Abstract
The stromal microenvironment plays a crucial role in tumor development and progression. One of the most potent activators of stromal cells is the platelet-derived growth factor (PDGF). To investigate the role of PDGF in epithelial tumor development we stably transfected immortal nontumorigenic human keratinocytes with the PDGF-B cDNA. Transfected HaCaT cells overexpressed PDGF-B but remained negative for the PDGF receptors α and β (mRNA). Thus, they did not exhibit autocrine growth stimulation in vitro, but proliferation of cocultured fibroblasts was enhanced and this effect was inhibited by a neutralizing antibody to PDGF-BB. After subcutaneous injection into nude mice the transfected cells maintained high PDGF expression and formed progressively enlarging, rapidly proliferating cysts, classified as benign tumors. During early tumor development (up to 2 months), PDGF-B transfectants induced marked mesenchymal cell proliferation and angiogenesis, yet this effect vanished at later stages (2–6 months) concomitantly with increased epithelial cell proliferation and enhanced tumor growth. These results demonstrate that an activated stromal environment can promote tumorigenic conversion of nontumorigenic keratinocytes by inducing sustained epithelial hyperproliferation. This effect is apparently caused by a dual action of PDGF-BB: (i) PDGF-BB can promote tumor growth by inducing angiogenesis and stroma formation, and (ii) PDGF-activated stromal cells maintain elevated keratinocyte proliferation via a paracrine mechanism. Thus, PDGF, a major factor activated in wound healing, may play an important role as an endogenous promoter in epithelial tumor formation.
The significance of the microenvironment for tumor development has been well documented in experimental in vivo systems. Both the composition of the extracellular matrix and the functional state of the mesenchymal cells seem to play an important role for tumor cell growth, invasion, and metastasis (1, 2). Several reports have demonstrated growth-promoting effects of activated stromal cells on tumor cells (3–5), but their precise contribution to epithelial tumor development and progression is much less understood.
The platelet-derived growth factor (PDGF) is a potent mitogen and chemoattractant for mesenchymal cells and plays an important role in wound healing (reviewed in ref. 6). PDGF is a 30-kDa dimer of the polypeptide chains A and B, linked by disulfide bonds. The PDGF isoforms (PDGF-AA, PDGF-AB, and PDGF-BB) exert their effects by interacting with two tyrosine kinase receptors. The α-receptor (PDGF-αR) binds all three isoforms, whereas the β-receptor (PDGF-βR) only binds PDGF-BB with high affinity. In wounds, the expression of the PDGF-βR is up-regulated in connective tissue (7, 8) and epithelial cells (9) concomitantly with an increased production of PDGF, indicating a powerful role of PDGF acting via paracrine and autocrine mechanisms. PDGF-BB is also up-regulated in a number of tumor cell lines (10, 11), which do not express the PDGF receptors suggesting its paracrine role in tumorigenesis (12). However, while a PDGF autocrine loop for tumors of mesenchymal origin has been well established (13), there is mainly circumstantial evidence in support of its paracrine function. The only direct evidence for that comes from the study of PDGF-B-transfected melanoma cells devoid of PDGF-receptors. These PDGF-B-overexpressing tumors induce a well-vascularized connective tissue and, as a consequence, demonstrate accelerated growth rate (14).
In this report we demonstrate that activated stromal cells induce tumorigenic conversion of stably nontumorigenic immortalized human keratinocytes (HaCaT) (15). Stromal cells, stimulated by the continuous overexpression of PDGF-BB by stably transfected HaCaT cells sustain keratinocyte proliferation, most probably by a paracrine mechanism leading to the formation of benign epithelial tumors. This indicates that tumorigenic progression of intrinsically nontumorigenic epithelial cells can be induced solely by altering the microenvironment. We suggest that PDGF present at injury sites and in certain chronic inflammatory diseases may significantly contribute to early stages of epithelial skin tumor formation and possibly other carcinomas associated with wounding and inflammation.
MATERIALS AND METHODS
cDNAs.
The expression vector pcDNA1 (Invitrogen), containing the human PDGF-B cDNA (0.7 kb HindIII-BamHI fragment) under the transcriptional control of the cytomegalovirus promoter and a gene encoding geneticin resistance (14), was a gift from B. Westermark (University Hospital, Uppsala, Sweden). Human PDGF-α and -β receptor cDNAs (16) were provided by L. Claesson-Welsh (Ludwig Institute for Cancer Research, Uppsala, Sweden). The plasmid containing cDNA for glyceraldehyde-3-phosphate dehydrogenase was purchased from American Type Culture Collection.
Antibodies.
A mouse mAb against BrdUrd was obtained from BioCell Consulting (Rheinach, Switzerland), fluorescein isothiocyanate-conjugated anti-mouse IgG from Sigma. The goat anti-human PDGF-BB antibody was obtained from R & D Systems and the mAb against collagen IV from Institut Pasteur (Lyon, France). The rat anti-mouse platelet/endothelial cell adhesion molecule IgG (anti-CD31 antibody) was a gift from E. Dejana (Instituto Mario Negri, Milan). Both staining procedures give identical results to visualize blood vessels. When combined with anti-BrdUrd staining the anti-collagen IV labeling was better preserved and thus this procedure was preferred (17). Respective secondary antibodies were purchased from Dianova (Hamburg, Germany).
Transfection.
The human keratinocyte cell line HaCaT was grown in 4 × MEM supplemented with 5% fetal calf serum as described (18). Cells (passage 30) were transfected with the expression vector containing PDGF-B cDNA or with the vector alone using the Ca2+-phosphate precipitation method as described (19). Clones were selected in media containing 400 μg/ml geneticin (GIBCO/BRL), isolated with cloning rings, and individually expanded.
Molecular Analysis of Transfected Cells.
Selected clones were examined for integration of the plasmids by Southern blot analysis. Genomic DNA was isolated by the SDS lysis method, digested overnight at 37°C with either BamHI or HindIII restriction endonucleases, and electrophoresed in 0.8% agarose gels (10 μg DNA per lane). Gels were blotted onto Hybond-N+ filters (Amersham) and hybridized according to the manufacturer’s instructions. The 32P-labeled PDGF-B probe was prepared using a random priming kit (Amersham). Filters were exposed to Kodak XAR5 films for 6 days.
Expression of mRNA for PDGF-B and its receptors was analyzed by Northern blot hybridization. Total cellular RNA was isolated by the guanidinium thiocyanate method (20). Samples were electrophoresed in 1% formaldehyde gels (20 μg RNA per lane), and gels blotted onto Hybond-N+ filters. The 32P-labeled probes were prepared using a random priming kit and blots hybridized according to the manufacturer’s instructions (Amersham). Exposure times were 5 days for PDGF-B and PDGF receptors -α and -β, and 1 day for the glyceraldehyde-3-phosphate dehydrogenase probe.
Assay of Mitogenic Activity.
To assay the secretion of active PDGF-BB, the mitogenic activity of medium conditioned by transfected cells was determined in cocultures of normal human adult dermal fibroblasts and either control- or PDGF-B-transfected HaCaT cells. Fibroblasts were seeded at 4 × 103 cells per cm2 on the bottom of six multiwell tissue culture plates (Falcon) and transfected HaCaT cells at 4.6 × 104 cells per cm2 on the corresponding filter inserts (3.0 μm pore size) in 4 × MEM supplemented with 5% fetal calf serum. Cells were allowed to attach for 12 hr, washed three times with serum-free 4 × MEM and shifted to supplemented keratinocyte defined medium (Promo Cell, Heidelberg) containing 0.5 mM Ca2+ and 0.5% fetal calf serum. In parallel experiments, a neutralizing antibody to PDGF-BB (R & D Systems) was added to the medium (2 μg/ml). Medium was changed every 2 days. After 5 days cells were trypsinized and the number of fibroblasts and transfected cells determined. The experiment was repeated twice with duplicate samples.
In Vitro Growth Rate of Transfected Cells.
PDGF transfectants were plated in 24 multiwell plates (3 × 104 cells per well), trypsinized, and counted 15 hr later to assess the plating efficiency. Thereafter, cells were counted twice daily (three wells per cell line) over a 5-day period.
Tumorigenicity Test.
Each side of the back of 7- to 9-week-old nude mice (Swiss/c-nu/nu/backcrosses) was s.c. injected with 5 × 106 cells in 100 μl culture medium, eight animals per cell line. Tumor formation was assayed weekly over an observation period of 6 months by measuring the two maximal diameters. Tumors were removed when >100 mm2 and in two further series also after 1, 2, 4, 6, and 8 weeks and 1, 2, 3, and 4 weeks, respectively. Tissues were either fixed in buffered formalin for histological examination or embedded in Tissue-Tek (Miles) and frozen in liquid nitrogen for preparation of cryostat sections. For labeling of proliferating cells, mice received tail-vein injections of BrdUrd and 2-deoxycytidine (65 mM each) in saline (100 μl) 2 hr before sacrifice.
Indirect Immunofluorescence.
The staining procedure was performed as described (19). For staining of CD31 and collagen IV, cryostat sections were fixed in acetone and 80% methanol (17). For BrdUrd, sections were additionally incubated in 2 M HCl for 10 min. For staining of PDGF-BB, sections were fixed in 4% paraformaldehyde. Dilutions were 1:20 for anti-PDGF-BB, 1:100 for anti-BrdUrd and anti-collagen IV and 1:4 for the anti-CD31 antibody.
In Situ Hybridization.
The in situ hybridization was performed as described (21). In brief, 35S-labeled RNA probes were prepared using T3 or T7 RNA polymerase (for antisense and sense, respectively) according to the manufacturer’s instructions (Boehringer Mannheim). Cryostat sections were fixed in 4% paraformaldehyde, pretreated, hybridized, and washed at high stringency as described (21). For autoradiography, slides were coated with NTB2 film emulsion and exposed for 3 weeks. After developing, the sections were counterstained with hematoxylin and eosin.
RESULTS
Integration of PDGF-B cDNA in HaCaT Cells.
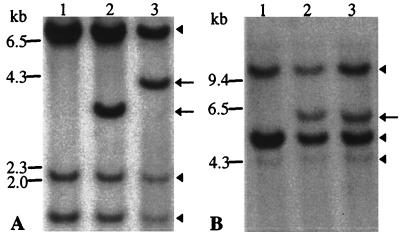
We stably transfected the immortalized but nontumorigenic skin keratinocyte cell line HaCaT with the PDGF-B cDNA in an expression vector (14). Cell clones, selected in medium containing geneticin and ring-isolated, were individually expanded and examined for the integration of plasmids. Southern analysis of the vector-transfected HaCaT cells (HaCaT/neo) revealed three bands corresponding to the endogenous PDGF-B gene (Fig. 1 A and B, lane 1), as in the parental HaCaT cells (data not shown). One additional band in the individual clones transfected with the PDGF-B cDNA (HaCaT/PDGF-B) demonstrated integration of the transfected PDGF-B gene (Fig. 1 A and B, lanes 2 and 3). Appearance of an extra band after the restriction digest with either BamHI or HindIII confirmed the integrity of the transfected gene. The pattern of integration (different BamHI fragment size) clearly distinguishes the two clones selected for further examination (clones 17 and 18) as independent transfection events.
Figure 1.
Southern analysis of the PDGF-B gene integration in HaCaT cells. Genomic DNA was digested with (A) BamHI or (B) HindIII. Three bands of identical pattern in each lane (arrowheads), correspond to the endogenous PDGF-B gene. An additional band in lanes 2 and 3 (arrows) represents the incorporated gene. Lanes: 1, HaCaT/neo; 2, HaCaT/PDGF-B/cl.17; 3, HaCaT/PDGF-B/cl.18.
Expression of PDGF-B and Its Receptors in HaCaT Cells.
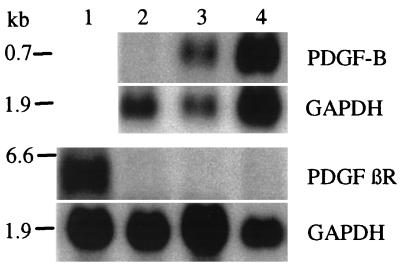
Since only the protein-coding part of the PDGF-B cDNA was used for transfection (14), transcription from the expression vector was to yield an mRNA of ≈0.7 kb that would be readily distinguishable from the endogenous PDGF-B transcripts (3.8 kb) (22). Northern blot analysis of the HaCaT/PDGF-B cells (clones 17 and 18) revealed abundant expression of a transcript of the expected size, whereas HaCaT/neo cells were negative (Fig. 2). Neither of the cell lines expressed mRNAs for the PDGF receptors β (Fig. 2) and α (data not shown). Parental and vector-transfected HaCaT cells were completely devoid of PDGF-B mRNA expression in vivo (data not shown).
Figure 2.
Northern analysis of PDGF-B and PDGF βR expression in cells transfected with PDGF-B (HaCaT/PDGF-B) or the vector only (HaCaT/neo). Hybridization with the PDGF-B probe revealed a 0.7 kb transcript corresponding to the transfected PDGF-B. All cell clones were negative for the PDGF βR, while this was positive in normal human dermal fibroblasts used as a control. Hybridization with a probe for glyceraldehyde-3-phosphate dehydrogenase indicates amounts of loaded RNA. Lanes: 1, fibroblasts; 2, HaCaT/neo; 3, HaCaT/PDGF-B/cl.17; 4, HaCaT/PDGF-B/cl.18.
PDGF-BB is not an Autocrine Growth Factor for HaCaT Cells.
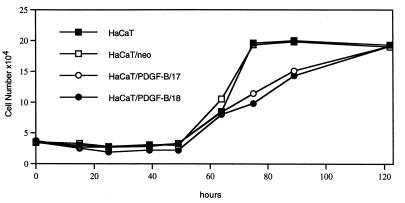
Due to the absence of the PDGF receptor autocrine effects on proliferation of HaCaT/PDGF-B cells were not to be expected. In fact, the PDGF-B overexpressing transfectants showed similar growth kinetics as the parental HaCaT and the HaCaT/neo cells (Fig. 3). In addition, administration of a neutralizing antibody to PDGF-BB did not affect the growth rate of HaCaT/PDGF-B cells (data not shown).
Figure 3.
In vitro growth kinetics of PDGF-B-transfected (clone 17 and 18) vs. control-transfected (HaCaT/neo) and parental HaCaT cells. Cells were plated in 24-multiwell plates and counted twice daily in triplicate and recorded as means of 3 measurements exhibiting standard deviations of <10%. Note the absence of stimulation in the growth of the PDGF-B transfected cells.
PDGF-B-Transfected HaCaT Cells Stimulate Proliferation of Fibroblasts.
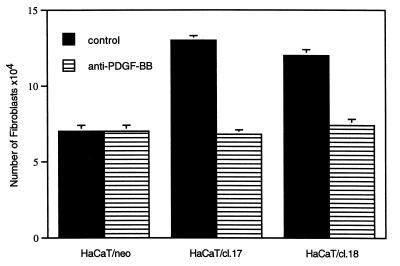
The ability of HaCaT/PDGF-B cells to secrete functionally active PDGF-BB was tested by means of their capacity to stimulate fibroblast proliferation in transfilter cocultures. When cocultured with HaCaT/neo cells, fibroblasts showed a 75% increase in cell number, whereas coculture with HaCaT/PDGF-B cells resulted in further increase in fibroblast number of 270% and 200% for clones 17 and 18, respectively, over 5 days (Fig. 4). The addition of a neutralizing antibody to PDGF-BB completely abrogated this enhanced growth-stimulatory effect (Fig. 4), indicating that the major mitogenic factor acting on fibroblasts and released by HaCaT/PDGF-B cells into the culture medium is indeed PDGF-BB.
Figure 4.
PDGF-B-transfected cells secrete a mitogenic activity for fibroblasts. Fibroblasts plated at 4 × 103 cells/cm2 (in multiwell plates) were cocultured with control-transfected (HaCaT/neo) or PDGF-B-transfected (clones 17 and 18) HaCaT cells (4 × 104 cells/cm2) (on filter inserts) in supplemented keratinocyte defined medium containing 0.5 mM Ca2+ and 0.5% fetal calf serum. Parallel cell cultures received a neutralizing antibody to PDGF-BB (2 μg/ml). The number of fibroblasts was determined after 5 days. Bars represent the mean of duplicate samples of two independent experiments ± SD.
Overexpression of PDGF-BB Renders HaCaT Cells Tumorigenic.
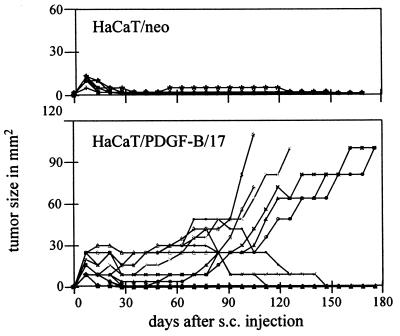
For the analysis of tumorigenicity, each cell type (HaCaT/neo and HaCaT/PDGF-B, clones 17 and 18) was subcutaneously injected into nude mice. Control-transfected cells showed no evidence of tumor growth over an observation period of 6 months (Fig. 5). After injection they formed small nodules that regressed rapidly within a few weeks, a feature identical in parental HaCaT cells (18, 23). In contrast, nodules formed by PDGF-B transfectants persisted for 6–8 weeks, and subsequently enlarged progressively (Fig. 5). In fact, five out of eight mice injected with clone 17, and seven out of eight mice injected with clone 18, developed tumors. The tumors obtained by both clones exhibited a similar growth profile (data for clone 18 not shown).
Figure 5.
Tumorigenicity of HaCaT/PDGF-B cells after s.c. injection into nude mice. Control-transfected cells (HaCaT/neo) showed no tumor growth, whereas HaCaT/PDGF-B cells (clone 17 shown as representative) developed tumors that progressed after a latency period of 6–8 weeks. Individual tumor sizes are given as the product of the two maximal diameters (mm2).
Morphology of Tumors.
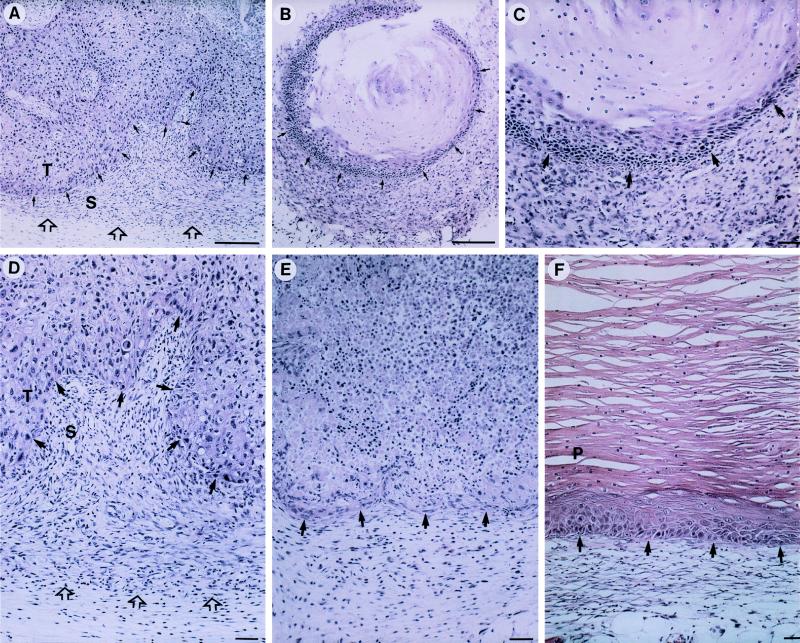
Histological examination of the nodules formed by HaCaT/PDGF-B cells 1 week after s.c. injection revealed the presence of numerous vital epithelial cells and only few necrotic foci. The nodules were surrounded by a cell-rich and well-vascularized stroma (Fig. 6 A and D). At the same time, only a small rim of vital HaCaT/neo cells was left, surrounding large necrotic areas. In contrast to HaCaT/PDGF-B cells, there was no prominent activation of the surrounding stromal tissue (Fig. 6E). While nodules of HaCaT/neo cells subsequently regressed, those formed by HaCaT/PDGF-B cells persisted with about the same size until 6–8 weeks after inoculation (see Fig. 5). Histological examination revealed the formation of benign cysts lined by a hyperplastic to slightly dysplastic, stratified, and keratinized epithelium (Fig. 6C). As a result of extensive keratinization, the nodules were filled with horn material. The newly developed cell-rich and well-vascularized stroma was still pronounced in 4- to 6-week-old nodules. The phenotype of progressively growing tumors (2- to 6-months old) was further clearly benign (Fig. 6F). A multilayered, hyperproliferative, and keratinizing epithelium with well-defined margins to the stroma surrounded a cyst filled with keratinized material. In comparison to cysts up to 6 weeks old, there was a remarkable decrease in the cellularity of the adjacent stroma (compare Fig. 6 C and F). This change in the composition of tumor stroma from early to late tumors was particularly evident when mesenchymal cell proliferation and vascular density were examined (Fig. 7 A and B). In the incipient nodules up to 3 weeks the surrounding mesenchyme was actively proliferating and rich in blood vessels, while only a few cells in the adjacent epithelium exhibited BrdUrd incorporation (Fig. 7A). In tumors of 4 weeks and older proliferative activity and vessel density decreased in the surrounding stroma of the enlarging tumors. This stromal reduction in cellularity and vascularization was accompanied by a drastic increase in proliferation in the multilayered epithelium as documented by the large number of BrdUrd-labeled cells in the two to three lowermost cell layers (Fig. 7b).
Figure 6.
Histology of s.c. tumors formed by PDGF-B-transfected cells. (A, D) Nodule formed by HaCaT/PDGF-B cells 1 week after inoculation. Note numerous vital epithelial cells (T = tumor cells) and pronounced stroma (S) restricted to the immediate vicinity of the tumor; (D) higher magnification of (A) with arrow heads delineating the border of the activated stroma to the host tissue. (E) Control-transfected cells (1 week after s.c. injection) display atrophy and provoke a weak stromal reaction. (B, C) At 4 weeks HaCaT/PDGF-B cells had formed benign epidermal cysts filled with horn material. Note pronounced stroma around the epithelial cysts in (C; higher magnification of B. (F) Part of a progressively growing tumor at 4 months, lined with a hyperplastic, stratified epithelium that exhibits prominent parakeratosis (p). Note considerably weakened stroma reaction as compared with A–D. (Bar = 50 μm.)
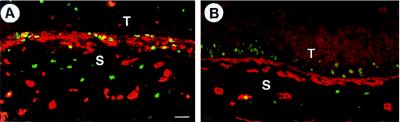
Figure 7.
Proliferation and angiogenesis in early and late s.c. HaCaT/PDGF-B tumors. 2–3 week-old nodules exhibit intense proliferation (BrdUrd-labeled nuclei, green) and increased vascularization (anti-collagen IV stained basement membranes of blood vessels, in red) in the activated stroma. Epithelial cell layers are vital but slowly proliferating (A). The keratinizing multilayered epithelium of progressively enlarging tumors (13 weeks, B) shows many proliferating (BrdUrd positive) cells in the lower layers, while the adjacent stroma cells are not labeled and vessel density is decreased (T = tumor cells; S = stroma). (Bar = 50 μm.)
In Vivo Expression of Transfected PDGF-B.
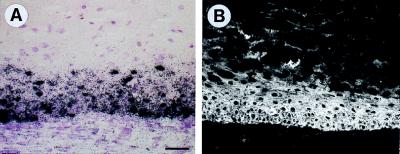
Continuously, strong expression of PDGF-B mRNA and protein in tumors was confirmed by in situ hybridization and immunofluorescence, respectively. Both RNA and protein were detected in all vital epithelial cells of early and late cystic tumors (Fig. 8).
Figure 8.
High expression of (A) PDGF-B mRNA (in situ hybridization) and (B) protein (immunofluorescence) in the epithelial rim of a 4-month-old tumor of HaCaT/PDGF-B cells (compare Fig. 6F). Bar = 50 μm.
DISCUSSION
The microenvironment plays a decisive role for tumor growth and metastatic spreading as shown in a variety of experimental systems (1, 2, 5). Moreover, regenerating and rapidly proliferating organs specifically enhance growth of transplanted tumor cells, suggesting paracrine growth regulation by diffusible factors. Those factors may promote tumor cell growth either directly through their receptor-mediated mechanism or indirectly by modifying the microenvironment. This may be achieved by modulation of the extracellular matrix, altering the cellular constituents of the stroma, and by inducing angiogenesis. Here we demonstrate that activated stromal cells not only support tumor growth but may induce tumorigenic conversion of epithelial cells. Stromal-cell activation occurs through mesenchymal growth factors such as PDGF overexpressed in the epithelial cells. In this “custom-making” process epithelial cells induce host cells to produce specific growth factors that in turn enhance epithelial cell proliferation. These findings further support the hypothesis that stromal cells are not passive companion cells but can play a crucial role in epithelial tumor development.
PDGF is primarily known as a mitogen and chemoattractant for mesenchymal cells. Accordingly, its role in the development of mesenchymal tumors, particularly gliomas and sarcomas, has been extensively studied (6, 13). In epithelial carcinogenesis, the role of PDGF is essentially unknown, but autocrine and paracrine functions have been postulated. In human carcinoma cell lines, expression of mesenchymal growth factors, such as PDGF, has been frequently observed as well as a potent stimulatory activity of their conditioned media on stromal fibroblasts (10, 11). In addition to direct growth stimulation, PDGF is a potent inducer of growth factor production in fibroblasts such as insulin-like growth factor (24, 25) and keratinocyte growth factor (26), both known as epithelial cell mitogens.
In this study we demonstrate that overexpression of PDGF-BB induces tumorigenic conversion of nontumorigenic though immortal human keratinocytes and provide several lines of evidence that this is based on a double paracrine mechanism. Integration and transcription of the transfected PDGF-B gene were verified by Southern and Northern blot analysis, respectively. The production of a functionally active protein was confirmed by the stimulation of fibroblast proliferation by conditioned medium of HaCaT/PDGF-B cells. This mitogenic effect was abolished by a neutralizing antibody to PDGF-BB, indicating that the major mitogenic effect on fibroblasts in the HaCaT/PDGF-B-conditioned media is caused by PDGF-BB. Several PDGF-BB isoforms have been identified so far; the 30-kDa species is a secreted form, whereas the 24-kDa and 32–34-kDa products are found in the cell and at the cell surface, respectively (6, 27). The fact that HaCaT/PDGF-B cells stimulated proliferation of fibroblasts in a transfilter coculture experiment, where close contact between the two cell types was prevented, confirms the production of a secreted PDGF-BB isoform.
Northern analysis revealed that HaCaT/PDGF-B cells were devoid of PDGF-receptor expression. In accordance, they do not show accelerated growth in culture compared with control-transfected cells, which also rules out an intracellular loop. Furthermore, addition of a neutralizing antibody to PDGF-BB in the transfilter experiment did not reduce the growth rate of HaCaT/PDGF-B cells. Taken together, these data strongly support the conclusion that HaCaT/PDGF-B cells are not growth-stimulated by PDGF-BB via an autocrine mechanism.
With regard to the fact that PDGF-BB is frequently expressed in tumor cells, and in view of its cognate receptors (particularly the β-chain) on stromal cells, its paracrine role in tumorigenesis by contributing to tumor stroma development has been suggested (6, 12). In epithelial tumors, this appositional expression of PDGF-BB and its receptor has been documented in basal cell carcinoma of the skin (12), but also in breast (28), lung (29), and colorectal cancer (30). Here we report that human keratinocytes transfected with PDGF-B induce a highly proliferative and well-vascularized stroma after s.c. injection into nude mice. This is in concert with data of others obtained with melanoma cells (14). Moreover, we show that the stroma reaction was pronounced only within the first 2–6 weeks after s.c. injection, indicating that the mitogenic and chemotactic effect of PDGF-BB is transient. This was clearly not due to a decrease in expression of the transfected PDGF, since both mRNA and protein expression in tumors remained high during the entire 6-month observation period.
It has long been recognized that tumor stroma undergoes evolution with time, the changes closely resembling events during wound healing. While stroma matures in central parts of a tumor, new provisional stroma is continually deposited at the periphery, and in this sense tumors are considered wounds that do not heal (31). In the process of maturation cellularity and vascularity decrease, the resulting fibrous connective tissue closely resembling scar tissue of healed wounds (31, 32). PDGF-BB is known to accelerate wound healing, and its overactivity seems to be involved in the development of various fibrotic conditions, for example in hypertrophic scars and scleroderma of the skin (33, 34). Hypothetically, consistent with its function in wound healing, PDGF-BB is likely able to initiate, but not maintain the formation of a provisional stroma in the absence of other factors responsible for the persistent deposition of a provisional matrix and neovascularization (e.g., vascular endothelial growth factor, ref. 32). Overactivity of PDGF-BB would result in the formation of a poorly vascularized fibrous tissue, the typical stromal surroundings of a benign tumor. In this regard, benign tumors may be considered as “wounds that overheal.”
Consistent with our observation that PDGF can initiate but not maintain the process of tumor stroma formation, we found that angiogenesis triggered by HaCaT/PDGF-B cells was arrested at later stages (2–6 months) of tumor development, implying that PDGF-BB can initiate but not maintain angiogenesis. It has been recently reported that PDGF-receptors are expressed only on angiogenic, i.e., activated, endothelial cells in vitro (35, 36), indicating that the response to PDGF-BB depends on a specific microenvironment. Deposition of extracellular matrix components stimulated by PDGF-BB (6) may, in analogy to fibroblasts (37, 38), eventually render endothelial cells nonresponsive to PDGF and other angiogenic factors. In support of this idea we detected high levels of vascular endothelial growth factor in HaCaT/PDGF-B tumors but no expression of vascular endothelial growth factor receptor type 2 (flk-1) on endothelial cells (data not shown). As demonstrated recently vascular endothelial growth factor receptor 2 expression is essential for maintained angiogenesis and its down-regulation is associated with reduced vascular density in stroma of benign tumors (17).
Here we demonstrate a novel role of PDGF as an indirect tumor promoter functioning via activation of stromal cells, which in turn stimulate keratinocytes to continued proliferation and eventual tumor formation. This double paracrine mechanism demonstrated in the HaCaT model system may have significance for the carcinogenesis process in skin and other epithelia. The HaCaT cell line used in this study carries mutations characteristic of uv exposure in both alleles of the p53 tumor suppressor gene (39), as frequently detected in skin carcinomas (40). This is considered an initiating event in skin carcinogenesis (23). Nevertheless, long-term in vitro passaging of HaCaT cells carrying numerous genetic changes did not lead to a progression to tumorigenicity, indicating a stable nontumorigenic phenotype of these cells (15). However, activation of the host environment in vivo was sufficient to induce tumor promotion by the same cells, indicating the critical role of the cellular environment in determining the fate of genetically predisposed cells in carcinogenesis (reviewed in ref. 1). A large body of evidence demonstrates that host cells, especially fibroblasts, enhance tumor growth in experimental models (1, 4, 41). Beyond that, we show that an activated stromal environment alone can induce tumor formation by intrinsically nontumorigenic epithelial cells.
All immortalized nontumorigenic human cells proliferate indefinitely in vitro, however their growth is suppressed in vivo, indicating endogenous negative growth control. During progression tumor cells acquire increasing degrees of resistance to these inhibitory homeostatic growth influences exerted by adjacent normal cells (5). As demonstrated here, these growth constraints can be obliterated by continuous enhancement of keratinocyte proliferation through a paracrine mechanism. This may be achieved by the PDGF-stimulated production of epithelial cell mitogens such as insulin-like growth factor and keratinocyte growth factor in activated fibroblasts (24–26). In addition, activated fibroblasts may promote epithelial proliferation by the modulation of extracellular matrix and release of bound growth factors. Although rapidly proliferating cells are considered at greater risk for further conversion to malignancy, we could not perceive a single case of progression to malignancy after s.c. injection of the HaCaT/PDGF-B transfectants within a 6-month observation period. Moreover, direct retransplantation of a HaCaT/PDGF-B tumor into other nude mice and follow-up for another 6 months did not reveal progression to malignancy.
In summary, the present data add to the emerging picture that tumor and stromal cells interact via a complex humoral network resulting in reciprocal growth stimulation comparable to the situation in wound healing. Several reports alluded to the association between wounding and carcinogenesis in carcinogen-initiated and transgenic mouse skin (reviewed in ref. 42). Transgenic mice with targeted expression of activated Ha-ras oncogene (43) or TGF-α (44) in skin keratinocytes, develop papillomas only upon wounding. Since PDGF is likely to be present in large quantities in wounds (25) as well as in inflammatory conditions (6), we suggest that elevated levels of PDGF-BB in wounds and inflammatory diseases may promote tumor formation by an epigenetic mechanism and act as an endogenous promoter.
Acknowledgments
We thank Dr. Bengt Westermark (Uppsala, Sweden) for providing the PDGF-B expression vector, Dr. Lena Claesson-Welsh (Uppsala, Sweden) for providing cDNAs for PDGF receptors, and Heinrich Steinbauer for his expert technical assistance. We gratefully acknowledge the expert secretarial assistance of Martina Kegel and stylistic improvements by Brigitte Nagel-Plagens. These studies were in part supported by the European Commission Project AIR2-CT93-0860. M.S. was a recipient of a fellowship of the Boehringer Ingelheim Fonds.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PDGF, platelet-derived growth factor; PDGF-βR, PDGF receptor-β chain; HACaT, immortal nontumorigenic human keratinocytes.
References
- 1.Fidler I J. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 2.Fridman R, Giaccone G, Kanemoto T, Martin G R, Gazdar A F, Mulshine J L. Proc Natl Acad Sci USA. 1990;87:6698–6702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieweke M H, Bissell M J. Crit Rev Oncog. 1994;5:297–311. doi: 10.1615/critrevoncog.v5.i2-3.90. [DOI] [PubMed] [Google Scholar]
- 4.Gregoire M, Lieubeau B. Cancer Metastasis Rev. 1995;14:339–350. doi: 10.1007/BF00690602. [DOI] [PubMed] [Google Scholar]
- 5.Cornil J, Theodorescu D, Man S, Herlyn J, Jambrosic J, Kerbel R S. Proc Natl Acad Sci USA. 1991;88:6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heldin C-H, Westermark B. In: The Molecular and Cellular Biology of Wound Repair. Clark R A F, editor. New York: Plenum; 1996. pp. 249–273. [Google Scholar]
- 7.Reuterdahl C, Sundberg C, Rubin K, Funa K, Gerdin B. J Clin Invest. 1993;91:2065–2075. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terracio L, Rönnstrand L, Tingström A, Rubin K, Claesson-Welsh L, Funa K, Heldin C-H. J Cell Biol. 1988;107:1947–1957. doi: 10.1083/jcb.107.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniades H N, Galanopoulos T, Neville-Golden J, Kiritsy C P, Lynch S E. Proc Natl Acad Sci USA. 1991;88:565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peres R, Betsholtz C, Westermark B, Heldin C-H. Cancer Res. 1987;47:3425–3429. [PubMed] [Google Scholar]
- 11.Bronzert D A, Pantazis P, Antoniades H N, Kasid A, Davidson N, Dickson R B, Lippman M E. Proc Natl Acad Sci USA. 1987;84:5763–5767. doi: 10.1073/pnas.84.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponten F, Ren Z, Nister M, Westermark B, Ponten J. J Invest Dermatol. 1994;102:304–309. doi: 10.1111/1523-1747.ep12371787. [DOI] [PubMed] [Google Scholar]
- 13.Westermark B, Heldin C-H. Cancer Res. 1991;51:5087–5092. [PubMed] [Google Scholar]
- 14.Forsberg K, Valyi-Nagy I, Heldin C-H, Herlyn M, Westermark B. Proc Natl Acad Sci USA. 1993;90:393–397. doi: 10.1073/pnas.90.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boukamp P, Popp S, Altmeyer S, Hülsen A, Fasching C, Cremer T, Fusenig N E. Genes Chromosomes Cancer. 1997;19:201–214. doi: 10.1002/(sici)1098-2264(199708)19:4<201::aid-gcc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Claesson-Welsh L, Eriksson A, Westermark B, Heldin C-H. Proc Natl Acad Sci USA. 1989;86:4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig N E. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 18.Boukamp P, Petrusevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boukamp P, Stanbridge E J, Foo D Y, Cerutti P A, Fusenig N E. Cancer Res. 1990;50:2840–2847. [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Moorman A F M, De-Boer P A J, Vermeulen J L M, Lamers W H. Histochem J. 1992;25:251–266. doi: 10.1007/BF00159117. [DOI] [PubMed] [Google Scholar]
- 22.Rao C D, Igarashi H, Chiu I M, Robbins K C, Aaronson S A. Proc Natl Acad Sci USA. 1986;83:2392–2396. doi: 10.1073/pnas.83.8.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boukamp P, Peter W, Pascheberg U, Altmeier S, Fasching C, Stanbridge E J, Fusenig N E. Oncogene. 1995;11:961–969. [PubMed] [Google Scholar]
- 24.Cullen K J, Smith H S, Hill S, Rosen N, Lippman M E. Cancer Res. 1991;51:4978–4985. [PubMed] [Google Scholar]
- 25.Clemmons D R. J Endocrinol Metab. 1984;58:850–856. doi: 10.1210/jcem-58-5-850. [DOI] [PubMed] [Google Scholar]
- 26.Brauchle M, Angermeyer K, Hübner G, Werner S. Oncogene. 1994;9:3199–3204. [PubMed] [Google Scholar]
- 27.LaRochelle W J, May-Siroff M, Robbins K C, Aaronson S A. Genes Dev. 1991;5:1191–1199. doi: 10.1101/gad.5.7.1191. [DOI] [PubMed] [Google Scholar]
- 28.Coltrera M D, Wang J, Porter P L, Gown A M. Cancer Res. 1995;55:2703–2708. [PubMed] [Google Scholar]
- 29.Vignaud J M, Marie B, Klein N, Plenat F, Pech M, Borrelly J, Martinet N, Duprez A, Martinet Y. Cancer Res. 1994;54:5455–5463. [PubMed] [Google Scholar]
- 30.Hsu S, Huang F, Friedman E. J Cell Physiol. 1995;165:239–245. doi: 10.1002/jcp.1041650204. [DOI] [PubMed] [Google Scholar]
- 31.Dvorak H F. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 32.Yeo T-K, Dvorak H F. In: Diagnostic Immunopathology. Colvin R B, Bhan A K, McCluskey R T, editors. New York: Raven; 1995. pp. 685–697. [Google Scholar]
- 33.Haisa M, Okochi H, Grotendorst G R. J Invest Dermatol. 1994;103:560–563. doi: 10.1111/1523-1747.ep12396856. [DOI] [PubMed] [Google Scholar]
- 34.Yamakage A, Kikuchi K, Smith E A, LeRoy E C, Trojanowska M. J Exp Med. 1992;175:1227–1234. doi: 10.1084/jem.175.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battegay E J, Rupp J, Iruela-Arispe L, Sage E H, Pech M. J Cell Biol. 1994;125:917–928. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marx M, Perlmutter R A, Madri J A. J Clin Invest. 1994;93:131–139. doi: 10.1172/JCI116936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Clark R A. J Cell Biol. 1996;132:239–249. doi: 10.1083/jcb.132.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y-C, Grinnell F. J Cell Biol. 1993;122:663–672. doi: 10.1083/jcb.122.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehman T A, Rama M, Boukamp P, Stanek J, Bennett W P, Welsh J A, Metcalf R A, Stampfer M R, Fusenig N E, Rogan E M, Reddel R, Harriss C C. Oncogene. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler A, Jonason A S, Leffel D J, Simon J A, Sharma H W, Kimmelman J, Remington L, Jacks T, Brash D E. Nature (London) 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 41.Camps J L, Chang S-M, Hsu T C, Freeman M R, Hong S-J, Zhau H E, von Eschenbach A C, Leyland W K. Proc Natl Acad Sci USA. 1990;87:75–79. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arbeit J M. Cancer Surv. 1996;26:7–34. [PubMed] [Google Scholar]
- 43.Bailleul B, Surani M A, White S, Barton S C, Brown K, Blessing M, Jorcano J, Balmain A. Cell. 1990;62:697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- 44.Vassar R, Fuchs E. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]