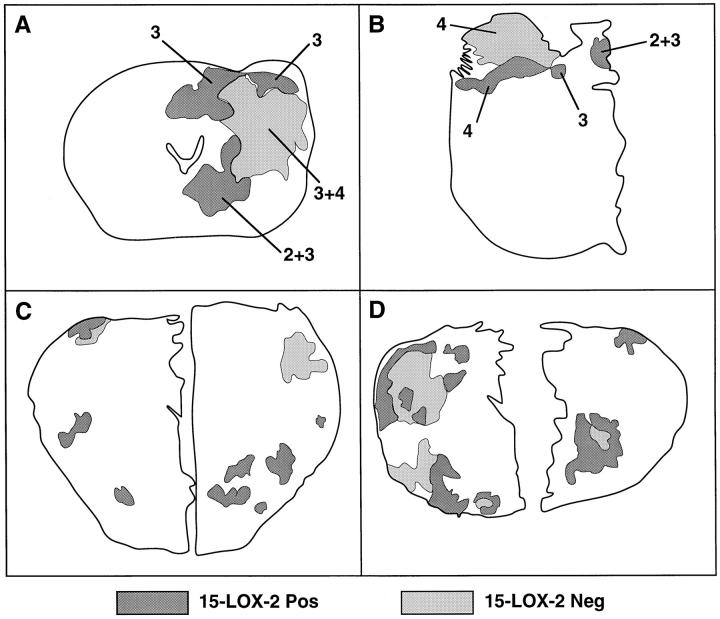
Correlation of reduced 15-LOX-2 with other known prognostic factors in prostate cancer, such as grade and stage, and clinical outcome will require analysis of a much larger number of cases, which currently is in progress. However, possible correlation of reduced 15-LOX-2 immunostaining with increased tumor grade or biological aggressiveness was indicated in several individual cases. In one case of a Gleason 3 + 3 = 6 adenocarcinoma with absent immunostaining in greater than one-half of the tumor assessed by immunohistochemistry, there was strong residual 15-LOX-2 immunopositivity in a Gleason pattern 2 tumor focus in the transition zone (Figure 4, C and D) ▶ . In two cases of overall Gleason 7 adenocarcinoma (one 3 + 4 and one 4 + 3), areas of Gleason pattern 2 and 3 were 15-LOX-2 immunopositive, whereas other areas of Gleason patterns 3 and 4 were negative, including in one case areas where the tumor was penetrating beyond the prostate capsule (Figure 4, E–G) ▶ . These two cases were among those assessed by HPLC, the tumor tissue for which was taken from the posterior peripheral zone (see also Figure 5, A and B ▶ ). Lack of 15-HETE formation from this tumor tissue likely correlates with the reduced 15-LOX-2 immunostaining in more peripheral (and higher-grade) portions of the tumor. Another example of possible tumor heterogeneity with regard to 15-LOX-2 expression was seen in a case with a mixed ductal and acinar pattern. Whereas the more peripherally located acinar carcinoma was largely 15-LOX-2 immunopositive, the more centrally located ductal carcinoma was negative (Figure 4, H and I) ▶ . Examples of the degree of heterogeneity of loss of 15-LOX-2 immunostaining within individual tumors are shown in Figure 5 ▶ , in which parts A–C correspond to the tumor cases analyzed by HPLC (as shown in Figure 3 ▶ ). Figure 5D ▶ shows an extreme example of the multifocal or heterogeneous nature of 15-LOX-2 negativity seen in some cases with only partial loss of 15-LOX-2 immunostaining.
Figure 5.
Heterogeneity of absent 15-LOX-2 immunostaining in prostate tumors. Immunostained entire whole-mount sections or right and/or left halves from four representative cases are outlined, with posterior toward top of figure. Benign glands stained uniformly, and stroma was negative as described in Results. Shaded outlines correspond only to actual tumor portions, with positive staining in dark gray and absent immunostaining areas in lighter shade. Numbers correspond to Gleason pattern of indicated areas (in tumors with more than one pattern). A through C correspond to cases A through C in Figure 3 ▶ (in which portions were also examined by HPLC assay). A: Gleason 3 + 4 = 7, overall 44.2% 15-LOX-2 negative. B: Gleason 4 + 3 = 7, overall 60.6% 15-LOX-2 negative. C: Gleason 3 + 3 = 6, overall 40% 15-LOX-2 negative. D: Gleason 3 + 3 = 6, overall 45% 15-LOX-2 negative.
Discussion
As discussed more fully below, there have been a few previous reports on the occurrence of AA-metabolizing enzymes in benign prostate and prostatic neoplasia. These include the arachidonate 5-lipoxygenase, 11 12-lipoxygenase, 10 and reticulocyte type of 15-lipoxygenase (15-LOX-1). 12 In this study we have used assays of enzyme activity and immunohistochemistry to document expression of the recently described 15-lipoxygenase enzyme 15-LOX-2 8 in benign human prostate epithelium and its reduction in prostate adenocarcinoma. One clear advantage of the methodological approach in our study is the ability to correlate enzyme protein with actual functional activity.
Characterization of the 15-lipoxygenase metabolism and its designation as 15-LOX-2 is based on the following lines of evidence. 1) Previous multitissue Northern blots demonstrated 15-LOX-2 mRNA expression in benign prostate, 8 and we have recently detected 15-LOX-2 mRNA in individual benign prostates on Northern Blots (S. B. Shappell, unpublished observations). 2) Analysis of lipoxygenase product formation by HPLC identifies the specific formation of 15-HETE in all of our incubations of benign prostate. Typically, the reticulocyte type of 15-lipoxygenase, 15-LOX-1, forms a mixture of 15-HETE and 12-HETE, 17 whereas 15-LOX-2 oxygenates AA solely at C-15. 8 In our experiments the characteristics of 15-HETE formation matched the catalytic activities of 15-LOX-2 (eg, Figure 1 ▶ and Figure 3, A and C ▶ ). The exception, when both 12- and 15-HETE formation was evident (Figure 3B) ▶ , might be accounted for by co-expression of 12-LOX and a 15-lipoxygenase. 3) An additional feature of the 15-lipoxygenase metabolism in prostate, again characteristic of 15-LOX-2, was the greater formation of 15-HETE from AA than of 13-hydroxyoctadecadienoic acid (13-HODE) from linoleic acid in side-by-side incubations of the same benign prostate tissue (not shown); this correlates with the substrate specificity of 15-LOX-2 8 and contrasts with the known preference of 15-LOX-1 for linoleic over arachidonic acid. 18 4) The antibody used in the current immunohistochemical studies specifically detects 15-LOX-2 and does not cross-react with 15- LOX-1, 19 12S-LOX, or 5-LOX (results herein). Furthermore, there was complete suppression of the positive reactions in the immunohistochemical staining by pre-absorption with purified 15-LOX-2 protein. The specificity of our antibody for 15-LOX-2 versus 15-LOX-1 was also supported by the staining of the prostatic epithelium with concomitant lack of staining of inflammatory cells, including macrophages, which might be expected to stain for 15-LOX-1. 20 Although inflammation was variable and generally mild in the sections examined, one benign prostate examined by both enzyme assay and immunostaining was a cystoprostatectomy specimen containing granulomatous inflammation secondary to previous BCG instillation therapy for bladder transitional cell carcinoma. In addition to formation of large amounts of 15-HETE on incubation of this prostate tissue with AA, by immunohistochemistry, the anti-15-LOX-2 antibody uniformly stained benign prostate epithelium but did not stain the large numbers of histiocytes (macrophages) forming the granulomas (not shown).
Few studies have examined the possible significance of AA metabolism or the presence of cyclooxygenase or lipoxygenase enzymes in benign prostate and prostatic neoplasia. Chaudry et al 9 reported prostaglandin E2 (PGE2) synthesis to be 10-fold higher in carcinoma tissue compared with benign prostatic hyperplasia (BPH) tissue. Whether the enzymes responsible for PGE2 synthesis were epithelial, stromal, or vascular was not elucidated. Increased formation of PGE2 has been demonstrated previously in human cancer cell lines, including human prostate cancer cell lines. 21
More recently, multiple reports have described the presence of lipoxygenase enzymes in prostate cancer and prostatic carcinoma cell lines. Gao et al 10 reported that 12-lipoxygenase (12-LOX) mRNA was elevated in 46 of 122 (38%) prostate tumor samples compared with normal tissue from the same patient. They found that 12-LOX mRNA was more likely to be elevated in tumor versus control tissue in patients with extracapsular extension (T3), positive lymph nodes, higher-grade lesions (Gleason score ≥ 8), and positive surgical margins. By in situ hybridization they detected 12-LOX mRNA within epithelial cells of the prostate, including basal cells of normal prostate tissue. Secretory cells were generally negative in normal tissue. This basal cell distribution of 12S-LOX in benign prostate tissue contrasts with the apical cell distribution of 15-LOX-2 in benign prostate demonstrated by immunohistochemistry herein. In contrast to benign glands, Gao et al reported that tumor epithelial cells demonstrated heterogeneous and occasionally marked expression of 12-LOX mRNA. 10 Biological effects of 12-HETE include promotion of tumor cell adhesion and endothelial cell contraction, indicating a potential contribution to tumor cell metastasis 2,22,23 and possible modulation of tumor growth by induction of angiogenesis. 24 12-LOX enzyme activity has not been reported in actual prostate tissues or prostate cancers, however. We did not observe detectable 12-HETE accumulation in most incubations of benign prostate tissue with exogenous arachidonic acid. We also did not observe 12-HETE formation in similar incubations with prostate adenocarcinoma. However, although the cases examined for 15-LOX-2 immunostaining included multiple examples of high-grade (Gleason ≥ 8) and T3 carcinoma, only one of the cases examined by HPLC was positive for extracapsular extension (T3), and none had positive surgical margins or lymph node metastases. Hence, the possibility of detecting 12-HETE formation and possible simultaneous decreased 15-HETE and increased 12-HETE formation under the experimental conditions used will require examination of higher-grade and higher-stage lesions.
A possible role for 5-LOX in modulation of growth of the prostate cancer cell line PC-3 was reported by Ghosh and Myers. 11 Arachidonic acid stimulated PC-3 cell growth, which was inhibited by selective 5-LOX inhibitors but not by 12-LOX or cyclooxygenase inhibitors. Furthermore, 5-HETE was produced from exogenous AA in PC-3 cells. 11 More recently, these investigators and others have demonstrated induction of apoptosis in PC-3 and LNCaP cell lines by inhibitors of 5-LOX and 5-LOX-activating protein (FLAP). 25,26 Again, we did not observe 5-HETE production from exogenous AA in benign prostate or a limited number of fresh-frozen prostate tumors. Given the known chromosomal and other possible genetic abnormalities of established prostate carcinoma cell lines, whether AA metabolic pathways in these cultured cells generated from individual tumors reflect similar alterations in actual cancers in vivo will require correlative studies, with analysis of high-grade and high-stage cancers, perhaps including metastatic tumors.
Spindler et al 12 recently provided evidence for a possible 15-lipoxygenase in the human prostate cancer cell lines LNCaP and PC-3, which they indicated as the same as the rabbit reticulocyte 15-lipoxygenase (designated herein as 15-LOX-1). These findings may require re-evaluation in light of the recent discovery of 15-LOX-2. However, based on the evidence we have obtained in human prostate cancer samples, transformed prostate carcinoma cell lines, such as LNCaP and PC-3 cells, would not be expected to express 15-LOX-2. The reported detection of 13-HODE in a single specimen of human prostate carcinoma by frozen-section immunohistochemistry (and its absence in surrounding benign epithelium) 12 does not correlate with the patterns of 15-LOX-2 expression observed in the series of samples we have examined, although 15-LOX-2 was still present to some degree by immunohistochemistry in a significant percentage of tumor cases. Certainly the origins of immunodetectable 13-HODE in tumor tissue might include its production through non-enzymatic mechanisms.
The biological significance of 15-LOX-2 expression in benign prostatic epithelium is not clear. The uniform expression in prostate apical or secretory cells suggests a possible role of this novel enzyme in secretory function. Reduced expression in atrophic prostate glands and prostate adenocarcinoma may thus parallel less secretory differentiation. However, given our demonstration of the loss of this protein and its associated enzymatic activity in prostate carcinoma, it is possible that reduction or loss of 15-LOX-2 and 15-HETE formation is a crucial event in the development or progression of prostate adenocarcinoma. In this regard, it is noteworthy that the murine homologue of 15-LOX-2, a recently identified 8S-lipoxygenase (8-LOX), is up-regulated in benign squamous papilloma of mouse skin induced by phorbol ester treatment and lost with progression to frank squamous cell carcinoma with additional treatments. 19,27 It is possible that the products of these related enzymes (15S-HETE and 8S-HETE, respectively) regulate aspects of cellular proliferation or differentiation and that the loss of this function is associated with development or progression of a malignant phenotype. The recent description of the activation of transcription-regulating PPAR receptor subtypes (peroxisome proliferator-activated receptors) by various AA-related products, including HETEs, indicates one potential mechanism of action. 28-31 This is a particularly intriguing hypothesis in light of the evidence that the synthetic activators of PPAR, the aromatic fatty acids phenyl acetate and phenyl butyrate, inhibit prostate cancer cell line growth in vitro and show activity against hormone-insensitive prostate cancer in vivo. 32-34 More recently, Kubota et al 35 demonstrated expression of PPARγ in PC-3, DU-145, and LNCaP cells and inhibition of PC-3 cell proliferation by PPARγ ligands, including the thiazolidinedione troglitazone and 15-deoxy-Δ12,14-prostaglandin J2. The recent demonstration of activation of PPARγ-dependent transcription by a variety of oxidized lipids, including specifically 15-HETE, 36 suggests a possible role of reduced 15-HETE activation of PPARγ in prostate carcinogenesis or progression. Whether this signaling pathway or some other mechanism of cell regulation is affected by alterations of prostate 15-LOX-2 expression remains to be further clarified.
Acknowledgments
We thank Kim Johnson for help in fresh tissue procurement, Jean McClure and Brent Weedman for help with figures, and Caroline Nolen and the VUMC Histology Laboratory for cutting prostate whole mounts for immunohistochemical staining.
Footnotes
Address reprint requests to Dr. Scott B. Shappell, Department of Pathology, C-3322 Medical Center North, Vanderbilt University Medical Center, Nashville, TN 37221-2562. E-mail: scott.shappell@mcmail.vanderbilt.edu.
Supported in part by National Institutes of Health grant GM-53638 (A.R. Brash). S.B. Shappell is the recipient of a Department of Defense Prostate Cancer Research Program New Investigator Award (PC970260).
References
- 1.Marnett LJ: Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res 1992, 52:5575-5589 [PubMed] [Google Scholar]
- 2.Tang DG, Honn KV: 12-Lipoxygenase, 12(S)-HETE, and cancer metastasis. Ann NY Acad Sci 1994, 744:199-215 [DOI] [PubMed] [Google Scholar]
- 3.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN: Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 1997, 99:2254-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith WL, Garavito RM, DeWitt DL: Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem 1996, 271:33157-33160 [DOI] [PubMed] [Google Scholar]
- 5.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN: Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 1987, 237:1171-1176 [DOI] [PubMed] [Google Scholar]
- 6.Lewis RA, Austen KF, Soberman RJ: Leukotrienes and other products of the 5-lipoxygenase pathway. Mech Dis 1990, 323:645-655 [DOI] [PubMed] [Google Scholar]
- 7.Funk CD: The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta 1996, 1304:65-84 [DOI] [PubMed] [Google Scholar]
- 8.Brash AR, Boeglin WE, Chang MS: Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci USA 1997, 94:6148-6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudry AA, Wahle KWJ, McClinton S, Moffat LEF: Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer 1994, 57:176-180 [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Grignon DJ, Chbihi T, Zacharek A, Chen YQ, Sakr W, Porter AT, Crissman JD, Pontes JE, Powell IJ, Honn KV: Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology 1995, 46:227-237 [DOI] [PubMed] [Google Scholar]
- 11.Ghosh J, Myers CE: Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem Biophys Res Commun 1997, 235:418-423 [DOI] [PubMed] [Google Scholar]
- 12.Spindler SA, Sarkar FH, Sakr WA, Blackburn ML, Bull AW, LaGattuta M, Reddy RG: Production of 13-hydroxyoctadecadienoic acid (13-HODE) by prostate tumors and cell lines. Biochem Biophys Res Commun 1997, 239:775-781 [DOI] [PubMed] [Google Scholar]
- 13.Boeglin WE, Kim RB, Brash AR: A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proc Natl Acad Sci USA 1998, 95:6744-6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC: A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem 1984, 136:175-179 [DOI] [PubMed] [Google Scholar]
- 15.Hammer RD, Scott M, Shahab I, Casey TT, Cousar JB, Macon WR: Latent membrane protein antibody reacts with normal hematopoietic precursor cells and leukemic blasts in tissues lacking Epstein-Barr virus genome by polymerase chain reaction. Am J Clin Pathol 1996, 106:469-474 [DOI] [PubMed] [Google Scholar]
- 16.Ingram CD, Brash AR: Characterization of HETEs and related conjugated dienes by UV spectroscopy. Lipids 1988, 23:340-344 [DOI] [PubMed] [Google Scholar]
- 17.Bryant RW, Bailey JM, Schewe T, Rapoport SM: Positional specificity of a reticulocyte lipoxygenase: conversion of arachidonic acid to 15S-hydroperoxy-eicosatetraenoic acid. J Biol Chem 1982, 257:6050-6055 [PubMed] [Google Scholar]
- 18.Soberman RJ, Harper TW, Betteridge D, Lewis RJ, Austen KF: Characterization and separation of the arachidonic acid 5-lipoxygenase and linoleic acid ω-6 lipoxygenase (arachidonic acid 15-lipoxygenase) of human polymorphonuclear leukocytes. J Biol Chem 1985, 260:4508-4515 [PubMed] [Google Scholar]
- 19.Jisaka M, Kim RB, Boeglin WE, Nanney LB, Brash AR: Molecular cloning and functional expression of a phorbol ester-inducible 8S-lipoxygenase from mouse skin. J Biol Chem 1997, 272:24410-24416 [DOI] [PubMed] [Google Scholar]
- 20.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, Steinberg D: Gene expression in macrophage-rich human atherosclerotic lesions: 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest 1991, 87:1146-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard WC, Alley MC, Mclemore TL, Boyd MR: Profiles of prostaglandins in sixteen established cell lines derived from human lung, colon, prostate, and ovarian tumors. Cancer Res 1988, 48:4770-4775 [PubMed] [Google Scholar]
- 22.Honn KV, Tang DG, Grossi I, Duniec ZM, Timar J, Renaud C, Leithauser M, Blair IA, Johnson CR, Diglio CA, Kimler VA, Taylor JD, Marnett LJ: Tumor derived 12(S)-hydroxyeicosatetraenoic acid induces microvascular endothelial cell retraction. Cancer Res 1994, 54:565-574 [PubMed] [Google Scholar]
- 23.Chen YQ, Duniec ZM, Liu B, Hagmann W, Gao X, Shimoji K, Marnett LJ, Johnson CR, Honn KV: Endogenous 12S-HETE production by tumor cells and its role in metastasis. Cancer Res 1994, 54:1574-1579 [PubMed] [Google Scholar]
- 24.Nie D, Hillman GG, Geddes T, Tang K, Pierson C, Grignon DJ, Honn KV: Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res 1998, 58:4047-4051 [PubMed] [Google Scholar]
- 25.Ghosh J, Myers CE: Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci USA 1998, 95:13182-13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson KM, Seed T, Vos M, Mulshine J, Meng J, Alrefai W, Ou D, Harris JE: 5-Lipoxygenase inhibitors reduce PC-3 cell proliferation and initiate nonnecrotic cell death. Prostate 1998, 37:161-173 [DOI] [PubMed] [Google Scholar]
- 27.Lehmann WD, Stephan M, Furstenberger G: Profiling assay for lipoxygenase products of linoleic and arachidonic acid by gas chromatography-mass spectrometry. Anal Biochem 1992, 204:158-170 [DOI] [PubMed] [Google Scholar]
- 28.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA: Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem 1995, 270:23975-23983 [DOI] [PubMed] [Google Scholar]
- 29.Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W: The PPARα-leukotriene B4 pathway to inflammation control. Nature 1996, 384:39-43 [DOI] [PubMed] [Google Scholar]
- 30.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM: 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83:803-812 [DOI] [PubMed] [Google Scholar]
- 31.Kliewer SA, Lenhard JM, Wilson TM, Patel I, Morris DC, Lehmann JM: A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 1995, 83:813-819 [DOI] [PubMed] [Google Scholar]
- 32.Pineau T, Hudgins WR, Liu L, Chen L, Sher T, Gonzalez FJ, Samid D: Activation of a human peroxisome proliferator-activated receptor by the antitumor agent phenylacetate and its analogs. Biochem Pharmacol 1996, 52:659-667 [DOI] [PubMed] [Google Scholar]
- 33.Thibault A, Cooper MR, Figg WD, Venzon DJ, Sartor AO, Tompkins AC, Weinberger MS, Headless DJ, McCall NA, Samid D, Myers CE: A phase I and pharmacokinetic study of intravenous phenylacetate in patients with cancer. Cancer Res 1994, 54:1690-1694 [PubMed] [Google Scholar]
- 34.Thibault A, Samid D, Cooper MR, Figg WD, Tompkins AC, Patronas N, Headless DJ, Kohler DR, Venzon DJ, Myers CE: Phase I study of phenylacetate administered twice daily in patients with cancer. Cancer 1995, 75:2932-2938 [DOI] [PubMed] [Google Scholar]
- 35.Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP: Ligand for peroxisome proliferator-activated receptor γ (Troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res 1998, 58:3344-3352 [PubMed] [Google Scholar]
- 36.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM: Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998, 93:229-240 [DOI] [PubMed] [Google Scholar]