Abstract
We have previously demonstrated that tamoxifen inhibits the growth of human cholangiocarcinoma cells in culture and inhibits tumor growth when cells are injected into nude mice. However, the mechanism of action of tamoxifen remains unknown. Here we demonstrate that tamoxifen and trifluoperazine, both potent calmodulin antagonists, induce apoptosis in vitro, probably acting via the Fas system, in human cholangiocarcinoma cells. Human cholangiocarcinoma cell lines heterogeneously express Fas antigen on their surface. Fas-negative and Fas-positive surface-expressing cells were isolated, cloned, and cultured. Fas antibody, tamoxifen, and trifluoperazine induced dose-dependent apoptosis only in Fas-positive cells; Fas-negative cells were unaffected. Furthermore, apoptosis induced by tamoxifen in Fas-positive cells was blocked by an inhibitory Fas antibody. Tamoxifen was not acting through an anti-estrogenic mechanism, because neither Fas-negative nor Fas-positive cells expressed estrogen receptors and the pure anti-estrogen compound, ICI 182780, did not induce apoptosis in either cell line. Fas-negative cells, but not Fas-positive cells, were able to produce tumors when subcutaneously injected into nude mice. These findings suggest Fas may be a candidate oncogene involved in the pathogenesis of cholangiocarcinoma. Furthermore, the similarity between the pro-apoptotic effects of tamoxifen and trifluoperazine support an underlying molecular mechanism for Fas-mediated apoptosis that involves calmodulin.
The antiestrogen tamoxifen (TMX) is commonly accepted as effective treatment for estrogen receptor (ER)-positive as well as some ER-negative breast cancers. 1-5 TMX inhibits tumor growth of human cholangiocarcinoma cells in culture and when injected into nude mice. 6 An anti-tumorigenic effect of TMX has also been reported for pancreatic cancer, 7 malignant gliomas, 8,9 and other tumors. 10 TMX therapy not only significantly improves the prognosis in a number of malignancies, but also has been used therapeutically in a number of other diseases such as osteoporosis, 11,12 atherosclerosis, 13,14 rheumatoid arthritis, and other autoimmune diseases. 15-17 Although TMX has widespread clinical use, it is clear that not all of its effects can be attributed to the competitive interaction with the estrogen receptor. TMX has a wide variety of other pharmacological activities including stimulation of transforming growth factor β (TGF-β), 18-20 up-regulation of nuclear factor κB (NF-κB), 21 calmodulin antagonism, 22,23 blockade of various chloride channels, 24 and inhibition of protein kinase C. 25
The Fas/APO-1 (CD95) and Fas ligand system is a key regulator of apoptosis (programmed cell death). 26-28 The Fas/APO-1 (CD95) cell surface receptor is a member of the tumor necrosis factor receptor (TNFR) superfamily. 29-31 Fas is expressed in various human organs and cells including lymphocytes, heart, lung, kidney, and ovary. 27,32,33 The expression level of Fas in cells may modulate cell death in both normal and pathological states. In normal cell populations at steady state, the rates of cell proliferation and cell death approximate each other. In cancer, however, increases in cell number predominate over cell death. Malignancy may not be associated exclusively with enhanced cell proliferation, but may also be linked to decreased cell death. 34,35
The failure of cells to undergo apoptosis, which may be involved in the pathogenesis of cancer, could be attributed to a deficiency of Fas expression or function. Many malignant cells express Fas. 36-38 In comparison to normal cells, some malignant tumors are characterized by abnormal phenotypes of Fas expression including abnormal expression of functional Fas, 39 mutant Fas incapable of intracellular signaling, 40 cellular release of soluble Fas, 41 and deficiency of Fas transduction pathway. 42 The aberrant expression of Fas by various tumor cells has attracted interest in Fas as a potential target for induction of apoptosis in the cancer therapy. 43,44
We have demonstrated that TMX inhibits the growth of human cholangiocarcinoma cells in cell culture and when cells are implanted into nude mice. 6 However, the molecular mechanism of TMX-induced growth inhibition of human cholangiocarcinoma remains unclear. In related investigations, we have demonstrated that TMX and TFP are able to inhibit Fas antibody-induced apoptosis in T cells transfected with HIV envelope glycoprotein gp160, which contains two calmodulin binding domains, and the accelerated spontaneous apoptosis in peripheral blood mononuclear cells from patients with AIDS. 45-47 These studies suggest that TMX might be involved in Fas-mediated apoptosis. In view of the abnormal Fas expression in malignant tumors and the ability of TMX to modulate Fas-mediated apoptosis in AIDS, we considered the possibility that Fas is involved in TMX-induced apoptotic cell death in human cholangiocarcinoma cells. In this study we have confirmed that Fas is variably expressed in cultured human cholangiocarcinoma cells, have shown that TMX and TFP stimulate apoptosis only in Fas-positive cells, and have found that Fas-negative cells, but not Fas-positive cells, are tumorigenic in nude mice.
Materials and Methods
Reagents
TMX and TFP were purchased from Sigma (St. Louis, MO). ICI 182780 was a gift from Dr. Alan Wakeling (Zeneca Pharmaceuticals, Macclesfield, UK). They were dissolved in DMSO at a concentration of 20 mmol/L as a stock solution and freshly diluted to required concentrations before each experiment. Apoptosis-inducing human Fas monoclonal antibody (CH11, IgM) and apoptosis-inhibitory human Fas monoclonal antibody (GH4, IgG) were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY).
Cell Culture and Isolation of Subpopulations
Human cholangiocarcinoma cells (SK-ChA-1) were provided by Dr. A. Knuth (Ludwing Institute for Cancer Research, London, UK). Cells were grown in RPMI1640 (Life Technologies, Inc., Gaithersburg, MD) supplemented with 2 mmol/L L-glutamine, penicillin (5 U/ml), streptomycin (5 μg/ml) and 10% heat-inactivated fetal calf serum (FCS) (complete medium). Cells were incubated at 37°C in 95% air/5% CO2.
Fas-negative and Fas-positive subpopulations were isolated by flow cytometry. The human cholangiocarcinoma cells were rinsed in cold phosphate-buffered saline (PBS) (8 g/liter NaCl, 0.2 g/liter KCl, 1.44 g/liter Na2HPO4 and 0.24 g/liter KH2PO4), once in 1:500 Versene (GIBCO, Gaithersburg, MD), incubated for 3 minutes at 37°C, and harvested into complete medium containing 10% FCS by vigorous pipetting. The cells were centrifuged at 1200 rpm for 5 minutes at 4°C, resuspended (10 7 cells/50 μl) in complete medium and labeled with 20 μl commercial PE-conjugated anti-human Fas antibody (PharMingen, San Diego, CA) at 4°C for 30 minutes and then washed with RPMI 1640 medium twice. Murine PE-IgG1 was used as an isotype control. The stained cells were sorted into Fas-negative and Fas-positive subsets. Fas-negative and Fas-positive cells were continuously cultured in RPMI 1640 complete medium for 2 weeks.
Cloning of Fas-Negative and Fas-Positive Cells
The sorted Fas-negative and Fas-positive cholangiocarcinoma cells were diluted to 1000 cells/ml. Cells (1, 3, and 5 μl) were added into each well containing 200 μl medium in a 96-well plate and then incubated for 1 week. A single cell per well was selected and grown in the medium until enough cloned cells were available for study.
Mice
Six- to eight-week-old athymic (nu/nu) female Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA) for tumor inoculation. All animals were maintained in a sterile environment; cages, bedding, food, and water were autoclaved and animals were maintained on a daily 12-hour light/12-hour dark cycle.
Determination of Cell Death
Cell pellets were resuspended in 1 ml PBS (pH 7.4) and a 0.1-ml aliquot was stained with an equal volume of 4% trypan blue for 5 minutes followed by cell counting. Blue-stained dead cells and unstained living cells were counted.
Assays for Apoptosis
Chromatin DNA Fragmentation Assay
Cell pellets were treated with 0.5 ml lysis buffer (10 mmol/L Tris-HCl, 400 mmol/L NaCl, 2 mmol/L Na2EDTA, pH 8.2, and 50 μl 10% sodium dodecyl sulfate) and 50 μl proteinase K (10 mg/ml) and incubated at 37°C overnight. DNA was extracted with the same volume of phenol two times and precipitated with two volumes of 100% ethanol at −20°C. After centrifugation at 3300 × g, the pellet was dissolved in 40 μl of Tris-EDTA buffer and incubated with 1 μl RNase (0.5 mg/ml) at 37°C for 1 hour followed by electrophoresis on a 1.0% agarose gel. The agarose gel was stained with ethidium bromide and the resulting DNA fragmentation pattern was revealed by UV illumination.
TUNEL Staining
Cells (105/200 μl PBS) were collected by cytospinning onto poly-l-lysine-precoated slides and fixed in 10% formalin for 1 hour. After rinsing with water, cells were incubated with 20 μg/ml proteinase K for 15 minutes and the slides washed four times with water. Endogenous peroxidase was blocked by methanol containing 1% hydrogen peroxide and the slides were washed with water. They were subsequently immersed in TdT buffer (30 mmol/L Trizma base, pH 7.2, 140 mmol/L sodium cacodylate, 1 mmol/L cobalt chloride) containing TdT (0.3 μl) and digitonigen-modified dUTP added and incubated in a humidified atmosphere at 37°C for 1 hour. The reaction was terminated by washing the slides with PBS. After the slides were incubated in 10% FCS in PBS for 30 minutes and dried, they were covered with 1:10 diluted alkaline phosphatase conjugated anti-digitonigen antibody (Boehringer Mannheim, Indianapolis, IN) and incubated at 24°C for 1 hour. The slides were then washed with PBS and stained with NBT/BCIP at 24°C for approximately 30 minutes. The apoptotic index was determined by light microscopy by counting 500 cells and was expressed as percentage of positive cells.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was extracted using RNAzol reagent (Biotecx Lab, Inc., Houston, TX). cDNA was generated using RNA PCR Core Kit reagents (Clontech Laboratories, Palo Alto, CA) and a 4800 GeneAmp thermocycler (Perkin-Elmer, Foster City, CA). For human Fas, the cDNA primers were 5′-CAGCTCTTCCACCTACAG-3′ (forward) and 5′-TCATGCTTCTCCCTCTTTCACATGG-3′ (reverse). Reaction conditions were denaturing at 94°C for 1 minute annealing at 52°C for 1 minute, and extension at 72°C for 1 minute for 30 cycles. Agarose gel electrophoresis confirmed the 500-bp DNA product for Fas. Human estrogen receptor DNA were amplified using 5′ primer, 5′-CAAGCCCGCTCATGATCA-3′ and the 3′ primer 5′-TGTGTAGAGGGCATGGTG-3′ and GPDH control primers 5′-TGAAGGTCGGTGTGAACGGTATTTGGC-3′ and 5′-CA-TGTAGGCCATGAGGTCCACCAC-3′. The reaction conditions were denaturing at 94°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 2 minutes for 30 cycles. The PCR products on 1% agarose gel electrophoresis were 350 and 450 bp, respectively.
Immunohistochemical Staining of Estrogen Receptor
Cholangiocarcinoma cells (1 × 105/1 ml RPMI 1640 complete medium) were collected by cytospinning on poly-l-lysine-precoated slides and washed with PBS (pH 7.6) twice, fixed by 3% paraformaldehyde for 30 minutes, and stained using a Vectastain Universal Elite ABC Kit (Vector Laboratories, Burlingame, CA) using the manufacturer’s protocol. The fixed cells were subsequently incubated with anti-human estrogen receptor monoclonal antibody for 30 minutes diluted in biotinylated secondary antibody for 30 minutes and Vectastain elite ABC reagent for 30 minutes. After each incubation the slides were washed three times and examined microscopically.
Tumor Xenograft in Nude Mice
Cloned Fas-negative and Fas-positive cultured cholangiocarcinoma cells (1 × 106/ml) were trypsinized, washed, and resuspended in Dulbecco’s PBS (Cellgro). Mice were anesthetized with isofluorane inhalation and 5 × 106/0.2 ml/site were inoculated subcutaneously into the flanks of mice using a 22-gauge needle. Two weeks were allowed for tumor engraftment after which tumor sizes were measured using a caliper. After 4–6 weeks, tumors were removed, fixed, and embedded in paraffin. Sections were cut and stained with hematoxylin and eosin for microscopic analysis.
Results
TMX Induces Apoptosis in Human Cholangiocarcinoma Cells
The effect of various concentrations of TMX on apoptosis of human cholangiocarcinoma cells was determined using nick end labeling (TUNEL) staining and DNA fragmentation. The data presented in Figure 1 ▶ show that treatment with 5, 10, and 20 μmol/L TMX for 48 hours resulted in a dose-dependent increase of apoptosis as indicated by the increase in number of dark TUNEL-positive apoptotic cells (Figure 1A) ▶ and the increase in DNA fragmentation (Figure 1B) ▶ . Controls, with dimethylsulfoxide alone, showed no increase in TUNEL-positive cells or an increase in DNA fragmentation. The apoptotic indices from TUNEL staining were 3%, 11%, 26%, and 42% for cells incubated with 0, 5, 10, and 20 μmol/L TMX, respectively.
Figure 1.

Apoptosis induced by TMX in human cholangiocarcinoma cells. A: TUNEL staining: human cholangiocarcinoma cells cultured for 2 days in the absence or presence of TMX at different concentrations (5, 10, and 20 μmol/L). Cells were stained with terminal DNA polymerase (TUNEL) as described in Materials and Methods. Dark blue cells indicate apoptotic cells. B: DNA fragmentation: cells were treated with the concentrations of TMX as indicated for 48 hours. Genomic DNA was isolated from 10 5 cells as described in the methods followed by 1% agarose gel electrophoresis and ethidium bromide staining.
Isolation of Fas-Negative and Fas-Positive Subpopulations
To determine whether human cholangiocarcinoma cells expressed Fas antigen, cells were stained with PE-conjugated Fas monoclonal antibody and the percentage of Fas-positive cells was determined by flow cytometry. Flow cytometric analysis revealed that approximately 20% of cells were Fas-positive, indicating that the cultured human cholangiocarcinoma cells heterogeneously express Fas (Figure 2A ▶ , panel 1). Fas-negative and Fas-positive subsets were separated by flow cytometric sorting. The sorted Fas-positive and Fas-negative cells were continually incubated in RPMI 1640 complete medium for 1–2 weeks. Using this technique, Fas-negative and Fas-positive cell populations were 80% and 60% pure, respectively (Figure 2A ▶ , panel 2).
Figure 2.
Isolation of Fas-negative and Fas-positive cholangiocarcinoma populations. A1: Fas expression of cholangiocarcinoma cells before flow cytometric sorting. Cells were incubated with PE-conjugated anti-human Fas monoclonal antibody at 4°C for 30 minutes and washed with FACS butter (PBS, 5% FCS). The surface Fas antigen of cells was measured by flow cytometry compared with the control incubated with PE-conjugated anti-mouse IgG. A2: Isolation of Fas-positive and Fas-negative subsets by flow cytometric sorting. Cells were labeled as described above and sorted by flow cytometry into cells (10%) at the far left side of PE fluorescence peak (Fas-negative) and cells (10%) at the far right side of PE fluorescence peak (Fas-positive). These cells were incubated in RPMI 1640 complete medium for 1 week. Fas antibody (0.1 μg/ml) was added in the medium of the Fas-negative cells. Fas expression of Fas-negative (solid line) and Fas-positive (dotted line) were measured by flow cytometry. A3: Cloned Fas-negative and Fas-positive cell lines. The sorted Fas-negative and sorted Fas-positive cells were diluted and cells grown from a single cell were subsequently transferred onto tissue culture plates to generate cloned cells. The figure shows Fas expression of a Fas-negative clone (solid line) and a Fas-positive clone (dotted line). Fluorescence intensity is plotted on the x-axis; cell counts on the y-axis. B: Fas mRNA determined by RT-PCR. The mRNAs of sorted and cloned Fas-negative and Fas-positive cells (as indicated) were prepared using RNAzon kit and RT-PCR of Fas in sorted (lane 1) and cloned Fas-negative cells (lanes 2–3), as well as sorted (lane 4) and cloned Fas-positive cells (lanes 5–6) were performed. Fas was amplified using the primers of Fas (500 bp, see Materials and Methods). The 500-bp Fas DNA band is marked.
Although the cell sorting technique isolated Fas-negative and Fas-positive cells, there was still overlap in Fas expression between these two populations. To further improve the purification of cells, several clones of Fas-negative and Fas-positive cells were generated by diluting to single cells and then re-expanding by cell division over 3–4 weeks. Fas expression on cells of these clones was then determined by flow cytometry. The data shown in Figure 2A ▶ , panel 3, are typical of 12 isolated clones. Fas expression of the Fas-negative clone was less than 15%, and Fas expression of the Fas-positive clone increased to 80%. To further confirm the difference in two populations, RT-PCR of Fas was performed on sorted and cloned cell populations. The data in Figure 2B ▶ show a high level of Fas PCR product only in Fas-positive cells compared to no PCR product in Fas-negative cells. Both cloned Fas-positive and Fas-negative cells grew in serum-free medium (data not shown), indicating that they are both transformed.
Sensitivity of Fas-Positive and Fas-Negative Cells to Fas Antibody
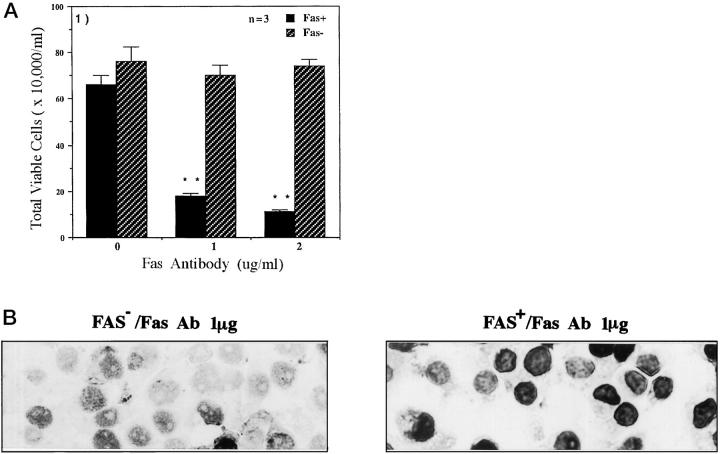
Fas-negative and Fas-positive human cholangiocarcinoma cells responded differently upon activation with Fas antibody. Quantitation of trypan blue staining, presented in Figure 3A ▶ , shows that Fas antibody markedly stimulated cell death only in Fas-positive cells. Associated microscopic morphological changes were consistent with characteristics of apoptosis including cell shrinkage, nuclear condensation, cell rounding, detachment from the monolayer, and plasma membrane blebbing (data not shown). Apoptosis was confirmed by TUNEL assay. Figure 3B ▶ shows that less than 8% of Fas-negative cells underwent apoptosis upon stimulation of Fas antibody. By contrast, Fas antibody induced apoptosis in more than 70% of Fas-positive cells.
Figure 3.
Fas antibody-induced apoptosis in Fas-negative and Fas-positive cells. A: Fas-negative (hatched bars) and Fas-positive (closed bars) cells (5 × 105/well) were seeded in 6-well plate for 12 hours and then exposed to 0, 1, and 2 μg (1 mg/ml) Fas antibody at 37°C for 16 hours and harvested in 1 ml PBS. Cell survival was measured with 4% trypan blue and counted under a light microscope. Each bar represents the mean ± SE of triplicate counts (**, P < 0.01). B: TUNEL assay. Fas-negative and Fas-positive cells were treated with 1 μg/ml Fas antibody at 37°C for 16 hours and TUNEL staining performed. The dark TUNEL-positive cells in Fas-negative and Fas-positive cells are 6.8% and 73.4%, respectively.
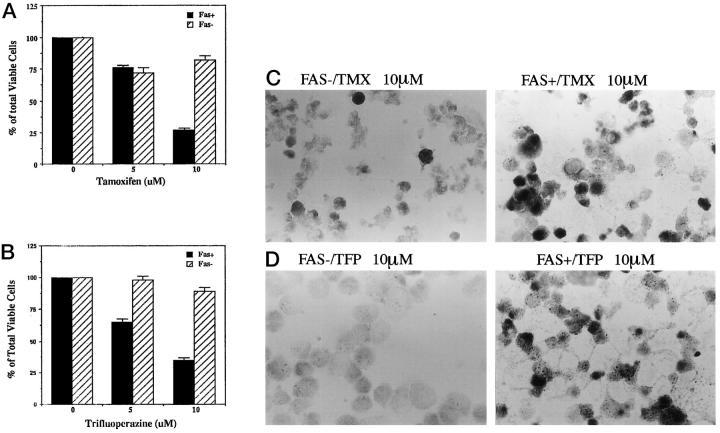
Induction of Apoptosis by TMX and TFP in Fas-Negative and Fas-Positive Cells
Although TMX is anti-estrogenic, it is also a very potent calmodulin antagonist, being equally as potent as the commonly used calmodulin antagonist, trifluoperazine (TFP). TFP has also been used as a chemotherapeutic agent for some malignancies. 3 We, therefore, tested the effects of TMX and TFP on apoptosis in Fas-negative and Fas-positive human cholangiocarcinoma cells. Figure 4, A and B ▶ , show that both TMX and TFP induced concentration-dependent cell death mainly in the Fas-positive population. TMX stimulated apoptotic cell death in Fas-positive cells, the percentage of viable cells being 75% and 26% at 5 and 10 μmol/L TMX, respectively, (Figure 4A ▶ , solid bars). TMX has a minimal effect on cell death (25% reduction in viable cells) in the Fas-negative population (Figure 4A ▶ , hatched bars). TFP has a similar effect on Fas-positive cells (Figure 4B ▶ , hatched bars). TFP at 5 μmol/L and 10 μmol/L induced cell death in Fas-positive cells with a decrease of the percentage of total viable cells to 62% and 28%, respectively. TFP, like TMX, has no or minimal effect on Fas-negative cells (Figure 4B ▶ , hatched bars). TUNEL assay was performed as described in methods on Fas-negative and Fas-positive cells treated with 10 μmol/L TMX (Figure 4C) ▶ or 10 μmol/L TFP (Figure 4D) ▶ . Results confirm the trypan blue staining experiments showing that apoptosis is stimulated by both TMX and TFP primarily in the Fas-positive cells.
Figure 4.
Apoptotic cell death induced by TMX and TFP in Fas-negative and Fas-positive cells. A: Fas-negative and Fas-positive cells were incubated in the presence of DMSO alone as controls, 5 μmol/L and 10 μmol/L TMX at 37°C for 36 hours. Cells were harvested in 1 ml PBS and then stained with 4% trypan blue and counted under a light microscope. Each bar is given as mean ± SE of triplicate counts. B: Cells were treated with TFP and assayed as in A. C and D: Apoptosis in Fas-positive and Fas-negative cells treated with 10 μmol/L TMX (C) and 10 μmol/L TFP (D) detected by TUNEL assay.
TMX-Induced Apoptosis Is Blocked by an Inhibitory Fas Antibody
To further demonstrate that TMX-induced apoptosis is mediated by the Fas pathway, cells were pretreated for 5 hours in the presence or absence of 2 μg inhibitory Fas antibody, which binds Fas but does not induce apoptosis, and then exposed to 10 μmol/L TMX. Figure 5 ▶ shows that TMX treatment of Fas-positive cells reduced the percentage of total viable cells to 54% compared with the control cells (100%). This apoptosis induced by TMX treatment could be reversed by addition of inhibitory Fas antibody (91%). In contrast, the percentage of total viable cells in TMX-treated Fas-negative cells did not change in the presence and absence of inhibitory antibody. The protective effect of inhibitory Fas antibody on TMX-induced apoptotic cell death in Fas-positive cells indicates that TMX-induced apoptosis is likely to be mediated through Fas/APO-1 (CD95) system.
Figure 5.
TMX-induced cell death is blocked by an inhibitory Fas antibody. Cloned Fas-positive and Fas-negative cells were pre-incubated with or without 2 μl (1 mg/ml) inhibitory Fas antibody at 37°C for 5 hours and then exposed to 10 μmol/L TMX for 16 hours. Cells were harvested and detected apoptotic cells by TUNEL assay.
TMX-Induced Apoptotic Cell Death Is Not Due to its Antiestrogenic Properties
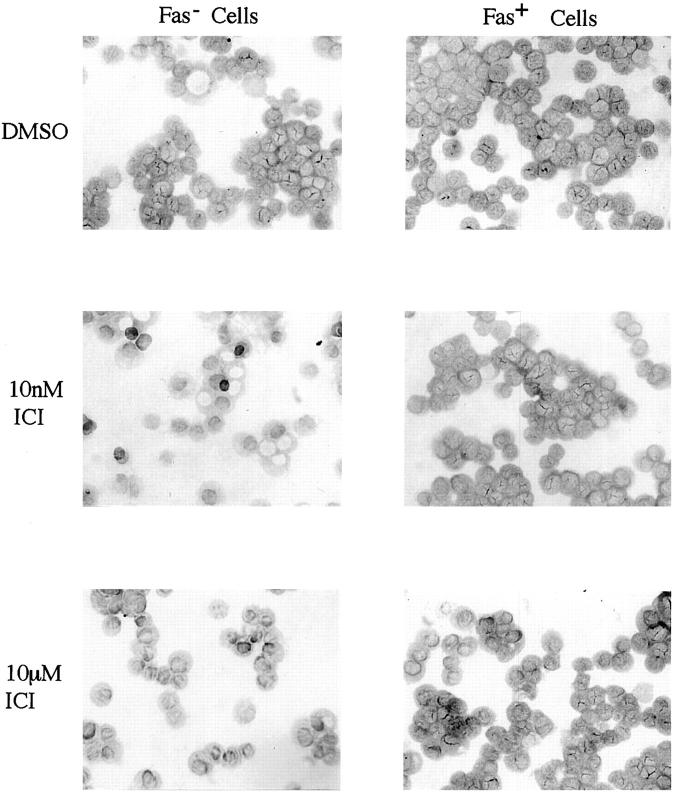
In the previous studies, we determined that the estrogen receptor was not expressed in untreated human cholangiocarcinoma cells cultured in RPMI 1640 medium containing phenol red using RT-PCR, Northern blot, and immunohistochemistry. 6 We confirmed these data in both the Fas-positive and Fas-negative cell lines using immunohistochemistry (data not shown) and RT-PCR (Figure 6) ▶ . Only the positive control (breast cancer MCF-7 cells) contains the 350 bp estrogen receptor product (Figure 6 ▶ , lane 2). In contrast, both Fas-negative (lane 3) and Fas-positive (lane 4) cells are negative for the estrogen receptor. The migration of the glyceraldehyde-3-phosphate dehydrogenase (GPDH) housekeeper product is also labeled in Figure 6 ▶ . Furthermore, to assess a possible functional role of the estrogen receptor in Fas-mediated apoptosis, we tested the effect of ICI 182780, a pure anti-estrogenic compound on apoptosis. ICI 182780 did not induce apoptosis at low (10 nmol/L) and high (10 μmol/L) concentrations in both Fas-negative and Fas-positive cells (Figure 7) ▶ .
Figure 6.
RT-PCR for estrogen receptors in human cholangiocarcinoma cells. The mRNAs of Fas-negative, Fas-positive cholangiocarcinoma cells and MCF-7 breast cancer cells as a positive control were isolated using RNAzon kit and RT-PCR for the estrogen receptor was performed as described in Materials and Methods. PCR products were electrophoresed on 1% agarose gel and stained with ethidium bromide. Lane 1 is DNA marker. Lane 2 represents the positive control MCF-7 breast cancer cells. Lane 3 represents Fas-negative cells. Lane 4 represents Fas-positive human cholangiocarcinoma cells. Migration of GPDH housekeeping products (450 bp) and estrogen products (350 bp) are labeled.
Figure 7.
The effect of ICI 182780 on apoptosis in cholangiocarcinoma cells. A total of 10 5 cells per 1 ml medium were seeded into 12-well plate and incubated for 12 hours. ICI at 10 nmol/L and 10 μmol/L were added into wells and incubated for 24 hours. Cells were TUNEL-stained. Left panels: Fas-negative cells; ICI at 10 nmol/L (upper) and 10 μmol/L (bottom). Right panels: Fas-positive cells; ICI at 10 nmol/L (upper) and ICI at 10 μmol/L (bottom).
Growth of Fas-Negative and Fas-Positive Cell Xenografts in Nude Mice
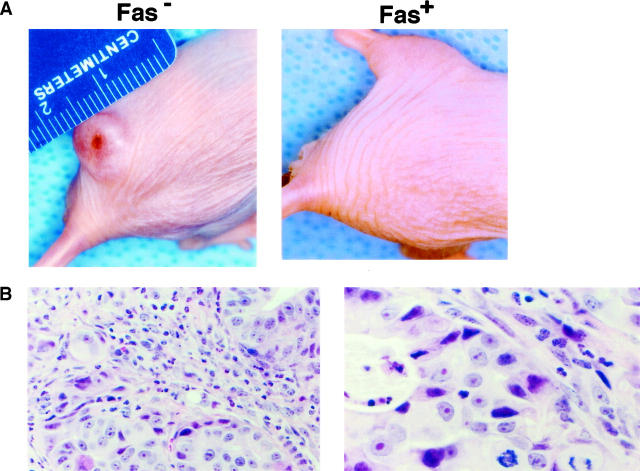
We determined the tumorigenicity of cloned Fas-negative and Fas-positive cholangiocarcinoma cells in nude mice. Cultured Fas-negative and Fas-positive cells (5 × 106) were subcutaneously injected into six female nude mice and growth of tumors determined. After 2 weeks, Fas-negative cells grew in all six nude mice forming tumors measuring 1–2 cm in greatest diameter (Figure 8A ▶ , Fas-negative). In contrast, Fas-positive cells did not form tumors in any of the six animals (Figure 8A ▶ , Fas-positive). Representative microscopic sections of H and E stains of the tumors is shown in Figure 8B ▶ , at both low and high powers. As can be seen, the tumors are characteristic adenocarcinomas, forming glandular structures and demonstrating a high degree of nuclear pleomorphism and prominent nucleoli. RT-PCR for Fas performed on four of the tumors confirmed that they continued to be Fas-negative (data not shown).
Figure 8.
Tumorigenesis of Fas-negative and Fas-positive human cholangiocarcinoma cells in nude mice. Fas-negative and Fas-positive cells (5 × 106) were inoculated subcutaneously into the flanks of nude mice in a total volume of 0.2 ml/site. Two weeks were allowed for tumor engraftment. The tumor engraftment rate of Fas-negative cells was 100% (n = 6) compared to 0% for Fas-positive cells (n = 6). A: A typical tumor in an animal injected with Fas-negative cells (left) and lack of tumor in the animal injected with Fas-positive cells (right). B: Representative hematoxylin and eosin staining of Fas-negative tumor. Original magnification, ×250 (left) and ×1000 (right).
Discussion
Cholangiocarcinoma is a highly malignant tumor of the biliary tree with less than 10% 5-year overall survival. 50-53 Currently, there are minimal opportunities for medical or surgical cure; therefore, new modalities of treatment are needed. 6 Here we show that cultured human cholangiocarcinoma cells heterogeneously express Fas, a receptor known to mediate apoptosis. The majority of cells (80%) fail to express Fas or only weakly express this receptor. This lack of expression of Fas, a major inducer of apoptotic cell death, may result in the failure of human cholangiocarcinoma to respond to current treatments and be responsible, in part, for the poor prognosis of this malignancy. 28,36,54 To further explore these hypotheses, we isolated and cloned Fas-negative and Fas-positive subpopulations of cultured human cholangiocarcinoma cells. The stability of Fas expression on Fas-negative and Fas-positive cells was also characterized by incubating cells for 2 to 6 weeks. Our data show that the percentage of Fas expressing cells in Fas-positive and Fas-negative clones remained at 90% and 10%, respectively, during the period of 6 weeks (data not shown). Therefore, these stable cell lines, one expressing Fas and the other not expressing Fas, provide an excellent model for studying the molecular mechanisms of Fas-mediated apoptosis. Using this two-cell model, we compared the sensitivities of apoptosis induction in vitro by Fas antibody, TMX, and TFP. Fas-negative cells are resistant, whereas Fas-positive cells are sensitive, to apoptosis induced by all three reagents. Furthermore, when Fas-negative and Fas-positive cells were subcutaneously inoculated into nude mice, Fas-negative, but not Fas-positive, cholangiocarcinoma cells produced tumors. These studies indicate that the deficiency of Fas expression may be associated with the pathogenesis of tumors and their resistance to anti-tumor drugs. Understanding the underlying molecular events and responses to therapeutic agents may lead to new therapeutic modalities.
TMX is an anti-cancer drug widely used in the treatment of breast cancer and other malignancies that do not express estrogen receptor. 55-58 It has previously been found to have an inhibitory effect on the growth of human cholangiocarcinoma in vitro and in vivo. 6 The molecular basis for the anti-tumor effect of TMX is not well understood. However, it may result not only from competitive interaction with the estrogen receptor, but also from its effects on numerous other potential cellular targets including calmodulin, protein kinase C, chloride channels, and secretion of TGF-β. 18-25 The relative importance of these various targets likely depends upon numerous variables, including the species studied, the target organ, and the amount of TMX used. In this study, TMX induces dose-dependent apoptosis in cultured Fas-positive, but not Fas-negative, human cholangiocarcinoma cells, which is blocked by an inhibitory Fas antibody, suggesting that TMX-induced apoptosis may be involved in a Fas-dependent mechanism. The apoptosis-inducing effect of TMX is duplicated by TFP, a classical calmodulin antagonist. Both agents induce apoptosis in Fas-positive cholangiocarcinoma cells at concentrations that inhibit calmodulin-dependent processes. 59-60 The lack of estrogen receptors and of an effect by the pure anti-estrogenic compound, ICI 182780, on cultured Fas-negative and Fas-positive cells indicate that the molecular mechanism by which TMX stimulates apoptosis in Fas-positive cells could not be explained simply by anti-estrogen effects of TMX. Our data support the concept that the pro-apoptotic effect of TMX on Fas-positive cholangiocarcinoma cells is due to calmodulin antagonism. However, it is interesting that TMX has a 25% effect on cell death in Fas negative cells, possibly reflecting contamination with Fas-positive cells.
There are several potential molecular sites of action for TMX and TFP as calmodulin antagonists for activating apoptosis. 61 However, the specific sites have not yet been identified. Calmodulin antagonism is the likely key common event and both Ca2+ and calmodulin are involved at various molecular levels in apoptotic signaling. For example, a recently discovered calcium/calmodulin-dependent serine/threonine kinase, DAP kinase, has been shown to increase tumor necrosis factor α-mediated apoptosis. 59 Interestingly, expression of DAP kinase was inversely related to the metastatic capability of carcinoma cells. Calcineurin, a calmodulin/Ca2+-dependent phosphatase, has been implicated in mediating apoptosis, either directly or by binding BCL-2. 62 Alterations in intracellular Ca2+, which would be expected to be affected by calmodulin antagonists have been implicated at several sites in the apoptotic signaling pathways, including mediating Ca2+-activated endonucleases, Ca2+-activated proteases, 63 nuclear scaffold proteases, 64 and Ca2+-dependent transglutaminases. 65
The minimum concentration of TMX required to induce apoptosis in vitro in Fas-positive human cholangiocarcinoma is 1–5 μmol/L. This effective concentration of 5 μmol/L in vitro raises the question of a possible link between TMX dose and treatment response in vivo. The commonly used dose of TMX in current clinical therapy is 10–20 mg per day, which achieves a highly variable steady-state plasma concentration of TMX 0.13–0.35 μmol/L. 66 Doses of TMX as high as 460 mg/day have been administered to treat patients with advanced solid tumors. Using this dose, steady-state plasma concentrations of TMX and its active metabolite, N-desmethyl TMX, concentrations greater than 5.0 μmol/L were achieved in 82% of patients, and concentrations greater than 10 μmol/L in 18% of patients. 67 Although there are a number of factors such as tumor location, vascularity, stage of disease, and age that influence the concentration of TMX at the site of the tumor, plasma concentrations can be achieved that are similar to those required in our in vitro studies to induce apoptosis of cholangiocarcinoma cells.
The potential importance of Fas expression in carcinogenesis is emphasized by the tumorigenic capability of only the Fas-negative cells when injected into nude mice. Fas-positive cells did not produce any tumors, suggesting that Fas-positive cells, but not Fas-negative cells, were killed when injected subcutaneously. Fas ligand (the natural ligand for Fas) may be the in vivo biological mediator stimulating apoptosis, thus preventing growth of tumors. Fas ligand is expressed on thyroid, 68 various epithelial cells, 69 and cornea, 70 and is also present in a soluble form. 71 Therefore, endogenous Fas ligand is a likely natural mechanism for killing the Fas-positive cholangiocarcinoma cells, resulting in their failure to grow and produce tumors. Alternatively, the Fas phenotype may be associated with other endogenous cellular factors that promote tumorigenesis and lack the Fas-positive phenotype. Fas ligand expression on tumor cells may also provide protection of the cells from immune killing. Evidence is now accumulating that many tumors, including colon carcinoma, melanoma, hepatocellular carcinoma, pancreatic carcinoma, and astrocytoma, may express Fas ligand. These Fas ligand-expressing tumor cells may have two functions. First, they may deliver a death signal to Fas-expressing T lymphocytes to escape immune system through Fas-Fas ligand interaction. To date, evidence in vivo has been obtained to support this in a murine melanoma model, which had decreased growth in lpr mice (expressing minimal or no Fas) compared with normal or gld mice (defect in Fas ligand). 52 Second, Fas-expressing tumor cells may also be activated in vivo by some unknown mechanisms to kill Fas-positive tumor cells (suicide apoptosis), leaving only Fas-negative tumor cells. Consistent with this hypothesis, some tumors spontaneously regress and often have large lymphocytic infiltrates, supporting the concept of a crucial involvement of the Fas system in tumorigenesis. 53
In conclusion, the data show that TMX stimulates apoptotic cell death in human cholangiocarcinoma cells and this is likely mediated through the Fas/APO-1 (CD95) signaling pathway via a calmodulin-dependent mechanism. The heterogeneous expression of Fas surface protein on cholangiocarcinoma cells may be useful prospectively to predict both malignant potential and responsiveness to therapy. These hypotheses will be explored in future experiments focused on underlying molecular mechanisms, tumorigenesis, and therapy.
Acknowledgments
We thank Dr. Rob Hardy for his helpful discussion and outstanding suggestions and Marsha Moore for her expert editorial assistance.
Footnotes
Address reprint requests to Jay M. McDonald, M.D., Professor and Chair, Department of Pathology, University of Alabama at Birmingham, 701 South 19th Street, 509 LHRB, Birmingham, Alabama 35294-0007.
Supported in part by National Institutes of Health grants CA72823, CA72823-S, and Veterans Affairs Merit Review (all to J. M. M.).
References
- 1.Perry RR, Kang Y, Greaves B: Effect of tamoxifen on growth and apoptosis of estrogen-dependent and -independent human breast cancer cells. Ann Surg Oncol 1995, 2:238-245 [DOI] [PubMed] [Google Scholar]
- 2.Kang Y, Cortina R, Perry RR: Role of c-myc in tamoxifen-induced apoptosis estrogen-independent breast cancer. J Natl Cancer Inst 1996, 88:279-284 [DOI] [PubMed] [Google Scholar]
- 3.Frankfurt OS, Sugarbaker EV, Robb JA, Villa L: Synergistic induction of apoptosis in breast cancer cells by tamoxifen and calmodulin inhibitors. Cancer Lett 1995, 97:149-154 [DOI] [PubMed] [Google Scholar]
- 4.Love RR: Tamoxifen therapy in primary breast cancer: biology, efficacy, side effects. J Clin Oncol 1989, 7:8013-8015 [DOI] [PubMed] [Google Scholar]
- 5.Butta A, Maclennan K, Flanders KC, Sacks NP, Smith I, McKinna A, Dowsett M, Wakefield LM, Sporn MB, Baum M, Colletta AA: Introduction of transforming growth factor β1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res 1992, 52:4261-4264 [PubMed] [Google Scholar]
- 6.Sampson LK, Vickers SM, Ying W, Phillips JO: Tamoxifen-mediated growth inhibition of human cholangiocarcinoma. Cancer Res 1997, 57:1743-1749 [PubMed] [Google Scholar]
- 7.Taylor OM, Benson EA, McMahon MJ: Clinical trial of tamoxifen in patients with irresectable pancreatic adenocarcinoma. The Yorkshire Gastrointestinal Tumor Group. Br J Surg 1993, 80:384-386 [DOI] [PubMed] [Google Scholar]
- 8.Pollack IF, Randall MS, Kristofik MP, Kelly RH, Selker RG, Vertosicky FT: Effects of tamoxifen on DNA synthesis and proliferation of human malignant glioma lines in vitro. Cancer Res 1990, 50:7134-7138 [PubMed] [Google Scholar]
- 9.Couldwell WT, Weiss MH, DiGiorgio LM, Weiner LP, Hinton DR, Ehsesmann LR, Corti PS, Apuzzo MLJ: Clinical and radiographic response in a minority of patients with recurrent malignant gliomas treated with high-dose tamoxifen. Neurosurgery 1993, 32:485-490 [DOI] [PubMed] [Google Scholar]
- 10.Gelmann EP: Tamoxifen for the treatment of malignancies other than breast and endometrial carcinoma. Semin Oncol 1997, 24(suppl 1):S65-S70 [PubMed] [Google Scholar]
- 11.Ward RL, Morgan G, Falley D, Kelly PJ: Tamoxifen reduces bone turnover and prevents lumbar spine proximal femoral bone loss in early postmenopausal women. Bone Miner 1993, 22:87-94 [DOI] [PubMed] [Google Scholar]
- 12.Recker RR: Clinical review 41: current therapy for osteoporosis. J Clin Endocrinol Metab 1993, 76:14-16 [DOI] [PubMed] [Google Scholar]
- 13.Grainger DJ, Witchell CM, Metcalfe JC: Tamoxifen elevates transforming growth factor-β suppresses diet-induced formation of lipid lesions in mouse aorta. Nat Med 1995, 1:1067-1073 [DOI] [PubMed] [Google Scholar]
- 14.Reckless J, Metcalfe JC, Grainger DJ: Tamoxifen decreases cholesterol seven-fold and abolishes lipid lesion development in apolipoprotein E knock out mice. Circulation 1997, 95:1542-1548 [DOI] [PubMed] [Google Scholar]
- 15.Sthoeger Z, Dayan M, Zinger H, Kalush F, Mor G, Zlatman YA, Kohen F, Mozes E: Treatments with tamoxifen and an antiestradiol antibody have beneficial effects on experimental SLE via cytokine modulation. Ann N Y Acad Sci 1997, 815:367-368 [DOI] [PubMed] [Google Scholar]
- 16.Dayan M, Zinger H, Kalush F, Mor G, Amir-Zaltzman Y, Kohen F, Sthoeger Z, Mozes E: The beneficial effects of treatment with tamoxifen and anti-estradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology 1997, 90:101-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grainger DJ, Metcalfe JC: Tamoxifen: teaching an old drug new tricks. Nat Med 1996, 2:381-385 [DOI] [PubMed] [Google Scholar]
- 18.Santambrogio L, Hochwald GM, Saxene B, Len CH, Martz JE, Carlino JA, Ruddle NH, Paladino MA, Gold LI, Thorbecke GJ: Studies on the mechanisms by which transforming growth factor-β protects against allergic encephalomyelitis-antagonism between TGF-β and tumor necrosis factor. J Immunol 1993, 151:1116-1127 [PubMed] [Google Scholar]
- 19.Benson JR, Baum M: Modulation of transforming growth factor β expression and induction of apoptosis by tamoxifen in ER positive and ER negative breast cancer cells. Br J Cancer 1995, 72:1441-1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce DF, Gorska AE, Jr, Chytil A, Meise KS, Page DL, Coffey RJ, Moses HL, Jr: Mammary tumor suppression by transforming growth factor β1 transgene expression. Proc Natl Acad Sci USA 1995, 92:4254-4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein B, Yang MX: Repression of the interleukine-6 promoter by estrogen receptor is mediated by NFκB and C/EBPB. Mol Cell Biol 1995, 15:4971-4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopes MCF, Vale MGP, Carvalho AP: Ca2(+)-dependent binding of tamoxifen to calmodulin isolated from bovine brain. Cancer Res 1990, 50:2753-2758 [PubMed] [Google Scholar]
- 23.MacNeil S, Dawson RA, Crocker G, Tucker WF, Bittiner B, Singleton JG, Hunter T, Tierney DF: Antiproliferative effects on keratinocytes of a range of clinically used drugs with calmodulin antagonist activity. Br J Dermatol 1993, 128:143-150 [DOI] [PubMed] [Google Scholar]
- 24.Nilius B, Prenen J, Szucs G, Wei L, Tanzi F, Voets T, Droogmans G: Calcium-acitated chloride channels in bovine pulmonary artery endothelial cells. J Physiol 1997, 498:381-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brian CA, Liskamp RM, Solomon DH, Weinstein IB: Inhibition of protein kinase C by tamoxifen. Cancer Res 1985, 45:2462-2465 [PubMed] [Google Scholar]
- 26.Yonehara S, Ishii A, Yonehara M: A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med 1989, 169:1747-1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trauth BC, Klas C, Peter AMJ, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH: Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989, 245:301-305 [DOI] [PubMed] [Google Scholar]
- 28.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima SI, Sameshima M, Hase A, Seto Y, Nagata S: The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66:233-243 [DOI] [PubMed] [Google Scholar]
- 29.Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, LiWever M, Richards S, Dhein J, Trauth BC, Ponstingl H, Krammer PH: Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem 1992, 267:10709-10715 [PubMed] [Google Scholar]
- 30.Itoh N, Nagata S: A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem 1993, 268:10932-10937 [PubMed] [Google Scholar]
- 31.Tartaglia LA, Ayres TM, Wong GHW, Goeddel DV: A novel domain within the 55 kDa TNF receptor signals cell death. Cell 1993, 74:845-853 [DOI] [PubMed] [Google Scholar]
- 32.Watanabe-Fukenaga R, Brannan CI, Itoh N, Yonehara S, Copeland NG, Jenkins NA, Nagata S: The cDNA structure, expression and chromosomal assignment of the mouse Fas antigen. J Immunol 1992, 125:871-876 [PubMed] [Google Scholar]
- 33.Watanabe-Fukenaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S: Lymphoproliferation disorders in mice explained by defects in Fas antigen that mediated apoptosis. Nature 1992, 356:314-317 [DOI] [PubMed] [Google Scholar]
- 34.Wu JX: Apoptosis and antiogenesis: two promising tumor markers in breast cancer. Anticancer Res 1996, 16:2233-2240 [PubMed] [Google Scholar]
- 35.Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267:1456-1462 [DOI] [PubMed] [Google Scholar]
- 36.Trauth BC, Klas C, Peter AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH: Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989, 245:301-305 [DOI] [PubMed] [Google Scholar]
- 37.Dhein J, Daniel PT, Trauth BC, Oehm A, Moller P, Krammer PH: Induction of apoptosis by monoclonal anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J Immunol 1992, 149:3166-3173 [PubMed] [Google Scholar]
- 38.Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, Schmidt A, Debatin KM, Krammer PH, Moller P: Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest 1993, 69:415-429 [PubMed] [Google Scholar]
- 39.Midis GP, Shen Y, Owen-Schaub LB: Elevated soluble Fas (sFas) levels in nonhematopoietic human malignancy. Cancer Res 1996, 56:3870-3874 [PubMed] [Google Scholar]
- 40.Wang GHW, Goeddel DV: Fas antigen and p55 TNF receptor signal apoptosis through distinct pathway. J Immunol 1994, 152:1751-1755 [PubMed] [Google Scholar]
- 41.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD: Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 1994, 263:1759-1762 [DOI] [PubMed] [Google Scholar]
- 42.Owen-Schaub LB, Radinsky R, Kruzel E, Berry K, Yonehara S: Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological response. Cancer Res 1994, 54:1580-1586 [PubMed] [Google Scholar]
- 43.O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: a molecular mechanism of tumor immune privilege. Mol Med 1997, 3:294-300 [PMC free article] [PubMed] [Google Scholar]
- 44.Debatin KM, Beltinger C, Bohler T, Fellenberg J, Friesen C, Fulda S, Herr I, Los M, Scheuerpflug C, Sieverts H, Stahnke K: Regulation of apoptosis through CD95 (APO-I/Fas) receptor-ligand interaction. Biochem Soc Trans 1997, 25:405-410 [DOI] [PubMed] [Google Scholar]
- 45.Radding W, Pan ZQ, Hunter E, Johnston P, Williams JP, McDonald JM: Expression of HIV-1 envelope glycoprotein alters cellular calmodulin. Biochem Biophys Res Commun 1996, 218:192-197 [DOI] [PubMed] [Google Scholar]
- 46.Pan ZQ, Radding W, Zhou T, Hunter E, Mountz JD, McDonald JM: Role of calmodulin in HIV-potentiated Fas-mediated apoptosis. Am J Pathol 1996, 149:903-910 [PMC free article] [PubMed] [Google Scholar]
- 47.Pan G, Zhou T, Radding W, Saag MS, Mountz JD, McDonald JM: Calmodulin antagonists inhibit apoptosis of CD4+ T-cells from patients with AIDS. Immunopharmacol 1998, 40:91-103 [DOI] [PubMed] [Google Scholar]
- 48.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Kloppel G, Lemoine NR: Loss of membrane E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol 1994, 174:243-248 [DOI] [PubMed] [Google Scholar]
- 49.Janckwski JA, Newham PM, Kemic O, Hirano S, Takeichi M, Pignatelli M: Differential expression of E-cadherin in normal, metaplastic and dysplastic oesophageal mucosa: a purative biomarker. Int J Oncol 1994, 4:441-448 [DOI] [PubMed] [Google Scholar]
- 50.Taoka H, Kawarada Y: Intrahepatic bile duct carcinoma (cholangiocarcinoma). J Jpn Surg Soc 1997, 98:484-490 [PubMed] [Google Scholar]
- 51.Chou FF, Sheen-Chen SM, Chen YS, Chen MC, Chen CL: Surgical treatment of cholangiocarcinoma. Hepato-Gastroenterol 1997, 44:760-765 [PubMed] [Google Scholar]
- 52.Leung JT, Kuan R: Intraluminal brachytherapy in the treatment of bile duct carcinomas. Australas Radiol 1997, 41:151-154 [DOI] [PubMed] [Google Scholar]
- 53.Kawamura K, Grabowski D, Krivacic K, Hidaka H, Ganapathi R: Cellular events involved in the sensitization of etoposide-resistant cells by inhibitors of calcium-calmodulin-dependent processes: role for effects on apoptosis, DNA cleavable complex, and phosphorylation. Biochem Pharmacol 1996, 52:1903-1909 [DOI] [PubMed] [Google Scholar]
- 54.Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 55.Couldwell WT, Hinton DR, He S, Chen TC, Sebat I, Weiss MS, Law RE: Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS Lett 1994, 345:43-46 [DOI] [PubMed] [Google Scholar]
- 56.Kang Y, Cortina R, Perry RR: Role of c-myc in tamoxifen-induced apoptosis in estrogen-independent breast cancer cells. J Natl Cancer Inst 1996, 88:279-284 [DOI] [PubMed] [Google Scholar]
- 57.Perry RR, Kang Y, Greaves B: Effects of tamoxifen on growth and apoptosis of estrogen-dependent -independent human breast cancer cells. Ann Surg Oncol 1995, 2:238-245 [DOI] [PubMed] [Google Scholar]
- 58.Vertosick FT, Jr, Selker RG, Rall MS, Kristofik MP, Rehn T: A comparison of the relative chemosensitivity of human gliomas to tamoxifen and n-desmethyltamoxifen in vitro. J Neurooncol 1994, 19:97-103 [DOI] [PubMed] [Google Scholar]
- 59.Inbal B, Cohen O, Polak-Charcon S, Kopolovic J, Vadai E, Eisenbach L, Kimchi A: DAP kinase links the control of apoptosis to metastasis. Nature 1997, 390:180-184 [DOI] [PubMed] [Google Scholar]
- 60.Maeda S, Suzuki A, Lin KH, Inagaki H, Saito T: DNA fragmentation induced in high-cell-density culture of primary rat hepatocytes is an active process dependent on energy availability, gene expression, and calmodulin. J Biochem 1995, 118:161-1165 [DOI] [PubMed] [Google Scholar]
- 61.McConkey DJ, Orrenius S: Breakthrough views: the role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun 1997, 239:357-366 [DOI] [PubMed] [Google Scholar]
- 62.Baffy G, Miyashita T, Williamson JR, Reed JC: Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced BCL-2 oncoprotein production. J Biol Chem 1993, 268:6511-6519 [PubMed] [Google Scholar]
- 63.Squier MKT, Miller ACK, Malkinson AM, Cohen JJ: Calpain activation in apoptosis. J Cell Physiol 1994, 159:229-237 [DOI] [PubMed] [Google Scholar]
- 64.Grimm LM, Goldberg AL, Poirier GG, Schwartz LM, Osborne BA: Proteasomes play an essential role in thymocyte apoptosis. EMBO J 1996, 15:3835-3844 [PMC free article] [PubMed] [Google Scholar]
- 65.Melino G, Annicchiarico-Petruzzeli M, Piredda L, Candi E, Gentile V, Davies PJ, Piacentini M: Tissue transglutaminase apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol 1994, 14:6584-6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Catherino WH, Jordan VC: A risk-benefit assessment of tamoxifen therapy. Drug Saf 1993, 8:381-397 [DOI] [PubMed] [Google Scholar]
- 67.Millward MJ, Lien EA, Robinson A, Cantwell BMJ: High-dose (480 mg/day) tamoxifen with etoposide: a study of a potential multi-drug resistance modulator. Oncology 1994, 51:79-83 [DOI] [PubMed] [Google Scholar]
- 68.Martinez-Lorenzo MJ, Alava MA, Anel A, Naval J: Release of preformed Fas ligand in soluble form is the major factor for activation-induced death of Jurkat T cells. Immunology 1996, 89:511-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mori T, Xu JP, Mori E, Sato E, Saito S, Guo MW: Expression of Fas-Fas ligand system associated with atresia through apoptosis in murine ovary. Horm Res 1997, 48(suppl 3):11-19 [DOI] [PubMed] [Google Scholar]
- 70.Wilson SE, Li Q, Weng J, Barry-Lane PA, Jester JV, Liang Q, Wordinger RJ: The Fas-Fas ligand system and other modulators of apoptosis in the cornea. Invest Ophthalmol Vis Sci 1996, 37:1582-1592 [PubMed] [Google Scholar]
- 71.Martinez-Lorenzo MJ, Alava MA, Anel A, Pineiro A, Naval J: Release of performed Fas ligand in soluble form is the major factor for activation-induced death of Jurkat T cells. Immunology 1996, 89:511-517 [DOI] [PMC free article] [PubMed] [Google Scholar]